Abstract
Controlled environment crop production recommendations often use the daily light integral (DLI) to quantify the light requirements of specific crops. Sole-source electric lighting, used in plant factories, and supplemental electric lighting, used in greenhouses, may be required to attain a specific DLI. Electric lighting is wasteful if not provided in a way that promotes efficient photochemistry. The quantum yield of photosystem II (ΦPSII), the fraction of absorbed light used for photochemistry, decreases with increasing photosynthetic photon flux density (PPFD). Thus, we hypothesized that the daily photochemical integral (DPI), the total electron transport through photosystem II (PSII) integrated over 24 h, would increase if the same DLI was provided at a lower PPFD over a longer photoperiod. To test this, ΦPSII and the electron transport rate (ETR) of lettuce (Lactuca sativa ‘Green Towers’) were measured in a growth chamber at DLIs of 15 and 20 mol m−2 d−1 over photoperiods ranging from 7 to 22 h. This resulted in PPFDs of 189 to 794 μmol m−2 s−1. The ΦPSII decreased from 0.67 to 0.28 and ETR increased from 55 to 99 μmol m−2 s−1 as PPFD increased from 189 to 794 μmol m−2 s−1. The DPI increased linearly as the photoperiod increased, but the magnitude of this response depended on DLI. With a 7-h photoperiod, the DPI was ≈2.7 mol m−2 d−1, regardless of DLI. However, with a 22-h photoperiod, the DPI was 4.54 mol m−2 d−1 with a DLI of 15 mol m−2 d−1 and 5.78 mol m−2 d−1 with a DLI of 20 mol m−2 d−1. Our hypothesis that DPI can be increased by providing the same DLI over longer photoperiods was confirmed.
Keywords: photochemistry, daily photochemical integral, electron transport, quantum yield of photosystem II, chlorophyll fluorescence, photoperiod
1. Introduction
The controlled environment agriculture industry, including indoor plant factories and greenhouses, in the United States (U.S.) spends $600 million annually on the electricity required for horticultural lighting [1]. To reduce these energy costs, it is important to understand how efficiently plants use the light they receive. Only then can strategies be developed to provide lighting when needed, with the intensity needed, to assure that the light can effectively enhance plant growth.
Light (photons) is necessary for photosynthesis; however, light must be provided at appropriate intensities. Too much light may damage plants [2] and too little light will inhibit growth [3]. To understand how efficiently plants use the light they receive, it is critical to study the process of photosynthesis and the light reactions in particular. The energy from photons absorbed by photosynthetic pigments in plants has one of three fates: (1) drive the light reactions of photosynthesis (photochemistry), (2) dissipated as heat (non-photochemical quenching of chlorophyll fluorescence), or (3) re-emitted as light from chlorophyll a (chlorophyll fluorescence). These three fates compete, thus, an increase in one process must be accompanied by a decrease in one or both of the other processes [4,5].
Measuring chlorophyll fluorescence is widely used to study the light reactions of photosynthesis. It provides a non-invasive method to quantify the quantum yield of photosystem II (ΦPSII), a unitless measure of the efficiency with which absorbed photons are used to drive photochemistry [6]. It can be used to quantify photochemical responses to photosynthetic photon flux density (PPFD). As the PPFD increases, a larger portion of the photosystem II (PSII) reaction centers become closed (unable to accept additional excitation energy). This occurs because once the electron acceptor QA in PSII has accepted an electron, it cannot accept another until the first one is transferred to the electron carrier QB. The decrease in “open” reaction centers with increasing PPFD results in a decrease in ΦPSII [5]. To safely dissipate much of the excess light energy, plants upregulate heat dissipation, which further lowers ΦPSII [4,5].
Under natural lighting conditions in a greenhouse, ΦPSII of ‘Green Towers’ lettuce decreased asymptotically from 0.81 to ≈0.22 as the PPFD increased from 0 to 1500 µmol m−2 s−1 [7], while in a separate study ΦPSII of ‘Little Gem’ lettuce decreased to only ≈0.35 at a PPFD of 1500 µmol m−2 s−1 [8]. For lettuce ‘Tiberius,’ grown under a PPFD of 200 µmol m−2 s−1, ΦPSII decreased to ≈0.22 at a PPFD of 1400 µmol m−2 s−1 [9]. This is consistent with our findings that there are substantial differences in the response of ΦPSII and electron transport rate (ETR) to PPFD among lettuce cultivars (unpublished). In addition, leaves acclimate to the light environment they are exposed to: leaves acclimated to high light levels have higher ΦPSII and ETR than leaves acclimated to lower light levels, when measured at the same PPFD [10].
The ΦPSII, combined with a known PPFD, can be used to calculate the electron transport rate (ETR) through PSII as ΦPSII × PPFDabsorbed × 0.5 (two photons are needed to move one electron through the entire electron transport chain) [4,6,11]. Oftentimes, PPFDabsorbed is estimated as 0.84 × PPFD, assuming leaves absorb 84% of the photons that strike the leaf surface, which is a common leaf absorptance coefficient for C3 plants [12].
Electron transport rate, in turn, can be used to determine the daily photochemical integral (DPI), defined as the ETR integrated over a 24-h period. Under greenhouse conditions, without supplemental light, the DPI increased asymptotically with increasing DLI. Because those data were collected under natural sun light conditions, potential interactions between photoperiod, PPFD, and DLI could not be determined [7]. Our goal was to quantify the effect of PPFD and photoperiod on DPI, while maintaining a static DLI. Because ΦPSII increases with lower PPFDs, we hypothesized that the DPI would be greater when the same DLI was provided over longer photoperiods at lower PPFDs. To better understand the physiological basis of treatment effects on ΦPSII, we also measured ΦNPQ (the quantum yield of non-photochemical energy dissipation in response to light exposure) and ΦNO (the quantum yield of other, non-light induced energy dissipation processes). Combined, ΦPSII, ΦNPQ, and ΦNO account for the fate of all photons absorbed by light-harvesting antennae surrounding PSII. In addition, we quantified the upregulation of non-photochemical quenching, as compared to the leaf in its dark-adapted state (non-photochemical quenching (NPQ), which is linearly related to heat dissipation) [4]. A better understanding of the relationship between ΦPSII, PPFD, photoperiod, DLI, and DPI can lead to the development of more efficient lighting strategies.
2. Results
2.1. Time Course of ΦPSII, ΦNO, and ΦNPQ
Photochemical responses of ‘Green Towers’ lettuce were measured at two DLIs (15 and 20 mol m−2 d−1), each applied across photoperiods of 7, 10, 13, 16, 19, and 22 h. The PPFD was constant throughout the entire photoperiod and ranged from 189 to 794 µmol m−2 s−1, depending on the photoperiod and DLI combination (Table 1).
Table 1.
Photosynthetic photon flux density (PPFD) during photochemical measurements. Plants were measured during photoperiods ranging from 7 to 22 h and PPFD was adjusted to ensure a daily light integral (DLI) of 15 or 20 mol m−2 d−1.
| Photoperiod (h) | 15 mol m−2 d−1 | 20 mol m−2 d−1 |
|---|---|---|
| —PPFD (µmol m−2 s−1)— | ||
| 7 | 595 | 794 |
| 10 | 416 | 555 |
| 13 | 320 | 427 |
| 16 | 260 | 347 |
| 19 | 219 | 292 |
| 22 | 189 | 252 |
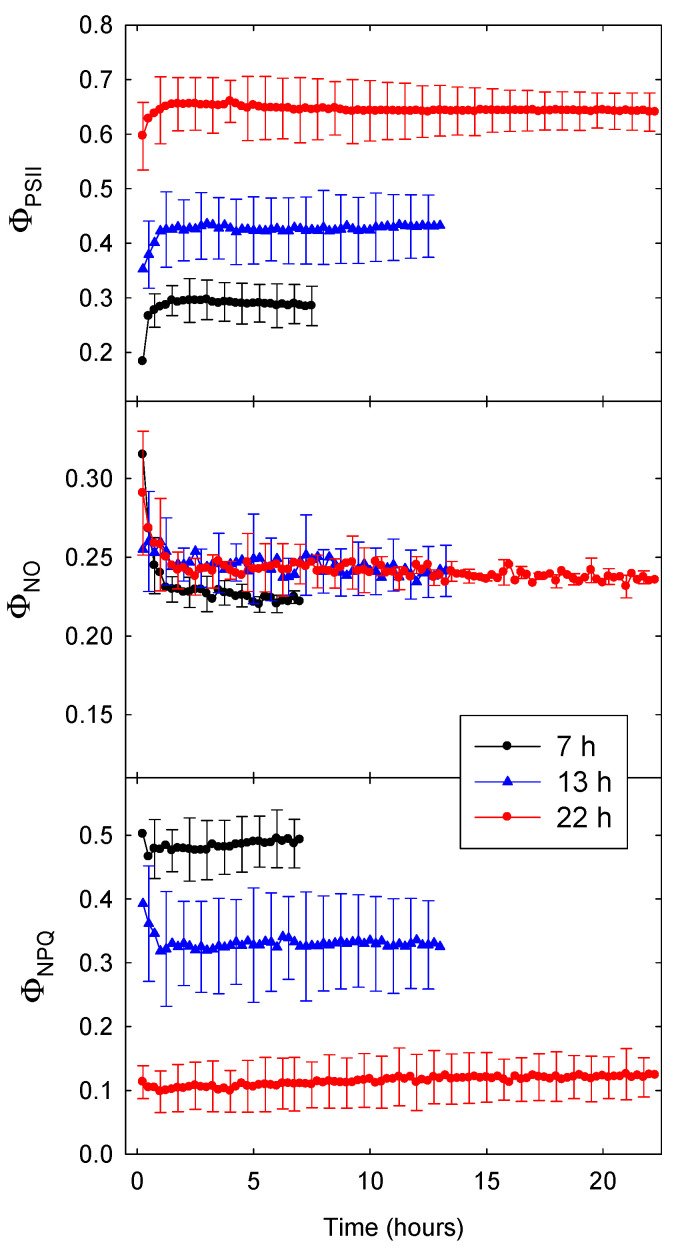
Figure 1 shows ΦPSII, ΦNO, and ΦNPQ data of lettuce for three different photoperiods tested at a DLI of 20 mol m−2 d−1. The ΦPSII gradually increased, and ΦNO and ΦNPQ decreased, during the first hour into the photoperiod. All three quantum yields changed little during the remainder of the photoperiod. With a 22-h photoperiod (and PPFD of 252 µmol m−2 s−1), ΦPSII was much higher than with shorter photoperiods and higher PPFD. Differences in ΦPSII among lighting treatments were largely due to increased energy dissipation (ΦNPQ) in response to the higher PPFDs associated with shorter photoperiods; ΦNO was slightly lower with a 7-h photoperiod (and PPFD of 794 µmol m−2 s−1), but treatment differences in ΦNO were small compared to those in ΦNPQ.
Figure 1.
The quantum yield of photosystem II (ΦPSII, top), the quantum yield of non-light-induced energy dissipation (ΦNO, middle), and the quantum yield of light-induced energy dissipation (ΦNPQ, bottom) of lettuce (Lactuca sativa ‘Green Towers’) under three different photoperiods (7, 13, and 22 h) with a daily light integral (DLI) of 20 mol m−2 d−1. Data are the means of three replications ± SD.
2.2. Time Course of the Quantum Yield of NPQ and ETR
The NPQ parameter is a measure of upregulation of energy dissipation relative to the dark-adapted state. Since all plants in this study were exposed to similar environmental conditions before measurements were taken, NPQ data can be directly compared among different lighting treatments [4]. Consistent with treatment effects on ΦNPQ, NPQ was about five times higher with a 7-h photoperiod (and PPFD of 794 µmol m−2 s−1) than with a 22-h photoperiod (and a PPFD of 252 µmol m−2 s−1). With 13- and 22-h photoperiods, NPQ stabilized within an hour, while NPQ kept increasing slowly throughout the 7-h photoperiod (Figure 2).
Figure 2.
Non-photochemical quenching (NPQ, top) and electron transport rate (ETR, bottom) of lettuce (Lactuca sativa ‘Green Towers’) under three different photoperiods (7, 13, and 22 h) with a daily light integral (DLI) of 20 mol m−2 d−1 over the course of the photoperiod. The area under each ETR curve represents the daily photochemical integral (DPI). Data are the means of three replications ± SD.
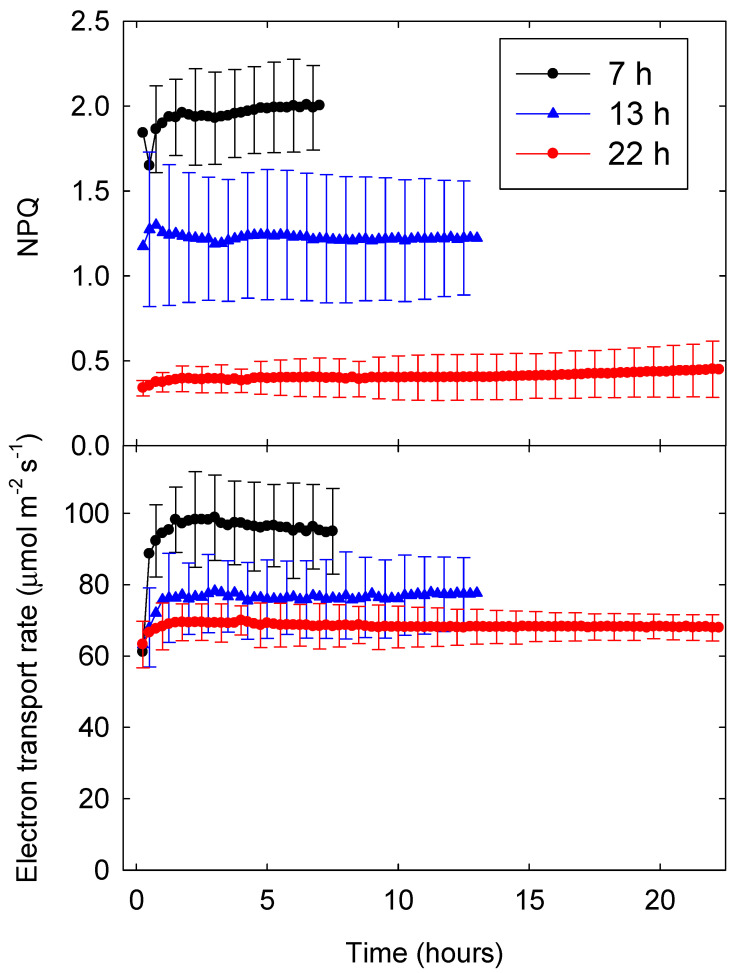
Instantaneous electron transport rates stabilized after about one hour and were higher with shorter photoperiods and higher PPFD (Figure 2 and Figure 3). The area under each ETR curve represents the DPI, which decreased with shorter photoperiods.
Figure 3.
The quantum yield of photosystem II (ΦPSII = 0.792 − 0.000663 × PPFD) (A) and the electron transport rate [ETR = −10.1 + 112.1 × (1 − e−0.00463 × PPFD)] (B) of lettuce (Lactuca sativa ‘Green Towers’), averaged over the entire photoperiod, as a function of photosynthetic photon flux density (PPFD) Data points represent the mean ± SD (n = 3). Plants were measured under two daily light integrals (DLI), 15 and 20 mol m−2 d−1, but DLI had no effect on the relationship between PPFD and these parameters.
2.3. ΦPSII and ETR as a Function of PPFD
Independent of DLI, the ΦPSII decreased linearly (r2 = 0.77, p < 0.001) from 0.67 to 0.29 as PPFD increased from 189 to 794 µmol m−2 s−1 (Figure 3A). The trend was the same for plants exposed to either DLI, 15 or 20 mol m−2 d−1. The ΦPSII decreased by 0.663 per mmol m−2 s−1 increase in PPFD.
Electron transport rate increased in an asymptotic manner from 55 µmol m−2 s−1 at a PPFD of 189 µmol m−2 s−1 to 99 µmol m−2 s−1 as PPFD increased to 794 µmol m−2 s−1 (r2 = 0.58, p < 0.001) (Figure 3B). The asymptote of the ETR was 102 µmol m−2 s−1. As was the case for ΦPSII, the trend was the same for both DLIs.
2.4. Daily Photochemical Integral
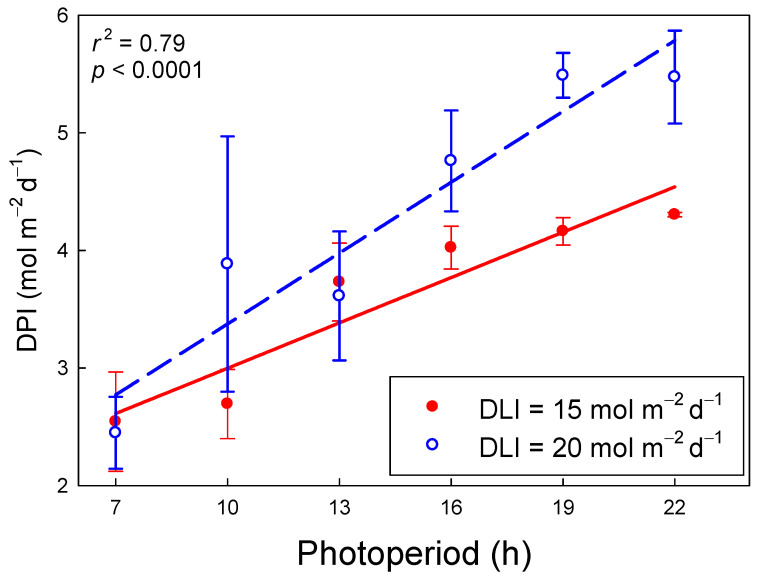
The DPI increased linearly with longer photoperiods, but this increase depended on the DLI. As the photoperiod increased from 7 to 22 h, DPI increased from 2.6 to 4.5 mol m−2 d−1 (74% increase) and from 2.8 to 5.8 mol m−2 d−1 (109% increase) for DLIs of 15 and 20 mol m−2 d−1, respectively (r2 = 079, p < 0.001; Figure 4). With a 7-h photoperiod, the DPI under the two DLIs was similar, but as the photoperiod got longer, differences in DPI among the two DLIs increased. Under a 22-h photoperiod, a DLI of 20 mol m−2 d−1 resulted in a 27% greater DPI than a DLI of 15 mol m−2 d−1. The effect of photoperiod on DPI was greater for a DLI of 20 mol m−2 d−1 because of the wider range of measurement PPFDs (253–794 µmol m−2 s−1) and thus ΦPSII values (0.28–0.64), compared to the PPFDs (189–595 µmol m−2 s−1) and ΦPSII values (0.39–0.68) for a DLI of 15 mol m−2 d−1 (Figure 3).
Figure 4.
The daily photochemical integral (DPI) of lettuce (Lactuca sativa ‘Green Towers’) as a function of photoperiod and daily light integral (DLI) [DPI = 1.508 + (0.00953 × photoperiod × DLI)]. Plants measured under longer photoperiods, or under the same photoperiod but at a lower DLI, received a lower photosynthetic photon flux density (PPFD) (Table 1). There was a significant interaction (p < 0.001) between DLI and photoperiod as indicated by the different slopes of the regression lines.
3. Discussion
3.1. Time Course of ΦPSII and ETR
It took approximately 1 h for ΦPSII and ETR to stabilize regardless of photoperiod and PPFD (Figure 1 and Figure 2). Plants typically reach steady-state photosynthesis after 5 to 10 min in the light [13]. By comparison, our data suggest it takes longer for ΦPSII and ETR to reach a steady state. The longer time required for photochemistry to stabilize is likely due to complex regulation of the light reactions, which involves multiple processes at different time scales. First, when dark-adapted plants with fully open reaction centers are exposed to light, there is a rapid (≈1 s) increase in chlorophyll fluorescence (the Kautsky effect). This increase in fluorescence, and decrease in ΦPSII, occurs because of a reduction of the electron acceptor QA, resulting in temporary closure of some of the PSII reaction centers [4]. In response to continued light exposure, plants upregulate heat dissipation [14]. This results in non-photochemical quenching of chlorophyll fluorescence and less excitation energy being directed towards the PSII reaction centers, with plants typically reaching a steady-state within 15 to 20 min, although this is species-dependent [4].
Photochemistry produces adenosine triphosphate (ATP) and reduced compounds (ferredoxin, nicotinamide adenine dinucleotide phosphate—NADPH) that are used in multiple metabolic processes. Because levels of ATP and NADPH are relatively stable in light-exposed leaves, photochemistry needs to be in balance with the usage of ATP, ferredoxin, and NADPH [15]. Photochemistry not only supports carbon fixation in the Calvin-Benson-Bassham (CBB) cycle, but also processes such as photorespiration [16], nitrate [17] and sulfate reduction [18], and the Mehler reaction [19]. Steady-state electron transport cannot be reached until all these processes have reached steady state, which requires time. For example, regulation of nitrate reduction depends on both CBB cycle activity and photochemistry. Carbohydrate accumulation, resulting from CBB cycle activity, induces upregulation of nitrate reductase mRNA transcript levels [20], presumably followed by increased levels of nitrate reductase. In turn, nitrate reductase activity is regulated by NADPH [20]. Thus, both the amount and activity of nitrate reductase are directly or indirectly dependent on photochemistry. The interplay among the various processes that depend on the light reactions may explain the relatively slow stabilization of photochemistry.
Differences in ΦPSII were largely due to upregulation of energy dissipation in response to higher PPFD, as indicated by differences in ΦNPQ and NPQ among treatments; differences in ΦNO among treatments were small (Figure 1 and Figure 2). Similar effects of short-term changes in PPFD have been reported for tobacco: ΦNO was ≈0.25 and changed little, while ΦNPQ increased from 0 to ≈0.6 in response to PPFD increasing from 0 to 900 µmol m−2 s−1 [21]. This raises the question whether it may be possible to reduce the increase in ΦNPQ (and thus NPQ) in response to increasing PPFD (see Section 3.5).
3.2. Φ PSII and ETR as a Function of PPFD
The ΦPSII decreased linearly as PPFD increased (Figure 3), which is consistent with previous studies. Weaver and van Iersel [7] reported that the ΦPSII of ‘Green Towers’ lettuce grown and measured in a greenhouse under natural light decreased exponentially as PPFD increased from 0 to ≈1500 µmol m−2 s−1. These plants were exposed to a wider range of PPFDs than those in the current study, which may explain the differences in trends, linear versus exponential, between the two studies. When comparing their data across the range of PPFDs used in this study (189 to 794 µmol m−2 s−1), they too saw a roughly linear decline in ΦPSII from ≈0.6 to ≈0.3; further declines in ΦPSII at higher PPFDs were small, with a ΦPSII of ≈0.22 at a PPFD of 1500 µmol m−2 s−1 [7]. Zhen and van Iersel [10] found that the ΦPSII of sweet potato (Ipomea batatas), lettuce, and pothos (Epipremnum aureum), grown in a greenhouse but measured in a growth chamber under precisely-controlled PPFDs, decreased exponentially with increasing PPFD, and the rate of decrease was species-dependent. In addition to differences among species, the greenhouse light levels strongly impacted ΦPSII; plants that were grown under shade showed a more rapid decrease in ΦPSII with increasing PPFD. This indicates that the photochemistry of plants can acclimate to different lighting conditions.
Two processes contribute to the decreasing ΦPSII with increasing PPFD. First, in response to increased light, a larger fraction of PSII reaction centers will be in a closed state and unable to accept additional excitation energy [4,5]. Second, in response to increased light, plants upregulate photoprotective processes, which result in the dissipation of absorbed light energy in the form of heat. Although the exact mechanisms of heat dissipation are still under debate, they depend on a pH gradient across the thylakoid membrane and may involve the xanthophyll cycle and PsbS protein [14,22,23,24].
The asymptotic increase in ETR with increasing PPFD (Figure 3) also is consistent with previous findings. Weaver and van Iersel [7] likewise found that ETR of ‘Green Towers’ lettuce responded to PPFD with an exponential rise to a maximum but reported a higher asymptote (121 µmol m−2 s−1). Zhen and van Iersel [10] found that the asymptote of the ETR curve depended on the light intensity plants received prior to the measurements, with plants grown in shade having lower ETR asymptotes than those grown in full sun. Different growing conditions and the ability of plants to acclimate to different light conditions may explain the difference in asymptotes between the current study and that reported by Weaver and van Iersel [7]. Plants in this study were grown in a growth chamber with constant PPFD of ≈250 μmol m−2 s−1 and a DLI of 12.6 mol m−2 d−1, while Weaver and van Iersel [7] grew plants in a greenhouse with an average DLI of 13.9 mol m−2 d−1 and much higher peak PPFDs. It is plausible that those greenhouse-grown plants had a greater photochemical capacity. It is important to note that all plants in our study were grown under the same lighting conditions, then transferred, for one day, to another set of lighting conditions for measurement. Therefore, plants in this study were not able to acclimate to the measurement lighting conditions.
3.3. Daily Photochemical Integral
The increase in DPI with longer photoperiods, with lower PPFDs and equal DLI, was expected, because of the increase in ΦPSII with lower PPFD. Since DPI is a relatively new concept, first introduced by Weaver and van Iersel [7], there have not been prior reports of photoperiod effects on DPI. Under sunlight only, with plants exposed to natural diurnal fluctuations in PPFD and a photoperiod of ≈12 h, the DPI at a DLI of 15 mol m−2 d−1 was 2.75 mol m−2 d−1 and at a DLI of 20 mol m−2 d−1 was 3.0 mol m−2 d−1, only 9% higher, despite a 33% increase in DLI [7]. Based on our regression analysis, we would expect DPIs of 3.2 and 4.3 mol m−2 d−1 with a 12-h photoperiod and DLIs of 15 and 20 mol m−2 d−1, respectively. The higher DPIs in our study are at least partly the result of maintaining a stable DLI throughout the photoperiod. Because of the asymptotic relationship between PPFD and ETR, maintaining a constant PPFD results in a higher DPI than variable light levels, assuming the DLI is the same [7]. At a DLI of 17 mol m−2 d−1, a 12-h photoperiod, and constant PPFD, Weaver and van Iersel [7] estimated the DPI of lettuce to be 3.5 mol m−2 d−1, consistent with our findings. Weaver and van Iersel [7] also simulated the effect of different photoperiods, all with constant PPFD on DPI. Based on these simulations, using a DLI of 17 mol m−2 d−1, the DPI is expected to increase from ≈2.8 to 4.4 mol m−2 d−1, (57%) as the photoperiod increases from 8 to 22 h. We observed substantially greater increases in DPI with longer photoperiods (74 and 109% at DLIs of 15 and 20 mol m−2 d−1). The greater increase in the current study may be the result of the constant PPFD during the ΦPSII and ETR measurements. Fluctuating PPFD levels require continuous changes in regulation of xanthophyll cycle pigments and the interconversion of violaxanthin and zeaxanthin occurs relatively slowly, over several minutes. Under fluctuating light levels, this can negatively impact electron transport and photosynthesis following a decrease in PPFD [25].
We found a substantially greater increase in DPI with longer photoperiods at a DLI of 20 mol m−2 d−1, compared a DLI of 15 mol m−2 d−1. As a matter of fact, at a 7-h photoperiod, the DPI was similar with both DLIs, a result of the response of ΦPSII to PPFD; the 33% higher PPFD (794 vs. 595 μmol m−2 s−1) with the 7-h photoperiod was offset by a 27% lower ΦPSII (0.28 vs. 0.39 at DLIs of 20 and 15 mol m−2 d−1, respectively). However, with a 22-h photoperiod, ΦPSII at a DLI of 20 mol m−2 d−1 (and PPFD of 252 μmol m−2 s−1) was only 5% lower than that at a DLI of 15 mol m−2 d−1 (and PPFD of 189 μmol m−2 s−1), despite the 33% greater PPFD.
3.4. Photoperiod and Crop Growth
Longer photoperiods with lower PPFD have been suggested as a strategy to increase photosynthetic efficiency and crop growth in controlled environments [26]. This study confirms that delivering the same DLI over longer photoperiods with lower PPFDs indeed results in greater DPIs in lettuce. Other studies have shown that longer photoperiods with lower PPFDs do translate into more growth. In a growth trial conducted in a growth chamber, Palmer [27] observed an 18% increase in lettuce shoot dry weight as photoperiod increased from 10 to 20 h with a DLI of 16 mol m−2 d−1. Weaver and van Iersel [8] found that for greenhouse-grown lettuce, dry weight increased by 28% when the photoperiod increased from 12 to 21 h, even though all plants received the same DLI (17 mol m−2 d−1). In a greenhouse trial with Rudbeckia fulgida var. sullivantii ‘Goldsturm,’ Elkins and van Iersel [28] determined that shoot and root dry weight increased linearly from 0.23 to 0.30 g/plant (30%) and 0.071 to 0.088 g/plant (24%), respectively, as photoperiods increased from 12 to 21 h, while maintaining a DLI of 12 mol m−2 d−1.
Much larger increases in DPI (74% to 109%) were observed in this study in response to longer photoperiods, as compared to growth responses (18% to 30%) in other studies. Plants in this study were grown under identical lighting conditions with relatively low PPFD, then transferred for one day to another set of lighting conditions for measurement. Therefore, our plants were not acclimated to the measurement PPFD. If, for example, plants that were measured at a high PPFD and short photoperiod also had been grown at high PPFD, acclimation to high PPFD likely would have resulted in greater photochemical capacity [10], resulting in higher ΦPSII, ETR, and DPIs. This would have made treatment differences smaller. Future work should study how acclimation affects DPI. Nonetheless, the combination of our findings with growth chamber [27] and greenhouse studies [8,28] indicates growth rates can be increased with longer photoperiods and lower PPFD.
3.5. Increasing ΦPSII, ETR, and DPI
Since ΦPSII decreases and ETR increases in response to increasing PPFD, there is an inherent tradeoff between efficient photochemistry and rapid electron transport. This is likely inevitable, because higher PPFD results in a larger fraction of closed PSII reaction centers and upregulation of photoprotective processes. The decrease in ΦPSII with increasing PPFD is largely caused by an upregulation of ΦNPQ (Figure 1). That raises the question of whether this upregulation of photoprotective processes can be reduced. Photoprotective processes are necessary to prevent excessive photoinhibition. Photoinhibition results from excess excitation energy directed towards reaction centers, and most commonly PSII. Excess excitation energy can lead to damage and degradation of the D1 protein in PSII [29]. Although plants can repair this damage, the repair cycle is slow, typically in the order of hours [24]. Photoinhibition results in a decrease of active PSII reaction centers, and thus, reduces the photochemical capacity of leaves. It is therefore not reasonable to suggest that photoprotective processes are not required. However, is it possible to reduce ΦNPQ without inducing excessive photoinhibition?
Plants have evolved under widely fluctuating light conditions, with those fluctuations coming over the course of minutes, hours, days, and seasons. As a result, plants evolved a wide range of photoprotective processes [29]. However, in controlled environmental agriculture it is possible to provide environmental conditions, including light, that are steady and near-optimal for crop growth. Many cultivars grown in controlled environment agriculture were bred for field production, where crops need to yield well under a wide range of environmental conditions; thus, desirable genotypes need to have enough plasticity to achieve phenotypic stability in a range of environments [30]. However, in controlled environment agriculture, the environment can be controlled, and breeding efforts can focus on developing genotypes optimized for that specific environment.
Desirable traits for plants grown in controlled environment agriculture include fast growth, preferably under relatively low PPFD, and high levels of desirable secondary compounds. To achieve fast growth, plants need to form a relatively large canopy quickly so they can intercept the provided light efficiently, and they need to be able to use that provided light efficiently for the production of biomass. Selecting for high ΦPSII seems like a logical target in the breeding of cultivars that can grow well under relatively low light.
Interestingly, the importance of ΦPSII in determining how efficiently plants use light energy to produce biomass is commonly overlooked. Multiple review articles have described the processes involved in the light use efficiency of biomass production in detail, but without addressing the importance of ΦPSII [31,32]. Since energy losses associated with the decrease in ΦPSII in response to increasing PPFD can be large, methods to increase ΦPSII deserve more attention. Such high ΦPSII could potentially be achieved in different ways. One option would be to breed cultivars with a high ratio of photosynthetic reaction centers relative to the size of the light-harvesting antennae. This would increase the overall capacity for electron transport, while reducing the amount of excitation energy directed towards individual reaction centers. Such an approach has been effective in the micro-algae Dunaliella salina [33]. This could potentially be coupled with breeding efforts to downregulate light-induced energy dissipation, allowing a larger fraction of the absorbed photons to be used for electron transport. In field-grown tobacco (Nicotiana tabacum), transforming plants to respond to fluctuating light levels more quickly by speeding up the conversion between violaxanthin and zeaxanthin and overexpressing PsbS increased CO2 assimilation and dry matter production by 15% [25]. This same approach is not likely to benefit plants in plant factories where light levels do not fluctuate, but it indicates that photoprotective mechanisms in crops may not be optimal for maximum growth. Cultivars developed for controlled environments are more likely to benefit from sustained low levels of heat dissipation, coupled with a large capacity for photochemistry. The response of ΦPSII and ΦNPQ are not pre-determined properties of plants; there are large differences among species and plants have the ability to acclimate to their light environment [10]. We have also seen variability among cultivars of the same species (unpublished results); this indicates that there is unexplored genetic variability that can be used as the basis for breeding to increase ΦPSII, ETR, and DPI.
3.6. Using DPI to Optimize Lighting Strategies
Lighting recommendations for greenhouse crops are often based on DLI [34,35], regardless of how that DLI is provided. However, crop light use efficiency and growth are affected not only by DLI, but also by how that light is provided over the course of the photoperiod. To account for this, newer lighting control approaches can account for the physiological responses of the crop, such as photosynthesis. For example, the ‘DynaLight Desktop’ control system for high-pressure sodium lights accounts for weather forecasts and variable electricity prices, with the goal of achieving a specific daily photosynthesis integral (similar to DPI, but based on CO2 assimilation per unit leaf area, rather than electron transport) [36,37]. This approach resulted in similar crop carbon gain as lighting control based on a PPFD threshold, but with 25% less electricity use. However, under short natural photoperiods, flowering of the long-day crops Campanula portenschlagiana ‘Blue Get Mee’ and Campanula cochlearifolia ‘Blue Wonder’ was poor and erratic. Thus, control algorithms should also account for photoperiodic flowering responses of crops [38]. The availability of dimmable LED fixtures provides new opportunities for lighting control systems. Taking advantage of the ability of these lights to respond to ambient sunlight conditions in real-time, the lighting strategy can be optimized to achieve a target DPI in a specific photoperiod. When electricity prices do not vary, such a control strategy would result in maintaining a constant PPFD throughout the photoperiod. This was estimated to result in a 7–10% reduction in electricity use, as compared to turning lights on and off based on a PPFD threshold [39]. This approach can be further optimized by including sunlight predictions and the ability to account for variable electricity prices [40].
3.7. Conclusions
To our knowledge, this is the first study to quantify DPI over different photoperiods with the same DLI. Lettuce DPI greatly increased with longer photoperiods and lower PPFDs because plants exhibited decreased ΦPSII with increasing PPFD. The short-term physiological responses found in this study are consistent with results from longer-term growth chamber and greenhouse studies where growth was measured [8,28]. This has practical implications for controlled environment agriculture. Longer photoperiods with lower PPFD can increase growth and reduce capital expenses, because fewer light fixtures would need to be installed to provide the appropriate PPFD and DLI. The relationships between PPFD, photoperiod, DLI, DPI, and electricity prices should be taken into account in the development of better lighting control algorithms to assure that lighting is provided to optimize crop growth with the least amount of supplemental light or electricity costs possible. There also appear to be unexplored possibilities to breed cultivars that are better adapted to controlled environment agriculture conditions.
4. Materials and Methods
4.1. Plant Material
Lettuce (Lactuca sativa ‘Green Towers’) seeds were sown in 15-cm diameter round pots filled with a soilless growing medium (Fafard 3B; SunGro Horticulture, Agawam, MA, USA). The seeds were germinated indoors on an ebb-and-flow bench under white light-emitting diode (LED) arrays (Fat Jeff; Aurora, St. Petersburg, FL, USA) with a PPFD of 230 μmol m−2 s−1 plus 10 μmol m−2 s−1 of far-red light (700–800 nm). They were fertigated, as needed, with 100 mg L−1 N water-soluble fertilizer solution (15N–2.2P–12.5K, Peters Excel 15–5–15 Cal-Mag Special; ICL Fertilizers, Dublin, OH, USA). After 10 d, the plants were thinned to one plant per pot, moved to a growth chamber (E15; Conviron, Winnipeg, Manitoba, Canada), and grown under cool-white fluorescent light with a PPFD of ≈250 μmol m−2 s−1, a 14-h photoperiod, ambient CO2, ≈37% relative humidity, and a constant air temperature of 22.7 °C. Plants were watered using the same fertilizer solution. New seeds were sown every 5 d to maintain a steady supply of plants of similar age. Thirty-six different lettuce plants were used during the study period from 16 January to 6 February 2018.
4.2. Experimental Setup
Two DLIs (15 and 20 mol m−2 d−1) were each applied across six photoperiod treatments (7, 10, 13, 16, 19, and 22 h) to evaluate the effects on ΦPSII, ETR, and DPI (Table 1). To measure these parameters, individual plants were moved daily into a second growth chamber (E15, Conviron) that was divided into two separate sections with identical measurement setups. Chlorophyll fluorescence measurements were collected using a modified version of the system described in van Iersel et al. [41]. Each section of the growth chamber was lit using custom-made LED arrays (PhytoSynthetix, Boulder, CO, USA), with the spectral distribution shown in Figure 5. The fixtures were powered by dimmable drivers. The drivers in each section were connected to separate dataloggers (CR1000; Campbell Scientific, Logan, UT, USA). A quantum sensor (LI-190; LI-COR Biosciences, Lincoln, NE, USA) and a chlorophyll fluorometer (MiniPam; Heinz Walz, Effeltrich, Germany) with a leaf clip were placed in each section, and the analog outputs from each were recorded by the datalogger. The datalogger was also connected to the serial communication port of the fluorometer, allowing the datalogger to trigger fluorometer measurements. The PPFDs required to reach the target DLI ranged from 189 to 794 µmol m−2 s−1, depending on the DLI and photoperiod. To control the PPFD, we programmed the target DLI and photoperiods into the datalogger program before each test. From that information, the datalogger calculated the required PPFD (DLI divided by photoperiod in seconds). Then, based on readings from the quantum sensor, the datalogger sent a voltage signal to the dimmable driver, using an analog output module (SDM-AO4A; Campbell Scientific) to adjust the output from the LED fixtures to achieve the required PPFD. With this setup, we could achieve precise control over the PPFD and DLI; the standard deviation of the mean PPFD for all 36 tests averaged 0.1 µmol m−2 s−1, while the DLI in each run was within 0.001 mol m−2 d−1 of the target DLI.
Figure 5.
Normalized photon flux density (PFD) of the custom-made light-emitting diode (LED) arrays used for photochemical measurements.
Two mature plants, approximately four weeks old, were selected each day data were collected. One plant was placed in each section and a fully expanded, uppermost leaf was chosen for chlorophyll fluorescence measurements. The leaf clip was attached to the leaf and the plant was kept in the dark for a minimum of 30 min to adapt to the dark, after which the dark-adapted steady-state (Fo) and maximum fluorescence (Fm) were measured. After the lights came on, steady-state (Ft) and maximum (Fm’) fluorescence were measured every 15 min using the approach described by van Iersel et al. [41] throughout the entire photoperiod. Immediately after Fm’ was measured, the actinic light was turned off and far-red LEDs (peak at 735 nm) were turned on to oxidize the plastoquinone pool and open all PSII reaction centers, after which F0′ was measured. Using Fm, Fm’, and Ft, ΦPSII, NPQ, and ETR were calculated [4]. The ETR was calculated assuming a leaf absorptance of 0.84 [12]. Both ΦNO and ΦNPQ were calculated assuming a lake model organization of light harvesting antennae and reaction centers [21]. During the measurement period, air temperature was 22.8 ± 0.2 °C (mean ± sd), vapor pressure deficit was 1.7 ± 0.2 kPa, and CO2 level was ambient inside the growth chamber.
4.3. Experimental Design and Data Analysis
The six photoperiods were randomized between days. Once the photoperiod for a specific day was determined, the DLIs of 15 and 20 mol m−2 d−1 were randomly assigned to the two sections of the growth chamber. Each treatment combination (DLI × photoperiod) was repeated three times. SigmaPlot (version 11.0; Systat Software, San Jose, CA) was used to analyze all the data. The effect of PPFD on ΦPSII and ETR were tested using linear and asymptotic rise to a maximum regression analyses [7]. To account for potential effects of DLI on the relationship between PPFD and ΦPSII or ETR, the DLI was included in the initial regression model as well, but was not significant.
The effect of photoperiod and DLI on DPI was tested using multiple regression, with photoperiod, DLI, and their interaction included in the initial model. Non-significant effects (main effects of photoperiod and DLI) were then removed using backward selection (α = 0.05).
Acknowledgments
We thank Michael Martin for his technical assistance with data collection and PhytoSynthetix, LLC for donating the light fixtures.
Author Contributions
Conceptualization, M.W.v.I.; methodology, C.E. and M.W.v.I.; formal analysis, C.E. and M.W.v.I.; investigation, C.E. and M.W.v.I.; data curation, C.E; writing—original draft preparation, C.E.; writing—review and editing, C.E. and M.W.v.I.; funding acquisition, M.W.v.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by USDA-NIFA-SCRI Award Number 2018-51181-28365 Project ‘Lighting Approaches to Maximize Profits.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Stober K., Lee K., Yamada M., Pattison M. Energy Savings Potential of SSL in Horticultural Applications. U. S. Dept. Energy; Washington, DC, USA: 2017. [Google Scholar]
- 2.Barber J., Andersson B. Too much of a good thing: Light can be bad for photosynthesis. Trends Biochem. Sci. 1992;17:61–66. doi: 10.1016/0968-0004(92)90503-2. [DOI] [PubMed] [Google Scholar]
- 3.Fankhauser C., Batschauer A. Shadow on the plant: A strategy to exit. Cell. 2016;164:15–17. doi: 10.1016/j.cell.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell K., Johnson G.N. Chlorophyll fluorescence: A practical guide. J. Expt. Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 5.Baker N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- 6.Genty B., Briantais J., Baker N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta. 1989;990:87–92. doi: 10.1016/S0304-4165(89)80016-9. [DOI] [Google Scholar]
- 7.Weaver G., van Iersel M.W. Photochemical characterization of greenhouse-grown lettuce (Lactuca sativa L. ‘Green Towers’) with applications for supplemental lighting control. HortScience. 2019;54:317–322. doi: 10.21273/HORTSCI13553-18. [DOI] [Google Scholar]
- 8.Weaver G., van Iersel M.W. Longer photoperiods with adaptive lighting control can improve growth of greenhouse-grown ‘Little Gem’ lettuce (Lactuca sativa) HortScience. 2020;55:573–580. doi: 10.21273/HORTSCI14721-19. [DOI] [Google Scholar]
- 9.Zou J., Zhang Y., Zhang Y., Bian Z., Fanourakis D., Yang Q., Li T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hort. 2019;257:108725. doi: 10.1016/j.scienta.2019.108725. [DOI] [Google Scholar]
- 10.Zhen S., van Iersel M.W. Photochemical acclimation of three contrasting species to different light levels: Implications for optimizing supplemental lighting. J. Am. Soc. Hort. Sci. 2017;142:346–354. doi: 10.21273/JASHS04188-17. [DOI] [Google Scholar]
- 11.Baker N.R., Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Expt. Bot. 2004;55:1607–1621. doi: 10.1093/jxb/erh196. [DOI] [PubMed] [Google Scholar]
- 12.Björkman O., Demmig B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta. 1987;170:489–504. doi: 10.1007/BF00402983. [DOI] [PubMed] [Google Scholar]
- 13.Kalaji H.M., Schansker G., Ladle R.J., Goltsev V., Bosa K., Allakhverdiev S.I., Brestic M., Bussotti F., Calatayud A., Dabrowski P., et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosyn. Res. 2014;122:121–158. doi: 10.1007/s11120-014-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demmig-Adams B., Cohu C.M., Muller O., Adams W.W. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosyn. Res. 2012;113:75–88. doi: 10.1007/s11120-012-9761-6. [DOI] [PubMed] [Google Scholar]
- 15.Geiger D.R., Servaites J.C. Diurnal regulation of photosynthetic carbon metabolism in C3 plants. Annu. Rev. Plant Biol. 1994;45:235–256. doi: 10.1146/annurev.pp.45.060194.001315. [DOI] [Google Scholar]
- 16.Krall J.P., Edwards G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 1992;86:180–187. doi: 10.1111/j.1399-3054.1992.tb01328.x. [DOI] [Google Scholar]
- 17.Tischner R. Nitrate uptake and reduction in higher and lower plants. Plant Cell Environ. 2000;23:1005–1024. doi: 10.1046/j.1365-3040.2000.00595.x. [DOI] [Google Scholar]
- 18.Takahashi H., Kopriva S., Giordano M., Saito K., Hell R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011;62:157–184. doi: 10.1146/annurev-arplant-042110-103921. [DOI] [PubMed] [Google Scholar]
- 19.Polle A. Mehler reaction: Friend or foe in photosynthesis? Bot. Acta. 1996;109:84–89. doi: 10.1111/j.1438-8677.1996.tb00546.x. [DOI] [Google Scholar]
- 20.Lillo C. Light regulation of nitrate reductase in green leaves of higher plants. Physiol. Plant. 1994;90:616–620. doi: 10.1111/j.1399-3054.1994.tb08822.x. [DOI] [Google Scholar]
- 21.Kramer D.M., Johnson G., Kiirats O., Edwards G.E. New fluorescence parameters for the determination of Q(A) redox state and excitation energy fluxes. Photosynth. Res. 2004;79:209–218. doi: 10.1023/B:PRES.0000015391.99477.0d. [DOI] [PubMed] [Google Scholar]
- 22.Horton P. Optimization of light harvesting and photoprotection: Molecular mechanisms and physiological consequences. Philos. Trans. R. Soc. B. 2012;367:3455–3465. doi: 10.1098/rstb.2012.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murchie E.H., Lawson T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Expt. Bot. 2013;64:3983–3998. doi: 10.1093/jxb/ert208. [DOI] [PubMed] [Google Scholar]
- 24.Ruban A.V. Quantifying the efficiency of photoprotection. Philos. Trans. R. Soc. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kromdijk J., Glowacka K., Leonelli L., Gabilly S.T., Iwai M., Niyogi K.K., Long S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science. 2016;354:857–861. doi: 10.1126/science.aai8878. [DOI] [PubMed] [Google Scholar]
- 26.van Iersel M.W. Optimizing LED lighting in controlled environment agriculture. In: Gupta S.D., editor. Light Emitting Diodes for Agriculture: Smart Lighting. Springer; Singapore: 2017. pp. 59–80. [DOI] [Google Scholar]
- 27.Palmer S. Effects of different photoperiods with constant daily light integral on growth and photosynthesis of mizuna, lettuce, and basil. HortScience. 2018;53:S247. [Google Scholar]
- 28.Elkins C., van Iersel M.W. Longer photoperiods with the same daily light integral improve growth of Rudbeckia seedlings in a greenhouse. HortScience. 2020 doi: 10.21273/HORTSCI14902-20. in press. [DOI] [Google Scholar]
- 29.Ruban A.V. Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015;66:7–23. doi: 10.1093/jxb/eru400. [DOI] [PubMed] [Google Scholar]
- 30.Folta K.M. Breeding new varieties for controlled environments. Plant Biol. 2019;21(Suppl. S1):6–12. doi: 10.1111/plb.12914. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X.-G., Long S.P., Ort D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008;19:153–159. doi: 10.1016/j.copbio.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Amthor J.S. From sunlight to phytomass: On the potential efficiency of converting solar radiation to phyto-energy. New Phytol. 2010;188:939–959. doi: 10.1111/j.1469-8137.2010.03505.x. [DOI] [PubMed] [Google Scholar]
- 33.Neidhardt J., Benemann J.R., Zhang L., Melis A. Photosystem-II repair and chloroplast recovery from irradiance stress: Relationship between chronic photoinhibition, light-harvesting chlorophyll antenna size and photosynthetic productivity in Dunaliella salina (green algae) Photosynth. Res. 1998;56:175–184. doi: 10.1023/A:1006024827225. [DOI] [Google Scholar]
- 34.Torres A.P., Lopez R.G. Measuring Daily Light Integral in a Greenhouse. Purdue Extension; West Lafayette, IN, USA: 2010. p. 7. [Google Scholar]
- 35.Both A.J., Albright L.D., Langhans R.W., Reiser R.A., Vinzant B.G. Hydroponic lettuce production influenced by integrated supplemental light levels in a controlled environment agriculture facility: Experimental results. Acta Hortic. 1997;418:45–51. doi: 10.17660/ActaHortic.1997.418.5. [DOI] [Google Scholar]
- 36.Kjaer K.H., Ottosen C.O., Jorgensen B.N. Cost-efficient light control for production of two campanula species. Sci. Hortic. 2011;129:825–831. doi: 10.1016/j.scienta.2011.05.003. [DOI] [Google Scholar]
- 37.Sørensen H.K., Fanourakis D., Tsaniklidis G., Bouranis D., Nejad A.R., Ottosen C.-O. Using artificial lighting based on electricity price without a negative impact on growth, visual quality or stomatal closing response in Passiflora. Sci. Hort. 2020;267:109354. doi: 10.1016/j.scienta.2020.109354. [DOI] [Google Scholar]
- 38.Kjaer K.H., Ottosen C.O., Jorgensen B.N. Timing growth and development of Campanula by daily light integral and supplemental light level in a cost-efficient light control system. Sci. Hortic. 2012;143:189–196. doi: 10.1016/j.scienta.2012.06.026. [DOI] [Google Scholar]
- 39.Weaver G.M., van Iersel M.W., Velni J.M. A photochemistry-based method for optimising greenhouse supplemental light intensity. Biosyst. Eng. 2019;182:123–137. doi: 10.1016/j.biosystemseng.2019.03.008. [DOI] [Google Scholar]
- 40.Mosharafian S., Afzali S., Velni J.M., van Iersel M.W. Development and implementation of a new optimal supplemental lighting control strategy in greenhouses; Proceedings of the ASME 2020 Dynamic Systems and Control Conference; 5–7 October 2020; in press. [Google Scholar]
- 41.van Iersel M.W., Weaver G., Martin M.T., Ferrarezi R.S., Mattos E., Haidekker M. A chlorophyll fluorescence-based biofeedback system to control photosynthetic lighting in controlled environment agriculture. J. Am. Soc. Hort. Sci. 2016;141:169–176. doi: 10.21273/JASHS.141.2.169. [DOI] [Google Scholar]