Abstract
With more than 700 described species, leeches include morphological, physiological, and behavioral diversity and occur in terrestrial and aquatic habitats, including freshwater, estuarine, and marine ecosystems. Leeches inhabit a number of extreme environments, including extremes in temperature, moisture, salinity, pressure, light, and pollution. In some cases, leeches in extreme environments have specialized morphological, physiological, or behavioral adaptations to survive these conditions, yet unique adaptations are not apparent in some species. Leeches that inhabit inhospitable habitats occur in more than one branch or family of leech phylogeny suggesting that there have been independent invasions of environments with extreme conditions. Herein, we review examples of leeches that live in extreme conditions and the exceptional biology that has contributed to leeches being the most extreme annelids.
Keywords: Sanguijuela, Sangsue, Blutegel, Sanguessuga, Hirudinea, Hirudinida
Graphical abstract
Highlights
-
•
Leeches occur in terrestrial, freshwater, estuarine, and marine ecosystems.
-
•
Leeches inhabit extremes in temperature, moisture, salinity, pressure, light, and pollution.
-
•
Not all leech species living in inhospitable conditions have unique adaptations.
-
•
There were multiple independent invasions of leeches into extreme environments.
1. Introduction
Most people think of leeches as uniformly black, slimy worms that feed on the blood of mammals in equally scummy ponds and swamps. While this thought is undeniably true, it is not representative of the morphological, physiological, and behavioral diversity that enables leeches to survive and even thrive in extreme habitats.
There are more than 700 species of leeches, with many notable examples of diversity and interesting evolutionary transitions in habitat preference, feeding behavior, and morphological adaptations (Borda and Siddall, 2004; Sket and Trontelj, 2008; Phillips and Siddall, 2009; Phillips et al., 2010; Oceguera-Figueroa, 2012; Oceguera-Figueroa et al., 2011; Govedich and Moser, 2015; Tessler et al., 2018). Leeches are found on all continents and seas, except terrestrial Antarctica, and they can be found in freshwater, estuarine, and marine aquatic ecosystems, as well as moist terrestrial ecosystems. Leech classification is arranged so that families and genera tend to include members with similar ecological and physiological tolerances. Based on the sister group, Branchiobdellida or crayfish worms, being an exclusively freshwater lineage, leeches likely had a freshwater ancestor. Within leeches, the Oceanobdelliformes (Piscicolidae and Ozobranchidae) represent a single invasion of marine habitats with few secondary transitions (e.g. Myzobdella lugubris) to brackish environments (Tessler et al., 2018; Phillips et al., 2019). Glossiphoniidae is the only lineage within Rhynchobdellida (proboscis-bearing leeches) to exclusively inhabit only freshwater, albeit with some temporary salinity tolerance (Sawyer, 1974). Arhynchobdellida (jawed leeches) is posited to have had a terrestrial ancestor with a single transition to freshwater followed by multiple secondary transitions to terrestrial environments (Borda and Siddall, 2004). The Arhynchobdellida are found in terrestrial and freshwater habitats, and include the Haemadipsidae that is one of the two exclusively terrestrial families of leeches.
The last three decades have brought forth the addition of DNA sequence data to phylogenetic assessments of leech evolution and this has changed much of the classification scheme set in the mid-20th century based on morphology, feeding preference, and geographic distributions (Richardson, 1969b, 1975; Sawyer, 1986). Many taxa at the familial level have been established as monophyletic groups using DNA sequence data (Borda and Siddall, 2004; Utevsky and Trontelj, 2004; Siddall et al., 2005; Williams and Burreson, 2006; Phillips et al., 2010; Oceguera-Figueroa et al., 2011; Nakano et al., 2012) and higher order relationships are being tested with next generation sequencing data (using 5 nuclear and mitochondrial loci by Tessler et al., 2018, and anchored hybrid enrichment by Phillips et al., 2019). With the current taxonomic framework and technological capabilities, the group is primed for synthetic approaches to better understand the placement of these unusual taxa in a phylogenetic and ecological context.
Leeches inhabit a number of extreme environments including extremes in temperature, moisture, salinity, pressure, light, and pollution. These environments are considered challenging for many organisms, and test the physiological limits of these soft-bodied worms. How are leeches that survive extreme environments different from their relatives that live in more hospitable conditions? What are some of the extreme adaptations of leeches that set the group apart from other clitellates? Herein, we address why each environment is extreme for leeches, the morphological, physiological, and behavioral adaptations of leeches to survive extreme conditions, and extreme adaptations of leeches that inhabit ecologically inhospitable conditions.
2. Tolerance of ecological extremes
2.1. Terrestrial habitats
Terrestrial environments are some of the most extreme for leeches and pose a high risk of desiccation. Leeches that live in these environments have morphological and behavioral adaptations to survive these dry conditions (Fig. 1), such as the ability to produce large quantities of mucus. All leeches produce mucus from mucus glands distributed throughout the body and it is important in a number of biological functions for the leech, including prevention of desiccation (Sawyer, 1986). Even aquatic species can tolerate some time out of water, thanks to their mucus covering, and in one study, specimens of Placobdella parasitica survived the loss of up to 70.4% of their body weight, especially if refrigerated during recovery (Hall, 1922).
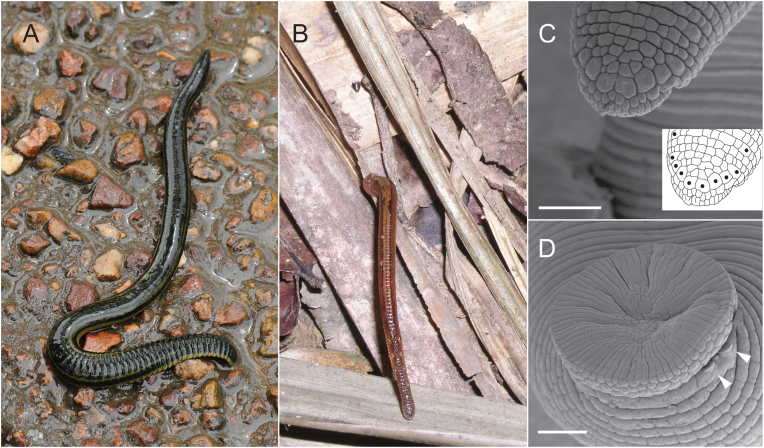
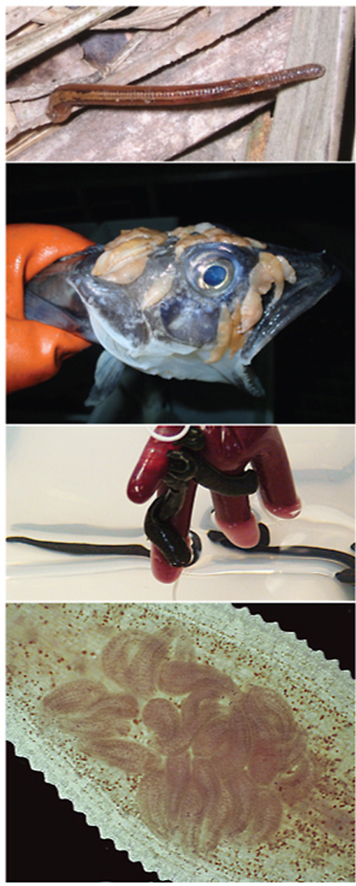
Fig. 1.
Terrestrial leeches. A) Orobdella sp. out of water after a rainstorm in the Philippines. Leech is estimated to be more than 25 cm in length. Image credit: Will Reeves. B) Haemadipsa zeylanica pursuing the photographer as a host on the Vietnamese forest floor. Leech size approximately 4 cm in length. C) SEM image of the head of a haemadipsid leech. The inset depicts an outline of the same image with the eye spots marked by black dots. D) Caudal sucker of a haemadipsid leech with friction rays on the sucker surface. White arrows indicate the two flaps of the auricle. Scale bars in C and D = 0.5 mm.
Many leeches in several different families occur globally in moist terrestrial habitats. The most infamous of these, the blood-sucking terrestrial leeches or “land leeches”, are of the family Haemadipsidae found in tropical and sub-tropical habitats of the Indian subcontinent, southeast and northeast Asia, Australia, and Indo-Pacific islands (Borda and Siddall, 2011, Fig. 1B). Lesser known terrestrial leeches include some North American species of Haemopidae, members of Xerobdellidae in Central and South America, members of Cylicobdellidae in South America, Americobdella valdiviana in southern Chile, the Kinabalu Giant Red Leech Mimobdella buettikoferi in Borneo, and Semiscoloides congolensis in Central Africa (Sawyer, 1986; Borda and Siddall, 2004; Borda et al., 2008). Terrestrialism evolved multiple times in the evolution of arhynchobdellid leeches and phylogenies based on molecular data have suggested that the Hirudiniformes had a terrestrial ancestor (Apakupakul et al., 1999; Borda and Siddall, 2004). This is supported by the behaviors of members of Hirudiniformes laying spongy cocoons out of water and internal insemination, by even the aquatic species.
Members of Haemopidae are predatory on terrestrial and aquatic worms and are distributed in Europe and North America. These are some of the largest leeches, epitomized by the species Haemopis grandis that can reach a total length of up to 300 mm (Klemm, 1982). Most species in the family are aquatic, with only four species (Haemopis elegans, Haemopis terrestris, Haemopis septagon, and Haemopis ottorum, although H. ottorum is a junior synonym of H. septagon sensu Sawyer, 2019) recognized as terrestrial or amphibious (Grosser, 2004; Wirchansky and Shain, 2010). Other Haemopis species remain in the water during the day, but come onto the shoreline at night or after rainfall to predate on wandering earthworms. Some Haemopis species can be found in humid environments or recently after rainfall in moist leaf litter or under logs and debris. Potentially, their large body size assists in preventing desiccation rather than having specialized morphological structures.
Like their aquatic relatives, leeches in terrestrial environments excrete ammonia as a waste product. It is excreted from nephridiopores arranged submarginally along the ventral side of the body. Some terrestrial leeches have modified the terminal set of nephridiopores to assist in maintaining moisture and increasing suction on the caudal sucker. In haemadipsid leeches, the terminal set of nephridiopores open onto each respiratory auricle, specialized structures arranged laterally at the base of the caudal sucker (Fig. 1D). The respiratory auricles are thought to channel liquid waste from the nephridiopores to the caudal sucker to maintain moisture on the sucker surface and increase suction. In addition, the caudal sucker of haemadipsids is textured with friction rays that also contribute to increased suction (Sawyer, 1986, Fig. 1D). Alternately, members of Xerobdellidae possess a medioventral common pore at the base of the caudal sucker that likely serves the similar function of wetting the caudal sucker to facilitate attachment in terrestrial environments (Borda et al., 2008).
Haemadipsid leeches move differently than leeches that live in aquatic environments. In general, haemadipsids engage in more inchworm crawling than vermiform crawling like other leeches, especially when the substrate is sandy or dusty. They also clean their tail suckers by wiping them against the ventral surface of their body, a behavior that seems to be unique to the family (Sawyer, 1986). Some predaceous terrestrial leeches, such as Haemopis terrestris (Haemopidae), are adept at swimming under ideal conditions, yet strictly terrestrial leeches, such as the haemadipsids, are unable to swim and if dropped in water sink to the bottom and then crawl out.
Terrestrial blood-feeding leeches are attracted to stimuli that signal the presence of a potential host, such as movement of the substrate and ground vibrations, air currents, shadows, and other sensory cues. Likewise, hungry blood-feeding aquatic leeches move towards water disturbance which could potentially be prey, while some leech species respond positively to rheotaxis of water currents (Sawyer, 1986). This response is similar to terrestrial leeches responding positively to air currents, particularly warm moist air similar to the breath of a mammal, although there is conflicting evidence that terrestrial leeches respond positively to CO2 as in breath (Stammers, 1950; Keegan et al., 1968).
2.2. Oxygen and respiration
In some environments, such as springs, lakes, ox-bow ponds/billabongs, swamps, and marshes, the water chemistry may be unique or ranging toward the extreme in terms of dissolved oxygen, temperature, and salinity. Leeches living in these systems must then be able to survive in a wide range of temperatures and oxygen conditions. In deep lakes that become stratified or covered in ice, extremes may occur annually with summer temperatures being high and oxygen levels being at or above (hyperoxia) saturation in the epilimnion, but low or depleted below in the hypolimnion during the warmer summer months. During the winter, the water below the ice may be at or near freezing and at the bottom of these lakes, with temperatures at or near 4 °C, oxygen may become depleted, leading to hypoxic or even anoxic conditions. (Davies and Govedich, 2001; Govedich et al., 2010). Leeches have been shown to be both conformers, taking oxygen from the water in proportion to its availability, and regulators, taking oxygen from the water at a constant level over a range of oxygen concentrations (Mann, 1956; Wrona and Davies, 1984; Govedich et al., 2010). In most leeches, oxygen consumption occurs through the body surface (Mann, 1962) and the leech can increase dorso-ventral undulations of the body to increase the rate of oxygen uptake. In the Piscicolidae and Ozobranchidae, there are various body coelom modifications, pulsatile vesicles, and gills that assist with oxygen uptake (Mann, 1962; Sawyer, 1986). Leeches with pulsatile vesicles can increase the pulsations when more oxygen is needed. Under low oxygen conditions, leeches may behaviorally compensate by ventilating (undulating to draw water across their body), or they may have metabolic and physiological adaptations to these low oxygen conditions. Some leeches have been able to survive anoxic conditions for several days or even weeks (Sladacek and Kosel, 1984; Davies et al., 1987) at lower temperatures (less than 21 °C) suggesting that this aids overwintering. Hyperoxia (200–300%) conditions may also lead to increased mortality, with individuals able to survive for several days or even weeks (Davies and Gates, 1991a, 1991b). Even though leeches can survive both anoxic and hyperoxic conditions, reductions in the number of mitochondria and ribosomes in neurons have been observed, suggesting that neurological damage may be occurring under these conditions (Singhal and Davies, 1987; Singhal et al., 1988; Davies and Govedich, 2001; Govedich et al., 2010).
2.3. Oceans and polar regions
Most leeches are not tolerant of salt or estuarine water, however, in spite of this there are marine leeches that are primarily parasitic on teleost and elasmobranch fishes (Family Piscicolidae; Fig. 2) or marine and freshwater turtles (Family Ozobranchidae) (Sawyer, 1986; Utevsky et al., 2019; Burreson, 2020). Colonizing the oceans, members of the Family Piscicolidae are surmised to have originated from freshwater ancestors and then in several instances, recolonized freshwaters in the Holoarctic (Utevsky and Trontelj, 2004; Williams and Burreson, 2006). Species in the Family Ozobranchidae (Ozobranchus spp.) show a similar pattern with most members being marine and a few exceptions inhabiting freshwater (Bogabdella spp. and Ozobranchus jantseanus) (Richardson, 1969a; Sawyer, 1986; Oceguera-Figueroa, 2020).
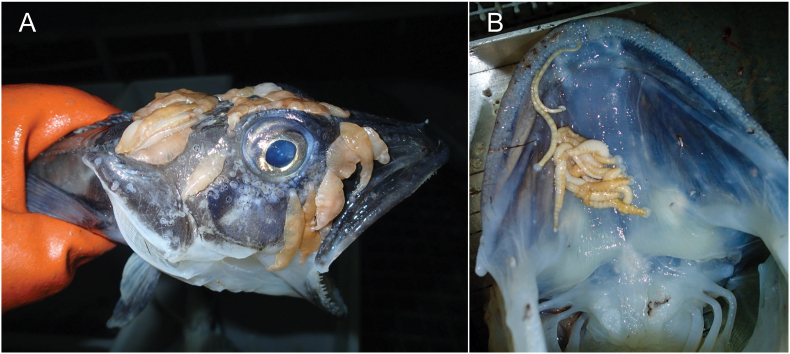
Fig. 2.
Leeches in polar regions. A) Channichthyid fish with several leeches (Trulliobdella bacilliformis) attached to the head region. B) View of the interior upper jaw of a channichthyid fish with leeches (Nototheniobdella sawyeri) attached. Image credits: Alex Dornburg.
Marine piscicolids are most abundant in the polar regions and temperature plays an important role in their salinity tolerance (Sawyer, 1986; Utevsky and Trontelj, 2004; Glasby et al., 2009). Most piscicolids can tolerate salinities only at low temperatures with more dissolved oxygen, however Branchellion spp. occur in temperate and tropical environments and bear lateral external projections, or gills, that assist with respiration at higher temperatures (Sawyer, 1986). Similarly, members of the Ozobranchidae possess external digitiform gills and the number of pairs of gills varies by species. The gills of individuals at rest are in constant slow motion with coelomic fluid being pumped through, and these gills likely play a role in its temperature tolerance (Sawyer, 1986). In the laboratory, the freshwater ozobanchid Ozobranchus jantseanus survived at −196 °C for 24 h and up to 32 months at −90 °C (Suzuki et al., 2014). The specimens of O. jantseanus in the laboratory experiment also survived repeated freeze-thaw cycles at temperature ranges of 20 °C to −100 °C. At the other extreme, the marine piscicolid Zeylanicobdella arugamensis can survive at temperatures ranging from 20 °C to 40 °C (Kua et al., 2014). The temperature tolerance of these species is not only exceptional for all leeches, but also for all other members of their respective families.
According to the World Register of Deep-Sea Species (http://www.marinespecies.org/deepsea/), the deep sea occurs at depths greater than 500 m (Burreson, 2016). At a depth of 500 m, the pressure is approximately 50 atm or 50 times greater than the pressure at sea level. There are several examples of deep-sea leeches: Bathybdella sawyeri occurs at 2447–2623 m depth at the Galápagos Rift and the Southeast Pacific Rise and Galatheabdella bruuni has been found at depths of 3880–4400 m in the Tasman Sea (Richardson and Meyer, 1973; Burreson, 1981; Burreson and Segonzac, 2006). However, the leech that occurs at the greatest depth is Johanssonia extrema described by Utevsky et al. (2019) that was collected at a depth of 8728.8 m in the Kuril-Kamchatka Trench. Occurring at that depth, J. extrema withstands pressure that is over 870 times greater than that at sea level.
There are extraordinary examples of leech species with unusual or unique morphology that occur in polar and deep-sea environments. Megaliobdella szidati is a very large leech (up to 34 cm in length) covered in magnificent papillae that easily distinguish it from all other leeches and occurs in the Southern Oceans south of 35° latitude (Meyer and Burreson, 1990). Ceratobdella quadricornuta is one of the ‘tentacled’ leeches and possesses four well-developed finger-like tentacles surrounding the oral sucker, presumably sensory structures, and parasitizes the Antarctic starry skate Raja georgiana in the Scotia Sea (Sawyer, 1972; Utevsky and Gordeev, 2015). Ambulobdella shandikovi has limb-like dorsal and ventrolateral tubercles, considered most likely to be sensory structures but could play a role in locomotion (Utevsky and Utevsky, 2018). Deep-sea leeches are almost always collected by chance and their behavior has not been systematically studied. How these unique and unusual morphological adaptations or structures assist these species in surviving at these extreme pressures in the deep sea or extreme temperatures of the polar regions compared to their relatives living at much less extreme pressures and temperatures has not been determined. Also, physiological adaptations have yet to be evaluated in these organisms.
2.4. Extreme darkness
In addition to surface waters, leeches have been found living deep within cave systems around the world. Troglobitic leeches, such as Erpobdella mestrovi (=Croatobranchus mestrovi) and Haemopis caeca, live deep within caves in eastern Europe and have lost much of their body coloration as well as the ability to detect light (Manoleli et al., 1998; Sket et al., 2001), relying instead on mechanoreception and chemoreception to locate prey. Other leech species living in caves connected to surface waters may retain the ability to detect light and their coloration suggests that these species are recent or temporary inhabitants of caves. For example, Motobdella montezuma has been found living in the cave systems associated with Montezuma Well, which still has close ties to the surface waters and M. montezuma has not lost the ability to detect light (these leeches are negatively phototactic). Their ability to find prey in dark cave systems is likely due to their reliance on mechanoreception and the ability to detect minute vibrations produced by prey species (Blinn and Davies, 1989; Blinn et al., 1988; Govedich et al., 1998).
2.5. Extreme pH and pollution
Leeches are found in a diverse range of aquatic systems with a variety of different water chemistries including Montezuma Well, a collapsed travertine spring mound located in Central Arizona. Montezuma Well is unique in having very high levels of CO2 > 550 mg/L, a high alkalinity >600 mg/L CaCO3, and high levels of arsenic >110 mg/L (Cole and Barry, 1973). The unique water chemistry of Montezuma Well reduces the diversity of animals that can live in the Well and has eliminated most aquatic vertebrates (no fish or amphibians are present). Despite this, Montezuma Well is the home of several endemic leech species such as Motobdella montezuma, Helobdella blinnii, and other undescribed Helobdella species (Govedich et al., 1998; Beresic-Perrins et al., 2017). Not only are these leeches capable of surviving under these conditions, they have filled the open niches typically occupied by vertebrates, with M. montezuma as one of the top predators, feeding almost exclusively on amphipods using mechanoreception to detect and capture prey (Blinn et al., 1986, 1987; 1988, 1990; Blinn and Davies, 1989; Govedich et al., 1998).
3. Extreme physiological and behavioral tolerance or life history adaptations
3.1. Leech senses
Leeches have a number of sensory structures that can be used for finding potential prey or hosts and also for locating suitable habitats. These include photoreceptors (eyes), sensillae used as chemoreceptors (for chemicals in the air and water), and sensillae used as mechanoreceptors (for vibrations or sounds). All three senses are used together by leeches to locate potential prey and hosts. Leeches have simple eyes made of small photoreceptors, typically located on the dorsal surface of the first few segments of the body (Fig. 1C), with a few species having photoreceptors also on the posterior sucker. The simple eyes are capable of detecting light and dark and potentially some movement, though they are probably not capable of forming high resolution images. Chemoreception is another important sense for leeches, allowing them to detect chemicals released by potential prey/hosts and predators. Sensillae that contain chemoreceptors are often located along the length of the body of leeches, but in many species, these sensillae may be concentrated near the anterior end. Land leeches, in particular, are well known for using CO2 to find hosts and have chemoreceptors located along the anterior margin of the head that allows them to move in the direction of a potential host. Mechanoreception or the ability to detect vibrations or sound is important for both aquatic and land leeches. Sensillae used for mechanoreception are typically located anteriorly and may be quite large in some species such as M. montezuma (Davies et al., 1985). This predaceous leech is known for using a unique form of mechanoreception, similar to passive sonar, to detect its prey while swimming in the water column (Blinn et al. 1987, 1990). This behavior has been linked to the presence of large surface sensillae containing numerous cilia used to detect variations in vibration frequency (Blinn et al. 1986, 1988, 1990). In contrast, Erpobdella punctata, a common North American erpobdellid leech, does not have these large sensillae and does not depend on mechanoreception for prey detection (Blinn and Davies, 1989). Mechanoreception is also found in other leech species and assists in detecting potential prey and hosts to varying degrees.
3.2. Extreme feeding
The blood-feeding behavior of many leech species is their most well-known characteristic, notably the medicinal leeches exemplified by Hirudo medicinalis Linnaeus, 1758. Counter to popular belief, around one-half of the known leech species feed on blood with the rest being predaceous on a variety of different invertebrates (Moser et al., 2009). Blood-feeding leeches either protrude their proboscis into host tissue with the assistance of secreted proteolytic enzymes or make an incision with their teeth and jaws and feed on the pooled blood (Sawyer, 1986; Moser et al., 2009). Blood-feeding leeches are assisted in blood-feeding by their numerous salivary secretions which contain spreading factors that increases the permeability of the skin, histamine-like secretions that dilate blood vessels, secretions that inhibit the inflammatory response, and anticoagulants (Sawyer, 1986; Moser et al., 2009). Few leech anticoagulants have been extensively studied and all attack various pathways of the clotting cascade. Salivary secretions have been characterized through transcriptome sequencing and used to predict several anticoagulant-producing gene regions (Min et al., 2010; Kvist et al., 2014, 2017). Functional tests of these sequences uncovered hirudin-like factors (HLFs) that are structurally similar to the gene region responsible for hirudin, yet the products have very low or no anticoagulant properties (Müller et al., 2017, 2019). Hirudin, the most abundant anticoagulant of the medicinal leech H. medicinalis, is the most well-known and the only leech anticoagulant to be used in medicine. It is a very powerful anticoagulant that targets thrombin (Sohn et al., 2001). Hementin, the anticoagulant in the Giant Amazon leech Haementeria ghilianii, dissolves fibrinogen-formed blood clots, while Theromyzon tessulatum has a different thrombin-inhibitor, theromin (Sawyer et al., 1991; Salzet et al., 2000). Similar gene structure among hirudins, hirudin-like factors (HLFs), and decorsins indicates a single origin of bloodfeeding in arhynchobdellid leeches, while anticoagulants of glossiphoniid leeches seem to represent convergent evolution (Müller et al., 2019).
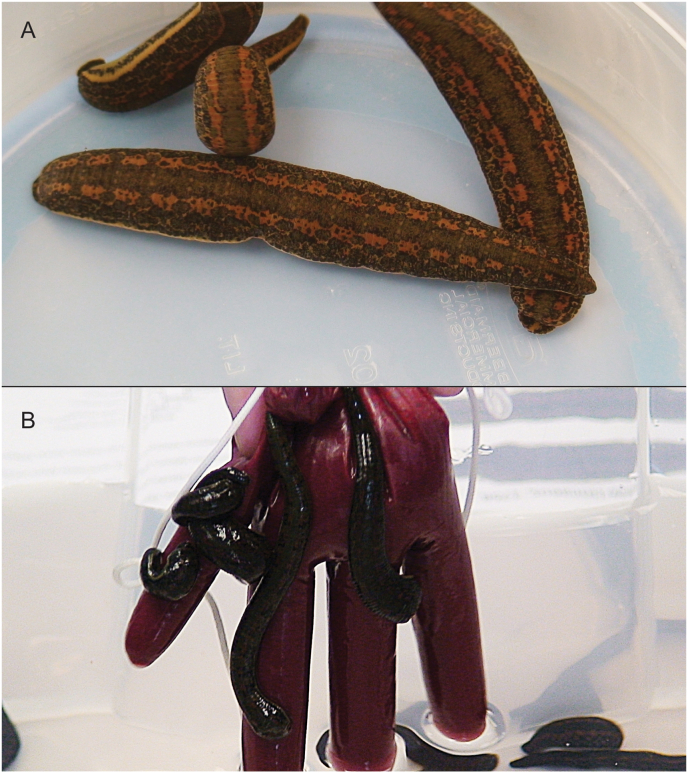
Blood-feeding leeches usually attach themselves to a host and feed until their engorgement, up to an elevenfold increase in body weight (Mann, 1962), followed by detachment (Fig. 3). The leech then can take several months to digest the bloodmeal, even though the salivary glands renew the supply of anticoagulants and salivary secretions within one week for unexpected feeding opportunities (Lemke et al., 2016; Sawyer, 1986). The digestive tract of blood-feeding leeches has branching lateral extensions, cecae, to maximize storage and surface area for absorption. Lent (1986) found individuals of H. medicinalis ingest 900 percent of their body weight in a single feeding and afterwards did not feed for an entire year. Ceca stretch receptors contribute to this remarkable feeding ability. In addition, blood-feeding desensitizes the leech to external stimuli. Once feeding was established, Galun and Kindler (1968) found that leeches would still feed after being cut and would feed for twice as long as leeches that were not cut. Li et al. (2018) found that individuals of Hirudo nipponia can withstand an internal fluid pressure approximately 6 times atmospheric pressure.
Fig. 3.
Extreme feeding. A) Hirudo verbana, a commercially important and frequently traded species of European medicinal leech. B) Several individuals of Hirudo verbana feeding on blood inside a nitrile rubber glove.
3.3. Hosts and orifices
Parasitism exhibited by leeches has driven some of the most dramatic morphological and behavioral adaptations within the annelids. Some leeches take the host-parasite association a step beyond feeding from the external surface of their host to entering and feeding inside the orifices. Members of Praobdellidae are characterized by feeding primarily from mucous membranes, particularly of mammals, but also from the skin of amphibians. This behavior is considered equally unnerving by both academic and public audiences. One explanation for targeting these tissues is that praobdellids have fewer teeth than other blood-feeding jawed leeches (0–40 compared to in excess of 100 in some species) and these are soft tissues with the blood vessels close to the surface (Phillips et al., 2010). Dinobdella ferox of South Asia frequently infests humans and livestock and reaches up to 8–10 inches in length, yet has jaws without teeth (Harding and Moore, 1927). Most human infestations occur unknowingly to the person while swimming, working in water, or by drinking unfiltered water, while the leeches invade the mouth, throat, nasal passages, and under the eyelids (Phillips et al., 2010). Leeches that retreat behind the eyeball to the recesses of the ocular orbit are quite tricky to remove, although hypertonic saline has been successfully used (Partyka and Fogg, 2009). Praobdellids tend to remain in orifices once the host leaves the water and will exit when the host's orifice is in humid or wet conditions, such as the next swimming trip or the shower (Lai, 2019). The leech may bite and feed multiple times within the orifice and can remain there for weeks to months (Harding and Moore, 1927; Lai, 2019). By seeking refuge in orifices, praobdellids have been dispersed through inhospitable environments via vertebrate migration (e.g. ungulates) and global airline travel (e.g. tourists and travelers).
Praobdellid leeches are not the only leeches known to enter hosts. Medicinal leeches (Hirudo spp.) applied as a treatment have been known to inadvertently migrate during therapy to invade orifices and surgical incisions (Park, 1993; Conroy et al., 2006) and in one case to tunnel through the bite wound (Flurry et al., 2011), despite not being members of Praobdellidae. In some cases, leeches have been reported as long-term endoparasites. Mann and Tyler (1963) reported infestations of the coelomic spaces of frogs in Papua New Guinea by the haemadipsid Philaemon cf. grandidieri. One specimen of P. cf. grandidieri was approximately the same length as the frog host and Mann and Tyler (1963) postulated that the leech entered the host when it was very small. Mann and Tyler (1963) also thought that the leech must take small bloodmeals to prevent exsanguinating the host. Theromyzon is a glossiphoniid genus of leeches that have a preference for feeding in the nasal cavities of aquatic birds (Davies et al., 2008). In addition to the nares, Theromyzon cooperi has been reported to feed in the eye socket of Rock Pigeons Columba livia (Oosthuizen et al., 1985). Heavily infested aquatic birds are lethargic with labored breathing and a heavy infestation can sometimes lead to paralysis (Bartonek and Trauger, 1975; Oosthuizen et al., 1985). Another glossiphoniid leech, Placobdelloides jaegerskioeldi inhabits one of the most extreme environments of all of the leeches that invade orifices, the rectum of the hippopotamus (Oosthuizen and Davies, 1994). Adult P. jaegerskioeldi have papilla-bearing tubercules that are postulated to provide traction against the anal-wall of the hippopotamus (Oosthuizen and Davies, 1994). This species is also one of the few glossiphoniid leeches that can actively swim and swims (even upstream) to its hippopotamus host (Oosthuizen and Davies, 1994).
3.4. Leech reproduction and parental care
Leeches are hermaphrodites, with each individual having both male and female reproductive organs, and leeches have been shown to have the ability to self-fertilize. The reproductive system of leeches is composed of paired testisacs and ovisacs, thin-walled structures derived from the coelom, which produce either spermatozoa or eggs. Mating in leeches ranges from mutual internal insemination, where the penis of each individual is inserted into the vagina of the other, to sperm exchange using hypodermic implantation of spermatophores (small sperm-filled sacs), which are attached to any location on the recipient's body (Salas-Montiel et al., 2017). The spermatophore, once attached, then releases sperm into the recipient's body and the sperm migrate to the ovisacs and fertilize the eggs. Once the eggs have been fertilized, they are released into a cocoon secreted by the clitellum. Hypodermic implantation may not be mutual and often individuals who are becoming reproductively mature will act as a male exclusively for a short time and will mate with as many individuals as possible. Hypodermic implantation also allows leeches to self-fertilize, if suitable partners are not available (Govedich et al., 2003; Govedich, 2004; Tan et al., 2004).
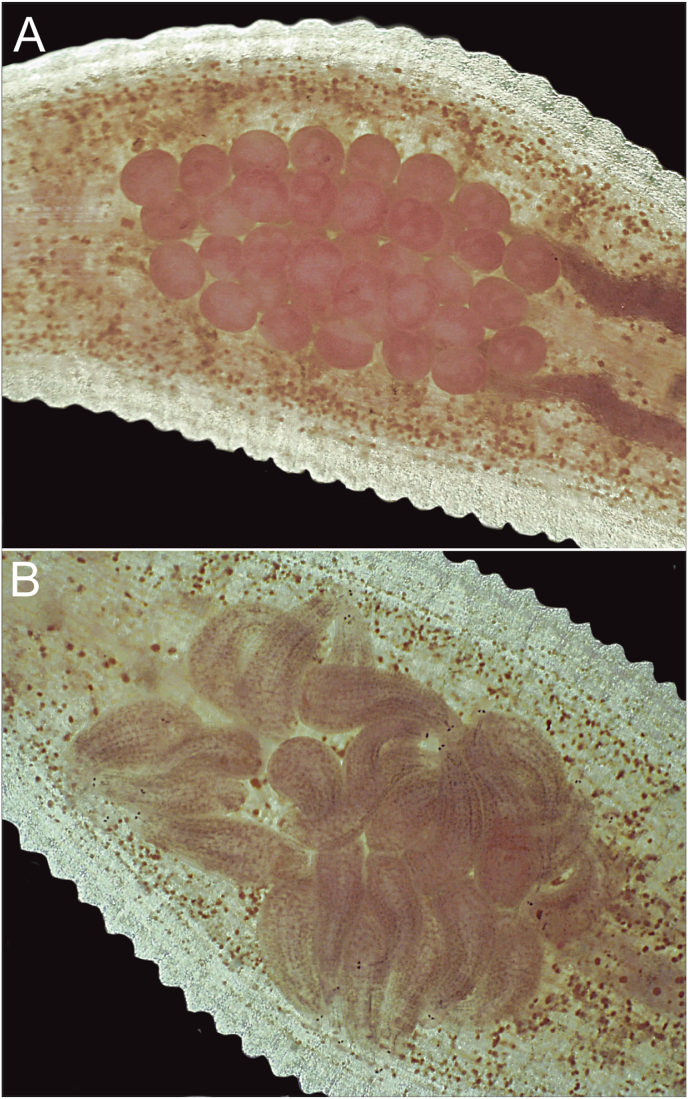
Leeches produce cocoons that may be thin and membranous, thick and leathery, or even have an almost plastic-like appearance. These cocoons help to support and protect the developing eggs and young. Most leech groups will abandon their cocoons once they have been produced, however, prior to abandonment, a nutrient fluid is provided inside the cocoon for the developing eggs and young. This stored energy nourishes the eggs and subsequent embryos and typically lasts until the developing young are large enough to emerge from the cocoon to fend for themselves. Juveniles are fully capable of caring for themselves after they leave the cocoon (Govedich et al., 2003; Govedich, 2004). Leeches in the family Glossiphoniidae, utilize a very different strategy, and care for their eggs and young for an extended period of time (Fig. 4). Parental care in this family includes both pre- and post-hatching parental care behaviors that range from the brooding of egg clusters (cocoons) in external nests, to brooding the developing eggs and young on the parent's body, to keeping eggs and developing young within an internal marsupial-like pouch (e.g. Marsupiobdella africana). All of these strategies allow the parents to protect the developing eggs from predators and at the same time allows them to ventilate the eggs by undulating their body, drawing oxygenated water across the eggs. Following hatching, parents will continue to protect and ventilate the young leeches, who attach themselves to the ventral surface of the parent immediately after hatching. Parents may also begin to provide food for their young either by capturing and providing prey (snails, oligochaetes, mosquito larvae, etc.) or by transferring nutrients across the body wall to the developing young in a manner reminiscent of a “placenta” (Sawyer, 1986; Kutschera and Wirtz, 1986a, Kutschera and Wirtz, 1986b; Kutschera, 1989, 1992; De Eguileor et al., 1994; Davies et al., 1997 Govedich, 2004; Paez et al., 2004). Parents have also been observed to starve themselves in order to feed their young when food is scarce (Govedich and Bain, unpublished data). Within the family Glossiphoniidae some species have also been observed living together in aggregations with individuals sharing food resources and even caring for juveniles that are not their own (Govedich, 2004). Living in aggregations is likely to reduce feeding costs and at the same time reduce the risk of predation. One outcome of living together and having large broods is sibling rivalry, where developing individuals compete with each other for limited food resources (Burd et al., 2006).
Fig. 4.
Leech parental care. A) Light microscopy image of a glossiphoniid leech with pink circular eggs gathered on its ventral side for protection. B) Light microscopy image of a glossiphoniid leech with leech hatchlings gathered on the ventral side of the parent leech. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Barbronia weberi is a well-known globally invasive leech species. Individuals are capable of self-reproduction and producing cocoons every other day for up to three months. Some individuals can produce more than 100 cocoons. Each cocoon is typically attached to a solid substrate including aquatic vegetation, rocks, or the sides of aquaria. Each cocoon may contain one to four small eggs, surrounded by transparent nutrient fluid. After hatching, juveniles remain within the cocoon for up to a month utilizing the stored nutrients. After leaving the cocoon, young B. weberi are capable of feeding independently and become reproductively mature within four months of hatching (Govedich et al., 2003). This rapid production of cocoons along with the ability for self-reproduction have contributed to the introduction and successful establishment of B. weberi in many new habitats worldwide (Sawyer, 2020).
3.5. Evolutionary considerations
In some cases, leeches in extreme environments have specialized morphological, physiological, or behavioral adaptations to survive these conditions, yet unique adaptations are not apparent in some species. Leeches that inhabit inhospitable habitats occur in more than one branch or family of leech phylogeny suggesting that there have been independent invasions of habitats with extreme conditions. The origins and evolution of adaptations and traits can be investigated with phylogeny and ancestral state reconstruction using morphology and molecular data, with the multiple evolutionary origins of terrestrialism and feeding preference having been paid the most attention. Functional genomics and transcriptomics will offer new insight into the genetic mechanisms underlying the traits that make leeches different from other annelids and enable leeches to survive in inhospitable conditions and habitats.
4. Conclusion
Leeches are the most extreme annelids. Polychaetes have many feeding behaviors and ecological adaptations, although the group is entirely aquatic. “Oligochaeta” has members that have invaded many different environments including marine, freshwater, terrestrial, and even glaciers (Mesenchytraceus species), and some species exhibit predatory behavior rather than feeding on detritus. In a fanciful competition between leeches and oligochaetes, leeches would easily dominate their annelid relatives since predatory leeches eat oligochaetes whole, as in the case of haemopids and M. buettikoferi, the Kinabalu Giant Red Leech, and leeches are demonstrably more robust and harder to kill when subjected to external trauma (Li et al., 2018).
Declaration of competing interest
The authors certify that we have no competing financial or non-financial interests.
We have no conflicts of interest to disclose.
Acknowledgements
The authors thank Bonnie Bain for thoughtful comments on an early version of the manuscript, and Freya Goetz for assistance with the figures and the illustration in the inset of Fig. 1. Special thanks goes to the Smithsonian Libraries for assistance with acquiring literature. Thank you to all of the dedicated leech researchers and collectors for their incredible discoveries and braving the extremes to collect and document leech diversity.
Contributor Information
Anna J. Phillips, Email: phillipsaj@si.edu.
Fredric R. Govedich, Email: govedich@suu.edu.
William E. Moser, Email: moserw@si.edu.
References
- Apakupakul K., Siddall M.E., Burreson E.M. Higher level relationships of leeches (Annelida: Clitellata: Euhirudinea) based on morphology and gene sequences. Mol. Phylogenet. Evol. 1999;12:350–359. doi: 10.1006/mpev.1999.0639. [DOI] [PubMed] [Google Scholar]
- Bartonek J.C., Trauger D.L. Leeches (Hirudinea) infestations among waterfowl near Yellowknife, Northwest Territories. Can. Field Nat. 1975;89:234–243. [Google Scholar]
- Beresic-Perrins R.K., Govedich F.R., Banister K., Bain B.A., Rose D., Shuster S.M. Helobdella blinni sp. n. (Hirundinida, Glossiphoniidae) a new leech species inhabiting Montezuma Well, Arizona, USA. ZooKeys. 2017;661:137–155. doi: 10.3897/zookeys.661.9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinn D.W., Davies R.W. The evolutionary importance of mechanoreception in three erpobdellid leech species. Oecologia. 1989;79:6–9. doi: 10.1007/BF00378232. [DOI] [PubMed] [Google Scholar]
- Blinn D.W., Davies R.W., Dehdashti B. Specialized pelagic feeding by Erpobdella montezuma (Hirudinea) Holarctic Ecol. 1987;10:235–240. [Google Scholar]
- Blinn D.W., Dehdashti B., Runck C., Davies R.W. The importance of prey size and density in an endemic predator-prey couple (leech Erpobdella montezuma-amphipod Hyalella montezuma) J. Anim. Ecol. 1990;59:187–192. [Google Scholar]
- Blinn D.W., Pinney C., Wagner V.T. Intraspecific discrimination of amphipod prey by a freshwater leech through mechanoreception. Can. J. Zool. 1988;66:427–430. [Google Scholar]
- Blinn D.W., Wagner V.T., Grim J.N. Surface sensilla of the predaceous fresh-water leech Erpobdella montezuma: possible importance in feeding. Trans. Am. Microsc. Soc. 1986;105:21–30. [Google Scholar]
- Borda E., Siddall M.E. Arhynchobdellida (Annelida: Oligochaeta: Hirudinida): phylogenetic relationships and evolution. Mol. Phylogenet. Evol. 2004;30:213–225. doi: 10.1016/j.ympev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Borda E., Siddall M.E. Insights into the evolutionary history of Indo-Pacific bloodfeeding terrestrial leeches (Hirudinida: Arhynchobdellida: haemadipisdae) Invertebr. Systemat. 2011;24:456–472. [Google Scholar]
- Borda E., Oceguera-Figueroa A.F., Siddall M.E. On the classification, evolution and biogeography of terrestrial haemadipsoid leeches (Hirudinida: Arhynchobdellida: Hirudiniformes) Mol. Phylogenet. Evol. 2008;46:142–154. doi: 10.1016/j.ympev.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Burd M., Govedich F.R., Bateson L. Sibling competition in a brood-tending leech. P. Roy. Soc. B-Biol. Sci. 2006;237:2461–2466. doi: 10.1098/rspb.2006.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burreson E.M. A new deep-sea leech, Bathybdella sawyeri, n. gen., n. sp., from thermal vent areas on the Galápagos Rift. Proc. Biol. Soc. Wash. 1981;94:483–491. [Google Scholar]
- Burreson E.M. A new deep-sea fish leech (Hirudinida: Piscicolidae) from the North east pacific ocean. Comp. Parasitol. 2016;83:88–91. [Google Scholar]
- Burreson E.M. Marine and estuarine leeches (Hirudinida : Ozobranchidae and Piscicolidae) of Australia and New Zealand with a key to the species. Invertebr. Systemat. 2020;34:235–259. [Google Scholar]
- Burreson E.M., Segonzac M. Morphological variability of Bathybdella sawyeri (Hirudinida: Piscicolidae) from hydrothermal vents on the Galápagos Rift and the south east pacific Rise. Zootaxa. 2006;1286:15–21. [Google Scholar]
- Cole G.A., Barry W.T. Montezuma well, Arizona, as a habitat. J. Ariz. Acad. Sci. 1973;8:7–13. [Google Scholar]
- Conroy F.J., Whitaker I.S., Jivan S., Majumder S. The prevention of migration during leech therapy. Plast. Reconstr. Surg. 2006;117:2539. doi: 10.1097/01.prs.0000219989.66879.90. [DOI] [PubMed] [Google Scholar]
- Davies R.W., Gates T.E. The effects of different oxygen regimes on the feeding and vertical distribution of Nephelopsis obscura (Hirudinoidea) Hydrobiologia. 1991;211:51–56. [Google Scholar]
- Davies R.W., Gates T.E. Intra- and interspecific differences in the response of two lentic species of leeches to seasonal hyperoxia. Can. J. Fish. Aquat. Sci. 1991;48:1124–1127. [Google Scholar]
- Davies R.W., Govedich F.R. Annelida: Euhirudinea, and Acanthobdellidae. In: Thorp J.H., Covich A.P., editors. Ecology and Classification of North American Freshwater Invertebrates. Academic Press; San Diego and New York: 2001. pp. 465–504. [Google Scholar]
- Davies R.W., Govedich F.R., Moser W.E. Leech parasites of birds. In: Atkinson C.T., Thomas N.J., Hunter D.B., editors. Parasitic Diseases of Wild Birds. Wiley-Blackwell; Ames: 2008. pp. 501–514. [Google Scholar]
- Davies R.W., McLoughlin N.J., Oosthuizen J.H. The life-cycle and feeding of the African freshwater leech Helobdella conifera (Glossiphoniidae) S. Afr. J. Zool. 1997;32:1–4. [Google Scholar]
- Davies R.W., Singhal R.N., Blinn D.W. Erpobdella montezuma (Hirudinoidea: Erpobdellidae), a new species of freshwater leech from North America. Can. J. Zool. 1985;63:965–969. [Google Scholar]
- Davies R.W., Yang T., Wrona F.J. Inter- and intra-specific differences in the effects of anoxia on erpobdellid leeches using static and flow-through systems. Holarctic Ecol. 1987;10:149–153. [Google Scholar]
- De Eguileor M., Daniel E., Giordana B., Lanzavecchia G., Valvassori R. Trophic exchanges between parent and young during development of Glossiphonia complanata (Annelida, Hirudinea) J. Exp. Zool. 1994;269:389–402. doi: 10.1002/jez.1402690502. [DOI] [PubMed] [Google Scholar]
- Flurry M., Natoli N.B., Mesa J.M., Moyer K.E. Tunneling of a leech into a free flap breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2011;64:1687–1688. doi: 10.1016/j.bjps.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Galun R., Kindler S.H. Regulation of feeding in leeches. Experientia. 1968;24:1140. [Google Scholar]
- Glasby C.J., Read G.B., Lee K.E., Blakemore R.J., Fraser P.M., Pinder A.M., Erséus C., Moser W.E., Burreson E.M., Govedich F.R., Davies R.W., Dawson E.W. Phylum Annelida: bristleworms, earthworms, leeches. In: Gordon D.P., editor. New Zealand Inventory of Biodiversity, Volume 1, Kingdom Animalia: Radiata, Lophotrochozoa, and Deuterostomia. Canterbury University Press; Christchurch: 2009. pp. 312–358. [Google Scholar]
- Govedich F.R. Tender loving leeches. Aust. Sci. 2004;25:16–22. [Google Scholar]
- Govedich F.R., Moser W.E. Clitellata: Hirudinida and Acanthobdellida. In: Thorp J., Rogers D.C., editors. Ecology and General Biology: Thorp and Covich's Freshwater Invertebrates. Academic Press; New York and London: 2015. pp. 565–588. [Google Scholar]
- Govedich F.R., Bain B.A., Burd M., Davies R.W. Life history of the invasive Asian freshwater leech Barbronia weberi (Blanchard, 1897) (Euhirudinea: Erpobdellidae) in Australia. Hydrobiologia. 2003;510:125–129. [Google Scholar]
- Govedich F.R., Bain B.A., Moser W.E., Gelder S.R., Davies R.W., Brinkhurst R.O. Annelida (Clitellata): Oligochaeta, Branchiobdellida, Hirudinida, Acanthobdellida. In: Thorp J.H., Covich A.P., editors. Ecology and Classification of North American Freshwater Invertebrates. Academic Press; New York and London: 2010. pp. 385–436. [Google Scholar]
- Govedich F.R., Blinn D.W., Keim P., Davies R.W. Phylogenetic relationships of three genera of Erpobdellidae (Hirudinoidea), with a description of a new genus, Motobdella, and species, Motobdella sedonensis. Can. J. Zool. 1998;76:2161–2171. [Google Scholar]
- Grosser C. Haemopis elegans (Hirudinea: Haemopidae) – ein wiederentdecktes europäisches Egeltaxon. Lauterbornia. 2004;52:77–86. [Google Scholar]
- Hall F.G. The vital limit of exsiccation of certain animals. Biol. Bull. 1922;42:31–51. [Google Scholar]
- Harding W.A., Moore J.P. The Fauna of British India, Including Ceylon and Burma. 1927. Hirudinea; pp. 1–302. (London) [Google Scholar]
- Keegan H.L., Toshioka S., Suzuki H. Blood sucking asian leeches of families hirudidae and Haemadipsidae. 406th medical laboratory special report. Bio-Med. Rep. 406 Med. Lab. 1968;16:1–130. [Google Scholar]
- Klemm D.J. United States Environmental Protection Agency, Environmental and Support Laboratory; Cincinnati, Ohio: 1982. Leeches (Annelida: Hirudinea) of North America. EPA-600/3-82/025. [Google Scholar]
- Kua B.C., Choong F.C., Leaw Y.Y. Effect of salinity and temperature on marine leech, Zeylanicobdella arugamensis (De Silva) under laboratory conditions. J. Fish. Dis. 2014;37 doi: 10.1111/jfd.12087. 210-207. [DOI] [PubMed] [Google Scholar]
- Kutschera U. Reproductive behaviour and parental care of the leech Helobdella californica (Hirudinea: Glossiphoniidae) Zool. Anz. 1989;222:122–128. [Google Scholar]
- Kutschera U. Reproductive behaviour and parental care of the leech Helobdella triserialis (Hirudinea: Glossiphoniidae) Zool. Anz. 1992;228:74–81. [Google Scholar]
- Kutschera U., Wirtz P. A leech that feeds its young. Anim. Behav. 1986;34:941–942. [Google Scholar]
- Kutschera U., Wirtz P. Reproductive behaviour and parental care of Helobdella striata (Hirudinea, Glossiphoniidae): a leech that feeds its young. Ethology. 1986;72:132–142. [Google Scholar]
- Kvist S., Oceguera-Figueroa A.F., Tessler M., Jiménez-Armenta J., Freeman R.M., Giribet G., Siddall M.E. When predator becomes prey: investigating the salivary transcriptome of the shark-feeding leech Pontobdella macrothela (Hirudinea: Piscicolidae) Zool. J. Linn. Soc. 2017;179:725–737. [Google Scholar]
- Kvist S., Brugler M.R., Goh T.G., Giribet G., Siddall M.E. Pyrosequencing the salivary transcriptome of Haemadipsa interrupta (Annelida: Clitellata: Haemadipsidae): anticoagulant diversity and insight into the evolution of anticoagulant capabilities in leeches. Invertebr. Biol. 2014;133:74–98. [Google Scholar]
- Lai Y.-T. Beyond the epistaxis: voluntary nasal leech (Dinobdella ferox) infestation revealed the leech behaviours and the host symptoms through the parasitic period. Parasitology. 2019;146:1477–1485. doi: 10.1017/S0031182019000751. [DOI] [PubMed] [Google Scholar]
- Lemke S., Müller C., Hildebrandt J. Be ready at any time: postprandial synthesis of salivary proteins in salivary gland cells of the haematophagous leech Hirudo verbana. J. Exp. Biol. 2016;219:1139—1145. doi: 10.1242/jeb.135509. [DOI] [PubMed] [Google Scholar]
- Lent C. New medical and scientific uses of the leech. Nature. 1986;323:494. doi: 10.1038/323494a0. [DOI] [PubMed] [Google Scholar]
- Li S., Zhang Y., Dou X., Zuo P., Liu J. Hard to be killed: load-bearing capacity of the leech Hirudo nipponia. J. Mech. Behav. Biomed. Mater. 2018;86:345–351. doi: 10.1016/j.jmbbm.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Mann K.H. A study of the oxygen consumption of five species of leech. J. Exp. Biol. 1956;33:615–626. [Google Scholar]
- Mann K.H. Pergamon Press; New York: 1962. Leeches (Hirudinea) Their Structure, Physiology, Ecology and Embryology. [Google Scholar]
- Mann K.H., Tyler M.J. Leeches as endoparasites of frogs. Nature. 1963;197:1224–1225. [Google Scholar]
- Manoleli D.G., Klemm D.J., Sarbu S.M. Haemopis caeca (Annelida: Hirudinea: ahrynchobdella: Haemopidae), A new species of troglobitic leech from a chemoautotrophically based groundwater ecosystem in Romania. Proc. Biol. Soc. Wash. 1998;111:222–229. [Google Scholar]
- Meyer M.C., Burreson E.M. Some leeches (Hirudinea: Piscicolidae) of the Southern Oceans. Biology of the antarctic seas XXI. Antarct. Res. 1990;52:219–236. [Google Scholar]
- Min G.S., Sarkar I.N., Siddall M.E. Salivary transcriptome of the North American medicinal leech, Macrobdella decora. J. Parasitol. 2010;96:1211–1221. doi: 10.1645/GE-2496.1. [DOI] [PubMed] [Google Scholar]
- Moser W.E., Govedich F.R., Klemm D.J. Annelida, Hirudinida (leeches) In: Likens G.E., editor. vol. 2. Elsevier Press; Oxford: 2009. pp. 116–123. (Encyclopedia of Inland Waters). [Google Scholar]
- Müller C., Haase M., Lemke S., Hildebrandt J.-P. Hirudin and hirudin-like factors in Hirudinidae: implications for function and phylogenetic relationships. Parasitol. Res. 2017;116:313–325. doi: 10.1007/s00436-016-5294-9. [DOI] [PubMed] [Google Scholar]
- Müller C., Lukas P., Lemke S., Hildebrandt J.-P. Hirudin and decorsins of the North American medicinal leech Macrobdella decora: gene structure reveals homology to hirudin and hirudin-like factors of Eurasian medicinal leeches. J. Parasitol. 2019;105:423–431. [PubMed] [Google Scholar]
- Nakano T., Ramlah Z., Hikida T. Phylogenetic position of gastrostomobdellid leeches (Hirudinida, Arhynchobdellida, Erpobdelliformes) and a new family for the genus Orobdella. Zool. Scripta. 2012;41:177–185. [Google Scholar]
- Oceguera-Figueroa A.F. Molecular phylogeny of the New World bloodfeeding leeches of the genus Haementeria and reconsideration of the biannulate genus Oligobdella. Mol. Phylogenet. Evol. 2012;62:508–514. doi: 10.1016/j.ympev.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Oceguera-Figueroa A.F. Class Clitellata: subclass Hirudinida. In: Damborenea C., Rogers D.C., Thorp J., editors. Keys to Neotropical and Antarctic Fauna. Academic Press; New York and London: 2020. pp. 463–474. [Google Scholar]
- Oceguera-Figueroa A.F., Phillips A.J., Pacheco-Chaves B., Reeves W.K., Siddall M.E. Phylogeny of macrophagous leeches (Hirudinea, Clitellata) based on molecular data and evaluation of the barcoding locus. Zool. Scripta. 2011;40:194–203. [Google Scholar]
- Oosthuizen J.H., Davies R.W. The biology and adaptations of the hippopotamus leech Placobdelloides jaegerskioeldi (Glossiphoniidae) to its host. Can. J. Zool. 1994;72:418–422. [Google Scholar]
- Oosthuizen J.H., le R., Fourie F. Mortality amongst waterbirds caused by the African duck leech. Theromyzon cooperi. S. Afr. J. Wildl. Res. 1985;15:98–106. [Google Scholar]
- Paez D., Govedich F.R., Bain B.A., Kellett M., Burd M. Costs of parental care on hunting behaviour of Helobdella papillornata (Euhirudinea: Glossiphoniidae) Hydrobiologia. 2004;519:185–188. [Google Scholar]
- Park A. The case of the disappearing leech. Br. J. Plast. Surg. 1993;46:543. doi: 10.1016/0007-1226(93)90236-5. [DOI] [PubMed] [Google Scholar]
- Partyka C., Fogg T. Leech on the eye: a novel use for hypertonic saline. Emerg. Med. Australasia (EMA) 2009;21:84–85. doi: 10.1111/j.1742-6723.2009.01156.x. [DOI] [PubMed] [Google Scholar]
- Phillips A.J., Siddall M.E. Poly-paraphyly of Hirudinidae: many lineages of medicinal leeches. BMC Evol. Biol. 2009;9:246. doi: 10.1186/1471-2148-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.J., Arauco-Brown R., Oceguera-Figueroa A., Gomez G.P., Beltrán M., Siddall M.E. Tyrannobdella rex n. gen. n. sp. and the evolutionary origins of mucosal leech infestations. PloS One. 2010;5 doi: 10.1371/journal.pone.0010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.J., Dornburg A., Zapfe K.L., Anderson F.E., James S.W., Erséus C., Moriarty Lemmon E., Lemmon A.R., Williams B.W. Phylogenomic analysis of a putative missing link sparks reinterpretation of leech evolution. Genome Biol. Evol. 2019;11:3082–3093. doi: 10.1093/gbe/evz120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson L.R. The family Ozobranchidae redefined, and a novel ozobranchiform leech from murray river turtles (class Hirudinoidea; order rhynchobdelliformes) Proc. Linn. Soc. N. S. W. 1969;94:61–80. [Google Scholar]
- Richardson L.R. A contribution to the Systematics of the Hirudinid leeches, with description of new families, genera, and species. Acta Zool. Acad. Sci. Hungar. 1969;15:97–149. [Google Scholar]
- Richardson L.R. A contribution to the general zoology of the land-leeches (Hirudinea: haemadipsoidea Superfam. Nov.) Acta Zool. Acad. Sci. Hungar. 1975;21:119–152. [Google Scholar]
- Richardson L.R., Meyer M.C. deep-Sea fish leeches (rhynchobdellae: Piscicolidae) Galathea Rep. 1973;12:113–126. [Google Scholar]
- Salas-Montiel R., Phillips A.J., Contreras-Mirón S., Oceguera-Figueroa A. Prevalence, abundance, and intensity of implanted spermatophores in the leech Haementeria officinalis (Glossiphoniidae: Hirudinidae) from Guanajuato, Mexico. J. Parasitol. 2017;103:47–51. doi: 10.1645/16-56. [DOI] [PubMed] [Google Scholar]
- Salzet M., Chopin V., Baert J., Matias I., Malecha J. Theromin, a novel thrombin inhibitor. J. Biol. Chem. 2000;40:30774–30780. doi: 10.1074/jbc.M000787200. [DOI] [PubMed] [Google Scholar]
- Sawyer R.T. A new species of ‘tentacled’ marine leech from the subantarctic Marion and Crozet Islands. Hydrobiologia. 1972;40:345–354. [Google Scholar]
- Sawyer R.T. Leeches (Annelida: Hirudinea) In: Hart C.W.Jr, Fuller S.L.H., editors. Pollution Ecology of Freshwater Invertebrates. Academic Press; New York: 1974. pp. 81–142. [Google Scholar]
- Sawyer R.T. vols. I–III. Clarendon Press; Oxford: 1986. (Leech Biology and Behaviour). [Google Scholar]
- Sawyer R.T. Observations on the terrestrial leech Haemopis septagon sawyer & shelley, 1976 (Annelida: Hirudinea) from the outer banks, North Carolina, USA, with a revision of the species. Zootaxa. 2019;4658:275–296. doi: 10.11646/zootaxa.4658.2.4. [DOI] [PubMed] [Google Scholar]
- Sawyer R.T. Reproduction without cross-fertilisation in the invasive Asian leech Barbronia weberi (Blanchard, 1897) (Hirudinea: Arhynchobdellida) Aquat. Invasions. 2020;15:271–281. [Google Scholar]
- Sawyer R.T., Powell Jones C., Munro R. The biological function of Hementin in the proboscis of the leech Haementaria ghilianii. Blood Coagul. Fibrinolysis. 1991;2:153–159. doi: 10.1097/00001721-199102000-00023. [DOI] [PubMed] [Google Scholar]
- Siddall M.E., Budinoff R.B., Borda E. Phylogenetic evaluation of systematics and biogeography of the leech family Glossiphoniidae. Invertebr. Systemat. 2005;19:105–112. [Google Scholar]
- Singhal R.N., Davies R.W. Histopathology of hyperoxia in Nephelopsis obscura (Hirudinoidea: Erpobdellidae) J. Invertebr. Pathol. 1987;50:33–39. [Google Scholar]
- Singhal R.N., Sarnat H.B., Davies R.W. Effect of anoxia and hyperoxia on the neurons in the leech Nephelopsis obscura (Erpobdellidae): ultrastructural studies. J. Invertebr. Pathol. 1988;52:409–418. doi: 10.1016/0022-2011(89)90078-5. [DOI] [PubMed] [Google Scholar]
- Sket B., Trontelj P. Global diversity of leeches (Hirudinea) in freshwater. Hydrobiologia. 2008;595:129–137. [Google Scholar]
- Sket B., Dovč P., Jalžić B., Kerovec M., Kučinić M., Trontelj P. A cave leech (Hirudinea, Erpobdellidae) from Croatia with unique morphological features. Zool. Scripta. 2001;30:223–229. [Google Scholar]
- Sladacek V., Kosel V. Indicator value of freshwater leeches (Hirudinea) with a key to the determination of European species. Acta Hydrochim. Hydrobiol. 1984;12:451–461. [Google Scholar]
- Sohn J., Kang H., Rao K.J., Kim C.H., Choi E.S., Chung B.H., Rhee S.K. Current status of the anticoagulant hirudin: its biotechnological production and clinical practice. Appl. Microbiol. Biotechnol. 2001;57:606–613. doi: 10.1007/s00253-001-0856-9. [DOI] [PubMed] [Google Scholar]
- Stammers F.M.G. Observations on the behaviour of land-leeches (genus Haemadipsa) Parasitology. 1950;40:237–246. doi: 10.1017/s0031182000018084. [DOI] [PubMed] [Google Scholar]
- Suzuki D., Miyamoto T., Kikawada T., Watanabe M., Suzuki T. A leech capable of surviving exposure to extremely low temperatures. PloS One. 2014;9:1–5. doi: 10.1371/journal.pone.0086807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.N., Govedich F.R., Burd M. Social group size, potential sperm competition, and reproductive investment in a hermaphroditic leech, Helobdella papillornata (Euhirudinea: Glossiphoniidae) J. Evol. Biol. 2004;17:574–580. doi: 10.1111/j.1420-9101.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- Tessler M., de Carle D., Voiklis M.L., Gresham O.A., Neumann J.S., Cios S., Siddall M.E. Worms that suck: phylogenetic analysis of Hirudinea solidifies the position of Acanthobdellida and necessitates the dissolution of Rhynchobdellida. Mol. Phylogenet. Evol. 2018;127:129–134. doi: 10.1016/j.ympev.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Utevsky A., Gordeev I. New tentacled leech Ceratobdella quadricornuta n. g., n. sp. (Hirudinida: Piscicolidae) parasitic on the starry skate Raja georgiana Norman from the Scotia Sea, Antarctica. Syst. Paraistol. 2015;91:203–210. doi: 10.1007/s11230-015-9570-3. [DOI] [PubMed] [Google Scholar]
- Utevsky S.Y., Trontelj P. Phylogenetic relationships of fish leeches (Hirudinea, Piscicolidae) based on mitochondrial DNA sequences and morphological data. Zool. Scripta. 2004;33:375–385. [Google Scholar]
- Utevsky S., Kovalchuk A., Kovalchuk N., Utevsky A., Chernyshev A.V. A new species of the genus Johanssonia Selensky, 1914 (Hirudinea: Piscicolidae) collected in the Kuril-Kamchatka Trench at the greatest depth ever recorded for fish leeches. Prog. Oceanogr. 2019;176:1–8. [Google Scholar]
- Utevsky A., Utevsky A. New Antarctic deep-sea weird leech (Hirudinida: Piscicolidae): morphological features and phylogenetic relationships. Syst. Parasitol. 2018;95:849–861. doi: 10.1007/s11230-018-9816-y. [DOI] [PubMed] [Google Scholar]
- Williams J.I., Burreson E.M. Phylogeny of the fish leeches (Oligochaeta, Hirudinida, Piscicolidae) based on nuclear and mitochondrial genes and morphology. Zool. Scripta. 2006;35:627–639. [Google Scholar]
- Wirchansky B.A., Shain D.H. A new species of Haemopis (Annelida: Hirudinea): evolution of North American terrestrial leeches. Mol. Phylogenet. Evol. 2010;54:226–234. doi: 10.1016/j.ympev.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Wrona F.J., Davies R.W. An improved flow-through respirometer for aquatic macroinvertebrate bioenergetic research. Can. J. Fish. Aquat. Sci. 1984;41:380–385. [Google Scholar]