Abstract
Endogenous and exogenous signals are perceived and integrated by plants to precisely control defense responses. As a crucial environmental cue, light reportedly plays vital roles in plant defenses against necrotrophic pathogens. Phytochrome-interacting factor (PIF) is one of the important transcription factors which plays essential roles in photoreceptor-mediated light response. In this study, we revealed that PIFs negatively regulate plant defenses against Botrytis cinerea. Gene expression analyses showed that the expression level of a subset of defense-response genes was higher in pifq (pif1/3/4/5) mutants than in the wild-type control, but was lower in PIF-overexpressing plants. Chromatin immunoprecipitation assays proved that PIF4/5 binds directly to the ETHYLENE RESPONSE FACTOR1 (ERF1) promoter. Moreover, genetic analyses indicated that the overexpression of ERF1 dramatically rescues the susceptibility of PIF4-HA and PIF5-GFP transgenic plants, and that PIF controls the resistance to B. cinerea in a COI1- and EIN2-dependent manner. Our results provide compelling evidence that PIF, together with the jasmonate/ethylene pathway, is important for plant resistance to B. cinerea.
Keywords: Arabidopsis, Botrytis cinerea, defense response, PIF, ethylene, jasmonic acid
1. Introduction
As sessile organisms, stress-resistant plants employ complex defense mechanisms to counter the adverse effects of multiple pathogens and/or herbivorous insects. The precisely controlled immunity is vital for the survivability of plants in the interaction with pathogens. According to the lifestyles, plant pathogens can be divided into biotrophs, which obtain nutrition from living host cells, and necrotrophs, which feed on the dead plant tissue [1]. As one of the typical broad host-range necrotrophic pathogens which can infect plants with no host specificity, Botrytis cinerea has been found to cause huge economical losses in agricultural production and is used as a model fungus to study the interaction between plants and pathogens.
There has been considerable progress in characterizing the molecular mechanisms underlying immune signaling networks against B. cinerea over the past several years [2]. Specifically, the plant hormones jasmonate (JA) and ethylene (ET) play crucial roles in plant defenses against necrotrophic pathogens [1,2]. Jasmonate is a lipid-derived compound, perceived by the F-box protein CORONATINE INSENSITIVE1 (COI1), which forms the SCFCOI1 E3 ubiquitin ligase with SKP1 and CULLIN1 to mediate the degradation of JAZ proteins [3,4,5,6]. JA-insensitive coi1 mutant plants have compromised defense response against B. cinerea [7,8,9,10]. Activated JA signaling in plants is often associated with increased resistance against B. cinerea, as well as growth inhibition [11,12,13]. Moreover, JA has been showed to repress hypocotyl elongation in a COI1-, JAZ-, and MYCs-dependent manner [14]. Additionally, JAZs also contribute to the synergism between JA and ET signaling in response to necrotrophic pathogens by directly binding to EIN3/EIL1 [15], which are two major transcription factors affecting ET signaling. They function downstream of EIN2, which interacts with the ethylene receptor ETR1 to positively regulate ET signaling [16,17,18]. ET-insensitive ein2 mutant plants are impaired in resistance to B. cinerea [7,8,19]. Following a Botrytis cinerea infection, JA and ET production are rapidly and simultaneously induced, leading to the activation of downstream defense-associated genes, such as ERF1, OCTADECANOID-RESPONSIVE ARABIDOPSIS AP2/ERF-DOMAIN PROREIN 59 (ORA59), and PLANT DEFENSIN 1.2 (PDF1.2), thereby enhancing plant disease resistance [11,12]. Moreover, simultaneous mutation of ethylene response factor ERF5 and ERF6 makes plants more susceptible against B. cinerea via compromised JA-induced defense gene expression [20].
In A. thaliana, a group of basic helix–loop–helix (bHLH) transcription factors called Phytochrome-interacting factor (PIFs) function downstream of phyB. The PIFs belong to subfamily 15 of the bHLH superfamily [21,22]. There are eight PIF proteins in Arabidopsis: PIF1, PIF2, PIF3, PIF4, PIF5, PIF6, PIF7, and PIF8 [23]. Recent functional analyses have provided some evidence that PIFs participate in a wide range of physiological processes, including seed germination, flowering, senescence, and shade avoidance [24,25,26,27,28,29,30,31,32]. As transcription factors, PIFs bind and regulate a large number of gene to regulate plant development and growth. For example, PIF negatively controls seed germination by decreasing gibberellin levels and increasing abscisic acid levels or by orchestrating ABA signaling in darkness [24,32,33]. Furthermore, PIF4 positively affects thermomorphogenesis and thermosensory-activated flowering [28,34], but negatively influences plant immunity [35]. Another study confirmed that PIF3/4/5 function as positive regulators during age-triggered and dark-induced leaf senescence [29]. Furthermore, PIF1/3/4/5 act redundantly to positively regulate the shade-avoidance syndrome by directly suppressing MIR156 expression [30]. The pifq (pif1/3/4/5) plants show differential phenotypes when compared to wild-type in the process of growth, development, and resistance, including constitutive photomorphogenesis and less sensitivity to ABA-inhibited seed germination and primary root growth in darkness [32], attenuated shade-avoidance syndrome [30], and enhanced resistance to DC3000 [35].
Recently, some results have reported that the expression of PIF3 and PIF7 is suppressed during a B. cinerea infection [36], and that Phytochrome B integrates light hints into the jasmonate signaling pathway, to prioritize plant growth over defense, under competition environment [37], suggesting that PIFs have a role in regulating plant defense response. In this study, we demonstrated that PIFs redundantly control plant defenses against B. cinerea by modulating the expression of a subset of defense-response genes, among which ERF1 can be directly bound and repressed by them. We also uncovered that the PIF-controlled defense is closely associated with JA/ET signaling.
2. Results
2.1. The Effect of PIF Mutations on Resistance to B. cinerea
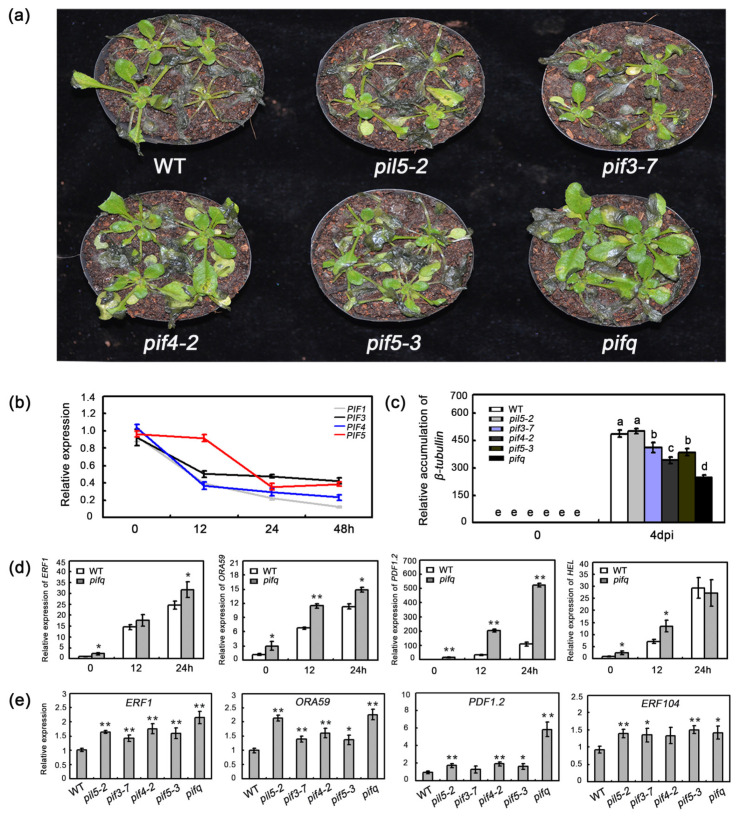
The PIFs, which belong to the bHLH transcription factor family, negatively regulate phyB-mediated light signaling. Previous investigations that used a method of time series of transcriptomic analysis with high-resolution demonstrated that the expression of PIF3 and PIF7 is suppressed during B. cinerea infection [36]. To further investigate the function of PIFs in regulation of plant defense against B. cinerea, we first examined their expression, including PIF1, PIF3, PIF4, and PIF5, after treating plants with B. cinerea. As shown in Figure 1b, complementary to previous reports, we found that, in addition to PIF3, B. cinerea inoculation also greatly repressed the expression of PIF1, PIF4, and PIF5. To determine whether PIFs function in plant defenses against B. cinerea, we examined the WT plants, several single mutants (pil5-2, pif3-7, pif4-2, and pif5-3), and the pifq mutant. At six days post-inoculation, the symptom development of the pil5-2, pif3-7, and pif5-3 plants was similar to that of the WT plants. In contrast, the pif4-2 and pifq mutants had slightly and significantly less extensive disease symptoms, respectively, when compared with the WT plants (Figure 1a). Similarly, the pif4-2 and pifq mutant plants had less pathogen biomass, when compared with the WT plants at three days post-inoculation (Figure 1c). Additionally, the pifq plants accumulated considerably less β-tubulin mRNA than the pif single mutants and the WT plants (Figure 1c). These results implied that PIFs function redundantly and negatively, to regulate plant defenses against B. cinerea.
Figure 1.
Phytochrome-interacting factor (PIF) proteins redundantly regulate plant defense response. (a) The disease phenotypes of pif mutant plants. The WT (Col-0) and pif plants were grown on soil for 28 days, under 12 h light/12 h dark conditions, and then inoculated with B. cinerea spores (5 × 105 spores/mL); representative plants were photographed at six days post-inoculation (dpi). The experiments were repeated three times, with similar results. (b) The expression of PIFs was downregulated upon Botrytis inoculation. (c) The relative biomass of Botrytis in pif plants. RNA was isolated from spray-inoculated plants at 0 and 4 dpi, and the relative β-tubulin gene transcripts were examined. Error bars indicate SD of three independent experiments. The different letters above columns indicate significant differences (one-way ANOVA; p < 0.05). (d) The relative expression of ERF1, ORA59, PDF1.2, and HEL in WT and pifq after Botrytis inoculation. (e) The basal expression of ERF1, ORA59, PDF1.2, and ERF104 in 14-day-old soil grown pif mutant under LD condition. For (d,e), error bars indicate SD of three independent experiments. Asterisks indicate Student’s t-test significant differences (* p < 0.05, ** p < 0.01).
2.2. The Effect of PIFs in Regulating the Basal and Pathogen-Induced Expression of Defense-Related Gene
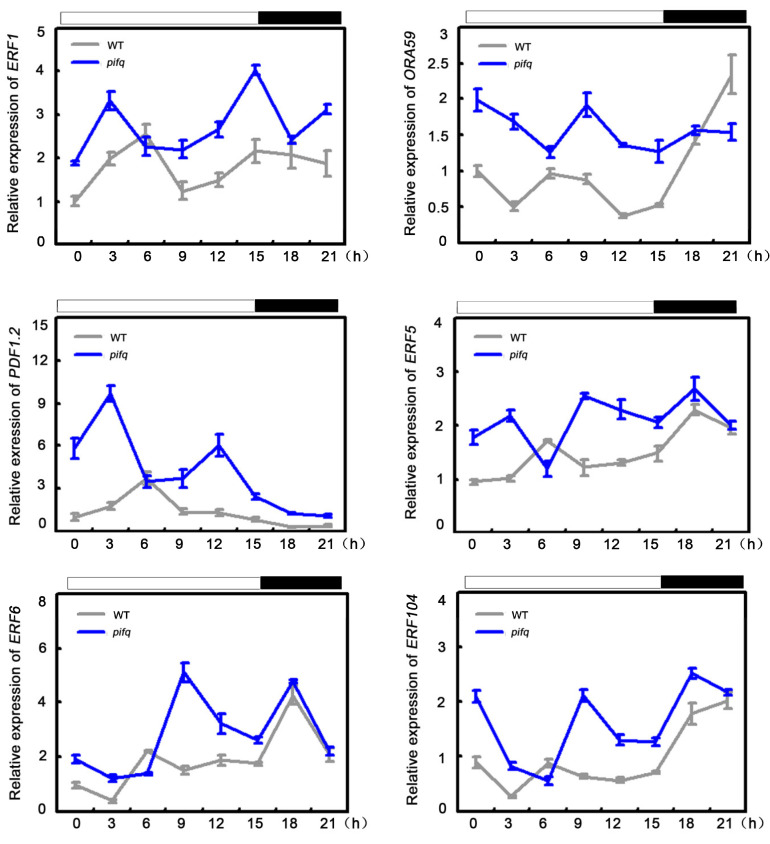
To defend against necrotrophic pathogens, plants activate a set of defense-related genes, including ERF1, ORA59, PATHOGEN INDUCIBLE PLANT DEFENSIN (PDF1.2), and HEVEIN-LIKE PROTEIN (HEL) [11,12,38]. To explore the molecular basis of the altered responses of the pifq mutants to the necrotrophic fungal pathogen, we first analyzed the expression of several defense-related genes in these plants, after an infection by B. cinerea. The ERF1, ORA59, PDF1.2, and HEL expression levels were higher in the pifq plants than in the WT plants, following the B. cinerea infection (Figure 1d). As shown in Figure 1d, it is obvious that pifq showed significantly high basal expression of these four genes. Thus, we hypothesized that pif mutants might have a more powerful basal defense. To further investigate this possibility, we tested the expression of defense-related genes in pif plants. The pif mutants we used showed a high expression level of defense-response genes, including ERF1, ORA59, PDF1.2, and ERF104 (Figure 1e). Moreover, previous studies proved that the expression levels and the protein abundance of PIFs are affected by diurnal conditions [39,40,41,42]. Because of their functional redundancy, we then used pifq mutants to examine the expression levels of several defense-related genes during a 24 h period. The expression levels of defense-associated genes, such as ERF1, ORA59, PDF1.2, ERF5, and ERF6, were higher in the pifq plants than in the WT plants at the ZT0~ZT3 and ZT9~ZT12 time-points (Figure 2). Considering the clearly close correlation between high expression of defense response genes under both conditions with and without B. cinerea treatment and the largely resistant phenotypes after its infection in pifq plants, we concluded that PIFs regulate defense-associated genes expression to control plant resistance against B. cinerea.
Figure 2.
The basal expression of defense-response genes in WT and pifq plants. The plants were grown on soil, under LD condition, for 14 days. The plants for RNA isolation were harvested at given times from ZT0. IPP2 gene was used as an internal control. Error bars indicate SD of three independent RNA extracts.
2.3. The Effect of PIF Overexpressions on Resistance to B. cinerea
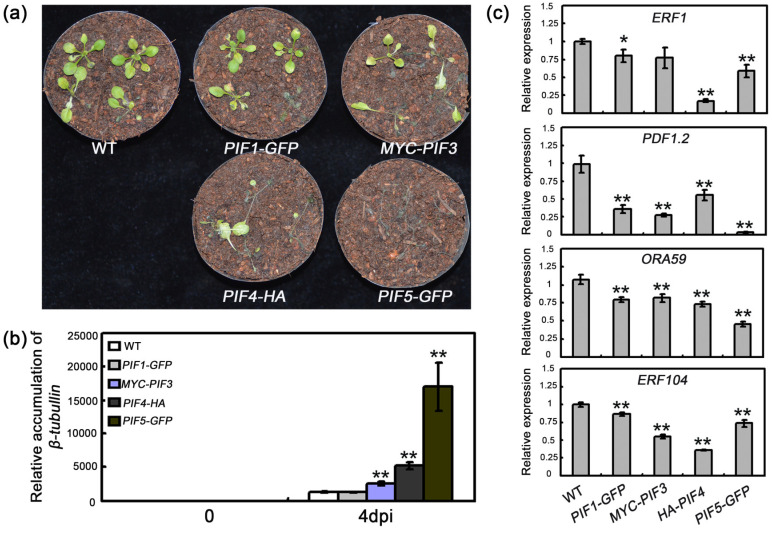
To further characterize the role of PIFs in defense responses to B. cinerea, we also examined the basal expression of defense-associated genes in transgenic plants overexpressing PIF1-GFP, MYC-PIF3, PIF4-HA, or PIF5-GFP. As shown in Figure 3c, these overexpression plants had lower expression of ERF1, ORA59, PDF1.2, and ERF104, as compared to WT. Then we compared the pathogen growth in these transgenic plants with that in WT plants. At four days after an inoculation with B. cinerea, we observed a rapid increase in the necrotic symptoms and β-tubulin mRNA accumulation in the plants overexpressing MYC-PIF3, PIF4-HA, or PIF5-GFP (Figure 3a,b). Thus, the constitutive overexpression of PIF genes decreased the resistance of transgenic plants to B. cinerea and accelerated disease symptom development, suggesting that PIFs play an important role in plant defenses against B. cinerea.
Figure 3.
The PIF overexpression plants were more susceptible against Botrytis than WT. (a) The disease symptoms of PIF overexpression plants. The plants were grown under 12 h light/12 h dark conditions, on soil, for 14 days, and then inoculated with 1 × 105 spores/mL B. cinerea spores. (b) The relative biomass of Botrytis in PIF overexpression plants. Error bars indicate SD of three independent experiments. (c) The basal expression of ERF1, ORA59, PDF1.2, and ERF104 was analyzed in 14-day-old soil grown PIF overexpression plants under LD condition. Error bars indicate SD of three independent experiments. For (b,c), asterisks indicate Student’s t-test significant differences (* p < 0.05, ** p < 0.01).
2.4. Expression Profiling to Identify the Potential PIF-Involved Pathway to Control Defense Response
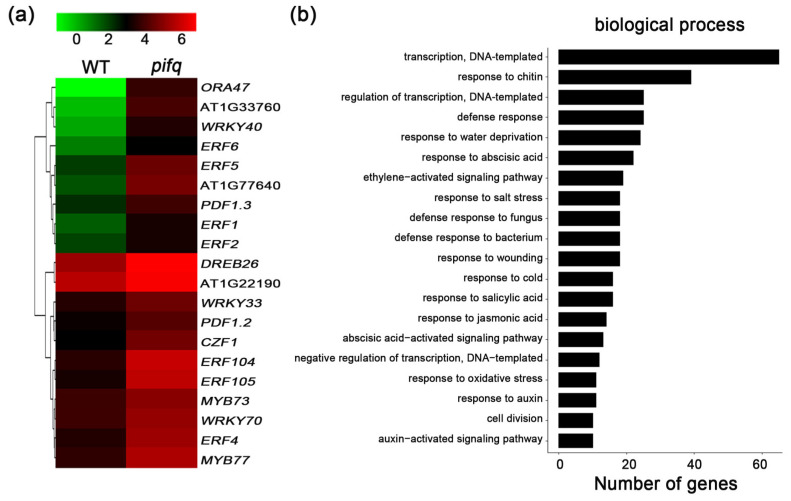
The obvious differences in the symptom development of the pifq mutant and PIF-overexpressing plants compelled us to further investigate the molecular basis of the altered responses. The basal expression levels of the defense-associated genes were higher and lower in the pifq and PIF-overexpressing plants, respectively (Figure 1e and Figure 3c). Thus, we completed a genome-wide transcriptomic analysis to compare the gene expression profiles between WT and pifq plants. The analysis revealed 409 differentially expressed genes of the pifq plants with more than two-fold changes in expression, including 137 downregulated genes and 272 upregulated genes (Supplementary Materials Data S1). Consistent with the enhanced disease resistance of the pifq plants, JA- and ET-related defense genes were among the 272 upregulated genes, including ERF1, ERF5, ERF6, WRKY33, PDF1.2, and PDF1.3 (Figure 4a; Supplementary Materials Data S1). A gene ontology (GO) analysis functionally annotated genes with representative GO terms associated with defense responses to fungi or JA and the ethylene-activated signaling pathway (Figure 4b), suggesting that PIFs might regulate plant defenses against B. cinerea by modulating the transcription of defense-associated genes and that the function of PIFs to regulate defense response might be highly involved in the JA and ET signaling pathway.
Figure 4.
The PIFs-regulated genes involved in defense response. (a) The hierarchical cluster analysis of select upregulated transcripts that are involved in jasmonate/ethylene (JA/ET)-related defense or response to chitin/fungus. (b) Gene ontology analysis of the upregulated genes in pifq.
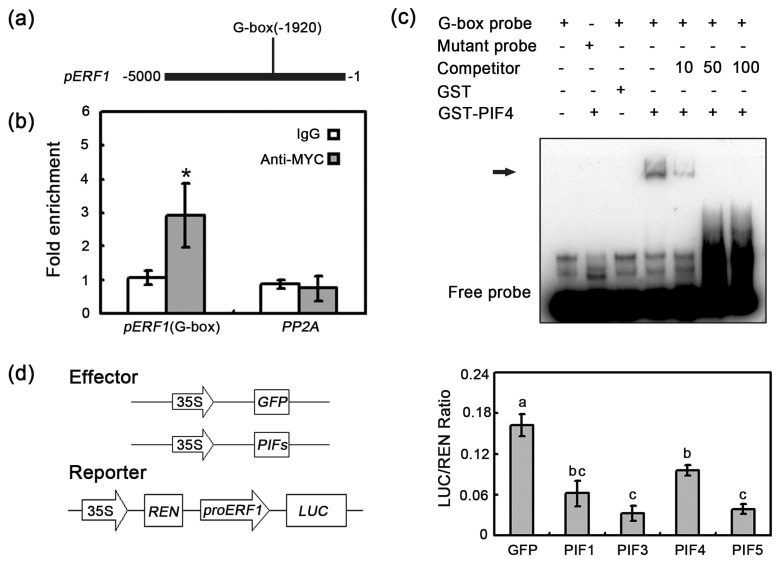
2.5. In Vivo Interaction between PIF4/5 and the ERF1 Promoter
Previous reports indicated that PIFs function by binding directly to the G-box (CACGTG) of their target gene promoters [30,34,43,44,45]. Given the existence of a G-box in the ERF1 promoter (Figure 5a) and the negative regulation of ERF1 expression by PIFs, we assumed that PIFs suppress ERF1 expression by binding directly to the promoter. To confirm this assumption, we first conducted chromatin immunoprecipitation (ChIP) assays with transgenic lines expressing pPIF4-PIF4-MYC in pifq background and PIF5-HA under the control of the cauliflower mosaic virus (CaMV) 35S promoter. The ChIP-qPCR results further demonstrated that PIF4 and PIF5 can bind to the ERF1 promoter via the G-box (Figure 5b and Supplementary Materials Figure S1a). Furthermore, to examine whether ERF1 is directly targeted by PIF4 and PIF5 in vitro, gel electrophoresis mobility shift assay (EMSA) was performed. The GST-PIF4/5 specifically bound to the ERF1 promoter region containing the normal G-box sequence (Figure 5c and Supplementary Materials Figure S1b). Additionally, with increasing concentrations, the unlabeled competitor greatly inhibited the binding of GST-PIF4/5. These observations indicated that PIFs can bind directly to the ERF1 promoter.
Figure 5.
PIF4 directly binds to ERF1 promoter and suppresses its expression. (a) Schematic diagram of the ERF1 promoter. The solid line indicates the position of G-box in the promoter. (b) ChIP-qPCR, using pPIF4-PIF4-MYC/pifq plants, shows that PIF4 binds to the ERF1 promoter region containing the G-box in vivo. The error bars indicate the SD of three independent experiments. Asterisks indicate Student’s t-test significant differences (* p < 0.05). (c) EMSA assay shows that GST-PIF4 bHLH recombinant protein binds to the promoter of ERF1 in vitro. DNA fragments of ERF1 promoter were synthesized with normal G-box motif (G-box probe) and mutated G-box motif (mutant probe), and labeled with biotin. The GST protein was used as negative control. (d) The diagram of the reporter and effectors used in the protoplast transfection assays. Transient expression assays indicate that PIFs repress ERF1 expression. The error bars indicate SD of three independent experiments. The different letters above columns indicate significant differences (one-way ANOVA; p < 0.05).
To confirm the negative regulatory functions of PIFs, we performed transient expression assays in WT A. thaliana mesophyll protoplasts, using a dual-luciferase reporter plasmid. As a reporter, the ERF1 promoter was fused to the firefly luciferase (LUC) gene, whereas the Renilla luciferase (REN) gene was placed under the control of the CaMV 35S promoter. The effector constructs contained PIF genes or GFP driven by the constitutive CaMV 35S promoter. The coexpression of PIF genes with the proERF1-LUC reporter significantly decreased the LUC/REN ratio relative to the effects of GFP (Figure 5d). This result further supported that PIFs act as negative regulators to repress the expression of ERF1 and suggested that there is a possibility that PIFs control plant defense response through directly inhibiting the expression of other defense-related genes.
2.6. Association of PIF-Regulated Defense with JA/ET Signaling
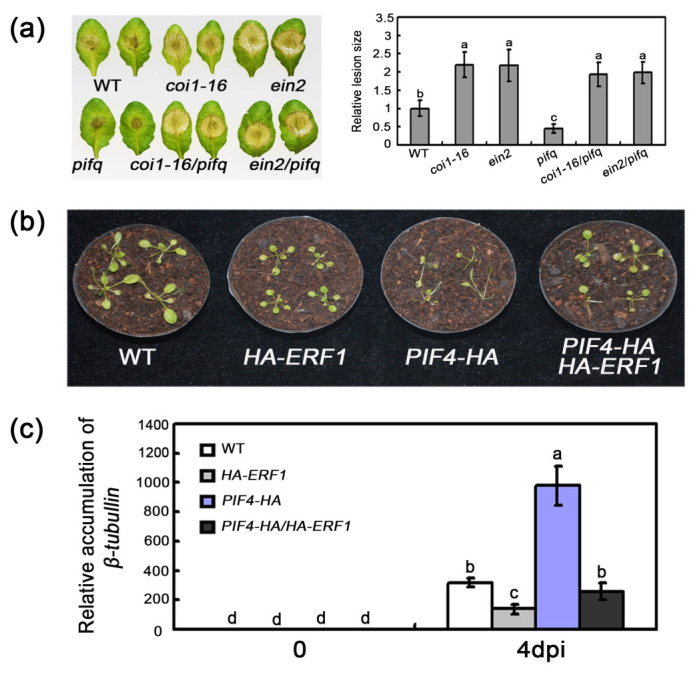
Having ascertained that PIFs regulate the expression of several JA/ET-related defense genes following an infection by B. cinerea and the GO terms regarding JA/ET response enriched in transcriptome analysis, we examined whether the enhanced defense response of pifq plants is also associated with JA/ET signaling pathways. Specifically, coi1-16 and ein2 mutants were crossed with pifq plants, to generate coi1-16/pifq and ein2/pifq quintuple mutants. The defense responses of these quintuple mutants were similar to those of the coi1-16 and ein2 plants, with larger lesions compared with the pifq plants (Figure 6a), indicating that PIF-regulated defense response against B. cinerea relies on JA and ET signaling. Additionally, we explored the genetic relationship between PIF4/5 and ERF1. Plants overexpressing PIF4-HA and PIF5-GFP were crossed with a transgenic plant carrying the HA-ERF1 construct. The overexpression of ERF1 significantly decreased the disease susceptibility and pathogen biomass of the PIF4-HA and PIF5-GFP transgenic plants (Figure 6b,c and Supplementary Materials Figure S2). Thus, our results provide evidence that the PIF-mediated defense is dependent on JA and ET signaling pathway.
Figure 6.
The functional relationship between PIFs and JA/ET in defense response. (a) The disease symptoms of drop-inoculated detached leaves were taken at 3 dpi, and the relative lesion size of pathogen on leaves were also measured. Error bars indicate SD of more than 15 leaves. (b) HA-ERF1 can partially rescue the enhanced susceptibility of PIF4-HA plants against Botrytis. (c) The B. cinerea β-tubulin mRNA accumulation analysis. Error bars indicate SD of three independent experiments. For (a,c), the different letters above columns indicate significant differences (one-way ANOVA; p < 0.05).
3. Discussion
Previous studies proved that phyB negatively regulates PIF transcription factors, at the protein level, by enhancing their degradation and by sequestering them from their target promoters [46]. Thus, loss-of-function phyB mutants show exaggerated PIF-mediated growth [47,48,49]. Another study also revealed that phyb mutants exhibit downregulated expression of JA-inducible genes, such as HEL, ERF1, and PDF1.2, and compromised resistance to B. cinerea [49,50,51]. Additionally, PIF4 was recently reported to positively regulate the temperature-induced suppression of defense responses to Pto DC3000 [35]. Therefore, deciphering the possible roles of PIFs in defense responses to necrotrophic pathogens will provide new insights into our understanding of the phyB-PIF module-mediated plant defenses.
Pathogen attack leads to extensive transcriptional changes and the production of specialized metabolites contributing to the establishment of effective plant defenses. Transcription factors critically influence plant innate immunity. Notably, the ethylene response factor transcription factors, such as ERF1 and ORA59, are key integrators of JA and ET defense signaling pathways [52]. In the current study, the expression levels of several ERF genes, including ERF1 and ORA59, were downregulated in 35S:PIF transgenic plants and upregulated in pifq mutants. This expression model is consistent with the disease resistance of these lines (Figure 1, Figure 2 and Figure 3), suggesting that PIFs negatively regulate ERF gene expression. Furthermore, a genetic analysis demonstrated that 35S:ERF1 can dramatically enhance disease resistance of PIF4-HA and PIF5-GFP transgenic plants (Figure 6b,c and Supplementary Materials Figure S2). All of these results, together, suggest that PIFs act upstream of ERF1 to negatively regulate the resistance to this necrotrophic pathogen. Our transcriptome sequencing analysis also indicated that the expression levels of the defense-response genes are widely upregulated. The GO functional annotations revealed that categories associated with JA/ET signaling are enriched among the 409 differentially expressed genes in pifq plants, implying the PIF-mediated defense against B. cinerea is closely related to JA/ET signaling (Figure 4). Similarly, mutations to COI1 and EIN2 in pifq plants dramatically compromised the resistance to B. cinerea (Figure 6a), further indicating that PIFs may be incorporated in the JA/ET pathway to control plant resistance to B. cinerea.
Numerous studies have demonstrated that the PIF proteins perform their biological functions by directly binding to the G-box (CACGTG) in their target promoters [30,34,43,44,45]. The ERF1 promoter contains a G-box cis-elements. Our EMSA and ChIP experiments revealed that PIF4/5 can bind directly to the G-box in the ERF1 promoter (Figure 5b,c and Supplementary Materials Figure S1), suggesting that ERF1 is a direct target of PIF. The opposite expression patterns of ERF1 in PIF mutants and overexpressing lines, as well as the downregulation of LUC expression in the transient expression assays (Figure 1e, Figure 3c and Figure 5d), further suggest that PIFs are negative regulators of ERF1 expression. Thus, our results provide evidence that PIFs may function as negative regulators of plant defenses against B. cinerea via the direct inhibition of ERF1 expression. To more thoroughly characterize the biological functions of PIFs and their possible signaling pathways in defense responses to B. cinerea, their downstream target genes will need to be identified. Moreover, the 409 differentially expressed genes in pifq plants may include other PIF targets. Previous studies indicated that PIF proteins can function as both positive and negative regulators [24,28,29,30,32,33,34,35]. Thus, PIFs extensively participate in the fine-tuning and tight control of the complex signaling and transcriptional networks that mediate plant growth and stress responses by functioning as positive and negative regulators.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Arabidopsis thaliana ecotype Columbia-0 was used in this study. The T-DNA insertion mutants pif1 (SALK_131872C), pif3-7 (CS66042), pif4-2 (CS66043), pif5-3 (CS66044), and pif1/3/4/5 (CS66049) were purchased from Arabidopsis Biological Resource Center. The overexpression transgenic plants involved in this study have been described: MYC-PIF3 [53], PIF4-HA, PIF5-HA [30], HA-ERF1 [54], coi1-16, and ein2. To generate PIF1-GFP and PIF5-GFP overexpression plants, Col-0 was transformed with p35S:PIF1-GFP and p35S:PIF5-GFP constructs. The coi1-16/pif1/3/4/5, ein2/pif1/3/4/5, phyb/pif1/3/4/5, PIF4-HA/HA-ERF1, and PIF5-GFP/HA-ERF1 were prepared by genetic crossing. The Arabidopsis seeds were sterilized in 10% bleach and sown on 1/2 MS medium with 0.5% agar and 1% sucrose (pH 5.8). After stratification for 3 days, at 4 °C, seeds were then transferred to growth chamber and grown under 16 h light/8 h dark (LDs) or 12 h light/12 h dark at 22 °C. Primers designed for mutant identification or clones are listed in Supplementary Materials Table S1.
4.2. Pathogen Infection
Botrytis cinerea (B05.10) was grown on Potato Dextrose Agar, under 12 h light/12 h dark, at 21 °C. B. cinerea spores’ collection and inoculation of plants were performed as previously described [10]. The inoculated plants were maintained in a dark and high-humidity environment. After 3 to 7 days, the symptom development could be observed. B. cinerea growth was quantified by qRT-PCR of total RNA isolated from the inoculated plants. For drop inoculation, leaves of 28-day-old plants grown on soil were inoculated with a single 8 μL drop of a suspension of 5 × 105 spores/mL, in Sabouraud maltose broth (SMB) buffer. The lesion sizes of B. cinerea infected leaves were measured, using ImageJ.
4.3. Expression Analysis
For reverse-transcription PCR analysis, total RNA was extracted from Arabidopsis seedlings, using the TRIzol reagent (Invitrogen, Waltham, MA, USA). Then, cDNA was synthesized from 1 μg of total RNA, according to the reverse-transcription protocol (Takara, Beijing, China). The cDNA was subjected to qPCR, using the SYBR Premix Ex Taq (Takara, Beijing, China), on a Roche LightCycler 480. ACTIN2 or IPP2 was amplified as the reference gene. The 2−ΔΔCt method was used for the calculation of relative expression levels. At least three biological replicates were conducted for each experiment. The gene-specific primers are provided in Supplementary Materials Table S1.
4.4. RNA Sequencing
The rosettes of 14-day-old WT and pifq plants grown on soil were collected. The total RNA was extracted from Arabidopsis seedlings, using the Trizol reagent (Invitrogen, Waltham, MA, USA). Shanghai OE Biotech Co. provided the supports of RNA sequencing and data analysis. TruSeq Stranded mRNA LTSample Prep Kit (Illumina, San Diego, CA, USA) was used to construct the libraries. Then the sequencing was performed, using Illumina HiSeqTM 2500. Trimmomatic was used to filter the raw sequencing reads. The differential genes were analyzed by DESeq, with p < 0.05 and fold change > 2 as the threshold. The GOseq was used to perform GO enrichment analysis of the differential genes. The hierarchical cluster of selected genes was constructed by using R package pheatmap.
4.5. Gel Mobility Shift Assay
For EMSA assay, PIF4 bHLH domain and the full-length PIF5 coding sequences were cloned into pGEX-TX-1 vector and transformed into E. coli strain transetta (DE3) (TransGen, Beijing, China). The recombinant proteins were induced at 37 °C for 3 h, with 0.1 mM IPTG, and purified with Glutathione-agarose beads (TransGen, Beijing, China). The 5′ terminal biotin-labeled DNA fragments were synthesized. The EMSA assay was performed, using Chemiluminescent EMSA Kit (Beyotime, Shanghai, China), following the manufacturer’s protocol.
4.6. Chromatin Immunoprecipitation Assay
For ChIP analyses, 18-day-old soil-grown plants, under LD conditions, were harvested at ZT0 (zeitgeber time) and cross-linked with 1% formaldehyde. The ChIP experiment was performed by using the EpiQuik Plant Chromatin Kit (Epigentek, Farmingdale, NY, USA). The normal mouse lgG was used as the negative control. The mouse anti-MYC and anti-HA antibody (Santa Cruz Biotechnology, Dallas, TX, USA) were used to immunoprecipitate the immunocomplexes. The purified chromatin fragments were subjected to qPCR. The primers used in the ChIP experiments are listed in Supplementary Materials Table S1.
4.7. Protoplast Transfection Assays
To generate reporter and effector constructs, the 5 kb promoter sequence of ERF1 was amplified by PCR and cloned into the pGreenII 0800-LUC vector; the corresponding CDS sequences of PIF1, PIF3, PIF4, PIF5, and GFP were cloned into pGreenII 62-SK vectors. Protoplast isolation from Col-0 and PEG mediated transformation was performed as described [55], and a total of 11 ug plasmid (1 ug reporter plasmid, and 10 ug of each effector plasmid) was used to perform the transformation. To measure the LUC and Renilla luciferase activities, a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) was used, and the luciferase activities were analyzed by GloMax-96 Microplate Luminometer (Promega, Madison, WI, USA). All the experiments were performed at least three biological times. The primers used are listed in Supplementary Materials Table S1.
Acknowledgments
We thank Hongwei Guo (Southern University of Science and Technology), Haiyang Wang (Chinese Academy of Agricultural Sciences), and Chengbin Xiang (University of Science and Technology of China) for sharing research materials. We also thank Qing-Ming Qin (Jilin University) for sharing the Botrytis cinerea (B05.10) strain, as well as the Central Laboratory of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences for technical supports.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/9/1246/s1. Figure S1. PIF5 directly binds to ERF1 promoter. Figure S2. HA-ERF1 can partially rescue the enhanced susceptibility of PIF5-GFP against Botrytis. Table S1. Primers used in this study. Data S1. The differentially expressed genes in pifq plants.
Author Contributions
D.Y. and L.C. designed the experiments; S.X., S.W., H.Z., M.M., Y.C., and D.L. performed the experiments; L.C., and S.X. drafted the manuscript; D.Y., L.C., and H.W. revised the manuscript; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R & D plan (2016YFD0101006), Natural Science Foundation of China (31671275), Candidates of the Young and Middle Aged Academic Leaders of Yunnan Province (2015HB094), and Yunnan Fundamental Research Projects (grant nos. 2017FB047 and 2019FA010).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 2.Mengiste T. Plant Immunity to Necrotrophs. Annu. Rev. Phytopathol. 2012;50:267–294. doi: 10.1146/annurev-phyto-081211-172955. [DOI] [PubMed] [Google Scholar]
- 3.Xie D.X., Feys B.F., James S., Nieto-Rostro M., Turner J.G. COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- 4.Xu L., Liu F., Lechner E., Genschik P., Crosby W.L., Ma H., Peng W., Huang D., Xie D. The SCF(COI1) ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell. 2002;14:1919–1935. doi: 10.1105/tpc.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- 6.Yan J., Zhang C., Gu M., Bai Z., Zhang W., Qi T., Chen Z., Peng W., Luo H., Nan F., et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell. 2009;21:2220–2236. doi: 10.1105/tpc.109.065730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penninckx I.A.M.A., Eggermont K., Terras F.R.G., Thomma B.P.H.J., DeSamblanx G.W., Buchala A., Métraux J.P., Manners J.M., Broekaert W.F. Pathogen-lnduced Systemic Activation of a Plant Defensin Gene in Arabidopsis Follows a Salicylic Acid-lndependent Pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penninckx I.A.M.A., Thomma B.P.H.J., Buchala A., Métraux J.P., Broekaert W.F. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laluk K., Luo H.L., Chai M.F., Dhawan R., Lai Z.B., Mengiste T. Biochemical and Genetic Requirements for Function of the Immune Response Regulator BOTRYTIS-INDUCED KINASE1 in Plant Growth, Ethylene Signaling, and PAMP-Triggered Immunity in Arabidopsis. Plant Cell. 2011;23:2831–2849. doi: 10.1105/tpc.111.087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y., Yu D. The WRKY57 transcription factor affects the expression of Jasmonate ZIM-domain genes transcriptionally to compromise Botrytis cinerea resistance. Plant Physiol. 2016;171:2771–2782. doi: 10.1104/pp.16.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzo O., Piqueras R., Sánchez-Serrano J.J., Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pré M., Atallah M., Champion A., De Vos M., Pieterse C.M.J., Memelink J. The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 2008;147:1347–1357. doi: 10.1104/pp.108.117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Q., Yoshida Y., Major I.T., Wang K., Sugimoto K., Kapali G., Havko N.E., Benning C., Howe G.A. JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2018;115:10768–10777. doi: 10.1073/pnas.1811828115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi R., Yan J., Xie D. Light promotes jasmonate biosynthesis to regulate photomorphogenesis in Arabidopsis. Sci. China Life Sci. 2020;63:943–952. doi: 10.1007/s11427-019-1584-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Z., An F., Feng Y., Li P., Xue L., Mu A., Jiang Z., Kim J.M., To T.K., Li W., et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:12539–12544. doi: 10.1073/pnas.1103959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao Q., Rothenberg M., Solano R., Roman G., Terzaghi W., Ecker J.R. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/S0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- 17.Alonso J.M., Hirayama T., Roman G., Nourizadeh S., Ecker J.R. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- 18.Bisson M.M.A., Bleckmann A., Allekotte S., Groth G. EIN2, the central regulator of ethylene signalling, is localized at the ER membrane where it interacts with the ethylene receptor ETR1. Biochem. J. 2009;424:1–6. doi: 10.1042/BJ20091102. [DOI] [PubMed] [Google Scholar]
- 19.Thomma B.P.H.J., Eggermont K., Tierens K.F.M.J., Broekaert W.F. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;121:1093–1102. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moffat C.S., Ingle R.A., Wathugala D.L., Saunders N.J., Knight H., Knight M.R. ERF5 and ERF6 Play Redundant Roles as Positive Regulators of JA/Et-Mediated Defense against Botrytis cinerea in Arabidopsis. PLoS ONE. 2012;7:e35995. doi: 10.1371/journal.pone.0035995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey P.C., Martin C., Toledo-Ortiz G., Quail P.H., Huq E., Heim M.A., Jakob M., Werber M., Weisshaar B. Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell. 2003;15:2497–2502. doi: 10.1105/tpc.151140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toledo-Ortiz G., Huq E., Quail P.H. The Arabidopsis basic/helix -loop-helix transcription factor family. Plant Cell. 2003;15:1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee N., Choi G. Phytochrome-interacting factor from Arabidopsis to liverwort. Curr. Opin. Plant Biol. 2017;35:54–60. doi: 10.1016/j.pbi.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Oh E., Yamaguchi S., Huc J., Yusukeb J., Jung B., Paik I., Leed H.S., Sun T.P., Kamiya Y., Choi G. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell. 2007;19:1192–1208. doi: 10.1105/tpc.107.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D.H., Yamaguchi S., Lim S., Oh E., Park J., Hanada A., Kamiya Y., Choi G. SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell. 2008;20:1260–1277. doi: 10.1105/tpc.108.058859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh E., Kang H., Yamaguchi S., Park J., Lee D., Kamiya Y., Choi G. Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell. 2009;21:403–419. doi: 10.1105/tpc.108.064691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J., Lee N., Kim W., Lim S., Choi G. ABI3 and PIL5 collaboratively activate the expression of SOMNUS by directly binding to its promoter in imbibed Arabidopsis seeds. Plant Cell. 2011;23:1404–1415. doi: 10.1105/tpc.110.080721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar S.V., Lucyshyn D., Jaeger K.E., Alós E., Alvey E., Harberd N.P., Wigge P.A. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature. 2012;484:242–245. doi: 10.1038/nature10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y., Yang C., Gao S., Zhang W., Li L., Kuai B. Age-triggered and dark-induced leaf senescence require the bHLH transcription factors PIF3, 4, and 5. Mol. Plant. 2014;7:1776–1787. doi: 10.1093/mp/ssu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Y., Liu Y., Wang H., Ma X., Wang B., Wu G., Wang H. Phytochrome-interacting factors directly suppress MIR156 expression to enhance shade-avoidance syndrome in Arabidopsis. Nat. Commun. 2017;8:348. doi: 10.1038/s41467-017-00404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R., Yang C., Jiang Y., Li L. A PIF7-CONSTANS-Centered Molecular Regulatory Network Underlying Shade-Accelerated Flowering. Mol. Plant. 2019;12:1587–1597. doi: 10.1016/j.molp.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Qi L., Liu S., Li C., Fu J., Jing Y., Cheng J., Li H., Zhang D., Wang X., Dong X., et al. PHYTOCHROME-INTERACTING FACTORS Interact with the ABA Receptors PYL8 and PYL9 to Orchestrate ABA Signaling in Darkness. Mol. Plant. 2020;13:414–430. doi: 10.1016/j.molp.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Oh E., Yamaguchi S., Kamiya Y., Bae G., Chung W.I., Choi G. Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 2006;47:124–139. doi: 10.1111/j.1365-313X.2006.02773.x. [DOI] [PubMed] [Google Scholar]
- 34.Sun J., Qi L., Li Y., Chu J., Li C. PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genet. 2012;8:e1002594. doi: 10.1371/journal.pgen.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gangappa S.N., Berriri S., Kumar S.V. PIF4 coordinates thermosensory growth and immunity in Arabidopsis. Curr. Biol. 2017;27:243–249. doi: 10.1016/j.cub.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Windram O., Madhou P., McHattie S., Hill C., Hickman R., Cooke E., Jenkins D.J., Penfold C.A., Baxter L., Breeze E., et al. Arabidopsis defense against Botrytis cinerea: Chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. Plant Cell. 2012;24:3530–3557. doi: 10.1105/tpc.112.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernández-Milmanda G.L., Crocco C.D., Reichelt M., Mazza C.A., Köllner T.G., Zhang T., Cargnel M.D., Lichy M.Z., Fiorucci A.S., Fankhauser C., et al. A light-dependent molecular link between competition cues and defence responses in plants. Nat. Plants. 2020;6:223–230. doi: 10.1038/s41477-020-0604-8. [DOI] [PubMed] [Google Scholar]
- 38.Berrocal-Lobo M., Molina A., Solano R. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 2002;29:23–32. doi: 10.1046/j.1365-313x.2002.01191.x. [DOI] [PubMed] [Google Scholar]
- 39.Nozue K., Covington M.F., Duek P.D., Lorrain S., Fankhauser C., Harmer S.L., Maloof J.N. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448:358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- 40.Nusinow D.A., Helfer A., Hamilton E.E., King J.J., Imaizumi T., Schultz T.F., Farré E.M., Kay S.A. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature. 2011;475:398–402. doi: 10.1038/nature10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soy J., Leivar P., González-Schain N., Sentandreu M., Prat S., Quail P.H., Monte E. Phytochrome-imposed oscillations in PIF3 protein abundance regulate hypocotyl growth under diurnal light/dark conditions in Arabidopsis. Plant J. 2012;71:390–401. doi: 10.1111/j.1365-313X.2012.04992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soy J., Leivar P., Monte E. PIF1 promotes phytochrome-regulated growth under photoperiodic conditions in Arabidopsis together with PIF3, PIF4, and PIF5. J. Exp. Bot. 2014;65:2925–2936. doi: 10.1093/jxb/ert465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez-García J.F., Huq E., Quail P.H. Direct targeting of light signals to a promoter element-bound transcription factor. Science. 2000;288:859–863. doi: 10.1126/science.288.5467.859. [DOI] [PubMed] [Google Scholar]
- 44.Hornitschek P., Lorrain S., Zoete V., Michielin O., Fankhauser C. Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J. 2009;28:3893–3902. doi: 10.1038/emboj.2009.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hornitschek P., Kohnen M.V., Lorrain S., Rougemont J., Ljung K., López-Vidriero I., Franco-Zorrilla J.M., Solano R., Trevisan M., Pradervand S., et al. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 2012;71:699–711. doi: 10.1111/j.1365-313X.2012.05033.x. [DOI] [PubMed] [Google Scholar]
- 46.Park E., Kim Y., Choi G. Phytochrome B Requires PIF Degradation and Sequestration to Induce Light Responses across a Wide Range of Light Conditions. Plant Cell. 2018;30:1277–1292. doi: 10.1105/tpc.17.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leivar P., Quail P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen M., Chory J. Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 2011;21:664–671. doi: 10.1016/j.tcb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Wit M., Spoel S.H., Sanchez-Perez G.F., Gommers C.M.M., Pieterse C.M.J., Voesenek L.A.C.J., Pierik R. Perception of low red:far-red ratio compromises both salicylic acid- and jasmonic acid-dependent pathogen defences in Arabidopsis. Plant J. 2013;75:90–103. doi: 10.1111/tpj.12203. [DOI] [PubMed] [Google Scholar]
- 50.Moreno J.E., Tao Y., Chory J., Ballaré C.L. Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc. Natl. Acad. Sci. USA. 2009;106:4935–4940. doi: 10.1073/pnas.0900701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cerrudo I., Keller M.M., Cargnel M.D., Demkura P.V., de Wit M., Patitucci M.S., Pierik R., Pieterse C.M.J., Ballaré C.L. Low red/far-red ratios reduce Arabidopsis resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acid-independent mechanism. Plant Physiol. 2012;158:2042–2052. doi: 10.1104/pp.112.193359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang P.Y., Catinot J., Zimmerli L. Ethylene response factors in Arabidopsis immunity. J. Exp. Bot. 2016;65:1231–1241. doi: 10.1093/jxb/erv518. [DOI] [PubMed] [Google Scholar]
- 53.Zhong S., Shi H., Xue C., Wang L., Xi Y., Li J., Quail P.H., Deng X.W., Guo H. A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr. Biol. 2012;22:1530–1535. doi: 10.1016/j.cub.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao J.L., Miao Z.Q., Wang Z., Yu L.H., Cai X.T., Xiang C.B. Arabidopsis ERF1 Mediates Cross-Talk between Ethylene and Auxin Biosynthesis during Primary Root Elongation by Regulating ASA1 Expression. PLoS Genet. 2016;12:e1005760. doi: 10.1371/journal.pgen.1005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoo S.D., Cho Y.H., Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.