Abstract
Introduction
Diabetes Mellitus is a chronic disease and a global epidemic. It is a known fact that co-morbidities, including Diabetes Mellitus, pose a higher risk of infection by COVID-19. Additionally, the outcomes following infection are far worse than in people without such co-morbities.
Factors contributing to the development of type 2 diabetes mellitus (T2DM) have long been established, yet this disease still bestows a substantial global burden. The aim was to provide a comprehensive review of the burden of diabetes pre-COVID-19 and the additional impact sustained by the diabetes population and healthcare systems during the COVID-19 pandemic, while providing recommendations of how this burden can be subsided.
Methodology
Literature searches were carried out on ‘Google Scholar’ and ‘PubMed’ to identify relevant articles for the scope of this review. Information was also collected from reliable sources such as the World Health Organisation and the International Diabetes Federation.
Results
T2DM presented with economic, social and health burdens prior to COVID-19 with an significant ‘Disability Adjusted Life Years’ impact. Whilst people with diabetes are more susceptible to COVID-19, enforcing lockdown regulations set by the Public Health department to reduce risk of infection brought about its own challenges to T2DM management. Through recommendations and adapting to new methods of management such as telehealth, these challenges and potential consequences of mismanagement are kept to a minimum whilst safeguarding the healthcare system.
Conclusion
By understanding the challenges and burdens faced by this population both evident pre-covid and during, targeted healthcare can be provided during the COVID-19 pandemic. Furthermore, implementation of targeted action plans and recommendations ensures the care provided is done in a safe and effective environment.
Electronic supplementary material
The online version of this article (10.1007/s40200-020-00656-4) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, Type 2 diabetes mellitus, Public Health, Telehealth, Management
Introduction
Diabetes Mellitus is a chronic disease resulting from the reduction in the body’s response to insulin production by the pancreas either due to an increase in insulin resistance or due to decreased insulin production [1]. In the past three decades, incidence of diabetes has quadrupled worldwide [2]. Diabetes has also classified as the 7th leading cause of death globally in 2016 [3]. According to the recent data published by the International Diabetes Federation, 463 million adults (20–79 years) suffered from diabetes in 2019. If not adequately controlled, the global diabetes prevalence is expected to increase by approximately 51% in 2045 [4]. The contributing factors for the development of type 2 diabetes (T2DM) can be broadly divided into genetic and environmental factors. Some specific risk factors include, obesity, smoking, leading a sedentary lifestyle, age and also the presence of Metabolic Syndrome [5, 6]. The pathophysiology and the underlying risk factors have long been established, yet the incidence of diabetes is still on a progressive incline [7] The 2019 coronavirus SARS-COV2 pandemic has further increased the burden on the diabetes population, those at risk of dyglcyaemic changes as well as the healthcare services [8]. The aim of this article was to provide a comprehensive review of the burden of diabetes pre-COVID-19 and the additional impact sustained by the diabetes population and healthcare systems during the COVID-19 pandemic, while providing recommendations of how this burden can be subsided.
Methodology
A literature search was carried out in July 2020 through ‘PubMed’ and ‘Google Scholar’ using the keywords ‘T2DM’, ‘COVID-19’ and ‘Public Health’. Articles were then filtered using several inclusion criteria including; English language, Human studies and Literature type. The latter mainly included Systematic reviews, Meta- Analysis and literature reviews. The authors then filtered the resulting articles by title and abstract and the remaining 65 articles which fitted the aim of this review were thus considered. Additionally, information was also collected from reliable reports such as those of the World Health Organization (WHO) and International Diabetes Federation (IDF).
Results
Burden of T2DM diabetes
Having an understanding of the impact of T2DM at an individual, community and population level is paramount for public health authorities and policymakers alike. The burden of a disease can be quantified in terms of the quality of life, morbidity, premature mortality, economic and healthcare impact [9].
Disability adjusted life years (DALYs)
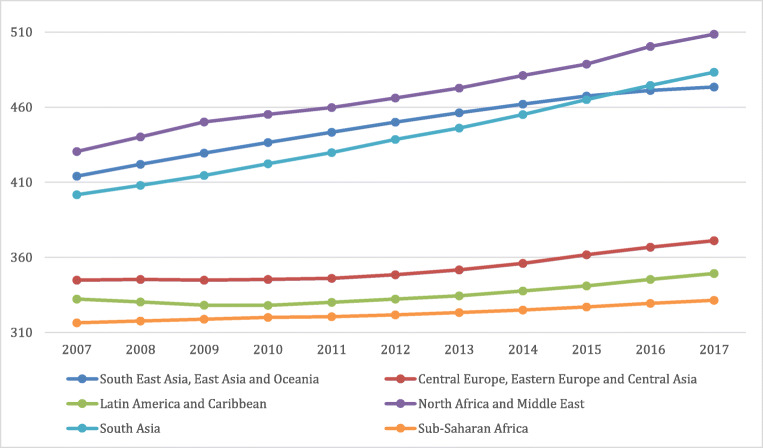
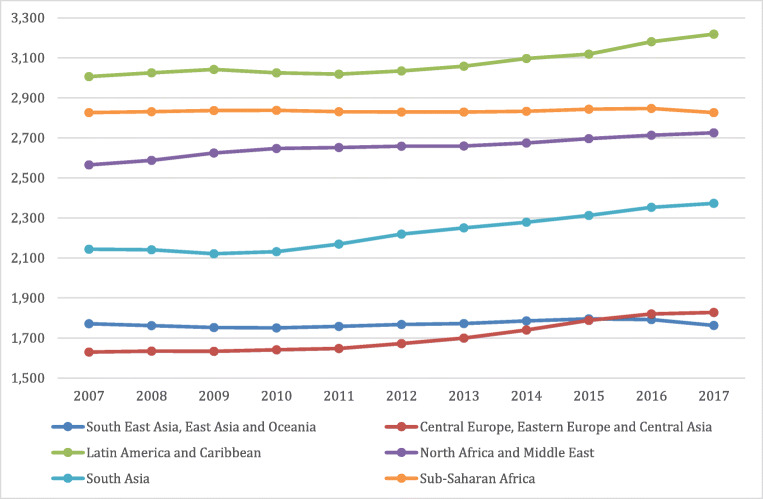
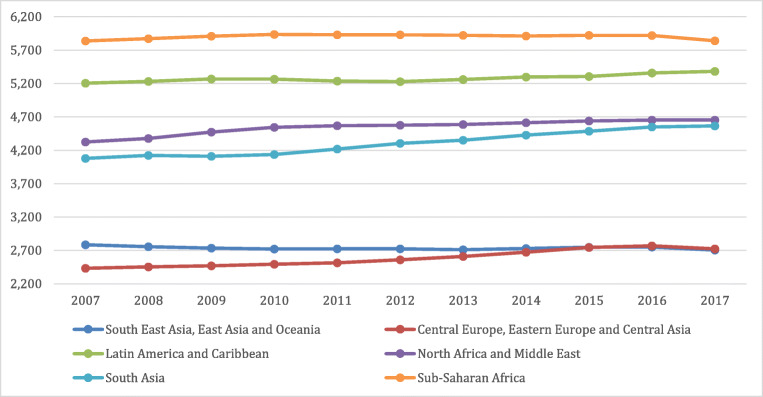
Public health policies and plans for provision of services all depend on the general population’s state of health and comorbid diseases which change over time. Developed in the 1990s, the DALYs metric is used to gauge the total burden of a disease by considering the number of years lost to a disease, premature mortality, or disability. It is also used to compare health and life expectancy globally. Such a calculation gives policy makers a better understanding of the overall duration of life in comparison to duration spent in poor or good health [9]. A global observation of the incline in DALYs across 10 years comparing individuals at different age categories (15–49 years, 50–69 years vs 70+ years) adapted from the Global Burden of Disease website can be seen in Figs. 1, 2 and 3 respectively. [10]. As seen in the graphs, the higher incidence of T2DM at a younger age range is contributing to the increase in DALYs. This will place a strain on healthcare costs and economic healthcare services as well as decreased work productivity and increased likelihood of early retirement or mortality. This will ultimately be a burden on the country’s economy.
Fig. 1.
Global DALYs Ages 15–49 [10]
Fig. 2.
Global DALYs Ages 50–69 [9]
Fig. 3.
Global DALYs Ages 70+ [9]
The universal rise in life expectancy has left policy makers questioning whether individuals maintain a good quality of life during these additional years, as reported by the Global Burden of Disease (GBD) Study and illustrated in Figs. 1, 2 and 3. Such evaluations are extremely relevant to decisions involving extension of retirement ages and health care stipulations. Namely to increase efforts for risk prevention of non-communicable diseases such as T2DM from early stage of the disease. Great inequalities between the burden of a disease and healthy life expectancy are present globally irrespective of a country’s quintile on the socio-demographic index or between sexes [11]. This implies that quantity is more prevalent than quality of life worldwide. The disabling outcomes of a disease such as T2DM has considerable implications for the health care system plans and disbursements [12].
Economic status and healthcare
T2DM presents with economic, social and health burdens not only for the individual but also for families and careers as well as the healthcare system. Additionally, employment is another social factor which is often impacted, leading to further strain on the country’s economy [13].
A country’s ability to prevent T2DM lies in the presence of an identification and targeting strategy aimed at high risk individuals. This is dependent on the infrastructure and human resources available with a consequential effect on the management plan of the diagnosed individuals [13]. Furthermore, statistical data regarding epidemiology would be essential for health care providers in the identification of the risk factors contributing to T2DM at a country level. This would aid in surveilling, diagnosing, monitoring as well as treating T2DM. In previous studies diabetes was reported to be more common with individuals with high socio-economic status [14–16]. In contrast, a recent study reported that a higher T2DM prevalence was associated with individuals having a lower socio-economic status due to limited access to health care and [17]. Moreover, this factor was also observed in low and middle income Asian countries experiencing fast economic advancement [16].
Susceptibility of COVID-19 among diabetes population
Coronaviruses are enveloped viruses known to cause respiratory infections in humans. Whilst most of these viruses are harmless and cause mild symptoms, a novel virus known as SARS (severe acute respiratory syndrome) - COV 2 as well as COVID-19 emerged in December of 2019, which proved to be more harmful than the previously known coronaviruses [18].
It is now a known fact that co-morbidities such as obesity, diabetes mellitus (DM), hypertension as well as advanced age all increase the chances of being infected with COVID-19 [19]. Additionally, reports from the Centres for Disease Control and Prevention stated that patients with diabetes and metabolic syndrome might be 10 times more likely to die due to COVID-19 [20].
There are several possible mechanisms which can make diabetic patients more susceptible to COVID-19. Some of these mechanisms include; impaired macrophage activity; impaired neutrophil recruitment and cytokine storm. However, the one mechanism which seems to be considered most is the increased viral load due to the virus entering the cells efficiently. In fact, the receptor which this virus uses is the Angiotensin-converting enzyme 2(ACE2) receptor which can be found expressed by various tissues including lungs, kidneys, pancreas and the heart [18, 21]. Firstly, the SARS CoV-2 Spike protein bind to the ACE2 cell surface where the S protein is then primed by the cellular proteases such as TMPRSS1 and furin. Priming involves cleaving the S protein at the S1/S2 domains, allowing the virus to fuse to the cell surface [18]. Virions are then taken up into endosomes where the SARS CoV 2 is cleaved and possibly activated by Cathepsin L [22] . Inside the cell SARS CoV 2 replicates itself whilst ACE catalyzes the conversion of Angiotensin I to Angiotensin II and ACE 2 converts Angiotensin II to Ang 1–7 [23]. Since ACE 2 receptors are also found in the pancreas, the entry of coronavirus in the pancreatic cells may result in acute beta cell dysfunction [21]. Finally this may lead to a state of acute hyperglycaemia which if left uncontrolled predisposes the diabetic individual to a greater risk of infection and also a higher chance of mortality [24, 25].
Certain medications prescribed to diabetic patients such as GLP-1 agonists, Angiotensin Receptor Blockers (ARB’s) and Angiotensin converting enzyme inhibitors (ACEI’s) are thought to upregulate ACE2 expression [26]. ACEI initially inhibits the Angiotensin converting enzyme (ACE) leading to decreased angiotensin I levels. This possibly causes a negative feedback loop that ultimately upregulates more ACE2 receptor which can now interact with the decreased Angiotensin I substrate available [27, 28]. Additionally, evidence of a 5-fold increase in ACE2 levels with lisinopril and a 3-fold increase in ACE2 levels with losartan was also published [26, 29]. Therefore, due to the ACE 2 receptor being expressed in various tissues as well as due to the upregulation of ACE2 receptor there is thus an increase in potential binding sites for SARS-CoV-2. This mechanism takes place in patients with diabetes and/or hypertension since they usually take ACEI or ARB’s. Hence, infection by COVID-19 may be more severe in these patients [30, 31]. Whilst the above mentioned mechanism seems to confirm that ARB’s and ACEI’s upregulate ACE2 expression, other studies which contradict this have been published. These studies claim that the administration of these medicines is actually beneficial to patients infected with COVID19 [32–35].
Challenges faced by diabetics during COVID-19
Lack of exercise
One of the many mitigation legislations put forward by governments along with public health authorities to contain the spread of COVID-19 was to institute social-distancing restrictions along with the closure of gyms and parks [36]. Furthermore, the population was advised to limit going out of their homes unnecessarily [25, 37]. Exercise has long been established to be an important requisite as part of the diabetes management and prevention plans [38] . Several studies carried out over the years found that lifestyle interventions including 150 min/week of physical activity and diet-induced weight loss of 5–7% reduced the risk of progression from impaired glucose tolerance (IGT) to Type 2 diabetes by 58% [39–41].
A systematic review and meta-analysis carried out on structured exercise interventions also concluded that structured exercise programs had a statistically and clinically significant beneficial effect on glycaemic control [42, 43]. Consequently, the World Health Organization (WHO) released a guideline called ‘Stay Physically Active during Quarantine’ which contains possible ways to stay active during COVID-19. The use of online classes and videos were encouraged as were frequent walking breaks around the house [44].
Increase in weight
The mandated lockdowns resulted in the limited access to fresh fruit and vegetables. Individuals including those with diabetes might have resorted to the consumption of long shelf-life canned or packaged foods that are typically high in calories and/or fats, with a potential increase in the consumption of carbohydrates [45–47]. Such food consumption increases the risk of weight gain and impose a higher cardiovascular, thrombotic and respiratory complications [48, 49]. The concurrent presence of obesity within the diabetes population poses additional detrimental effects on the functioning capabilities of the lungs lead to a decrease forced expiratory volume (FEV) and forced vital capacity (FVC) [50]. Additionally, it has been hypothesized that pulmonary lipofibroblasts together with normal adipocytes play a role in the pathogenic response of COVID-19. This is believed to be brought about by the increased expression of the ACE-2 receptors which turns the adipocytes into reservoirs for the virus. Moreover, the adipocytes aid in the transdifferentiation of lipofibroblasts into myofibroblasts leading to pulmonary fibrosis. Consequently, the presence of fibrosis leads to severe outcomes of the COVID-19 infection among the diabetes-obese population [51].
Mental stress and quality of sleep
A recurrent issue during lockdown appeared to be an increased ‘mental stress’ and changes in sleeping habits [52]. Anxiety mainly stemmed from contracting the virus, being restricted to the place of residence for a long period of time and also not being able to meet with loved ones [53]. The increased levels of anxiety were reported by more than 80% of the participants from North India who stated that they were worried about COVID-19, out of which 12.5% reported difficulties in sleeping [54]. Another study carried out in China reported that 53.8% of participants sustained a moderate to severe impact on their mental health due to COVID-19 pandemic [55]. Fig. 1 in the supplement material is a guideline released by the National Diabetes Service Scheme (Australia) intended in helping with management of worries and anxiety related to COVID-19 and diabetes [56]. Similarly, The European country of Malta also released a set of recommendations to help the local diabetic population in managing their condition as well as to reduce anxiety related to COVID-19 [57] The National Health Service (NHS) also published ‘GUIDANCE FOR: Supporting people with Diabetes during the COVID-19 pandemic’ which compiles informative websites that the diabetic population might need to access during these difficult times [58].Apart from these guidelines, a number of countries including the European country of Malta, set up designated helpline to provide aid to all those experiencing mental health issues including the diabetes population [36, 56–58].
Whilst COVID-19 and the subsequent stress can be a source of sleep disturbance, one has to also take into account diet; lifestyle and diseases [55]. In fact, shorter sleep duration and unstable sleeping patterns have been linked to obesity and cardiovascular problems [59, 60]. An association was also found between sleep disorders and patients with T2DM, where increased rates of insomnia, excessive sleeping during the day and a more frequent use of sleep medications were reported [61]. These changes in sleeping patterns may be due to the T2DM itself as well as due to complications which come with it such as polyuria and peripheral nephropathy [62].
Lack of vitamin D
Lockdown restrictions challenged individuals including those with diabetes with inadequate Vitamin D levels due to low sunlight exposure during this pandemic [25].
Vitamin D deficiency can lead to an increased mortality and morbidity due to COVID–19 [63] Vitamin D supplementation is not only thought to decrease the risk of infection but it is also being suggested as a cure for infection patients [64] Vitamin D has numerous mechanisms through which it decreases the risks of microbial infections and death. These mechanisms can be grouped into three main categories; physical barrier, cellular natural immunity and adaptive immunity [65].
Glycemic control
It was observed that infected elderly with diabetes had an elevated fasting blood glucose as opposed to their HbA1C which remained stable [66]. However, during the acute phase of the COVID-19 infection it is essential that strict glucose control is maintained to prevent the occurrence of complications [66, 67].
Public Health recommendations for diabetic patients
A number of healthcare recommendations and guidelines have been issued during these unprecedent times by different stakeholders including the Institute for Healthcare Excellence on managing the diabetic population pre-COVID-19 [68]. Examples of these recommendations can be found as part of the supplement material (Supplement Tables 1, 2 and 3).
Change in healthcare services due to COVID-19
Individuals with diabetes are not always able to self-cafe and modify drug doses, especially those in marginalised and disadvantaged populations as well as elderly deprived of social support. These populations are dependent on health professionals [69]. In such cases, where no designated point of reference is available, managing their own condition can place further psychological stress on the patients, which might have been the case during the COVID-19 lockdown periods. Complications arising from poorly managed blood glucose such as Diabetic Ketoacidosis raises the risk for morbidity and mortality. This will not only put a strain on an individual and the family unit but also on the health care system [70]. Most outpatient services were temporary halted during the pandemic whilst those that continued their services were challenged due to staff reduction as these were deployment to frontline duties or illnesses [36]. Hence, ensuring that delivery of care does not cease during this pandemic was a great feat.
Virtual care was a tool employed by many countries in an attempt to continue provision of service whilst also preventing nosocomial exposure to COVID-19. Telehealth was consequently beneficial for countries, such as USA,UK and India, when providing a service in distant locations with shortage of staff [71–73]. Using such technologies enabled imparted education to individuals with diabetes about changes in insulin dosing as well as general self-care. The ongoing communication empower individuals and allow them to independently manage their condition. Studies carried out prior to the pandemic indicated that virtual communication can successfully lower HbA1c [74]. Practitioners through telemedicine can further emphasize the importance of controlling glucose levels as well as relate the potentially improved outcomes of COVID-19 if encountered [75].
However, such a tool is not always viable due to limited accessibility, acceptance and knowledge on the use of technology. In fact, some individuals still requested to be seen in the traditional face-to-face setting [76]. Moreover, practitioners in developing countries should always consider financial implications of therapies on an individual. Simple treatment regimens and low-cost therapy should ideally be prescribed especially to underprivileged populations [75]. The guidelines observed in Supplement Fig. 3 have formulated by the British National Health System (NHS) to assess the risk of COVID-19 susceptibility before setting up an outpatient assessment or follow up [77].
Recommendations for changes in management
Healthcare professionals can potentially encounter clients who are awaiting result or have been confirmed as COVID-19 positive. Hence it is essential to encourage staff to wear PPEs whilst also adhering to recommended sanitisation procedures; especially in aerosol generating practices. Such procedures should also be enforced in hospital routine activities such as waste, food, utensil and laundry handling. Bornstein et al., compiled a list of guidelines for healthcare workers to follow when dealing with diabetic patients in different scenarios. These guidelines can be found in Fig. 2 in the supplementary material [78]. Easy and practical recommendations that were compiled by Wang et al., (2020) that can be relayed to patients are listed in Fig. 3 which can be accessed in the supplement material [77].
Management of non-Communicable Diseases during COVID-19
The extensive impacts on health revealed by this pandemic has demonstrated the vulnerability of individuals with non-communicable diseases (NCDs) [79]. A study carried out in Italy showed that 96% of patients that died in hospitals had previous comorbidities, with T2DM being second highest amongst hypertension, malignant tumours, cardiac and respiratory diseases [80]. The link between NCD and COVID-19 mortality has also been made in USA, China and Spain [79, 81, 82].
Measures undertaken for NCDs included quarantine and physical distancing. This could potentially result in poor management of the condition by both the patient - through behavioural risk factors - and the healthcare professional [83]. Rescheduling of routine medical tests and appointments can further hinder management as well as limited access to primary healthcare centres, pharmacies and transport. All these factors will make it tougher to ensure continuity of care. Research from other pandemics indicates that exacerbation of NCDs occurs without proper healthcare management [84]. This is due to stress that is brought about by changes in routine, uncertain economic situations and new regulations which will ultimately raise rates of disability, morbidity and mortality in patients with NCDs [79].
The importance of T2DM management to avoid serious repercussions on health and overall economy is not a new concept. Hence it is important to equip patients with the right knowledge about the current pandemic and its possible effects on their overall health. It is crucial, now more than ever, to ensure that patients have direct contact with a healthcare practitioner to mitigate any queries or concerns that they may have. This will ultimately empower individuals to adhere to recommendations whilst also avoiding extra stressors which may exacerbate hyperglycaemic effects such as kidney failure, amputation, nerve damage and heart disease [85].
Conclusion
T2DM has been a global burden for decades; however, additional burden has been imposed with the onset of COVID-19 pandemic. Consequently, at a global level, healthcare systems as well as the diabetes population were impacted during this pandemic. Mitigation restrictions that were aimed to curb the spread may have imposed a higher burden on the diabetes population. Having an understanding of the different challenges and subsequent burden faced by this vulnerable population will enable healthcare professional, healthcare provision and policy makers to provide targeted action plans.
Electronic supplementary material
(DOCX 102 kb)
Funding
No funding was received to conduct this study.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Justine Sciberras, Email: justine.sciberras.15@um.edu.mt.
Lara Maria Camilleri, Email: lara.m.camilleri.14@um.edu.mt.
Sarah Cuschieri, Email: sarah.cuschieri@um.edu.mt.
References
- 1.Health P., “Diabetes: a National Public Health Priority - proposal for a National Strategy for diabetes,” Consult. Doc., 2015.
- 2.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 3.W. health O. (WHO), “Diabetes: Key Facts,” 2020. [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- 4.International Diabetes Federation, “Global Fact Sheet 2019,” IDF Diabetes Atlas, no. 9th Edition, 2019.
- 5.Hull R. L. Utzschneider K. M. Kahn S E, “Mechanisms linking obesity to insulinresistance and type 2 diabetes.,” Div. Metab. Endocrinol. Nutr. Dep. Med. VA Puget Sound Heal. Care Syst. Univ. Washington, 1660 South Columbian Way, Seattle, Washingt. 98108, USA., 2006.
- 6.A. American et al., “Diagnosis and managment of the metabolic syndrome AHA / NHLBI Scientific Statement,” pp. 2735–2752, 2006.
- 7.Cuschieri S. Type 2 diabetes – an unresolved disease across centuries contributing to a public health emergency. Diabetes Metab Syndr Clin Res Rev. 2019;13(1):450–453. doi: 10.1016/j.dsx.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Pal R., Bhadada S. K., “COVID-19 and diabetes mellitus: An unholy interaction of two pandemics,” no. January, 2020. [DOI] [PMC free article] [PubMed]
- 9.McGrath R, Al Snih S, Markides K, Hall O, Peterson M. The burden of health conditions for middle-aged and older adults in the United States: disability-adjusted life years. BMC Geriatr. 2019;19(1):100. doi: 10.1186/s12877-019-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.I. for H. M. and E. (IHME), “GBD compare data visualization,” IHME Website, 2020.
- 11.Dickman SL, Himmelstein DU, Woolhandler S. Inequality and the health-care system in the USA. Lancet. 2017;389(10077):1431–1441. doi: 10.1016/S0140-6736(17)30398-7. [DOI] [PubMed] [Google Scholar]
- 12.Kyu HH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roglic G., Global report on diabetes. World Health Organization, 2016.
- 14.Fagg J, Valabhji J. How do we identify people at high risk of type 2 diabetes and help prevent the condition from developing? Diabet Med. 2019;36(3):316–325. doi: 10.1111/dme.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley L, Cowan M. World Health Organization noncommunicable diseases country profiles. Geneva: Switz. WHO Libr. Cat. Data; 2014. [Google Scholar]
- 16.Wu H., Meng X., Wild S. H., Gasevic D., Jackson C. A., Socioeconomic status and prevalence of type 2 diabetes in mainland China, Hong Kong and Taiwan: a systematic review, J. Glob. Health, vol. 7, no. 1, 2017. [DOI] [PMC free article] [PubMed]
- 17.Anjana RM, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5(8):585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 18.Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Metab. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajpal A., Rahimi L., Ismail-Beigi F., Factors leading to high morbidity and mortality of COVID-19 in patients with type 2 diabetes, J. Diabetes, 2020. [DOI] [PMC free article] [PubMed]
- 20.Bornstein SR, Dalan R, Hopkins D, Mingrone G, Boehm BO. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020;16(6):297–298. doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J-K, Lin S-S, Ji X-J, Guo L-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann M, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wösten-Van Asperen RM, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 24.Yang JK, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 25.Cuschieri S., Grech S., COVID-19 and diabetes: The why, the what and the how, J. Diabetes Complications, p. 107637, 2020. [DOI] [PMC free article] [PubMed]
- 26.Ferrario CM, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 27.Amraei R, Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9(7):1–18. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rico-Mesa JS, White A, Anderson AS. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep. 2020;22(5):20–23. doi: 10.1007/s11886-020-01291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins J., Preventing a covid-19 pandemic, BMJ, vol. 368, no. February, pp. 1–2, 2020. [DOI] [PubMed]
- 30.Lei F, George K, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet. 8(April):e21, 20AD. [DOI] [PMC free article] [PubMed]
- 31.Drucker DJ. Coronavirus infections and type 2 diabetes—shared pathways with therapeutic implications. Endocr. Rev. 2020;41(3):bnaa011. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mcmurray J. J. V, Pfeffer M. A., Ph D., Solomon S. D., “Spe ci a l R e p or t Renin – Angiotensin – Aldosterone System Inhibitors in Patients with Covid-19,” pp. 1653–1659, 2020. [DOI] [PMC free article] [PubMed]
- 34.Pranata R, et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14(5):983–990. doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam K. W. et al., “Continued In-Hospital Angiotensin-Converting Enzyme Inhibitor and Angiotensin II Receptor Blocker Use in Hypertensive COVID-19 Patients Is Associated With Positive Clinical Outcome,” J. Infect. Dis., vol. 11794, 2020. [DOI] [PMC free article] [PubMed]
- 36.Cuschieri S., “COVID-19 panic, solidarity and equity—the Malta exemplary experience,” J. Public Heal., no. May, 2020. [DOI] [PMC free article] [PubMed]
- 37.Kipps C., Hamer M., Hill N., Lorgelly P., Enforced inactivity in the elderly and diabetes risk: initial estimates of the burden of an unintended consequence of COVID-19 lockdown, medRxiv, 2020.
- 38.Sato Y, Nagasaki M, Kubota M, Uno T, Nakai N. Clinical aspects of physical exercise for diabetes/metabolic syndrome. Diabetes Res Clin Pract. 2007;77(3 SUPPL):87–91. doi: 10.1016/j.diabres.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 39.Cuschieri S, et al. Prevalence of obesity in Malta. Obes Sci Pract. 2016;2(4):466–470. doi: 10.1002/osp4.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eriksson KF, Lindgärde F. No excess 12-year mortality in men with impaired glucose tolerance who participated in the Malmo preventive trial with diet and exercise. Diabetologia. 1998;41(9):1010–1016. doi: 10.1007/s001250051024. [DOI] [PubMed] [Google Scholar]
- 41.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JH, Lee YE. Effects of exercise on glycemic control in type 2 diabetes mellitus in Koreans: the fifth Korea National Health and nutrition examination survey (KNHANES V) J Phys Ther Sci. 2015;27(11):3559–3564. doi: 10.1589/jpts.27.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nieman DC. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and Meta-analysis. Yearb Sport Med. 2012;2012:214–215. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 44.W. H. Organization . Stay physically active during self-quarantine. Denmark: World Health Organization Copenhagen; 2020. [Google Scholar]
- 45.Wicaksana A. L., Hertanti N. S., Ferdiana A., Pramono R. B., Diabetes management and specific considerations for patients with diabetes during coronavirus diseases pandemic: a scoping review, Diabetes Metab. Syndr. Clin. Res. Rev., 2020. [DOI] [PMC free article] [PubMed]
- 46.Pellegrini M, et al. Changes in weight and nutritional habits in adults with obesity during the ‘lockdown’ period caused by the COVID-19 virus emergency. Nutrients. 2020;12(7):1–11. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz-Roso MB, et al. Covid-19 confinement and changes of adolescent’s dietary trends in Italy, Spain, Chile, Colombia and Brazil. Nutrients. 2020;12(6):1–18. doi: 10.3390/nu12061807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braekkan S, Siegerink B, Lijfering W, Hansen JB, Cannegieter S, Rosendaal F. Role of obesity in the etiology of deep vein thrombosis and pulmonary embolism: current epidemiological insights. Semin Thromb Hemost. 2013;39(5):533–540. doi: 10.1055/s-0033-1343355. [DOI] [PubMed] [Google Scholar]
- 49.Van Dam RM, Seidell JC. Carbohydrate intake and obesity. Eur J Clin Nutr. 2007;61:S75–S99. doi: 10.1038/sj.ejcn.1602939. [DOI] [PubMed] [Google Scholar]
- 50.Sattar N., McInnes I. B., McMurray J. J. V, Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms, Circulation, 2020. [DOI] [PubMed]
- 51.Kruglikov I. L., Scherer P. E., The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections, Obesity, 2020. [DOI] [PMC free article] [PubMed]
- 52.Marelli S. et al., Impact of COVID-19 lockdown on sleep quality in university students and administration staff, J. Neurol., no. 0123456789, 2020. [DOI] [PMC free article] [PubMed]
- 53.Banerjee M., Chakraborty S., Pal R., Diabetes self-management amid COVID-19 pandemic, Diabetes Metab. Syndr. Clin. Res. Rev., 2020. [DOI] [PMC free article] [PubMed]
- 54.Ghosh A., Arora B., Gupta R., Anoop S., Misra A., Effects of nationwide lockdown during COVID-19 epidemic on lifestyle and other medical issues of patients with type 2 diabetes in North India, Diabetes Metab. Syndr. Clin. Res. Rev., 2020. [DOI] [PMC free article] [PubMed]
- 55.Wang C, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int. J. Environ. Res. Public Health. 2020;17(5):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Diabetes Service Scheme, Managing worry about COVID-19 and diabetes, no. March, 2020.
- 57.M. for Health and Malta, “Covid19 outbreak – Recommendations on self-care for people living with diabetes,” vol. 21, no. 1, pp. 1–9, 2020.
- 58.Skinner T, Speight J. Supporting people with diabetes during a pandemic. Diabet Med. 2020;37(7):1155–1156. doi: 10.1111/dme.14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayes JF, Balantekin KN, Altman M, Wilfley DE, Taylor CB, Williams J. Sleep patterns and quality are associated with severity of obesity and weight-related behaviors in adolescents with overweight and obesity. Child Obes. 2018;14(1):11–17. doi: 10.1089/chi.2017.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hasler G, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27(4):661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 61.Kryger M., Salamon E, Skomro RP, Ludwig S, Sleep complaints and restless legs syndrome in adult type 2 diabetics, vol. 2, no. 5, pp. 417–422, 2001. [DOI] [PubMed]
- 62.A. D. Association Comprehensive medical evaluation and assessment of comorbidities. Diabetes Care. 2017;40(January):S25–S32. doi: 10.2337/dc17-S006. [DOI] [PubMed] [Google Scholar]
- 63.Singh SK, Jain R, Singh S. Vitamin D deficiency in patients with diabetes and COVID-19 infection: Diabetes Metab. Syndr. Clin. Res. Rev; 2020. [DOI] [PMC free article] [PubMed]
- 64.Grant WB, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rondanelli M. et al., Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds - Practical Advice on Dosages , Evidence-based Complement. Altern. Med., vol. 2018, 2018. [DOI] [PMC free article] [PubMed]
- 66.Xue T. et al., Blood glucose levels in elderly subjects with type 2 diabetes during COVID-19 outbreak: a retrospective study in a single center, Available SSRN 3566198, 2020.
- 67.Naruse K. Does glycemic control rescue type 2 diabetes patients from COVID-19-related deaths? J Diabetes Investig. 2020;11(4):792–794. doi: 10.1111/jdi.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown P. Type 2 diabetes risk identification and prevention: NICE public health guidance 38 in practice. Diabetes Prim. Care. 14(5):2012.
- 69.Myers AK, et al. Perceptions of insulin pen use and technique in black and Hispanic/Latino patients with type 2 diabetes: a qualitative study. J Racial Ethn Heal Disparities. 2020:1–9. [DOI] [PubMed]
- 70.Peters AL, Garg SK. The silver lining to COVID-19: avoiding diabetic ketoacidosis admissions with telehealth. Diabetes Technol Ther. 2020;22(6):449–453. doi: 10.1089/dia.2020.0187. [DOI] [PubMed] [Google Scholar]
- 71.Jones MS, Goley AL, Alexander BE, Keller SB, Caldwell MM, Buse JB. Inpatient transition to virtual care during COVID-19 pandemic. Diabetes Technol Ther. 2020;22(6):444–448. doi: 10.1089/dia.2020.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joshi R., Atal S., Fatima Z., Balakrishnan S., Sharma S., Joshi A., Diabetes care during COVID-19 lockdown at a tertiary care centre in India, Diabetes Res. Clin. Pract., vol. 166, 2020. [DOI] [PMC free article] [PubMed]
- 73.Wake D. J. et al., Endocrinology in the time of COVID-19: Remodelling Diabetes Services and Promoting Innovation, Eur. J. Endocrinol., vol. 1, no. aop, 2020. [DOI] [PMC free article] [PubMed]
- 74.Zhai Y., Zhu W., Cai Y., Sun D., Zhao J., Clinical-and cost-effectiveness of telemedicine in type 2 diabetes mellitus: a systematic review and meta-analysis, Medicine (Baltimore)., vol. 93, no. 28, 2014. [DOI] [PMC free article] [PubMed]
- 75.Misra A, Bloomgarden Z. Diabetes during the COVID-19 pandemic: a global call to reconnect with patients and emphasize lifestyle changes and optimise glycemic and blood pressure control: J. Diabetes; 2020. [DOI] [PMC free article] [PubMed]
- 76.Anjana R. M. et al., “ACCEPTABILITY AND UTILISATION OF NEWER TECHNOLOGIES AND EFFECTS ON GLYCEMIC CONTROL IN TYPE 2 DIABETES–LESSONS LEARNT FROM LOCKDOWN,” Diabetes Technol. Ther., no. ja, 2020. [DOI] [PubMed]
- 77.Wang W, Lu J, Gu W, Zhang Y, Liu J, Ning G. Care for diabetes with COVID-19: advice from China. J Diabetes. 2020;12(5):417–419. doi: 10.1111/1753-0407.13036. [DOI] [PubMed] [Google Scholar]
- 78.Bornstein S. R. et al., Practical recommendations for the management of diabetes in patients with COVID-19, lancet Diabetes Endocrinol., 2020. [DOI] [PMC free article] [PubMed]
- 79.Kluge HHP, et al. Prevention and control of non-communicable diseases in the COVID-19 response. Lancet. 2020;395(10238):1678–1680. doi: 10.1016/S0140-6736(20)31067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Istituto Superiore di Sanità, “Characteristics of SARS-CoV-2 patients dying in Italy. Report based on available data on April 29, 2020. COVID-19.,” 2020.
- 81.Richardson S, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area: Jama; 2020. [DOI] [PMC free article] [PubMed]
- 82.Novel CPERE. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua liu xing bing xue za zhi= Zhonghua liuxingbingxue zazhi. 2020;41(2):145. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 83.Venema V., Coronavirus: should I worry about my lockdown eating?, BBC News, vol. 25, 2020.
- 84.Slama S, et al. Care of non-communicable diseases in emergencies. Lancet. 2017;389(10066):326–330. doi: 10.1016/S0140-6736(16)31404-0. [DOI] [PubMed] [Google Scholar]
- 85.da Gama CAP, Guimarães DA, Cardoso CS, da Silva J, Oliveira CDL. Difficulties to treatment adherence according to the perception of people living with type 2 diabetes. Int J Health Sci (Qassim) 2019;7(2):29–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 102 kb)