Abstract
Anthocyanins, betalains, riboflavin, carotenoids, chlorophylls and caramel are the basic natural food colorants used in modern food manufacture. Betalains, which are composed of red–violet betacyanin and yellow betaxanthins, are water-soluble pigments that color flowers and fruits. Betalains are pigments primarily produced by plants of the order Caryophyllales. Because of their anti-inflammatory, cognitive impairment, anticancer and anti-hepatitis properties, betalains are useful as pharmaceutical agents and dietary supplements. Betalains also exhibit antimicrobial and antimalarial effects, and as an example, betalain-rich Amaranthus spinosus displays prominent antimalarial activity. Studies also confirmed the antidiabetic effect of betalains, which reduced glycemia by 40% without causing weight loss or liver impairment. These findings show that betalain colorants may be a promising alternative to the synthetic dyes currently used as food additives.
Keywords: betalain, cancer, natural product, beetroot, Beta vulgaris
1. Introduction
Vegetables and phytochemicals markedly decrease the risk of different degenerative and chronic diseases [1] such as colorectal cancer, which is a significant cause of death worldwide; however, only few individuals use a traditional diet including fruits and vegetables. Recently, the use of natural food colorants has increased mainly owing to their low toxicity, environmental safety and renewable vegetable origin [2]. In addition to enhancing the appearance of food, natural colorants have bioactive properties that protect the plant in which they are contained against environmental stimuli caused by infections with fungi, insects or microorganisms. Furthermore, some colorants may be beneficial to human health [3]. Food colorants such as betalains have chemoprotective effects that combat oxidative stress and balance oxidants and antioxidants in the body. The Chenopodiaceae family includes two classes of vegetables, containing Swiss chard (Beta vulgaris L. var. cicla; BVc) and beetroot (B. vulgaris var. rubra L.; BVr), which have been a part of the traditional western diet [4].
The powder or extract form of betanin, a natural pigment, is an antioxidant used in the food industry. The antioxidant activity of betanin in biologic lipid environments has been indicated in human macromolecules such as membranes, low-density lipoproteins (LDL) and whole cells [5]. Moreover, betanin exerts anti-inflammatory effect and protects hepatic functions in human cells. The compound regulates redox signaling pathways mediated by the inflammatory response in cultured endothelial cells and exerts antiproliferative effects on human tumor cell lines [6]. Specifically, in both healthy liver and tumoral human hepatic cell lines, betanin induces the translocation of the an antioxidant response element called erythroid 2-related factor 2 (Nrf2) from the cytosol to the nuclear compartment, known to conduct mRNA and protein levels of detoxifying/antioxidant enzymes including GSTM, GSTP, GSTA (glutathione S-transferases), GSTT, HO– (hemeoxygenase-1) and NQO1 (NAD(P)H quinone dehydrogenase 1), thereby, exerting hepatoprotective and anticarcinogenic effects [7,8]. Various studies related to isolation of betanin, involving comprehensive steps and procedures to extract the purified compound from plant sources including complex food matrices such as beets. Among the purification studies of betanin, it is said that chromatographic methods, including high-efficiency liquid chromatography, applying reverse phase columns provide most efficient results [9]. However, no studies have been found evaluating the stability of this molecule during storage conditions or its antioxidant ability after purification and during storage [10].
In the food industry, synthetic antioxidants are added to fatty foods, especially meats, for delaying oxidative processes that cause sensorial changes, decreases in nutritional values and formation of secondary compounds that are potentially harmful to health during storage 11]. However, the synthetic antioxidants butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) may cause harmful effects to human health, as they have been reported to be potential tumor promoters succeeding long-term administration to animals [11]. Therefore, these synthetic antioxidants have been replaced with natural antioxidants extracted from food [11,12].
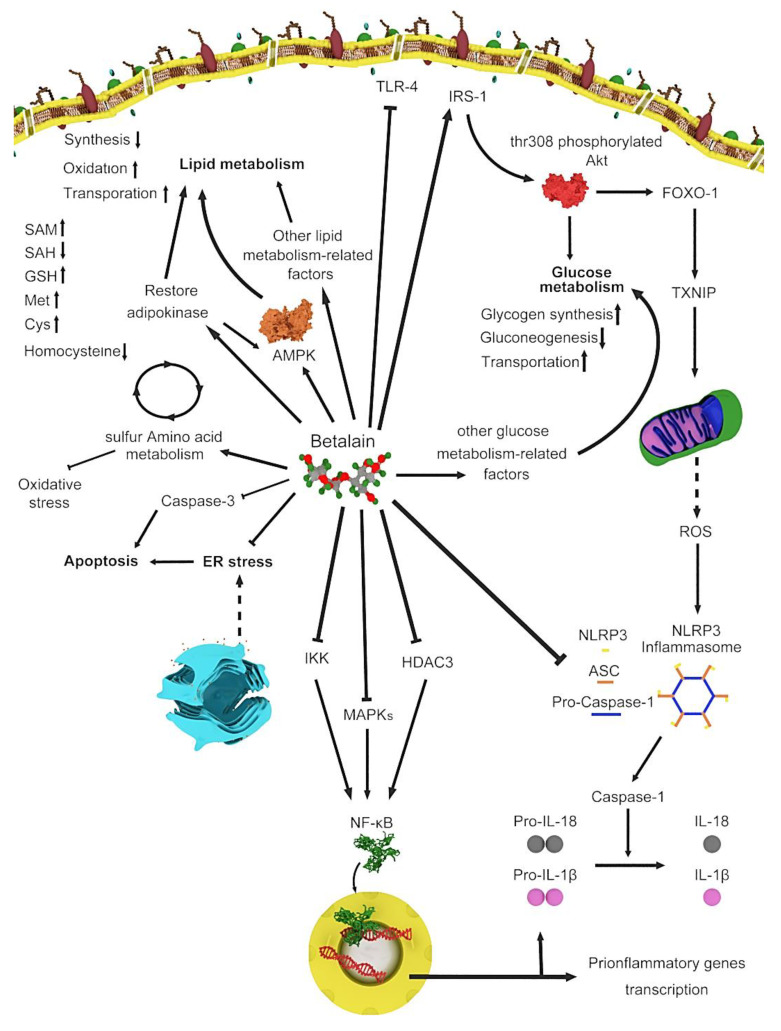
Betalain-rich extracts from food sources has been investigated for the antitumoral potential in animal models and cancer cell lines [4,5,6]. Betanin, the original nutritional betacyanin, show significant inhibition to the growth of tumor cells of the stomach, breast, lung, colon and central nervous system [7]; induce apoptosis in K562 human myeloid leukemia cells; and weakly exhibit epigenome-regulated gene expression in MCF-7 breast cancer cells. However, the potential antiproliferative, chemopreventive and epigenetic activities of betaxanthins are yet to be investigated [8]. Recently, the focus has shifted to the usage of natural products to improve human health instead of prevention diseases [9]. Thus, the number of studies on the application of betalains in medical sciences is increasing. Therefore, a narrative review of the therapeutic uses of betalains and the genes involved in betalain metabolism (Figure 1) may help future investigations regarding the advantages of natural products. Because of the importance of issue, few interesting reviews articles have been published very recently [11,12,13]. In this review, we tried to offer updated data in different therapeutic classification with focus to the molecular mechanisms of betalains.
Figure 1.
Various genes and routes involved in betalain metabolism.
2. Taxonomy
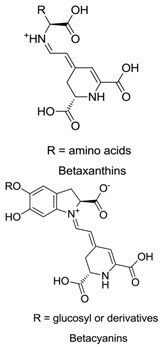
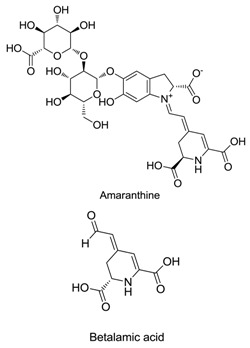
Betalains contain two classes of pigments, namely yellow betaxanthins and red betacyanins [14]. Red beetroot (BVr) extract is a group of betalains with significant antioxidant activity, which is attributed to betalamic acid, as determined by the 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and ferric reducing antioxidant power methods [15]. Beet is taxonomically classified as the genus Beta of the subfamily Chenopodiaceae, subclass Caryophyllidae and class Dicotyledonae [16]. Based on its morphologic characteristics, the genus Beta includes two groups, namely cultivated and wild maritime beets. In the wild maritime group, a unique species called sea beets (B. vulgaris maritima) is the ancestral form of all the remaining species. The cultivated group consists of sugar beets (B. vulgaris saccharifera), leaf beets (B. vulgaris cicla), forage beets (B. vulgaris crassa) and garden beets (B. vulgaris rubra) [16]. A list of betalain-producing plants is provided in Table 1.
Table 1.
Betalain-producing plant species.
| Family | Species | Common Name or Representative | Chemical Structures | Betalains | References |
|---|---|---|---|---|---|
| Achatocarpaceae Aizoaceae | – | – | – | – | [17] |
| Amaranthaceae | Amaranthus spinosus |
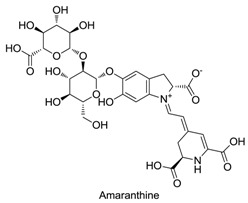
|
Amaranthine, isoamaranthine | [18] | |
| Gomphrena globosa | Spiny amaranth |
|
Betaxanthins and several betacyanins | [19] | |
| Celosia argentea (var. plumose and var. cristata) | Feathered amaranth and common cockscomb |
|
Amaranthine, betalamic acid, dopamine-derived betacyanins | [20] | |
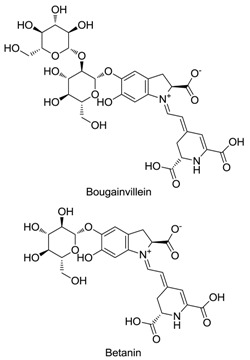
| Cactaceae | Hylocereus polyrhizus | Red-purple pitaya |
|
Betacyanins (10 kinds), bougainvillein-r-I, betanin, isobetanin, phyllocactin, isophyllocactin, hylocerenin | [21,22] |
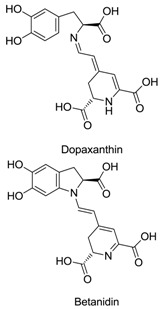
| Aizoaceae | Lampranthus productus | Ice plant |
|
Dopaxanthin, betanidin | [23] |
| Nyctaginaceae | Boerhavia erecta | Erect spiderling |
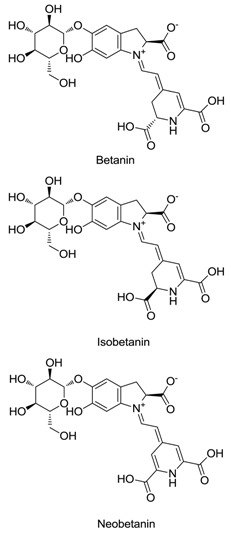
|
Betanin, isobetanin, neobetanin | [24] |
| Portulacaceae | Portulaca grandiflora | Moss rose |
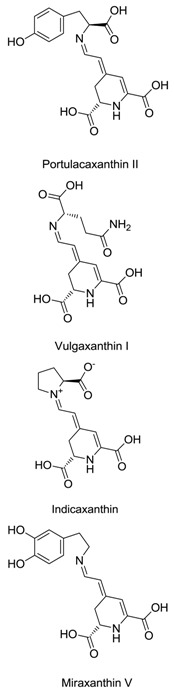
|
Dopaxanthin, portulacaxanthin II, vulgaxanthin I, miraxanthin V, indicaxanthin | [25,26] |
3. Therapeutic Effect of Betalains
3.1. Antidiabetic Activity
Yanardag et al. [27] identified a low level of blood sugar in diabetic rats administered BVc extract. Further investigation found that glycemia in these rats was reduced to 40% without causing weight loss or hepatic impairment [28].
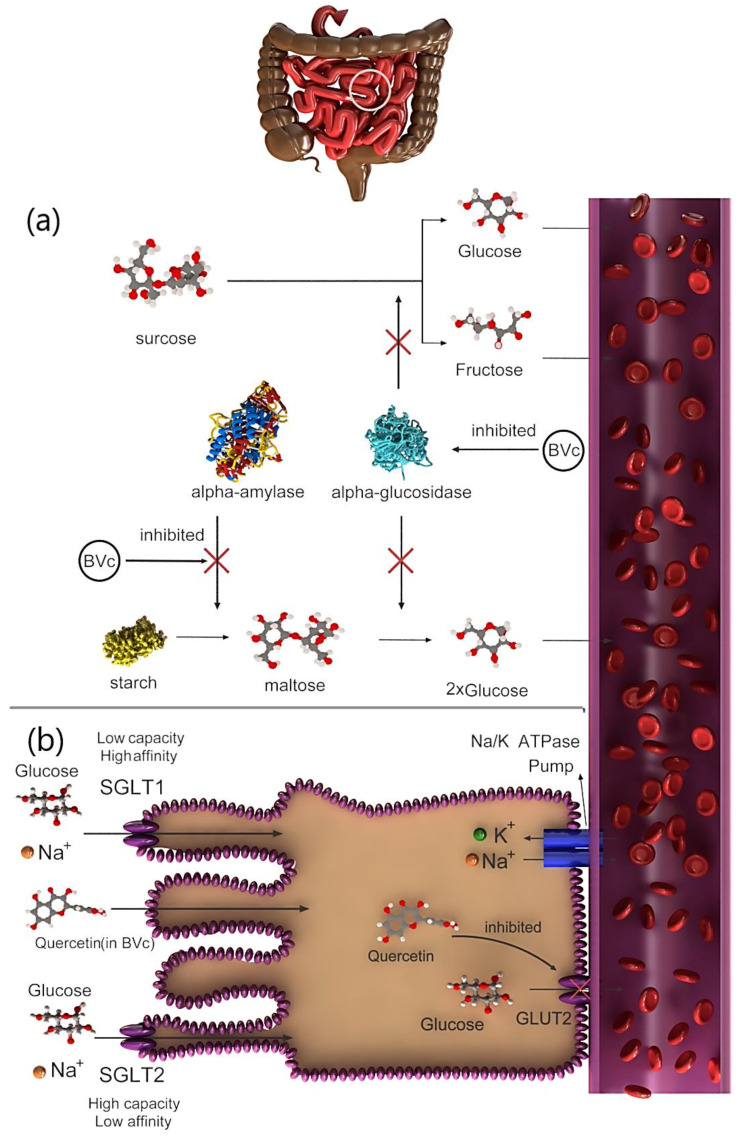
The hypoglycemic action of the extract is attributed to saponins, which prevent glycogenolysis and gluconeogenesis [29]. Therefore, the molecular pathways that affect this hypoglycemic mechanism must be thoroughly examined. Several studies showed that the hypoglycemic activity of the BVc extract, which is mediated via prevention of glucose transporters, may be caused by flavonoids. For example, quercetin, which is found in BVc, exhibited antidiabetic effects by preventing the action of the intestinal glucose transporter GLUT2 (Figure 2b) [30]. Another hypoglycemic mechanism is the inhibition of α-amylase and α-glucosidase activities by flavonoids [31]. For example, two flavanol glycosides isolated from Salsola kali actively inhibited α-amylase activity [32]. The digestion and absorption of carbohydrates can be delayed by inhibiting α-amylase activity, consequently suppressing postprandial hyperglycemia (Figure 2a) [33]. Vitexin-2-O-glycoside, a C-glycosyl flavone-containing vitexin found in the leaves and seeds of BVc, strongly inhibits α-glucosidase (Figure 2a) [34]. This finding suggests that α-glucosidase inhibition serves as the primary mechanism underlying the hypoglycemic effect previously observed in diabetic rats [28,34]. In 2014, more than 1.9 billion adults worldwide were estimated to be overweight and more than 600 million of whom were obese. Intake of high-energy foods with low fat is the leading inducer of obesity and overweightness [35]. Type 2 diabetes is a form of chronic diabetes triggered by hyperglycemia, and it leads to impaired insulin secretion, insulin action or both. In contrast, obesity is characterized by chronic low-grade inflammation in the adipose tissue, liver and skeleton, leading to areas of hypoxia in adipose tissue [36]. Nutritional therapy and glucose monitoring, including diet control, are suggested as interventions to monitoring the blood sugar in type 2 diabetes. The antihypertensive activities of quinoa and amaranth have been evaluated using laboratory enzymatic methods, and their anti-obesity effects have been studied in obese and hyperglycemic mouse models [37]. Glucosidase and pancreatic lipase are essential enzymes for breaking down complex carbohydrates and absorption of triglyceride lipids. The use of bioactive agents in foods to control both enzymes may have potential benefits in regulating blood sugar and weight and consequently manage obesity and type 2 diabetes. The phenolic content of quinoa inhibits α-glucosidase and pancreatic lipase activities [38]. The most common phytoecdysteroid, 20HE, is extracted from quinoa seeds, and it significantly reduces fasting blood sugar in obese mice. In addition, mice fed 20HE-enriched quinoa decreased mRNA levels of various genes related to inflammation (monocyte chemotactic protein 1, CD68) and reduced insulin resistance [37]. Quinoa diet is known to show reversed effects of HF-induced depletion of unbroken proteins in mice. In the study, male Wistar rats fed with amaranth seeds was shown to have significantly lower plasma MDA levels and higher antioxidant enzyme activity than the control rats. Amaranth seeds can act as a medium to protect against obesity caused by fructose and diabetes [39]. Amaranth seed and its oil showed reduced serum glucose level and increased serum insulin levels in rats with diabetes. Therefore, amaranth seed is useful for correcting blood sugar level and preventing diabetic side effects. However, the components responsible for its anti-obesity and antidiabetic activities are still unknown. The anti-obesity and antidiabetic activities of quinoa and amaranth have been investigated mostly in in vitro and in vivo experiments, whereas clinical studies have been limited. Therefore, the effect of diets containing quinoa and amaranth must be investigated in humans [37].
Figure 2.
Mechanism of hypoglycemic function of Beta vulgaris var. cicla L. (BVc) by (a) alpha-amylase and alpha-glucosidase to control glucose in diabetic patients and (b) inhibiting glucose transporter isoform 2 (GLUT2).
3.2. Cardiovascular Disease (CVD)
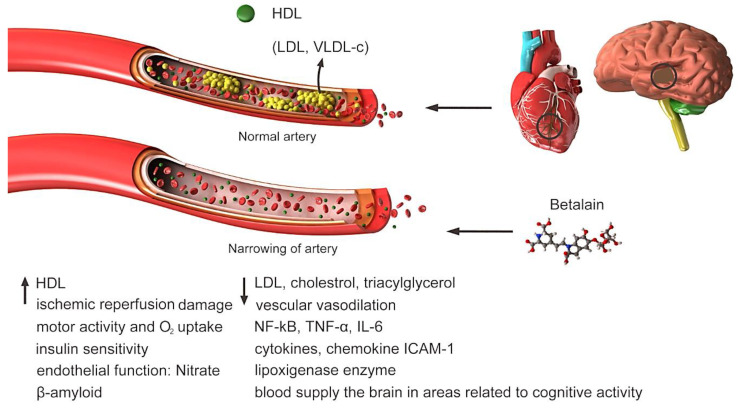
CVD is the leading cause of death and disability worldwide. Unhealthy diet is considered as one of the most significant risk factors for CVD [40]. Total cholesterol, LDL cholesterol (LDL-c), and triglyceride concentrations are risk markers of CVD (Figure 3). The effects of dietary quinoa (quinoa contains a marked concentration of betalain: 630.4 mg/100 g dry portion) on risk parameters of CVD were evaluated after 30 days of consumption in 22 students between 18 and 45 years old. Approximately 42.2% and 40.7% of the individuals had hypotension and decreased body weight, respectively [41]. Diet and foods containing extruded amaranth oil reduced total cholesterol, LDL-c and triglycerides by approximately 50%. Previously, very-low-density lipoprotein cholesterol (VLDL-c) concentrations were compared between hypercholesterolemia and control rabbits [42]. Among the hypercholesterolemia rabbits, those with remarkably low heart rate variability (HRV; total power (TP) 400 ms2) were assigned to the resistance-deficient group (Group 1), whereas those with a slightly higher HRV (TP > 400 ms2) were assigned to the low resistance group (Group 2). Regional and national level athletes with TP ranging from 3500 to 7000 ms2 were allocated to Group 3 [43]. Administration of amaranth oil at 18 mL per day for three weeks significantly lowered total cholesterol, triglycerides, LDL and VLDL-c in the subjects [37]. An LDL greater than 130 mg/dL, high-density lipoprotein (HDL) cholesterol lower than 35 mg/dL and total blood cholesterol greater than 200 mg/dL are indicators of high cholesterol, thereby marking a high risk of CVD development [44]. Both amaranth and quinoa seeds contain good quality of lutein, polyunsaturated fatty acids and tocopherols [45,46]; however, further research is required to confirm the effectiveness of these ingredients for CVD treatment in humans. In a previous prospective and double-blind study, postmenopausal women that consumed 25 g of quinoa flakes daily showed a decrease in total cholesterol and LDL-c, as well as an increase in GSH, which decreased their risk of CVD development [47].
Figure 3.
Highly concentrated low-density lipoprotein (LDL) is associated with increased cholesterol deposition in the walls of blood vessels and atherosclerosis (susceptibility to cardiovascular disease) and should be tried to reduce its level in the blood. Clogged or narrowed arteries can block blood flow to the heart, brain or other organs. This can lead to stroke, heart attack or even heart failure. Betalain reduces blood LDL, increases HDL and vascular vasodilation. Other factors that increase or decrease under the influence of betalain are shown in Figure.
3.3. Hepatitis
In a human study, a supplement containing uncooked red beet juice reduced non-HDL-c, LDL-c and total cholesterol [48]. In AML mice, treatment with betanin decreased LDL levels [49]. The consumption of a non-lipid diet increased serum TC, TC/HDL-c ratio, triglyceride (TAG) and atherogenic index, but decreased short-chain fatty acid (SCFA) production in rats [50]. However, the use of red beetroot (RBR) crisps inhibited the growth of TC and TAG, resulting in a higher probability of elevated total SCFA pool. The prescription of 3% RBR crisps also reduced the level of hepatic TC. Collectively, these findings suggest that the consumption of RBR crisps reduces metabolic changes in rats with dietary dyslipidemia [51]. However, another study on rats revealed that although RBR intake alleviated the concentration of SCFAs, it also caused the accumulation of long-chain fatty acids [52].
3.4. Antimicrobial and Antiviral Activities
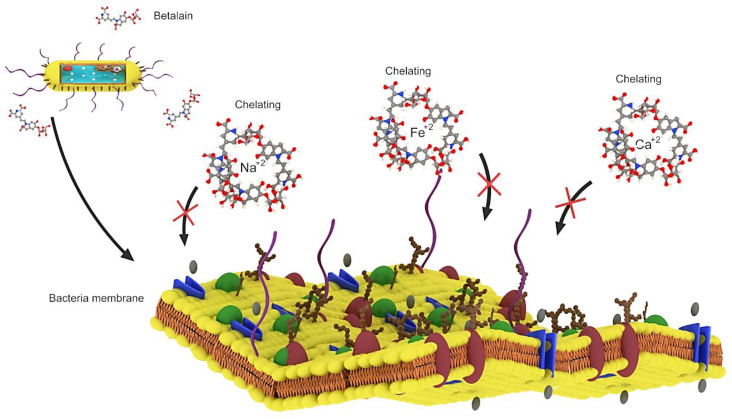
Betalains exhibit antimicrobial and antimalarial effects, whereas betalain-rich Amaranthus spinosus shows prominent antimalarial activity in mice owing to its high levels of betanin and amaranthine, which can chelate the required inner cations (Fe+2, Ca+2 and Mg+2) and block the intracellular transport of choline in parasites [53]. Extracts of Opuntia matudae, which contain betalains, prevent the growth of Escherichia coli O157: H7 (Figure 4) [54]. Beetroot pomace induced a decrease in the growth of Staphylococcus aureus, Salmonella typhimurium and Bacillus cereus [55]. However, beetroot pomace was unable to prevent the growth of Gram-negative bacteria (Pseudomonas aeruginosa, E. coli, Citrobacter freundii, Enterobacter cloacae, Salmonella typhimurium, Citrobacter youngae), with C. freundii and S. typhimurium showing the highest susceptibility to beetroot pomace [56]. Betalain-rich extracts from red pitahaya exerted a broad-spectrum antimicrobial activity by preventing the growth of Gram-positive bacteria (Escherichia faecalis, B. cereus, Listeria monocytogenes and S. aureus) at 7.8 mg/mL, Gram-negative bacteria (E. cloacae, Proteus vulgaris, Proteus mirabilis, P. aeruginosa, Salmonella typhi Ty2, Yersinia enterocolitica, Klebsiella pneumonia, Enterobacter aerogenes and E. coli) at 15.6–62.5 mg/mL, yeasts (Rhizoctonia solani and Candida albicans) at 125–250 mg/mL and molds (Aspergillus flavus, Cladosporium herbarium, Fusarium oxysporum and Botrytis cinerea) at 500 mg/mL [57]. The antimicrobial activity of betalains is speculated to be caused by their negative effects on the function, structure and penetration of the microbial cell membrane, ultimately causing cell death [56]. Although betalains are known to exert broad-spectrum antimicrobial activity, only their microbial prevention mechanism was reported. The basic molecular and cellular mechanism underlying the antimicrobial effect of betalains will be highlighted in future studies [56].
Figure 4.
Betalain by chelating Fe+2, Ca+2 and Mg+2 ions which are among the basic needs of bacteria, betalain prevents them from entering the bacteria, resulting in the death of the bacteria.
3.5. Cognitive Impairment
Most of the people with cognitive impairment diseases such as dementia and Alzheimer’s disease suffer from cerebral circulatory disorders. Nitrate, which is metabolized and produced in beet nitric oxide (NO), has the ability to improve circulatory problems [58,59]. In a study of 75-year-old volunteers on a diet containing red beet juice, a significant increase in blood flow was observed by magnetic resonance imaging (MRI) of the brain in areas related to cognitive activity [60]. However, in other studies there were conflicting results related to the design and study groups selected. The betanine compound in red beet extract has been shown to help reduce the accumulation of inappropriate proteins in the brain (a process associated with Alzheimer’s disease). Ming and the authors showed that betanine is a promising compound for inhibiting adverse reactions in the brain that are involved in the progression of Alzheimer’s disease. Beta-amyloid is an adhesive fragment of a protein or peptide that accumulates in the brain and disrupts the connection between nerve synapses. This damage becomes more severe when amyloid beta binds to metals such as Fe and Cu. Metals lead to errors in the process of accumulation and accumulation of beta-amyloid protein, creating masses that cause inflammation and oxidation and ultimately the destruction of nerve cells. When betanine was added to the Cu-bound amyloid beta protein, oxidation was reduced by up to 90% and the folding abnormalities in the proteins stopped. Therefore, it seems that the main mechanism of betanine is the reduction of oxidation, which slows down the accumulation of beta-amyloid protein [61].
In Parkinson’s disease, 70–50% of dopaminergic neurons are significantly breaks down in the black liver [62]. As mentioned earlier, L-dopa is an intermediate compound in the red beet pigment production process. L-dopa is a major drug in the treatment of Parkinson’s disease that converts dopamine through the enzyme tyrosine hydroxylase [26,63]. In the Parkinson’s model of rats induced by tacrine, haloperidol and reserpine, administration of red beet (100, 200 and 300 mg/kg po) can protect against behavioral changes and its beneficial effects against Parkinson’s disease with antioxidant activity and possibly Show dopaminergic activity [64]. Of course, what has been mentioned requires more research.
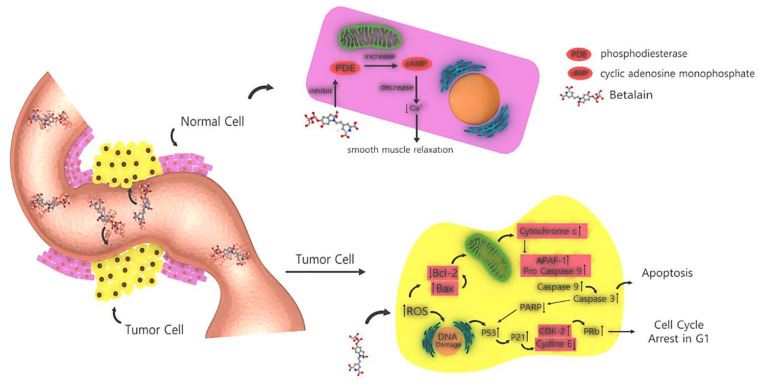
3.6. Anticancer Activity
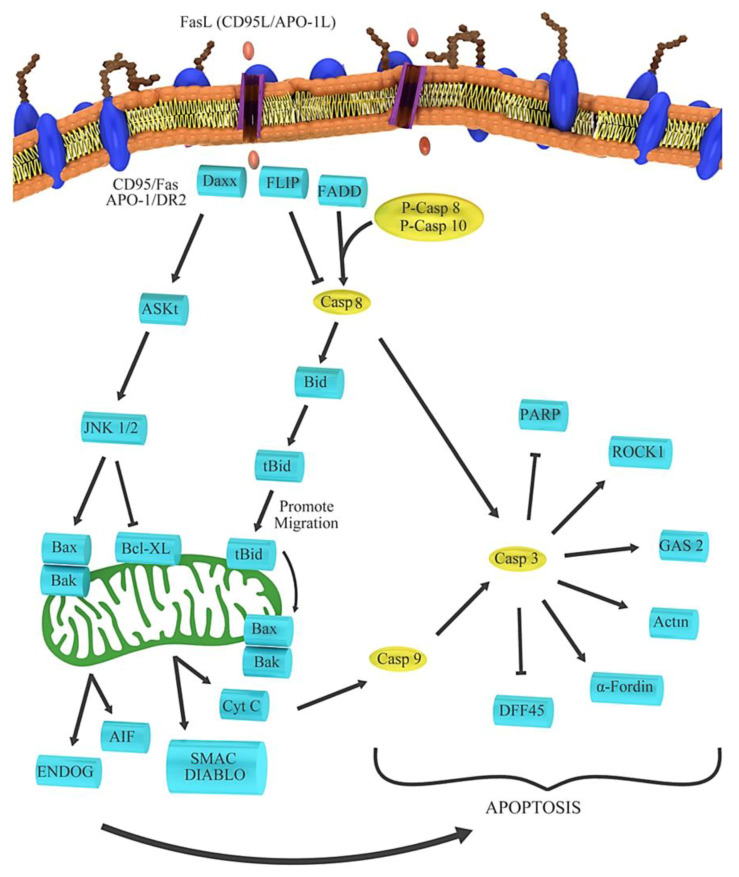
There has been a growing interest in the anticancer properties of beets and the use of beet products or their ingredients as dietary supplements for cancer prevention [65]. Recent studies on betalain and their in vitro outcomes against cancer are presented in Table 2. Among the different atypical mechanisms underlying the chemopreventive attributes of beetroot at the cellular level, the anti-inflammatory, antioxidant, proapoptotic, antiproliferative and free radical-scavenging mechanisms have been investigated. Previous studies have shown marked increases in BAX, caspase 9, caspase 3, cytochrome and ROS as well as decreases in BCL2 and PARP, causing DNA damage and ultimately leading to apoptosis. This process is shown in Figure 5 [66]. Beetroot is known for its high antioxidant activity, which is attributable to its pigments (i.e., betalains) [67]. The red components (betacyanins) of beetroot contain 75–95% betanin, which is considered to be its main pigment and the indicator of its phytochemical activity [68]. Although it is hypothesized that betanin is responsible for the beneficial effects of beet or beet fruit juice, cytotoxicity analysis revealed that the p53 wild-type cancer cell lines (B16F10 and MCF-7) are highly sensitive to 40 μM of the betanin/isobetanin mixture (as indicated by inhibited proliferation and low cell resistance), whereas cancer cell lines (e.g., HT-29) expressing the less mutated p53 (MDA-MB-231) are not sensitive to this mixture at the same concentration [69]. Because the effect of a betanin-rich extract was similar in both 2D and 3D culture conditions, the betanin/isobetanin concentrate was further found to inhibit the formation of a cluster, a cell structure that is resistant to apoptosis in cancer cell proliferation [70]. In the above process, MDA-MB-231 and B16F10 are metastatic cells cultured in the independent state of the anchor owing to the activation of the ERK signaling pathway. [71,72]. Therefore, the detection of an efficient molecule in anoikis-resistant cancer cells would be a promising objective in a future study. The inhibition of cell proliferation by mixture confirmed its anticancer properties and its effect on various cellular cycles. In MCF-7 cells, betanin/isobetanin extract reduced the number of G1-phase cells and increased the number of S-phase cells. It was also observed in MCF-7 cells treated with resveratrol or riproximin [73,74]. Betanin/isobetanin extract also contains cell cycle regulators, such as resveratrol and riproximin, which regulate the levels of cyclin A2 and cyclin B1 in MCF-7 cells. MDA-MB-231 cells cultured in 2D were arrested at the G1 phase after treatment with betanin/isobetanin extract. However, when these cells were cultured as aggregates, these molecules did not significantly affect the cell cycle progression. When assessing the cellular toxicity of red beet extract in MCF-7 cells, Cappadocia et al. found that the IC50 of the extract was 600 µmol (after 72 h of exposure) [66,75]. Reddy et al. [15] found inhibition of MCF-7 cell growth after treatment with betanin concentrate for 48 h (294 μM IC50). Overall, our findings were consistent with the results of the above studies. Betanin purified from raw beet extract significantly inhibited the growth of MCF-7, inducing cell death at very low concentrations (below 40 µM) [69]. Because the survival of MCF-7 cells was severely reduced by treatment with the betanin/isobetanin mixture, the nature of cell death was investigated. Using different methods, it was found that treatment with betanin causes apoptosis in 2DMCF-7 cells [69]. The expression of apoptotic proteins (bad, TRAILR4, FAS and phosphorylated p53) was dramatically increased and mitochondrial membrane potential was markedly altered [69]. Betanin decreases the number of small endothelial CD31 vessels and increases the expression of caspase 3, indicating that its inhibitory effects on lung tumor is mediated through induction of apoptosis and inhibition of angiogenesis. Betanin also caused apoptosis by activating caspases 3, 7, 9 and PARP in human lung cancer cell lines. Our data suggest that betanin significantly inhibits lung tumor growth in A/J mice and acts as a carcinogen in human lung cancer [76]. Previously, fluorescence-activated cell sorting analysis showed induction of apoptosis and increased activity of caspases 3 and 8 [76]. RTqPCR assay showed that the combination of XVX + BC can increase the expression level of proapoptotic BAX and decrease the expression of anti-apoptotic anti-BIRC5 (survivin) and pro-survival CTNNB1 (β-catenin) [77]. The most obvious effect of BC was an increase in caspase 8 activity, which led to the induction of external apoptosis [77]. In the APO-1 pathway, the apoptotic genes activate caspase 8. After that, the apoptotic pathway is activated by caspase 3 and other genes involved in apoptosis, as shown in Figure 6. In another study, treatment with betanin/isobetanin resulted in a significant reduction in the proliferation and survival of cancer cells, changes in mitochondrial membrane potential (via both internal and external apoptosis pathways) and the formation of autophagous vesicles in MCF-7-treated cells [50]. In addition to a significant increase in the protein expression of Bad, TRAILR4, FAS and p53, the treatment led to autophagic cell death. The researchers concluded that betanin/isobetanin treatment may be useful for the treatment of cancer, especially in functional p53 tumors. Although betanin-rich extract does not affect normal cell lines [69], betanin increased the proliferation of chronic human myeloid leukemia cell line (K562) in a dose- and time-dependent manner [50]. In addition, treatment with 40 mM betanin resulted in cells entering the phase below G0/G1 (28.4% of cells); the activation of apoptotic processes such as chromatin condensation, cellular contraction, membrane hemorrhage, DNA fragmentation and poly ribs (ADP) cleavage polymerization; reduction of membrane potential; regulation of Bcl-2; and release of cytochrome c into the cytosol [50]. Using confocal microscopy, betanin was observed to enter cells and induce apoptosis in K562 cells via intrinsic pathways [78].
Table 2.
Outcome and purpose of recent in vitro studies on cancer therapy of betalain.
| Source of betalain | Type of study | Applications | Outcomes | Ref. |
|---|---|---|---|---|
| Celosia argentea var. plumosa | In vitro | Production of betalains | Production of dihydroxylated betalains in the cells during eight days of culture | [95] |
| Lepismium lorentzianum, Lepismium lumbricoides, Rhipsalis floccosa and Pfeiffera ianthothele | In vitro | Antimutagenic | Significant antimutagenic effect for L. lumbricoides and weak effect for P. ianthothele and R. floccosa | [96] |
| Opuntia spp. | In vitro (various cell lines) |
Anticancer | Among the cancer lines tested, the viability of prostate and colon cells was the most affected | [97] |
| Beta vulgaris (beetroot) | In vitro Lung cancer (A549), human prostate (PC-3) and breast (MCF-7 and MDA-MB-231) cancer cell lines |
Anticancer | Beetroot ingestion can be used to prevent cancer Betanin may contribute to the cytotoxicity and chemo preventive activities of beetroot extract when used alone or in combination with doxorubicin to mitigate the toxic side effects of the latter |
[76,98] |
| Ex vivo (Rat skin and lung tissues) | ||||
| Opuntia spp. | In vitro Human colon cancer cell line (HT29) |
Antiproliferative | An unexpected increase in intracellular ROS accumulation in HT29 cells suggested that cancer cell death may be induced by the pro-oxidant effect | [99] |
| O. ficus-indica | In vitro Chronic myeloid leukemia cell line (K562) |
Anticancer | Betanin induced apoptosis in K562 cells through the intrinsic pathway and this was mediated by the release of cytochrome c from the mitochondria into the cytosol as well as by PARP cleavage | [78] |
Figure 5.
How betalain affects cancer cells and induces apoptosis in them vs. how betalain affects normal cells.
Figure 6.
Processes involved in the Apo-1 route.
4. Approaches to Enhance the Oral Bioavailability and Stability of Betalains
Bioavailability is defined as phytochemical percentage of a drug that enters the bloodstream [79]. The bioavailability of betalains has been reported in several animal and human studies. Netzel et al. [80] and Frank et al. [81] studied the pharmacokinetics of betalains in healthy humans after the ingestion of beet root juice. Postconsumption, betacyanins were immediately found in the urine; however, the amount of unmetabolized betalains excreted in urine was found to be significantly low. As the pigment content in urine accounted for 0.5–0.9 of the dose administered, the researchers concluded that renal clearance does not significantly aid in the systemic elimination of betalains [80]. It was hypothesized that other elimination pathways were involved, such as biliary excretion, enterohepatic circulation and metabolism, including metabolism by contributors such as intestinal bacteria [81]. Tesoriere et al. [82] simulated the gastric, oral and intestinal digestion of betalains in vitro by comparing various types of food consisting of pigment content. Their findings indicated that the food matrix prevented the degradation of betanin/isobetanin in the gastric environment. Furthermore, loss of betacyanins during digestion was observed in the small intestine, with differences observed for foods containing pigments and those containing purified betalains. Results showed that betalamic acid accumulation was observed after the degradation of purified betalains, however, this was not occur during the digestion of food containing betalains [82]. Therefore, the researchers concluded that the bioavailability of dietary betalains heavily depends on the chemical stability of the digestive tract; however, other factors such as the type of food matrix can alter the bioaccessibility of digestive enzymes [82]. Intestinal bacteria also participates actively in the metabolism of betalains and interfere with their absorption and bioavailability [83]. Tesoriere et al. [84] examined the permeability of red beet indicaxanthin and betanin in Caco-2 monolayer cells. Indicaxanthin was found to have a higher permeability coefficient than betanin. Further, the key step in the absorption of betalains was attributed to multidrug resistance-associated protein-2 (MRP-2), which controls the efflux of phytochemicals via a dose-dependent activity [84].
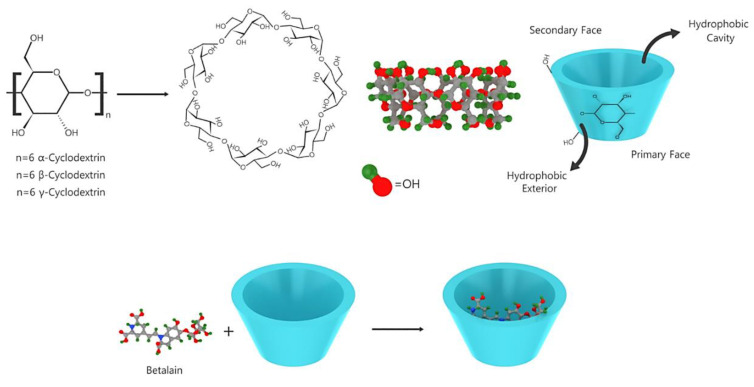
β-cyclodextrin and glucose oxidase contribute to betalain stabilization via the adsorption of free water and the removal of dissolved oxygen, respectively [85,86]. Interestingly, phenolic antioxidants and tocopherol did not exhibit any stabilizing effect on betalain [87]. Because of the conjugated dienes in the 1,7-diazaheptamethine structure, betalains absorb UV and visible lights [88]. Previously, structural implication on the fluorescence of betaxanthins has been reported [89]. In addition to the use of antioxidants, the metal chelating agent EDTA and inclusion complexes containing maltodextrin and β-cyclodextrin, encapsulation is an efficient method to stabilize and ease the administration of betalain. As shown in Figure 7, the effect of encapsulation on the stabilization and improvement of the bioavailability of polyphenols has been previously investigated [90,91].
Figure 7.
Betalain encapsulated by cyclodextrin.
Collectively, these data confirm the high availability of betalains in the human body, with betaxanthin showing greater bioavailability than betacyanin. However, further research is necessary to elucidate the specific content of betalain metabolites in plasma, urine and bile [92]. Owing to its high bioavailability and health-protective effect, betaxanthin has been employed as a food supplement to enhance the quality of processed food products [93]. Studies regarding the stability and bioaccessibility of betalains under simulated digestive conditions propose that digestive stability manage the bioaccessibility of betaxanthins, whereas additional factors relevant to the food matrix and food processing affect betacyanin bioaccessibility. Previously, the radical-scavenging activity and stability of betalains under simulated human gastrointestinal tract conditions have been examined [94]. When the pH value was less than three and the concentration of bile salts was increased to 4%, betalains were relatively stable and their radical-scavenging activities decreased from 75% to 38%. Similarly, the antiradical activities and stabilities of betanin under different pH, temperature, and light conditions have been previously examined [90].
5. Conclusions and Future Trends
Numerous studies have revealed the health benefits of betalains arising from their high antioxidant capacity (Table 2 and Table 3). Although betalains were previously restricted to plants belonging to the order Caryophyllales and some fungal species, the present study revealed the first betalain-producing bacteria as well as the main steps involved in the pigment formation. Moreover, our findings indicate that the biosynthesis of betalain can be extended to prokaryotes. Betalains are formed through decisive steps in the biosynthesis of beta-beta; these include the condensation of the beta-chromatin chromophore, betalamic acid, with cyclo-dopa and amino acids or amino acids alone or those involved in the formation of the corresponding aldimine from the red-purple beta and yellow betaxanthins. Because of their use as food colorants, antiseptics and radioactive radicals to protect against stress-related disorders, betalain enzymes have attracted the attention of researchers. However, future studies on pure beta-lysine are needed to elucidate more thoroughly its precise biologic functions.
Table 3.
Pharmacological benefits of betalains.
| Therapeutic Application | Source of Betalain | Outcomes | References | |
|---|---|---|---|---|
| Species | Active Components/Parts | |||
| Antidiabetic | Beet | – | Experiments have shown a 40% reduction in glycemia, without weight loss and liver dysfunction. The action of hypoglycemia mechanism for the extract is experimentally attributed to saponins that inhibit glycogenolysis and gluconeogenesis. | [100] |
| – | Reduce serum glucose, lipid profile, ALT, AST, TNF-α, IL-1β, IL-6, MDA and increase in hepatic TAO and GST in rats. | [101] | ||
| Red beetroot | Betavulgaroside I, II, III and IV | Reduce blood glucose in rats. | [102] | |
| Betalains | Reduce blood glucose levels in mice. | [103] | ||
| Betanin | Prevent induction of diabetes by alloxan in mice; reduce cardiogenic fibrosis in rats. | [104,105,106] | ||
| Apigenin | Increase insulin level in mice. | [107] | ||
| Luteolin | Increase insulin level in mice. | [108] | ||
| Quercetin | Decrease blood glucose level in rats. | [109] | ||
| Kaempferitrin | Increase antioxidant and hypoglycemic effects in rats. | [110] | ||
| Epicatechin | Revive insulin-producing cells in rats. | [111] | ||
| – | Inhibit absorption and digestion of glucose in intestine in mice. | [103] | ||
| Aqueous extract | Increase glucose disposal in skeletal myocytes and glucose absorption through GLUT4 transporters in mice. | [112] | ||
| Chard | Aqueous chard extract | Increase number and volume of secretion of insulin-producing cells in humans. | [28] | |
| Cardiovascular disease | Red beetroot | Nitrate in red beet – | Reduce the blood pressure and LDL cholesterol in humans. | [113] |
| – | Reduce serum total cholesterol and triacylglycerol levels in rats. | [51] | ||
| Pulp | Reduce cholesterol and triglycerides in rats. | [114] | ||
| – | improve in early vascular dysfunction and Reduce LDL cholesterol levels, increase HDL cholesterol levels; reduce oxidative stress; invert injury to brachial endothelial artery, improve function of the muscles and increase strength; reduce systolic blood pressure (4–5 mmHg); increase antithrombotic, antiadhesive effects; reduce blood pressure and improve brachial artery blood flow in humans. | [115,116,117] | ||
| Ethanol extract of stalks and leaves | Reduce oxidative stress, blood glucose and cholesterol in liver in mice. | [118] | ||
| Fiber content in the red beet | Reduce cholesterol and the number of tumors of colon cancer in rats. | [119] | ||
| Betanin | Temporarily increase heart rate and blood pressure in rats; increase SIRT1 and reduce LOX1 and hs-CRP in humans. | [50,120] | ||
| Anti-hepatitis | Boerhavia diffusa L. | Spongy roots decoction | – | [121] |
| B. diffusa | Root extract | According to studies, B. diffusa showed the potential to cure infectious hepatitis by antiviral mechanism. In the study, B. diffusa root extract (5 mg/mL) showed antiviral potency by inhibiting surface antigen as well as inhibiting HBV (hepatitis B virus). | [122] | |
| Antibacteria | B. diffusa | Methanolic extract | The ethanolic extract of whole plant of B. diffusa has antimicrobial activity against bacterial strains Bacillus subtilis UC564, Staphylococcus aureus 15 ML296, Staphylococcus aureus ML329 and Salmonella typhi DI at 2000 µg/mL. | [121] |
| Opuntia matudae | Extract of whole plant | Opuntia matudae extract has the potential to inhibit the growth four strains of E. coli O157:H7 and could provide a natural means of controlling pathogenic contamination. | [54] | |
| Hylocereus polyrhizus | Subfractionation extract | Flesh and peels extract have wide range of antimicrobials spectrum to prevent the growth of all pathogenic bacteria and/or human food spoilage, molds and yeasts. | [57] | |
| Cognitive improvement | Red beetroot | – | Increase blood supply the brain in areas related to cognitive activity in humans. | [60] |
| Alzheimer’s disease | Betanin | Reduce accumulation of β-amyloid protein in humans. | [123] | |
| Parkinson’s | Methanolic extract | Increase antioxidant activity and possible dopaminergic activity in rats. | [65] | |
| Anticancer | Table 2 | |||
Author Contributions
Conceptualization, H.H. and K.H.K.; formal analysis, E.M. and S.M.-R.; data curation, E.M. and S.M.-R.; writing—original draft preparation, E.M. and S.M.-R.; writing—review and editing, J.S.Y., J.W.H., H.H. and K.H.K.; supervision, H.H. and K.H.K.; funding acquisition, H.H. and K.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2018R1A2B2006879 and 2019R1A5A2027340) and Tabriz University of Medical Science (Grant No. 58628).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zhang Y.J., Gan R.Y., Li S., Zhou Y., Li A.N., Xu D.P., Li H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rymbai H., Sharma R., Srivastav M. Bio-colorants and its implications in health and food industry—A review. Int. J. Pharmacol. Res. 2011;3:2228–2244. [Google Scholar]
- 3.Jimenez-Garcia S.N., Vazquez-Cruz M.A., Guevara-Gonzalez R.G., Torres-Pacheco I., Cruz-Hernandez A., Feregrino-Perez A.A. Current approaches for enhanced expression of secondary metabolites as bioactive compounds in plants for agronomic and human health purposes—A review. Pol. J. Food Nut. Sci. 2013;63:67–78. [Google Scholar]
- 4.Gautam S., Saxena S., Kumar S. Fruits and vegetables as dietary sources of Antimutagens. J. Food Chem. Nanotechnol. 2016;2:97–114. [Google Scholar]
- 5.Oroian M., Escriche I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015;74:10–36. doi: 10.1016/j.foodres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Ramiro I., Ramos S., Bravo L., Goya L., Martín M.Á. Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur. J. Nutr. 2012;51:881–892. doi: 10.1007/s00394-011-0269-1. [DOI] [PubMed] [Google Scholar]
- 8.Numazawa S., Ishikawa M., Yoshida A., Tanaka S., Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am. J. Physiol. Cell Physiol. 2003;285:C334–C342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 9.Strack D., Vogt T., Schliemann W. Recent advances in betalain research. Phytochemistry. 2003;62:247–269. doi: 10.1016/s0031-9422(02)00564-2. [DOI] [PubMed] [Google Scholar]
- 10.Tonon R.V., Brabet C., Hubinger M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010;43:907–914. [Google Scholar]
- 11.Rahimi P., Abedimanesh S., Mesbah-Namin S.A., Ostadrahimi A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food. Sci. Nutr. 2019;59:2949–2978. doi: 10.1080/10408398.2018.1479830. [DOI] [PubMed] [Google Scholar]
- 12.Gengatharan A., Dykes G.A., Choo W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT Food Sci. Technol. 2015;64:645–649. doi: 10.1016/j.lwt.2015.06.052. [DOI] [Google Scholar]
- 13.Choo W.S. Betalains: Application in functional foods, Bioact. Food. Springer; Cham, Switzerland: 2019. pp. 1471–1498. [Google Scholar]
- 14.Zou D.-M., Brewer M., Garcia F., Feugang J.M., Wang J., Zang R., Liu H., Zou C. Cactus pear: A natural product in cancer chemoprevention. Nutr. J. 2005;4:25. doi: 10.1186/1475-2891-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy M.K., Alexander-Lindo R.L., Nair M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 2005;53:9268–9273. doi: 10.1021/jf051399j. [DOI] [PubMed] [Google Scholar]
- 16.Wang L., Zhang J., Song M., Tian B., Li K., Liang Y., Han J., Wu Z. A shell-crosslinked polymeric micelle system for pH/redox dual stimuli-triggered DOX on-demand release and enhanced antitumor activity. Colloids Surf. B Biointerfaces. 2017;152:1–11. doi: 10.1016/j.colsurfb.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Clement J., Mabry T. Pigment evolution in the Caryophyllales: A systematic overview. Bot. Acta. 1996;109:360–367. doi: 10.1111/j.1438-8677.1996.tb00584.x. [DOI] [Google Scholar]
- 18.Stintzing F.C., Schieber A., Carle R. Identification of betalains from yellow beet (Beta vulgaris L.) and cactus pear [Opuntia ficus-indica (L.) Mill.] by high-performance liquid chromatography− electrospray ionization mass spectrometry. J. Agric. Food Chem. 2002;50:2302–2307. doi: 10.1021/jf011305f. [DOI] [PubMed] [Google Scholar]
- 19.Kugler F., Stintzing F.C., Carle R. Identification of betalains from petioles of differently colored Swiss chard (Beta vulgaris L. ssp. cicla [L.] Alef. Cv. Bright Lights) by high-performance liquid chromatography–electrospray ionization mass spectrometry. J. Agric. Food Chem. 2004;52:2975–2981. doi: 10.1021/jf035491w. [DOI] [PubMed] [Google Scholar]
- 20.Schliemann W., Cai Y., Degenkolb T., Schmidt J., Corke H. Betalains of Celosia argentea. Phytochemistry. 2001;58:159–165. doi: 10.1016/S0031-9422(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 21.Kugler F., Graneis S., Schreiter P.P.-Y., Stintzing F.C., Carle R. Determination of free amino compounds in betalainic fruits and vegetables by gas chromatography with flame ionization and mass spectrometric detection. J. Agric. Food Chem. 2006;54:4311–4318. doi: 10.1021/jf060245g. [DOI] [PubMed] [Google Scholar]
- 22.Stintzing F.C., Schieber A., Carle R. Betacyanins in fruits from red-purple pitaya, Hylocereus polyrhizus (Weber) Britton & Rose. Food Chem. 2002;77:101–106. [Google Scholar]
- 23.Gandía-Herrero F., García-Carmona F., Escribano J. Purification and characterization of a latent polyphenol oxidase from beet root (Beta vulgaris L.) J. Agric. Food Chem. 2004;52:609–615. doi: 10.1021/jf034381m. [DOI] [PubMed] [Google Scholar]
- 24.Stintzing F.C., Kugler F., Carle R., Conrad J. First 13C-NMR Assignments of Betaxanthins. Helv. Chim. Acta. 2006;89:1008–1016. doi: 10.1002/hlca.200690077. [DOI] [Google Scholar]
- 25.Gandía-Herrero F., Escribano J., García-Carmona F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta. 2005;222:586–593. doi: 10.1007/s00425-005-0004-3. [DOI] [PubMed] [Google Scholar]
- 26.Gandía-Herrero F., Escribano J., García-Carmona F. Betaxanthins as substrates for tyrosinase. An approach to the role of tyrosinase in the biosynthetic pathway of betalains. Plant Phys. 2005;138:421–432. doi: 10.1104/pp.104.057992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanardag R., Çolak H. Effect of chard (Beta vulgaris L. var. cicla) on blood glucose levels in normal and alloxan-induced diabetic rabbits. Pharm. Pharmacol. Commun. 1998;4:309–311. [Google Scholar]
- 28.Bolkent S., Yanardag R., Tabakoglu-Oguz A., Ozsoy-Saçan O. Effects of chard (Beta vulgaris var. cicla L.) extract on pancreatic B cells in streptozotocin-diabetic rats: A morphological and biochemical study. J. Ethnopharmacol. 2000;73:251–259. doi: 10.1016/S0378-8741(00)00328-7. [DOI] [PubMed] [Google Scholar]
- 29.Massiot G., Dijoux M.G., Lavaud C., Men-Olivier L.L., Connolly J.D., Sheeley D.M. Seco-glycosides of oleanolic acid from Beta vulgaris. Phytochemistry. 1994;37:1667–1670. doi: 10.1016/S0031-9422(00)89589-8. [DOI] [PubMed] [Google Scholar]
- 30.Song J., Kwon O., Chen S., Daruwala R., Eck P., Park J.B., Levine M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and glucose. J. Biol. Chem. 2002;277:15252–15260. doi: 10.1074/jbc.M110496200. [DOI] [PubMed] [Google Scholar]
- 31.Yilmazer-Musa M., Griffith A.M., Michels A.J., Schneider E., Frei B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J. Agric. Food Chem. 2012;60:8924–8929. doi: 10.1021/jf301147n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tundis R., Loizzo M.R., Statti G.A., Menichini F. Inhibitory effects on the digestive enzyme α-amylase of three Salsola species (Chenopodiaceae) in vitro. Pharmazie. 2007;62:473–475. [PubMed] [Google Scholar]
- 33.Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin. Investig. Med. 1995;18:303–311. [PubMed] [Google Scholar]
- 34.Li H., Song F., Xing J., Tsao R., Liu Z., Liu S. Screening and structural characterization of α-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MS n and SORI-CID FTICR MS. J. Am. Soc. Mass Spectrom. 2009;20:1496–1503. doi: 10.1016/j.jasms.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y.S., Kim J.W., Osborne O., Sasik R., Schenk S., Chen A., Chung H., Murphy A., Watkins S.M., Quehenberger O., et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157:1339–1352. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Y., Tsao R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.20160076. [DOI] [PubMed] [Google Scholar]
- 38.Tang Y., Zhang B., Li X., Chen P.X., Zhang H., Liu R., Tsao R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J. Agric. Food Chem. 2016;64:1712–1719. doi: 10.1021/acs.jafc.5b05761. [DOI] [PubMed] [Google Scholar]
- 39.Pasko P., Barton H., Zagrodzki P., Iżewska A., Krosniak M., Gawlik M., Gawlik M., Gorinstein S. Effect of amaranth seeds in diet on oxidative status in plasma and selected tissues of high fructose-fed rats. Plant Foods Hum. Nutr. 2010;65:146–151. doi: 10.1007/s11130-010-0164-6. [DOI] [PubMed] [Google Scholar]
- 40.Record N.B., Onion D.K., Prior R.E., Dixon D.C., Record S.S., Fowler F.L., Cayer G.R., Amos C.I., Pearson T.A. Community-wide cardiovascular disease prevention programs and health outcomes in a rural county, 1970–2010. J. Am. Med. Assoc. 2015;313:147–155. doi: 10.1001/jama.2014.16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farinazzi-Machado F.M.V., Barbalho S.M., Oshiiwa M., Goulart R., Junior O.P. Use of cereal bars with quinoa (Chenopodium quinoa W.) to reduce risk factors related to cardiovascular diseases. Food Sci. Technol. 2012;32:239–244. [Google Scholar]
- 42.Plate A.Y.A., Arêas J.A.G. Cholesterol-lowering effect of extruded amaranth (Amaranthus caudatus L.) in hypercholesterolemic rabbits. Food Chem. 2002;76:1–6. doi: 10.1016/S0308-8146(01)00238-2. [DOI] [Google Scholar]
- 43.Yelisyeyeva O., Semen K., Zarkovic N., Kaminskyy D., Lutsyk O., Rybalchenko V. Activation of aerobic metabolism by Amaranth oil improves heart rate variability both in athletes and patients with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2012;118:47–57. doi: 10.3109/13813455.2012.659259. [DOI] [PubMed] [Google Scholar]
- 44.Martirosyan D.M., Miroshnichenko L.A., Kulakova S.N., Pogojeva A.V., Zoloedov V.I. Amaranth oil application for coronary heart disease and hypertension. Lipids Health Dis. 2007;6:1. doi: 10.1186/1476-511X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Y., Li X., Chen P.X., Zhang B., Liu R., Hernandez M., Draves J., Marcone M.F., Tsao R. Assessing the fatty acid, carotenoid, and tocopherol compositions of amaranth and quinoa seeds grown in Ontario and their overall contribution to nutritional quality. J. Agric. Food Chem. 2016;64:1103–1110. doi: 10.1021/acs.jafc.5b05414. [DOI] [PubMed] [Google Scholar]
- 46.Tang Y., Li X., Chen P.X., Zhang B., Hernandez M., Zhang H., Marcone M.F., Liu R., Tsao R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015;174:502–508. doi: 10.1016/j.foodchem.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 47.De Carvalho F.G., Ovídio P.P., Padovan G.J., Jordão Junior A.A., Marchini J.S., Navarro A.M. Metabolic parameters of postmenopausal women after quinoa or corn flakes intake—A prospective and double-blind study. Int. J. Food Sci. Nutr. 2014;65:380–385. doi: 10.3109/09637486.2013.866637. [DOI] [PubMed] [Google Scholar]
- 48.Asgary S., Afshani M.R., Sahebkar A., Keshvari M., Taheri M., Jahanian E., Rafieian-Kopaei M., Malekian F., Sarrafzadegan N. Improvement of hypertension, endothelial function and systemic inflammation following short-term supplementation with red beet (Beta vulgaris L.) juice: A randomized crossover pilot study. J. Hum. Hypertens. 2016;30:627–632. doi: 10.1038/jhh.2016.34. [DOI] [PubMed] [Google Scholar]
- 49.Chen H., Xu Y., Wang J., Zhao W., Ruan H. Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation, oxidative stress-myeloperoxidase/low-density lipoprotein in rat. Int. J. Clin. Exp. Pathol. 2015;9:10139–10147. [PMC free article] [PubMed] [Google Scholar]
- 50.Rahimi P., Mesbah-Namin S.A., Ostadrahimi A., Separham A., Jafarabadi M.A. Betalain-and betacyanin-rich supplements’ impacts on the PBMC SIRT1 and LOX1 genes expression and Sirtuin-1 protein levels in coronary artery disease patients: A pilot crossover clinical trial. J. Funct. Foods. 2019;60:103401. doi: 10.1016/j.jff.2019.06.003. [DOI] [Google Scholar]
- 51.Wroblewska M., Juskiewicz J., Wiczkowski W. Physiological properties of beetroot crisps applied in standard and dyslipidaemic diets of rats. Lipids Health Dis. 2011;10:178. doi: 10.1186/1476-511X-10-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Budinsky A., Wolfram R., Oguogho A., Efthimiou Y., Stamatopoulos Y., Sinzinger H. Regular ingestion of Opuntia robusta lowers oxidation injury. Prostaglandins Leukot. Essent. Fatty Acids. 2001;65:45–50. doi: 10.1054/plef.2001.0287. [DOI] [PubMed] [Google Scholar]
- 53.Hilou A., Nacoulma O., Guiguemde T. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J. Ethnopharmacol. 2006;103:236–240. doi: 10.1016/j.jep.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Hayek S.A., Ibrahim S.A. Antimicrobial activity of Xoconostle pears (Opuntia matudae) against Escherichia coli O157: H7 in laboratory medium. Int. J. Microbiol. 2012;2012:368472. doi: 10.1155/2012/368472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vulić J.J., Ćebović T.N., Čanadanović V.M., Ćetković G.S., Djilas S.M., Čanadanović-Brunet J.M., Velićanski A.S., Cvetković D.D., Tumbas V.T. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 2013;4:713–721. doi: 10.1039/c3fo30315b. [DOI] [PubMed] [Google Scholar]
- 56.Čanadanović-Brunet J.M., Savatović S.S., Ćetković G.S., Vulić J.J., Djilas S.M., Markov S.L., Cvetković D.D. Antioxidant and antimicrobial activities of beet root pomace extracts. Czech J. Food Sci. 2011;29:575–585. doi: 10.17221/210/2010-CJFS. [DOI] [Google Scholar]
- 57.Tenore G.C., Novellino E., Basile A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods. 2012;4:129–136. doi: 10.1016/j.jff.2011.09.003. [DOI] [Google Scholar]
- 58.Clifford T., Howatson G., West D.J., Stevenson E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015;7:2801–2822. doi: 10.3390/nu7042801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hobbs D.A., Kaffa N., George T.W., Methven L., Lovegrove J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012;108:2066–2074. doi: 10.1017/S0007114512000190. [DOI] [PubMed] [Google Scholar]
- 60.Presley T.D., Morgan A.R., Bechtold E., Clodfelter W., Dove R.W., Jennings J.M., Kraft R.A., King S.B., Laurienti P.J., Rejeski W.J., et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24:34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hadipour E., Taleghani A., Tayarani-Najaran N., Tayarani-Najaran Z. Biological effects of red beetroot and betalains: A review. Phytother. Res. 2020;34:1847–1867. doi: 10.1002/ptr.6653. [DOI] [PubMed] [Google Scholar]
- 62.Mosley R.L., Benner E.J., Kadiu I., Thomas M., Boska M.D., Hasan K., Laurie C., Gendelman H.E. Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin. Neurosci. Res. 2006;6:261–281. doi: 10.1016/j.cnr.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dorszewska J., Prendecki M., Lianeri M., Kozubski W. Molecular effects of L-dopa therapy in Parkinson’s disease. Curr. Genom. 2014;15:11–17. doi: 10.2174/1389202914666131210213042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nade V.S., Kawale L.A., Zambre S.S., Kapure A.B. Neuroprotective potential of Beta vulgaris L. in Parkinson’s disease. Indian J. Pharmacol. 2015;47:403–408. doi: 10.4103/0253-7613.161263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zielińska-Przyjemska M., Olejnik A., Dobrowolska-Zachwieja A., Łuczak M., Baer-Dubowska W. DNA damage and apoptosis in blood neutrophils of inflammatory bowel disease patients and in Caco-2 cells in vitro exposed to betanin. Adv. Hyg. Exp. Med. 2016;70:265–271. doi: 10.5604/17322693.1198989. [DOI] [PubMed] [Google Scholar]
- 66.Kapadia G.J., Rao G.S. Red Beet Biotechnology. Springer; Berlin, Germany: 2013. Anticancer effects of red beet pigments; pp. 125–154. [Google Scholar]
- 67.Allegra M., Furtmüller P.G., Jantschko W., Zederbauer M., Tesoriere L., Livrea M.A., Obinger C. Mechanism of interaction of betanin and indicaxanthin with human myeloperoxidase and hypochlorous acid. Biochm. Biophys. Res. Commun. 2005;332:837–844. doi: 10.1016/j.bbrc.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 68.Gliszczyńska-Świgło A., Szymusiak H., Malinowska P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. 2006;23:1079–1087. doi: 10.1080/02652030600986032. [DOI] [PubMed] [Google Scholar]
- 69.Nowacki L., Vigneron P., Rotellini L., Cazzola H., Merlier F., Prost E., Ralanairina R., Gadonna J.P., Rossi C., Vayssade M. Betanin-enriched red beetroot (Beta vulgaris L.) extract induces apoptosis and autophagic cell death in MCF-7 cells. Phytother. Res. 2015;29:1964–1973. doi: 10.1002/ptr.5491. [DOI] [PubMed] [Google Scholar]
- 70.Hirschhaeuser F., Menne H., Dittfeld C., West J., Mueller-Klieser W., Kunz-Schughart L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 71.Fukazawa H., Noguchi K., Murakami Y., Uehara Y. Mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitors restore anoikis sensitivity in human breast cancer cell lines with a constitutively activated extracellular-regulated kinase (ERK) pathway. Mol. Cancer Ther. 2002;1:303–309. [PubMed] [Google Scholar]
- 72.Goundiam O., Nagel M.D., Vayssade M. Growth and survival signalling in B16F10 melanoma cells in 3D culture. Cell Biol. Int. 2010;34:385–391. doi: 10.1042/CBI20090147. [DOI] [PubMed] [Google Scholar]
- 73.Pervaiz A., Zepp M., Adwan H., Berger M.R. Riproximin modulates multiple signaling cascades leading to cytostatic and apoptotic effects in human breast cancer cells. J. Cancer Res. Clin. Oncol. 2016;142:135–147. doi: 10.1007/s00432-015-2013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joe A.K., Liu H., Suzui M., Vural M.E., Xiao D., Weinstein I.B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- 75.Kapadia G.J., Azuine M.A., Subba Rao G., Arai T., Iida A., Tokuda H. Cytotoxic effect of the red beetroot (Beta vulgaris L.) extract compared to doxorubicin (Adriamycin) in the human prostate (PC-3) and breast (MCF-7) cancer cell lines. Anticancer Agents Med. Chem. 2011;11:280–284. doi: 10.2174/187152011795347504. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Q., Pan J., Wang Y., Lubet R., You M. Beetroot red (betanin) inhibits vinyl carbamate-and benzo (a) pyrene-induced lung tumorigenesis through apoptosis. Mol. Carcinog. 2013;52:686–691. doi: 10.1002/mc.21907. [DOI] [PubMed] [Google Scholar]
- 77.Scarpa E., Emanuelli M., Frati A., Pozzi V., Antonini E., Diamantini G., Di Ruscio G., Sartini D., Armeni T., Palma F. Betacyanins enhance vitexin-2-O-xyloside mediated inhibition of proliferation of T24 bladder cancer cells. Food Funct. 2016;7:4772–4780. doi: 10.1039/C6FO01130F. [DOI] [PubMed] [Google Scholar]
- 78.Sreekanth D., Arunasree M., Roy K.R., Reddy T.C., Reddy G.V., Reddanna P. Betanin a betacyanin pigment purified from fruits of Opuntia ficus-indica induces apoptosis in human chronic myeloid leukemia Cell line-K562. Phytomedicine. 2007;14:739–746. doi: 10.1016/j.phymed.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Toutain P.L., Bousquet-Melou A. Bioavailability and its assessment. J. Vet. Pharmacol. Ther. 2004;27:455–466. doi: 10.1111/j.1365-2885.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 80.Netzel M., Stintzing F., Quaas D., Strass G., Carle R., Bitsch R., Bitsch I., Frank T. Renal excretion of antioxidative constituents from red beet in humans. Food Res. Int. 2005;38:1051–1058. doi: 10.1016/j.foodres.2005.03.016. [DOI] [Google Scholar]
- 81.Frank T., Stintzing F.C., Carle R., Bitsch I., Quaas D., Straß G., Bitsch R., Netzel M. Urinary pharmacokinetics of betalains following consumption of red beet juice in healthy humans. Pharmacol. Res. 2005;52:290–297. doi: 10.1016/j.phrs.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 82.Tesoriere L., Fazzari M., Angileri F., Gentile C., Livrea M.A. In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J. Agric. Food Chem. 2008;56:10487–10492. doi: 10.1021/jf8017172. [DOI] [PubMed] [Google Scholar]
- 83.Rechner A.R., Smith M.A., Kuhnle G., Gibson G.R., Debnam E.S., Srai S.K.S., Moore K.P., Rice-Evans C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004;36:212–225. doi: 10.1016/j.freeradbiomed.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 84.Tesoriere L., Gentile C., Angileri F., Attanzio A., Tutone M., Allegra M., Livrea M. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur. J. Nutr. 2013;52:1077–1087. doi: 10.1007/s00394-012-0414-5. [DOI] [PubMed] [Google Scholar]
- 85.Savolainen K., Kuusi T. The stability properties of golden beet and red beet pigments: Influence of pH, temperature, and some stabilizers. Z. Lebensm. Unters. Forsch. 1978;166:19–22. doi: 10.1007/BF01122999. [DOI] [PubMed] [Google Scholar]
- 86.Czapski J. Heat stability of betacyanins in red beet juice and in betanin solutions. Eur. Food Res. Technol. 1990;191:275–278. [Google Scholar]
- 87.Attoe E.L., Von Elbe J.H. Degradation kinetics of betanin in solutions as influenced by oxygen. J. Agric. Food Chem. 1982;30:708–712. [Google Scholar]
- 88.Gandía-Herrero F., Escribano J., García-Carmona F. Structural implications on color, fluorescence, and antiradical activity in betalains. Planta. 2010;232:449–460. doi: 10.1007/s00425-010-1191-0. [DOI] [PubMed] [Google Scholar]
- 89.Khan M.I., Giridhar P. Plant betalains: Chemistry and biochemistry. Phytochemistry. 2015;117:267–295. doi: 10.1016/j.phytochem.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 90.Fang Z., Bhandari B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010;21:510–523. [Google Scholar]
- 91.Krishnaiah D., Nithyanandam R., Sarbatly R. A critical review on the spray drying of fruit extract: Effect of additives on physicochemical properties. Crit. Rev. Food Sci. Nutr. 2014;54:449–473. doi: 10.1080/10408398.2011.587038. [DOI] [PubMed] [Google Scholar]
- 92.Clifford T., Constantinou C.M., Keane K.M., West D.J., Howatson G., Stevenson E.J. The plasma bioavailability of nitrate and betanin from Beta vulgaris rubra in humans. Eur. J. Nutr. 2017;56:1245–1254. doi: 10.1007/s00394-016-1173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han J., Gao C., Yang S., Wang J., Tan D. Betanin attenuates carbon tetrachloride (CCl 4)-induced liver injury in common carp (Cyprinus carpio L.) Fish. Physiol. Biochem. 2014;40:865–874. doi: 10.1007/s10695-013-9892-5. [DOI] [PubMed] [Google Scholar]
- 94.Banning A., Deubel S., Kluth D., Zhou Z., Brigelius-Flohé R. The GI-GPx gene is a target for Nrf2. Mol. Cell. Biol. 2005;25:4914–4923. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guadarrama-Flores B., Rodriguez-Monroy M., Cruz-Sosa F., Garcia-Carmona F., Gandia-Herrero F. Production of dihydroxylated betalains and dopamine in cell suspension cultures of Celosia argentea var. plumosa. J. Agric. Food Chem. 2015;63:2741–2749. doi: 10.1021/acs.jafc.5b00065. [DOI] [PubMed] [Google Scholar]
- 96.Zampini I.C., Ordóñez R., Giannini N.P., Blendinger P.G., Isla M.I. Nutraceutical properties and toxicity studies of fruits from four Cactaceae species grown in Argentine Northwestern. Food Res. Int. 2011;44:2345–2351. [Google Scholar]
- 97.Chavez-Santoscoy R.A., Gutierrez-Uribe J.A., Serna-Saldívar S.O. Phenolic composition, antioxidant capacity and in vitro cancer cell cytotoxicity of nine prickly pear (Opuntia spp.) juices. Plant Foods Hum. Nutr. 2009;64:146–152. doi: 10.1007/s11130-009-0117-0. [DOI] [PubMed] [Google Scholar]
- 98.Harlev E., Nevo E., Solowey E., Bishayee A. Cancer preventive and curative attributes of plants of the Cactaceae family: A review. Planta Med. 2013;79:713–722. doi: 10.1055/s-0032-1328632. [DOI] [PubMed] [Google Scholar]
- 99.Serra A.T., Poejo J., Matias A.A., Bronze M.R., Duarte C.M.M. Evaluation of Opuntia spp. derived products as antiproliferative agents in human colon cancer cell line (HT29) Food Res. Int. 2013;54:892–901. doi: 10.1016/j.foodres.2013.08.043. [DOI] [Google Scholar]
- 100.Ninfali P., Angelino D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia. 2013;89:188–199. doi: 10.1016/j.fitote.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Abd El-Ghffar E.A., Hegazi N.M., Saad H.H., Soliman M.M., El-Raey M.A., Shehata S.M., Barakat A., Yasri A., Sobeh M. HPLC-ESI- MS/MS analysis of beet (Beta vulgaris) leaves and its beneficial properties in type 1 diabetic rats. Biomed. Pharmacoter. 2019;120:109541. doi: 10.1016/j.biopha.2019.109541. [DOI] [PubMed] [Google Scholar]
- 102.Yoshikawa M., Murakami T., Kadoya M., Matsuda H., Muraoka O., Yamahara J., Murakami N. Medicinal foodstuffs. III. Sugar beet. (1): Hypoglycemic oleanolic acid oligoglycosides, betavulgarosides, I, II, III, and IV, from the root of Beta vulgaris L. (Chenopodiaceae) Chem. Pharm. Bull. 1996;44:1212–1217. doi: 10.1248/cpb.44.1212. [DOI] [PubMed] [Google Scholar]
- 103.Lugo-Radillo A., Delgado-Enciso I., Peña-Beltrán E. Betanidin significantly reduces blood glucose levels in BALB/c mice fed with an atherogenic diet. Nat. Prod. Bioprospect. 2012;2:154–155. doi: 10.1007/s13659-012-0034-z. [DOI] [Google Scholar]
- 104.Yamashita T.S., Katsunori Effects of beet red inhibiting experimental diabetes in vivo-studies using ALS strain mice. Foods Food Ingred. J. Jpn. 2008;213:122. [Google Scholar]
- 105.Han J., Ma D., Zhang M., Yang X., Tan D. Natural antioxidant betanin protects rats from paraquat-induced acute lung injury interstitial pneumonia. Biomed. Res. Int. 2015;2015:608174. doi: 10.1155/2015/608174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han J., Tan C., Wang Y., Yang S., Tan D. Betanin reduces the accumulation and cross-links of collagen in high-fructose-fed rat heart through inhibiting non-enzymatic glycation. Chem. Biol. Interact. 2015;227:37–44. doi: 10.1016/j.cbi.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 107.Panda S., Kar A. Apigenin (4′,5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan-induced diabetic mice. J. Pharm. Pharmacol. 2007;59:1543–1548. doi: 10.1211/jpp.59.11.0012. [DOI] [PubMed] [Google Scholar]
- 108.Zarzuelo A., Jiménez I., Gámez M.J., Utrilla P., Fernadez I., Torres M.I., Osuna I. Effects of luteolin 5-O-beta-rutinoside in streptozotocin-induced diabetic rats. Life Sci. 1996;58:2311–2316. doi: 10.1016/0024-3205(96)00231-7. [DOI] [PubMed] [Google Scholar]
- 109.Shetty A.K., Rashmi R., Rajan M.G.R., Sambaiah K., Salimath P.V. Antidiabetic influence of quercetin in streptozotocin-induced diabetic rats. Nutr. Res. 2004;24:373–381. doi: 10.1016/j.nutres.2003.11.010. [DOI] [Google Scholar]
- 110.de Sousa E., Zanatta L., Seifriz I., Creczynski-Pasa T.B., Pizzolatti M.G., Szpoganicz B., Silva F.R. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(alpha)-dirhamnoside from Bauhinia forficata leaves. J. Nat. Prod. 2004;67:829–832. doi: 10.1021/np030513u. [DOI] [PubMed] [Google Scholar]
- 111.Chakravarthy B.K., Gupta S., Gode K.D. Functional beta cell regeneration in the islets of pancreas in alloxan induced diabetic rats by (-)-epicatechin. Life Sci. 1982;31:2693–2697. doi: 10.1016/0024-3205(82)90713-5. [DOI] [PubMed] [Google Scholar]
- 112.Ul Kabir A., Samad M.B., Ahmed A., Jahan M.R., Akhter F., Tasnim J., Hasan S.M., Sayfe S.S., Hannan J.M. Aqueous fraction of Beta vulgaris ameliorates hyperglycemia in diabetic mice due to enhanced glucose stimulated insulin secretion, mediated by acetylcholine and GLP-1, and elevated glucose uptake via increased membrane bound GLUT4 transporters. PLoS ONE. 2015;10:e0116546. doi: 10.1371/journal.pone.0116546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kerley C.P., Dolan E., Cormican L. Nitrate-rich beetroot juice selectively lowers ambulatory pressures and LDL cholesterol in uncontrolled but not controlled hypertension: A pilot study. Ir. J. Med. Sci. 2017;186:895–902. doi: 10.1007/s11845-016-1551-2. [DOI] [PubMed] [Google Scholar]
- 114.Rabeh M.N., Marwa I.E. Antihypercholesterolemic effects of beet (Beta vulgaris L.) root waste extract on hypercholesterolemic rats and its antioxidant potential properties. J. Nutr. 2014;13:500. doi: 10.3923/pjn.2014.500.505. [DOI] [Google Scholar]
- 115.Velmurugan S., Gan J.M., Rathod K.S., Khambata R.S., Ghosh S.M., Hartley A., Van Eijl S., Sagi-Kiss V., Chowdhury T.A., Curtis M., et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2015;103:25–38. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holy B., Nnamdi Isaac N., Ojoye Ngoye B. Post-prandial effect of beetroot (Beta vulgaris) juice on glucose and lipids levels of apparently healthy subjects. Eur. J. Pharm. Med. Res. 2017;4:60–62. [Google Scholar]
- 117.Singh A., Verma S., Singh V., Nanjappa C., Roopa N., Raju P.S., Singh S.N. Beetroot juice supplementation increases high density lipoprotein-cholesterol and reduces oxidative stress in physically active individuals. J. Pharm. Nutr. Sci. 2015;5:179–185. doi: 10.6000/1927-5951.2015.05.03.2. [DOI] [Google Scholar]
- 118.Lorizola I.M., Furlan C.P.B., Portovedo M., Milanski M., Botelho P.B., Bezerra R.M.N., Sumere B.R., Rostagno M.A., Capitani C.D. Beet stalks and leaves (Beta vulgaris L.) protect against high-fat diet-induced oxidative damage in the liver in mice. Nutrients. 2018;10:872. doi: 10.3390/nu10070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bobek P., Galbavý S., Mariássyová M. The effect of red beet (Beta vulgaris var. rubra) fiber on alimentary hypercholesterolemia and chemically induced colon carcinogenesis in rats. Nahrung. 2000;44:184–187. doi: 10.1002/1521-3803(20000501)44:3<184::AID-FOOD184>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 120.Krantz C., Monier M., Wahlström B. Absorption, excretion, metabolism and cardiovascular effects of beetroot extract in the rat. Food Cosmet. Toxicol. 1980;18:363–366. doi: 10.1016/0015-6264(80)90190-X. [DOI] [PubMed] [Google Scholar]
- 121.Patil K.S., Bhalsing S.R. Ethnomedicinal uses, phytochemistry and pharmacological properties of the genus Boerhavia. J. Ethnopharmacol. 2016;182:200–220. doi: 10.1016/j.jep.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 122.Mohan K., Paramasivam R., Chandran P., Veerasami V., Gnanasekaran A., Shanmugam A., Murugesan K. Inhibition of Hepatitis B virus DNA polymerase and modulation of TH. J. Pharm. Res. 2011;4:1044–1046. [Google Scholar]
- 123.Gilgun-Sherki Y., Melamed E., Offen D. Antioxidant treatment in Alzheimer’s disease: Current state. J. Mol. Neurosci. 2003;21:1–11. doi: 10.1385/JMN:21:1:1. [DOI] [PubMed] [Google Scholar]