Abstract
Social partners tend to coordinate their behaviors in time. This “interactional synchrony” is associated with a host of positive social outcomes, making it ripe for study in autism spectrum disorder (ASD). Twenty children with ASD and 17 typically developing (TD) children participated in conversations with familiar and unfamiliar adults. Conversations were rated for movement synchrony and verbal synchrony, and mothers completed measures regarding children’s everyday social and communication skills. Children with ASD exhibited less interactional synchrony, with familiar and unfamiliar partners, than TD peers. Beyond group-level differences, interactional synchrony negatively correlated with autism symptom severity, and predicted dimensional scores on social and communication measures. Results suggest that disrupted interactional synchrony may be associated with impaired social functioning in ASD.
Keywords: Interactional synchrony, social reciprocity, nonverbal communication, movement, verbal communication
During social interaction, partners unconsciously coordinate their behaviors with one another across communicative modalities. This includes interpersonal matching of discrete behaviors, such as facial expressions and body movements (Chartrand & Lakin, 2013). It also includes the phenomenon of interactional synchrony, a dynamic process in which the timing of partners’ behaviors becomes coordinated (Bernieri & Rosenthal, 1991). Social coordination has been linked to increased rapport (Chartrand & Bargh, 1999; Hove & Risen, 2009; Ramseyer & Tschacher, 2011), cooperation (Wiltermuth & Heath, 2009), empathy (Sonnby-Borgström, Jönsson, & Svensson, 2003; Stel & Vonk, 2010), social cognition (Miles, Nind, Henderson, & Macrae, 2010), and smoother conversation (Chartrand & Bargh, 1999; Garrod & Pickering, 2004; Stel & Vonk, 2010). The instinct to coordinate may therefore be adaptive, playing a critical role in positive social interaction (Lakin, Jefferis, Cheng, & Chartrand, 2003). Interactional synchrony has also been implicated as fundamental for normative social development (Feldman, 2007; Harrist & Waugh, 2002). From early infancy, children and caregivers mutually coordinate movements and vocalizations in time (Condon & Sander, 1974; Jaffe, Beebe, Feldstein, Crown, & Jasnow, 2001). Thus, children appear to be attuned to temporal coordination in their social environments, and to naturally organize their own behavior accordingly.
Social partners’ inclination to synchronize with one another may help explain why neurotypical individuals are often able to achieve reciprocal social exchanges without much conscious effort. In turn, if natural interpersonal contingencies become disrupted within an interaction (e.g., in individuals with social communication difficulties), partners may feel less connected and the interaction may feel more challenging. Consider the experience of participating in a video call with a poor internet connection. Delays and non-contingencies between communication efforts result in interactions that feel more difficult or awkward. Similarly, a failure to engage in interactional synchrony during everyday interactions may have deleterious effects on social connectedness, understanding of others’ communicative cues, and relationship quality.
The association between interactional synchrony and social functioning makes it ripe for investigation in conditions characterized by social psychopathology, such as autism spectrum disorder (ASD). ASD is a neurodevelopmental disorder defined by core impairments in social interaction and communication, as well as patterns of restricted and/or repetitive behaviors (American Psychiatric Association, 2013). One feature of ASD is deficits in establishing and maintaining social-emotional reciprocity, often in both verbal and nonverbal domains. Verbally, individuals with ASD have difficulty with back-and-forth conversation in which partners are equal participants. Nonverbally, they have difficulty reciprocally using and responding to facial and body cues (American Psychiatric Association, 2013). The implicit social contingencies afforded by interactional synchrony may underlie social-emotional reciprocity in neurotypical individuals. In turn, quantifying atypicality in this process may offer a lens into fundamental reciprocity impairments in ASD.
Research has begun to document atypical interactional synchrony in ASD. Several studies using structured (i.e., non-naturalistic) social motor synchrony tasks (e.g., where partners simultaneously rock in chairs or swing pendulums) suggest that children with ASD are less likely than typically developing (TD) peers to coordinate movements in time with caregivers (Fitzpatrick et al., 2016, 2018; Marsh et al., 2013) and experimenters (Fitzpatrick et al., 2013, 2017a). This research has also begun to link reduced social motor synchrony in ASD to poorer social skills, including joint attention and theory of mind (Fitzpatrick et al., 2017b). However, there is a paucity of literature on interactional synchrony in ASD during natural conversations, which hinders understanding of how previous results generalize to real-world social functioning. There is some evidence that adolescents with ASD fail to coordinate temporal patterns of speech and silences with conversation partners in the same way as TD children (Feldstein, Konstantareas, Oxman, & Webster, 1982). Similarly, there is evidence that the contingent patterning of vocal interactions over time between young children with ASD and adults is disrupted relative to young TD children (Warlaumont, Richards, Gilkerson, & Oller, 2014). Nonverbally, one study using automated video analysis has suggested that children with ASD achieve higher than chance levels of body movement synchrony during unstructured conversations, but that lower synchrony is associated with more severe ASD symptoms in the domain of restricted behavior (Romero et al., 2018).
Further characterization of interactional synchrony and its relationship to social functioning, in children with and without ASD, can inform understanding of the role of this phenomenon in typical and atypical development. Herein, our goal is to critically expand upon prior research by quantifying interactional synchrony within natural conversations, and across different communication modalities. We also expand on previous research by examining the influence of familiarity among conversation partners on interactional synchrony. Previous studies with neurotypical individuals suggest that synchrony is higher among familiar dyads or those with pre-existing rapport, compared to unfamiliar dyads (Latif, Barbosa, Vatiokiotis-Bateson, Castelhano, & Munhall, 2014; McIntosh, 2006). In contrast, in ASD, Feldstein and colleagues (1982) found that youth with ASD failed to achieve synchrony in the timing of their speech cues with both their parents and unfamiliar adults.
Several approaches for quantifying interactional synchrony during conversations have been widely used with neurotypical populations, including micro-coding of discrete behaviors (e.g., Condon & Ogston, 1967), computational analysis from videos or sensors (see Delaherche et al., 2012), and behavioral rating or macro-coding approaches (e.g., Bernieri, Reznick, & Rosenthal, 1988). We used a behavioral rating approach in the current study, as we were specifically interested in whether there are differences in interactional synchrony in children with ASD that are perceivable to observers, even when unaware of children’s diagnostic status. By leveraging human perceptions, we were able to study whether differences in interactional synchrony are a salient aspect of how social interactions of children with ASD are experienced by others. Moreover, while behavioral rating approaches are less granular and objective than other more recent methods (see Schmidt & Fitzpatrick, 2016), they focus directly on the social dyad as a system, rather than on specific individual behaviors, allowing for a more qualitative, gestalt measure of an interaction as opposed to simple statistical co-occurrence.
Verbal and nonverbal (i.e., movement) synchrony were rated in children with and without ASD, as they engaged in two conversations: one with a familiar adult and one with an unfamiliar adult. We then examined relationships between interactional synchrony and standardized measures of social and communication skills, in order to investigate the role of interactional synchrony in broader, day-to-day social functioning. We predicted that children with ASD would exhibit reduced interactional synchrony relative to TD peers in both verbal and nonverbal domains. Based on previous literature, we further predicted that children with ASD would exhibit similar degrees of interactional synchrony with familiar and unfamiliar partners, while TD children would exhibit higher interactional synchrony with familiar partners relative to unfamiliar partners. Across both groups, we predicted that higher ratings of interactional synchrony would be significantly associated with better scores on standardized social and communication measures.
METHODS
Participants
Participants were 37 children aged 9–16, including 20 children with ASD and 17 TD children (see Table 1 for participant characterization). Participants were recruited largely through their participation in a larger ongoing study in our laboratory, or through our laboratory’s recruitment database of previous participants who agreed to be re-contacted for future studies. All children in the ASD group met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5; American Psychiatric Association, 2013) criteria for ASD, and met diagnostic cutoffs on the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al., 2012) and Autism Diagnostic Interview – Revised (Rutter, Le Couteur, & Lord, 2003). Diagnostic testing was also conducted with the TD group to confirm that they did not have ASD; all TD children failed to meet ASD criteria on the ADOS-2 and Social Communication Questionnaire, Lifetime Form (SCQ; Rutter, Bailey, & Lord, 2003), as well as per clinician judgment based on DSM-5.
Table 1.
Participant Characterization
| ASD M (SD) Range |
TD M (SD) Range |
t or χ2 | p | |
|---|---|---|---|---|
| n | 20 | 17 | ||
| Gender (M:F) | 19:1 | 15:2 | 0.56 | .45 |
| Age (years) | 13.80 (1.38) 11–16 |
14.32 (2.02) 9–16 |
−0.93 | .36 |
| Full Scale IQ | 108.50 (14.15) 84–133 |
114.71 (12.68) 84–136 |
−1.39 | .17 |
| Verbal IQ | 110.05 (17.58) 78–138 |
116.76 (13.62) 87–132 |
−1.28 | .21 |
| Nonverbal IQ | 105.20 (11.92) 78–122 |
110.06 (13.93) 83–138 |
−1.14 | .26 |
| ADOS-2 Calibrated Severity Score | 6.45 (1.54) 4–9 |
1.0 (0.00) 1–1 |
14.58 | < .001 |
| SCQ Score | 21.90 (6.94) 6–35 |
1.47 (1.63) 0–7 |
11.85 | < .001 |
| SSIS-RS Total Social Skills Score | 83.20 (12.30) 55–110 |
105.12 (13.62) 70–122 |
−5.14 | < .001 |
| CCC-2 General Communication Composite | 82.50 (12.05) 60–116 |
111.29 (6.54) 100–121 |
−8.80 | < .001 |
Note: ASD: autism spectrum disorder; TD: typically developing; ADOS-2: Autism Diagnostic Observation Schedule, Second Edition; SCQ: Social Communication Questionnaire; SSIS-RS: Social Skills Improvement System – Rating Scales; CCC-2: Children’s Communication Checklist, Second Edition.
Participants in both groups had fluent English language and Full Scale IQs of 80 or above on the Wechsler Abbreviated Scale of Intelligence, Second Edition (Wechsler, 2011) or corresponding short form of the Wechsler Intelligence Scale for Children, Fourth Edition (Wechsler, 2003). Exclusion criteria for both groups included uncorrected vision, hearing, or motor impairments of a degree that would impact a conversational task, and/or current diagnosis or diagnostic history of a tic disorder, neurological disorder or abnormality, or other disorder or injury affecting facial or body motility. TD children also did not have a first or second degree relative with ASD per caregiver report.
Groups were matched on mean chronological age, mean verbal and nonverbal cognitive ability, and gender ratio. Due to equipment failure, the unfamiliar interaction was not recorded for one participant with ASD, excluding him from some analyses. Group matching was not affected by this exclusion.
The biological mother of each child also participated as familiar interaction partner. Clinically significant ASD symptoms were ruled out in mothers with the Adult Self-Report Form of the Social Responsiveness Scale, Second Edition (SRS-2; Constantino & Gruber, 2012); all mothers fell within the normative range (Total T-score < 60).
Visit Procedures
This research was prospectively reviewed and approved by the University of Rochester Research Subjects Review Board. Written informed consent was obtained from mothers for their child’s and own participation. All children provided written or verbal assent.
Children participated in two 10-minute conversations, the first with their mother (familiar) and the second with a female research assistant (RA; unfamiliar). Mother-child interactions always took place prior to RA interactions, because RA-child interactions were less likely to be biased by mother-child interactions than vice-versa, given that children and mothers are already accustomed to having natural conversations with one another. Three different RAs served as unfamiliar interaction partners. All were Caucasian females in their early twenties, with similar levels of experience interacting with children with ASD through their participation in research studies in the lab. RAs were not given any specific conversational guidelines related to this task, and instead were simply instructed to converse naturally. They were made aware that some of the children they would interact with would have ASD and some would not. RAs were assigned to visits by another RA who was responsible for screening and scheduling families. Each RA interacted with an equal number of participants over the course of the study, and there was no statistically significant effect of assigned RA on either movement or verbal synchrony.
Conversation partners were seated facing one another four feet apart and asked to collaboratively plan a two-week vacation, a modification of a task that has been used to measure interactional synchrony in adolescents (Bernieri, Davis, Rosenthal, & Knee, 1994). To keep the second conversation somewhat novel, one dyad was instructed to plan a warm-weather vacation and the other to plan a cold-weather vacation. Assignment of vacation type by dyad was randomized, with equal representation across groups. The task was intended to be positive and cooperative, as the goal was to elicit a two-way conversation rather than to successfully plan the vacation. Thus, to alleviate potential anxiety or executive functioning burden, dyads were told to have fun and not to worry about logistical (e.g., monetary) constraints. Additionally, a printed list of prompts for discussion was provided on a table beside the interaction partners, in case they needed additional ideas for conversation topics (e.g., what clothes to bring, what activities to plan). All children actively participated in their two conversations and completed both tasks in full without evident difficulty. Interactions were recorded with a wall-mounted, high-definition digital video camera, positioned to capture a full-body side view of both partners.
Mothers completed two standardized rating scales regarding children’s daily functioning. The Parent form of the Social Skills Improvement System-Rating Scales (SSIS-RS; Gresham & Elliot, 2008) assessed general social skills. The SSIS-RS is a widely used and validated assessment targeting broad social skills existing on a continuum across all children; that is, it measures general social competence as a separate construct from ASD-related social symptoms. The Children’s Communication Checklist, Second Edition (CCC-2; Bishop, 2006) assessed dimensional general communication and pragmatic language skills. See Table 1 for group-level SSIS-RS and CCC-2 scores.
Rating Procedures
Video recordings were rated for interactional synchrony by three female RAs. Raters were blind to diagnostic status and were not the same RAs who served as unfamiliar interaction partners. Raters were trained through review of a manual developed for this project, followed by consensus rating of pilot participants. Because we were interested in capturing perceptions of interactional synchrony across observers, all three raters rated every interaction. Therefore, the purpose of the training was to ensure that raters understood the procedures and scales, rather than to achieve an a priori interrater reliability threshold. These procedures are consistent with previous work measuring human perceptions of interactional synchrony (Bernieri et al., 1988; 1994), in which raters were oriented to the rating scheme but intentionally not trained extensively on how to assign ratings, in order to capture more gestalt perceptions.
Interactions were rated for two types of interactional synchrony: movement synchrony and verbal synchrony. Movement synchrony was rated using a system of four ratings developed by Bernieri and colleagues, which focuses specifically on the form and timing of movements. This has been the most consistently used human judgment approach for measuring nonverbal interactional synchrony, shown to be valid and reliable through studies of infants, adolescents, and adults (Bernieri, 1988; Bernieri et al., 1994, 1988; Cappella, 1997; Kimura & Daibo, 2006). Verbal synchrony was rated using two ratings from the developmental synchrony literature that focus on qualities of verbal reciprocity (Lindsey, Colwell, Frabutt, Chambers, & MacKinnon-Lewis, 2008; Melby & Conger, 2001). Rating descriptions with examples, exactly as provided to raters, are provided in Table 2.
Table 2.
Interactional Synchrony Ratings
| Rating | Description | |
|---|---|---|
| Movement Synchrony | Simultaneous Movement1 | Degree to which partners engage in simultaneous movement; quantity and/or degree of movement that appears to begin or end at the same moment. For example, if a mother begins to turn her head at the precise moment that a child lifts an arm, it is an instance of simultaneous movement. (1–9 scale) |
| Tempo Similarity1 | Degree to which partners have similar tempos of behavior or seem to be “marching to the beat of the same drummer.” Assume that all people have built-in tempos or speeds at which their behavior is set (much like the tempo an orchestra follows at a concert). Assess the degree to which these two tempos or speeds are similar between the two people. (1–9 scale) | |
| Coordination/Smoothness1 | Degree to which the partners’ flows of behaviors are coordinated and smooth, intertwine, or mesh evenly and smoothly. Assume you are viewing a choreographed dance rather than a social interaction. How coordinated and smooth do the movements appear across partners? (1–9 scale) | |
| Posture Congruence1 | Degree to which partners match one another’s postures and bodily behaviors. Note that this rating focuses less on synchronized movements over time, and more on similarity between static postures and behaviors. Consider posture similarities across all parts of the body (e.g., arms, legs, trunks). (1–9 scale) | |
| Verbal Synchrony | Conversational Equality2 | Extent to which partners are equal participants in exchanging verbal information; degree to which both partners initiate conversational topics and respond to the conversation of their partner. High scores indicate that there is an equal participation on the part of both partners in the conversation. Low scores indicate that one partner maintains conversational dominance. Note that this rating should focus exclusively on the dyadic quality of verbal communication, based on the content of the statements. It is not meant to tap a broader range of non-verbal and verbal exchanges like many of the other codes. (1–5 scale) |
| Silence/Pause3 | The presence of tense or uncomfortable gaps and pauses in the ongoing conversation between the two partners. Do not simply code the absence of verbal interaction between members of a dyad. To be coded here, one or more partners must display evidence of tension or discomfort. Duration of silence, as well as the context of the interaction, are indicators of intensity. (1–9 scale) |
Interactions were rated in randomized order. Raters never watched both interactions for the same child in the same day, nor consecutively rated interactions involving the same unfamiliar adult. Raters viewed each interaction twice in full. The first viewing was silent and focused exclusively on movement synchrony ratings. The second viewing included audio and focused on verbal synchrony ratings. This safeguarded against perceptions of movement synchrony being confounded by conversational content or verbal reciprocity, and is consistent with previous studies in which movement ratings were completed without sound (Bernieri et al., 1988; 1994; Cappella, 1997; Kimura & Daibo, 2006). Also consistent with the previous studies using these movement ratings, interactions were rated in one-minute intervals; RAs assigned a score for every rating each minute. If the presentation was mixed with respect to a particular rating during the one-minute interval, raters assigned the value that best represented the average across the interval. One-minute interval ratings were subsequently averaged to yield one mean score for each rating, from each rater, across the full interaction.
Reliability and Data Reduction
Interrater reliability was measured with two-way random, consistency, average-measures intraclass correlations (ICC), conducted on the mean scores for each rating, separately by dyad type, after averaging across all 10 one-minute intervals. Across all ratings for both dyad types, ICCs ranged from .81 to .96. Average ratings across raters were therefore used in further analyses.
To ensure that movement synchrony and verbal synchrony ratings captured distinct subtypes of interactional synchrony, exploratory factor analysis with varimax rotation was run on all ratings. Results confirmed that ratings were best represented by two factors, together accounting for 70.1% of total variance. As expected, the first factor (movement synchrony) included Simultaneous Movement, Tempo Similarity, Coordination/Smoothness, and Posture/Congruence for both dyads (loadings ranged from .59–.82). The second factor (verbal synchrony) included Conversational Equality and Silence/Pause for both dyads (loadings .58–.94).
Consistent with some previous studies (Bernieri et al., 1994; Cappella, 1977), all four movement synchrony ratings were highly correlated in familiar dyads (rs .53–.80, ps < .01). In unfamiliar dyads (as in Bernieri, 1988; Bernieri et al., 1988; Kimura & Daibo, 2006), Simultaneous Movement, Tempo Similarity, and Coordination/Smoothness were significantly correlated (rs .50–.67, ps < .01), but Postural Congruence and Simultaneous Movement were not, r(34) = .24, p = .15. Because Postural Congruence showed an inconsistent pattern in its relationship with other ratings, and has shown similar inconsistency in prior literature, it was not included in the composite nor analyzed as a separate construct. Given their high correlations, the other three movement synchrony ratings were z-transformed and averaged to form a composite score for each dyad, as analyzing them separately would yield redundant results. The two verbal synchrony ratings were also significantly correlated in familiar, r(35) = .64, p < .001, and unfamiliar dyads, r(34) = .60, p < .001, and thus were also z-transformed and averaged to form composite scores.
RESULTS
Group Differences in Interactional Synchrony
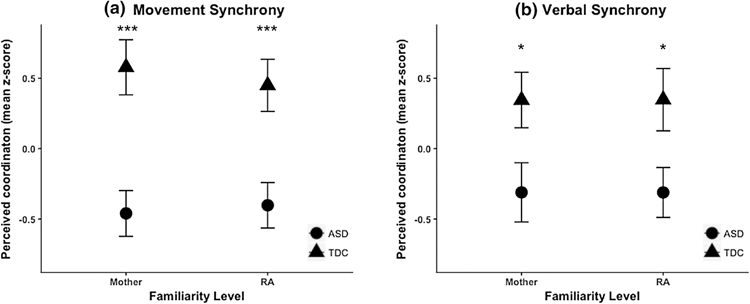
Movement synchrony and verbal synchrony composite scores were analyzed within separate 2 × 2 mixed design ANOVAs, with between-subjects factor of diagnostic group (ASD, TD), and within-subjects factor of familiarity level (mother, RA). Group differences, by familiarity level, are depicted in Figure 1. As predicted, the ANOVA for movement synchrony yielded a significant main effect of group, with a large effect size, F(1,34) = 17.59, p < .001, ηp2 = .34. Children with ASD (M = −0.43, SD = 0.16) were rated as displaying significantly less movement synchrony with interaction partners than TD children (M = 0.51, SD = 0.16), collapsed across familiarity levels. Within each familiarity condition, group comparisons also yielded large effect sizes (familiar: Cohen’s d = 1.42; unfamiliar: Cohen’s d = 1.16). Neither the familiarity effect, F(1,34) = 0.11, p = .74, ηp2 < .01, nor the familiarity by group interaction, F(1,34) = 0.79, p = .38, ηp2 = .02, were significant.
Figure 1.
Interactional synchrony across diagnostic groups and familiarity levels. Group mean z-scores (± SEM) for (A) composite movement synchrony ratings, and (B) composite verbal synchrony ratings.
*p < .05, ***p ≤ .001
Similarly, the ANOVA for verbal synchrony yielded a significant main effect of group, with a large effect size, F(1,34) = 6.47, p = .016, ηp2 = .16 (see Figure 1). Children with ASD (M = −0.31, SD = 0.18) displayed significantly less verbal synchrony with interaction partners than TD children (M = 0.35, SD = 0.19). Effect sizes for group comparisons were medium within each familiarity condition (familiar: Cohen’s d = 0.75; unfamiliar: Cohen’s d = 0.78). Again, there was neither a familiarity effect, F(1,34) < 0.001, p = .99, ηp2 < .001, nor a group by familiarity interaction, F(1,34) < 0.001, p = .99, ηp2 = < .001.
Association with Autism Severity
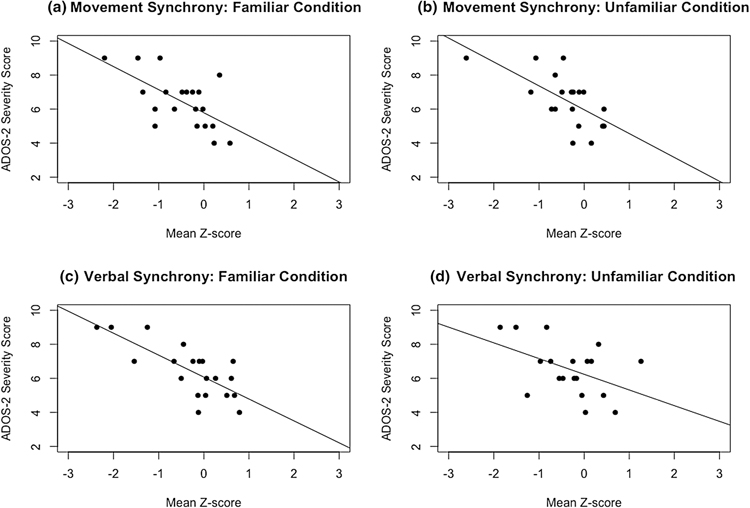
Pearson correlations were performed between standardized calibrated severity scores (CSS) from the ADOS-2 (Hus & Lord, 2014; Lord et al., 2012) and the movement synchrony and verbal synchrony composite scores. These were only conducted in the ASD group, as TD CSSs all fell at the bottom of the scale. Scatterplots are presented in Figure 2. Movement synchrony was significantly negatively correlated with ASD symptom severity, in familiar, r(18) = −.62, p = .003, and unfamiliar, r(17) = −.64, p = .003, dyads (see Figure 2). The same was true for verbal synchrony (familiar: r(18) = −.75, p < .001; unfamiliar: r(17) = −.46, p = .047).
Figure 2.
Scatterplots with regression lines relating interactional synchrony to autism symptom severity in children with ASD only. Mean movement synchrony z-scores are significantly negatively related to autism severity in the: (A) familiar condition (p = .003), and (B) unfamiliar condition (p = .003). Mean verbal synchrony z-scores are significantly negatively related to autism severity in the: (C) familiar condition (p < .001), and (D) unfamiliar condition (p = .047).
Association with Dimensional Social and Communication Skills
Linear regressions were conducted with movement synchrony and verbal synchrony composite scores entered as a set of predictors of scores on standardized rating scales of social and communication skills. We only analyzed synchrony scores from unfamiliar dyads, because behavior with an unfamiliar person (vs. highly familiar person) is more representative of broader skills, and because everyday social communication was rated by mothers, so using synchrony scores from mothers’ interactions would have restricted interpretation to the mother-child relationship.
In order to analyze interactional synchrony as a dimensional construct spanning children with typical and atypical social communication, we conducted the first analysis on the combined sample (ASD and TD), with standardized Total Social Skills scores from the SSIS-RS regressed onto the set of synchrony composites. We then conducted the same analysis with standardized General Communication Composite scores from the CCC-2 as the dependent variable. In both cases, the model with both predictors (movement synchrony and verbal synchrony) explained a significant portion of skill variance, and movement synchrony, but not verbal synchrony, emerged as a significant independent predictor (see Table 3).
Table 3.
Relationships between Interactional Synchrony and Dimensional Social and Communication Ability
| ASD + TD | TD Only | ASD Only | ||||
|---|---|---|---|---|---|---|
| Social Skills | Comm. Skills | Social Skills | Comm. Skills | Social Skills | Comm. Skills | |
| Movement Synchrony | .53*** | .55*** | .69** | .68* | .11 | .22 |
| Verbal Synchrony | .19 | .05 | .08 | −.25 | .03 | −.13 |
| Model F | 11.32*** | 8.18*** | 8.45** | 3.62* | .10 | .52 |
| R2 | .41 | .33 | .55 | .34 | .01 | .06 |
Note. Social (SSIS-RS) and communication (CCC-2) skill standard scores were regressed onto the set of composite scores for movement synchrony and verbal synchrony. Effects are presented as standardized beta coefficients for ease of comparison.
ASD: autism spectrum disorder; TD: typically developing
p ≤ .05,
p ≤ .01,
p ≤ .001
Regression analyses were then repeated within each group, to evaluate whether children with ASD and TD children showed similar relationships between interactional synchrony and everyday social and communication skills. The TD group demonstrated the same pattern as the overall sample: movement synchrony, but not verbal synchrony, predicted both social skills and communication skills (see Table 3). Significant associations between interactional synchrony and social or communication skills were not found within the ASD group alone.
DISCUSSION
Interactional Synchrony Distinguishes Children with ASD from TD Children
Our first goal was to quantify the degrees to which children with and without ASD engage in interactional synchrony, the tendency to dynamically coordinate behaviors in time with social partners. To our knowledge, ours was the first study to simultaneously measure interactional synchrony in ASD within natural conversations, with both familiar and unfamiliar non-expert partners, and across both verbal and nonverbal domains. We are aware of just one other study to date that has directly analyzed movement synchrony in ASD within a conversational context, and that study used expert clinician interaction partners and did not include a TD comparison group (Romero et al., 2018). As we predicted, children with ASD were perceived as significantly less synchronous with social partners than TD peers, suggesting alteration in ASD in a social process that has been established as automatic and fundamental from early infancy in typical development. By leveraging human perceptions, we showed that differences in interactional synchrony are salient even to observers unaware of children’s diagnostic status.
Interactional synchrony was measured in terms of both verbal and nonverbal behavior. Verbal synchrony was rated significantly lower in children with ASD, meaning that there was less balanced give and take between conversation partners and more atypicality in the length of silences between conversational turns. This result was not surprising, given known difficulties with back and forth conversation in ASD. Still, this finding is valuable in that it substantiates these rating procedures, within brief unstructured conversations with non-expert interaction partners, as sensitive to characteristic difficulties with reciprocal conversation. Although social reciprocity deficits are required for an ASD diagnosis, they can be difficult to concretely assess, and are often not explicitly measured at the level of a social dyad. In general, clinical ASD assessment relies largely on scoring specific behaviors on the part of the individual on the autism spectrum, rather than quantifying the dynamics of natural back and forth social interaction. Targeting verbal reciprocity more directly, as a dyadic construct in the context of typical conversations (as opposed to expert clinician measures or questionnaires), may offer a meaningful metric for measuring symptom severity and/or change over time.
Movement synchrony was also perceived as significantly lower in the ASD group relative to the TD group. In other words, raters detected distinct differences in the way children with ASD moved their bodies in relation to interaction partners, within a natural conversational free of movement demands. This finding is novel, because while movement differences have been increasingly recognized in ASD (Fournier, Hass, Naik, Lodha, & Cauraugh, 2010), disruptions in the back-and-forth coordination of body movements are not thought of as a salient symptom in the same way as disrupted coordination of verbal cues. It is therefore particularly interesting that our results indicate even more pronounced differences in movement synchrony than in verbal synchrony, when comparing participants with ASD to TD peers. If these movement differences are tangible within conversations, they may greatly contribute to why interactions in ASD can feel “off” or awkward to social partners. Indeed, research in neurotypical populations has consistently demonstrated that coordinated body movement influences social connectedness. We extend that link by showing that a clinical disorder defined by social connectedness deficits may also be characterized by reduced movement synchrony.
Contrary to expectations, familiarity level with an interaction partner (mothers vs. RAs) did not impact either verbal synchrony or movement synchrony in children with or without ASD. This pattern of similar interactional synchrony across partners reflects that the tendency to synchronize may be a stable trait, present across social contexts, which is consistent with research characterizing it as a ubiquitous process that occurs even among unfamiliar individuals (e.g., Chartrand & Bargh, 1999). However, it should be noted that the environment and task were consistent across partners, and provided more structure than most daily social encounters. Different types of social situations (e.g., less structured, less cooperative, less supportive partner, i.e., peers) may elicit more variability in interactional synchrony.
Interactional Synchrony Predicts Dimensional Social and Communication Ability
Our second objective was to examine interactional synchrony as a dimensional construct, by testing whether it was predictive of social and communication skills spanning typical and atypical populations. In children with ASD, ratings of both verbal synchrony and movement synchrony were significantly negatively correlated with overall ASD symptom severity, indicating a relationship between poorer interactional synchrony and individual differences in core ASD symptomatology. This association is particularly noteworthy with respect to movement synchrony. While nonverbal communication impairments are a diagnostic criterion for ASD, global movement differences, including movement synchrony, may be an under-recognized symptom. Indeed, clinicians have spontaneously identified motor differences as a key behavior in identifying “frank” ASD presentations (de Marchena & Miller, 2017). Movement synchrony may offer a quantifiable behavior that specifically associates with individual variability in ASD severity, and thus a means to capture fundamental social reciprocity impairments.
Our results also indicate a relationship between interactional synchrony and broader social and communication ability in the general population. Across ASD and TD groups, interactional synchrony predicted higher scores on standardized parent-report measures of everyday social communication. This includes a measure (SSIS-RS) of social skills and competencies including cooperation, helping, and affiliative behaviors, which are the same types of prosocial behaviors linked to interactional synchrony in adult samples (Chartrand & Lakin, 2013; van Baaren, Holland, Kawakami, & van Knippenberg, 2004; Wiltermuth & Heath, 2009). It also includes a general measure of language skills (CCC-2), which is consistent with research linking interactional synchrony to language in early childhood (Harrist & Waugh, 2002), as well as evidence that coordinated speech among partners results in less effortful conversation (Garrod & Pickering, 2004). Our results therefore help affirm a dimensional relationship between interactional synchrony and social and communication skills, spanning normative and non-normative ranges of functioning. They also extend the empirical link between interactional synchrony and social communication to school-age children and adolescents, an age group in which interactional synchrony has been relatively understudied (Harrist & Waugh, 2002).
Of particular interest, only movement synchrony emerged as a significant independent predictor of parent-reported social and communication skills, including within the TD group alone. In other words, a brief snapshot of children’s movement synchrony was predictive of mothers’ ratings of their broader social functioning in everyday environments. Establishing an explicit association between movement synchrony and dimensional social and communication ability, including within a TD sample, further highlights movement synchrony as an important social process in its own right, above and beyond more widely emphasized language-based features of social interaction. Previous literature has suggested that interactional synchrony provides an early foundation for a cascade of positive social developmental outcomes (Feldman, 2007; Harrist & Waugh, 2002). When considered through this lens, our preliminary results begin to implicate diminished movement synchrony as one mechanism through which social communication impairments in ASD may occur. A predisposition toward movement synchrony may serve as a pathway for typical social communication development; given the strong link between interactional synchrony and positive social outcomes, its presence during early interactions may cause children to seek more interaction, resulting in increased opportunities to hone social communication skills. In turn, it is possible that a lack of interactional synchrony with social partners in children with ASD may lead to interactions that feel more awkward and less socially rewarding, making children less motivated to seek social opportunities, and thereby disrupting developmental trajectories. Additionally, TD peers or relatives engaged in conversation with a child with ASD might experience a reduced sense of connectedness or poorer interaction quality due to a lack of synchrony, which may negatively affect their engagement level during future interactions with that child. Investigating these possible pathways in larger, longitudinal samples is an important future direction.
Limitations
This study’s rigorous characterization and behavioral rating procedures constrained its sample size, which precludes generalization of our results to all children with or without ASD, as well as our ability to fully capture the heterogeneity in social interaction skills within the autism spectrum. In particular, the small sample size requires that correlation and regression analyses measuring dimensional constructs be interpreted as preliminary rather than conclusive. Furthermore, because of the task’s high verbal demands, this study focused on older children and adolescents who all had relatively strong cognitive and language skills. In a way, this makes observed group differences particularly meaningful; our findings suggest that atypical interactional synchrony may explain why interactions with individuals with ASD can feel less reciprocal, even when other clinical features are milder. Still, the restricted nature of our sample prevents us from drawing conclusions about interactional synchrony across the full autism spectrum. In future research, it will be important to determine whether atypical interactional synchrony is representative of the full spectrum, through procedures adapted for younger children and those with lower language skills and/or more significant autism symptoms. In addition, this study included very few females and a somewhat broad age range, and was underpowered to test for sex or age differences. We controlled for both sex and age by matching across ASD and TD groups, and age was not significantly correlated with either movement or verbal synchrony in our sample. However, we recognize that these factors may influence interactional synchrony in ways that are not captured herein. Finally, unfamiliar RA interaction partners were aware of the study objectives and hypotheses, which could have subconsciously influenced their behavior. While this is unlikely given the similar results across RAs and mothers (who were naïve to the focus on synchrony), staff would ideally be naïve to specific study goals.
Clinical Implications
A principal implication of this study is that coordinated movement may serve an adaptive role within social interaction, and provide a lens for understanding conditions associated with social impairment like ASD. We found that how children move their bodies in time with social partners is tied to their ability to get along with others, joining a growing literature championing the centrality of movement abnormalities in ASD, and the role of movement in social communication more generally (Cook, 2016; Dowd, Rinehart, & McGinley, 2010; West, 2018). More attention should be given to how movement may shape typical and atypical social functioning. For example, shared cognitive and affective states may be embodied in shared movement or bodily positions (i.e., interactional synchrony), meaning that a failure by individuals with ASD to coordinate movements with others may affect their ability to attain a shared experience (see Eigsti, 2013; Winkielman, McIntosh, & Oberman, 2009 for relevant discussions).
Our results also speak to the importance of studying social communication behaviors within their natural context of dyadic interaction. ASD is defined by social interaction deficits, yet, research has primarily focused on one-sided behaviors of individuals with ASD, with less emphasis on dynamic, bi-directional behaviors. Measuring interpersonal processes, as opposed to individual behaviors, may yield differences in ASD that are more robust and more generalizable to real-world functioning (García-Pérez, Lee, & Hobson, 2007; Northrup & Iverson, 2015).
Finally, results carry implications for intervention for ASD and other social communication difficulties. While early synchronous behaviors within child-caregiver interactions are already considered a valuable intervention target (Landa, Holman, O’Neill, & Stuart, 2011; Siller & Sigman, 2002), this study suggests that the social use of movement may be a particularly promising treatment target, with the potential to positively impact social communication skills across diagnoses and levels of impairment.
Acknowledgments
This research was supported in part by the National Institute on Deafness and Other Communication Disorders (R01 DC009439). We would like to thank the families that participated. We would also like to thank Paul Allen, Laura Soskey Cubit, Jessica Keith, Kim Schauder, and our undergraduate research assistants for their many invaluable contributions, and Ashley de Marchena and Heather Nuske for feedback on manuscript drafts.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest. All authors declare that they have no conflict of interest.
Ethical Approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent. Informed consent was obtained from all individual participants included in this study.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Bernieri FJ (1988). Coordinated movement and rapport in teacher-student interactions. Journal of Nonverbal Behavior, 12(2), 120–138. 10.1007/BF00986930 [DOI] [Google Scholar]
- Bernieri FJ, Davis JM, Rosenthal R, & Knee CR (1994). Interactional synchrony and rapport: Measuring synchrony in displays devoid of sound and facial affect. Personality and Social Psychology Bulletin, 20(3), 303–311. 10.1177/0146167294203008 [DOI] [Google Scholar]
- Bernieri FJ, Reznick JS, & Rosenthal R (1988). Synchrony, pseudosynchrony, and dissynchrony: Measuring the entrainment process in mother-infant interactions. Journal of Personality and Social Psychology, 54(2), 243–253. 10.1037/0022-3514.54.2.243 [DOI] [Google Scholar]
- Bernieri FJ, & Rosenthal R (1991). Interpersonal coordination: Behavior matching and interactional synchrony In Fundamentals of Nonverbal Behavior (pp. 401–432). New York, NY, USA: Cambridge University Press. [Google Scholar]
- Bishop DVM (2006). Children’s Communication Checklist-2. San Antonio, TX: Pearson. [Google Scholar]
- Cappella JN (1977). Behavioral and judged coordination in adult informal interactions: Vocal and kinesic indicators. Journal of Personality and Social Psychology, 72(1), 119–131. [Google Scholar]
- Chartrand TL, & Bargh JA (1999). The chameleon effect: The perception–behavior link and social interaction. Journal of Personality and Social Psychology, 76(6), 893–910. 10.1037/0022-3514.76.6.893 [DOI] [PubMed] [Google Scholar]
- Chartrand TL, & Lakin JL (2013). The antecedents and consequences of human behavioral mimicry. Annual Review of Psychology, 64(1), 285–308. 10.1146/annurev-psych-113011-143754 [DOI] [PubMed] [Google Scholar]
- Condon WS, & Ogston WD, (1967). A segmentation of behavior. Journal of Psychiatric Research, 5, 221–235. [Google Scholar]
- Condon WS, & Sander LW (1974). Neonate movement is synchronized with adult speech: Interactional participation and language acquisition. Science, 183(4120), 99–101. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social Responsiveness Scale, Second Edition. Torrance, CA: Western Psychological Services. [Google Scholar]
- Cook J (2016). From movement kinematics to social cognition: The case of autism. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371(1693). 10.1098/rstb.2015.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaherche E Chetouani M, Mahdhaoui A, Saint-Georges C, Viaux S, & Cohen D (2012). Interpersonal synchrony: A survey of evaluation methods across disciplines. IEEE Transactions on Affective Computing, 3(3), 349–365. 10.1109/T-AFFC.2012.12 [DOI] [Google Scholar]
- de Marchena A, & Miller J (2017). “Frank” presentations as a novel research construct and element of diagnostic decision-making in autism spectrum disorder. Autism Research, 10(4), 653–662. 10.1002/aur.1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd AM, Rinehart NJ, & McGinley J (2010). Motor function in children with autism: Why is this relevant to psychologists? Clinical Psychologist, 14(3), 90–96. 10.1080/13284207.2010.525532 [DOI] [Google Scholar]
- Eigsti I-M (2013). A review of embodiment in autism spectrum disorders. Frontiers in Psychology, 4, 1–10. 10.3389/fpsyg.2013.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R (2007). Parent-infant synchrony and the construction of shared timing; Physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 48(3–4), 329–354. 10.1111/j.1469-7610.2006.01701.x [DOI] [PubMed] [Google Scholar]
- Feldstein S, Konstantareas M, Oxman J, & Webster CD (1982). The chronography of interactions with autistic speakers: An initial report. Journal of Communication Disorders, 15(6), 451–460. 10.1016/0021-9924(82)90018-1 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick P, Diorio R, Richardson MJ, & Schmidt RC (2013). Dynamical methods for evaluating the time-dependent unfolding of social coordination in children with autism. Frontiers in Integrative Neuroscience, 7, 1–13. 10.3389/fnint.2013.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P, Frazier JA, Cochran DM, Mitchell T, Coleman C, & Schmidt RC (2016). Impairments of social motor synchrony evident in autism spectrum disorder. Frontiers in Psychology, 7 10.3389/fpsyg.2016.01323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P, Romero V, Amaral JL, Duncan A, Barnard H, Richardson MJ, & Schmidt RC (2017a). Evaluating the importance of social motor synchronization and motor skill for understanding autism. Autism Research, 10, 1687–1699. 10.1002/aur.1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P, Romero V, Amaral JL, Duncan A, Barnard H, Richardson MJ, & Schmidt RC (2017b). Social motor synchronization: Insights for understanding social behavior in autism. Journal of Autism and Developmental Disorders, 47, 2092–2107. 10.1007//s10803-017-3124-2 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick P, Frazier JA, Cochran D, Mitchell T, Coleman C, & Schmidt RC (2018). Relationship between theory of mind, emotion recognition, and social synchrony in adolescents with and without autism. Frontiers in Psychology, 9, 1337 10.3389/fpsyg.2018.01337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, & Cauraugh JH (2010). Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders, 40(10), 1227–1240. 10.1007/s10803-010-0981-3 [DOI] [PubMed] [Google Scholar]
- García-Pérez RM, Lee A, & Hobson RP (2007). On intersubjective engagement in autism: A controlled study of nonverbal aspects of conversation. Journal of Autism and Developmental Disorders, 37(7), 1310–1322. 10.1007/s10803-006-0276-x [DOI] [PubMed] [Google Scholar]
- Garrod S, & Pickering MJ (2004). Why is conversation so easy? Trends in Cognitive Sciences, 8(1), 8–11. 10.1016/j.tics.2003.10.016 [DOI] [PubMed] [Google Scholar]
- Gresham FM, & Elliot SN (2008). Social Skills Improvement System - Rating Scales. Minneapolis, MN: Pearson. [Google Scholar]
- Harrist AW, & Waugh RM (2002). Dyadic synchrony: Its structure and function in children’s development. Developmental Review, 22(4), 555–592. 10.1016/S0273-2297(02)00500-2 [DOI] [Google Scholar]
- Hove MJ, & Risen JL (2009). It’s all in the timing: Interpersonal synchrony increases affiliation. Social Cognition, 27(6), 949–960. 10.1521/soco.2009.27.6.949 [DOI] [Google Scholar]
- Hus V, & Lord C (2014). The Autism Diagnostic Observation Schedule, Module 4: Revised algorithm and standardized severity scores. Journal of Autism and Developmental Disorders, 44(8), 1996–2012. 10.1007/s10803-014-2080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe J, Beebe B, Feldstein S, Crown CL, & Jasnow MD (2001). Rhythms of dialogue in infancy: coordinated timing in development. Monographs of the Society for Research in Child Development, 66(2), i–viii, 1–132. [PubMed] [Google Scholar]
- Kimura M, & Daibo I (2006). Interactional synchrony in conversations about emotional episodes: A measurement by the “between participants pseudosynchrony experimental paradigm.” Journal of Nonverbal Behavior, 30, 115–126. [Google Scholar]
- Lakin JL, Jefferis VE, Cheng CM, & Chartrand TL (2003). The chameleon effect as social glue: Evidence for the evolutionary significance of nonconscious mimicry. Journal of Nonverbal Behavior, 27(3), 145–162. 10.1023/A:1025389814290 [DOI] [Google Scholar]
- Landa RJ, Holman KC, O’Neill AH, & Stuart EA (2011). Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: a randomized controlled trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 52(1), 13–21. 10.1111/j.1469-7610.2010.02288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif N, Barbosa AV, Vatiokiotis-Bateson E, Castelhano MS, & Munhall KG (2014). Movement coordination during conversation. PLOS ONE, 9(11), 10.1371/journal.pone.0113316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey EW, Colwell MJ, Frabutt JM, Chambers JC, & MacKinnon-Lewis C (2008). Mother-child dyadic synchrony in European American and African American families during early adolescence: Relations with self-esteem and prosocial behavior. Merrill-Palmer Quarterly, 54(3), 289–315. [Google Scholar]
- Lord C, Rutter ML, DiLavore PS, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule, Second Edition. Los Angeles: Western Psychological Services. [Google Scholar]
- Marsh KL, Isenhower RW, Richardson MJ, Helt M, Verbalis AD, Schmidt RC, & Fein D (2013). Autism and social disconnection in interpersonal rocking. Frontiers in Integrative Neuroscience, 7 10.3389/fnint.2013.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh D (2006). Spontaneous facial mimicry, liking and emotional contagion. Polish Psychological Bulletin, 37(1), 31–42. [Google Scholar]
- Melby JN, & Conger RD (2001). The Iowa Family Interaction Rating Scales: Instrument summary In Family Observational Coding Systems: Resources for Systemic Research (pp. 33–58). Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Miles L, Nind L, Henderson Z, & Macrae CN (2010). Moving memories: Behavioral synchrony and memory for self and others. Journal of Experimental Social Psychology, 46(2), 457–460. 10.1016/j.jesp.2009.12.006 [DOI] [Google Scholar]
- Northrup JB, & Iverson JM (2015). Vocal Coordination During Early Parent-Infant Interactions Predicts Language Outcome in Infant Siblings of Children with Autism Spectrum Disorder. Infancy: The Official Journal of the International Society on Infant Studies, 20(5), 523–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseyer F, & Tschacher W (2011). Nonverbal synchrony in psychotherapy: Coordinated body movement reflects relationship quality and outcome. Journal of Consulting and Clinical Psychology, 79(3), 284–295. 10.1037/a0023419 [DOI] [PubMed] [Google Scholar]
- Romero V, Fitzpatrick P, Roulier S, Duncan A, Richardson MJ, & Schmidt RC (2018). Evidence of embodied social competence during conversation in high functioning children with autism spectrum disorder. PLoS ONE, 13(3). 10.1371/journal.pone.0193906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter ML, Bailey A, & Lord C (2003). Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Rutter ML, Le Couteur A, & Lord C (2003). Autism Diagnostic Interview - Revised. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Schmidt RC, & Fitzpatrick P (2016). The origin of the ideas of interpersonal synchrony and synergies In Passos P, Davis K, & Chow JY (Eds.), Interpersonal coordination and performance in social systems (pp. 17–31). New York, NY: Routledge. [Google Scholar]
- Siller M, & Sigman M (2002). The behaviors of parents of children with autism predict the subsequent development of their children’s communication. Journal of Autism and Developmental Disorders, 32(2), 77–89. [DOI] [PubMed] [Google Scholar]
- Sonnby-Borgström M, Jönsson P, & Svensson O (2003). Emotional empathy as related to mimicry reactions at different levels of information processing. Journal of Nonverbal Behavior, 27(1), 3–23. 10.1023/A:1023608506243 [DOI] [Google Scholar]
- Stel M, & Vonk R (2010). Mimicry in social interaction: Benefits for mimickers, mimickees, and their interaction. British Journal of Psychology, 101(2), 311–323. 10.1348/000712609X465424 [DOI] [PubMed] [Google Scholar]
- van Baaren RB, Holland RW, Kawakami K, & van Knippenberg A (2004). Mimicry and prosocial behavior. Psychological Science, 15(1), 71–74. 10.1111/j.0963-7214.2004.01501012.x [DOI] [PubMed] [Google Scholar]
- Warlaumont AS, Richards JA, Gilkerson J, & Oller DK (2014). A social feedback loop for speech development and its reduction in autism. Psychological Science, 25(7), 1314–1324. 10.1177/0956797614531023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children, Fourth Edition. San Antonio, TX: Pearson. [Google Scholar]
- Wechsler D (2011). Wechsler Abbreviated Scale of Intelligence, Second Edition. San Antonio, TX: Pearson. [Google Scholar]
- West KL (2018). Infant motor development in autism spectrum disorder: A synthesis and meta-analysis. Child Development, 0(0), 1–18. 10.1111/cdev.13086 [DOI] [PubMed]
- Wiltermuth SS, & Heath C (2009). Synchrony and cooperation. Psychological Science, 20(1), 1–5. 10.1111/j.1467-9280.2008.02253.x [DOI] [PubMed] [Google Scholar]
- Winkielman P, McIntosh DN, & Oberman L (2009). Embodied and disembodied emotion processing: Learning from and about typical and autistic individuals. Emotion Review, 1(2), 178–190. 10.1177/1754073908100442 [DOI] [Google Scholar]