Abstract
This study investigates updated information in different search engines on the distribution, phytochemistry, pharmacology, and toxicology of Brugmansia suaveolens (Solanaceae) using the extracts or chemical compounds at present. This plant has been used in traditional medicine in different cultures as a hallucinatory, analgesic, aphrodisiac, nematicide, sleep inducer, and muscle relaxant, as well as a treatment for rheumatism, asthma, and inflammation. The flowers, fruits, stems, and roots of the plant are used, and different chemical compounds have been identified, such as alkaloids, volatile compounds (mainly terpenes), coumarins, flavonoids, steroids, and hydrocarbons. The concentration of the different compounds varies according to the biotic and abiotic factors to which the plant is exposed. The toxic effect of the plant is mainly attributed to atropine and scopolamine, their averages in the flowers are 0.79 ± 0.03 and 0.72 ± 0.05 mg/g of dry plant, respectively. Pharmacological studies have shown that an aqueous extract exhibits the antinociceptive effect, at doses of 100 and 300 mg/kg i.p. in mice. On the other hand, the ethanolic extract at 1000 mg/L, showed a nematocidal activity in vitro of 64% against Meloidogyne incognita in 72 h. Likewise, it showed a 100% larvicidal activity at 12.5 mg/L against Ancylostoma spp. In another study, the lethal activity of shrimp in brine from an ethanolic extract showed an LC50 of 106 µg/mL at double serial concentrations of 1000–0 (µg/mL). Although there are pharmacological and phytochemical studies in the plant, they are still scarce, which has potential for the examination of the biological activity of the more than one hundred compounds that have been reported, many of which have not been evaluated.
Keywords: alkaloids, terpenoid, scopolamine, antinociceptive, nematicide, toxicity
1. Introduction
Brugmansia suaveolens (Humb. and Bonpl. ex Willd.) Bercht. and J.Presl is widely distributed in the world both as a spontaneous species and as an ornamental plant [1], mainly in areas with climates ranging from tropical and subtropical to temperate [2]. It belongs to the Solanaceae family, and according to The Plant List, 12 are recognized in this genus, including hybrids and a subspecies (Brugmansia arborea, B. × candida, B. × cubensis, B. × dolichocarpa, B. × insignis, B. longifolia, B. pittieri, B. × rubella, B. sanguinea, B. sanguinea subsp. vulcanicola, B. suaveolens, and B. versicolor) [1]. The objective of this review is to present complete and updated information on the current research regarding the distribution, phytochemistry, pharmacology, and toxicology of B. suaveolens, in order to identify its therapeutic potential and open new research opportunities. The most salient data were searched using the keyword “Brugmansia suaveolens” in Google Scholar, ScienceDirect, Wiley, Taylor and Francis, and PubMed. The synonyms, according to The Plant List, of B. suaveolens (Humb. and Bonpl. ex Willd.) Bercht. and C. Presl, are Brugmansia albidoflava (Lem.) Verschaff. ex Bosse (unresolved), Datura albidoflava Lem. (synonym), Datura arborea Mart. (synonym), Datura gardneri Hook. (synonym), Datura suaveolens Humb. and Bonpl. ex Willd. (synonym), Datura suaveolens f. albidoflava (Lem.) Voss (synonym), Datura suaveolens var. macrocalyx Sendtn. (synonym), Pseudodatura suaveolens Zijp (unresolved), and Stramonium arboreum Moench (unresolved) [1].
Brugmansia suaveolens is known by various names in different areas of the world, such as in Mexico (Floripondio or florifundio) [3], (Toloache de castilla) [4], Argentina (Floripón) [5], Peru (Misha Colambo (Snake)) [6], (Floripondio) [7], (Toe, Toe de flor blanca) [8], Brazil (Trombeteira or Cartucheira) [9], (Trompeta de Ángel) [10], Sweden (Angel’s Trumpet) [11], Sir Lanka (Attana) [12], and in Indonesia (Kecubung Bunga Kuning and Kecubung Bunga Putih) [13], (Cubung) [14], Pakistan (Shaitani ganti/Bel Boti) [15], and Butan (Gangmeto) [16]. In many other countries, B. suaveolens is better known by the name Angel’s Trumpet.
2. Botany
2.1. Taxonomical Classification
Brugmansia suaveolens, was discovered by Alexander von Humbold and Aimé Bonpland (Humb. Bonpl. Ex Willd.). It was first formally described by Friederich von Berchtold and Jan Presl, and published in Hortus suburbanus Londinensis [1] (Table 1).
Table 1.
Taxonomical classification of Brugmansia suaveolens.
| Kingdom | Plantae |
|---|---|
| Subkingdom | Tracheobionta |
| Superdivision | Spermatophyta |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Order | Solanales |
| Family | Solanaceae |
| Subfamilia | Solanoideae |
| Tribe | Datureae |
| Genus | Brugmansia |
| Species | B. suaveolens [1] |
Brugmansia suaveolens belongs to the group of woody plants [17], considered shrubs or small trees (Figure 1), with a length ranging from 1 to 6 m high. The petiole is 2–5 cm long; the leaf lamina, with the widest end below the middle part known as an elliptical 15–30 cm long and 5–12 cm wide; the corolla is formed by a tube with lobes of 25–30 cm long; and the basal half is a narrow tubular shape and abruptly expands to form extended lobes 10–15 cm long. The color of the flower is white or reddish. The fruit is narrow at the end and wide in the middle part, and is 20 cm long with a 2.5 cm diameter. Flowering begins in January and from April to November. It bears fruit from May to June and in October [3].
Figure 1.
Brugmansia suaveolens.
2.2. Distribution
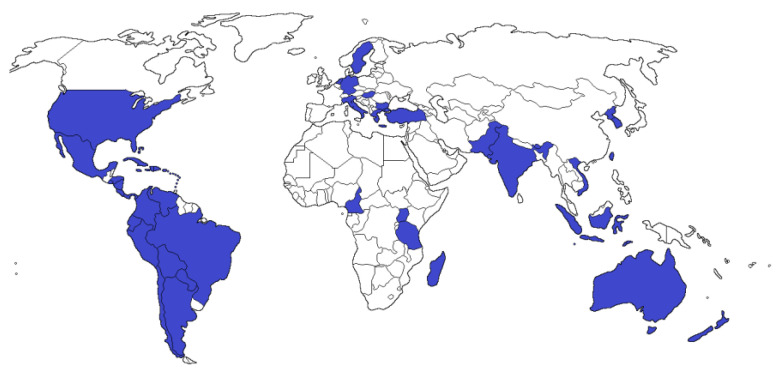
Brugamnsia suaveolens is widely distributed around the world (Figure 2), including in the USA [2], Mexico [18], Honduras, Nicaragua, Panama, El Salvador, Paraguay, and the Antilles [3], Argentina [19], Bolivia [20], Costa Rica [21], Colombia [22], Ecuador [23], Venezuela [24], Peru [6], Chile [25], Brazil [18], Korea [26], Vietnam [27], Taiwan [28], India [29], Indonesia [30], Butan [16], Sri Lanka [12], Pakistan [15], Turkey [31], Australia [32], New Zealand [33], Cameroon, Madagascar, Tanzania [3], Uganda [34], Italy, Bulgaria [35], Netherlands [35], Germany [36], Hungary [37], Greece [38], and Sweden [39].
Figure 2.
Global distribution map of Brugmansia suaveolens.
2.3. Ethnobotany
Many of these species are mainly appreciated as ornamentals, because of their ease of cultivation and the production of their characteristic flower smell at dusk [3], reaching its maximum peak at 21:00 [22]. B. suaveolens is also used in traditional medicine [3], despite being documented in the literature that its greatest use is as a hallucinogenic in shamanic rituals in some populations of Latin America [6], among them being some ethnic groups from the Amazon of Peru and Ecuador [40]. In the Inga people of Colombia, it is used externally to ward off the evil spirits that cause insomnia [41]. However, the first instances of its medicinal use were the Spanish in colonial times, where these plants were used for the treatment of rheumatism, infections, and asthma [42].
It is used to calm toothache [3]; to treat inflammation from trauma [6]; reduce general body inflammation [14]; for sores; to heal wounds without scars [6]; for treating pain in general [9], especially chest pain [43]; to treat abscesses, dermatitis, and fungal infections of the skin [44]; for snake bites; as an aphrodisiac [45]; for diarrhea [15]; gonorrhea; and for loss of appetite [14]. The flower buds are used to treat eye pain [46] and coughing [4].
3. Phytochemistry
Chemical studies of this medicinal species date back to 1996 [47]. Such studies were the first qualitative on groups of compounds, where they were identified as amines, carbohydrates [48], alkaloids, phenolic compounds, flavonoids, steroids, terpenoids, tannins, anthraquinone glycosides, saponins, and triterpenes. The quantification of the alkaloids (5.903 ± 0.01333 mg/g), phenolic compounds (3.435 ± 0.0110 mg/g), and flavonoids (9.945 ± 0.0256 mg/g) was also carried out from the ethanolic extract of the flowers [49]. The concentrations of such compounds can vary, as in living flowers, they show a continuous change in the profile of their volatile compounds, which depend on intrinsic (genetic) and external factors (light, temperature, and water stress). In the case of the cut flowers, they suffer faster deterioration and a loss of volatile compounds [22]. Other factors that also affect it are attacks from pathogens (viruses, bacteria, fungi, and nematodes) and herbivores. The Marvin program was used to draw the structures of organic chemical compounds [50].
3.1. Alkaloids
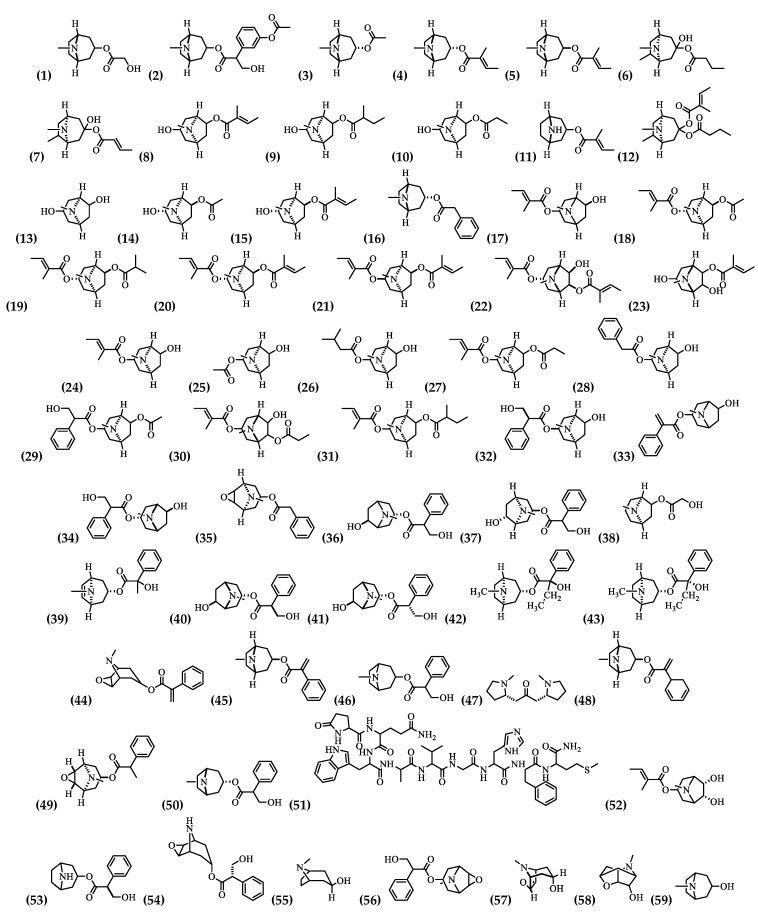
Tropane alkaloids have anticancer activity [51]. Therefore, this group should be more studied in this regard. However, chemically, it is one of the most studied, where 59 alkaloids have been identified in the mature flowers, as well as in the immature flowers and fruits, corolla, flowers, roots, and flower nectar (Table 2 and Figure 3).
Table 2.
Alkaloids from Brugmansia suaveolens.
| No. | Compound Name | Parts Used |
|---|---|---|
| (1) | 3-(Hydroxyacetoxy)-tropane | Roots [35] |
| (2) | 3-(3′Acetoxytropoyloxy)-tropane | Flowers, Roots [35] |
| (3) | 3α-Acetoxytropane | Root cultivation [36] |
| (4) | 3α-Tigloyloxytropane | Flowers and Roots [35] |
| (5) | 3β-Tigloyloxytropane | Flowers [35] |
| (6) | 3-Hydroxy-6-methylbutyryloxy-tropane | Roots [35] |
| (7) | 3-Hydroxy-6-methyl-butenoyl-oxytropane | Flowers [47] |
| (8) | 3-Hydroxy-6-tigloyloxytropane | Root cultivation [36] |
| (9) | 3-Hydroxy-6-(2-methyl butyryloxy)-tropane | Root cultivation [36], Flowers, Roots [35] |
| (10) | 3-Hydroxy-6-propionyl-oxytropane | Flowers [47] |
| (11) | 3-Tigloyloxynortropane | Flowers [35] |
| (12) | 3-Tigloyloxy-6-methylbutyryloxytropane | Flowers, Roots [35] |
| (13) | 3,6-Dihydroxytropane | Flowers [47] |
| (14) | 3α-Hydroxy-6β-acetoxytropane | Flowers, Roots [35] |
| (15) | 3α-Hydroxy-6β-tigloyloxytropane | Flowers, Roots [35] |
| (16) | 3α-Phenylacetoxytropane | Roots [35] |
| (17) | 3α-Tigloyloxy-6β-hydroxytropane | Flowers, Roots [35] |
| (18) | 3α-Tigloyloxy-6β-acetoxytropane | Flowers [35] |
| (19) | 3α-Tigloyloxy-6β-isobutyryloxytropane | Flowers, Roots [35] |
| (20) | 3α,6β-Ditigloyloxytropane | Flowers, Roots [35] and Root cultivation [36] |
| (21) | 3β,6β-Ditigloyloxytropane | Roots [35] |
| (22) | 3α,6β-Ditigloyloxy-7β-hydroxytropane | Flowers [35] |
| (23) | 3,6-dihydroxy-7-tigloyloxytropane | Flowers [35] |
| (24) | 3-Tigloyloxy-6-hydroxytropane | Root cultivation [36] |
| (25) | 3-Acetoxy-6-hydroxytropane | Root cultivation [36] |
| (26) | 3-Isovaleryloxy-6-hydroxytropane | Roots [35] |
| (27) | 3-Tigloyloxy-6-propionyloxytropane | Flowers, and Roots [35] |
| (28) | 3-Phenylacetoxy-6-hydroxytropane | Flowers [35] |
| (29) | 3-Tropoyloxy-6-acetoxytropane | Flowers [35] |
| (30) | 3-Tigloyloxy-6-propionyloxy-7-hydroxytropane | Flowers, Roots [35] |
| (31) | 3-Tigloyloxy-6-(2-methylbutyryloxy)-tropane | Root cultivation [36] and Roots [35] |
| (32) | 7-Hydroxyhyoscyamine | Flowers [35] |
| (33) | 6-Hydroxyapoatropine | Flowers [47], Root cultivation [36], Roots [35] |
| (34) | 6-Hydroxyhyoscyamine | Flowers, Roots [35] |
| (35) | 3-Phenylacetoxy-6,7-epoxytropane | Flowers [52] |
| (36) | 6β-Hydroxyhyoscyamine | Root cultivation [36] |
| (37) | 7β-Hydroxyhyoscyamine | Root cultivation [36] |
| (38) | 6-Hydroxyacetoxytropane | Flowers, Roots [35] |
| (39) | 6,7-Dehydronoratopine | Flowers [47] |
| (40) | 6R-Hydroxyhyoscyamine | Flowers [47] |
| (41) | 6S-Hydroxyhyoscyamine | Flowers [47] |
| (42) | 6R-Hydroxynorhyoscyamine | Flowers [47] |
| (43) | 6S-Hydroxynorhyoscyamine | Flowers [47] |
| (44) | Aposcopolamine | Flowers [47], Root cultivation [36], and Roots [35] |
| (45) | Apoatropine | Flowers [47], Root cultivation [36], and Roots [35] |
| (46) | Atropine | Root cultivation [36], and Corolla [53] |
| (47) | Cuscohygrine | Root cultivation [36] |
| (48) | Dihydroapoatropine | Flowers [47] |
| (49) | Dihydroaposcopolamine | Flowers [47] |
| (50) | Hyoscyamine | Root cultivation [36], Corolla [53], Roots [35], and Flowers [47] |
| (51) | Litorine | Flowers, and Roots [35] |
| (52) | Meteloidine | Flowers [35] |
| (53) | Norhyoscyamine | Flowers [47] |
| (54) | Norscopolamine | Flowers [47] |
| (55) | Pseudotropine | Root cultivation [36], Flowers, Roots [35] |
| (56) | Scopolamine | Ripe flowers, and immature flowers and fruits [54], Corolla [53], Flowers [47], Roots [35], and Flowers nectar [37] |
| (57) | Scopine | Root cultivation [36], Flowers [47] |
| (58) | Scopoline | Root cultivation [36] |
| (59) | Tropine | Flowers, and Roots [35] |
Figure 3.
Structure of alkaloids from Brugmansia suaveolens.
3.2. Volatile Compounds
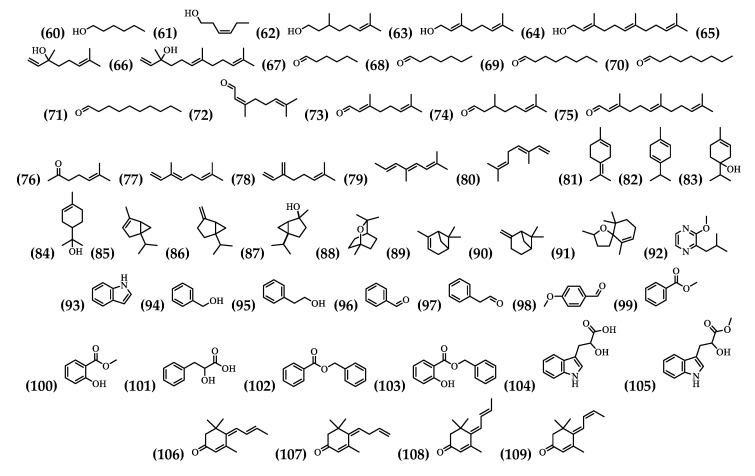
In the flowers and leaves, 50 volatile compounds have been identified and most of these compounds are found in the flowers (Table 3 and Figure 4).
Table 3.
Volatile Compounds from Brugmansia suaveolens.
| No. | Compound Name | Parts Used | No. | Compound Name | Parts Used |
|---|---|---|---|---|---|
| (60) | Hexanol | Flowers [22] | (85) | α-Tujene | Flowers [22] |
| (61) | (Z)-3-Hexen-1-ol | Flowers [22] | (86) | Sabinene | Flowers [22] |
| (62) | Citronellol | Flowers [22] | (87) | trans-Sabinene hydrate | Flowers [22] |
| (63) | Geraniol | Flowers [22] | (88) | 1,8-cineol | Flowers [22] |
| (64) | (trans, trans)-Farnesol | Flowers [22] | (89) | α-Pinene | Flowers [22] |
| (65) | Linalool | Flowers [21] | (90) | β-Pinene | Flowers [22] |
| (66) | (E)-Nerolidol | Flowers [21] | (91) | Theaspirane A | Flowers [21] |
| (67) | Hexanal | Flowers [22] | (92) | 2-Isobutyl-3-methoxypyrazine | Flowers [21] |
| (68) | Heptanal | Flowers [21] | (93) | Indole | Flowers [22] |
| (69) | Octanal | Flowers [21] | (94) | Benzyl alcohol | Flowers [22] |
| (70) | Nonanal | Flowers [22] | (95) | Phenethyl alcohol | Flowers [22] |
| (71) | Decanal | Flowers [22] | (96) | Benzaldehyde | Flowers [22] |
| (72) | Neral | Flowers [22] | (97) | Phenylacetaldehyde | Flowers [21] |
| (73) | Geranial | Flowers [22] | (98) | 4-Methoxy benzaldehyde | Flowers [22] |
| (74) | Citronellal | Flowers [22] | (99) | Methyl benzoate | Flowers [22] |
| (75) | Farnesal | Flowers [22] | (100) | Methyl salicylate | Flowers [22] |
| (76) | 6-Methyl hept-5-en-2-one | Flowers [22] | (101) | 3-phenyl lactic acid | Leaves [29] |
| (77) | cis-β-Ocimene | Flowers [22] | (102) | Benzyl benzoate | Flowers [22] |
| (78) | β-Myrcene | Flowers [22] | (103) | Benzyl salicylate | Flowers [22] |
| (79) | Allo-ocimene | Flowers [22] | (104) | 3-(3-indolyl) lactic acid | Leaves [29] |
| (80) | trans-β-Ocimene | Flowers [22] | (105) | Indole-3-lactic acid methyl ester | Leaves [29] |
| (81) | Terpinolene | Flowers [22] | (106) | Megastigmatrienone I | Flowers [21] |
| (82) | γ-Terpinene | Flowers [21] | (107) | Megastigmatrienone II | Flowers [21] |
| (83) | Terpinen-4-ol | Flowers [22] | (108) | Megastigmatrienone III | Flowers [21] |
| (84) | α-Terpineol | Flowers [22] | (109) | Megastigmatrienone IV | Flowers [21] |
Figure 4.
Structure of Volatile Compounds from Brugmansia suaveolens.
3.3. Phenolic Compounds, Coumarin, and Flavonoids
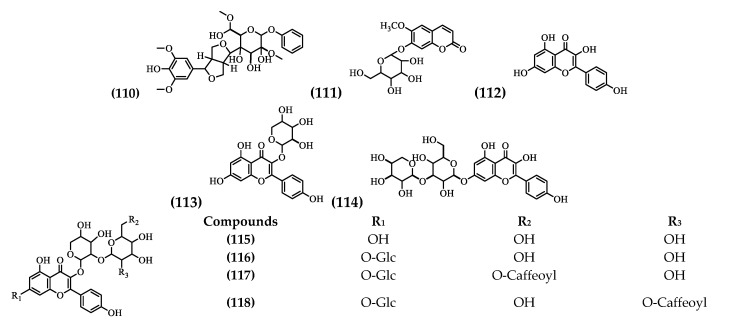
A glycosylated phenolic compound, a coumarin, and seven flavonoids have been identified, in the flowers and leaves (Table 4 and Figure 5).
Table 4.
Phenolic Compounds, Coumarin, and Flavonoids from Brugmansia suaveolens.
| No. | Compound Name | Parts Used |
|---|---|---|
| (110) | Acanthoside B | Flowers [55] |
| (111) | Scopoletin 7-O-β-d-galactopyranoside | Flowers [55] |
| (112) | Kaempferol | Flowers [55] |
| (113) | Kaempferol 3-O-α-l-arabinopyranoside | Leaves [29] |
| (114) | kaempferol 3-O-α-l-arabinopyranosyl-7-O-β-d-glucopyranoside | Leaves [29] |
| (115) | Kaempferol 3-O-β-d-glucopyranosyl-(1′′′→2″)-O-α-l-arabinopyranoside | Leaves [56] |
| (116) | Kaempferol 3-O-β-d-glucopyranosyl-(1′′′→2″)-O-α-l-arabinopyranoside-7-O-β-d-glucopyranoside | Leaves [56] |
| (117) | Kaempferol 3-O-β-d-[6′′′-O-(E-caffeoyl)]-glucopyranosyl-(1′′′→2″)-O-α-l-arabinopyranoside-7-O-β-d-glucopyranoside | Leaves [56] |
| (118) | Kaempferol 3-O-β-d-[2′′′-O-(E-caffeoyl)]-glucopyranosyl-(1′′′→2″)-O-α-l-arabinopyranoside-7-O-β-d-glucopyranoside | Leaves [56] |
Figure 5.
Structure of Phenolic Compounds, Coumarin, and Flavonoids from Brugmansia suaveolens.
3.4. Steroids
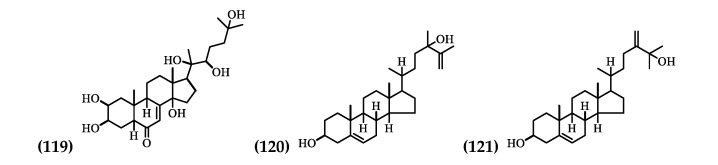
Three Steroids have been identified in the flowers and leaves (Table 5 and Figure 6).
Table 5.
Steroids from Brugmansia suaveolens.
Figure 6.
Structure of Steroids from Brugmansia suaveolens.
3.5. Hydrocarbons
The presence of four hydrocarbons in B. suaveolens has been identified only in the flowers (Table 6 and Figure 7).
Table 6.
Hydrocarbons from Brugmansia suaveolens.
Figure 7.
Structure of the hydrocarbons from Brugmansia suaveolens.
4. Pharmacological Activity
Brugmansia suaveolens is reported in traditional medicine in many Latin American countries; however, the first studies are from 22 years ago [4]. As there are very few pharmacological investigations of the plant, this is still an opportunity for future investigations.
4.1. Antinociceptive
The aqueous extract of B. suaveolens flowers was administered at doses of 100 and 300 mg/kg i.p. They significantly inhibited (p < 0.05) the induced contortions and increased the percentage of inhibition by acetic acid to 0.6% (3.0 ± 0.8 and 94.9%, and 0.6 ± 0.5 and 99%, respectively). Diclofenac 5 mg/kg i.p (43.4 ± 3.5 and 25.8%) was used as a positive control. An increase in the latency time was observed in the formalin test (20 µL of 2.5%); in the first phase (0–5 min), with a dose of 100 mg/kg (15.6 ± 4.2 s and 63.3%) and 300 mg/kg (0.3 ± 0.3 s and 98.6%) and diclofenac (43.6 ± 7.0 s and 0%), and the second phase (20–25 min) with a dose of 100 mg/kg (7.5 ± 2.8 s and 82.2%) and 300 mg/kg (0.0 ± 0.0 s and 100%) and diclofenac (7.0 ± 2.8 s and 69.6%) in male Swiss albino mice. An increase in the latency time was also observed in the hot plate and tail dip tests [9].
In another study of the aqueous extract of flowers of B. suaveolens on the probable antinociceptive mechanism of the 300 mg/kg dose, a mechanism on benzodiazepine receptors was found. Flumazenil (5 mg/kg, i.p.) was used as an antagonist [57].
4.2. Antimicrobial
The antibacterial activity of the aqueous extract of B. suaveolens flowers against Bacillus thurigiensis was evaluated in one study and showed no activity [58].
4.3. Nematicide
The ethanolic extract of flowers at a concentration of 1000 mg/L, showed a 64% in vitro nematocidal activity against Meloidogyne incognita within 72 h. [49]. In another study of the ethanolic extract of aerial parts (flowers, and flowers and stems), a 100% larvicidal activity at a dilution of 12.5 mg against Ancylostoma spp was shown [59].
4.4. Cytotoxicity
Studies of the cytotoxic evaluation of the aqueous extract of B. suaveolens were carried out in the Brine-shrimp model (Artemia sp., Artemiidae) during 24 h, the concentrations of 1000, 500, 250, 125, 62.5, 31.25, and 0 (µg/mL) were evaluated. An LC50 of 106 µg/mL was obtained [7].
4.5. Muscle Relaxer
Brugmansia suaveolens ethanol extract inhibits rabbit smooth muscle contractility at 100% at a concentration of 75.5 g/mL [4].
5. Toxicity
There are several factors (climatic and seasonal) that can increase or decrease the concentration of the alkaloids associated with the toxicity of the plant. It has been documented that it has been involved in poisoning in many parts of the world, and other species, such as B. candida, B. sanguinea, and B. × candida, are considered toxic in some places like Mexico, especially their seeds. It is documented that an intake of 4 to 5 g of raw leaf, or just a seed, can cause a child to die [3]. Among the most toxic compounds are atropine and scopolamine, and their averages in the flowers are 0.79 ± 0.03 and 0.72 ± 0.05 mg/g of dry plant, respectively; these concentrations will increase if the plant is fertilized with organic fertilizer (6 kg/m2 per year) [44]. In another study, a scopolamine concentration of 149.80 ± 6.01 µg/mL was determined in the flower nectar [37]. The plant parts that are the most involved in poisoning are flowers (77.5%), leaves (13.4%), fruits (4.5%), stem (2.3%), and root (2.3%) [60].
The signs and symptoms of Angel’s Trumpet poisoning are mydriasis, dry mouth, delirium, reddened skin [61], dry skin [60], agitation/aggressiveness, reduced bowel sounds [61], ileal paralysis, drowsiness [62], visual hallucinations, tachycardia, urinary retention, fever, increased systolic blood pressure, a Glasgow Coma Scale (GCS) of <12, [61], vertigo [60], decreased temperature and difficulty breathing prior to coma [3].
Unusual poisoning occurred in a five-year-old boy who consumed flowers, and as a consequence, unilateral tonic pupils and Guillain-Barré syndrome were observed [31].
6. Conclusions
This review details the distribution and ethnomedical, phytochemical, pharmacological, and toxicological uses of B. suaveolens in the world. The scientific investigations that have been carried out to date are scarce, the analgesic, cytotoxic, nematicidal, and antimicrobial activity has been studied. However, regarding it’s chemistry, it is important to highlight that 125 compounds have been reported and identified, and a high percentage are not associated with pharmacological activity. Ethnomedical uses reported around the world include its uses to treat pain, insomnia, rheumatism, infections, asthma, inflammation, sores, wounds, abscesses, dermatitis, snakebites, loss of appetite, coughs, and as an aphrodisiac. From this empirical and traditional knowledge in different countries, the scientific validation of this plant species emerges as a great area of opportunity, which provides an opportunity for interdisciplinary collaboration between different research groups.
Acknowledgments
This work was supported by Secretaría de Educación Publica (SEP-PROMEP), Mexico. Photograph by M. en C. Gabriel Flores Franco, curator of the CIByC herbarium of the Autonomous University of the State of Morelos (UAEM).
Author Contributions
Writing—original draft preparation, V.L.P., D.O.S.-S., D.A.-M., C.S.-L., and R.A.-V.; writing—review and editing, V.L.P., D.O.S.-S., D.A.-M., C.S.-L., and R.A.-V.; supervision, V.L.P., D.O.S.-S., D.A.-M., C.S.-L., and R.A.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Secretaría de Educación Pública (SEP-PROMEP) and Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico under number ON.551-6/18-7513.
Conflicts of Interest
The authors declare that they have no conflicts of interest. The funding sponsors contributed the scholarship payment, and had no role in the study design, collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.The Plants List. “Brugmansia suaveolens”. [(accessed on 18 August 2020)];2020 Available online: http://www.theplantlist.org./tpl1.1/record/kew-26840192020.
- 2.Chellemi D.O., Webster C.G., Baker C.A., Annamalai M., Achor D., Adkins S. Widespread occurrence and low genetic diversity of Colombian datura virus in Brugmansia suggest an anthropogenic role in virus selection and spread. Plant Dis. 2011;95:755–761. doi: 10.1094/PDIS-09-10-0654. [DOI] [PubMed] [Google Scholar]
- 3.Monroy-Ortiz C., Monroy R. Las Plantas, Compañeras de Siempre: La Experiencia en Morelos. 1st ed. UAEM, Centro de Investigaciones Biológicas de la Conabio Conanp; Cuernavaca, Morelos, Mexico: 2006. [Google Scholar]
- 4.Encarnación-Dimayuga R., Altamirano L., Maki K.A. Screening of medicinal plants from Baja California Sur (Mexico) by their effects on smooth muscle contractility. Pharm. Biol. 1998;36:124–130. doi: 10.1076/phbi.36.2.124.4606. [DOI] [Google Scholar]
- 5.Furlan V., Kujawska M., Hilgert N.I., Pochettino M.L. To what extent are medicinal plants shared between country home gardens and urban ones? A case study from Misiones, Argentina. Pharm. Biol. 2016;54:1628–1640. doi: 10.3109/13880209.2015.1110600. [DOI] [PubMed] [Google Scholar]
- 6.De Feo V. The ritual use of Brugmansia species in traditional Andean medicine in northern Peru. Econ. Bot. 2004;58:S221–S229. doi: 10.1663/0013-0001(2004)58[S221:TRUOBS]2.0.CO;2. [DOI] [Google Scholar]
- 7.Bussmann R.W., Malca G., Glenn A., Sharon D., Nilsen B., Parris B., Dubose D., Ruiz D., Saleda J., Martinez M., et al. A townesmith toxicity of medicinal plants used in traditional medicine in Northern Peru. J. Ethnopharmacol. 2011;137:121–140. doi: 10.1016/j.jep.2011.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanz-Biset J., Cañigueral S. Plants as medicinal stressors, the case of depurative practices in Chazuta valley (Peruvian Amazonia) J. Ethnopharmacol. 2013;145:67–76. doi: 10.1016/j.jep.2012.09.053. [DOI] [PubMed] [Google Scholar]
- 9.Parker A., Goulart Peraza G., Sena J., Sinnott Silva E., Flores Soares M.C., Cezar Vaz M.R., Badiale Furlong L., Muccillo-Baisch A.L. Antinociceptive effects of the aqueous extract of Brugmansia suaveolens flowers in mice. Biol. Res. Nurs. 2007;8:234–239. doi: 10.1177/1099800406293984. [DOI] [PubMed] [Google Scholar]
- 10.Lucinda N., Inoue-Nagata A.K., Kitajima E.W., Nagata T. Complete genome sequence of Brugmansia suaveolens mottle virus, a potyvirus from an ornamental shrub. Arch. Virol. 2010;155:1729–1732. doi: 10.1007/s00705-010-0798-6. [DOI] [PubMed] [Google Scholar]
- 11.Hudák J., Walles B., Vennigerholz F. The transmitting tissue in Brugmansia suaveolens L.: Ultrastructure of the stylar transmitting tissue. Ann. Bot. 1993;71:177–186. doi: 10.1006/anbo.1993.1022. [DOI] [Google Scholar]
- 12.Jayawickreme K.P., Janaka K.V.C., Subasinghe S. Unknowing ingestion of Brugmansia suaveolens leaves presenting with signs of anticholinergic toxicity: A case report. J. Med. Case Rep. 2019;13:1–4. doi: 10.1186/s13256-019-2250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oktavia A.I., Indriani S., Jati B. Ethnobotanical study of toxic plants in Ngadiwono Village, Tosari District, Pasuruan Regency, East Java. Indones. J. Environ. Sustain. Dev. 2017;8:83–88. doi: 10.21776/ub.jpal.2017.008.02.04. [DOI] [Google Scholar]
- 14.Batoro J., Siswanto D. Ethnomedicinal survey of plants used by local society in Poncokusumo district, Malang, East Java Province, Indonesia. Asian J. Med. Biol. Res. 2017;3:158–167. doi: 10.3329/ajmbr.v3i2.33563. [DOI] [Google Scholar]
- 15.IjazaI F., IqbalbI Z., Ur RahmancI I., AlamdI J., Mulk KhaneI S., Mujtaba ShahfI G., KhangI K., AfzalhI A. Investigation of traditional medicinal floral knowledge of Sarban Hills, Abbottabad, KP, Pakistan. J. Ethnopharmacol. 2016;179:208–233. doi: 10.1016/j.jep.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 16.Chetri B.K., Wangdi P., Penjor T. Ethnomedicinal practices in Kilikhar, Mongar. Asian Plant Res. J. 2018;1:1–13. doi: 10.9734/aprj/2018/v1i226275. [DOI] [Google Scholar]
- 17.Bye R., Sosa V. Molecular phylogeny of the jimsonweed genus Datura (Solanaceae) Syst. Bot. 2013;38:818–829. doi: 10.1600/036364413X670278. [DOI] [Google Scholar]
- 18.Safford W.E. Synopsis of the genus Datura. [(accessed on 25 March 2020)];J. Washingt. Acad. Sci. 1921 11:173–189. Available online: http://www.jstor.org/stable/24532461. [Google Scholar]
- 19.Kujawska M. Forms of medical pluralism among the Polish Community in Misiones, Argentina. Anthropol. Med. 2016;23:205–219. doi: 10.1080/13648470.2016.1180580. [DOI] [PubMed] [Google Scholar]
- 20.Hosking J.R., Conn B.J., Lepschi B.J., Barker C.H. Plant species first recognised as naturalised or naturalising for New South Wales in 2004 and 2005. Cunninghamia. 2011;12:85–114. [Google Scholar]
- 21.Anthony S.J., Zuchowski W., Setzer W.N. Composition of the floral essential oil of Brugmansia suaveolens. Rec. Nat. Prod. 2009;3:76. [Google Scholar]
- 22.Stashenko E.E., Martínez J.R. Sampling flower scent for chromatographic analysis. J. Sep. Sci. 2008;31:2022–2031. doi: 10.1002/jssc.200800151. [DOI] [PubMed] [Google Scholar]
- 23.Mosquera-Yuqui F., Garrido P., Flores F.J. Molecular characterization and complete genome of alstroemeria mosaic virus (AlMV) Virus Genes. 2020;56:87–93. doi: 10.1007/s11262-019-01712-9. [DOI] [PubMed] [Google Scholar]
- 24.Giraldo C.E., Barbosa E.P., Freitas A.V.L. Immature stages of Pagyris cymothoe cymothoe (Hewitson, 1855) (Lepidoptera, Danainae, Ithomiini) Trop. Zool. 2013;26:145–153. doi: 10.1080/03946975.2013.866010. [DOI] [Google Scholar]
- 25.Danton P., Perrier C., de Reyes G.M. Nouveau catalogue de la flore vasculaire de l’archipel Juan Fernández (Chili) Nuevo catálogo de la flora vascular del Archipiélago Juan Fernández (Chile) Acta Bot. Gall. 2006;153:399–587. doi: 10.1080/12538078.2006.10515559. [DOI] [Google Scholar]
- 26.Zhao F., Lim S., Yoo R.H., Lim H.S., Kwon S.Y., Lee S.H., Moon J.S. Complete genome sequence of a South Korean isolate of Brugmansia mosaic virus. Arch. Virol. 2013;58:2019–2022. doi: 10.1007/s00705-013-1693-8. [DOI] [PubMed] [Google Scholar]
- 27.Mai N.T. Quantitative analysis of scopolamine in Brugmansia suaveolens by HPLC-MS method. J. Multidiscip. Eng. Sci. Technol. 2017;4:8176–8179. [Google Scholar]
- 28.Lin T.J., Nelson L.S., Tsai J.L., Hung D.Z., Hu S.C., Chan H.M., Deng J.F. Common toxidromes of plant poisonings in Taiwan. Clin. Toxicol. 2009;47:161–168. doi: 10.1080/15563650802077924. [DOI] [PubMed] [Google Scholar]
- 29.Sajeli Begum A., Sahai M., Fujimoto Y., Asai K., Schneider K., Nicholson G., Suessmuth R. A new kaempferol diglycoside from Datura suaveolens Humb. & Bonpl. ex. Willd. Nat. Prod. Res. 2006;20:1231–1236. doi: 10.1080/14786410600905816. [DOI] [PubMed] [Google Scholar]
- 30.Suganda A.G., Nishiyama Y., Yamakawa T., Sugiyama N. Random amplified polymorphic DNA analysis to distinguish Brugmansia suaveolens, B. candida and B. versicolor. Plant Biotechnol. 2006;23:519–520. doi: 10.5511/plantbiotechnology.23.519. [DOI] [Google Scholar]
- 31.Sevketoglu E., Tatlı B., Tuğcu B., Demirelli Y., Hatipoglu S. An unusual cause of fulminant Guillain-Barré syndrome: Angel’s trumpet. Pediatr. Neurol. 2010;43:368–370. doi: 10.1016/j.pediatrneurol.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Haegi L. Taxonomic account of Datura L.(Solanaceae) in Australia with a note on Brugmansia Pers. Aust. J. Bot. 1976;24:415–435. doi: 10.1071/BT9760415. [DOI] [Google Scholar]
- 33.Sykes W.R. Checklist of dicotyledons naturalised in New Zealand 10. Polemoniales and Boraginaceae. N. Z. J. Bot. 1981;19:311–317. doi: 10.1080/0028825X.1981.10426384. [DOI] [Google Scholar]
- 34.Otim A.S., Kajobe R., Abila P.P., Kasangaki P., Echodu R. Important plants for honey production in four agro ecological zones of Uganda. Bee World. 2019;96:81–86. doi: 10.1080/0005772X.2019.1608892. [DOI] [Google Scholar]
- 35.Doncheva T., Berkov S., Philipov S. Comparative study of the alkaloids in tribe Datureae and their chemosystematic significance. Biochem. Syst. Ecol. 2006;34:478–488. doi: 10.1016/j.bse.2006.01.008. [DOI] [Google Scholar]
- 36.Zayed R., and Wink M. Induction of tropane alkaloid formation in transformed root cultures of Brugmansia suaveolens (Solanaceae) Z. Nat. C. 2004;59:863–867. doi: 10.1515/znc-2004-11-1216. [DOI] [PubMed] [Google Scholar]
- 37.Kerchner A., Darók J., Bacskay I., Felinger A., Jakab G. Protein and alkaloid patterns of the floral nectar in some solanaceous species. Acta Biol. Hung. 2015;66:304–315. doi: 10.1556/018.66.2015.3.6. [DOI] [PubMed] [Google Scholar]
- 38.Malandraki I., Papachristopoulou M., Vassilakos N. First report of potato spindle tuber viroid (PSTVd) in ornamental plants in Greece. New Dis. Rep. 2010;21:9. doi: 10.5197/j.2044-0588.2010.021.009. [DOI] [Google Scholar]
- 39.Vennigerholz F. The transmitting tissue in Brugmansia suaveolens: Immunocytochemical localization of pectin in the style. Protoplasma. 1992;171:117–122. doi: 10.1007/BF01403726. [DOI] [Google Scholar]
- 40.Bennett B.C. Hallucinogenic plants of the Shuar and related indigenous groups in Amazonian Ecuador and Peru. Brittonia. 1992;44:483–493. doi: 10.2307/2807199. [DOI] [Google Scholar]
- 41.Laferriere J.E. Medicinal plants of the Lowland Inga people of Colombia. Int. J. Pharmacogn. 1994;32:90–94. doi: 10.3109/13880209409082977. [DOI] [Google Scholar]
- 42.Schultes E.R., Plowman T. The ethnobotany of Brugmansia. J. Ethnopharmacol. 1979;1:147–164. doi: 10.1016/0378-8741(79)90004-7. [DOI] [PubMed] [Google Scholar]
- 43.Lalzarzovi S.T., Lalramnghinglova H. Traditional use of medicinal plants found within Aizawl city in Mizoram, India. Pleione. 2016;10:269–277. [Google Scholar]
- 44.Reis R.B., Bragagnolo F.S., Gianeti T.M.R., Rodrigues S.A., Funari C.S., Gonçalves G.G., Ming L.C. Brugmansia suaveolens leaf productivity and alkaloid contents under different doses of organic fertilizer. J. Agric. Sci. 2019;11:341–349. doi: 10.5539/jas.v11n3p341. [DOI] [Google Scholar]
- 45.Carlini E.A., Maia L.O. Plant and fungal hallucinogens as toxic and therapeutic agents. Toxinology. 2015;1:1–44. [Google Scholar]
- 46.Rohman F., Juma D.H., Utomo S.R., Arifah S.N., Putra W.E. Plants diversity as a medicinal plants by the Tengger Tribe, Bromo Tengger Semeru National Park, East Java, Indonesia. EurAsian J. Biosci. 2019;13:2293–2298. [Google Scholar]
- 47.Freitas A.V.L., Trigo J.R., Brown K.S., Witte L., Hartmann T., Barata L.E.S. Tropane and pyrrolizidine alkaloids in the ithomiines Placidula euryanassa and Miraleria cymothoe (Lepidoptera: Nymphalidae) Chemoecology. 1996;7:61–67. doi: 10.1007/BF01239482. [DOI] [Google Scholar]
- 48.Sakunthala P., Charles A., Kesavan D., Ramani V.A. Phytochemical screening and adsorption studies of Brugmansia suaveolens. Chem. Sci. Rev. Lett. 2013;29:319–322. [Google Scholar]
- 49.Nandakumar A., Vaganan M.M., Sundararaju P., Udayakumar R. Phytochemical analysis and nematicidal activity of ethanolic leaf extracts of Datura metel, Datura innoxia and Brugmansia suaveolens against Meloidogyne incognita. Asian J. Biol. 2017;2:1–11. doi: 10.9734/AJOB/2017/34241. [DOI] [Google Scholar]
- 50.Marvin, “MarvinSketch 18.04”. [(accessed on 25 March 2020)];2018 Available online: http://www.chemaxon.com.
- 51.Alam E.A. In vitro cultures for the production of some anticancer agents. Life Sci. J. 2013;10:297–310. [Google Scholar]
- 52.Pinto C.F., Salinas S., Flores-Prado L., Echeverría J., Niemeyer H.M. Sequestration of tropane alkaloids from Brugmansia suaveolens (Solanaceae) by the treehopper Alchisme grossa (Hemiptera: Membracidae) Biochem. Syst. Ecol. 2016;66:161–165. doi: 10.1016/j.bse.2016.03.015. [DOI] [Google Scholar]
- 53.Andreola B., Piovan A., Da Dalt L., Filippini R., Cappelletti E. Unilateral mydriasis due to Angel’s Trumpet. Clin. Toxicol. 2008;46:329–331. doi: 10.1080/15563650701378720. [DOI] [PubMed] [Google Scholar]
- 54.Alves M.N., Sartoratto A., Trigo J.R. Scopolamine in Brugmansia suaveolens (Solanaceae): Defense, allocation, costs, and induced response. J. Chem. Ecol. 2007;33:297–309. doi: 10.1007/s10886-006-9214-9. [DOI] [PubMed] [Google Scholar]
- 55.Mai N.T. Investigation on chemical constituents of the Brugmansia suaveolens flowers. J. Multidiscip. Eng. Sci. Technol. 2019;6:10021–10024. [Google Scholar]
- 56.Geller F., Murillo R., Steinhauser L., Heinzmann B., Albert K., Merfort I., Laufer S. Four new flavonol glycosides from the leaves of Brugmansia suaveolens. Molecules. 2014;19:6727–6736. doi: 10.3390/molecules19056727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muccillo-Baisch A.L., Parker A.G., Cardoso G.P., Cezar-Vaz M.R., Flores Soares M.C. Evaluation of the analgesic effect of aqueous extract of Brugmansia suaveolens flower in mice: Possible mechanism involved. Biol. Res. Nurs. 2010;11:345–350. doi: 10.1177/1099800409354123. [DOI] [PubMed] [Google Scholar]
- 58.Vilani A., Lozano E.R., Potrich M., de Gouvea A., Martins Costa Maia F., Angeli Alves L.F., Dall Agnol de Lima J. Activity of plant aqueous extracts on Bacillus thuringiensis and their interactions on Anticarsia gemmatalis (Lepidoptera: Erebinae) Semin. Ciências Agrárias. 2017;38:1051–1057. doi: 10.5433/1679-0359.2017v38n2p1051. [DOI] [Google Scholar]
- 59.Santos I.D., Souza F., Akisue G., da Coelho S.F.A., Coelho M.D.G. Evaluation of ovicidal and larvicidal activity of ten plant extracts against Ancylostoma spp. Rev. Patol. Trop. 2013;42:209–216. [Google Scholar]
- 60.Doan U.V.M., Wu L., Phua D.H., Mendez Rojas B., Yang C.C. Datura and Brugmansia plants related antimuscarinic toxicity: An analysis of poisoning cases reported to the Taiwan poison control center. Clin. Toxicol. 2019;57:246–253. doi: 10.1080/15563650.2018.1513527. [DOI] [PubMed] [Google Scholar]
- 61.Isbister G.K., Oakley P.A., Dawson H., Whyte I.M. Presumed Angel’s trumpet (Brugmansia) poisoning: Clinical effects and epidemiology. Emerg. Med. 2003;15:376–382. doi: 10.1046/j.1442-2026.2003.00477.x. [DOI] [PubMed] [Google Scholar]
- 62.Fuchs J., Rauber-Lüthy C., Kupferschmidt H., Kupper J., Kullak-Ublick G.A., Ceschi A. Acute plant poisoning: Analysis of clinical features and circumstances of exposure. Clin. Toxicol. 2011;49:671–680. doi: 10.3109/15563650.2011.597034. [DOI] [PubMed] [Google Scholar]