Abstract
Huaier, a sandy beige mushroom with anti-tumor effects, has been applied into Traditional Chinese Medicine for more than 1600 years. Previous studies showed that Huaier exerted its anti-tumor effects not only by direct action on tumor cells, but also indirectly by modulation of immune function. In the present study, we found that Huaier treatment significantly repressed tumor growth in mice with 4T1 breast cancer and resulted in significant accumulation of CD4+ T cells and mature dendritic cells (DCs) in the tumor microenvironment. In vitro experiments demonstrated that Huaier treatment promoted both DC2.4 and bone marrow derived DCs (BMDCs) to express costimulatory molecules, enhance production of IL-1β and IL-12p70, while it inhibited their phagocytic activities, suggesting that Huaier treatment promotes maturation of DCs. Furthermore, we found Huaier-treated DCs profoundly stimulated proliferation of alloreactive CD4+ T cells and drove them to differentiate into Th1 subset. Expression of PI3K, Akt, p-Akt, JNK, and p-JNK was up-regulated, while p-p38 MAPK was down-regulated in Huaier-treated BMDCs, suggesting that Huaier promotes maturation of DCs with potent ability to activate Th1 immune response via modulation of MAPK and PI3K/Akt signaling pathways. Our findings provide further evidence for the mechanisms underlying the anti-tumor activity of Huaier.
Keywords: Huaier, dendritic cells, breast cancer, Th1, cell signaling pathway
Introduction
Trametes robiniophila Murr, also known as Huaier, is a sandy beige mushroom applied in Traditional Chinese Medicine (TCM) for more than 1600 years.1 A polysaccharide, which consists of 18 monosaccharides and 18 kinds of amino acids, is supposed to be the mainly active ingredient in Huaier.2 Using as a complementary medicine, Huaier has gained increased attention due to its antitumor and immunomodulatory functions. It has been shown that Huaier can inhibit cancer cell proliferation and viability,3 inhibit cancer stem cells growth,4,5 induce cancer cell apoptosis3,6-10 and autophagy,11,12 activate cancer cell NLRP3-dependent pyroptosis,13 repress tumor growth,6,14-16 interfere with tumor angiogenesis,6,14,17 decrease metastasis rate,14,18,19 and prolong survival time of tumor-bearing host.20-22 Huaier also exerts potent anti-tumor effect via immunomodulation,23 such as increasing the proportion of CD4+ T cells and NK cells,14 regulate the polarization of macrophages24 and their functions,15,18,21 increase immune-stimulating cytokine secretion.14
Dendritic cells (DCs) are the main component of professional antigen-presenting cells (APCs) and provide a link between the innate and adaptive immune system.25 DCs exist in all tissues, sensing pathogens and other danger signals, endocytosing the antigen, processing and presenting it to naïve T lymphocytes.26,27 The unique ability of DCs to initiate adaptive immune response is attributed to the secretion of several cytokines and the expression of various cell surface molecules, such as major histocompatibility complex (MHC), costimulatory, and adhesion molecules.28,29 Increasing lines of evidence show that DCs are essential immune cells in immunotherapy against cancer.30
Recently, an in vivo study has highlighted that combined treatment of Huaier and DC-CIK on nude mice is more potent in killing tumor cells.31 However, given that DCs exert inevitable functions in both innate and adaptive immune responses, whether Huaier extractum can regulate DCs function has not yet been elucidated. In this study we investigated the regulatory effects of Huaier on DCs and found that Huaier promotes DC maturation, favors DCs to induce Th1 immune response and results in potent anti-tumor activity.
Materials and Methods
Reagents
Huaier extractum was donated by Qidong Gaitianli Pharmaceutical Co., Ltd. (Jiangsu, China). RPMI 1640 was purchased from Gibco-BRL (Rockville, IN, USA). Fetal bovine serum (FBS) was purchased from Zhejiang Tianhang Biotechnology Co., Ltd. (Zhejiang, China). Recombinant murine GM-CSF and IL-4 were brought from Peprotech (Rocky Hill, NJ, USA). Lipopolysaccharide (LPS), fluorescein isothiocyanate (FITC)-dextran and carboxylate-modified red fluorescent latex beads were purchased from Sigma-Aldrich (St Louis, MO, USA). Mouse CD4 (L3T4) and CD11c MicroBeads were purchased from Miltenyibiotec (Bergisch Gladbach, Germany). Cell Counting Kit-8 was purchased from Dojindo Laboratory (Kumamoto, Japan).
Preparation of Huaier Extractum
Two grams of Huaier extractum was dissolved in 5 ml RPMI 1640. The solution was filtered through a sterilized 0.22 μm filter to get the 400 mg/ml stock solution and was stored at 4°C for short-term storage.
Cell Culture
DC2.4, a cell line of dendritic cells originated from C57BL/6 mouse, was provided by Professor Dajing Xia (Zhejiang University School of Public Health). Cells were cultured in RPMI 1640 medium containing 10% FBS at 37°C in a humidified incubator under 5% CO2.
Mice and Treatment of 4T1 Breast Cancer Murine Model
Female C57BL/6 (H-2b) mice and BALB/c (H-2d) mice aged 6 to 8 weeks were purchased from SLC Laboratory Animal Co., Ltd. (Shanghai, China), and were housed in specific pathogen free conditions. For the in vivo experiment, female BALB/c mice were weighed and randomly divided into three groups (Control, H1 and H2, 5 mice in each group). 4T1 cells (1×106) were subcutaneously injected into the right armpit of each mouse. Seven days later, different treatments were given to each group. For group H1, 200 µl solution containing 25 mg Huaier extractum was given by gavage once a day. For group H2, the dose of Huaier extractum was raised to 50 mg/200 µl. For group Control, instead of Huaier solution, 200 µl water was given by gavage per day. The administration of gavage lasted for 21 days. Mice were sacrificed for the follow-up experiments. The largest diameter (LD) and the smallest diameter (SD) of tumors were measured. The tumor volume (TV) was calculated according to the previously reported formula14: TV (mm3) = (LD×SD2)/2.
Preparation of Bone Marrow Derived DCs
Bone marrow derived dendritic cells (BMDCs) were induced from mouse femur bone marrow cells as described elsewhere.32 Briefly, C57BL/6 mice derived erythrocyte-deleted marrow cells were seeded in six-well culture plates with RPMI 1640 supplemented with 10% FBS, GM-CSF (30 ng/ml) and IL-4 (10 ng/ml). Three days later, the non-adherent cells were removed with gentle washes and the adherent cells were replenished with RPMI 1640 supplemented with 10% FBS, GM-CSF (30 ng/ml) and IL-4 (10 ng/ml). On day 6, the non-adherent and loosely adherent cells were harvested and purified by CD11c MicroBeads according to the instructions of manufacturer.
Flow Cytometry Analysis
To analyze the immune cell components in the blood, spleen and tumor tissues, peripheral blood, spleens and tumors in 4T1 breast cancer-bearing mice were sampled. Single cell suspensions were made as previously reported.14 The cells were stained with Zombie Red Fixable Viability Kit (BioLegend, San Diego, California) to exclude dead cells. Live cells were then stained with the following fluorescein-conjugated monoclonal antibodies and analyzed by FACS: CD45-APC/Cy7, CD45-Pacific Blue, CD3-PE/Cy7, CD3-Percp/Cy5.5, CD4-PE, CD4-FITC, CD8-FITC, CD8-PE/Cy7 (BioLegend, San Diego, California). To determine the DCs in the 4T1 breast cancer microenvironment, single cells were stained with CD45-APC/Cy7, CD11c-FITC, CD40-PE/Cy7, CD54-APC, CD86-PE, MHC II-Percp/Cy5.5 (BioLegend, San Diego, California), and were analyzed on a BD FACSCanto II. To examine the in vitro influence of Huaier on maturation and function of DCs, 2×105 DCs (DC2.4 or BMDCs) were cultured in 24-well plates for 36 hours in the absence (group Control) or presence (group H) of 4 mg/ml Huaier. For group LPS, DCs were treated with 1 μg/ml LPS. The cells were harvested and used for subsequent experiments. For analysis of DC phenotype, the cells were stained with the indicated fluorescein-conjugated monoclonal antibodies (PE-conjugated anti-mouse CD40, CD54, CD86, MHC class I (H-2Kb), MHC class II (I-Ab), and FITC-conjugated anti-mouse CD80 (eBioscience, Thermo Fisher Scientific, Shanghai, China), and were analyzed on a FACS Calibur.
Quantitative Real-Time PCR
IL-1β and IL-12p40 mRNA expressions in DCs from the tumor microenvironment were analyzed by quantitative RT-PCR (qRT-PCR). Total RNA was extracted with Trizol reagent (Thermo Fisher Scientific), and cDNAs were synthesized using a cDNA synthesis kit (Takara) according to the manufacturer’s instructions. qRT-PCR was performed with a SYBR PCR kit (Takara) on an ABI 7500 Real-Time PCR Systems (Applied Biosystems). The data were normalized to the β-Actin expression level in each sample. Primer se-quences: Il-1β (F: 5’-GCAACTG TTCCTGAACTCA ACT-3’, R: 5’-ATCTTTTGGGGTCCGTCAACT-3’); Il-12p40 (F: 5’-GAGCACTCCCCATT CCTACTTCT-3’, R: 5’-CCCTCCTCTGTCTCCTTCATCTT-3’); β-Actin (F: 5’-AG TGTGACGTTGACATCCGT, R: 5’-GCAGCTCAGTA ACAGTCCGC-3’).
Analysis of DC Endocytosis
FITC-dextran and red fluorescent latex beads were used to determine the endocytosis ability of BMDCs, respectively. When red fluorescent latex beads were used, they were opsonized in 10% FBS for 30 minutes at 37°C and incubated with DCs from different groups at a 10:1 ratio for 3 hours at 37°C (4°C for negative control group).33,34 When FITC-dextran was used, however, 2×105 DCs per group were suspended in 1 ml RPMI 1640 supplemented with 1 mg/ml FITC-dextran and incubated at 37°C for 1 hour (4°C for negative control group).35 The fluorescence intensity of latex beads or dextran phagocytosed by DCs was determined by FACS.
Analysis for DCs to Stimulate CD4+ T Cell Proliferation
Capacity of DCs to stimulate T cell proliferation was analyzed by mixed leukocyte reaction (MLR). Briefly, BALB/c mice derived CD4+ T cells were prepared from spleen by using CD4 MicroBeads according to the manufacturer’s instruction. 1×105 CD4+ T cells were added to 96-well plates and co-incubated with 1×104 mitomycin C inactivated C57BL/6 mice derived BMDCs from different groups in a total volume of 200 μl for 96 hours under the condition of 5% CO2 at 37°C. The proliferation of CD4+ T cells was determined by CCK-8 kit according to the instruction of the manufacturer.
Analyses of Cytokine Secretions
The supernatants of different culture groups were collected and stored at −20°C for measurement of cytokine secretion levels. Concentrations of IL-1β, IL-4, IL-10, IL-12p70, and IFN-γ in the culture supernatants were measured by ELISA kits according to the instructions of the manufacturer. Commercial ELISA kits (eBioscience, Thermo Fisher Scientific, Shanghai, China) include IL-1 beta (Cat # 88-7013-22), IL-4 (Cat # 88-7044-88), IL-10 (Cat # 88-7105-22), IL-12p70 (Cat # 88-7121-88), and IFN-gamma (Cat # 88-7314-22) mouse ELISA Kit.
Western Blot Analyses
CD11c magnetic beads-purified C57BL/6 mice derived BMDCs (2×106 cells) were treated with 1 µg/ml LPS or 4 mg/ml Huaier extractum for 0, 15, 30, and 60 minutes, respectively. After treatment, DCs from different groups were harvested and lysed with lysis buffer. The lysates were centrifuged at 12,000× g for 20 minutes at 4°C, the supernatants were harvested and the protein concentrations were determined by BCA assay. Protein samples were fractionated on 12% Tris-glycine gels, followed by proteins transfer onto a PVDF membrane (Millipore, Bedford, MA, USA). The membranes were blocked with 5% non-fat dry milk and then probed with 1:500 to 1:1,000 diluted primary antibodies against the following mouse proteins (Cell Signaling Technology, Beverly, MA, USA), including Akt (Cat#4685), phosoho-Akt (Cat#4060), PI3K (Cat#4249), JNK (Cat#9252), phospho-JNK (Cat#4668), p38 (Cat#9212), phospho-p38 (Cat#4511), ERK (Cat#4695), phospho-ERK (Cat#4370), and GAPDH (Cat#51332), followed by HRP-labeled secondary antibodies at a 1:5,000 dilution. Antibody binding was visualized with a chemiluminescent substrate and visualized on autoradiography film.
Statistical Analysis
Data were presented as the mean ± standard deviation (SD). Comparisons within and across the groups were performed by Student’s t-test or ANOVA. The SPSS version 23.0 software and GraphPad Prism 6.0 software were used for statistical analysis. P < .05 was considered to be statistically significant.
Results
Huaier Significantly Suppressed the Tumor Progression and Improved the General Condition in Tumor-Bearing Mice
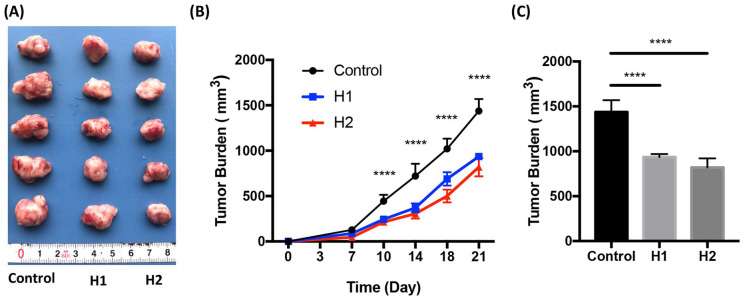
To examine the in vivo anti-tumor effect of Huaier extractum, the 4T1 breast cancer-bearing mice were treated for 21 days by gavage once a day with different doses of Huaier. Although there was no significant difference in tumor volumes between group H1 and group H2, the tumor volumes in the group H1 and H2 were significantly reduced when compared with that in the Control group (Figure 1A-C, P < .0001). Furthermore, mild hunched mouse stature and ruffled fur in tumor-bearing mice can be significantly ameliorated in the groups H1 and H2 10 days post Huaier treatment. These data suggested that Huaier treatment can suppress tumor growth and ameliorate the general conditions of breast cancer-bearing mice.
Figure 1.
Huaier inhibits tumor growth. 4T1 breast cancer-bearing mice were treated by gavage once a day for 21 days with different doses of Huaier. Tumor volumes were measured and analyzed. (A) Tumors from different groups; (B) Tumor growth tendency of each group; (C) The statistics of tumor burden in each group at day 21.
n = 5, ****P < .0001 compared with the H1 and H2.
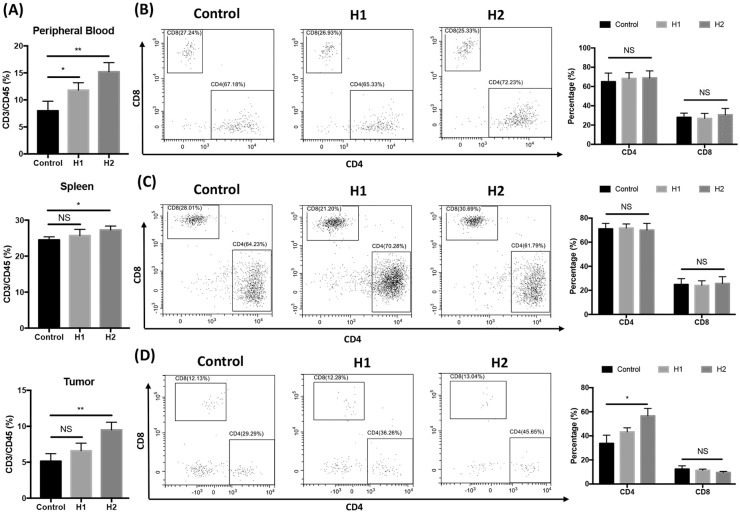
Huaier Treatment Resulted in CD4+ T Cells Accumulation in Tumor Microenvironment
Since CD4+ T cells participate in secreting multiple cytokines in the tumor microenvironment (TME) and CD8+ T cells have the direct cytotoxicity to tumor cells, both CD4+ T cells and CD8+ T cells are indispensable in anti-tumor effects.36 T cell subsets in peripheral blood, spleen and TME of 4T1 breast cancer-bearing mice were analyzed. CD3/CD45 percentages in peripheral blood, spleen and TME were significantly increased in group H2 (Figure 1A). Although proportions of both CD4+ T cells and CD8+ T cells were not profoundly affected in peripheral blood (Figure 2B) and spleen (Figure 2C), treatment with high dose of Huaier extractum (group H2) resulted in significant accumulation of CD4+ T cells, but not CD8+ T cells in TME (Figure 2D).
Figure 2.
Effects of Huaier on T cell subsets in different tissues of tumor-bearing mice. Percentages of CD3+ cells in peripheral blood, spleen and tumor of mice in different groups were shown (A). CD4+ and CD8+ T cell subsets in peripheral blood (B), spleen (C) and tumor microenvironment (D) of 4T1 breast cancer-bearing mice were analyzed by FACS. Representative FACS profiles in each group and the related statistical analyses are shown.
n = 5, *P < .05; **P < .01.
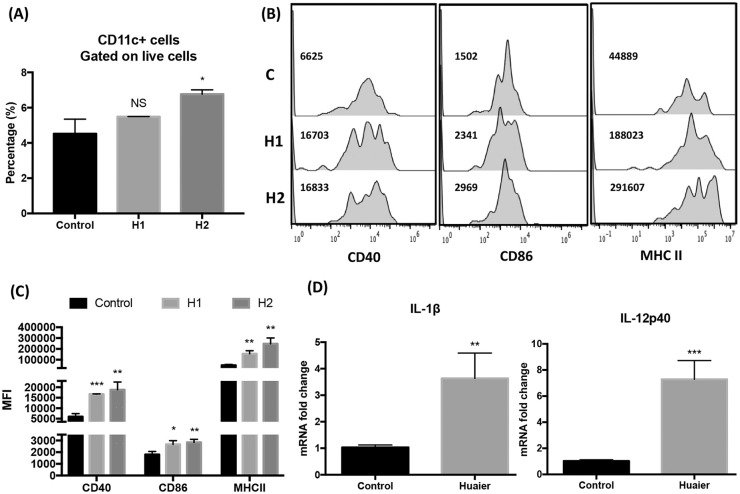
Huaier Extractum Promoted Accumulation of Mature Dendritic Cells in Breast Cancer Microenvironment
DCs are the important component of the immune system and have a unique ability to prime naïve T cells. They play a crucial role in the induction of anti-tumor immune response. To exert the priming effect, DCs undergo a maturation process, characterized by the up-regulation of surface markers, such as CD40, CD54, CD80, CD86 and the major histocompatibility complex (MHC) I and II,28 and by reducing phagocytosis.37,38 In order to examine the in vivo regulatory effects of Huaier on DCs, CD11c+ DCs in the 4T1 breast cancer microenvironment from different groups were analyzed for their expression of costimulatory molecules and MHC II as well as their mRNA expression of immunostimulatory cytokines IL-1β and IL-12. As shown in Figure 3, although significant increase in the percentage of CD11c+ cells can only be observed in group H2 (Figure 3A), DCs in breast cancer microenvironment from Huaier-treated groups expressed increased levels of costimulatory molecules, including CD40, CD86, and MHC II (Figure 3B and C), and mRNA of IL-1β and IL-12p40 (Figure 3D), when compared with those from the control group, suggesting that Huaier treatment induced mature DCs accumulation in the tumor microenvironment.
Figure 3.
Huaier treatment results in mature DC accumulation in 4T1 breast cancer microenvironment. (A) The percentage of CD11c+ DCs in TME from different groups; (B) FACS analyses of the expression of CD40, CD86, and MHC II of CD11c+ DCs in TME from different groups; (C) Statistical analyses of the mean fluorescence intensity in each group; (D) mRNA expression of IL-1β and IL-12p40 in CD11c+ DCs in TEM were analyzed by qRT-PCR.
n = 5, *P < .05, **P < .01, ***P < .001.
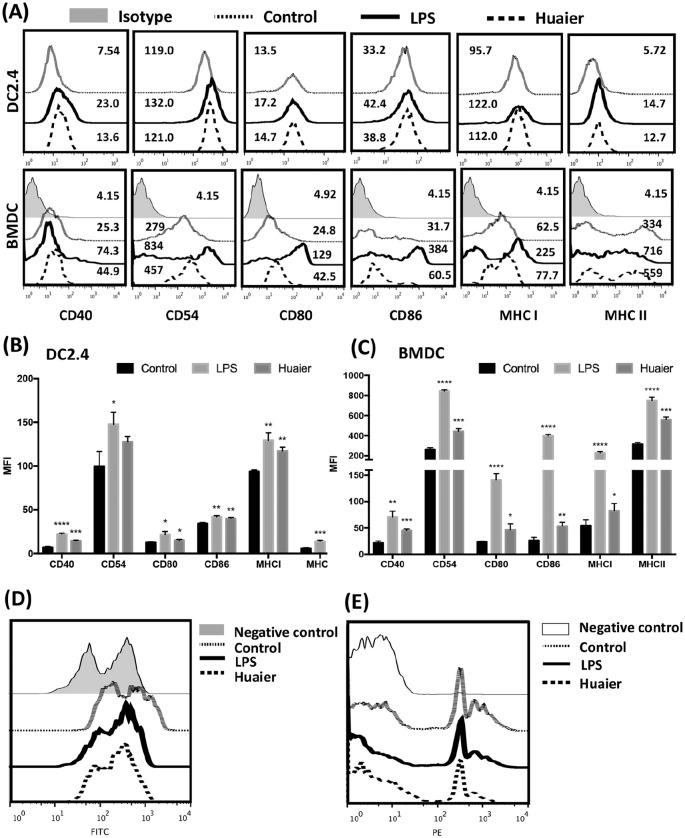
Huaier Favored Maturation of DCs In Vitro
Because in vivo experiments showed Huaier treatment promoted accumulation of mature DCs in TME, the in vitro regulatory effect of Huaier on DCs was examined. DC2.4 and BMDCs were treated with Huaier extractum and their expression of surface molecules was analyzed at first. As shown in Figure 4, Huaier up-regulated the expression of CD40, CD54, CD80, CD86, MHC I, and MHC II, leading to a mature phenotype of both DC2.4 and BMDCs (Figure 4A-C). Then, the influence of Huaier on phagocytic capacity of BMDC was examined. As shown in Figure 4D, DCs from control group retained the phagocytic capacity of FITC-dextran, while DCs treated with Huaier or LPS showed a significant decrease in phagocytic capacity. Moreover, when carboxylate-modified red fluorescent latex beads were used, the phagocytic capacity of Huaier treated DCs mirrored the same trend (Figure 4E). These data suggested that Huaier treatment promoted maturation of DCs in vitro.
Figure 4.
Huaier promotes maturation of both DC2.4 and BMDC. FACS analyses of CD40, CD54, CD80, CD86, MHC I, and MHC II expression in DC2.4 and BMDCs from different groups (A). Statistical analyses of costimulatory molecule expression in DC2.4 (B) and BMDC (C). Phagocytosis of FITC-dextran (D) and carboxylate-modified red fluorescent latex beads (E) by BMDCs from different groups.
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Huaier Enhanced Pro-Inflammatory Cytokines Secretion by DCs
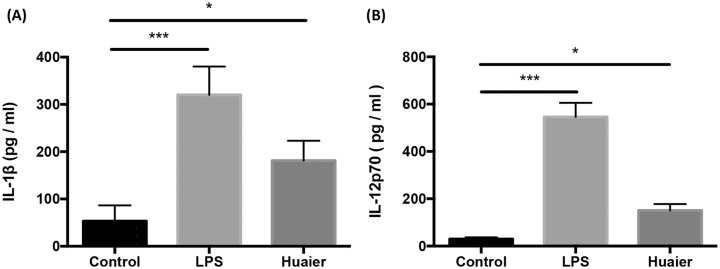
Previous studies have demonstrated that activated DCs produced a specific cytokine milieu participating actively in priming and polarizing naïve T cells.38,39 This cytokine milieu included IL-1, 2, 4, 6, 12, IFN-γ, TGF-β, and so on. Among all these cytokines, IL-1β and IL-12 played a central role in pro-inflammatory functions.40,41 Thus, we detected the secretion of IL-1β and IL-12p70 in Huaier treated BMDCs. In accordance with other reports,40,41 DCs in the group LPS significantly up-regulated the secretion of IL-1β and IL-12p70. However, DCs treated with Huaier also showed an increase in IL-1β and IL-12p70 secretion but milder compared to the LPS group (Figure 5A and B).
Figure 5.
Huaier enhances DCs to secrete IL-1β and IL-12p70. IL-1β (A) and IL-12p70 (B) secretion by BMDCs in different groups.
n = 3, *P < .05, ***P < .001.
Huaier-Treated DCs Biased to Drive Th1 Cell Differentiation
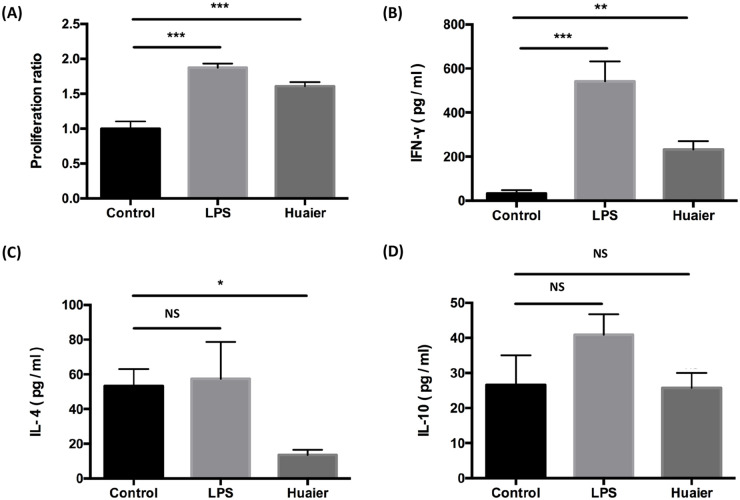
Since DC is the major source to prime naïve T cells, we speculated that Huaier promoted CD4+ T cell accumulation in TME might work, at least in part, via modulating DC functions. The capacity of DCs to stimulate CD4+ T cell proliferation was examined by MLR. As shown in Figure 6A, DCs from group LPS and group Huaier played an active role in CD4+ T cell proliferation. The proliferation ratio in group of Huaier was almost 1.6 times that in the control group (P < .001).
Figure 6.
Huaier enhances DCs to stimulate T cells proliferation and to induce Th1 polarization. (A) Proliferation of alloreactive CD4+ T cells co-cultured with BMDCs in different groups. Concentrations of IFN-γ (B), IL-4 (C) and IL-10 (D) in the supernatants of alloreactive CD4+ T cells co-cultured with BMDCs in different groups.
n = 3, NS: no significance, *P < .05, **P < .01, ***P < .001.
CD4+ T cells can differentiate into several lineages, such as Th1, Th2, Th17, and Treg.42 Th1 and Th2 clones are mainly discriminated by the secretion of cytokines.43 IFN-γ is the signature cytokine of Th1 cells,44 while Th2 cells mainly produce IL-4 and IL-10.45,46 Concentrations of IL-4, IL-10, and IFN-γ in the supernatants of T/DC co-culture system were detected by ELISA. Though not as drastic as the increase caused by DCs from LPS group, IFN-γ secretion was up-regulated in Huaier group (Figure 6B). Moreover, Huaier treated DCs suppressed the secretion of IL-4 (Figure 6C) but caused no significant change in IL-10 secretion (Figure 6D) when compared with the control DCs. These data suggested that Huaier-treated DCs significantly stimulate CD4+ T cell proliferation and favor naïve CD4+ T cells to differentiate into the Th1 subset.
Huaier Activated PI3K/Akt1 Pathway but Inhibited Phosphorylation of p38 MAPK in DCs
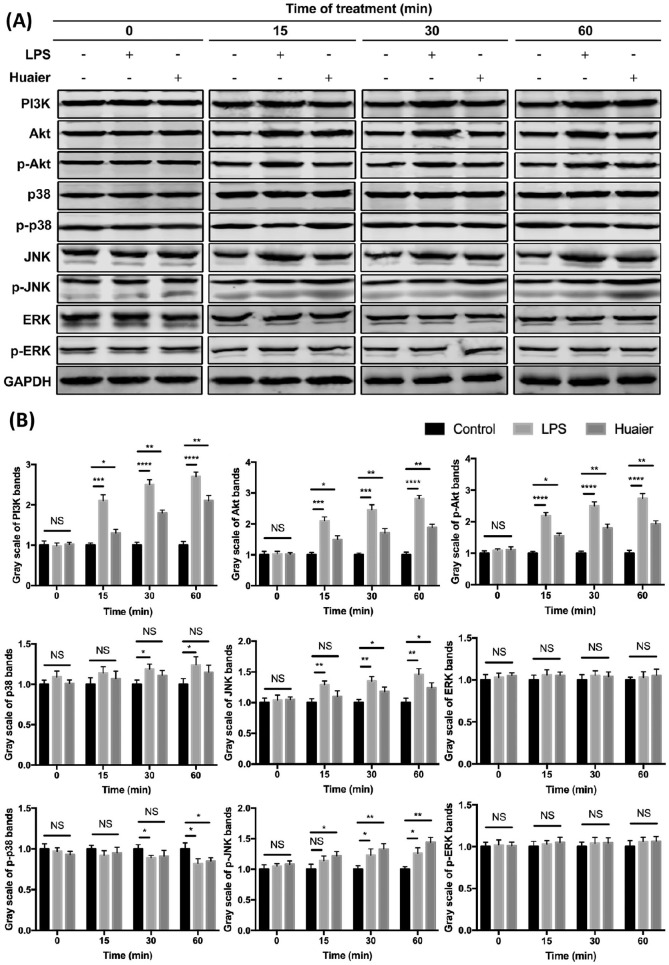
Previous studies showed that PI3K/Akt pathway is essential in DC survival and activation.47,48 The inhibition of PI3K or Akt expression resulted in DC apoptosis and the defect in immune stimulation.48 Moreover, it was also demonstrated that inhibition of p38 mitogen activated protein kinase (MAPK) during differentiation of DCs augmented the priming and activation of tumor-specific effector T cells,49 and the c-Jun NH2-terminal kinase (JNK) also played important role in the maturation of DCs, but activation of the extracellular signal-regulated protein kinases (ERK) in DCs functions more importantly in driving Th2 activation.50 Thus, protein levels of PI3K, Akt, p-Akt, p38, p-p38, JNK, p-JNK, ERK, and p-ERK in Huaier-treated DCs were examined. As shown in Figure 7, when compared with the control group, Huaier-treated DCs showed a significantly up-regulated expression of PI3K, Akt, and p-Akt; although total p38 protein was not affected, p-p38 was down-regulated in Huaier-treated DCs at 60 minutes; Huaier treatment also resulted in a mild increase in total JNK, and a profound increase in p-JNK; total ERK and p-ERK were not significantly affected by Huaier treatment. These data imply that Huaier not only favors DC survival and activation, but also enhances the ability of DCs to prime and activate T cells.
Figure 7.
Western blot analyses of signaling proteins in BMDCs from different groups. BMDCs were treated with 1 µg/ml LPS or 4 mg/ml Huaier extractum for 0, 15, 30, and 60 minutes. PI3K, Akt, p-Akt, p38, p-p38, JNK, p-JNK, ERK, p-ERK, and GAPDH expressions in treated BMDCs were analyzed by Western blot (A). Gray scale analyses of different protein bands were shown (B).
*P < .05, **P < .01, ***P < .001, ****P < .0001.
Discussion
Huaier is a kind of TCM that has been widely used in clinic for more than 1600 years. In general, Huaier can inhibit tumor proliferation, apoptosis, angiogenesis and metastasis.1,23 Clinical studies showed that Huaier could be used as an effective adjuvant in combined therapy for patients with hepatic carcinoma.20,51,52 Besides the direct anti-tumor effect, it can also promote the functions of several immune cells and modulate immune responses.14 Among the immune cells, DCs play an indispensable role in both the innate and the adaptive immunity because of their unique ability to prime naïve T cells and initiate the rapid and potent immune response toward infection and cancer. Immature DCs reside within peripheral non-lymphoid tissues where they actively capture and process antigen. Upon activation by antigen and other stimuli, DCs migrate from peripheral tissues, via afferent lymphatics, to secondary lymphoid tissues for antigen presentation. Maturing DCs undergo a rapid burst of cytokine synthesis and expression of costimulatory molecules, and relocate to the T cell zone of lymph nodes to scan, capture and prime antigen-specific naïve T cells, thereby activating T cells to mount a specific immune response.
Since Sipuleucel-T immunotherapy, a DC cancer vaccine, for prostate cancer has already achieved a considerable clinical effect,53 modalities that promote phenotypic and functional maturation of DCs are potentially effective immunotherapeutic strategies for treatment of cancers.
In the present study, we found, in accordance with other reports,6,14 that Huaier can significantly suppress tumor growth and ameliorate the general conditions of 4T1 breast cancer-bearing mice. Analyses of cellular constitution revealed that Huaier treatment resulted in CD4+ T cells and mature DCs accumulation in 4T1 breast cancer microenvironment, implying that the antitumor effects of Huaier might partly result from its modulatory effects on DCs. To verify this speculation, we explored the regulatory effects of Huaier on DCs in vitro. Our results demonstrated clearly that Huaier treatment resulted in phenotypic and functional maturation of both the DC cell line DC2.4 and BMDC, which was supported by the fact that Huaier treatment enhances DCs’ capability to express costimulatory molecules including CD40, CD54, CD80, CD86, and MHC II, and secrete IL-1β and IL-12p70 while it suppresses the phagocytic capacity of DCs. Most importantly, when co-cultured with alloreactive T cells, Huaier-treated BMDCs profoundly stimulate proliferation of T cells and drive T cells to differentiate into the Th1 subset as verified by the significantly increased production of IFN-γ and decreased secretion of IL-4 in the supernatants of T cells/Huaier-treated DCs co-culture.
It has been found that the MAPK signaling pathway is one of the most ancient signal transduction pathways which actively participates in all aspects of immune responses.54 There are three major groups in the MAPK family, p38, JNK and ERK. These three MAPKs regulate DC maturation and function differently.55 Inhibition of p38 MAPK during differentiation of DCs augmented the priming and activation of tumor-specific effector T cells and overcame regulatory T cell-mediated immunosuppression.49 Lines of evidence showed that tumor-derived suppressive factors could up-regulate p38 MAPK activity, thereby inhibiting the differentiation, maturation and functions of DCs56,57 and the p38 specific inhibitors SB202190 and SC203580 could restore the function of DCs in patients with multiple myeloma.56 JNK also plays important roles in DC maturation, but ERK activation in DC functions more importantly to induce Th2 activation.50 In our experiments, we showed that although total p38 MAPK, ERK and p-ERK remained unchanged, the p-p38 in Huaier-treated DCs was down-regulated when the cells were treated for 60 minutes. Meanwhile, significantly increased total JNK and p-JNK were observed. These data suggest that Huaier favors maturation of DCs with potent ability to activate Th1 immune response via modulation of MAPK signaling pathway.
Furthermore, it was reported that the PI3K/Akt pathway plays critical roles in DC survival and activation.47,48 Inhibition of PI3K or Akt expression resulted in DC apoptosis and a defect in immune stimulation.48 Our data showed that significantly up-regulated expression of PI3K, Akt, and p-Akt was observed in Huaier-treated DCs compared to the control group, suggesting that Huaier favors the survival and activation of DCs.
Taken together, the present study exploited systematically, for the first time, the regulatory effects of Huaier on the phenotypic and functional maturation of DCs, and found that Huaier promotes DCs maturation and favors them to induce Th1 immune response via modulation of MAPK and PI3K/Akt signaling pathways. Our findings provide further evidence to the mechanisms by which Huaier exerts its anti-tumor effects.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the grants from National Natural Science Foundation of China (No. 81872317, 81520108024).
ORCID iDs: Jun Pan  https://orcid.org/0000-0002-4031-5766
https://orcid.org/0000-0002-4031-5766
Jian Huang  https://orcid.org/0000-0003-3340-5007
https://orcid.org/0000-0003-3340-5007
References
- 1. Song X, Li Y, Zhang H, Yang Q. The anticancer effect of Huaier (Review). Oncol Rep. 2015;34(1):12-21. [DOI] [PubMed] [Google Scholar]
- 2. Luo Z, Hu X, Xiong H, et al. A polysaccharide from Huaier induced apoptosis in MCF-7 breast cancer cells via down-regulation of MTDH protein. Carbohydr Polym. 2016;151:1027-1033. [DOI] [PubMed] [Google Scholar]
- 3. Zhang N, Kong X, Yan S, Yuan C, Yang Q. Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci. 2010;101(11):2375-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang T, Wang K, Zhang J, et al. Huaier aqueous extract inhibits colorectal cancer stem cell growth partially via downregulation of the Wnt/beta-catenin pathway. Oncol Lett. 2013;5(4):1171-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang X, Zhang N, Huo Q, et al. Huaier aqueous extract inhibits stem-like characteristics of MCF7 breast cancer cells via inactivation of hedgehog pathway. Tumour Biol. 2014;35(11):10805-10813. [DOI] [PubMed] [Google Scholar]
- 6. Ren J, Zheng C, Feng G, et al. Inhibitory effect of extract of fungi of Huaier on hepatocellular carcinoma cells. J Huazhong Univ Sci Technolog Med Sci. 2009;29(2):198-201. [DOI] [PubMed] [Google Scholar]
- 7. Xu X, Wei Q, Wang K, et al. Anticancer effects of Huaier are associated with down-regulation of P53. Asian Pac J Cancer Prev. 2011;12(9):2251-2254. [PubMed] [Google Scholar]
- 8. Zhang F, Zhang Z, Liu Z. Effects of Huaier aqueous extract on proliferation and apoptosis in the melanoma cell line A875. Acta Histochem. 2013;115(7):705-711. [DOI] [PubMed] [Google Scholar]
- 9. Cui Y, Meng H, Liu W, Wang H, Liu Q. Huaier aqueous extract induces apoptosis of human fibrosarcoma HT1080 cells through the mitochondrial pathway. Oncol Lett. 2015;9(4):1590-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo Z, Hu X, Xiong H, et al. A polysaccharide from Huaier induced apoptosis in MCF-7 breast cancer cells via down-regulation of MTDH protein. Carbohydr Polym. 2016;151:1027-1033. [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Qi W, Li Y, et al. Huaier extract induces autophagic cell death by inhibiting the mTOR/S6K pathway in breast cancer cells. PLoS One. 2015;10(7):e0131771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qi W, Sun M, Kong X, et al. Huaier extract synergizes with tamoxifen to induce autophagy and apoptosis in ER-positive breast cancer cells. Oncotarget. 2016;7(18):26003-26015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie J, Zhuan B, Wang H, et al. Huaier extract suppresses non-small cell lung cancer progression through activating NLRP3-dependent pyroptosis. Anat Rec (Hoboken). Published online November 6, 2019. doi:10.1002/ar.24307 [DOI] [PubMed] [Google Scholar]
- 14. Li C, Wu X, Zhang H, et al. A Huaier polysaccharide inhibits hepatocellular carcinoma growth and metastasis. Tumour Biol. 2015;36(3):1739-1745. [DOI] [PubMed] [Google Scholar]
- 15. Yang A, Fan H, Zhao Y, et al. An immune-stimulating proteoglycan from the medicinal mushroom Huaier up-regulates NF-kappaB and MAPK signaling via Toll-like receptor 4. J Biol Chem. 2019;294(8):2628-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou C, Li J, Qian W, et al. Huaier extract restrains pancreatic cancer by suppressing Wnt/beta-catenin pathway. Biomed Pharmacother. 2020;127:110126. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Zhang N, Huo Q, Yang Q. Anti-angiogenic and antitumor activities of Huaier aqueous extract. Oncol Rep. 2012;28(4):1167-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zou Y, Xiong H, Xiong H, et al. A polysaccharide from mushroom Huaier retards human hepatocellular carcinoma growth, angiogenesis, and metastasis in nude mice. Tumour Biol. 2015;36(4):2929-2936. [DOI] [PubMed] [Google Scholar]
- 19. Hu Z, Yang A, Su G, et al. Huaier restrains proliferative and invasive potential of human hepatoma SKHEP-1 cells partially through decreased Lamin B1 and elevated NOV. Sci Rep. 2016;6:31298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lei JY, Yan LN, Zhu JQ, Wang WT. Hepatocellular carcinoma patients may benefit from postoperative huaier aqueous extract after liver transplantation. Transplant Proc. 2015;47(10):2920-2924. [DOI] [PubMed] [Google Scholar]
- 21. Sun Y, Sun T, Wang F, et al. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohydr Polym. 2013;92(1):577-582. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Wang X, Chen T. Efficacy of Huaier granule in patients with breast cancer. Clin Transl Oncol. 2019;21(5):588-595. [DOI] [PubMed] [Google Scholar]
- 23. Pan J, Yang C, Jiang Z, Huang J. Trametes robiniophila Murr: a traditional Chinese medicine with potent anti-tumor effects. Cancer Manag Res. 2019;11:1541-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Qi W, Song X, Lv S, Zhang H, Yang Q. Huaier extract suppresses breast cancer via regulating tumor-associated macrophages. Sci Rep. 2016;6:20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pearce EJ, Everts B. Dendritic cell metabolism. Nat Rev Immunol. 2015;15(1):18-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Merad M, Salmon H. Cancer: a dendritic-cell brake on antitumour immunity. Nature. 2015;523(7560):294-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245-252. [DOI] [PubMed] [Google Scholar]
- 29. Steinman RM. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai J Med. 2001;68(3):160-166. [PubMed] [Google Scholar]
- 30. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun WW, Dou JX, Zhang L, et al. Killing effects of Huaier Granule combined with DC-CIK on nude mice transplanted with colon carcinoma cell line. Oncotarget. 2017;8(28):46081-46089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnston SA, Voelz K, May RC. Cryptococcus neoformans thermotolerance to avian body temperature is sufficient for extracellular growth but not intracellular survival in macrophages. Sci Rep. 2016;6:20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Webster SJ, Daigneault M, Bewley MA, et al. Distinct cell death programs in monocytes regulate innate responses following challenge with common causes of invasive bacterial disease. J Immunol. 2010;185(5):2968-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stumbles PA, Thomas JA, Pimm CL, et al. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med. 1998;188(11):2019-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rakhra K, Bachireddy P, Zabuawala T, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18(5):485-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427(6970):154-159. [DOI] [PubMed] [Google Scholar]
- 38. van Panhuys N, Klauschen F, Germain RN. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization In Vivo. Immunity. 2014;41(1):63-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Medzhitov R, Janeway CA., Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91(3):295-298. [DOI] [PubMed] [Google Scholar]
- 40. Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166(9):5448-5455. [DOI] [PubMed] [Google Scholar]
- 41. Dissanayake D, Hall H, Berg-Brown N, et al. Nuclear factor-kappaB1 controls the functional maturation of dendritic cells and prevents the activation of autoreactive T cells. Nat Med. 2011;17(12):1663-1667. [DOI] [PubMed] [Google Scholar]
- 42. Becattini S, Latorre D, Mele F, et al. T cell immunity. Functional heterogeneity of human memory CD4(+) T cell clones primed by pathogens or vaccines. Science. 2015;347(6220):400-406. [DOI] [PubMed] [Google Scholar]
- 43. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hsieh CS, Heimberger AB, Gold JS, O’Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992;89(13):6065-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noben-Trauth N, Hu-Li J, Paul WE. IL-4 secreted from individual naive CD4+ T cells acts in an autocrine manner to induce Th2 differentiation. Eur J Immunol. 2002;32(5):1428-1433. [DOI] [PubMed] [Google Scholar]
- 47. Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96(3):1039-1046. [PubMed] [Google Scholar]
- 48. Park D, Lapteva N, Seethammagari M, Slawin KM, Spencer DM. An essential role for Akt1 in dendritic cell function and tumor immunotherapy. Nat Biotechnol. 2006;24(12):1581-1590. [DOI] [PubMed] [Google Scholar]
- 49. Lu Y, Zhang M, Wang S, et al. p38 MAPK-inhibited dendritic cells induce superior antitumour immune responses and overcome regulatory T-cell-mediated immunosuppression. Nat Commun. 2014;5:4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dillon S, Agrawal A, Van Dyke T, et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172(8):4733-4743. [DOI] [PubMed] [Google Scholar]
- 51. Zhao GS, Liu Y, Zhang Q, et al. Transarterial chemoembolization combined with Huaier granule for the treatment of primary hepatic carcinoma: Safety and efficacy. Medicine (Baltimore). 2017;96(29):e7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67(11):2006-2016. [DOI] [PubMed] [Google Scholar]
- 53. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422. [DOI] [PubMed] [Google Scholar]
- 54. Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55-72. [DOI] [PubMed] [Google Scholar]
- 55. Wei-guo Z, Hui Y, Shan L, et al. PPAR-gamma agonist inhibits Ang II-induced activation of dendritic cells via the MAPK and NF-kappaB pathways. Immunol Cell Biol. 2010;88(3):305-312. [DOI] [PubMed] [Google Scholar]
- 56. Wang S, Yang J, Qian J, Wezeman M, Kwak LW, Yi Q. Tumor evasion of the immune system: inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood. 2006;107(6):2432-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang S, Hong S, Yang J, et al. Optimizing immunotherapy in multiple myeloma: Restoring the function of patients’ monocyte-derived dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and neutralizing interleukin-6 in progenitor cells. Blood. 2006;108(13):4071-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]