Abstract
Iron (Fe) homeostasis is essential for plant growth and development, and it is strictly regulated by a group of transcriptional factors. Iron-related transcription factor 3 (OsIRO3) was previously identified as a negative regulator for Fe deficiency response in rice. However, the molecular mechanisms by which OsIRO3 regulate Fe homeostasis is unclear. Here, we report that OsIRO3 is essential for responding to Fe deficiency and maintaining Fe homeostasis in rice. OsIRO3 is expressed in the roots, leaves, and base nodes, with a higher level in leaf blades at the vegetative growth stage. Knockout of OsIRO3 resulted in a hypersensitivity to Fe deficiency, with severe necrosis on young leaves and defective root development. The iro3 mutants accumulated higher levels of Fe in the shoot under Fe-deficient conditions, associated with upregulating the expression of OsNAS3, which lead to increased accumulation of nicotianamine (NA) in the roots. Further analysis indicated that OsIRO3 can directly bind to the E-box in the promoter of OsNAS3. Moreover, the expression of typical Fe-related genes was significantly up-regulated in iro3 mutants under Fe-sufficient conditions. Thus, we conclude that OsIRO3 plays a key role in responding to Fe deficiency and regulates NA levels by directly, negatively regulating the OsNAS3 expression.
Keywords: rice, iron, Fe deficiency, transcription factor, OsIRO3, OsNAS3, nicotianamine
1. Introduction
Iron (Fe) is an indispensable micronutrient for plants and animals. It acts as a cofactor for a number of enzymes and plays an essential role in many metabolic processes [1]. Fe deficiency is one of the most prevalent nutrient deficiencies in the world; it affects more than one third of the global population [2]. Plants, which are the major Fe sources for humans, take up inorganic Fe from the soil. Although Fe is abundantly present in the earth’s crust, its bioavailability is very low due to the insolubility of inorganic Fe, especially in calcareous soils, which account for about 30% of the world’s cultivated soils [3]. Therefore, disclosing the mechanism underlying Fe homeostasis in plants is important to human health.
Higher plants use two major Fe uptake strategies under low-Fe conditions: Strategy I and Strategy II [3,4]. Non-grass plants, such as Arabidopsis, employ Strategy I, which mainly comprises three processes: (1) roots secrete protons into the rhizosphere that lower the pH by increasing the activity of the H+-ATPase 2(AHA2), resulting in higher solubility of the ferric (Fe3+) form; (2) Fe3+ is reduced to the ferrous (Fe2+) form by the plasma membrane protein ferric reduction oxidase 2 (FRO2); and (3) Fe2+ is taken up by iron-related transporter 1 (IRT1) [3,5,6,7]. In contrast, grass plants that use Strategy II and secrete mugineic acid family phytosiderophores (MAs) through transporter of mugineic acid 1 (TOM1) to bind to Fe3+, and then Fe3+-MA complexes are taken up into roots by yellow stripe 1 (YS1) or YS1-like (YSL) proteins [8,9,10,11].
To adapt to fluctuating environments, plants have a set of sophisticated regulatory systems at transcriptional and post-transcriptional levels. In Arabidopsis, the basic helix-loop-helix (bHLH) fer-like iron deficiency-induced transcription factor (FIT) forms hetero-dimers with subgroup Ib bHLH proteins AtbHLH38, AtbHLH39, AtbHLH100, and AtbHLH101, and these dimers positively regulate the major Strategy I-type Fe acquisition genes, including IRT1, FRO2, and AHA2 [12,13,14]. In addition to the FIT regulator network, the PYE (popeye) network also participates in Fe homeostasis [15]. Under Fe deficiency conditions, PYE is strongly induced in the pericycle. The pye mutant is sensitive to various low Fe growth conditions compared with wild type (WT). PYE can directly and negatively regulate the expression of three genes ferric reduction oxidase 3 (FRO3), nicotainamine synthase 4 (NAS4), and zinc-induced facilitator 1 (ZIF1) that are involved in Fe homeostasis [16]. PYE can interact with some subgroup IVc bHLH transcription factors (bHLH034/104/105/115). These bHLH transcription factors have been demonstrated to regulate Fe homeostasis by binding to the E-box in the promoter of bHLH38/39/100/101 and PYE [17,18,19,20]. Clade IVb bHLHs comprise three bHLHs (bHLH11, bHLH121, and PYE). bHLH11 negatively regulates FIT-dependent Fe uptake and modulates Fe levels in Arabidopsis [21]. bHLH121 functions as a master positive regulator of Fe homeostasis; it acts upstream of FIT in concert with ILR3 and its closest homologs [22,23,24]. These results suggest a number of bHLHs are critical for modulating Fe homeostasis in Arabidopsis.
In rice, a number of bHLH transcription factors have also been identified to regulate Fe homeostasis. Iron-related transcription factor 2 (OsIRO2), a homologue of Arabidopsis bHLH38/39/100/101, plays a critical role in Fe deficiency responses by positively regulating Strategy II-associated genes [25,26]. In rice, OsbHLH156, a homologue of FIT, interacts with OsIRO2 and positively regulates Strategy II Fe acquisition through localizing of OsIRO2 into the nucleus [27,28]. By contrast, plants that overexpress OsIRO3 are sensitive to Fe deficiency, and both Strategy I- and Strategy II-associated genes are suppressed in these plants, suggesting IRO3 is a negative regulator of Fe deficiency responses in rice [29]. Amino acid sequence analysis showed that OsIRO3 is a homology protein of PYE, suggesting the regulatory mechanism of PYE in Arabidopsis and OsIRO3 in rice was conserved. The expression of OsIRO2/OsIRO3 is regulated by the subgroup IVc bHLH transcription factor positive regulators of iron homeostasis (OsPRI1/OsbHLH060, OsPRI2/OsbHLH058, OsPRI3/OsbHLH059) [30,31,32]. Rice proteins OsPRI1/2/3 are homologies of bHLH034/104/105/115, which are positive regulators of iron homeostasis in Arabidopsis [29,30,31,32]. Considering that OsIRO2 is also a homology gene of bHLH038/039/100/101, the positive regulation network of Fe homeostasis between rice and Arabidopsis is also conserved. OsbHLH133 is induced by Fe-deficient conditions in rice, and it is an essential regulator of proper Fe distribution between roots and shoots [33].
OsIRO3 has been reported as a negative regulator of the Fe deficiency response in rice, mainly based on the phenotype and gene expression analysis gained from OsIRO3 overexpression lines [29]. However, the exact regulatory mechanism of OsIRO3 in Fe deficiency responses is still unclear, and the direct downstream genes of OsIRO3 are also unknown. In this study, we analyzed phenotypes of OsIRO3-knockout mutants under both Fe-deficient and Fe-sufficient conditions. Functional analysis revealed that in rice, OsIRO3 played an essential role for the response to Fe deficiency and for maintaining Fe homeostasis. Importantly, OsIRO3 can directly bind to the promoter of OsNAS3 and negatively regulate its expression.
2. Results
2.1. Expression Analysis of OsIRO3
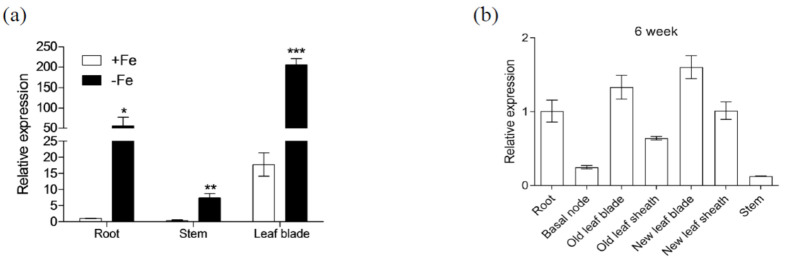
We performed quantitative real-time polymerase chain reaction (qPCR) analysis to investigate the expression pattern of OsIRO3. The expression levels of OsIRO3 in roots, stems, and shoots were significantly upregulated by Fe deficiency (Figure 1a). The transcript abundance of OsIRO3 was higher in leaf blades than in roots and stems under both Fe-sufficient and Fe-deficient conditions (Figure 1a). To investigate the tissue-specific OsIRO3 expression pattern, we examined its levels in different tissues from 6-week-old rice plants. OsIRO3 was highly expressed in roots, leaf blades, and leaf sheaths; it was also expressed in the stems and basal nodes (Figure 1b).
Figure 1.
Expression pattern of OsIRO3. (a) Relative expression of OsIRO3 in root, stem, and leaf blade under both Fe-sufficient conditions (+Fe) or Fe-deficient conditions (−Fe). Two-week-old plants were transferred to +Fe or −Fe for 7 d. The root, leaf blades, and stems were sampled for expression analysis. Asterisks above bars indicate significant differences (* p < 0.05, ** p < 0.01, *** p < 0.001) compared with the Fe-sufficient condition (+Fe), as determined by two-tailed Student’s t test. (b) Relative expression of OsIRO3 in different organs at vegetative growth stage. Different tissues of 6–week-old rice grown in solution were sampled for expression analysis. The expression level relative to the expression in +Fe root (a) or root (b) was shown. Data are given as the means ± SD of three biological replicates.
2.2. Knockout of OsIRO3 Leads to Hypersensitivity to Fe Deficiency
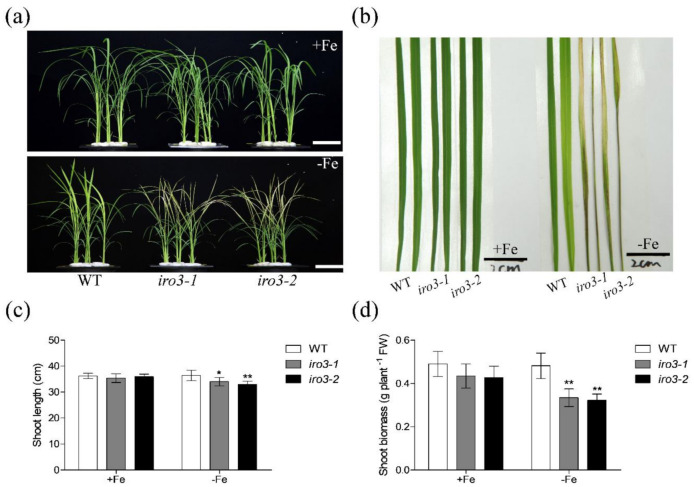
To further investigate the function of OsIRO3 in Fe homeostasis in rice, we used the CRISPR/Cas9 system to create loss-of-function mutants of OsIRO3. Two OsIRO3 gene sequences in the first exon were selected as mutation sites and were used in two independent rice transformations, respectively (Figure S1a). The homozygous iro3 mutants (i.e., iro3-1 and iro3-2) were identified by sequencing. Both mutants were affected by a frame shift due to one base insertion (Figure S1b), which resulted in the OsIRO3 protein lacking the basic helix-loop-helix (bHLH) domain due to the premature termination codon appearing in the N-terminal of OsIRO3 (Figure S2). Then, we compared the growth capacity of WT and OsIRO3-knockout mutants (iro3-1 and iro3-2) under both Fe-sufficient and Fe-deficient conditions (Figure S1). In the presence of Fe, the growth performances of the two iro3 mutants were similar to that of WT plants (Figure 2 and Figure 3). These results indicate that OsIRO3 affects neither the normal growth nor the basal metabolism of rice. However, the new leaves of iro3 mutants appeared severely necrotic after being grown under Fe-deficient conditions for 8 days, whereas WT leaves only showed chlorosis, which is typical of Fe deficiency (Figure 2a,b). The shoot height and fresh weight of both iro3 mutants were significantly lower than those of the WT under Fe-deficient conditions (Figure 2c,d). The necrosis appeared in iro3 mutant leaves might experience reactive oxygen species (ROS)-induced hypersensitive cell death. Under Fe-deficient conditions, we found the iro3 mutants accumulated higher superoxide (O2−) and hydrogen peroxide (H2O2) levels by Nitro blue tetrazolium (NBT) and 3,3′–diaminobenzidine (DAB) staining, respectively (Figure S3). These results indicate that the shoots of iro3 mutants are more sensitive to Fe deficiency than those of WT.
Figure 2.
Shoot phenotype analysis of iro3 mutants. (a) Shoot growth performance of wild type (WT) and iro3 mutants under +Fe or −Fe conditions. (b) The two newly developed leaves of WT and iro3 mutants under +Fe or −Fe conditions. (c) Shoot length of WT and iro3 mutants. (d) Shoot biomass of WT and iro3 mutants. Fourteen-day-old seedlings were transferred to Fe-deficient conditions (−Fe) or Fe-sufficient conditions (+Fe) for 8 d. The iro3-1 mutant contains an insertion of ‘A’; the iro3-2 mutant contains an insertion of ‘T’. Data are given as the means ± SD of six biological replicates. All data were compared with the WT. Asterisks indicate significant differences of WT and iro3 mutants based on two-tailed Student’s t test (* p < 0.05, ** p < 0.01). Bars = 5 cm in (a) and 2 cm in (b).
Figure 3.
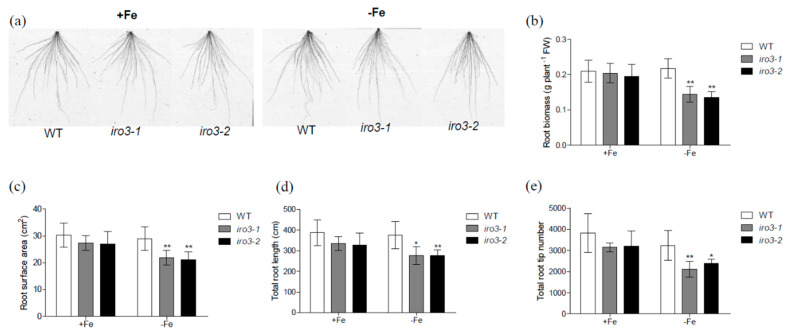
Root phenotype analysis of iro3 mutants. (a) Root growth performance of WT and iro3 mutants grown under +Fe or −Fe conditions. (b–e) Root parameters of WT and iro3 mutants grown under +Fe or −Fe conditions. Root biomass (b), root surface area (c), total root length (d), and total root tip number (e) of WT and iro3 mutants under +Fe and −Fe conditions. Fourteen-day-old seedlings were transferred to −Fe or +Fe for 8 d. The iro3-1 mutant contains an insertion of ‘A’; the iro3-2 mutant contains an insertion of ‘T’. Data are given as the means ± SD of six biological replicates. All data were compared with the WT. Asterisks indicate significant differences of WT and iro3 mutants based on two-tailed Student’s t test (* p < 0.05, ** p < 0.01).
We also found alterations in root morphology of the iro3 mutants in response to Fe deficiency compared with that of WT plants (Figure 3). There was significantly less root biomass in both iro3 mutants compared with that of WT under Fe-deficient conditions (Figure 3a,b). Furthermore, measurement of various root indices revealed that iro3 mutants had less root surface area and total root length and a lower total root tip number than those of WT plants under Fe-deficient conditions (Figure 3c–e). Similar to shoots, there was no difference between WT and the iro3 mutants’ roots under Fe-sufficient conditions (Figure 3). These results suggest that knockout of OsIRO3 leads to decreased tolerance of shoot and root responses to Fe deficiency. Hence, iro3 mutants are hypersensitive to Fe deficiency, and OsIRO3 is required for rice survival under Fe deficiency stress.
2.3. Fe Concentration Analysis in WT and iro3 Mutants
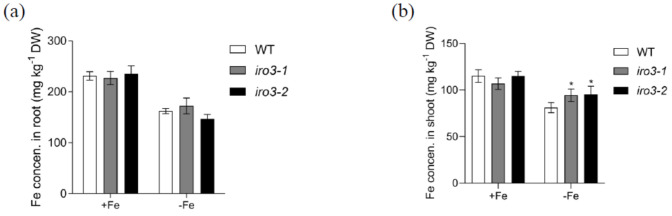
Next, to investigate whether knockout of OsIRO3 affects Fe uptake and transport in rice plants, we measured Fe concentration in WT and iro3 mutants under both Fe-sufficient and Fe-deficient conditions. Under Fe-sufficient conditions, there was no difference in root or shoot Fe concentration between WT and iro3 mutants (Figure 4). By contrast, under Fe-deficient conditions, while there was no difference in the roots, the Fe concentration was significantly higher in the shoots of mutant compared with those of WT plants (Figure 4a,b). These results suggest that OsIRO3 is involved in maintaining Fe accumulation in shoots under Fe-deficient conditions.
Figure 4.
Fe concentration in WT and iro3 mutants. (a) Root and (b) shoot Fe concentration of WT, iro3-1, and iro3-2 under +Fe or −Fe conditions. Fourteen-day-old seedlings of WT, iro3-1, and iro3-2 were transferred to nutrient solution containing 0 or 50 μM Fe (III)-EDTA and grown for 8 d. Root and shoot Fe content of WT, iro3-1, and iro3-2 were analyzed. Data are given as the means ± SD of four biological replicates. All data were compared with the WT. Significant differences from the mutant and WT are indicated by * p < 0.05, as determined by two-tailed Student’s t test.
2.4. Expression of Fe Homeostasis Genes in Roots of WT and iro3 Mutants
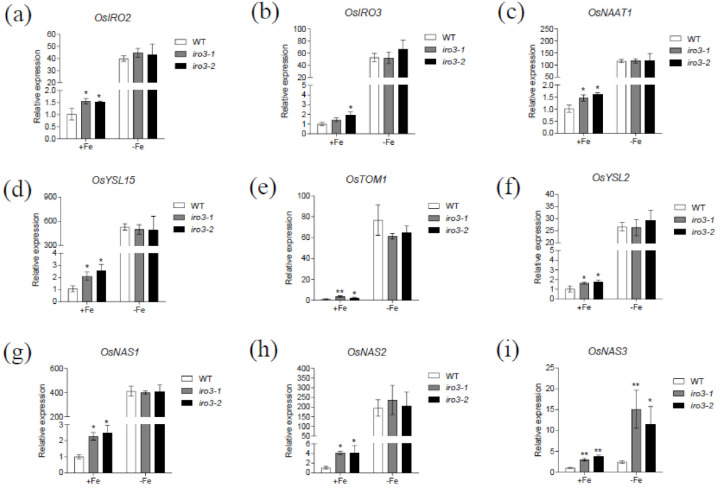
To further evaluate the role of OsIRO3 in maintaining Fe homeostasis, we examined the expression of Fe homeostasis genes in roots of iro3 mutant and WT plants under both Fe-sufficient and Fe-deficient conditions. We selected several representative Fe homeostasis genes for analysis, including genes encoding transcriptional factors OsIRO2 and OsIRO3, and genes involved in Strategy II Fe-uptake pathways in rice [3,21,29]. Most of these genes showed a similar expression pattern between the mutant and WT plants. Specifically, the expression of all evaluated Fe homeostasis genes was significantly upregulated in the iro3 mutants compared with that of the WT under Fe-sufficient conditions, while there was no difference in expression between WT and mutant plants under Fe-deficient conditions (Figure 5). Among the analyzed genes, OsNAS3 expression was higher in the iro3 mutants than that of WT plants under Fe-deficient conditions, which is different from other Fe homeostasis genes (Figure 5i). These results suggest that OsIRO3 negatively regulated Fe homeostasis genes under Fe-sufficient conditions, but negatively regulated OsNAS3 under both Fe-sufficient and Fe-deficient conditions.
Figure 5.
Expression of Fe deficiency responsive genes in the iro3 mutants. Fourteen-day-old seedlings grown in Fe-sufficient media were transferred to Fe-sufficient or Fe-deficient media for 4 days. Roots were sampled and used for RNA extraction. The expression of OsIRO2 (a), OsIRO3 (b), OsNAAT1 (c), OsYSL15 (d), OsTOM1 (e), OsYSL2 (f), OsNAS1 (g), OsNAS2 (h), and OsNAS3 (i) was determined by quantitative real-time RT-PCR. OsActin1 was used as the internal standard. Data are given as the means ± SD of three biological replicates. All data were compared with the WT. Significant differences from the mutant and WT are indicated by * p < 0.05, ** p < 0.01, as determined by two-tailed Student’s t test.
2.5. OsIRO3 Directly Binds to the Promoter of OsNAS3
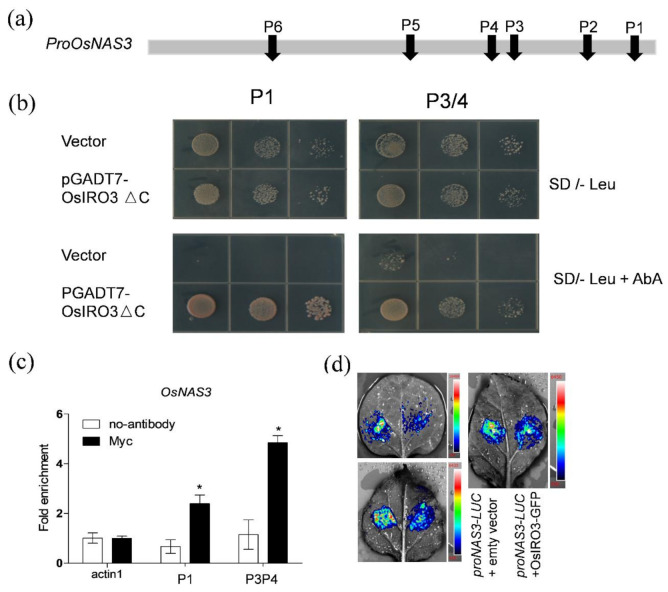
The expression pattern of OsNAS3 suggested that it may be directly regulated by OsIRO3. To confirm this, we analyzed the 1 kb sequences upstream of the OsNAS3 translation start sites, and identified six E-box motifs (P1–P6) that could be recognized by the bHLH transcription factor [34] (Figure 6a). Because the fragment containing P2/5/6 has self-activation in yeast one-hybrid assays, we only checked whether OsIRO3 binds to the fragment containing P1 or P3/4. Then, using a yeast one-hybrid assay, we found that OsIRO3 can bind to the fragment containing P1 or P3/4 in the OsNAS3 promoter (Figure 6b). To further confirm the potential association of OsIRO3 with the promoters of OsNAS3, we performed chromatin immunoprecipitation (ChIP)-qPCR experiments (Figure 6c). Our ChIP-qPCR assays using OsIRO3-Myc plants revealed an enrichment of OsIRO3-Myc recombinant protein on the E-box motifs (P1 and P3/4) in the OsNAS3 promoter (Figure 6c). Then, to verify the regulation effect of OsNAS3 by OsIRO3, we analyzed the expression of proOsNAS3-LUC reporter in Nicotiana benthamiana leaves under Pro35S:OsIRO3-GFP effector and empty vector, respectively (Figure 6d). The expression of OsIRO3-GFP in tabaco leaves was confirmed by examining the GFP signal through confocal observation (Figure S4). The Pro35S:OsIRO3-GFP effector dramatically decreased the luciferase (LUC) signal of the proOsNAS3-LUC reporter (Figure 6d). Collectively, OsIRO3 appears to directly bind to the E-box of the OsNAS3 promoter and negatively regulates its expression.
Figure 6.
OsIRO3 binds to the promoters of OsNAS3. (a) E-boxes in the promoter. The bar indicates the position of the E-box in the 1 kb sequence from the translation start site of OsNAS3. (b) Yeast-one-hybrid assays. The P1 and P3/4 sequence indicated in (a) were used as bait and OsIRO3 as prey. The representative growth status of yeast cells is shown on synthetic dextrose medium agar plates without Leu (SD/-Leu) with or without aureobasidin A (AbA). The AbA resistance was activated by prey proteins that specifically interact with the bait sequence. (c) ChIP-qPCR analyses of the DNA binding ratio of OsIRO3 to the promoters of OsNAS3. qPCR was used to quantify enrichment of the indicated promoters and a fragment of the OsActin1 promoter containing an E-box motif was used as a negative control. The DNA binding ratio indicates the targeted DNA fragment levels relative to the OsActin1 promoter fragment. Data are given as the means ± SD of three biological replicates. Significant differences from control (no-antibody) are indicated by * p < 0.05, as determined by two-tailed Student’s t test. (d) OsIRO3 repressed the activity of the OsNAS3 promoter in transient expression assays. The three pictures are representative of three experiments, respectively. The left side of tabaco leaves were co-expressed ProOsNAS3-LUC and empty vector, the right side of tabaco leaves were co-expressed ProOsNAS3-LUC and OsIRO3-GFP. In the color scale, “Red” represents a high LUC signal, while the color “Blue” represents the lowest LUC signal.
2.6. Nicotianamine (NA) Analysis in WT and iro3 Mutants
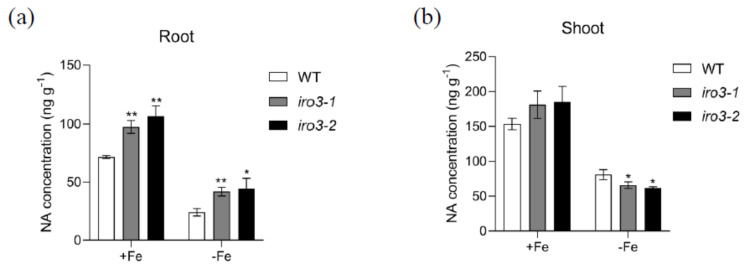
Given that the expression of an NA synthase–encoding gene, OsNAS3, was directly regulated by OsIRO3, we measured the NA concentration in WT and iro3 mutants under both Fe-sufficient and Fe-deficient conditions. In both conditions, iro3 mutants accumulated significantly higher levels of NA compared with WT plants (Figure 7a). This finding is consistent with the higher expression level of OsNAS3 in the roots of iro3 mutants compared with that of WT plants (Figure 5i). By contrast, under Fe-sufficient conditions, iro3 mutants accumulated higher but not significantly different levels of NA compared with that of WT plants, while the NA level was lower in the iro3 mutants compared with that of WT plants under Fe-deficient conditions (Figure 7b). These data suggest that OsIRO3 regulates Fe transport by affecting NA biosynthesis in rice.
Figure 7.
Nicotianamine (NA) concentration in WT and iro3 mutants. (a) Root NA concentration of WT and iro3 mutants under +Fe and −Fe conditions. (b) Shoot NA concentration of WT and iro3 mutants under +Fe and –Fe conditions. Two-week-old seedlings were transferred to Fe-deficient conditions (−Fe) and Fe-sufficient conditions (+Fe) for 4 d. Root and shoot samples were harvested and NA concentrations were measured according to the method described in the Materials and Methods section. Data are given as the means ± SD of three biological replicates. All data were compared with the WT. Significant differences from the wild type are indicated by * p < 0.05, ** p < 0.01, as determined by two-tailed Student’s t test.
3. Discussion
In plants, a number of bHLH transcription factors have been reported to be involved in modulating Fe homeostasis [35]. Among them, OsIRO3 in rice and PYE in Arabidopsis are negative regulators of iron homeostasis. However, OsIRO3 has been reported as a negative regulator in response to Fe deficiency only based on the functional analysis of OsIRO3 overexpression lines [29]. In this study, by using knockout mutants of OsIRO3, we demonstrated that OsIRO3 is essential for the rice response to Fe deficiency and modulates the NA level by directly regulating OsNAS3 expression.
Fe deficiency can induce some classical responses in plants, including chlorosis of new leaves, inhibition of growth development, and upregulation of Fe homeostasis genes [3,36,37]. We evaluated the role of OsIRO3 through the generation of the loss-of-function mutants of OsIRO3 (Figures S1 and S2). Rather than exhibiting the typical Fe deficient symptom (chlorosis), the iro3 mutants presented new leaves with severe necrosis (Figure 2a,b), with decreased shoot height and root and shoot biomass, and defective root development compared with those of WT plants (Figure 2 and Figure 3). Although both iro3 mutants and OsIRO3-overexpressing lines are sensitive to Fe deficiency, iro3 mutants contained significantly higher concentrations of Fe in the shoots under Fe-deficient conditions instead of the decreased Fe concentration observed in OsIRO3-overexpressing lines (Figure 4) [29]. This phenomenon also happened in the pye mutants [16]. Furthermore, Fe distribution in the shoots and young leaves of iro3 mutants were not obviously different compared with those of WT plants (Figures S5 and S6). Therefore, it is hard to explain the phenotype in the mutants under Fe-deficient conditions by Fe uptake and translocation alone. PYE can directly bind to the promoters of NAS4, FRO3, and ZIF1, which are important for Fe distribution in plant tissues, cells, and subcellular compartments [16]. Proper Fe distribution within plant cells and subcellular compartments and Fe bioavailability are essential for normal growth and development [3,16]. We consider that hypersensitivity of iro3 mutants to Fe deficiency could be due to disruption of Fe bioavailability and distribution homeostasis at the subcellular level. NA is an Fe chelator important for intercellular Fe homeostasis. In addition, NA is important for Fe bioavailability in plants [38,39]. In fact, we found that OsIRO3 repressed OsNAS3 expression by directly binding to the E-box of its promoter (Figure 6). OsNAS3 is an enzyme involved in the biosynthesis of NA. Although it had been reported that PYE could directly and negatively regulate NAS4 expression, the NA concentrations were not shown [16]. Here, we examined the NA concentration in WT and iro3 mutants (Figure 7). The accumulation of NA in iro3 mutants further supports the hypothesis that OsNAS3-dependent NA biosynthesis is an important cause of unbalance in Fe homeostasis in cells. Because Fe can exist in both the ferric (Fe3+) and the ferrous (Fe2+) forms in cells and can serve as an essential cofactor for components in the electron transport chain, impairment of Fe homeostasis can easily trigger the formation of harmful reactive oxygen species [1,40]. The phenomenon of new leaves of iro3 mutants under Fe-deficient conditions was similar to the programmed cell death (PCD) induced by ROS bursts [1,40]. In our study, we demonstrated that iro3 mutants accumulated more O2− and H2O2 content compared to that of WT plants (Figure S3). Thus, the necrosis that appeared in the mutants may be caused by ROS bursts as a result of impaired intercellular Fe homeostasis. In addition, as graminaceous plants, NA in rice can be further used for generation of dexomugineic acid (DMA), which is essential for Fe uptake, transport, and distribution, but was not present in Arabidopsis. So, the potential change to the DMA concentration in iro3 mutants is different from that of the pye mutants. Other uncharacterized target genes of OsIRO3 could also contribute to the hypersensitivity to Fe deficiency of iro3 mutants. Thus, potential target genes should be investigated in the future. In addition, OsIRO2, OsNAAT1, OsYSL15, OsTOM1, OsYSL12, OsNAS1, and OsNAS2 are important Fe homeostasis genes for Fe uptake, transport, and regulation [3,10,11,26,38,41]. The transcript abundance of these genes was significantly higher in the iro3 mutants compared with that of the WT plants. However, unlike OsNAS3, the expression of these Fe homeostasis genes was not affected in the iro3 mutants under Fe-deficient conditions (Figure 5). These data indicate that the expression of these typical Fe homeostasis genes was negatively regulated by OsIRO3 in an indirect manner. Recent studies have revealed that several subfamilies of bHLH transcription factors work together to regulate Fe homeostasis by forming homo- or heterodimers to regulate genes for Fe uptake and metabolism [14,18,22,27,35]. In Arabidopsis, bHLH34/104/105/115 facilitate Fe homeostasis by directly regulating the expression of bHLH38/39/100/101 (the homologues of OsIRO2) and PYE (the homologue of OsIRO3) [16,17,18,19,20,25]. Furthermore, bHLH34/104/105/115 can form homo-or heterodimers [19,20]. In addition, it has been reported that PYE can form heterodimers with bHLH105 (ILR3) as a negative regulator complex of some Fe-related genes, such as NAS4, FER, and NEET [42]. This finding suggests that the interaction between Fe-related bHLH transcription factors is critical for regulating the expression of Fe homeostasis genes, like bHLH105. However, whether OsPRI1, OsPRI2, and OsPRI3 can form homo- or heterodimers or form heterodimers with OsIRO3 is unknown. Therefore, more work should be done to analyze the regulatory relationship with Fe deficiency related to bHLH proteins in rice.
Above all, OsIRO3 negatively modulates Fe homeostasis; this conserved regulatory mechanism of PYE in Arabidopsis and OsIRO3 in rice was further confirmed by function analysis of iro3 mutants. Furthermore, we demonstrate OsNAS3 is a direct target gene of OsIRO3 and is negatively regulated by OsIRO3. The hypersensitivity to Fe deficiency of iro3 mutants and pye mutants indicated that the negative roles of OsIRO3 in rice and PYE in Arabidopsis under Fe-deficient conditions are very important.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Wild-type (WT) rice (Oryza sativa cv Nipponbare) and two iro3 mutants created by CRISPR/Cas9 [41] were used. For hydroponic experiments, plants were growth in a greenhouse at 25–30 °C. Rice seeds were soaked in water at 37 °C for 2 d. Germinated seeds were then transferred to a net floating on 0.5 mM CaCl2 solution. After 4 d, seedlings were transferred into half-strength Kimura B solution. The nutrient solution contained the macronutrients (NH4)2SO4 (0.18 mM), MgSO4·7H2O (0.27 mM), KNO3 (0.09 mM), Ca(NO3)2·4H2O (0.18 mM), and KH2PO4 (0.09 mM) and the micronutrients MnCl2·4H2O (0.50 μM), H3BO3 (3.00 μM), (NH4)6Mo7O24·4H2O (1.00 μM), ZnSO4·7H2O (0.40 μM), CuSO4·5H2O (0.20 μM), and Fe(III)-EDTA (50.00 μM). The pH was adjusted to 5.5, and the nutrient solution was renewed every 2 d. All experiments were repeated at least three times with three replicates each, and representative results of one experiment are shown.
4.2. Plasmid Construction for Plant Transformation
For generating iro3-1 and iro3-2 CRISPR/Cas9 mutants, two 20-bp target sequences, 5′- GCCATGGTGCCGTCGGAGAG -3′ and 5′-GCTGCCGACAAGCTCGTCCA -3′ at the first exon of OsIRO3, were used to design a gRNA spacer and fused to the U3 promoter at the BsaI site of pRGEB31 (Addgene, Watertown, MA, USA) as described [43]. Homozygous iro3 mutants were identified by sequencing. The coding sequence of OsIRO3 without a stop codon was amplified and fused in frame to the 5′ terminus of Myc in the plasmid of pGWB617 to generate the vector 35S-OsIRO3-Myc. These constructs were introduced into the Agrobacterium strain EHA105. Callus was induced from mature embryos of rice cultivar Nipponbare for Agrobacterium-mediated rice transformation [44].
4.3. Phenotypic Analysis of the OsIRO3 Knockout Lines
Two-week-old seedlings of WT and two iro3 mutants were grown in nutrient solution with 50 μM Fe (III)-EDTA or without Fe. After 8 d, root and shoot biomass and length were measured. Total root length, root surface area, and total root tip number were detected by a WinRHIZO root analysis instrument [45].
4.4. Fe Concentration Analysis
To compare Fe concentration in roots and shoots of WT and mutants, 14-day-old seedlings of WT, iro3-1, and iro3-2 were transferred into nutrient solution containing 0 or 50 μM Fe (III)-EDTA and grown for 8 d. Roots and shoots were sampled and dried at 80 °C. After 3 d, samples were digested with HNO3/HClO4 (87:13 v/v) at 100 °C for 1 h, 120 °C for 1 h, 140 °C for 1 h, 160 °C for 1 h, and 180 °C for 1 h. After dissolving samples in 2% HNO3, the concentrations of Fe were determined by ICP-MS (Perkin-Elmer NexION 300X, Waltham, MA, USA).
4.5. Real-Time PCR Analysis
To investigate the expression pattern of OsIRO3 in response to Fe deficiency, 2-week-old seedlings were exposed to solution without Fe for 1 week. Roots, shoots, and stems were sampled and subjected to RNA extraction. To further examine the expression pattern of different organs at different growth stages, different organs from plants grown in a paddy field were taken for RNA extraction.
For expression analysis of genes related to Fe homeostasis, WT and the iro3 mutants were grown in a nutrient solution with or without Fe for 4 d, roots were sampled and subjected to RNA extraction. Total RNA was extracted by using an RNA extraction kit (TaKaRa, Dalian, China). A cDNA Synthesis Kit (TaKaRa, Dalian, China) was used to synthesize first-strand cDNA. Quantitative RT-PCR was performed using the SYBR Green Supermix system on a Mastercycler ep realplex machine (Eppendorf, Germany). OsActin1 was used as the internal standard. The primers used for quantitative real-time PCR are listed in Table S1.
4.6. Yeast-One-Hybrid Assay
For yeast one hybrid assay, the fragments containing P1 and P3/4 of the OsNAS3 promoter were inserted into the pAbAi vector and the coding sequences of OsIRO3 were cloned into the pGADT7 prey vector. The plasmids were co-transferred into the Y1H Gold yeast strain according to the Matchmaker Gold Yeast One-Hybrid Library Screening System (Clontech). Yeast strains can grow well on the synthetic dextrose medium without Leu supplemented with 200 ng/mL aureobasidin A (AbA), which indicates interaction between prey protein and the bait sequence.
4.7. Transient Expression Assays in Tobacco
To investigate the regulatory role of OsIRO3 on OsNAS3 expression in tobacco, we constructed an effector vector Pro35S:IRO3-GFP and a reporter vector ProOsNAS3:LUC. Agrobacterium tumefaciens strain EHA105 was used. The corresponding constructs were co-transiently expressed in young leaves of tobacco by an Agrobacterium-mediated infiltration method as described previously [20]. Tobacco leaves were infected by agrobacterial cells containing plasmids by an infiltration buffer (10 mM MgCl2, 0.2 mM acetosyringone, and 10 mM MES, pH 5.6).
4.8. ChIP-qPCR Assay
Pro35S:OsIRO3-Myc transgenic lines were used for the ChIP assays according to previously described protocols [46,47]. Roots of Pro35S:OsIRO3-Myc transgenic plants were cross-linked with 1% (v/v) formaldehyde under vacuum for 30 min, and Gly was added to a final concentration of 0.125 mol L−1 to quench the cross-linking. Then, samples were immediately ground in liquid nitrogen for nuclei isolation, and the chromatin solution was then sonicated to shear the DNA into fragments of 100~1000 bp. Protein chromatin DNA complexes were isolated by Myc Antibody (Santa). To check OsIRO3-DNA binding efficiency, qPCR was performed according to the procedure described previously. pOsACTIN1 was used as the endogenous control.
4.9. Measurement of Nicotianamine (NA) Concentration
To determine the NA concentrations in WT and the iro3 mutants, 2-week-old seedlings were exposed to solution with or without Fe. After 4 d, root and shoot were sampled and stored at −20 °C before measurement. Then, 100 mg of samples were ground in liquid nitrogen and extracted with 500 μL of water at 80 °C for 30 min, followed by 10 min centrifugation (18,000× g). The supernatant solution was transferred to centrifuge tubes with filters (Amicon Ultra) and further centrifuged for 10 to 30 min at 18,000× g. The NA concentration in the supernatant solution was determined using a UPLC interconnected with a LTQ Orbitrap XL mass spectrometer (Thermo Scientific, Waltham, MA, USA) as described previously [48].
4.10. Bioimaging of Fe by μ-XRF
The new leaves were sampled from 14-day-old seedlings of both WT and iro3 mutants (iro3-2) after Fe deficiency treatment for 4 d. The new leaves were put on the 4% agarose for µ-XRF analysis. The high-resolution distribution analysis of Fe in the new leaves was analyzed using a micro X-ray Fluorescence (μ-XRF) Spectrometer (M4 Tornado) [49]. The Pixel size was 4 μm and Pixel time was 4 ms.
4.11. Measurement of ROS Level in New Leaves
Nitro blue tetrazolium (NBT) staining was used to detect O2−, and H2O2 was detected by 3,3′–diaminobenzidine (DAB) staining. The new leaves were sampled from 14-day-old seedlings of both WT and iro3 mutants after Fe deficiency treatment for 4 d. Then, the samples were cut into small pieces and were submerged in NBT solution (6 mM NBT prepared in 10 mM of sodium citrate, pH = 6) and DAB solution (1 mg/mL DAB solution, pH = 3.8) in a Petri dish (35 mm) using tweezers. Samples were then incubated at room temperature for 8 h under light [50]. After incubation, samples were discolored in 95% boiling ethanol until the chlorophyll was removed completely. The cleared leaves were then photographed.
4.12. Statistical Analyses
Statistical analysis was performed using SPSS ver. 20.0 for all the obtained data. For pairwise comparisons of WT and iro3 mutants, data were analyzed using one-way ANOVA followed by two-tailed Student’s t test.
Acknowledgments
We thank Wenli Zhang for technical assistance in ChIP assay.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/9/1095/s1, Figure S1: Gene editing of OsIRO3. Figure S2. The predicted truncated proteins in the iro3 mutants. Figure S3. Loss of OsIRO3 function results in increased of ROS level. Figure S4. Picture of OsIRO3-GFP signal in tabaco leaves. Figure S5. Fe distribution in shoot of iro3 mutants. Figure S6. Metal distribution in new leaves of WT and iro3-2. Table S1: Primers used in this study.
Author Contributions
Conceptualization, W.W., H.S., and L.Z.; methodology, W.W., J.Y., Y.M., and T.W.; formal analysis, W.W. and L.Z.; writing—original draft preparation, W.W.; writing—review and editing, W.W. and L.Z.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31470347 and 31770269).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Palmer C.M., Guerinot M.L. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 2009;5:333–340. doi: 10.1038/nchembio.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO/UNICEF/UNU . Iron Deficiency Anaemia: Assessment, Prevention and Control: A Guide for Programme Managers. WHO/UNICEF/UNU; Geneva, Switzerland: 2001. Document WHO/NHD/01.03. [Google Scholar]
- 3.Kobayashi T., Nishizawa N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012;63:131–152. doi: 10.1146/annurev-arplant-042811-105522. [DOI] [PubMed] [Google Scholar]
- 4.Marschner H., Römheld V. Strategies of plants for acquisition of iron. Plant Soil. 1994;165:261–274. doi: 10.1007/BF00008069. [DOI] [Google Scholar]
- 5.Eide D., Broderius M., Fett J., Guerinot M.L. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson N.J., Procter C.M., Connolly E.L., Guerinot M.L. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–696. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- 7.Simonetta S., Wolfgang S. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 2009;183:1072–1084. doi: 10.1111/j.1469-8137.2009.02908.x. [DOI] [PubMed] [Google Scholar]
- 8.Curie C., Panaviene Z., Loulergue C., Dellaporta S.L., Briat J.-F., Walker E.L. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- 9.Murata Y., Yamaji N., Ueno D., Nomoto K., Iwashita T. A specific transporter for iron(III)-phytosiderophore in barley roots. Plant J. 2006;46:563–572. doi: 10.1111/j.1365-313X.2006.02714.x. [DOI] [PubMed] [Google Scholar]
- 10.Inoue H., Kobayashi T., Nozoye T., Takahashi M., Kakei Y., Suzuki K., Nakazono M., Nakanishi H., Mori S., Nishizawa N.K. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J. Biol. Chem. 2009;284:3470–3479. doi: 10.1074/jbc.M806042200. [DOI] [PubMed] [Google Scholar]
- 11.Nozoye T., Nagasaka S., Kobayashi T., Takahashi M., Sato Y., Sato Y., Uozumi N., Nakanishi H., Nishizawa N.K. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J. Biol. Chem. 2011;286:5446–5454. doi: 10.1074/jbc.M110.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colangelo E.P., Guerinot M.L. The essential basic Helix-Loop-Helix protein FIT1 is required for the iron deficiency response. Plant Cell. 2004;16:3400–3412. doi: 10.1105/tpc.104.024315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Y., Wu H., Wang N., Li J., Zhao W., Du J., Wang D.-W., Ling H.-Q. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 2008;18:385–397. doi: 10.1038/cr.2008.26. [DOI] [PubMed] [Google Scholar]
- 14.Wu H., Chen C., Du J., Liu H., Cui Y., Zhang Y., He Y., Wang Y., Chu C., Feng Z., et al. Co-Overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis-Enhanced cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiol. 2012;158:790–800. doi: 10.1104/pp.111.190983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov R., Brumbarova T., Bauer P. Fitting into the harsh reality: Regulation of iron-deficiency responses in dicotyledonous plants. Mol. Plant. 2012;5:27–42. doi: 10.1093/mp/ssr065. [DOI] [PubMed] [Google Scholar]
- 16.Long T.A., Tsukagoshi H., Busch W., Lahner B., Salt D.E., Benfey P.N. The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. Plant Cell. 2010;22:2219–2236. doi: 10.1105/tpc.110.074096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selote D., Samira R., Matthiadis A., Gillikin J.W., Long T.A. Iron-Binding E3 ligase mediates iron response in plants by targeting basic Helix-Loop-Helix transcription factors. Plant Physiol. 2014;167:273–286. doi: 10.1104/pp.114.250837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J., Liu B., Li M., Feng D., Jin H., Wang P., Liu J., Xiong F., Wang J., Wang H.-B. The bHLH transcription factor bHLH104 interacts with IAA-LEUCINE RESISTANT3 and modulates iron homeostasis in Arabidopsis. Plant Cell. 2015;27:787–805. doi: 10.1105/tpc.114.132704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Zhang H., Ai Q., Liang G., Yu D. Two bHLH transcription factors, bHLH34 and bHLH104, regulate iron homeostasis in Arabidopsis thaliana. Plant Physiol. 2016;170:2478–2493. doi: 10.1104/pp.15.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang G., Zhang H., Li X., Ai Q., Yu D. bHLH transcription factor bHLH115 regulates iron homeostasis in Arabidopsis thaliana. J. Exp. Bot. 2017;68:1743–1755. doi: 10.1093/jxb/erx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanabe N., Noshi M., Mori D., Nozawa K., Tamoi M., Shigeoka S. The basic helix-loop-helix transcription factor, bHLH11 functions in the iron-uptake system in Arabidopsis thaliana. J. Plant Res. 2019;132:93–105. doi: 10.1007/s10265-018-1068-z. [DOI] [PubMed] [Google Scholar]
- 22.Gao F., Robe K., Bettembourg M., Navarro N., Rofidal V., Santoni V., Gaymard F., Vignols F., Roschzttardtz H., Izquierdo E., et al. The transcription factor bHLH121 interacts with bHLH105 (ILR3) and its closest homologs to regulate iron homeostasis in Arabidopsis. Plant Cell. 2019;32:508–524. doi: 10.1105/tpc.19.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S.A., LaCroix I.S., Gerber S.A., Guerinot M.L. The iron deficiency response in Arabidopsis thaliana requires the phosphorylated transcription factor URI. Proc. Natl. Acad. Sci. USA. 2019;116:24933–24942. doi: 10.1073/pnas.1916892116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei R., Li Y., Cai Y., Li C., Pu M., Lu C., Yang Y., Liang G. bHLH121 functions as a direct link that facilitates the activation of FIT by bHLH IVc transcription factors for maintaining Fe homeostasis in Arabidopsis. Mol. Plant. 2020;13:634–649. doi: 10.1016/j.molp.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Ogo Y., Itai R.N., Nakanishi H., Inoue H., Kobayashi T., Suzuki M., Takahashi M., Mori S., Nishizawa N.K. Isolation and characterization of IRO2, a novel iron-regulated bHLH transcription factor in graminaceous plants. J. Exp. Bot. 2006;57:2867–2878. doi: 10.1093/jxb/erl054. [DOI] [PubMed] [Google Scholar]
- 26.Ogo Y., Itai R.N., Nakanishi H., Kobayashi T., Takahashi M., Mori S., Nishizawa N.K. The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J. 2007;51:366–377. doi: 10.1111/j.1365-313X.2007.03149.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang S., Li L., Ying Y., Wang J., Shao J.F., Yamaji N., Whelan J., Ma J.F., Shou H. A transcription factor OsbHLH156 regulates Strategy II iron acquisition through localising IRO2 to the nucleus in rice. New Phytol. 2020;225:1247–1260. doi: 10.1111/nph.16232. [DOI] [PubMed] [Google Scholar]
- 28.Liang G., Zhang H., Li Y., Pu M., Yang Y., Li C., Lu C., Xu P., Yu D. Oryza sativa FER-LIKE FE DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (OsFIT/OsbHLH156) interacts with OsIRO2 to regulate iron homeostasis. J. Integr. Plant Biol. 2020;62:668–689. doi: 10.1111/jipb.12933. [DOI] [PubMed] [Google Scholar]
- 29.Zheng L., Ying Y., Wang L., Wang F., Whelan J., Shou H. Identification of a novel iron regulated basic helix-loop-helix protein involved in Fe homeostasis in Oryza sativa. BMC Plant Biol. 2010;10:166–174. doi: 10.1186/1471-2229-10-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H., Li Y., Yao X., Liang G., Yu D. Positive regulator of iron homeostasis1, OsPRI1, facilitates iron homeostasis. Plant Physiol. 2017;175:543–554. doi: 10.1104/pp.17.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi T., Ozu A., Kobayashi S., An G., Jeon J.-S., Nishizawa N.K. OsbHLH058 and OsbHLH059 transcription factors positively regulate iron deficiency responses in rice. Plant Mol. Biol. 2019;101:471–486. doi: 10.1007/s11103-019-00917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H., Li Y., Pu M., Xu P., Liang G., Yu D. Oryza sativa POSITIVE REGULATOR of IRON DEFICIENCY RESPONSE 2 (OsPRI2) and OsPRI3 are involved in the maintenance of Fe homeostasis. Plant Cell Environ. 2019;43:261–274. doi: 10.1111/pce.13655. [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Ying Y., Narsai R., Ye L., Zheng L., Tian J., Whelan J., Shou H. Identification of OsbHLH133 as a regulator of iron distribution between roots and shoots in Oryza sativa. Plant Cell Environ. 2013;36:224–236. doi: 10.1111/j.1365-3040.2012.02569.x. [DOI] [PubMed] [Google Scholar]
- 34.Toledo-Ortiz G. The arabidopsis Basic/Helix-Loop-Helix transcription factor family. Plant Cell. 2003;15:1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao F., Robe K., Gaymard F., Izquierdo E., Dubos C. The transcriptional control of iron homeostasis in plants: A tale of bHLH transcription factors? Front. Plant Sci. 2019;10:6. doi: 10.3389/fpls.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andresen E., Peiter E., Küpper H. Trace metal metabolism in plants. J. Exp. Bot. 2018;69:909–954. doi: 10.1093/jxb/erx465. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X., Zhang D., Sun W., Wang T. The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis. Int. J. Mol. Sci. 2019;20:2424. doi: 10.3390/ijms20102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoue H., Higuchi K., Takahashi M., Nakanishi H., Mori S., Nishizawa N.K. Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J. 2003;36:366–381. doi: 10.1046/j.1365-313X.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- 39.Becker R., Fritz E., Manteuffel R. Subcellular localization and characterization of excessive iron in the nicotianamine-less tomato mutant chloronerva. Plant Physiol. 1995;108:269–275. doi: 10.1104/pp.108.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verbon E.H., Trapet P.L., Striglis I.A., Kruijs S., Bakker P.A.H.M., Pieterse C.M. Iron and Immunity. Annu. Rev. Phytopathol. 2017;55:355–375. doi: 10.1146/annurev-phyto-080516-035537. [DOI] [PubMed] [Google Scholar]
- 41.Inoue H., Takahashi M., Kobayashi T., Suzuki M., Nakanishi H., Mori S., Nishizawa N.K. Identification and localisation of the rice nicotianamine aminotransferase gene OsNAAT1 expression suggests the site of phytosiderophore synthesis in rice. Plant Mol. Biol. 2008;66:193–203. doi: 10.1007/s11103-007-9262-8. [DOI] [PubMed] [Google Scholar]
- 42.Tissot N., Robe K., Gao F., Grant-Grant S., Boucherez J., Bellegarde F., Maghiaoui A., Marcelin R., Izquierdo E., Benhamed M., et al. Transcriptional integration of the responses to iron availability in Arabidopsis by the bHLH factor ILR3. New Phytol. 2019;223:1433–1446. doi: 10.1111/nph.15753. [DOI] [PubMed] [Google Scholar]
- 43.Xie K., Yang Y. RNA-Guided genome editing in plants using a CRISPR–cas9 system. Mol. Plant. 2013;6:1975–1983. doi: 10.1093/mp/sst119. [DOI] [PubMed] [Google Scholar]
- 44.Chen S., Jin W., Wang M., Zhang F., Zhou J., Jia Q., Wu Y.-R., Liu F., Wu P. Distribution and characterization of over 1000 T-DNA tags in rice genome. Plant J. 2003;36:105–113. doi: 10.1046/j.1365-313X.2003.01860.x. [DOI] [PubMed] [Google Scholar]
- 45.Pang W., Crow W.T., Luc J.E., McSorley R., Giblin-Davis R.M., Kenworthy K.E., Kruse J.K. Comparison of water displacement and WinRHIZO software for plant root parameter assessment. Plant Dis. 2011;95:1308–1310. doi: 10.1094/PDIS-01-11-0026. [DOI] [PubMed] [Google Scholar]
- 46.Lv Q.-D., Zhong Y., Wang Y., Wang Z., Zhang L., Shi J., Wu Z., Liu Y., Mao C., Yi K., et al. SPX4 negatively regulates phosphate signaling and homeostasis through its interaction with PHR2 in rice. Plant Cell. 2014;26:1586–1597. doi: 10.1105/tpc.114.123208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang X., Pan Q., Lin Y., Gu T., Li Y. A native chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in strawberry fruits. Plant Methods. 2020;16:10. doi: 10.1186/s13007-020-0556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Z., Zhang C., Ge Y. Sufficient sulfur supply promotes seedling growth, alleviates oxidation stress, and regulates iron uptake and translocation in rice. Biol. Plantarum. 2015;59:788–792. doi: 10.1007/s10535-015-0554-6. [DOI] [Google Scholar]
- 49.Dong C., He F., Berkowitz O., Liu J., Cao P., Tang M., Shi H., Wang W., Li Q., Shen Z., et al. Alternative splicing plays a critical role in maintaining mineral nutrient homeostasis in rice (Oryza sativa) Plant Cell. 2018;30:2267–2285. doi: 10.1105/tpc.18.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thordal-Christensen H., Zhang Z., Wei Y., Collinge D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.