Abstract
Depression is a leading cause of disability worldwide and current treatments are often inadequate for many patients. Increasing evidence indicates that inflammation contributes to susceptibility to depression. We hypothesized that targeting Toll-like receptor 4 (TLR4), one of the main signaling pathways for triggering an inflammatory response, would lessen stress-induced depression-like behaviors in male mice. TLR4 inhibition with the CNS-penetrating drug (+)-naloxone that is a TLR4 antagonist but is inactive at opiate receptors increased resistance to the learned helplessness model of depression and provided an antidepressant-like effect in the tail suspension test. (+)-Naloxone administration also reversed chronic restraint stress-induced impairments in social behavior and novel object recognition. These effects involved blockade of stress-induced activation of glycogen synthase kinase 3β (GSK3β), NF-κB, IFN regulatory factor 3 (IRF3) and nitric oxide production, and reduced levels of the cytokines tumor necrosis factor-α (TNFα) and interferon-β (IFNβ). These findings demonstrate that blocking TLR4 with (+)-naloxone effectively diminishes several detrimental responses to stress and raise the possibility that (+)-naloxone may be a feasible intervention for depression.
Keywords: Depression, Inflammation, (+)-naloxone, Toll-like receptor-4
1. Introduction
Major depressive disorder (MDD) is a debilitating disease with a prevalence of approximately 21% in women and 11% in men in the United States (Belmaker and Agam, 2008; Kessler et al., 2005). Treatment options for MDD are limited and are often ineffective, partly because of the insufficient understanding of the causes of MDD (Gaynes et al., 2009; Kennis et al., 2020).
In addition to genetic and environmental factors, increasing evidence has associated MDD with abnormally elevated inflammation (Dantzer et al., 2008; Medina-Rodriguez et al., 2018; Miller et al., 2009). Pertinent to this, stress is a major risk factor for MDD, and inflammation is a frequent response to stress (Maes et al., 1998; Miller and Raison, 2015, 2016). These findings have raised the possibility that targeting the immune system’s inflammatory actions may be a therapeutic option for treating MDD.
Toll-like receptor 4 (TLR4) is a key initiating component of the inflammatory system (Okun et al., 2011). TLR4 can activate inflammatory reactions via the protein myeloid differentiation primary response 88 (MyD88)-mediated intracellular signaling pathway and via a MyD88-independent pathway that signals through TIR‐domain‐containing adapter‐inducing IFN‐β (TRIF) protein, and each of these two signaling pathways contributes to inflammation (Okun et al., 2011). Activating TLR4 with the prototypical TLR4 agonist lipopolysaccharide (LPS) is a well-recognized means to induce behaviors in rodents that model symptoms of depression (Dantzer, 2018; Dantzer et al., 2008), and deletion of TLR4 promotes resilience towards stress-induced depressive-like behaviors in mouse models (Cheng et al., 2016). These findings raise the possibility that utilization of drugs that block TLR4 may provide antidepressant effects.
There are two stereoisomers of the drug naloxone, (−)-naloxone and (+)-naloxone, that are both antagonists of TLR4 (Hutchinson et al., 2008). TLR4 antagonism likely contributed to several effects of naloxone administration that involve inflammation that were reported prior to its identification as a TLR4 antagonist. For example, (−)-naloxone had an anti-inflammatory effect against sepsis in animal models (Law and Ferguson, 1988; Miller et al., 1986) and was neuroprotective by reducing inflammation and neuronal loss in focal cerebral ischemia in rats (Chen et al., 2001; Liao et al., 2003). (+)-Naloxone treatment promoted post-stroke recovery by reducing microglia activation and neuronal loss in rats (Anttila et al., 2018). Both naloxone stereoisomers reduced neurodegeneration and microglial activation induced by LPS (Liu et al., 2000). Although both naloxone stereoisomers block TLR4, (+)-naloxone is inactive at opiate receptors, which are potently blocked by (−)-naloxone (Iijima et al., 1978). Many years ago naloxone was demonstrated to reduce susceptibility to learned helplessness, a mouse model of depression (Hemingway and Reigle, 1987; Maier et al., 1980; Whitehouse et al., 1983). Preceding the discovery that naloxone is a TLR4 antagonist, this finding was interpreted as indicative of an opioid receptor-associated modulation of the development of learned helplessness. Subsequently, (+)-naloxone was found to block TLR4 by binding the co-receptor of TLR4, myeloid differentiation protein 2 (MD2), at the site bound by LPS, antagonizing TLR4-mediated signaling (Hutchinson et al., 2012; Northcutt et al., 2015). Overall, (+)-naloxone has similar anti-inflammatory and neuroprotective effects as (−)-naloxone, but (+)-naloxone does not block opioid receptors, unlike (−)-naloxone, avoiding the side effects of blocking opioid receptors.
These properties of (+)-naloxone provide the opportunity to test if a CNS-penetrant TLR4 antagonist that may be administered safely to human subjects was capable of modifying the susceptibility of mice to stress-induced depression-like behaviors. We found that administration of the TLR4 antagonist (+)-naloxone reduced the susceptibility of mice to learned helplessness, had an antidepressant-like effect in the tail suspension test, and diminished detrimental effects of chronic restraint stress on sociability and memory. These findings raise the possibility that TLR4 antagonism provided by (+)-naloxone may represent an alternative treatment for MDD patients.
2. Materials and methods
2.1. Mice and drug administration
C57BL/6 wild-type mice were obtained from Charles Rivers Laboratories. C57BL/6 TLR4 global knockout (TLR4−/−) mice were initially provided by Dr. Suzanne M. Michalek (University of Alabama at Birmingham). TLR4−/− mice were bred using the following paradigm: TLR4+/− x TLR4+/− to obtain 25% TLR4−/−, 25% wild-type, and 50% TLR4+/− mice, and male littermates were used at 8–12 weeks of age for experiments. Mice were housed in groups of 3–5 in the University of Miami vivarium in light and temperature controlled rooms and were treated in accordance with NIH and the University of Miami Institutional Animal Care and Use Committee regulations. The experiments were carried out with littermates that were randomized to their treatment groups. Where indicated, mice were treated intravenously (i.v.) via a tail vein with the TLR4 antagonists TAK-242 (3 mg/kg; Sigma-Aldrich; a dose previously reported to be effective in mice for attenuating ischemia and endotoxic shock (Hua et al., 2015; Sha et al., 2007)) or (+)-naloxone (5 mg/kg; provided by Dr. Kenner Rice; a dose previously used by the Watkins group (Northcutt et al., 2015)).
2.2. Learned helplessness
Only male mice were used because female rodents often fail to develop learned helplessness (Dalla et al., 2008). Mice were placed in one side of a Gemini Avoidance system shuttle box (San Diego Instruments, San Diego, CA, USA) with the gate between chambers closed and 180 inescapable foot shocks (IES) were delivered at an amplitude of 0.3 mA, a duration of 6–10 s per shock (averaging 8 s), and a randomized intershock interval of 5–45 s (Beurel et al., 2013). Twenty-four h after inescapable foot shocks, mice were tested with 30 escape trials with a 0.3 mA foot shock for a maximum duration of 24 s with the door of the chamber opening at the beginning of the foot shock administration to allow mice to escape. The trials in which the mouse did not escape within the 24 s time limit were counted as escape failures. Mice with greater than 15 escape failures were defined as learned helpless. Where indicated, mice were treated i.v. via a tail vein with the TLR4 antagonists TAK-242 (3 mg/kg) or (+)-naloxone (5 mg/kg) 1 h prior to initiation of the learned helplessness protocol.
2.3. Chronic restraint stress
For chronic restraint stress, male mice were restrained individually in a 50 mL ventilated conical tube for 2 h per day (~0900–1100) for 2 weeks, as we previously described (Beurel et al., 2013), and behavioral tests were carried out beginning after the last day of chronic restraint stress. Mice were treated with (+)-naloxone (5 mg/kg; i.v.) or saline 1 h after the last session of chronic restraint stress and 1 h after injection mice were tested for activity in a novel open field, and later habituated to the social interaction apparatus. The following day mice were treated again with (+)-naloxone (5 mg/kg; i.v.) 1 h prior to measuring social interactions and novel object recognition.
2.4. Open field activity
For open field activity measurements (Schwartz and Shechter, 2010), mice were placed in a Plexiglas open field instrument (Med Associates, St Albans, VT) outfitted with photo beam detectors under soft overhead lighting, and activity was monitored during 30 min using activity monitoring software (Med Associates). The number of beam breaks was measured automatically by the apparatus and were calculated for each 5 min period. Mice were exposed, or not, to chronic restraint stress and were treated with (+)-naloxone (5 mg/kg; i.v.) or vehicle 1 h after the last session of chronic restraint stress, and 1 h after injection mice were tested for activity in a novel open field.
2.5. Social interactions
Diminished social interaction is associated with depression and was measured as we previously described (Beurel et al., 2013). Social interactions were measured using a sociability apparatus, which is a rectangular, transparent, Plexiglas box (24 cm X 19 cm, 19 cm high) divided into three equal sized chambers with doors. Chambers 1 and 3 had a wire cage; Chamber 2 in the middle was empty. Mice were habituated individually by being placed in Chamber 2 and were allowed to freely explore the entire apparatus for 25 min the day prior to testing, and, separately, stimulus mice were habituated for 20 min to the wire cage in Chamber 1. Testing consisted of 5 min rehabituation followed by 10 min access to all chambers with an unfamiliar stimulus mouse (age- and sex-matched) in the wire enclosure in Chamber 1. Each session was videotaped and videos were analyzed for the number of nose contacts with the stimulus mouse.
2.6. Novel object recognition
Novel object recognition was measured as described previously (Pardo et al., 2016) by allowing each mouse individually to explore two identical objects for 5 min, and after a 5 min period in an opaque chamber, mice were allowed to explore an unused familiar object and a novel object for 5 min. Time spent exploring each object (sniffing or touching the object with its nose, vibrissa, mouth or forepaws) was quantified from video recordings.
2.7. Tail suspension test
The tail suspension test was measured in a separate cohort of mice that were not exposed to foot shocks or restraint stress. Mice were suspended by the tail on an automated tail suspension test cubicle (33 × 31.75 × 33 cm; Med Associates, St Albans, VT, USA) for a period of 6 min and the time they were moving was measured automatically by the apparatus (Liu et al., 2003). The immobile time during the last 4 min was used to determine the immobility time for each mouse. Mice were treated i.v. via a tail vein with the TLR4 antagonist (+)-naloxone (5 mg/kg) or vehicle 1 h prior to the tail suspension test.
2.8. Cytokine measurements
The hippocampus was dissected from non-shocked mice (control) or 12 h after exposure of mice to inescapable foot shocks in the learned helplessness protocol and homogenized in lysis buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton-100, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 5 μg/ml pepstatin, 1 mM phenylmethanesulfonyl fluoride, 1 mM sodium vanadate, 50 mM sodium fluoride, and 100 nM okadaic acid as previously described (Cheng et al., 2016). Plasma samples were also collected at the time of sacrifice. The cytokines TNFα, IL-6, IL-1β, IL-17A and IFN-β were measured by enzyme-linked immunosorbent assay (ELISA; eBioscience or Biolegend) using 150 μg protein or by the ProcartaPlex Multiplex Immunoassay (Invitrogen) using 25 μg protein, according to the manufacturer’s instructions. Cytokine concentrations were determined with a microplate reader (SpectraMax M3) or the multiplex assay reader (Magpix; Luminex).
2.9. Nitric oxide (NO) measurement
NO2−, a stable breakdown product of NO, was measured with a Griess Reagent System (Promega) following the manufacturer’s instructions.
2.10. Western blotting
Proteins (10–20 μg) from the hippocampus prepared as described above were resolved with SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with antibodies to IκBα (#9242, Cell Signaling Technology), active NF-κB (MAB3026, Millipore), phosphoSer9-GSK3β (#9336, Cell Signaling Technology), total GSK3β (05–412, Millipore), total IFN regulatory factor 3 (IRF3) (#4302S, Cell Signaling Technology), phospho-Ser385-IRF3 (#PA5–36775, Invitrogen), and iNOS (#610432, BD biosciences) and reblotted with β-actin (#A5441, Sigma Aldrich) to ensure loading consistency.
2.11. Data and statistical analyses
Statistical significance was analyzed with a one-way analysis of variance (ANOVA) with Dunnett post-hoc test for multiple comparisons or with Student’s t-test using Prism software. p < 0.05 was considered significant. Histograms represent mean ± SEM.
3. Results
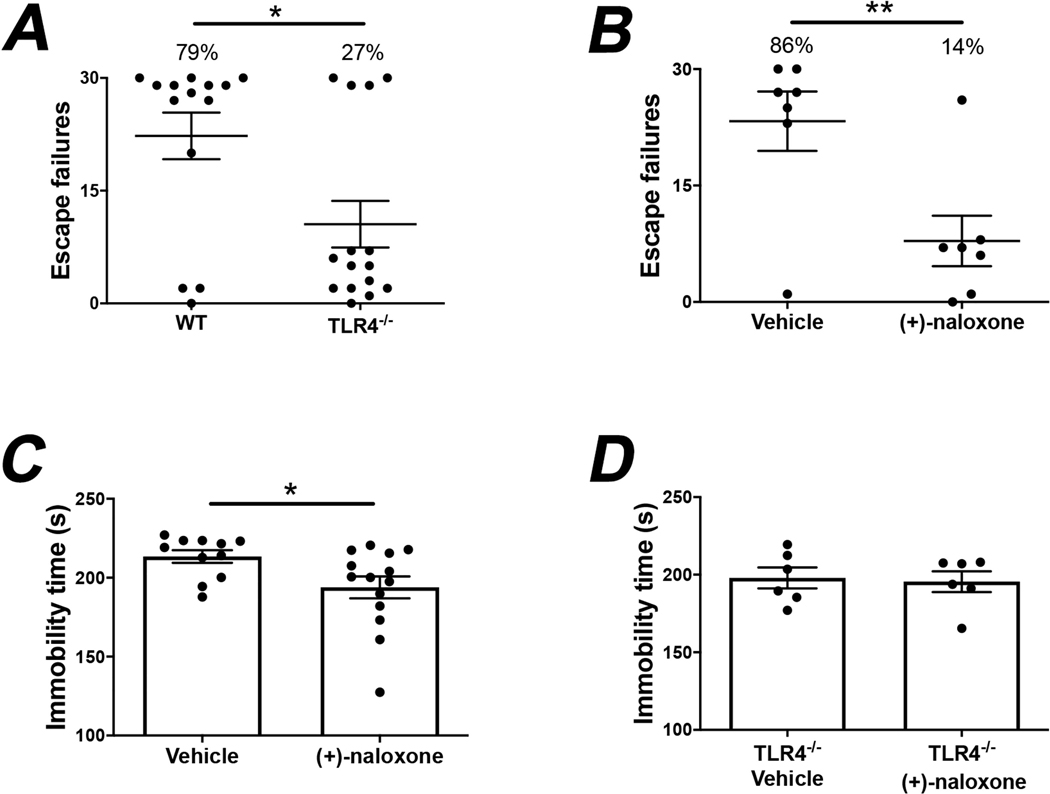
TLR4−/− mice displayed resilience to the learned helplessness model of depression, as only 27% of TLR4−/− mice developed learned helplessness whereas 79% of wild-type mice developed learned helplessness (Fig. 1A; unpaired t test, t = 2.671, p < 0.05), as we previously reported (Cheng et al., 2016). Therefore, we tested if administration of TLR4 antagonists decreases susceptibility to learned helplessness.
Fig. 1.
(+)-Naloxone administration reduces depressive-like behaviors in mice by blocking TLR4. (A) Wild-type (WT) and TLR4−/− mice were subjected to the learned helplessness (LH) paradigm and the number of failures to escape was recorded. Mice were considered learned helplessness if they failed to escape more than 15 out of 30 trials of escapable foot shocks. Each point represents the number of failures to escape for an individual mouse. Bars represent means ± SEM. Unpaired t test, t = 2.671, *p < 0.05, n = 14–15 mice/group. (B-C) Wild-type mice were administered (+)-naloxone (5 mg/kg; i.v.) or vehicle and separate cohorts of mice were subjected to (B) the learned helplessness paradigm or (C) the tail suspension test 1 h after treatment. The number of failures to escape or the immobility time, respectively, were measured. Mice were considered learned helplessness if they failed to escape more than 15 out of 30 trials. Each point represents an individual mouse. Bars represent means ± SEM. Unpaired t test, (B) t = 3.071, (C) t = 2.256, *p < 0.05, **p < 0.01, n = 7–14 mice/group. (D) TLR4−/− mice were subjected to the tail suspension test 1 h after administration of (+)-naloxone (5 mg/kg; i.v.) or vehicle and the immobility time was measured. n = 6 mice/group. Each point represents an individual mouse. Bars represent means ± SEM.
We tested the effects of blocking TLR4 with administration of the TLR4 antagonist (+)-naloxone. Administration of (+)-naloxone (5 mg/kg; i.v.) strongly reduced the percentage of learned helpless mice from 86% of vehicle-treated mice to 14% of (+)-naloxone-treated mice (Fig. 1B; Unpaired t test, t = 3.071, p < 0.01). Administration of (+)-naloxone (5 mg/kg) also provided an antidepressant-like effect in the tail suspension test (Liu et al., 2003), significantly reducing the immobility time (Fig. 1C; unpaired t test, t = 2.256, p < 0.05). In contrast to wild-type mice, (+)-naloxone did not reduce the immobility time in the tail suspension test when administered to TLR4−/− mice (Fig. 1D), verifying it acted via TLR4.
We also tested if administrating the TLR4 antagonist TAK-242 (Ii et al., 2006) was effective in diminishing susceptibility to learned helplessness. However, mice treated with TAK-242 (3 mg/kg; i.v.) displayed the same susceptibility to learned helplessness as mice treated with vehicle (Suppl. Fig. 1A). Therefore we tested if administered TAK-242 effectively inhibited TLR4 in vivo by measuring the plasma levels of TNFα. The stress of inescapable foot shocks used in the learned helplessness paradigm increased plasma TNFα levels and this increase was blocked in mice treated with 3 mg/kg TAK-242 (Suppl. Fig. 1B; one-way ANOVA, F(2,6) = 5.912, Dunnett post-hoc test, p < 0.05), confirming that TNFα in the plasma is induced by stress in a TLR4-mediated mechanism and that TAK-242 effectively inhibits TLR4 in vivo. However, foot shock stress also increased hippocampal TNFα levels, but this was not diminished by TAK-242 treatment (Suppl. Fig. 1C; one-way ANOVA, F(2,6) = 7.791, Dunnett post-hoc test, p < 0.05). This result indicates both that hippocampal increases in TNFα are at least partly independent of peripheral plasma TNFα increases, and that TAK-242 did not effectively block hippocampal TLR4 with the experimental parameters utilized, although it is possible that higher doses or chronic administration may have been more effective. Given the efficacy of (+)-naloxone, further experiments were focused on the effects of (+)-naloxone.
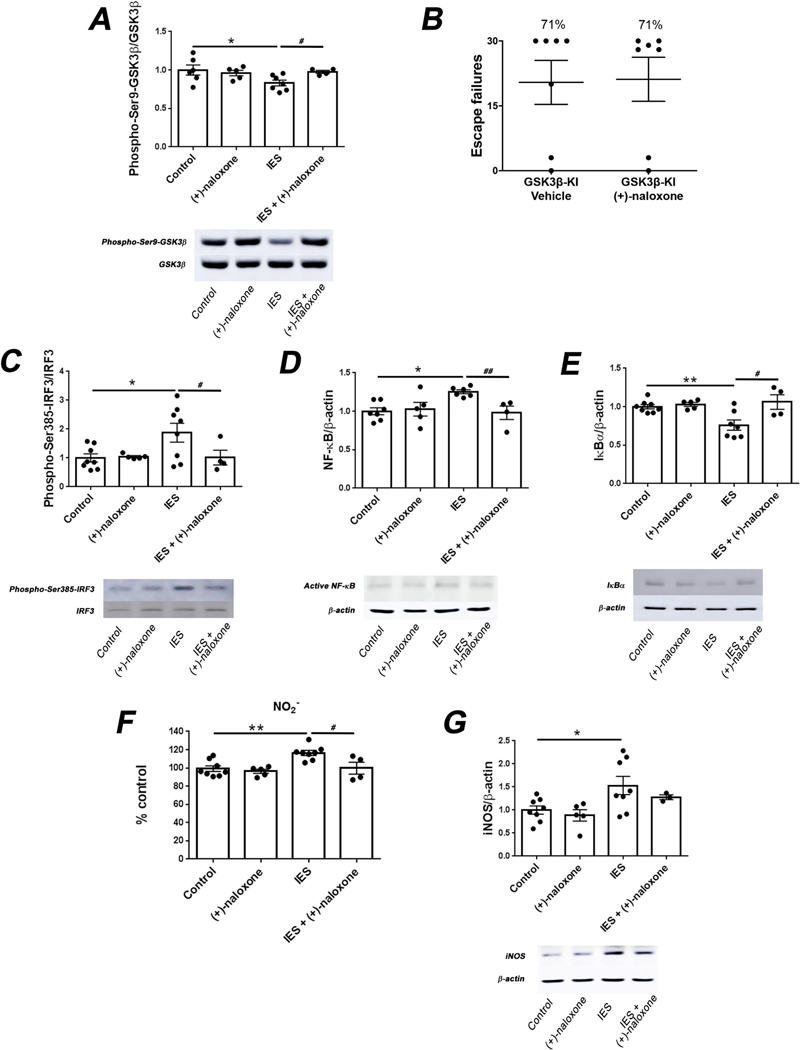
We previously reported that the susceptibility to learned helplessness induced by TLR4 involves an inflammatory cascade that includes activation of glycogen synthase kinase 3β (GSK3β) (Cheng et al., 2016). GSK3β activation is indicated by reduction of the inhibitory phosphorylation on serine-9 (Beurel et al., 2015). We found that treatment with (+)-naloxone completely blocked the activation of GSK3β following exposure to foot shocks (Fig. 2A; one-way ANOVA, F (3,18) = 3.004, Dunnett post-hoc test, p < 0.05, Student’s t-test, t = 2.728, p < 0.05), but did not alter GSK3β activation in control mice. The involvement of GSK3β on the effect exerted by naloxone was corroborated by using GSK3β knockin mice in which serine-9 is mutated to alanine to block the possibility of inhibition by serine-9 phosphorylation (McManus et al., 2005). Unlike in wild-type mice, (+)-naloxone administration did not reduce susceptibility to learned helplessness in GSK3β knockin mice (Fig. 2B), corroborating the conclusion that GSK3β inhibition is necessary for the resilience to learned helplessness exerted by (+)-naloxone treatment.
Fig. 2.
Administration of (+)-naloxone inhibits the TLR4 signaling pathway induced by stress. (A) Wild-type non-shocked (Control) mice or mice subjected to inescapable foot shocks (IES) were administered the TLR4 antagonist (+)-naloxone (5 mg/kg; i.v.) or vehicle 1 h prior to IES and sacrificed 12 h after IES. Hippocampal levels of phospho-Ser9-GSK3β and total GSK3β were measured. Each point represents an individual mouse. Bars represent means ± SEM. One-way ANOVA, F(3,18) = 3.004, Dunnett post-hoc test, *p < 0.05, compared to control mice, Student’s t-test, t = 2.728, #p < 0.05, IES vs. IES+ (+)-naloxone, n = 4–7 mice/group. (B) GSK3β knockin (GSK3β-KI) mice were treated with (+)-naloxone (5 mg/kg; i.v.) or vehicle and subjected to the learned helplessness paradigm 1 h after treatment. The number of failures to escape was recorded. Mice were considered learned helplessness if they failed to escape more than 15 out of 30 trials of escapable foot shocks. Each point represents the number of failures to escape for an individual mouse. Bars represent means ± SEM. n = 7 mice/group. (C-G) Wild-type non-shocked (Control) mice or mice subjected to inescapable foot shocks (IES) were administered (+)-naloxone (5 mg/kg; i.v.) or vehicle 1 h prior to IES and sacrificed 12 h after IES. Hippocampal levels of (C) phospho-Ser385-IRF3 and total IRF3, (D) active NF-κB and β-actin, (E) IκBα and β-actin, (F) NO2−, a stable breakdown product of NO, and (G) iNOS and β-actin were quantified. Each point represents an individual mouse. Bars represent means ± SEM. One-way ANOVA, (C) F(3,21) = 3.507, (D) F(3,18) = 4.620, (E) F(3,20) = 6.776, (F) F(3,21) = 7.994, (G) F(3,20) = 3.904, Dunnett post-hoc test, *p < 0.05, **p < 0.01, compared to control mice, Student’s t-test, (C) t = 4.917, (D) t = 3.578, (E) t = 2.673, (F) t = 2.817, #p < 0.05, ##p < 0.01, IES vs. IES + (+)-naloxone, n = 3–8 mice/group.
TLR4 activation can trigger a MyD88-dependent pathway that involves the activation of NF-kB followed by the induction of inflammatory cytokines (Hayden and Ghosh, 2012; Kawai and Akira, 2007; Kuzmich et al., 2017), and a MyD88-independent pathway that involves activation of interferon regulatory factor 3 (IRF3) and NF-kB leading to IFN-β production (Bagchi et al., 2007; Yamamoto et al., 2003). IRF3 activation requires phosphorylation on serine-385 (Lin et al., 1999). Inescapable foot shocks of the learned helplessness protocol increased hippocampal IRF3-serine385 phosphorylation and this was blocked by administration of (+)-naloxone (Fig. 2C; one-way ANOVA, F(3,21) = 3.507, Dunnett post-hoc test, p < 0.05, Student’s t-test, t = 4.917, p < 0.05), confirming blockade of the MyD88-independent pathway by (+)-naloxone. In addition, (+)-naloxone administration blocked the increase in the hippocampal levels of active NF-kB (Fig. 2D; one-way ANOVA, F(3,18) = 4.620, Dunnett post-hoc test, p < 0.05, Student’s t-test, t = 3.578, p < 0.01) and the decrease of the NF-kB inhibitor IkBα (Fig. 2E; one-way ANOVA, F (3,20) = 6.776, Dunnett post-hoc test, p < 0.01, Student’s t-test, t = 2.673, p < 0.05) that were induced by foot shocks, as previously reported (Cheng et al., 2016). In TLR4−/− mice there were no effects of foot shocks or (+)-naloxone treatment on the hippocampal levels of active NF-κB (Suppl. Fig. 2A) or IκBα (Suppl. Fig. 2B), confirming that these changes (Fig. 2) are mediated by TLR4. Foot shocks also increased the hippocampal levels of NO (Fig. 2F; one-way ANOVA, F (3,21) = 7.994, Dunnett post-hoc test, p < 0.01, Student’s t-test, t = 2.817, p < 0.05) and inducible nitric oxide synthase (Fig. 2G; one-way ANOVA, F(3,20) = 3.904, Dunnett post-hoc test, p < 0.05), and these increases were attenuated by (+)-naloxone administration. (+)-Naloxone administration did not change any of these molecules in control mice (Fig. 2C-G).
Consistent with MyD88-independent pathway activation, hippocampal IFNβ levels increased following foot shocks and this increase was blocked by (+)-naloxone administration (Fig. 3A; one-way ANOVA, F(2,17) = 4.969, Dunnett post-hoc test, p < 0.05). (+)-Naloxone administration also blocked foot shock-induced increases in the levels of TNFα in plasma (Fig. 3B; one-way ANOVA, F (2,14) = 3.479, Dunnett post-hoc test, p < 0.05) and hippocampus (Fig. 3C; one-way ANOVA, F(2,14) = 5.930, Dunnett post-hoc test, p < 0.05). However, (+)-naloxone did not block the foot shock-induced increases of several other hippocampal inflammatory cytokines IL-17A, IL-1β and IL-6 (Fig. 3D-F; one-way ANOVA, F(2,17) = 8.942 (D), F(2,16) = 6.960 (E), F(2,17) = 4.057 (F), Dunnett post-hoc test, p < 0.05) that were previously shown to be mediated by activation of TLR4 (Cheng et al., 2016), suggesting a preferential inhibition by (+)-naloxone of the TLR4-mediated MyD88-independent signaling pathway.
Fig. 3.
Administration of (+)-naloxone reduces the levels of IFNβ and TNFα induced by stress. (A-F) Wild-type non-shocked (Control) mice or mice subjected to inescapable foot shocks (IES) were administered the TLR4 antagonist (+)-naloxone (5 mg/kg; i.v.) or vehicle 1 h prior to IES and sacrificed 12 h after IES. (A) Hippocampal levels of IFNβ. Each point represents an individual mouse. Bars represent means ± SEM. One-way ANOVA, F(2,17) = 4.969, Dunnett post-hoc test, *p < 0.05, compared to control mice, n = 4–8 mice/group. (B) Plasma and (C) hippocampal levels of TNFα. Each point represents an individual mouse. Bars represent means ± SEM. One-way ANOVA, (B) F(2,14) = 3.479, (C) F(2,14) = 5.930, Dunnett post-hoc test, *p < 0.05, compared to control mice, n = 3–8 mice/group. Hippocampal levels of (D) IL-17A, (E) IL-1β and (F) IL-6. Each point represents an individual mouse. Bars represent means ± SEM. One-way ANOVA, (D) F (2,17) = 8.942, (E) F(2,16) = 6.960, (F) F(2,17) = 4.057, Dunnett post-hoc test, *p < 0.05, **p < 0.01, compared to control mice, n = 4–8 mice/group.
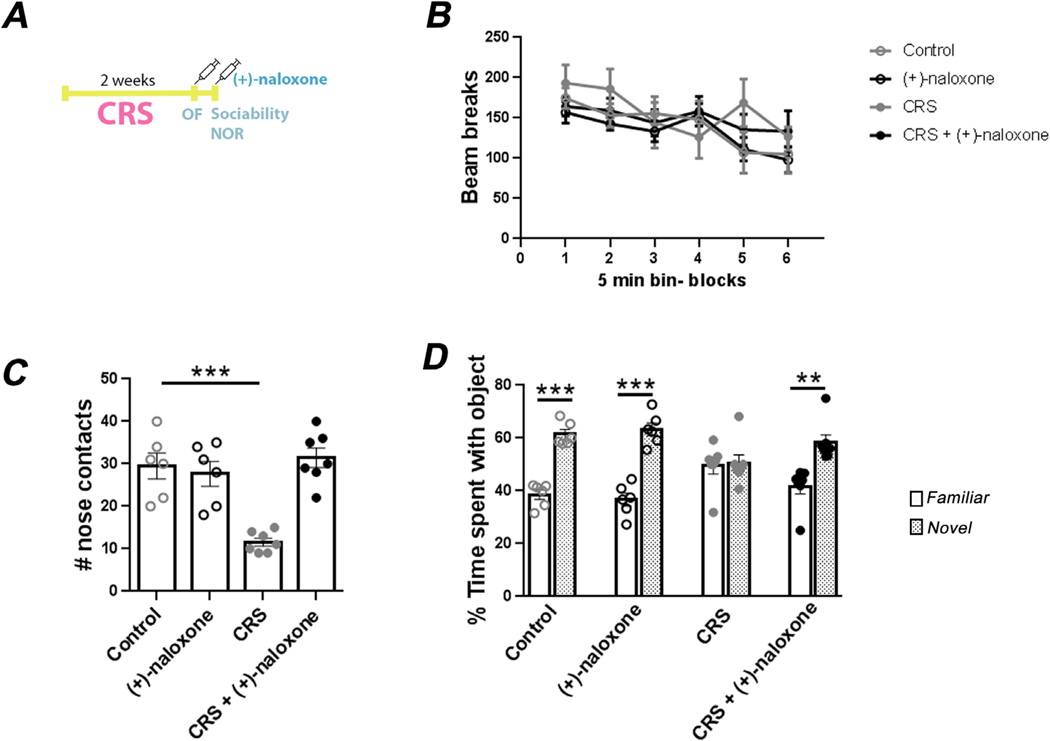
We further tested if pharmacological inhibition of TLR4 with (+)-naloxone improved behavioral impairments caused by another type of stress, chronic restraint stress, which has been widely used to induce behavioral impairments associated with depression (Chiba et al., 2012). After two weeks of restraint, activity in a novel open field, sociability, and learning in the novel object recognition test were studied in mice treated with (+)-naloxone or vehicle and compared to control groups of mice not exposed to chronic restraint stress, as described in Fig. 4A. Neither chronic restraint stress nor (+)-naloxone treatment altered locomotor activity in the novel open field (Fig. 4B). In the three chambered social interaction test, non-stressed mice treated with (+)-naloxone showed the same social interactions as non-stressed mice treated with vehicle (Fig. 4C). As expected, mice exposed to chronic restraint stress had a decreased number of nose contacts with the unfamiliar mouse (Fig. 4C; one-way ANOVA, F(3,22) = 15.95, Dunnett post-hoc test, p < 0.001). This impairment in social interaction was reversed by TLR4 inhibition with (+)-naloxone treatment (Fig. 4C), suggesting an antidepressant effect of (+)-naloxone since impaired social interactions is commonly associated with depression (Krishnan and Nestler, 2008). Learning and memory also can be impaired by chronic stress, is often associated with depression, and may be impaired by TLR4-mediated signaling (Burriss et al., 2008; Okun et al., 2012; Sun and Alkon, 2004). The novel object recognition test revealed that non-stressed control mice, with or without treatment with (+)-naloxone, spent ~60% of the time exploring the novel object and ~40% of the time exploring the familiar object (Fig. 4D; one-way ANOVA, F(7,44) = 13.94, Tukey post-hoc test, p < 0.001). Exposure to chronic restraint stress completely impaired novel object recognition, as these mice spent equal times exploring the two objects (Fig. 4D). Treatment with (+)-naloxone completely reversed the stress-induced impairment in novel object recognition (Fig. 4D; one-way ANOVA, F (7,44) = 13.94, Tukey post-hoc test, p < 0.01). Thus, TLR4 inhibition by (+)-naloxone restored chronic stress-induced impairments in sociability and novel object recognition.
Fig. 4.
Treatment with (+)-naloxone reverses chronic stress-induced impairments in sociability and novel object recognition. (A) Scheme of the experimental procedure involving 2 weeks of chronic restraint stress (CRS), administration of (+)-naloxone (5 mg/kg; i.v.) or vehicle, and behavioral testing of open field activity (OF), sociability, and novel object recognition (NOR). Control groups of mice were subjected to the same behavioral tests with (+)-naloxone or vehicle treatments without being exposed to CRS. (B) Locomotor activity was quantified automatically by the apparatus by counting the number of beam breaks in the open field test. Data represent means ± SEM, n = 6–7 mice/group. (C) The number of nose contacts were measured in the three chambered sociability test. Each point represents an individual mouse. Bars represent means ± SEM, one-way ANOVA, F(3,22) = 15.95, Dunnett post-hoc test, ***p < 0.001 compared to control mice, n = 6–7 mice/group. (D) The percentage of time exploring a familiar object versus a novel object was quantified in the novel object recognition test. Each point represents an individual mouse. Bars represent means ± SEM, one-way ANOVA, F(7,44) = 13.94, Tukey post-hoc test, **p < 0.01, ***p < 0.001, n = 6–7 mice/group.
4. Discussion
Identification of mechanisms and interventions for MDD is crucial for reducing the impact of depression on patients and their caregivers. Based on growing evidence that inflammation contributes to depression susceptibility and impairs therapeutic responses, we tested if antagonists of the major inflammatory signaling pathway mediated by TLR4 ameliorate stress-induced detrimental effects in mice.
Administration of the TLR4 antagonist TAK-242 (3 mg/kg) did not alter susceptibility to learned helplessness. This outcome appears likely due to insufficient in vivo blockade of TLR4, as indicated by its lack of effect on hippocampal TNFα levels even though plasma TNFα levels were reduced. Similarly, administration of TAK-242 (10 mg/kg) did not alter chronic social defeat stress-induced social avoidance and impaired performance in the light–dark box test, although it did improve behavior in the forced swim test (Zhang et al., 2020). Daily administration of TAK-242 (3 mg/kg) 30 min before stresses during chronic unpredictable mild stress significantly modified sucrose preference, open field activity and Morris water maze deficits (Wang et al., 2018). Thus, a higher dose than we used or chronic administration of TAK-242 may be more effective than the treatment that we employed that did not alter behaviors or the hippocampal TNFα level. Thus, for this study the utilization of TAK-242 was not informative for the main goal of testing if blocking TLR4 provides antidepressant effects. Nevertheless, these experiments provided two important pieces of information. First, they demonstrated that brain TNFα levels following stress can be elevated independently of plasma TNFα levels. Second, they demonstrated that diminishing peripheral TNFα is not sufficient to reduce susceptibility to learned helplessness.
Administration of the TLR4 antagonist (+)-naloxone effectively improved mouse behavioral responses to stress in four different measurements, learned helplessness, the tail suspension test, and chronic restraint stress-induced impairments in social interactions and memory. The antidepressant effect in the tail suspension test was confirmed to be mediated by blocking TLR4 because (+)-naloxone was ineffective in mice lacking TLR4, whereas TLR4 knockout mice are resistant to learned helplessness so (+)-naloxone could not be tested in learned helpless TLR4−/− mice (Cheng et al., 2016).
The antidepressant-like effect of (+)-naloxone in the learned helplessness model provided an opportunity to examine signaling pathways that influence susceptibility to learned helplessness. (+)-Naloxone-induced resilience towards learned helplessness was associated with a block of stress-induced activation of GSK3β. This result further substantiates our previous reports that stress activates GSK3β in a TLR4-dependent manner and that GSK3 inhibitors decrease inflammation and vulnerability to learned helplessness (Beurel and Jope, 2009; Cheng et al., 2016; Jope et al., 2017; Martin et al., 2005). Diminished GSK3 activation by (+)-naloxone treatment following stress may have contributed to the reductions of activation of NF-kB and IRF3. Substantial evidence that GSK3 promotes NF-kB activation was previously reviewed (Beurel et al., 2015), and evidence that GSK3 promotes IRF3 activation and IFN-β production has also been reported (Khan et al., 2015; Lei et al., 2010; Park et al., 2017; Qin et al., 2016), although GSK3 is only one factor in the regulation of these two signaling molecules and other signals attenuated by (+)-naloxone’s inhibition of TLR4 likely also contributed. Confirming previous evidence that the learned helplessness protocol increases the production of many cytokines (Cheng et al., 2016; Jope et al., 2017), we found that learned helplessness induction was associated with increased hippocampal levels of multiple cytokines, including TNFα, IL-17A, IL-6 and IL-1β. The present study demonstrated that also the MyD88-independent pathway is activated by stress with an increase in hippocampal IRF3 activation and the induction of IFNβ, a major cytokine induced by the TLR4-induced MyD88-independent signaling pathway (Okun et al., 2011). This fits well with reports of IFNβ treatment increasing the risk for depression (Arnett and Randolph, 2006; Goeb et al., 2003; Pandya and Patten, 2002), although these studies remain controversial (Schippling et al., 2016). Since of the cytokines measured only IFNβ and TNFα levels were reduced by (+)-naloxone treatment, it appears that (+)-naloxone preferentially blocks the TLR4-linked MyD88-independent signaling pathway more than the MyD88-dependent pathway. This finding extends to in vivo a previous report in cultured BV-2 microglial cells that (+)-naloxone attenuated LPS-induced activation of IRF3 and production of IFNβ, TNFα, and NO (Wang et al., 2016). Furthermore, we extended to in vivo the finding that (+)-naloxone reduced hippocampal iNOS and NO that were increased by exposure to the learned helplessness protocol.
Taken together, our present findings and previous reports indicate that stress activates TLR4 signaling that activates both the MyD88-dependent and MyD88–independent pathways, and that both of these contribute to the outcome of depression-like behaviors, since disruption of either pathway appears to be sufficient to reduce depression-like behaviors. These results fit with previous findings that (+)-naloxone is useful for suppressing inflammation underlying preterm birth in rodent models (Chin et al., 2016), protection against sepsis in animal models (Law and Ferguson, 1988; Miller et al., 1986), recovery from ischemic injury (Anttila et al., 2018), prevention of morphine-prolonged neuropathic pain (Grace et al., 2016), and reversion of acute and prolonged neuropathic pain (Lewis et al., 2012). Thus, (+)-naloxone may be a useful intervention to dampen inflammation and may be useful for increasing resilience to stress and diminishing susceptibility to depression.
Acknowledgements
This research was supported by a Merit Award from the Veterans Administration (BX003678) and grants from the NIH (MH104656, MH110415). The work of the Drug Design and Synthesis Section was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anttila JE, Albert K, Wires ES, Matlik K, Loram LC, Watkins LR, Rice KC, Wang Y, Harvey BK, Airavaara M, 2018. Post-stroke Intranasal (+)-Naloxone Delivery Reduces Microglial Activation and Improves Behavioral Recovery from Ischemic Injury. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett PA, Randolph JJ, 2006. Longitudinal course of depression symptoms in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 77, 606–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J, 2007. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J. Immunol 178, 1164–1171. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G, 2008. Major depressive disorder. N. Engl. J. Med 358, 55–68. [DOI] [PubMed] [Google Scholar]
- Beurel E, Grieco SF, Jope RS, 2015. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol. Ther 148, 114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS, 2013. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol. Psychiatry 73, 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS, 2009. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J. Neuroinflammation 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burriss L, Ayers E, Ginsberg J, Powell DA, 2008. Learning and memory impairment in PTSD: relationship to depression. Depress Anxiety 25, 149–157. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Liao SL, Chen WY, Hong JS, Kuo JS, 2001. Cerebral ischemia/reperfusion injury in rat brain: effects of naloxone. NeuroReport 12, 1245–1249. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Pardo M, Armini RS, Martinez A, Mouhsine H, Zagury JF, Jope RS, Beurel E, 2016. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav. Immun 53, 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H, 2012. Chronic restraint stress causes anxiety- and depression-like behaviors, down-regulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 39, 112–119. [DOI] [PubMed] [Google Scholar]
- Chin PY, Dorian CL, Hutchinson MR, Olson DM, Rice KC, Moldenhauer LM, Robertson SA, 2016. Novel Toll-like receptor-4 antagonist (+)-naloxone protects mice from inflammation-induced preterm birth. Sci. Rep 6, 36112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ, 2008. Females do not express learned helplessness like males do. Neuropsychopharmacology 33, 1559–1569. [DOI] [PubMed] [Google Scholar]
- Dantzer R, 2018. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev 98, 477–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ, 2009. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr. Serv 60, 1439–1445. [DOI] [PubMed] [Google Scholar]
- Goeb JL, Cailleau A, Laine P, Etcharry-Bouyx F, Maugin D, Duverger P, Gohier B, Rannou-Dubas K, Dubas F, Garre JB, 2003. Acute delirium, delusion, and depression during IFN-beta-1a therapy for multiple sclerosis: a case report. Clin. Neuropharmacol 26, 5–7. [DOI] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR, 2016. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA 113, E3441–E3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S, 2012. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26, 203–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway RB 3rd, Reigle TG, 1987. The involvement of endogenous opiate systems in learned helplessness and stress-induced analgesia. Psychopharmacology 93, 353–357. [DOI] [PubMed] [Google Scholar]
- Hua F, Tang H, Wang J, Prunty MC, Hua X, Sayeed I, Stein DG, 2015. TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J. Cereb. Blood Flow Metab 35, 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR, 2012. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci 32, 11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR, 2008. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur. J. Neurosci 28, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, Hazeki O, Kitazaki T, Iizawa Y, 2006. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol. Pharmacol 69, 1288–1295. [DOI] [PubMed] [Google Scholar]
- Iijima I, Minamikawa J, Jacobson AE, Brossi A, Rice KC, 1978. Studies in the (+)-morphinan series. 5. Synthesis and biological properties of (+)-naloxone. J. Med. Chem 21, 398–400. [DOI] [PubMed] [Google Scholar]
- Jope RS, Cheng Y, Lowell JA, Worthen RJ, Sitbon YH, Beurel E, 2017. Stressed and Inflamed, Can GSK3 Be Blamed? Trends Biochem. Sci 42, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S, 2007. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med 13, 460–469. [DOI] [PubMed] [Google Scholar]
- Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C, 2017. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol. Psychiatry 25, 321–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE, 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan KA, Do F, Marineau A, Doyon P, Clement JF, Woodgett JR, Doble BW, Servant MJ, 2015. Fine-Tuning of the RIG-I-Like Receptor/Interferon Regulatory Factor 3-Dependent Antiviral Innate Immune Response by the Glycogen Synthase Kinase 3/beta-Catenin Pathway. Mol. Cell. Biol 35, 3029–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ, 2008. The molecular neurobiology of depression. Nature 455, 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F, 2017. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines (Basel) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law WR, Ferguson JL, 1988. Naloxone alters organ perfusion during endotoxin shock in conscious rats. Am. J. Physiol 255, H1106–H1113. [DOI] [PubMed] [Google Scholar]
- Lei CQ, Zhong B, Zhang Y, Zhang J, Wang S, Shu HB, 2010. Glycogen synthase kinase 3beta regulates IRF3 transcription factor-mediated antiviral response via activation of the kinase TBK1. Immunity 33, 878–889. [DOI] [PubMed] [Google Scholar]
- Lewis SS, Loram LC, Hutchinson MR, Li CM, Zhang Y, Maier SF, Huang Y, Rice KC, Watkins LR, 2012. (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. J. Pain 13, 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SL, Chen WY, Raung SL, Chen CJ, 2003. Neuroprotection of naloxone against ischemic injury in rats: role of mu receptor antagonism. Neurosci. Lett 345, 169–172. [DOI] [PubMed] [Google Scholar]
- Lin R, Mamane Y, Hiscott J, 1999. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol. Cell. Biol 19, 2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Jiang JW, Wilson BC, Du L, Yang SN, Wang JY, Wu GC, Cao XD, Hong JS, 2000. Systemic infusion of naloxone reduces degeneration of rat sub-stantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J. Pharmacol. Exp. Ther 295, 125–132. [PubMed] [Google Scholar]
- Liu X, Peprah D, Gershenfeld HK, 2003. Tail-suspension induced hyperthermia: a new measure of stress reactivity. J. Psychiatr. Res 37, 249–259. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS, 1998. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 10, 313–318. [DOI] [PubMed] [Google Scholar]
- Maier SF, Davies S, Grau JW, Jackson RL, Morrison DH, Moye T, Madden JT, Barchas JD, 1980. Opiate antagonists and long-term analgesic reaction induced by inescapable shock in rats. J. Comp. Physiol. Psychol 94, 1172–1183. [DOI] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM, 2005. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol 6, 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR, 2005. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 24, 1571–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Rodriguez EM, Lowell JA, Worthen RJ, Syed SA, Beurel E, 2018. Involvement of innate and adaptive immune systems alterations in the pathophysiology and treatment of depression. Front. Neurosci 12, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2015. Are anti-inflammatory therapies viable treatments for psychiatric disorders? Where the rubber meets the road. JAMA Psychiatry 72, 527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Menke JA, Hansen NB, Zwick DL, Bickers RG, Nowicki PT, 1986. The effect of naloxone on the hemodynamics of the newborn piglet with septic shock. Pediatr. Res 20, 707–710. [DOI] [PubMed] [Google Scholar]
- Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomrenze MB, Galer EL, Kopajtic TA, Li CM, Amat J, Larson G, Cooper DC, Huang Y, O’Neill CE, Yin H, Zahniser NR, Katz JL, Rice KC, Maier SF, Bachtell RK, Watkins LR, 2015. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol. Psychiatry 20, 1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Barak B, Saada-Madar R, Rothman SM, Griffioen KJ, Roberts N, Castro K, Mughal MR, Pita MA, Stranahan AM, Arumugam TV, Mattson MP, 2012. Evidence for a developmental role for TLR4 in learning and memory. PLoS ONE 7, e47522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP, 2011. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 34, 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya R, Patten S, 2002. Depression in multiple sclerosis associated with interferon beta-1a (Rebif). Can. J. Psychiatry 47, 686. [DOI] [PubMed] [Google Scholar]
- Pardo M, Abrial E, Jope RS, Beurel E, 2016. GSK3beta isoform-selective regulation of depression, memory and hippocampal cell proliferation. Genes Brain Behav. 15, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Ko R, Lee SY, 2017. Reciprocal regulation of TLR2-mediated IFN-beta production by SHP2 and Gsk3beta. Sci. Rep 7, 6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Liu Q, Tian S, Xie W, Cui J, Wang RF, 2016. TRIM9 short isoform preferentially promotes DNA and RNA virus-induced production of type I interferon by recruiting GSK3beta to TBK1. Cell Res. 26, 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippling S, O’Connor P, Knappertz V, Pohl C, Bogumil T, Suarez G, Cook S, Filippi M, Hartung HP, Comi G, Jeffery DR, Kappos L, Goodin DS, Arnason B, 2016. Incidence and course of depression in multiple sclerosis in the multinational BEYOND trial. J. Neurol 263, 1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Shechter R, 2010. Systemic inflammatory cells fight off neurodegenerative disease. Nat. Rev. Neurol 6, 405–410. [DOI] [PubMed] [Google Scholar]
- Sha T, Sunamoto M, Kitazaki T, Sato J Ii, Iizawa MY, 2007. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur. J. Pharmacol 571, 231–239. [DOI] [PubMed] [Google Scholar]
- Sun MK, Alkon DL, 2004. Induced depressive behavior impairs learning and memory in rats. Neuroscience 129, 129–139. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H, Watkins LR, 2016. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br. J. Pharmacol 173, 856–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu J, Liu Y, Li Z, Li X, 2018. TLR4-NF-kappaB signal involved in depressive-like behaviors and cytokine expression of frontal cortex and hippocampus in stressed C57BL/6 and ob/ob mice. Neural Plast. 2018, 7254016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse WG, Walker J, Margules DL, Bersh PJ, 1983. Opiate antagonists overcome the learned helplessness effect but impair competent escape performance. Physiol. Behav 30, 731–734. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S, 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643. [DOI] [PubMed] [Google Scholar]
- Zhang K, Lin W, Zhang J, Zhao Y, Wang X, Zhao M, 2020. Effect of Toll-like receptor 4 on depressive-like behaviors induced by chronic social defeat stress. Brain Behav. 10, e01525. [DOI] [PMC free article] [PubMed] [Google Scholar]