Abstract
Objectives
In order to address the substantial increased risk of cardiovascular disease among people with schizophrenia, it is necessary to identify the factors responsible for some of that increased risk. We analysed the extent to which these risk factors were documented in primary care electronic medical records (EMR), and compared their documentation by patient and provider characteristics.
Design
Retrospective cohort study.
Setting
EMR database of the University of Toronto Practice-Based Research Network Data Safe Haven.
Participants
197 129 adults between 40 and 75 years of age; 4882 with schizophrenia and 192 427 without.
Primary and secondary outcome measures
Documentation of cardiovascular disease risk factors (age, sex, smoking history, presence of diabetes, blood pressure, whether a patient is currently on medication to reduce blood pressure, total cholesterol and high-density lipoprotein cholesterol).
Results
Documentation of cardiovascular risk factors was more complete among people with schizophrenia (74.5% of whom had blood pressure documented at least once in the last 2 years vs 67.3% of those without, p>0.0001). Smoking status was not documented in 19.8% of those with schizophrenia and 20.8% of those without (p=0.0843). Factors associated with improved documentation included older patients (OR for ages 70–75 vs 45–49=3.51, 95% CI 3.26 to 3.78), male patients (OR=1.39, 95% CI 1.33 to 1.45), patients cared for by a female provider (OR=1.52, 95% CI 1.12 to 2.07) and increased number of encounters (OR for ≥10 visits vs 3–5 visits=1.53, 95% CI 1.46 to 1.60).
Conclusions
Documentation of cardiovascular risk factors was better among people with schizophrenia than without, although overall documentation was inadequate. Efforts to improve documentation of risk factors are warranted in order to facilitate improved management.
Keywords: health informatics, cardiology, mental health, primary care, preventive medicine, schizophrenia & psychotic disorders
Strengths and limitations of this study.
This study analyses data from the University of Toronto Practice-Based Research Network Data Safe Haven, one of the world’s largest primary care electronic medical record databases.
It uses deidentified data from primary care charts to identify cardiovascular disease risk factors.
Strengths of the study include the sample size and the breadth of data included, from approximately 400 primary care clinics in Ontario, Canada.
Weaknesses include possible missing data resulting from the process of transferring data from primary care charts into a deidentified database, and the fact that the clinics included in the database are mainly urban and suburban academic clinics; these results may not necessarily be generalisable to all primary care settings.
Introduction
High-quality, comprehensive data are needed to understand health and how to improve it. Risk factors must be known and documented so that interventions can be planned and implemented.
One of the key challenges in primary care research has been the availability and quality of data. When Julian Tudor Hart conducted research on patients accessing care in his practice in Wales in the 1970s, it required laboriously searching through individual paper charts to collect necessary data.1 Today, electronic medical records (EMR) are widely used and can facilitate instant searches at the practice level as well as at local and national levels through databases that aggregate data from multiple practices. However, several studies have demonstrated that important data—for example, regarding cardiovascular risk factors such as smoking and whether someone has a diagnosis of hypertension—remain incomplete.2 3
People with serious mental illnesses, particularly those with schizophrenia, die 8–10 years earlier than those without these conditions.4 5 This is primarily due to higher rates of cardiovascular disease (CVD).5–7 While the long-term metabolic effects of antipsychotic medications used to treat schizophrenia are unclear, their use is associated with increased weight and blood glucose.8 9 Patients may also face challenges with self-care or accessing appropriate medical care.10 To date, there is sparse evidence about how to improve physical health status in these patients; a recent review of ‘collaborative care’ where both physical and mental health are attended to for these patients did not find any evidence of reductions in CVD risk.11
The primary prevention of CVD includes addressing risk factors such as tobacco use and hypertension; these are commonly managed in primary care. This is particularly true for people with serious mental illness, who are seen more frequently by family physicians than by psychiatrists.12 The prevalence of schizophrenia in the general adult population is 1%–3%, making it a relatively common condition.13 14 The prevalence and frequency of interaction strongly supports the important role played by family medicine in reducing the risk of CVD for people with mental illness. To do this effectively it is necessary to understand what that risk is and what variables should be focused on.
As a first step in establishing patients’ physical health status and identifying who to target for interventions to improve health, it is necessary to understand their health status. Whether data completeness concerns regarding CVD risk are general to all patients or whether they are more pronounced among those with serious mental illness is unknown.
Our study objectives were: to describe documentation of CVD risk factors (high-density lipoprotein (HDL), low-density lipoprotein, total cholesterol; blood pressure; smoking status) among patients with and without schizophrenia; and to explore patient and provider characteristics associated with sufficient documentation of these risk factors to calculate the Framingham Risk Score for patients with schizophrenia.
Methods
This is an observational retrospective cohort study design. We applied the Strengthening the Reporting of Observational Studies in Epidemiology checklist for reporting observational studies.15
Setting and data sources
We used data from the University of Toronto Practice-Based Research Network (UTOPIAN) Data Safe Haven, a primary care EMR database; data extracted as of 1 April 2018 were used for this project.16 The UTOPIAN Data Safe Haven contains EMR records from over 550 000 patients who access care in primary care practices in the Greater Toronto Area in Ontario, Canada. Physicians have consented to the provision of deidentified data, housed in a secure environment. These data are used for quality improvement and research purposes. The UTOPIAN database includes validated definitions for eight long-term conditions: osteoarthritis, diabetes, epilepsy, parkinsonism, dementia, hypertension, chronic obstructive pulmonary disease, depression.17 18 Neighbourhood-level income quintiles are also available from patient residential postal codes using Statistics Canada’s Postal Code Conversion Files.19 20
Study population
We included patients 40–75 years of age because Canadian guidelines recommend regular screening for CVD risk in this age range. There is no clear consensus on the recommended interval for screening, which varies from yearly to every 5 years.21–23 Guidelines suggest yearly CVD risk assessment for patients with schizophrenia24; however, these are not routinely followed in primary care practice and this increased frequency is consensus based and not necessarily supported by strong evidence. We therefore chose to look at a 2-year interval in which screening could have taken place, recognising that there may be some patients for whom it may be appropriate to screen less often. The most commonly used CVD risk assessment tool in Canadian primary care practice is the Framingham Risk Calculator,25 which includes the following items: (1) age, (2) sex, (3) smoking history, (4) presence of diabetes, (5) systolic blood pressure (SBP), (6) whether a patient is currently on medication to reduce blood pressure, (7) total cholesterol, and (8) HDL cholesterol. This is a validated risk stratification tool that establishes a patient’s risk of developing CVD (including coronary death, myocardial infarction, coronary insufficiency, angina, ischaemic stroke, haemorrhagic stroke, transient ischaemic attack, peripheral artery disease, heart failure) within the next 10 years. It is valid for patients 30–74 years of age.25
We identified patients who were 40–75 years of age as of 31 March 2018. We limited our cohort definition to those who had at least three primary care visits in the 2-year period between 1 April 2016 and 31 March 2018. To identify outcomes we looked at whether people had CVD risk factors as outlined above documented at least once in the above period. This definition ensured that we included patients likely to be routinely followed by the providers whose records are included in the database, and is consistent with our usual approach for studies using this database. We identified patients with schizophrenia using the same definition used in a previous study using the same database, using a combination of encounter diagnoses used for billing purposes as well as documentation of the condition in the EMR.26
Statistical analysis
We compared the documentation of CVD risk factors included in the Framingham Risk Calculator between those with and without schizophrenia using a χ2 test. P values derived from multiple hypothesis tests were adjusted using false discovery rates. In particular, the CVD risk factors included: HDL cholesterol, SBP, total cholesterol measured in the last 2 years of study follow-up and whether smoking status had ever been recorded. The relationship between the complete documentation of all Framingham elements was also assessed with respect to patient characteristics (age, sex, number of encounters in 2 years of study follow-up, diagnosis of schizophrenia, most recent body mass index (BMI) in the last 2 years of study follow-up), provider characteristics (age, sex) and geographical characteristics (income quintiles, rurality). A mixed-effects multilevel logistic regression was used to estimate unadjusted and adjusted ORs for the complete documentation of all Framingham elements (ie, calculable Framingham Score). Providers were specified as a random effect in the regression model.
All statistical analyses were generated using SAS software V.9.4 M4 (SAS Institute). A fixed nominal level of 0.05 was used to determine statistical significance in this study.
Patient and public involvement
There was no patient and public involvement in the design or conduct of this study.
Results
Cohort generation
Data from 376 physicians practising in 96 different clinic sites were included. In total, 197 309 patients were identified with age between 40 and 75 years old (as of 31 March 2018), recorded sex and had at least three visits in the 2 years of interest (figure 1). Out of 197 309 patients, 83 064 (40.4%) had adequate data to calculate a Framingham Risk Score using the most recent data available for HDL, SBP, and total cholesterol in the last 2 years and smoking status ever recorded. Of these, 4882 patients met the definition of schizophrenia and 2201 (43.8%) of these patients with schizophrenia had complete documentation to calculate the Framingham Risk Score.
Figure 1.
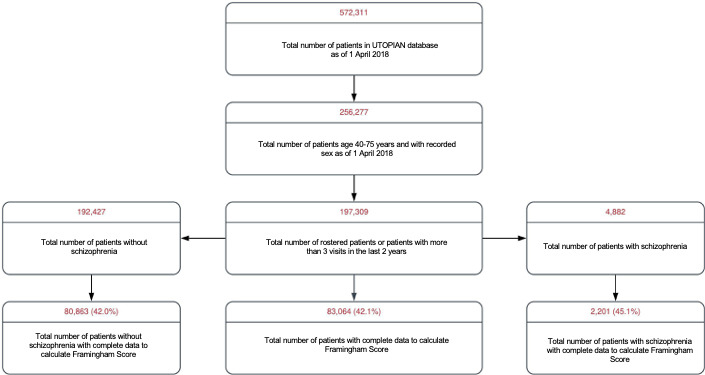
Distribution of Framingham risk factors among patients with and without schizophrenia. UTOPIAN, University of Toronto Practice-Based Research Network.
Individual Framingham data elements
We compared the presence of individual Framingham elements between 4882 patients with schizophrenia and 192 427 patients without, over a 2-year lookback window (1 April 2016 to 31 March 2018) (table 1). Framingham elements were documented more completely among those with schizophrenia: 25.5% of those with schizophrenia and 32.7% of those who had no documented blood pressure readings over the last 2 years (p<0.0001). 39.2% of those with schizophrenia and 42.1% of those without did not have any cholesterol readings (p<0.0001). There was no difference in documentation of smoking status between the two groups (p=0.084), with documentation missing in approximately 20% of all charts.
Table 1.
Distribution of Framingham factors among patients with and without schizophrenia
| Schizophrenia | P value* | ||||
| No | Yes | ||||
| n | Column % | n | Column % | ||
| Age range (years) | |||||
| 40–44 | 28 574 | 14.80 | 700 | 14.30 | – |
| 45–49 | 29 137 | 15.10 | 753 | 15.40 | |
| 50–54 | 30 939 | 16.10 | 784 | 16.10 | |
| 55–59 | 31 790 | 16.50 | 832 | 17.00 | |
| 60–64 | 27 061 | 14.10% | 707 | 14.50 | |
| 65–69 | 22 430 | 11.70% | 588 | 12.00 | |
| 70–75 | 22 496 | 11.70% | 518 | 10.60 | |
| Sex | |||||
| Female | 106 841 | 55.50 | 2539 | 52.00 | – |
| Male | 85 586 | 44.50 | 2343 | 48.00 | |
| HDL level (mmol/L) | |||||
| Missing | 79 437 | 41.30 | 1842 | 37.70 | <0.0001 |
| 0–0.89 | 8565 | 4.50 | 375 | 7.70 | |
| 0.9–1.19 | 27 925 | 14.50 | 866 | 17.70 | |
| 1.2–1.29 | 11 006 | 5.70 | 272 | 5.60 | |
| 1.3–1.59 | 28 313 | 14.70 | 703 | 14.40 | |
| 1.60+ | 37 181 | 19.30 | 824 | 16.90 | |
| Total cholesterol (mmol/L) | |||||
| Missing | 81 073 | 42.10 | 1916 | 39.20 | <0.0001 |
| 0–4.09 | 25 388 | 13.20 | 865 | 17.70 | |
| 4.1–5.19 | 39 801 | 20.70 | 1068 | 21.90 | |
| 5.2–6.19 | 30 009 | 15.60 | 663 | 13.60 | |
| 6.2–7.19 | 11 225 | 5.80 | 252 | 5.20 | |
| 7.2+ | 4931 | 2.60 | 118 | 2.40 | |
| Systolic blood pressure (mm Hg) | |||||
| Missing | 62 934 | 32.70 | 1245 | 25.50 | <0.0001 |
| 120 or less | 42 293 | 22.00 | 1445 | 29.60 | |
| 120–129 | 36 752 | 19.10 | 940 | 19.30 | |
| 130–139 | 28 764 | 14.90 | 725 | 14.90 | |
| 140–149 | 14 043 | 7.30 | 350 | 7.20 | |
| 150–159 | 5752 | 3.00 | 124 | 2.50 | |
| 160 or more | 1889 | 1.00 | 53 | 1.10 | |
| Smoking status | |||||
| Missing | 40 109 | 20.80 | 968 | 19.80 | 0.0843 |
| Non-smoker | 125 796 | 65.40 | 2633 | 53.90 | |
| Smoker | 26 522 | 13.80 | 1281 | 26.20 | |
| Type 2 diabetes mellitus | |||||
| No | 168 151 | 87.40 | 3953 | 81.00 | – |
| Yes | 24 276 | 12.60 | 929 | 19.00 | |
| Antihypertensive medication | |||||
| No | 140 415 | 73.00 | 3486 | 71.40 | – |
| Yes | 52 012 | 27.00 | 1396 | 28.60 | |
| Total | 192 427 | 100.00 | 4882 | 100.00 | – |
*P values compare the proportion of missing data and non-missing data with respect to schizophrenia (using Χ2 test with false discovery rate).
HDL, high-density lipoprotein.
Patient, provider and geographical characteristics as predictors of calculable Framingham Score
Individual patient characteristics between those who had complete documentation of Framingham Score factors and those who did not are found in tables 2 and 3. Unadjusted and adjusted ORs for the complete documentation of Framingham Score are found in online supplemental tables S1 and S2.
Table 2.
Calculable Framingham Score with respect to individual Framingham factors
| Calculable Framingham Score | Total | ||||
| No | Yes | ||||
| n | Row % | n | Row % | n | |
| Age range (years) | |||||
| 40–44 | 21 922 | 74.90 | 7352 | 25.10 | 29 274 |
| 45–49 | 20 367 | 68.10 | 9523 | 31.90 | 29 890 |
| 50–54 | 19 024 | 60.00 | 12 699 | 40.00 | 31 723 |
| 55–59 | 18 106 | 55.50 | 14 516 | 44.50 | 32 622 |
| 60–64 | 14 143 | 50.90 | 13 625 | 49.10 | 27 768 |
| 65–69 | 10 565 | 45.90 | 12 453 | 54.10 | 23 018 |
| 70–75 | 10 118 | 44.00 | 12 896 | 56.00 | 23 014 |
| Sex | |||||
| Female | 63 352 | 57.90 | 46 028 | 42.10 | 109 380 |
| Male | 50 893 | 57.90 | 37 036 | 42.10 | 87 929 |
| HDL level (mmol/L) | |||||
| Missing | 81 279 | 100.00 | – | – | 81 279 |
| 0–0.89 | 2408 | 26.90 | 6532 | 73.10 | 8940 |
| 0.9–1.19 | 7973 | 27.70 | 20 818 | 72.30 | 28 791 |
| 1.2–1.29 | 3092 | 27.40 | 8186 | 72.60 | 11 278 |
| 1.3–1.59 | 8069 | 27.80 | 20 947 | 72.20 | 29 016 |
| 1.60+ | 11 424 | 30.10 | 26 581 | 69.90 | 38 005 |
| Total cholesterol (mmol/L) | |||||
| Missing | 82 989 | 100.00 | – | – | 82 989 |
| 0–4.09 | 6336 | 24.10 | 19 917 | 75.90 | 26 253 |
| 4.1–5.19 | 11 400 | 27.90 | 29 469 | 72.10 | 40 869 |
| 5.2–6.19 | 8686 | 28.30 | 21 986 | 71.70 | 30 672 |
| 6.2–7.19 | 3167 | 27.60 | 8310 | 72.40 | 11 477 |
| 7.2+ | 1667 | 33.00 | 3382 | 67.00 | 5049 |
| Systolic blood pressure (mm Hg) | |||||
| Missing | 62 874 | 98.00 | 1305 | 2.00 | 64 179 |
| 120 or less | 18 185 | 41.60 | 25 553 | 58.40 | 43 738 |
| 120–129 | 14 338 | 38.00 | 23 354 | 62.00 | 37 692 |
| 130–139 | 10 431 | 35.40 | 19 058 | 64.60 | 29 489 |
| 140–149 | 5359 | 37.20 | 9034 | 62.80 | 14 393 |
| 150–159 | 2270 | 38.60 | 3606 | 61.40 | 5876 |
| 160 or more | 788 | 40.60 | 1154 | 59.40 | 1942 |
| Smoking status | |||||
| Missing | 41 077 | 100.00 | – | – | 41 077 |
| Non-smoker | 58 342 | 45.40 | 70 087 | 54.60 | 128 429 |
| Smoker | 14 826 | 53.30 | 12 977 | 46.70 | 27 803 |
| Type 2 diabetes mellitus | |||||
| No | 105 354 | 61.20 | 66 750 | 38.80 | 172 104 |
| Yes | 8891 | 35.30 | 16 314 | 64.70 | 25 205 |
| Antihypertensive medication | |||||
| No | 92 342 | 64.20 | 51 559 | 35.80 | 143 901 |
| Yes | 21 903 | 41.00 | 31 505 | 59.00 | 53 408 |
| Total | 114 245 | 57.90 | 83 064 | 42.10 | 197 309 |
HDL, high-density lipoprotein.
Table 3.
Calculable Framingham Score with respect to patient, provider and geographical characteristics
| Calculable Framingham Score | Total | ||||
| No | Yes | ||||
| n | Row % | n | Row % | n | |
| Schizophrenia | |||||
| No | 111 564 | 58.00 | 80 863 | 42.00 | 192 427 |
| Yes | 2681 | 54.90 | 2201 | 45.10 | 4882 |
| BMI level (kg/m2) | |||||
| Missing | 84 398 | 77.70 | 24 206 | 22.30 | 108 604 |
| 18.4 or less (underweight) | 378 | 42.90 | 503 | 57.10 | 881 |
| 18.5–24.9 (normal) | 9191 | 38.60 | 14 619 | 61.40 | 23 810 |
| 25–29.9 (overweight) | 10 622 | 32.60 | 21 935 | 67.40 | 32 557 |
| 30–34.9 (obese class I) | 5847 | 30.50 | 13 331 | 69.50 | 19 178 |
| 35–39.9 (obese class II) | 2306 | 30.40 | 5279 | 69.60 | 7585 |
| 40 or more (obese class III) | 1503 | 32.00 | 3191 | 68.00 | 4694 |
| Encounters (n) | |||||
| Missing | 42 673 | 86.00 | 6952 | 14.00 | 49 625 |
| 3–5 visits | 25 915 | 58.60 | 18 311 | 41.40 | 44 226 |
| 6–9 visits | 19 861 | 48.80 | 20 830 | 51.20 | 40 691 |
| ≥10 visits | 25 796 | 41.10 | 36 971 | 58.90 | 62 767 |
| Income quintiles | |||||
| Missing | 14 795 | 58.90 | 10 326 | 41.10 | 25 121 |
| 1 | 15 851 | 58.70 | 11 162 | 41.30 | 27 013 |
| 2 | 15 883 | 58.30 | 11 348 | 41.70 | 27 231 |
| 3 | 17 118 | 58.20 | 12 281 | 41.80 | 29 399 |
| 4 | 20 810 | 57.80 | 15 221 | 42.20 | 36 031 |
| 5 | 29 788 | 56.70 | 22 726 | 43.30 | 52 514 |
| Region | |||||
| Missing | 2284 | 73.60 | 819 | 26.40 | 3103 |
| Rural | 11 590 | 59.70 | 7833 | 40.30 | 19 423 |
| Urban | 100 371 | 57.40 | 74 412 | 42.60 | 174 783 |
| Provider age (years) | |||||
| Missing | 5880 | 50.60 | 5741 | 49.40 | 11 621 |
| 29–39 | 21 926 | 54.10 | 18 618 | 45.90 | 40 544 |
| 40–49 | 23 203 | 58.40 | 16 550 | 41.60 | 39 753 |
| 50–59 | 29 127 | 55.90 | 22 943 | 44.10 | 52 070 |
| 60+ | 34 109 | 64.00 | 19 212 | 36.00 | 53 321 |
| Provider sex | |||||
| Female | 52 950 | 54.20 | 44 655 | 45.80 | 97 605 |
| Male | 61 295 | 61.50 | 38 409 | 38.50 | 99 704 |
| Total | 114 245 | 57.90 | 83 064 | 42.10 | 197 309 |
BMI, body mass index.
bmjopen-2020-038013supp001.pdf (125.2KB, pdf)
Patients with schizophrenia did not have decreased adjusted odds for the complete documentation of all Framingham score factors as compared to patients without schizophrenia (OR=0.90, 95% CI 0.79 to 1.01, p=0.10) (figure 2). The adjusted odds for the complete documentation of Framingham factors increased with respect to the patient’s age (70–75 years vs 40–44 years, OR=3.51, 95% CI 3.26 to 3.78). Male patients had increased adjusted odds of calculable Framingham score as compared with female patients (male vs female, OR=1.39, 95% CI 1.33 to 1.45). An increase in the BMI level was associated with an increase in adjusted odds for calculable Framingham score (obese class III vs underweight, OR=2.00, 95% CI 1.66 to 2.43) (table 3). An increase in the total number of encounters also led to increased adjusted odds for the complete documentation of Framingham factors (more than 10 visits vs 3–5 visits in the last 2 years, OR=1.53, 95% CI 1.46 to 1.60).
Figure 2.
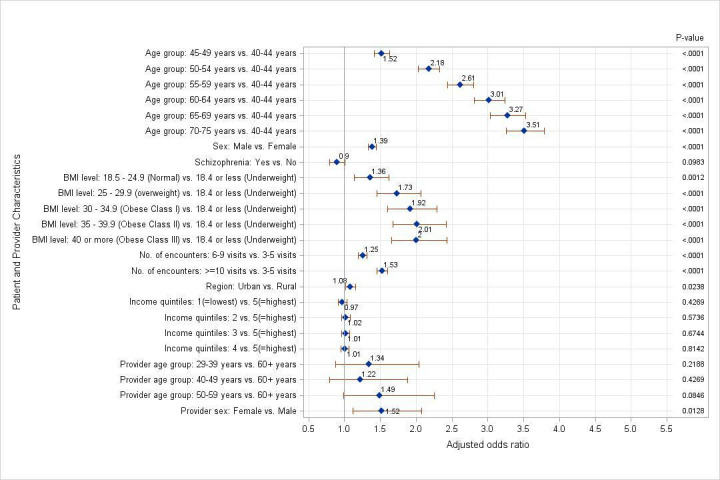
Adjusted ORs for calculable Framingham Score using random-effects multilevel logistic regression model. BMI, body mass index.
Patients residing in urban regions had higher adjusted odds for the complete documentation of Framingham factors as compared with patients residing in rural regions (OR=1.08, 95% CI 1.02 to 1.16). However, no significant differences in adjusted ORs were detected across the five levels of income quintiles (1 vs 5, OR=0.97, 95% CI 0.91 to 1.03, p=0.43). Female physicians had increased adjusted odds for the complete documentation of Framingham factors as compared with male physicians (OR=1.52, 95% CI 1.12 to 2.07). However, provider age did not contribute to increased or decreased adjusted odds for calculable Framingham Score (29–39 years vs 60+ years, OR=1.49, 95% CI 0.98 to 2.26, p=0.08).
Discussion
In this study of primary care EMRs from the UTOPIAN, we found better documentation of cardiovascular risk factors among people with schizophrenia as opposed to those without the condition. However, overall documentation was inadequate.
Other studies on preventive health for people with schizophrenia, such as those addressing cancer screening, have found lower rates of preventive care when compared with the general population.27 We actually found more complete documentation of some risk factors among people with schizophrenia when compared with those without, such as blood pressure. There are various recommendations for frequency of CVD risk screening in the general population; for example, Allan et al suggested every 5 years for men over 40 and women over 50.23 More complete documentation of risk factors would be expected based on guidelines suggesting more frequent CVD risk assessment among people who are on antipsychotic medication.14 To some extent, the present study demonstrates a promising finding, suggesting that patients with schizophrenia are receiving at least as good care from this perspective as those without the condition. It is, however, quite concerning that there are substantial gaps in documentation of particular risk factors such as smoking cessation. Nearly 20% of patients did not have smoking status documented in the chart. We suggest that if it is not documented, then it is extremely unlikely that smoking cessation has been addressed at a primary care visit. Smoking is highly prevalent among people with schizophrenia; Canadian estimates range from 47%28 to 78%.29 There are many effective interventions to support patients with schizophrenia to stop smoking.30 It is therefore essential to document smoking status for all patients with schizophrenia and to make smoking cessation a priority. We found several factors associated with what we assessed to be ‘appropriate’ documentation of risk factors sufficient for cardiovascular risk assessment, such as increasing number of clinical encounters, male sex, as well as increasing BMI and age.
Limitations of this study include the use of EMR data, which is known to have deficiencies around data quality and completeness.31 32 UTOPIAN, as part of the Canadian Primary Care Sentinel Surveillance Network, is disproportionally comprised of more providers in academic practices and has an older population than the Canadian average.33 These findings therefore may not be generalisable to all Canadian primary care settings. UTOPIAN contains data from multiple EMR vendors and as a consequence there is the possibility that some data may be missing as a result of errors in database formation; these data are extracted with the best available approaches and regular data cleaning attempts to minimise these errors. Other studies have found some deficiencies, particularly related to documentation of health conditions, in EMR data in the Canadian setting.3 There are no Canadian national standards for necessary elements of EMR documentation in primary care. In Ontario, laboratory results enter most physicians’ EMRs through the Ontario Laboratory Information System34 which is an automatic process, reducing the extent to which documentation is incomplete because of provider error. Primary care providers therefore receive test results from all other providers involved in a patient’s care, making primary care records an appropriate location to assess these parameters. Our focus on ‘documentation’ in this study is as a result of the practical principle that if something is not documented, it cannot be acted on; therefore, data documentation and completeness are being taken as a proxy for their consideration in clinical decision-making. We acknowledge that this approach may result in ‘overestimation’ of the extent to which CVD risk screening is occurring for patients with schizophrenia. It is possible to have all of the Framingham items documented in the medical record but not to have brought them together to estimate overall cardiovascular risk. However, given the primary conclusion that cardiovascular risk screening is inadequate in this sample, the study methods biasing towards ‘overestimation’, if anything, support this main finding. It is not possible from the data considered in this study to ascertain whether a provider has attempted to intervene towards smoking cessation, or whether someone has addressed blood pressure management. There are other risk stratification approaches available both for the general population (such as QRISK235) and specifically for people with serious mental illness (PRIMROSE36). We chose to focus on the Framingham assessment because it is the most commonly used in Canadian primary care and therefore would be most relevant to the study context.
In summary, we found slightly more complete documentation of cardiovascular risk factors and their management among people with schizophrenia as opposed to those without this condition. However, overall documentation of these risk factors remains incomplete. Adequate CVD risk assessment is essential to identifying and addressing risk factors, particularly among people with schizophrenia who have much higher mortality from CVD (and other conditions) than the general public. Efforts should be undertaken in primary care to improve data completeness and CVD risk assessment and management.
Supplementary Material
Footnotes
Twitter: @bradenoneill
Contributors: BO, SK, BA, FS, RM, MK and MG contributed to the initial conception of the study. BO, SK, BA and RM made substantial contributions to the statistical methodology, analysis and data interpretation. BO and SK wrote the first draft of the manuscript. BO, SK, BA, FS, MK and MG provided substantial revisions to the manuscript.
Funding: This study was funded by the Foundation for Advancing Family Medicine of the College of Family Physicians of Canada (2018 Janus Research Grant). BO completed this work during a Research Fellowship with the Medical Psychiatry Alliance, Toronto, Ontario. BO and MG receive salary support from North York General Hospital and the Department of Family and Community Medicine, University of Toronto, Ontario, Canada.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Ethics approval was obtained from the North York General Hospital Research Ethics Board (approval number 18-0006).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Data from this study are held by the University of Toronto Practice-Based Research Network; ethics approval for this study does not allow unrestricted public access to the data. Please contact the corresponding author for information on how to access.
References
- 1.Hart JT. The inverse care law. Lancet 1971;297:405–12. 10.1016/S0140-6736(71)92410-X [DOI] [PubMed] [Google Scholar]
- 2.Greiver M, Aliarzadeh B, Meaney C, et al. . Are we asking patients if they smoke? missing information on tobacco use in Canadian electronic medical records. Am J Prevent Med 2015;49:264–8. [DOI] [PubMed] [Google Scholar]
- 3.Singer A, Yakubovich S, Kroeker AL, et al. . Data quality of electronic medical records in Manitoba: do problem Lists accurately reflect chronic disease billing diagnoses? J Am Med Inform Assoc 2016;23:1107–12. 10.1093/jamia/ocw013 [DOI] [PubMed] [Google Scholar]
- 4.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psych 2015;72:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatov E, Rosella L, Chiu M, et al. . Trends in standardized mortality among individuals with schizophrenia, 1993-2012: a population-based, repeated cross-sectional study. CMAJ 2017;189:E1177–87. 10.1503/cmaj.161351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennekens CH, Hennekens AR, Hollar D, et al. . Schizophrenia and increased risks of cardiovascular disease. Am Heart J 2005;150:1115–21. 10.1016/j.ahj.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 7.Goldstein BI, Fagiolini A, Houck P, et al. . Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord 2009;11:657–62. 10.1111/j.1399-5618.2009.00735.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch L, Yang J, Bresee L, et al. . Second-generation antipsychotics and metabolic side effects: a systematic review of population-based studies. Drug Saf 2017;40:771–81. 10.1007/s40264-017-0543-0 [DOI] [PubMed] [Google Scholar]
- 9.Spertus J, Horvitz-Lennon M, Abing H, et al. . Risk of weight gain for specific antipsychotic drugs: a meta-analysis. NPJ Schizophr 2018;4:1–7. 10.1038/s41537-018-0053-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Hert M, Dekker JM, Wood D, et al. . Cardiovascular disease and diabetes in people with severe mental illness position statement from the European psychiatric association (EPA), supported by the European association for the study of diabetes (EASD) and the European Society of cardiology (ESC). Eur psychiatr 2009;24:412–24. 10.1016/j.eurpsy.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 11.Bullock HL, Waddell K, Wilson MG. Rapid synthesis: identifying and assessing core components of collaborative-care models for treating mental and physical health conditions. Hamilton, Canada: McMaster Health Forum, 2017. [Google Scholar]
- 12.Slomp M, Bland R, Patterson S, et al. . Three-year physician treated prevalence rate of mental disorders in Alberta. Can J Psychiatry 2009;54:199–203. 10.1177/070674370905400308 [DOI] [PubMed] [Google Scholar]
- 13.Government of Canada Chapter 3: Schizophrenia – A report on mental illnesses in Canada [online], Ottawa, 2012. Available: https://www.canada.ca/en/public-health/services/reports-publications/report-on-mental-illnesses-canada/schizophrenia.htm [Accessed 31 Jan 2018].
- 14.Saha S, Chant D, Welham J, et al. . A systematic review of the prevalence of schizophrenia. PLoS Med 2005;2:e141. 10.1371/journal.pmed.0020141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, et al. . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 16.University of Toronto Department of Family and Community Medicine University of Toronto practice based research network (utopian). Available: https://www.dfcm.utoronto.ca/landing-page/utopian [Accessed 31 Jan 2018].
- 17.CPCSSN Team Case definitions: Canadian primary care sentinel surveillance network (CPCSSN), version 2, 2019. Available: http://cpcssn.ca/research-resources/cpcssn-case-definitions-v2
- 18.Williamson T, Green ME, Birtwhistle R, et al. . Validating the 8 CPCSSN case definitions for chronic disease surveillance in a primary care database of electronic health records. Ann Fam Med 2014;12:367–72. 10.1370/afm.1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkins R. Use of postal codes and addresses in the analysis of health data. Health Rep 1993;5:157–77. [PubMed] [Google Scholar]
- 20.Pampalon R, Hamel D, Gamache P, et al. . A deprivation index for health planning in Canada. Chronic Dis Can 2009;29:178–91. [PubMed] [Google Scholar]
- 21.Genest J, McPherson R, Frohlich J, et al. . 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can J Cardiol 2009;25:567–79. 10.1016/S0828-282X(09)70715-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson TJ, Grégoire J, Hegele RA, et al. . 2012 update of the Canadian cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013;29:151–67. 10.1016/j.cjca.2012.11.032 [DOI] [PubMed] [Google Scholar]
- 23.Allan GM, Lindblad AJ, Comeau A, et al. . Simplified lipid guidelines: prevention and management of cardiovascular disease in primary care. Can Fam Physician 2015;61:857–67. [PMC free article] [PubMed] [Google Scholar]
- 24.Pringsheim T, Kelly M, Urness D, et al. . Physical health and drug safety in individuals with schizophrenia. Can J Psychiatry 2017;62:673–83. 10.1177/0706743717719898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Agostino RB, Vasan RS, Pencina MJ, et al. . General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 2008;117:743–53. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 26.O’Neill B, Kalia S, Aliarzadeh B, et al. . Agreement between primary care and hospital diagnosis of schizophrenia and bipolar disorder: a cross-sectional, observational study using record linkage. PLoS One 2019;14:e0210214 10.1371/journal.pone.0210214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martens PJ, Chochinov HM, Prior HJ, et al. . Are cervical cancer screening rates different for women with schizophrenia? A Manitoba population-based study. Schizophr Res 2009;113:101–6. 10.1016/j.schres.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 28.Johnson JL, Ratner PA, Malchy LA, et al. . Gender-specific profiles of tobacco use among non-institutionalized people with serious mental illness. BMC Psychiatry 2010;10:101. 10.1186/1471-244X-10-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerber GJ, Krupa T, Eastabrook S, et al. . Substance use among persons with serious mental illness in eastern Ontario. Can J Commun Ment Health 2003;22:113–28. 10.7870/cjcmh-2003-0008 [DOI] [PubMed] [Google Scholar]
- 30.Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev 2013;2:CD007253. 10.1002/14651858.CD007253.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiskopf NG, Weng C. Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research. J Am Med Inform Assoc 2013;20:144–51. 10.1136/amiajnl-2011-000681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birtwhistle R, Williamson T. Primary care electronic medical records: a new data source for research in Canada. CMAJ 2015;187:239–40. 10.1503/cmaj.140473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Queenan JA, Williamson T, Khan S, et al. . Representativeness of patients and providers in the Canadian primary care sentinel surveillance network: a cross-sectional study. CMAJ Open 2016;4:E28–32. 10.9778/cmajo.20140128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ontario Laboratory Information System eHealth Ontario. Available: https://www.ehealthontario.on.ca/en/for-healthcare-professionals/ontario-laboratories-information-system-olis
- 35.Hippisley-Cox J, Coupland C, Vinogradova Y, et al. . Performance of the QRISK cardiovascular risk prediction algorithm in an independent UK sample of patients from general practice: a validation study. Heart 2008;94:34–9. 10.1136/hrt.2007.134890 [DOI] [PubMed] [Google Scholar]
- 36.Zomer E, Osborn D, Nazareth I, et al. . Effectiveness and cost-effectiveness of a cardiovascular risk prediction algorithm for people with severe mental illness (primrose). BMJ Open 2017;7:e018181. 10.1136/bmjopen-2017-018181 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038013supp001.pdf (125.2KB, pdf)


