Introduction
Several decades ago, Bernardo Ochoa1 described a rare but potentially devastating inherited disease that is now called urofacial, or Ochoa, syndrome (UFS).2 UFS is characterized by 2 features. First, a so-called “non-neurogenic neurogenic” dyssynergic bladder in which functional bladder outflow obstruction causes incomplete voiding, vesicoureteric reflux (VUR), ascending urosepsis, pyelonephritis, and renal failure. Second, a grimace where the corners of the mouth become downturned on smiling, so that the face appears to be crying. Severe constipation is reported in approximately two-thirds of cases.1 Ochoa’s1 cohort was from Colombia, but UFS has subsequently been reported worldwide and cases totaled at least 150.2 UFS is an autosomal recessive disorder and most cases are caused by biallelic pathogenic variants in either of 2 genes: HPSE2 (Mendelian Inheritance in Man (MIM) 613469)3, 4, 5 encoding heparanase-2, which inhibits the enzymatic activity of classical heparanase, or LRIG2 (MIM 615112)6 encoding leucine-rich repeats and Ig-like domains, a protein that may modulate growth factor signaling. Heparanase 2 and LRIG2 proteins are present in pelvic ganglia and in bladder autonomic nerves emanating from these ganglia,5, 6, 7 and the patterns of bladder nerves are abnormal in mice carrying biallelic variants in either Hpse2 or Lrig2.5, 6, 7 Thus, UFS features a peripheral neuropathy affecting the bladder, whereas the cause of the grimace requires further study.
A minority of people with an apparent clinical diagnosis of UFS do not carry variants in HPSE2 or LRIG2.5, 6, 7 The current report draws attention to a different genetic syndrome, which can mimic UFS and be associated with renal failure. We describe one such individual with a heterozygous missense predicted pathogenic variant in early B-cell factor 3 (EBF3), a gene encoding a transcription factor and associated with hypotonia, ataxia, and developmental delay syndrome (HADDS; MIM 617330).8,9,S1 We proceeded to seek variants in EBF3 in a UK cohort with familial primary nonsyndromic VURS2 and reviewed the published literature of HADDS to determine whether other such individuals had renal tract disease.
Case Presentation
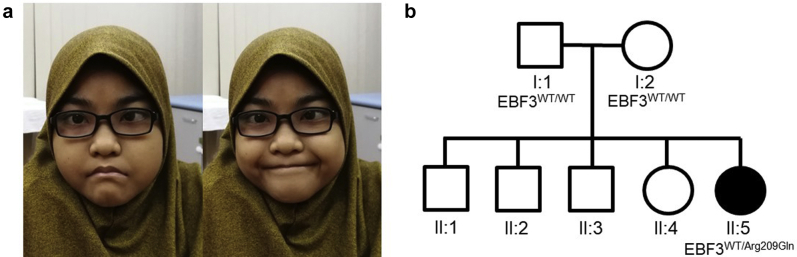
A 3-year-old Malaysian girl was assigned a presumptive clinical diagnosis of UFS. Antenatal history of the proband was unremarkable but, after a normal delivery, she developed sepsis neonatally followed by recurrent urinary tract infections throughout her childhood. In the first year of life, investigations demonstrated a neurogenic bladder (in the absence of spinal cord damage) with a thickened wall, bilateral VUR, and bilateral hydroureter and hydronephrosis, the latter persisting on scans throughout childhood. When her face was at rest, the ends of her mouth were downturned and they failed to become upturned on smiling (Figure 1a), so producing an “abnormal horizontal smile” rather than the classical UFS grimace that features a downturned mouth.
Figure 1.
Face and family tree of the index case. (a) Proband face at rest (left frame) and the horizontal smile (right frame). (b) Pedigree showing affected proband with clinically unaffected mother, father, and elder siblings. Only the index case, II:5, carried the EBF3 p.(Arg209Gln) variant.
The abnormal smile became less obvious as she grew up, perhaps due to improving myopathic face. She also suffered from constipation through childhood. Although the combination of renal tract disease, constipation, and abnormal smile were broadly compatible with UFS, other features were present that were atypical for UFS, including severe short stature and microcephaly; a deep philtrum, broad chin with midline cleft, and high arched palate; hypotonia with global developmental delay, being unable to walk independently and speak more than 2 words aged 3; tapering digits and fusion between the second and third toes; and a ventricular septal defect and patent ductus arteriosus that resolved spontaneously. Her brain magnetic resonance imaging scan was normal. These additional clinical features raised the possibility of a blended phenotype of UFS and another diagnosis co-occurring in the same individualS3 or of an alternative diagnosis with clinical overlap with UFS.
She died aged 17 years, after a brief illness featuring metabolic acidosis and acute kidney injury, with a plasma creatinine of 687 μM (7.77 mg/100 ml), treated with hemodialysis; blood cultures were negative and her urine showed a mixed growth of gram-negative rods.
Genetic Analyses of the Family
Parental consent was obtained for genetic analyses. Sanger sequencing of HPSE2 and LRIG2 exons failed to identify relevant pathogenic variants in these genes. Whole exome sequencing of the proband was then undertaken (see Supplementary Methods and Supplementary Figure S1), and no further variants were identified in other genes (BCN2, CHRM3, the smooth muscle genes ACTA2, ACTG2, MYH11, MYLK, MYL9, LMOD1, or MYOCD) implicated in congenital bladder outflow obstruction.S4–S6 Instead, we identified a heterozygous missense variant c.626G>A, p.(Arg209Gln) in EBF3 predicted to cause a missense change in a DNA-binding domain of the encoded transcription factor protein. This variant had been reported previously in an individual with HADDS,5, 6, 7 and it was absent from gnomAD control databases.S7 Sanger sequencing of parental DNA confirmed variant c.626G>A was de novo, being absent in the clinically unaffected nonconsanguineous parents (Figure 1b). The proband’s 4 siblings were also assessed as clinically unaffected, and so they were not tested for the variant. Of 8 in silico prediction tools used to consider the possible pathogenicity of p.(Arg209Gln), all but 1 predicted that the variant was deleterious (Supplementary Table S1).
Sequencing EBF3 Exons in a Cohort With Primary Nonsyndromic VUR
A previous linkage study using patient cohorts from the United Kingdom, the Republic of Ireland, and Slovenia indicated a susceptibility locus for familial primary nonsyndromic VUR on chromosome 10q26.S8 Given that EBF3 is located at the edge of the 9 Mb region, we hypothesized that variants in EBF3 may be enriched in such cases. Accordingly, we undertook Sanger sequencing of EBF3 exons in 80 individuals randomly selected from the UK cohortS2 but did not identify any predicted deleterious variants (Supplementary Table S2).
Discussion
Heterozygous variants in EBF3 result in the rare developmental disorder, HADDS.S9 The clinical features vary among individuals, and a subset of cases has urinary tract defects and facial features, including downturned corners of the mouth. Of the 30 cases with pathogenic EBF3 variants recorded in the literature,8,9,S1,S9–S11 10 (33%) were reported to have structural or functional urinary tract defects, including neurogenic bladder and VUR (Table 1). Given that VUR can be asymptomatic, and that it was not evident that all individuals with variants in EBF3 reported in the literature had undergone urinary tract investigations, this proportion may be an underestimate. Indeed, the proportion rises to 40% when cases with recurrent urinary tract infections are included (Table 1), and whether these additional cases have functional or structural defects of the urinary tract requires further investigation. Of these individuals with EBF3 variants and urinary tract disease, a subset had facial features (Table 1) that we believe, as in the current case, could have led to confusion with UFS. On the other hand, UFS disease is confined to the urinary tract and the face, whereas EBF3 variants resulting in HADDS can feature developmental delay and more widespread dysmorphology (Table 2), as evidenced by the current proband.
Table 1.
Pathogenic EBF3 variants associated with the renal tract and/or smiling, as reported in this study and in the published literature
| EBF3 coding variant | EBF3 protein variant | Renal tract anomaly | Facial features relevant to smiling | Reference |
|---|---|---|---|---|
| c.280_283del | p.(Glu94Lysfs∗37) | Neurogenic bladder, VUR, recurrent UTIs | Not recorded | Sleven et al.S1 |
| c.469_477dup | p.(His157_Ile159dup) | Recurrent UTIs | Not recorded | Harms et al.9 |
| c.471C>A | p.(His157Gln) | Neurogenic bladder, VUR | Not recorded | Tanaka et al.8 |
| c.487C>T | p.(Arg163Trp) | Atonic bladder, urethral stricture, bilateral VUR and hydronephrosis, recurrent UTIs | Downturned corners of the mouth | Blackburn et al.S9 |
| c.488G>A | p.(Arg163Gln) | Impaired bladder control | Triangular-shaped facies | Chao et al.S10 |
| c.488G>T | p.(Arg163Leu) | Incomplete bladder emptying, VUR | Triangular-shaped facies | Chao et al.S10 |
| c.512G>A | p.(Gly171Asp) | Neurogenic bladder, bilateral VUR | Facial asymmetry | Harms et al.9 |
| c.554+1G>T | - | VUR, recurrent UTIs, renal dysplasia | Normal | Sleven et al.S1 |
| c.579G>T | p.(Lys193Asn) | VUR | Downturned corners of the mouth, minimal facial expression | Sleven et al.S1 |
| c.616C>T | p.(Arg206∗) | Hydronephrosis, recurrent UTIs | Not recorded | Tanaka et al.8 |
| c.626G>A | p.(Arg209Gln) | Normal | Lack of social smile | Tanaka et al.8 |
| c.626G>A | p.(Arg209Gln) | Neurogenic bladder with bilateral VUR and hydronephrosis, recurrent UTIs | Abnormal horizontal smile | Proband in the current study |
| c.1402_1414del13 | p.(Thr464Profs∗10) | Recurrent UTIs | Not recorded | Tanaka et al.8 |
UTI, urinary tract infection; VUR, vesicoureteric reflux.
Each row represents a single nonrelated case. The renal tract features are as described in the original publications. In addition, facial features relevant to smiling are indicated.
Table 2.
Key teaching points
|
|
|
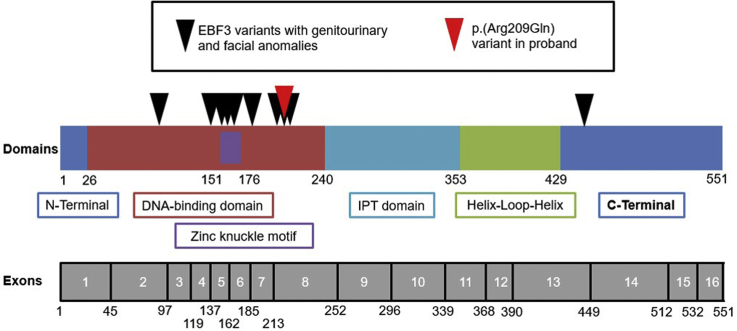
All but one of the known pathogenic variants in EBF3 associated with urinary tract disease, fall within the DNA-binding domain of EBF3, as does the variant identified in our proband (Figure 2). Variants in the DNA-binding domain, and more specifically the zinc knuckle motif, have previously been associated with a reduction in EBF3 binding affinity for DNA,9 and are therefore likely to alter downstream gene expression, compromising normal development.
Figure 2.
Domains and exons of the transcription factor protein encoded by EBF3. Numbers correspond to amino acids. Red arrowhead indicates the variant in the current proband. Black arrowheads point to positions of published variants associated with renal tract and facial anomalies, as detailed in Table 1. Zinc knuckle motif is contained within the DNA-binding domain IPT (Ig-like/plexins/transcription factor domain).
As outlined in the introduction, HPSE2 and LRIG2, the genes altered in UFS, are expressed in bladder autonomic nerves, and pathogenic variants in mutant mice lead to abnormal patterns of nerves in the bladder outflow tract and the organ’s body.5, 6, 7 Thus, UFS features a bladder peripheral neuropathy that correlates with the functional outflow obstruction in this disease. Future studies are required to understand how EBF3 affects the biology of the urinary tract. We note, however, that in the GUDMAP gene expression database,S12,S13 Ebf3 transcripts are detected in the embryonic mouse bladder and urethral region, and in the urorectal septum of the nascent organ (https://www.gudmap.org/id/Q-48NJ@2T7-SRMW-5H12). It is currently unclear whether they are expressed in particular tissue compartments such as the nerves, muscle, or urothelium.
Aristaless-Related Homeobox (ARX) is an upstream translational repressor of EBF3,S10,S14,S15 and ARX variants have been linked to a wide variety of developmental disorders, including epileptic encephalopathy (MIM 308350), Partington X-linked mental retardation syndrome (MIM 309510), and Proud syndrome (MIM 300004).5, 6, 7,S16,S17 There is overlap in the range of phenotypes of individuals with ARX variants and those with EBF3 variants. We speculate that the ARX-EBF3 interaction may lie upstream of HPSE2 and LRIG2, which are directly implicated in the pathobiology of UFS. Perhaps yet other genes associated with UFS-like disease lie downstream of the ARX-EBF3 pathway, likely with a restricted pattern of expression within key neurological pathways involved in innervation of bladder and facial muscle.
As recently reviewed,S4 the several decades search for genes implicated in primary nonsyndromic VUR, a common condition affecting at least 1% of infants, has yet to yield definitive answers, apart from TNXB1 in a small subset of familial cases.S18 The condition is most likely genetically heterogeneous, but parametric analyses of 460 affected families comprising 1062 affected individuals showed linkage to rs7907300 located at chromosome 10q24.S8 EBF3 is 1 of 69 genes that fall within the wider 9 Mb region surrounding rs7907300, making EBF3 a plausible candidate for conferring VUR susceptibility. Nevertheless, we failed to detect likely pathogenic variants in EBF3 in 80 index cases from the UK, themselves comprising approximately half of the total group analyzed in the 10q24 linkage study. Previously, we had analyzed the UK cohort by sequencing HPSE25, 6, 7 and LRIG2,5, 6, 7 with similar negative results. However, it should be noted that patients with VUR associated with neuropathic-type bladders were excluded from this particular collection.S2 In contrast, we have identified potentially pathogenic biallelic LRIG2 variants in 3 individuals with both severe bladder voiding dysfunction and renal failure.5, 6, 7,5, 6, 7 Thus, we suggest that the latter population may be enriched for individuals with Mendelian genetic causes of their urinary tract disease.
Conclusion
The association of urinary tract disease, especially a neuropathic bladder and VUR, with an abnormal smile should alert clinicians to the possibility of not only UFS, but also HADDS (Table 2). These insights have implications for genetic counseling given that UFS is autosomal recessive, whereas HADDS is a dominantly inherited disease. HPSE2 and LRIG2, the genes implicated in UFS, pattern bladder nerves. The mechanism whereby EBF3 affects urinary tract biology requires further study (Table 2).
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank the family for consent to publish the proband’s photo. We thank the UK VUR Study Group for providing index cases with primary nonsyndromic VUR. We acknowledge grant support from: the Wellcome Trust (PhD studentship to JRH); Kidney Research UK (project grant Paed_RP_002_20190925 to WGN and ASW); the Medical Research Council (project grant MR/L002744/1 to ASW and WGN, and MR/T016809/1 to ASW); Newlife Foundation (project grants 15-15/03 and 15-16/06 to WGN and ASW); the NIHR Academic Lecturer scheme (HMS); the Academy of Medical Sciences (HMS); and the Manchester NIHR BRC (IS-BRC-1215-20007 to WGN).
Footnotes
Supplementary Methods.
Supplementary References.
Table S1. In silico tools interrogating the possible pathogenicity of the EBF3 variant c.626G>A found in the index case.
Table S2. Variants detected in EBF3 sequencing of 80 index cases of the UK VUR cohort.
Figure S1. Filtering strategy used for whole exome sequencing data analysis.
Supplementary Material
References
- 1.Ochoa B. Can a congenital dysfunctional bladder be diagnosed from a smile? The Ochoa syndrome updated. Pediatr Nephrol. 2004;19:6–12. doi: 10.1007/s00467-003-1291-1. [DOI] [PubMed] [Google Scholar]
- 2.Newman W.G., Woolf A.S. Urofacial syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews®. University of Washington, Seattle; Seattle (WA): 1993. http://www.ncbi.nlm.nih.gov/books/NBK154138/ Available at: [Google Scholar]
- 3.Daly S.B., Urquhart J.E., Hilton E. Mutations in HPSE2 cause urofacial syndrome. Am J Hum Genet. 2010;86:963–969. doi: 10.1016/j.ajhg.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang J., Zhang S., Yang P. Loss-of-function mutations in HPSE2 cause the autosomal recessive urofacial syndrome. Am J Hum Genet. 2010;86:957–962. doi: 10.1016/j.ajhg.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuart H.M., Roberts N.A., Hilton E.N. Urinary tract effects of HPSE2 mutations. J Am Soc Nephrol. 2015;26:797–804. doi: 10.1681/ASN.2013090961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuart H.M., Roberts N.A., Burgu B. LRIG2 mutations cause urofacial syndrome. Am J Hum Genet. 2013;92:259–264. doi: 10.1016/j.ajhg.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts N.A., Hilton E.N., Lopes F.M. Lrig2 and Hpse2, mutated in urofacial syndrome, pattern nerves in the urinary bladder. Kidney Int. 2019;95:1138–1152. doi: 10.1016/j.kint.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka A.J., Cho M.T., Willaert R. De novo variants in EBF3 are associated with hypotonia, developmental delay, intellectual disability, and autism. Cold Spring Harb Mol Case Stud. 2017;3:a002097. doi: 10.1101/mcs.a002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harms F.L., Girisha K.M., Hardigan A.A. Mutations in EBF3 disturb transcriptional profiles and cause intellectual disability, ataxia, and facial dysmorphism. Am J Hum Genet. 2017;100:117–127. doi: 10.1016/j.ajhg.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.