Abstract
Molecular adjuvants are important for augmenting or modulating immune responses induced by DNA vaccination. Promising results have been obtained using IL-12 expression plasmids in a variety of disease models including the SIV model of HIV infection. We used a mouse model to evaluate plasmid IL-12 (pIL-12) in a DNA prime, recombinant adenovirus serotype 5 (rAd5) boost regimen specifically to evaluate the effect of IL-12 expression on cellular and humoral immunity induced against both SIVmac239 Gag and Env antigens. Priming with electroporated (EP) DNA+pIL-12 resulted in a 2–4-fold enhanced frequency of Gag-specific CD4 T cells which was maintained through the end of the study irrespective of the pIL-12 dose, while memory Env-specific CD4+T cells were maintained only at the low dose of pIL-12. There was little positive effect of pIL-12 on the humoral response to Env, and in fact, high dose pIL-12 dramatically reduced SIV Env-specific IgG. Additionally, both doses of pIL-12 diminished the frequency of CD8 T-cells after DNA prime, although a rAd5 boost recovered CD8 responses regardless of the pIL-12 dose. In this prime-boost regimen, we have shown that a high dose pIL-12 can systemically reduce Env-specific humoral responses and CD4T cell frequency, but not Gag-specific CD4+ T cells. These data indicate that it is important to independently characterize individual SIV or HIV antigen immunogenicity in multi-antigenic vaccines as a function of adjuvant dose.
Keywords: DNA, IL-12, Adenovirus, T cell, Antibody
1. Introduction
Despite improvements in the control of virus replication using HIV anti-retroviral therapies and additional efforts such as microbicide use and pre-exposure prophylaxis, an effective HIV vaccine remains the most viable solution to control the HIV pandemic. DNA vaccines are attractive due to their safety and ease of engineering and manufacturing [1]. First generation HIV based DNA vaccines failed to generate high levels of immunogenicity in humans [2], but subsequent progress on plasmid optimization, molecular adjuvants and improved delivery systems have all contributed to the increased efficacy of DNA vaccines and renewed interested in their application to HIV vaccine development [3–8]. In particular, the use of electroporation (EP) has significantly enhanced immune responses in animal models [9–13].
Co-delivery of DNA encoding molecular adjuvants with DNA encoding antigen is a strategy used to augment or modulate the immune response. A variety of cytokines and chemokines have been tested including GM-CSF, IL-2, IL-15, CCL25, CCL27 and IL-12 [14–18]. Co-delivery of plasmid IL-12 (pIL-12) enhances T cell responses in non-human primates (NHPs) [10,19–21], which may play a role in protection from SHIV challenge [20]. Additionally, the combination of delivery of DNA by EP and the inclusion of pIL-12 has been shown to enhance the potency of DNA vaccines in NHPs [10,21]. As a result we tested EP delivery of SIV DNA+pIL-12 followed by a rAd5 boost in the NHP SIV challenge model and observed an approximately 2log reduction in peak viral load and 4 log reduction in set point viral load following infection [22]. Analysis of the immune response when pIL-12 was included, demonstrated an increase in frequency of CD4+ T cell responses, which were characterized to be multifunctional and maintained following a rAd5 boost [22]. As a result of these studies, an analogous strategy is now being tested in phase I clinical trials (ClinicalTrials.gov Identifier: NCT01496989). The goals of this trial are to determine the safety of the vaccine regimen and to assess the effect of different levels of pIL-12 in the DNA prime.
As a guide for ongoing analysis of both NHP and human studies involving pIL-12, we evaluated the effect of pIL-12 on the immunogenicity of SIV Gag and Env DNA vaccines in the mouse model to determine the impact of murine pIL-12 on the humoral and cellular immune responses to both antigens. Specifically, we studied the effect of high and low dose pIL-12 on the immune responses induced against two co-delivered SIV immunogens and observed a differential impact on the maintenance of Gag versus Env-specific T cells at a high dose of pIL-12. Additionally, we describe the requirement of EP and low dose pIL-12 to generate a balanced cellular and humoral immune response to both antigens. These results suggest that pIL-12 dose optimization for each antigen individually, as well as in combination, may need to be conducted to achieve the desired outcome in future multi-antigen pre-clinical and clinical studies.
2. Materials and methods
2.1. Animals
C57BL/6 mice were purchased from the Jackson Laboratory and maintained at SUNY Downstate Medical Center under specific pathogen free conditions. The SUNY Downstate Institutional Animal Care and Use Committee reviewed and approved all experimental protocols.
2.2. Vectors
DNA and rAd5 vaccine immunogens used in this study were as previously described [22]. Briefly, immunogens were designed from the SIVmac239 sequence (GenBank accession number M33262). The Gag plasmid expressed myristoylated Gag protein and the Env plasmid expressed native gp160. Aldevron LLC (Fargo, ND) produced and purified large-scale plasmid DNA used for vaccination. Both DNA and rAd5 share the same insert sequences, which were optimized for expression in mammalian cells. rAd5 vectors were constructed in a ΔE1A, partial ΔE1B and ΔE3 genetic background using AdEasy adenoviral vector system (Stratagene, La Jolla, CA). The murine pIL-12 used in this study expressed the IL-12 p35 and p40 subunits from separate promoters to generate the optimal expression ratio of each subunit and generate maximal levels of bioactive IL-12 p70 [23].
2.3. Immunizations
Animals were anesthetized and the area over the tibialis anterior shaved before immunization with 30μg SIVmac239 Gag and Env DNA with or without pIL-12 (0.3 or 25μg). For electroporation, 4s after DNA injection, two electrical pulses for 40ms at 250mA were applied to the injection site using the TriGrid™ Delivery System (Ichor Medical Systems, Inc., San Diego, CA). Animals were primed with DNA twice, 4 weeks apart before an IM boost at week 8 with 1×108 particles of rAd5 vectors encoding SIVmac239 Gag and Env. Gag antigens were immunized into the left leg while Env antigens were immunized into the right leg.
2.4. Multiparameter flow cytometry
At various times following immunization, 1.5×106 leukocytes from the spleen were incubated with 2μg/ml anti-CD28 (BD Biosciences), 10μg/ml brefeldin A (Sigma–Aldrich), and 15-mer overlapping peptide pools or 2μg/ml each of the DD13 (DRFYKSLRAEQTD, CD4 epitope) [24] or AL11 (AAVKNWMTQTL, CD8) [25] SIV Gag peptides for 5h at 37°C before intracellular cytokine staining. Control stimulations were prepared for each animal by incubating leukocytes with only anti-CD28 and brefeldin A. For all T cell data, the control co-stimulation response was subtracted from the peptide-stimulated response. Cells were stained with LIVE/DEAD Fixable Violet Dead Cell Stain (Molecular Probes), CD3, CD4, IFNγ, IL-2, TNFα (BD Biosciences), and CD8 (BioLegend). Cells were resuspended in 0.5% paraformaldehyde before being acquired on a modified BD LSR II flow cytometer and analyzed using FlowJo software version 9.4 (Tree Star) and SPICE version 5.22 [26].
2.5. ELISA
Anti-SIV Env specific IgG in serum was quantified by ELISA. Briefly, serial dilutions of heat-inactivated serum were incubated in 96 well plates coated with 100ng/well of SIVmac239 gp140, SIVmac251 gp120 or SIVsmE660 gp140 (Immune Technology Corp., NY). Plates were blocked with 3% BSA before addition of serial diluted serum samples and detection of IgG with anti-mouse IgG-HRP (Jackson ImmunoResearch, PA).
2.6. Statistics
Statistical significance of T cell cytokine production was calculated in SPICE software using the Wilcoxon Signed Rank Test, bars represent the median response. Significance of antibody production was calculated by two-tailed Mann–Whitney test using PRISM software.
3. Results
3.1. The frequency and quality of Th1 CD4+ T cell responses are enhanced by electroporation of DNA vaccines and the inclusion of low dose plasmid IL-12
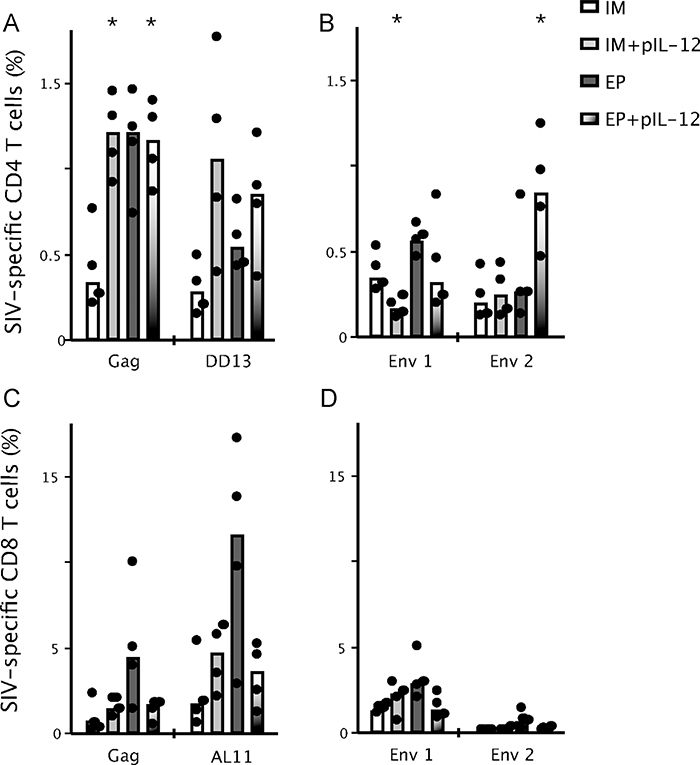
To determine the effect of using pIL-12 adjuvant and EP in the vaccine regimen, mice were immunized twice by IM injection or EP, 4 weeks apart with Gag plasmid DNA in the left leg and Env plasmid DNA in the right leg in the presence or absence of low dose pIL-12 (0.3μg). SIV-specific T cell responses were analyzed by peptide stimulation and intracellular cytokine staining two weeks following the second DNA immunization (Fig. 1). Compared to IM injection without molecular adjuvant, the Gag-specific CD4+ T cells stimulated with the peptide pool were enhanced 2–3-fold in animals immunized by EP with or without low dose pIL-12 or IM+pIL-12 (Fig. 1A). Comparing these data to cells stimulated with only the dominant CD4 H-2b Gag epitope, DD13 (included in the Gag pool), the effect of IL-12 was evident and it was clear and the CD4+ T cell response was dominated by responses directed toward the DD13 peptide in the pIL-12 groups (Fig. 1A). Examining the Env-specific CD4+ T cell response (Fig. 1B) showed that immunization by IM with pIL-12 significantly reduced the CD4+ T cell response in Env peptide pool 1 and had little effect on pool 2 compared to IM injection without pIL-12. EP had a modest effect on T cells stimulated with pool 1 but no effect on pool 2. EP with pIL-12 enhanced the frequency of responding CD4+ T cells in pool 2 only. An adjuvant effect of pIL-12 on Env-specific CD4+ T cells was not observed in the IM immunized animals as was seen with Gag.
Fig. 1.
Frequency of CD4+ (A and B) and CD8+ (C and D) SIV-specific cytokine secreting T cells following DNA immunization. Mice were immunized twice, 4 weeks apart with SIV Gag and Env DNA (30μg) by IM or EP +/− pIL-12 (0.3μg). Splenocytes were harvested two weeks after the second immunization, incubated ex vivo for 5h with Gag pooled peptides, the dominant Gag peptides: DD13 (CD4) and AL11 (CD8) (A and C) or Env pooled peptides (B and D), anti-CD28 and brefeldin A before being analyzed by flow cytometry for production of IFNγ, IL-2 and TNFα. The total frequency of cytokine secreting T cells (%) producing IFNγ, IL-2 and TNFα are shown and bars represent the median, n = 4 and data are representative of 2 experiments. *p < 0.05, significantly different from animals immunized by IM.
CD8+ T cells specific for SIV Gag (Fig. 1C) and Env (Fig. 1D) were mildly enhanced by EP delivery of DNA; however, animals immunized by EP with pIL-12 showed a 2–5-fold reduction as compared to those who were vaccinated by EP only. Both the dominant CD8 H-2b Gag epitope, AL11, and the Gag peptide pool responses (not including AL11) show the same trend of diminished CD8 T cell frequency when pIL-12 was included in EP delivery of the DNA (Fig. 1C).
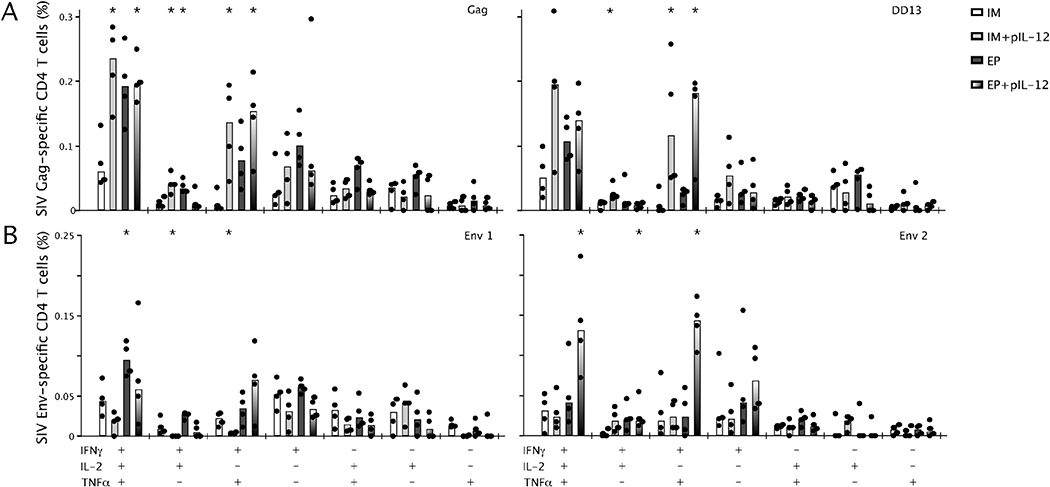
One measure of T cell immune response quality is the production of any combination of IFNγ, IL-2 or TNFα in response to specific antigen stimulation at the single cell level. Multifunctional T cells are capable of producing all three cytokines and of producing more of each cytokine on a per cell basis. The majority of the antigen specific Th1 CD4+ T cells that define the quality of the response can be subdivided into populations based on IFNγ secretion (single positive), IFNγ and TNFα (double positive) and IFNγ, TNFα and IL-2 (triple positive). When the quality of the Gag (Fig. 2A) and Env-specific (Fig. 2B) CD4+ T cell responses were quantified, triple positive Gag-specific CD4+ T cells were lowest after IM immunization and increased by IM+IL12, EP, or EP+IL12, although the positive effect of EP and IL12 did not appear to be additive (Fig. 2A). A model of Th1 differentiation proposes sequential loss of T cell functions, namely IL-2 and TNFα, upon multiple/prolonged antigen exposure [27,28], and we observed that pIL-12 might have enhanced differentiation of the Gag-specific CD4+ T cell response in this manner. This was consistent with detection of an increased frequency of double positive (IFNγ/TNFα) CD4+ T cells that appeared to be primarily in response to the dominant CD4 epitope DD13 (Fig. 2A).
Fig. 2.
Quality of SIV Gag-specific (A) and SIV Env-specific (B) CD4+ Th1 cells secreting IFNγ, IL-2 and TNFα in any combination after DNA immunization. Mice were immunized twice, 4 weeks apart with SIV Gag and Env DNA (30μg) by IM or EP +/− pIL-12 (0.3μg). Splenocytes were harvested two weeks after the second immunization, incubated ex vivo for 5h with Gag pooled peptides or the dominant peptide DD13 (A) or Env pool 1 and Env pool 2 peptides (B), anti-CD28 and brefeldin A before being analyzed by flow cytometry for production of IFNγ, IL-2 and TNFα. The frequency of cytokine secreting T cells (%) producing IFNγ, IL-2 and TNFα in any combination are shown with bars representing the median, n = 4 and data are representative of 2 experiments. *p < 0.05, significantly different from animals immunized by IM.
The frequency of multifunctional Env-specific CD4+ T cells was predominantly enhanced in the animals receiving EP+pIL-12 and these were directed toward epitopes in Env pool 2 (Fig. 2B). In contrast to the Gag-specific effects, vaccination with EP or IM+pIL-12 did not significantly increase numbers of multifunctional T cells, and instead, the maximum frequency of Env-specific multifunctional cells required the combination of EP+pIL-12.
3.2. High dose plasmid IL-12 dampens DNA-primed CD4+ Th1 and CD8+ T cell responses
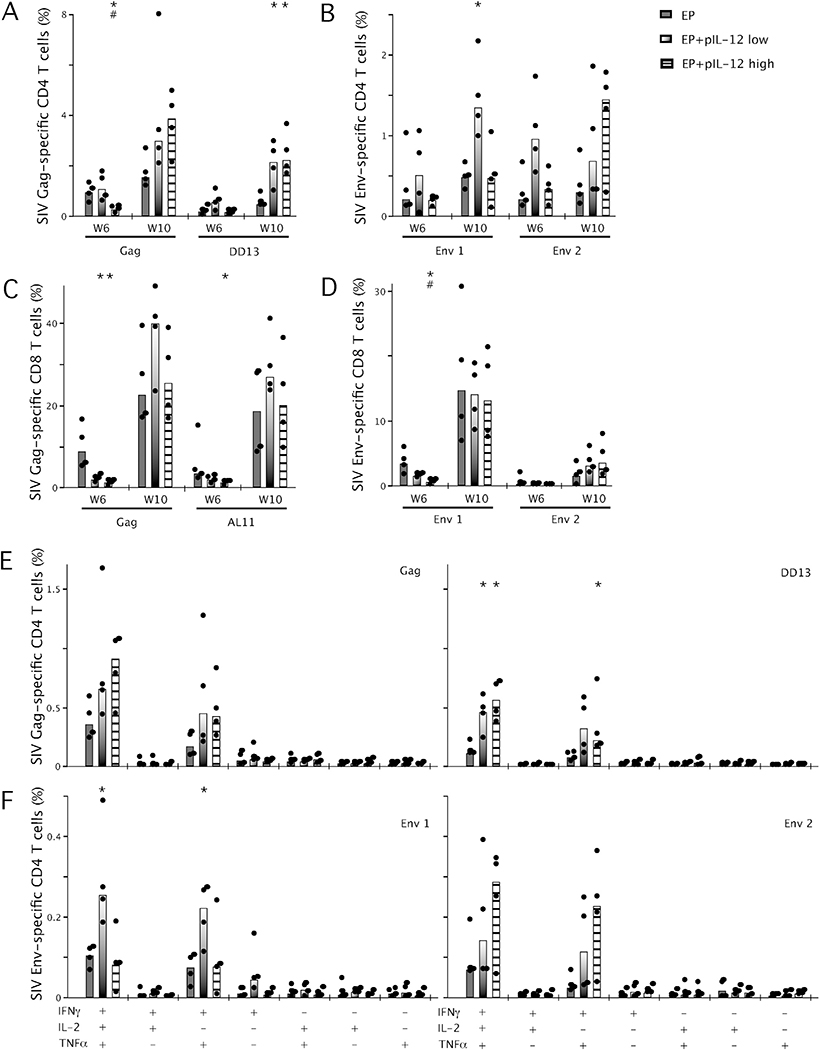
In earlier studies, high doses of pIL-12 (25–50μg) were required to induce an adjuvant effect with HIV Env DNA [16,29]. Therefore, we tested EP SIV Env DNA with both a low (0.3μg) and high dose (25μg) of pIL-12 in combination with a low dose pIL-12/Gag DNA immunization delivered to a separate vaccination site. The peak Gag responses induced by EP or EP plus a low dose of pIL-12 (Figs. 1 and 3) were abrogated using a higher dose of pIL-12 (Fig. 3A). However, this inhibitory effect did not appear to prevent CD4+ T cells from being boosted by a rAd5-Gag and rAd5-Env immunization at week 8 as seen by the expansion of both Gag and Env-specific CD4+ T cells at week 10. Additionally, as a result of the initial inhibition, the EP+pIL-12 high Gag-specific CD4+ T cells expanded to a greater extent that those in the low dose group with approximately a 16-fold expansion from week 6 to week 10 compared to a 3-fold expansion in the low dose group. The Env-specific response in pool 1 was enhanced by low dose pIL-12, and there was a trend for the response to peptides in pool 2 to be enhanced by high dose pIL-12 (Fig. 3B).
Fig. 3.
Effect of pIL-12 on the frequency of SIV Gag and Env-specific cytokine secreting T cells following EP immunization. Mice were immunized by EP twice, four weeks apart with Gag and Env plasmids and low dose (0.3μg) or high dose (25μg) pIL-12 before a boost with rAd5 vectors expressing Gag and Env four weeks after the second DNA immunization. Splenocytes were harvested at week 6 and week 10, incubated ex vivo for 5h with Gag pooled peptides or the dominant peptides DD13/AL11 (A/C) or Env pool peptides (B/D), anti-CD28 and brefeldin A before being analyzed by flow cytometry for combined production of IFNγ, IL-2 and TNFα. The frequency of multifunctional cytokine secreting Gag-specific (E) or Env-specific (F) CD4+ T cells (%) at week 10 producing IFNγ, IL-2 and TNFα in any combination are shown, bars represent the median, n =4 and data are representative of 2 experiments. *p < 0.05, significantly different from animals immunized by EP or #p < 0.05, significantly different from animals immunized by EP+pIL-12 low.
Gag-specific CD8+ T cell responses were inhibited by both low and high dose pIL-12 at week 6 compared to EP alone (Fig. 3C), while Env-specific CD8+ T cells were significantly reduced by high dose pIL-12 compared to low dose pIL-12 and EP alone (Fig. 3D). As observed with the CD4+ T cells, this inhibitory effect did not prevent the expansion of the CD8+ T cells to both antigens following a boost with rAd5. CD8+ T cells in both low and high dose groups expanded to a similar frequency as those in the EP only group.
The quality of the Gag-specific CD4+ T cell responses at week 10 revealed a trend toward increased multifunctional cells. Upon analysis of the dominant peptide response (DD13) within the pool, a significant increase in the frequency of multifunctional CD4+ T cells was observed in both high and low dose pIL-12 dose groups as compared to EP alone (Fig. 3E). The quality of the Env-specific CD4+ T cell response (Fig. 3F) was slightly different with low dose pIL-12 enhancing the multifunctional response to epitopes represented by pool 1 while the high dose pIL-12 was inhibitory. For pool 2, there was a trend toward both low and high dose pIL-12 enhancing a multifunctional response. The quality and frequency of multifunctional CD8+ T cells following the boost was similar among all groups with a mixed population of multifunctional cells (data not shown).
3.3. The frequency and quality of memory Th1 CD4+ T cell responses is enhanced by inclusion of pIL-12 in an EP but not an IM DNA prime
At the end of the study (week 14), the frequency of Gag-specific CD4+ T cell responses were approximately 2–3-fold higher in animals immunized with the combination of EP and low dose pIL-12 as compared to EP alone or IM +/− pIL-12 (Fig. 4A). This response was comprised of approximately 50% DD13 specific responses and 50% responses to other epitopes in the Gag peptide pool. A modest increase also was observed in the frequency of Env-specific CD4+ T cells with 2–3 animals in the EP+low dose pIL-12 group. Upon analysis of the quality of the Th1 Gag-specific immune response, a greater frequency of multifunctional memory CD4+ T cells were maintained in the EP+pIL-12 group as compared to other vaccine groups (Fig. 4B). Similar data was observed with the Env-specific CD4+ T cells (data not shown). The frequency of Gag-specific CD8+ T cells at week 14 were inhibited when pIL-12 was included in the IM vaccination (Fig. 4C). This effect was not however observed in Env-specific responses with the memory response being predominantly represented by peptides in pool 1. The quality of the CD8+ T cell response remained similar between all groups (data not shown).
Fig. 4.
Frequency of memory Gag and Env-specific CD4+ (A) or CD8+ (C) T cells secreting IFNγ, IL-2 and TNFα or the quality of the memory CD4+ Gag-specific T cell response (B). Mice were immunized twice, 4 weeks apart with SIV Gag and Env DNA (30μg) by IM or EP +/− pIL-12 (0.3μg) before a boost with rAd5 vectors expressing Gag and Env. Splenocytes were harvested at week 14, incubated ex vivo for 5h with Gag pooled peptides, the dominant DD13/AL11 peptides or Env pooled peptides, anti-CD28 and brefeldin A before being analyzed by flow cytometry for total production of IFNγ, IL-2 and TNFα (A/C) or IFNγ, IL-2 and TNFα in any combination (B), bars represent the median, n = 4 and data are representative of 2 experiments. *p < 0.05, significantly different from animals immunized by IM.
3.4. Multifunctional Gag and Env-specific memory T cell responses differ in their ability to be maintained when the prime includes high dose pIL-12
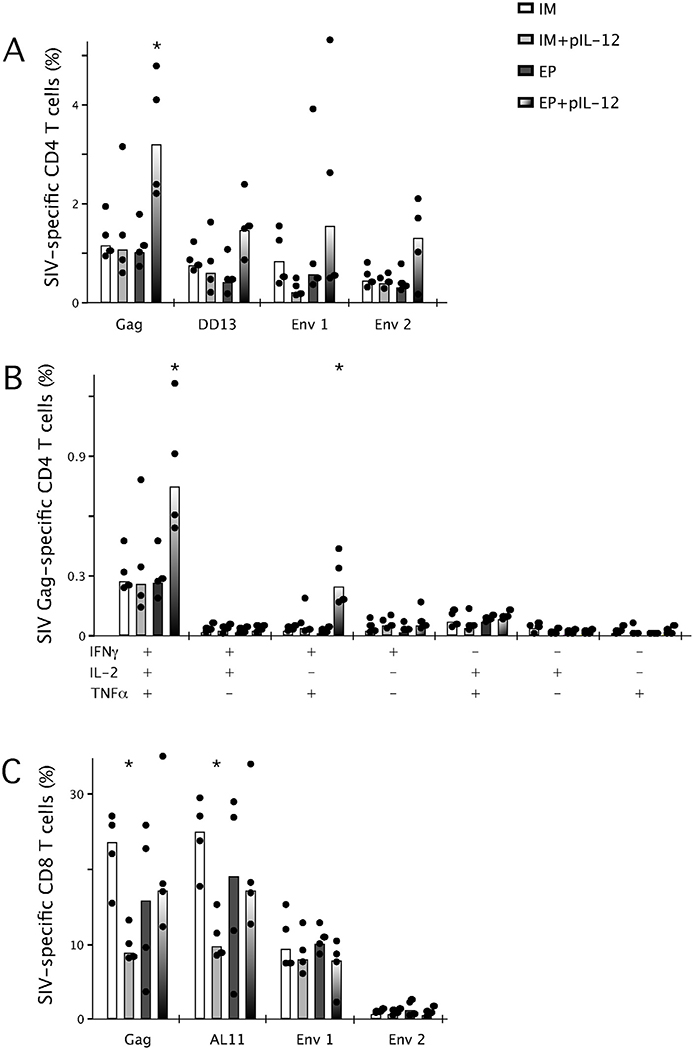
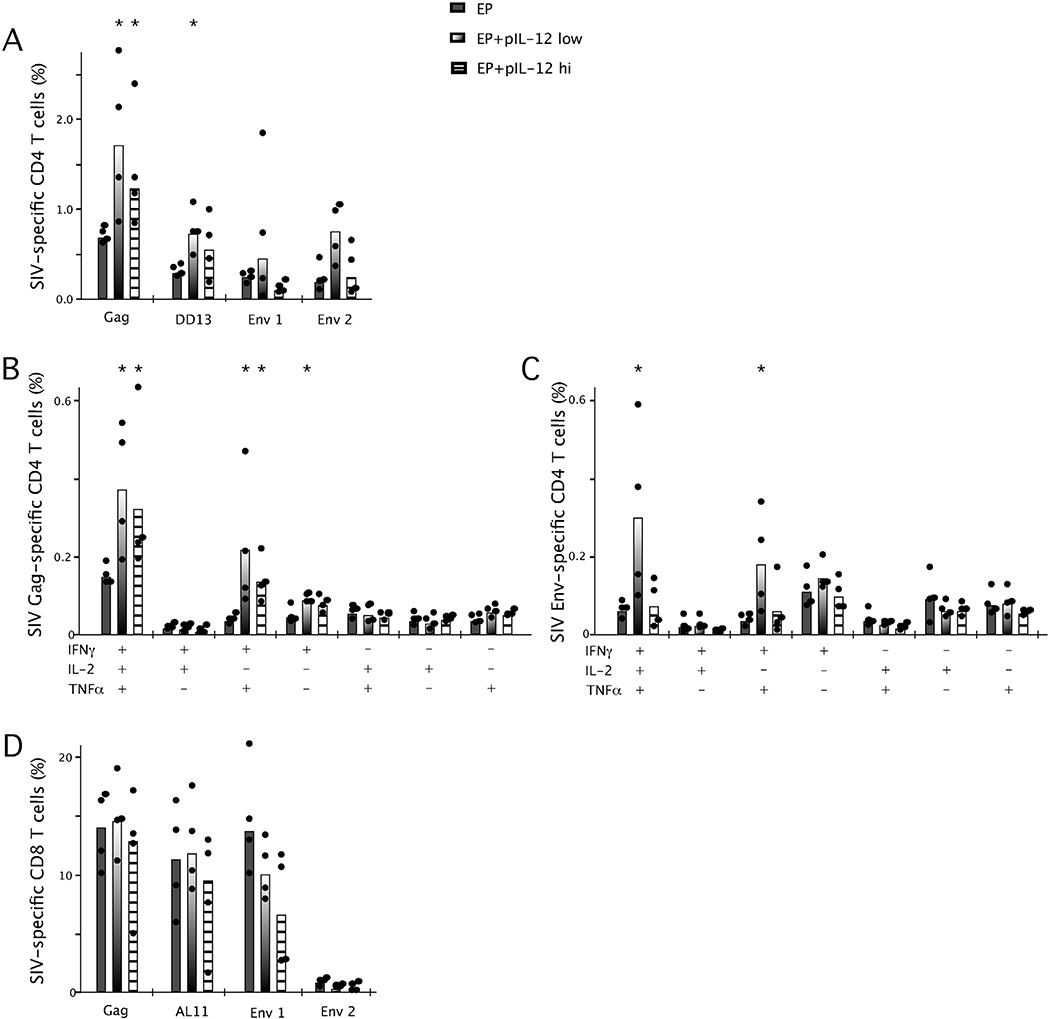
While the total frequency of Gag-specific CD4+ T cells induced with EP immunization and low or high dose pIL-12 remained enhanced at week 14 compared to EP immunization alone (Figs. 4A and 5A), there was a trend for high dose pIL-12 to reduce the frequency of Env-specific responses (Fig. 5A). The frequency of Env-specific T cells in the high dose pIL-12 group was similar to EP immunization without pIL-12. This became more apparent upon analysis of the quality of the Gag-specific (Fig. 5B) and Env-specific (Fig. 5C) immune responses. In both pIL-12 dose groups, the frequency of multifunctional Gag-specific CD4+ T cells were enhanced approximately 2-fold as compared to the EP immunization alone (Fig. 5B). Env-specific (Fig. 5C) responses, however, only demonstrated maintenance of multifunctional memory CD4+ T cells when the prime included low dose pIL-12, irrespective of the fact that these cells were boosted with Ad5 at week 10 (Fig. 3F).
Fig. 5.
Effect of high dose pIL-12 on the frequency (A) and quality (B/C) of memory CD4 and CD8 (D) SIV-specific cytokine secreting T cells. Mice were immunized twice, 4 weeks apart with SIV Gag and Env DNA (30μg) by EP with low dose (0.3μg) or high dose (25μg) pIL-12 before a boost with rAd5 vectors expressing Gag and Env. Splenocytes were harvested at week 14, incubated ex vivo for 5h with Gag pooled peptides, the dominant DD13/AL11 peptides or Env pooled peptides, anti-CD28 and brefeldin A before being analyzed by flow cytometry for total production of IFNγ, IL-2 and TNFα (A/D) or IFNγ, IL-2 and TNFα in any combination (B/C) with bars representing the median, n = 4 and data are representative of 2 experiments, *p < 0.05, significantly different from animals immunized by EP.
The frequency of Gag and Env-specific CD8+ T cells remained similar at the end of the study. Env-specific responses did however appear to be blunted in 2 out of 4 animals receiving the high dose pIL-12 (Fig. 5D). The quality of the CD8+ T cell response remained similar between all groups (data not shown).
3.5. Electroporation of SIVmac239 Env DNA with high dose pIL-12 inhibits SIV-specific IgG responses
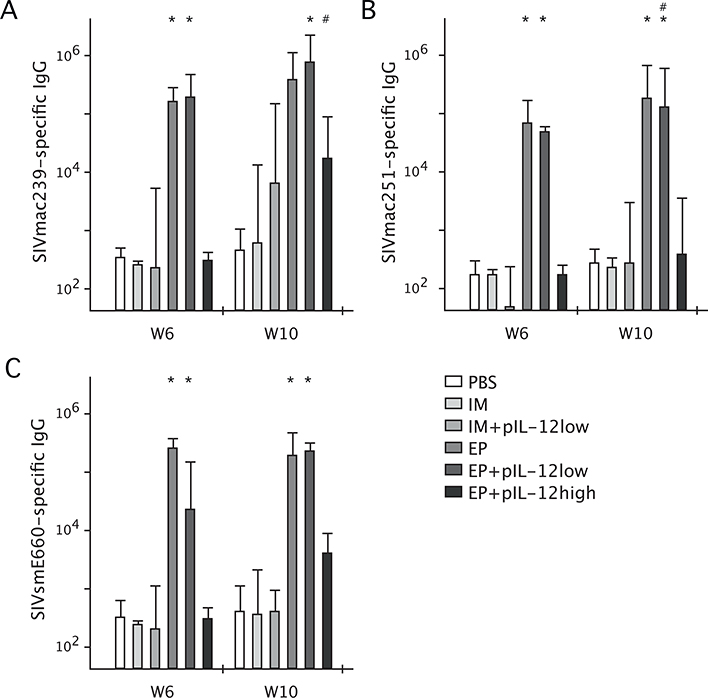
At week 6, 2 weeks following the second DNA immunization, the level of SIVmac239-Env specific IgG quantified by ELISA was significantly greater in EP and EP+low dose pIL-12 immunized animals as compared to EP+ high dose pIL-12 and all other groups (Fig. 6A). In addition, the median endpoint titers of animals vaccinated by EP +/− pIL-12 were approximately 2 logs higher than those immunized by IM +/− pIL-12 which were similar in level to the response of control PBS immunized animals. At week 10, 2 weeks following the rAd5 boost, the level of SIVmac239 Env-specific IgG was significantly greater in the EP+low dose pIL-12 group, but not the EP only group, as compared to the EP+high dose pIL-12 group. The median end point titers of animals vaccinated with EP +/− pIL-12 remained approximately 1–2logs higher than those immunized by IM +/− pIL-12. In addition, SIVmac239 Env-specific IgG was significantly boosted in the EP+high dose pIL-12 group from week 6 to week 10.
Fig. 6.
SIVmac239 (A) and cross reactive SIVmac251 (B) and SIVsmE660 (C) Env-specific IgG endpoint titers following DNA vaccination (week 6) and rAd5 boost (week 10) in mice immunized by IM or EP with SIV Env DNA +/− pIL-12. Serum was collected after vaccination and endpoint titers calculated by ELISA. Bars represent median endpoint titer, n = 4 and data are representative of 2 experiments. *p < 0.05, significantly different from animals immunized by EP+pIL-12 high, #p < 0.05, significantly different from week 6 to week 10.
Levels of cross-reactive SIVmac251 (Fig. 6B) and SIVsmE660 Env-specific IgG (Fig. 6C) showed a similar trend with EP titers being approximately 2 logs higher than IM or control animal titers. Additionally, endpoint titers of EP and EP+low dose pIL-12 immunized animals were significantly higher than the EP+high dose pIL-12 group. Following the rAd5 boost, cross reactive SIVmac251 and SIVsmE660 Env-specific antibodies remained approximately 2 logs higher in animals immunized by EP +/− low dose pIL-12 than IM +/− low dose pIL-12. Furthermore, cross-reactive IgG remained significantly higher in the EP +/− low dose pIL-12 immunized animals compared to the EP+high dose pIL-12 group. Finally, we observed that SIVmac251 cross-reactive IgG was boosted in the EP+low dose pIL-12, but not the EP only group comparing the titers at week 6 and week 10 (Fig. 6B).
4. Discussion
The primary objective of this study was to investigate, in the mouse model, how delivery of the molecular adjuvant pIL-12 alters the immune responses induced by DNA vaccination. We chose the mouse model for the ease and simplicity of analysis while recognizing that extrapolation to larger animals and humans should be done with caution. Nevertheless, we believe these studies can be useful in establishing general principles, help guide optimization of vaccine regimens, and suggest experiments that should be conducted in animal systems where efficacy endpoints can be measured. Our own findings in an NHP SIV challenge study examining a DNA prime/rAd5 boost regimen suggest a link between the use of pIL-12 adjuvant in the prime and control of SIV replication [22]. Others have also seen effects of IL-12 adjuvant on efficacy [10,19–21]. In our previous NHP study, there was a 2log reduction in peak viral load and a 4log reduction at set point following intrarectal SIV infection, which compared favorably to other vaccination strategies [30–32]. While the mechanism of efficacy is currently under investigation, the inclusion of pIL-12 resulted in an enhanced frequency of multifunctional CD4+ T cells. This occurred in the absence of enhanced antibody responses and may suggest that vaccine-induced SIV-specific CD4+ T cells may be advantageous in immune control of virus replication.
Both our NHP study and the murine studies reported here have demonstrated the T cell adjuvant effect of pIL-12 resulted in an increased frequency of multifunctional CD4+ T cells following a DNA prime and maintenance of these as memory cells following a rAd5 boost. In addition, when the quality of the Gag- and Env-specific CD4+ T cell response was examined, it was observed that pIL-12 had driven differentiation toward a greater frequency of cells that produce three cytokines (IFNγ, IL-2 and TNFα), as well as cells that produce both IFNγ and TNFα. Such multifunctional CD4+ T-cells have been described to be generated from a more differentiated Th1 T cell and have been shown to play a role in protection after certain viral and parasitic infections [27,33]. A greater frequency of such multifunctional T cells that can maintain the reservoir of memory cells and be mobilized quickly as effector cells is a desirable characteristic of a T cell vaccine [28].
While we observed an enhancement of the frequency and quality of Gag-specific CD4+ T cells following both IM and EP immunization with a low dose of pIL-12 (Fig. 2A), pIL-12 enhanced Env-specific CD4+ T cells only in animals immunized by EP (Fig. 2B). These data suggest that Env CD4+ T cell responses may require a higher dose of pIL-12 by IM immunization to see an adjuvant effect [16,29], and highlight the requirement of a full dose titration of the adjuvant with both antigens individually and in combination as it is possible that their requirements for pIL-12 dose are different. Thus, to establish and enhanced pool of memory CD4 cells specific for Gag and Env, a lower dose of pIL-12 is required in combination with EP.
When a higher dose of pIL-12 was introduced into the EP arm of the study, with the intention of enhancing Env-specific responses, a negative impact on the frequency of Gag, and to some extent, Env-specific CD4+ T cells, was observed (Fig. 3A and B). IL-12 is known to stimulate the synthesis of nitric oxide which is required for the immunostimulatory effects of IL-12; however, high levels of IL-12 can induce apoptosis and immunosuppression when high levels of nitric oxide and IFNγ and are induced, which likely explain the reduced frequency of SIV-specific CD4+ T cells observed at week 6 [29,34]. Additionally, in the EP+pIL-12 high dose group, pIL-12 was administered with the Env plasmid in the right leg, while the low dose of pIL-12 was immunized with the Gag plasmid in the left leg. However, high dose pIL-12 significantly reduced the Gag response 3–4-fold demonstrating a systemic effect of IL-12 (Fig. 3A). Nevertheless, the immunosuppressive effect of IL-12 did not prevent both Gag and Env-specific CD4+ T cells from being boosted by rAd5.
Examining the quality of the boosted CD4+ T cell response, it was apparent that epitopes represented by Env pool 1 were preferentially boosted by low dose pIL-12 but suppressed by the high dose, while epitopes in pool 2 showed a trend (3 out of 4 animals) toward also being enhanced by high dose pIL-12. This discrepancy perhaps reflects an enhanced breadth of response induced by the high dose IL-12 toward epitopes in pool 2 but without further epitope mapping this difference could simply be attributed to an enhanced magnitude of response. Notably, the high dose pIL-12 expanded population of Env pool 2 specific CD4+ T cells was not maintained (Fig. 5C), unlike Gag-specific responses (Fig. 5B), suggesting an exhaustion and potential death of these cells as a result of extended or excessive IL-12 stimulation and differentiation [28]. Once more, this highlights the potential benefits of conducting a pIL-12 dose titration with specific antigens individually and in combination to fully appreciate the effect of IL-12 on multiple antigens.
In addition to the inhibitory effect of pIL-12 on Th1 CD4+ T cells after DNA immunization, low dose pIL-12 inhibited the frequency of SIV-specific CD8+ T cells (Fig. 3C and D). Furthermore, Env-specific CD8+ T cells were inhibited by low dose pIL-12, and further suppressed by the high dose (Fig. 3D). This additional immunosuppressive effect could simply be a result of increased levels of nitric oxide and IFNγ, but it is possible that under both dose regimens, as a result of extended exposure to an inflammatory signal, CD8+ T cells could be driven to further differentiate into short-lived effector cells [35,36]. Of note, pIL-12 at either dose did not prevent the surviving CD8+ T cells from expanding upon a rAd5 boost, or from their maintenance through the end of the study. This may be an indication of the value of a heterologous prime-boost regimen for CD8+ memory T cell maintenance when pIL-12 is used during the prime. Both our NHP and murine study described here did not contain animals vaccinated only with DNA which could address the question of whether pIL-12 is detrimental in a homologous prime/boost setting for the maintenance of memory CD8+ T cells, which have been shown to have a potential role in protection from SIV infection [37–39].
Our previous NHP study contained all 9 SIVmac239 genes, which may be advantageous in eliciting broad T cell responses, which have been linked to protection in an SIV challenge model and are potentially important in the mechanism of control of viral load. Furthermore, in the vaccinated macaques, no difference was observed in the level of SIVmac239 Env-specific antibodies that was attributed to pIL-12. Nevertheless, we cannot rule out the possibility that SIV replication may have been suppressed after infection by antibodies that directly neutralized virus or induced cell-mediated mechanisms as described in HIV-1 infection [40,41]. While EP immunization alone is enough to enhance the generation of the antibody response compared to IM immunization as observed in the current murine study (Fig. 6) and by other investigators [10–13], an accumulative adjuvant effect of EP and low dose pIL-12 on antibody production was not observed here. We did however note that the EP+low dose pIL-12 group showed significantly higher SIVmac239-specific IgG titers compared to the high dose group while the EP alone group did not. In addition, the EP+low dose pIL-12 showed a significant increase in SIVmac251 cross-reactive IgG following the rAd5 boost while the EP alone group did not. This could be explained by a slightly higher titer of SIVmac239 antibodies but may also hint at an increase in breadth. A recent NHP study has demonstrated that pIL-12 may increase the breadth of the antibody response following SIVmac239 Env DNA immunization by EP 4 times. Significantly higher titers of IgG recognizing SIVmac251 were detected when pIL-12 was included in the vaccine regimen [42]. We did not observe this IL-12 induced effect, however there are several differences between the studies notably the animal species and DNA-only regimen. Nevertheless, we report significant levels of cross-reactive Env-specific IgG generated by EP immunization. The role and dose of pIL-12 on the antibody response in this model is currently under investigation as several studies have reported an adjuvant effect of protein IL-12 installed by the intranasal route on the enhancement of protective mucosal and systemic antibody responses [43–45]. Research on the role of IL-12 on B cell differentiation and development may additionally explain the immunosuppressive effect of high dose pIL-12 on systemic antibody responses observed in this study. A recent study reports that while IL-12 has an established role in B cell differentiation and function, IL-12 produced from DCs can inhibit B cell differentiation to germinal center cells and promote differentiation into short-lived plasmablasts [46].
In summary, here we show that mice, like NHPs, generated a multifunctional Th1 T cell phenotype as a result of the molecular adjuvant pIL-12. Additionally, this study demonstrates a clear difference between the cellular and humoral immune responses seen when using high and low dose pIL-12 during a DNA prime and this may have important implications for the use of IL-12 as an adjuvant in animal studies and clinical trials. The level of IL-12 present at the site of vaccination can be a function of the amount plasmid used, the use of electroporation (or other method for increasing expression), and/or the strength of the promoter driving the IL-12 gene. As a consequence, we believe that the present study suggests that dose response testing of antigens alone and in combination will be necessary to optimize immune responses and potentially the efficacy of a vaccine. Immunization with a high or inhibitory dose of a molecular adjuvant like pIL-12 however, may not be feasible in pre-clinical or clinical trials due to limiting concentrations of DNA or study costs. Additionally, these data bring attention to the effect that route, method of immunization, or the requirement of individual antigens for differing adjuvant dose within a multi-antigenic vaccine can have on both the humoral and cellular immune responses. If an HIV vaccine inducing a balanced cellular and humoral immune response is required for efficacy, one must take care to accurately study the adjuvant effect on each vaccine immunogen, so as to not bias the immune response in favor of a single antigen or a cellular over humoral immune response.
References
- [1].Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nature Reviews Genetics 2008;9(October (10)):776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. Journal of Infectious Diseases 1998;178(July (1)):92–100. [DOI] [PubMed] [Google Scholar]
- [3].Babiuk S, van Drunen Littel-van den Hurk S, Babiuk LA. Delivery of DNA vaccines using electroporation. Methods in Molecular Medicine 2006;127:73–82. [DOI] [PubMed] [Google Scholar]
- [4].Barouch DH, Santra S, Schmitz JE, Kuroda MJ, Fu TM, Wagner W, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 2000;290(October (5491)):486–92. [DOI] [PubMed] [Google Scholar]
- [5].Buchan S, Gronevik E, Mathiesen I, King CA, Stevenson FK, Rice J. Electroporation as a prime/boost strategy for naked DNA vaccination against a tumor antigen. Journal of Immunology 2005;174(May (10)):6292–8. [DOI] [PubMed] [Google Scholar]
- [6].Higgins D, Marshall JD, Traquina P, Van Nest G, Livingston BD. Immunostimulatory DNA as a vaccine adjuvant. Expert Review of Vaccines 2007;6(October (5)):747–59. [DOI] [PubMed] [Google Scholar]
- [7].Smith JM, Amara RR, Campbell D, Xu Y, Patel M, Sharma S, et al. DNA/MVA vaccine for HIV type 1: effects of codon-optimization and the expression of aggregates or virus-like particles on the immunogenicity of the DNA prime. AIDS Research and Human Retroviruses 2004;20(December (12)): 1335–47. [DOI] [PubMed] [Google Scholar]
- [8].Yan J, Yoon H, Kumar S, Ramanathan MP, Corbitt N, Kutzler M, et al. Enhanced cellular immune responses elicited by an engineered HIV-1 subtype B consensus-based envelope DNA vaccine. Molecular Therapy: The Journal of the American Society of Gene Therapy 2007;15(February (2)):411–21. [DOI] [PubMed] [Google Scholar]
- [9].Brave A, Nystrom S, Roos AK, Applequist SE. Plasmid DNA vaccination using skin electroporation promotes poly-functional CD4 T-cell responses. Immunology and Cell Biology 2011;89(March (3)):492–6. [DOI] [PubMed] [Google Scholar]
- [10].Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine 2008;26(June (25)):3112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. Journal of Virology 2007;81(May (10)):5257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 2004;22(June (19)):2489–93. [DOI] [PubMed] [Google Scholar]
- [13].Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. Journal of Immunology 2000;164(May (9)):4635–40. [DOI] [PubMed] [Google Scholar]
- [14].Kim JJ, Yang JS, Montaner L, Lee DJ, Chalian AA, Weiner DB. Coimmunization with IFN-gamma or IL-2, but not IL-13 or IL-4 cDNA can enhance Th1-type DNA vaccine-induced immune responses in vivo. Journal of Interferon and Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research 2000;20(March (3)):311–9. [DOI] [PubMed] [Google Scholar]
- [15].Kraynyak KA, Kutzler MA, Cisper NJ, Khan AS, Draghia-Akli R, Sardesal NY, et al. Systemic immunization with CCL27/CTACK modulates immune responses at mucosal sites in mice and macaques. Vaccine 2010;28(February (8)):1942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu R, Megati S, Roopchand V, Luckay A, Masood A, Garcia-Hand D, et al. Comparative ability of various plasmid-based cytokines and chemokines to adjuvant the activity of HIV plasmid DNA vaccines. Vaccine 2008;26(September (37)):4819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kathuria N, Kraynyak KA, Carnathan D, Betts M, Weiner DB, Kutzler MA. Generation of antigen-specific immunity following systemic immunization with DNA vaccine encoding CCL25 chemokine immunoadjuvant. Human Vaccines and Immunotherapeutics 2012;8(November (11)):1607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kalams SA, Parker S, Jin X, Elizaga M, Metch B, Wang M, et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PloS ONE 2012;7(1):e29231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, et al. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. Journal of Medical Primatology 2005;34(October (5–6)):262–70. [DOI] [PubMed] [Google Scholar]
- [20].Chong SY, Egan MA, Kutzler MA, Megati S, Masood A, Roopchard V, et al. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine 2007;25(June (26)):4967–82. [DOI] [PubMed] [Google Scholar]
- [21].Schadeck EB, Sidhu M, Egan MA, Chong SY, Piacente P, Masood A, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine 2006;24(May (21)):4677–87. [DOI] [PubMed] [Google Scholar]
- [22].Winstone N, Wilson AJ, Morrow G, Boggiano C, Chiuchiolo MJ, Lopez M, et al. Enhanced control of pathogenic Simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen. Journal of Virology 2011;85(September (18)):9578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jalah R, Rosati M, Ganneru B, Pilkington GR, Valentin A, Kulkarni V, et al. The p40 subunit of interleukin (IL)-12 promotes stabilization and export of the p35 subunit: implications for improved IL-12 cytokine production. Journal of Biological Chemistry 2013;288(March (9)):6763–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu J, Ewald BA, Lynch DM, Nanda A, Sumida SM, Barouch DH. Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies. Journal of Virology 2006;80(December (24)):11991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presenceofpre-existinganti-Ad5immunity.JournalofImmunology 2004;172(May (10)):6290–7. [DOI] [PubMed] [Google Scholar]
- [26].Roederer M, Nozzi JL, Nason MX, SPICE. Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry Part A: The Journal of the International Society for Analytical Cytology 2011;79(January (2)):167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature Medicine 2007;13(July (7)):843–50. [DOI] [PubMed] [Google Scholar]
- [28].Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature Reviews Immunology 2008;8(April (4)):247–58. [DOI] [PubMed] [Google Scholar]
- [29].Gherardi MM, Ramirez JC, Esteban M. Interleukin-12 (IL-12) enhancement of the cellular immune response against human immunodeficiency virus type 1 env antigen in a DNA prime/vaccinia virus boost vaccine regimen is time and dose dependent: suppressive effects of IL-12 boost are mediated by nitric oxide. Journal of Virology 2000;74(July (14)):6278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, et al. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. Journal of Virology 2005;79(December (24)):15547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Suh YS, Park KS, Sauermann U, Franz M, Norley S, Wilfingseder D, et al. Reduction of viral loads by multigenic DNA priming and adenovirus boosting in the SIVmac-macaque model. Vaccine 2006;24(March (11)):1811–20. [DOI] [PubMed] [Google Scholar]
- [32].Wilson NA, Keele BF, Reed JS, Piaskowski SM, MacNair CE, Bett AJ, et al. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. Journal of Virology 2009;83(July (13)):6508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006;107(June (12)):4781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lasarte JJ, Corrales FJ, Casares N, Lopez-Diaz de Cerio A, Qian C, Xie X, et al. Different doses of adenoviral vector expressing IL-12 enhance or depress the immune response to a coadministered antigen: the role of nitric oxide. Journal of Immunology 1999;162(May (9)):5270–7. [PubMed] [Google Scholar]
- [35].Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 2007;27(September (3)):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: Role of direct IL-12 signals for cell fate decision of CD8(+) T cells. European Journal of Immunology 2009;39(July (7)): 1774–83. [DOI] [PubMed] [Google Scholar]
- [37].Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nature Medicine 2009;15(March (3)):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu J, O’Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 2009;457(January (7225)):87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yamamoto T, Johnson MJ, Price DA, Wolinsky DI, Almeida JR, Petrovas C, et al. Virus inhibition activity of effector memory CD8(+) T cells determines simian immunodeficiency virus load in vaccinated monkeys after vaccine breakthrough infection. Journal of Virology 2012;86(May (10)):5877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Haynes BF, Moody MA, Liao HX, Verkoczy L, Tomaras GD. B cell responses to HIV-1 infection and vaccination: pathways to preventing infection. Trends in Molecular Medicine 2011;17(February (2)):108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Willey S, Aasa-Chapman MM. Humoral immunity to HIV-1: neutralisation and antibody effector functions. Trends in Microbiology 2008;16(December (12)):596–604. [DOI] [PubMed] [Google Scholar]
- [42].Jalah R, Patel V, Kulkarni V, Rosati M, Alicea C, Ganneru B, et al. IL-12 DNA as molecular vaccine adjuvant increases the cytotoxic T cell responses and breadth of humoral immune responses in SIV DNA vaccinated macaques. Human Vaccines and Immunotherapeutics 2012;8(November (11)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Albu DI, Jones-Trower A, Woron AM, Stellrecht K, Broder CC, Metzger DW. Intranasal vaccination using interleukin-12 and cholera toxin subunit B as adjuvants to enhance mucosal and systemic immunity to human immunodeficiency virus type 1 glycoproteins. Journal of Virology 2003;77(May (10)): 5589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Arulanandam BP, O’Toole M, Metzger DW. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. Journal of Infectious Diseases 1999;180(October (4)):940–9. [DOI] [PubMed] [Google Scholar]
- [45].Arulanandam BP, Lynch JM, Briles DE, Hollingshead S, Metzger DW. Intranasal vaccination with pneumococcal surface protein A and interleukin-12augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infection and Immunity 2001;69(November (11)):6718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kim SJ, Caton M, Wang C, Khalil M, Zhou ZJ, Hardin J, et al. Increased IL-12 inhibits B cells’ differentiation to germinal center cells and promotes differentiation to short-lived plasmablasts. Journal of Experimental Medicine 2008;205(September (10)):2437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]