Abstract
Commercial sparkling wine production represents a relatively low but important part of the Croatian wine production, especially in the Zagreb county. This study presents the results of volatile aroma compounds profile and organic acid composition of commercial sparkling wine samples from three vine-growing regions in Zagreb county. In total, 174 volatile aroma compounds were identified, separated between their chemical classes (aldehydes, higher alcohols, volatile phenols, terpenes, C13-norisoprenoids, lactones, esters, fatty acids, sulfur compounds, other compounds, other alcohols). Higher alcohols such as phenylethyl and isoamyl alcohol as well as 2-methyl-1-butanol, and esters such as diethyl succinate, ethyl hydrogensuccinate, and ethyl lactate had the strongest impact on the volatile compounds profile of Zagreb county sparkling wine. The presence of diethyl glutarate and diethyl malonate, compounds whose concentrations are influenced by yeast autolysis or caused by chemical esterification during the ageing process, was also noted. The influence of every single volatile aroma compound was evaluated by discriminant analysis using forward stepwise model. The volatile profiles of traditional sparkling wines from Croatia were presented for the first time. It is hoped the results will contribute to better understanding the quality potential and to evaluate possible differences on the bases of detected aroma concentrations and multivariate analysis.
Keywords: sparkling wines, volatile aroma compounds, Zagreb county, discriminant analysis
1. Introduction
According to International Organisation of Vine and Wine (OIV), in 2018, world sparkling wine production reached 20 million hectoliters with an overall increase of +57% since 2002. In global sparkling wine production, almost half of the total volume produced comes from Italy (27%) and France (22%), followed by Germany (14%), Spain (11%), and USA (6%) [1]. For the past ten years, Croatia has also recorded apparent increase in sparkling wines production, with the Zagreb County as one of the leading wine-growing counties. According to the Croatian Agency for Agriculture and Food data, in 2017, 885.80 hL of sparkling wines were produced in that area with a continuous upward trend. In Croatia almost all sparkling wines are produced by the traditional method, where marked influence can be connected to grape variety. Pjenušac is a quality sparkling wine (Protected Geographical Indication) elaborated by the traditional method that is defined by an excess pressure higher than 3.5 bar, primarily connected with presence of carbon dioxide in solution that is kept at the temperature of 20 °C, and for which alcoholic concentration of the cuvées used for their production have at least 9% volume (Council Regulation (EC), 1308/2013). In Zagreb county, ‘‘Pjenušac’’ is mainly produced from Riesling and Chardonnay grape cultivars and Pinot Noir between the red ones. However, there is also a great diversity of other grape varieties as Manzoni, Portugizer, Muller Thurgau, as well as Kraljevina and Plavec žuti, presenting a quality that can obtain high quality natural sparkling wines with their own personality and sensory profile. However, no work dealing with the influence of these grape cultivars for sparkling wine production have been done. The quality of sparkling wines is mainly influenced by their aroma composition and properties of single aroma compound present [2,3,4]. The sparkling wines aroma composition is formed by the interaction of different factors, such as grape variety and its maturity level, the production technology, the primary and secondary methods of wine fermentation, type of yeast strain used, storage temperature, ageing period, and ingredients used for liqueur d’expedition, level of oxygen during the process of disgorging, type of closure used, and levels of SO2 as well as CO2 [5,6]. According to Kemp et al. [6] wines used in the dosage solutions can have strong impact on volatile compounds concentrations, more than concentration of added sugar. An increase of ethyl esters, such as diethyl succinate, alcohols, and some varietal aromas, such as TDN (1,1,6-trimethyl-1,2-dihydronapthalene) and vitispirane, connected with fermentation in the bottle was noted by several authors [7,8,9], while concentrations of acetic acid esters and fatty acids diminish because of their clevage to the yeast cell walls [7,9]. In the work by Muñoz-Redondo et al. [10] some ester compounds were pointed out as markers of the second fermentation. Aroma changes can be further modified during the ageing on lees, so, therefore, the ageing time can determine the volatile aroma profile present in the sparkling wine [7,11,12]. Loyaux et al. [13] studied the aroma composition changes during the champagne ageing period and detected a slow decrease of isoamyl butyrate and hexyl acetate levels, as well as nerolidol concentrations and an increase in benzaldehyde and vitispirane levels. Over a period of 16 years, the concentrations of benzaldehyde increased up to 4 mg L−1. Environmental factors such as terrain structure, agro- and amphelo-pedological characteristics, climate and viticulture practices used, often described as “terroir”, can also have strong influence on grape composition and wine quality. Geographical origin also has an significant role in the differentiation of wines, since it can indicate the resemblance among wines coming from the one specific vine-growing region and the main differences among the ones coming from several viticultural regions [14,15]. Wine aroma precursors, as well as most wine components, are mainly accumulated during the grape maturation process in the vineyard. They can form a recognizable pattern in the grapes that can enhances the unique nature and specific structure of wines. Studies by Goldner et al. [16] and Vilanova et al. [17] have demonstrated differences in the sensory characteristics of Malbec and Albariño wines from different geographic origins. Robinson et al. [18] noted that the volatile aroma profile of certain type of wine can have marked impact in obtaining a geographical designation by forming a product with characteristics of specific vine-growing area. Nowadays, Voce et al. [19] carried out a comprehensive mapping of sparkling wines samples according to their volatile aroma compounds from Trentodoc and Franciacorta to determine regional features among them. The main target of this research was to define the volatile compounds profile in a relatively significant number of sparkling wine samples from three vine-growing regions in Zagreb county and to evaluate possible differences on the bases of detected aroma concentrations and multivariate analysis. From our experience, this work represents the first definition of the chemical structure of Zagreb county sparkling wines.
2. Results and Discussion
2.1. Composition of Organic Acids
Organic acid profile of Croatian sparkling wines from three Zagreb county vine-growing regions is presented in Table 1. There was no significant difference observed among the sparkling wines in terms of main organic acids as well as pH values. The most abundant acid was tartaric with an average concentration between 2.06 and 2.35 g L−1, values similar to ones published by Focea et al., Caliari et al. and Gallardo-Chacón et al. [20,21,22], but much higher compared to results published by Sartor et al. [23]. Conversely, malic acid concentrations were relatively low when compared to literature data by Caliari et al. and Sartor and al. [21,23], and ranged between 0.81 and 1.31 g L−1. It is well known that, in the sparkling wine elaboration process, the grapes must be usually harvested before they are completely matured [24]. The lactic acid concentrations varied according to the region, and could be influenced by grape composition, as well as by yeast activity, formed from malic acid degradation. The concentration of succinic acid, formed during the fermentation process, was lower compared to data obtained in previous studies [21,23]. Citric acid was present in all sparkling wines samples contrary to the data obtained by Caliari et al. and Sartor and al. [21,23] where it was not detected, but in agreement with work by Gallardo-Chacón et al. [22].
Table 1.
Organic acid composition (g L−1) of sparkling wines from different vine-growing regions.
| Parameters | Vine Growing Regions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plešivica (n = 19) | Zelina (n = 8) | Krašić (n = 3) | |||||||
| MIN | MAX | Mean ± SD | MIN | MAX | Mean ± SD | MIN | MAX | Mean ± SD | |
| Tartaric acid | 1.28 | 3.77 | 2.35 ± 0.70 | 1.72 | 2.91 | 2.23 ± 0.40 | 1.61 | 2.50 | 2.06 ± 0.62 |
| Malic acid | 0.12 | 1.61 | 0.81 ± 0.43 | 0.43 | 2.02 | 1.20 ± 0.55 | 0.99 | 1.62 | 1.31 ± 0.44 |
| Citric acid | 0.04 | 0.51 | 0.18 ± 0.12 | 0.06 | 0.59 | 0.26 ± 0.15 | 0.06 | 0.08 | 0.07 ± 0.01 |
| Succinic acid | 0.01 | 0.50 | 0.13 ± 0.14 | 0.01 | 0.28 | 0.11 ± 0.08 | 0.03 | 0.07 | 0.05 ± 0.02 |
| Lactic acid | 0.01 | 0.27 | 0.10 ± 0.09 | 0.07 | 0.36 | 0.16 ± 0.09 | 0.10 | 0.13 | 0.12 ± 0.09 |
| pH value | 2.89 | 3.56 | 3.16 ± 0.17 | 3.05 | 3.39 | 3.16 ± 0.10 | 3.20 | 3.45 | 3.33 ± 0.10 |
MIN-minimum value, MAX-maximum value, SD-standard deviation.
2.2. Volatile Compounds
One hundred and seventy-one volatile compounds presented in sparkling wines from three different Zagreb county vine-growing regions were detected, quantified and classified into several chemical classes (aldehydes, higher alcohols, volatile phenols, terpenes, C13-norisoprenoids, lactones, esters, fatty acids, sulfur compounds, other compounds, other alcohols). In Table 2, the average values of main volatile compounds chemical classes are presented, showing a significant difference among vine-growing regions while individual volatile compounds are presented in Table 3. The most abundant class was higher alcohols group with the highest concentrations of isoamyl and phenylethyl alcohol as well as 2-methyl-1-butanol. Comparing these compounds among vine-growing regions shows that sparkling wines from Zelina had significantly the highest concentrations. Data from the work by Torrens et al., Caliari et al., and Torchio et al. [9,25,26] also showed that major aromatic compound was phenylethyl alcohol, with OAV > 1 having influence on the sweet, rose and honey aroma structure of sparkling wines. The concentrations of higher alcohols not exceeding the amount of 300 mg L−1 can positively influenced the formation of wine complexity [27] which was not the case in our samples. Besides above mentioned compounds cis-3-hexene-1-ol, had also an impact on “green grass” odour profile of Zagreb county sparkling wines, especially in some samples from Plešivica vine-growing region. Representatives of alcohols that are also characterized by “green” and “herbaceous” notes, such as trans-1-hexanol, and cis-2-hexene-1-ol, and trans-3-hexen-1-ol, which are mostly synthetized during the pre-fermentation wine production process, were detected, but in concentrations under the defined odour threshold value in all sparkling wines samples analysed. Yeast contact and storage time on lees during sparkling wine production might have been the reason for relatively higher concentrations of 1-hexanol that ranged from 1612 to 2948 µg L−1, concentrations that are in agreement with data reported by [28]. As it can be seen from the Table 3, the presence of 1-hexanol was significantly the highest in sparkling wines from Krašić while there were no marked differences between other two regions. According to Alexandre et al. [29] and Benucci et al. [30], esters are the main class of aroma compounds released by the degradation of yeast cells having low perception thresholds and so positively contributing to the aroma of fruit as well as floral-like aroma of sparkling wine. Significantly, the highest amount of esters was detected in sparkling wines from Zelina while there was no marked difference between the other two vine-growing regions. Also, it can be seen that total esters concentration was more or less similar or something higher when compared to the data published by Benucci et al. [30]. Among esters presented in the analysed sparkling wines, the most common were diethyl succinate, ethyl hydrogensuccinate, and ethyl lactate, which is comparable with the results published in the work by Benucci et al. [30], while the ones with the OAV > 1 were ethyl butanoate, hexanoate, octanoate, ethyl-2-methylbutanoate, ethyl-3-methylbutanoate, isoamyl acetate, and isoamyl lactate. Comparable results were achieved by Voce et al. [19] in Ribolla Gialla sparkling wines where esters had an important role in volatile profile structure. Ethyl decanoate (floral) and 2-phenylethyl acetate (scent of rose) were detected in all sparkling wines in concentrations under the odour threshold values but according to Genovese et al. [31] these compounds can show synergistic effect even at low concentrations. The concentrations of 2-phenylethyl acetate published by Torchio et al. [26] were comparable with our data (23.40 to 28.73 µg L−1). According to Torrens et al. [9] and Riu-Aumatell et al. [11] diethyl succinate and ethyl lactate are considered as “ageing esters” whose concentrations can increase in contact with yeast cells during the second fermentation. For the development of cava, diethyl succinate can be used as a marker, mainly connected with the period of cava storage in the cellar [11]. In Zagreb county sparkling wines its concentrations were between 3917.45 µg L−1 (Krašić) up to 7430.69 µg L−1 (Zelina) which is compared to Ribolla Gialla wines (2555 µg L−1) higher but compared to concentrations published by Martinez-Garcia et al. [32] ranging between 8900 µg L−1 and 15,000 µg L−1 much lower. Among other ester compounds detected in Zagreb county sparkling wines isobutyl lactate, ethyl-2-hydroxy-3-methylbutanoate, diethyl hydroxysuccinate and isobutyl lactate concentrations were significantly higher in Zelina vine-growing region wines while ethyl vanillate, phenyl acetate and ethyl-hydroxyhexanoate concentrations were significantly the highest in wines from Plešivica vine-growing region. In analysed sparkling wines, the presence of diethyl glutarate and diethyl malonate, compounds, whose concentrations are influenced by yeast autolysis or caused by chemical esterification during the ageing process, was detected. By the use of chemometric analysis, diethyl malonate was pointed out as one of the most important compounds having strong influence in the Chardonnay wines differentiation [33]. In the work by Carlin et al. [34], the above mentioned compounds were also reported with concentrations of diethyl glutarate (5.8–7.3 µg L−1) similar to our data (12.0–19.9 µg L−1). Sparkling wines from Krašić stood out with significantly the highest concentration of diethyl malonate, while there was no significant difference in diethyl glutarate concentrations among tested wines. Terpens as a large group of wine aroma compounds primarly characterized by floral aroma are translocated from the grape to the must during the pressing and settling process in free volatile form or bound to sugars. In wines, according to Bordiga et al. [35] the transformation of the monoterpenes is linked to corresponding pyranic and furanic oxides or reduction by yeast membrane incorporation and acetylation [36]. Changes in the aroma characteristics during wine maturation were investigated by Oliveira et al. [37], showing a marked increase in monoterpenic oxides and decrease in monoterpenic alcohols. In our research, the presence of trans and cis linalool oxide, furan as well as geranyl acetate was detected in sparkling wines from all three vine-growing regions. Tetrahydrolinalool was significantly the most abundant terpene in sparkling wines from Plešivica and Zelina vine-growing region, while the significantly highest concentrations of nerol, terpene-4-ol and geraniol were detected in wines from Zelina while terpendiol II and hotrienol was most common in wines from Krašić. According to Caliari et al. [25] the main monoterpenes presented in their work were hotrienol, geraniol, linalool, citronellol, α- terpineol and the oxide forms of linalool. In all sparkling wines, the odour threshold value of linalool was above one. This corresponds to our data pointing out linalool, geraniol and hotrienol as the compounds with OAV > 1. Among C13-norisoprenoids compounds detected β-damascenone and TDN were the most common with the significantly highest total concentration above odour detection threshold in sparkling wines from Zelina vine-growing region sparkling wines. TDN originate from carotenoid degradation that is influenced by the ageing process linked to acid-catalysed reactions [9]. Also, according to Marais et al. [38], the TDN levels were remarkably higher in grapes that had more sunlight during maturation than in grapes from shaded locations. So, there is a reason to point out a potential impact of pruning level as well as leaf removal on carotenoid levels [34]. In the work by Francioli et al. [7], TDN was pointed out as a compound that, together with diethyl succinate and vitispirane, can discriminate cavas aged >20 months. A significant difference was also detected in total fatty acids concentrations probably being connected with the different grapes origin, the concentration of lipid substances in the must and differences in winemaking conditions used [19]. The most representative fatty acids similar to data published by Voce et al. [19] were hexanoic, octanoic and decanoic acid with the highest concentrations detected in Plešivica vine-growing region sparkling wines and average concentrations higher than their odour detection threshold. These acids, depending on the concentration, can have negative role in the development of wine sensory profile [9,21], but Shinohara’s [39] data pointed out that, if the concentrations are from 4 to 10 mg L−1, they can positively influenced wine aroma, while if their concentrations are more than 20 mg L−1 they can negatively influence the organoleptic profile of wines what was not the case in our study. Among sulfur compounds detected in analysed sparkling wines 4-methylthio-1-butanol was previously pointed by Rapp [27] as a potential contributor to wine aroma. Its formation can be linked to the degradation of amino acids containing sulfur or as a process connected to sulfur pesticides degradation that are used in the grape protection [40]. Another identified sulfur compound was 3-methylthio-1-propanol which at the contrary has negative influence to aroma mainly due to odour descriptor defined as boiled potatoes, but in our work with no direct impact because of relatively high odour detection threshold. Influence of ageing and storage on lees on the concentration of some fermentative sulfur compounds during sparkling wine production was investigated by Fedrizzi et al. [41], showing significant increments for 4-methylthio-1-butanol as well as 3-methylthio-1-propanol. This result supports the assumption of an analogue synthesis pathway starting from homomethionine as published in the work by Rapp et al. [42]. As can be seen from the results presented in Table 3, wines from Zelina had significantly the highest amount of 4-methylthio-1-butanol while wines from Plešivica stood out with significantly the highest concentrations of 3-methylthio-1-propanol. Among volatile components lactones, mainly γ-lactones and whiskey lactones can influence wine aroma by adding “fruity”, “coconut-like” and “peach-like” notes. Lactones mostly arise from cyclisation of the corresponding γ-hydroxycarboxylic acids, an unstable molecules that can be formed by glutamic acid deamination and decarboxylation process [22,39,43]. Lactones may also come from grapes, as is the case in Riesling, where they contribute to the varietal aroma [24]. The concentration of lactones in thirteen samples of sparkling wine were analysed by Kosmerl and Cegnar [44], with values between 15.0 and 57.5 µg L−1, and γ-nonalactone and γ-decalactone with levels below 4.7 µg L−1. In contrast, in Croatian sparkling wines values were much higher, ranging between 7.58 and 25.44 µg L−1 for γ-nonalactone and 1.93 and 57.19 µg L−1 for γ–decalactone while γ-octalactone concentrations were lower, between 1.06 and 1.80 µg L−1. Comparing average lactones concentrations between vine-growing regions significantly higher values were determined in sparkling wines from Plešivica primarily due to teh presence of γ-butyrolactone. Among others, significantly, the highest concentrations of γ-decalactone and γ-undecalactone were detected in sparkling wines from Krašić.
Table 2.
Average volatile compound concentrations (µg L−1) of sparkling wines produced in different vine-growing regions.
| Parameters | Vine Growing Regions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plešivica (n = 19) | Zelina (n = 8) | Krašić (n = 3) | |||||||
| MIN | MAX | Mean ± SD | MIN | MAX | Mean ± SD | MIN | MAX | Mean ± SD | |
| ∑ Aldehydes | 356.26 | 1902 | 842 ± 62 ab | 281.36 | 1161 | 546.77 ± 88.03 c | 978.02 | 1100 | 1039 ± 172 a |
| ∑ Higher alcohols | 38338 | 59964 | 49531 ± 5846 b | 44334 | 97754 | 75423 ± 20673 a | 42223 | 43706 | 42965 ± 1048 b |
| ∑ Volatile phenols | 84.0 | 448.1 | 181.6 ± 89.0 b | 17.1 | 257.1 | 84.5 ± 76.1 c | 304.5 | 458.0 | 381.3 ± 108.5 a |
| ∑ Terpenes | 188.6 | 1270 | 475.3 ± 242.0 b | 481.8 | 1044 | 760.0 ± 219.8 a | 609.9 | 800.6 | 705.3 ± 134.8 ab |
| ∑ C13-norisoprenoides | 0.3 | 13.1 | 3.9 ± 4.7 b | 4.1 | 13.2 | 8.1 ± 2.7 a | 0.1 | 1.5 | 0.84 ± 1.0 c |
| ∑ Lactones | 16.97 | 740.33 | 158.96 ± 218.0 a | 15.83 | 472.28 | 133.34 ± 179.8 b | 22.09 | 180.32 | 101.20 ± 111.8 b |
| ∑ Esters | 14990 | 30215 | 21658 ± 4660 b | 15604 | 85050 | 48045 ± 29098 a | 16456 | 23977 | 20217.3 ± 5318 b |
| ∑ Fatty acids | 6815 | 25035 | 15244 ± 5327 a | 183.8 | 8970 | 2137 ± 3324 b | 6915 | 10363 | 8639 ± 2438 ab |
| ∑ Other alcohols | 419.6 | 2381.8 | 947.1 ± 478.1 a | 140.2 | 815.0 | 302.4 ± 241.9 b | 637.0 | 769.0 | 703.0 ± 93.3 ab |
| ∑ Sulfur compounds | 27.64 | 195.5 | 103.8 ± 40.3 b | 96.4 | 932.9 | 574.9 ± 302.5 a | 30.2 | 65.5 | 47.9 ± 24.9 b |
| ∑ Other compounds | 102.69 | 3025 | 553.4 ± 750.0 a | 82.3 | 291.6 | 162.6 ± 79.9 a | 269.3 | 332.3 | 300.9 ± 44.5 a |
MIN-minimum value, MAX-maximum value, SD-standard deviation; Means mean ± S.D. are calculated only from samples in which analytes were quantified; Means with different superscript letters in the same row differ significantly (p ≤ 0.05).
Table 3.
Individual volatile compound concentrations (µg L−1) of sparkling wines produced in different vine growing regions.
| Parameters | ODT (µg L−1) | Odour Descriptor | OAV | Vine-Growing Regions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plešivica | Zelina | Krašić | ||||||||||
| MIN | MAX | Mean ± SD | MIN | MAX | Mean ± SD | MIN | MAX | Mean ± SD | ||||
| Aldehydes | ||||||||||||
| 2,4-Decadienal | 270 [45] | floral [46] | 0.03 | 8.80 | 2.19 ab ± 1.89 | n.d. | 3.67 | 1.47 b ± 1.23 | 2.72 | 3.28 | 3.00 a ± 0.23 | |
| 2,4-Heptadienal (E,E) | 0.05 | 23.61 | 6.30 a ± 5.70 | n.d. | 11.11 | 4.0 a ± 4.94 | 8.95 | 9.90 | 9.43 a ± 0.39 | |||
| 2,4-Heptadienal (Z,Z) | 0.07 | 9.35 | 3.75 a ± 2.52 | n.d. | 12.06 | 11.30 a ± 19.69 | 6.31 | 16.98 | 11.65 a ± 4.36 | |||
| 2,4-Nonadienal | 0.09 [47] | cucumber [46] | >1 | 0.12 | 4.73 | 1.70 a ± 1.26 | n.d. | 5.74 | 0.70 a ± 1.78 | 0.22 | 3.56 | 1.89 a ± 1.36 |
| 2-Heptenal | 4.6 [48] | green [46] | >1 | n.d. | 383.08 | 215.13 a ± 92.35 | n.d. | 153.46 | 17.1 b ± 48.23 | 147.88 | 156.95 | 152.42 a ± 3.70 |
| 5-Hydroxymethylfurfural | 100,000 [48] | almond [49] | 1.30 | 789.45 | 67.40 b ± 166.44 | 0.26 | 35.44 | 7.98 b ± 12.06 | 57.87 | 453.56 | 255.72 a ± 161.54 | |
| 5-Methyl-furfural | 16,000 [48] | bitter, almond, spice [49] | 2.38 | 35.45 | 10.28 a ± 7.29 | 2.75 | 13.96 | 7.57 b ± 3.40 | 7.21 | 15.69 | 11.45 a ± 3.46 | |
| Benzeneacetaldehyde | 4 [50] | 81.5 | 246.35 | 142.60 a ± 49.27 | n.d. | 318.1 | 70.92 a ± 109.14 | 110.87 | 202.25 | 156.56 a ± 37.31 | ||
| Benzaldehyde | 350 [51] | bitter, almond [52] | 8.71 | 78.56 | 27.23 a ± 17.87 | 2.91 | 50.96 | 20.38 a ± 14.50 | 30.9 | 35.25 | 33.08 a ± 1.78 | |
| Decanal | 0.1–2 [53] | >1 | n.d. | 138.25 | 25.71 a ± 42.02 | 0.89 | 117.58 | 55.23 a ± 34.39 | 0.25 | 51.77 | 26.01 a ± 21.03 | |
| Furfural | 770 [54] | burn, almond, yeast [55] | 5.24 | 423.08 | 125.89 a ± 108.98 | n.d. | 279.63 | 164.93 a ± 87.47 | 154.58 | 291.47 | 223.03 a ± 55.89 | |
| Heptanal | 3 [53] | n.d. | 3.05 | 0.84 a ± 0.75 | n.d. | 2.79 | 0.75 a ± 0.98 | 0.81 | 0.93 | 0.87 a ± 0.05 | ||
| Hexanal | 5 [56] | herbaceous [51] | >1 | 90.13 | 428.31 | 203.69 b ± 90.81 | 221.77 | 433.73 | 315.25 a ± 64.33 | 100.29 | 173.01 | 136.65 b ± 29.69 |
| Nonanal | 1 [50] | >1 | n.d. | 19.45 | 10.04 a ± 7.29 | n.d. | 17.38 | 3.65 b ± 2.77 | 15.12 | 19.82 | 17.47 a ± 6.77 | |
| Higher alcohols | ||||||||||||
| 1-Butanol | 150,000 [57] | medicinal [51] | 43.07 | 186.43 | 112.11 a ± 42.20 | 28.48 | 78.64 | 57.75 b ± 14.98 | 93.06 | 109.75 | 101.41 ab ± 6.81 | |
| 1-Decanol | 5000 [58] | pear, waxy, violet [58] | 0.44 | 1.75 | 0.69 b ± 0.32 | 0.46 | 6.17 | 3.83 a ± 2.08 | 0.42 | 0.67 | 0.55 b ± 0.10 | |
| 1-Heptanol | 425 [50] | oily [46] | 2.72 | 64.47 | 19.21 a ± 18.05 | 0.98 | 13.19 | 4.37 b ± 4.64 | 26.91 | 31.31 | 29.11 a ± 1.80 | |
| 1-Hexanol | 2500 [55] | grass just cut [51] | >1 | 677 | 3707 | 1874 b ± 788 | 471 | 3260 | 1612 b ± 848 | 2719 | 3177 | 2948 a ± 186 |
| 1-Methoxy-2-propanol | 8.34 | 82.26 | 19.82 a ± 15.70 | n.d. | 17.24 | 4.78 b ± 6.42 | 13.54 | 25.96 | 19.75 ab ± 5.07 | |||
| 1-Octadecanol | 0.41 | 0.81 | 0.59 a ± 0.10 | 0.58 | 3.17 | 2.24 a ± 1.09 | 0.7 | 15.94 | 8.32 a ± 6.22 | |||
| 1-Octanol | 110–130 [53] | chemical [51] | 3.20 | 8.37 | 5.29 b ± 1.47 | 5.16 | 8.65 | 6.74 a ± 1.23 | 5.43 | 5.92 | 5.68 ab ± 0.20 | |
| 1-Pentanol | 64,000 [39] | bitter, almond, balsamic [39] | 9.34 | 31.92 | 15.82 a ± 5.09 | 0.30 | 24.3 | 6.90 b ± 9.30 | 15.95 | 18.07 | 17.01 a ± 0.87 | |
| 2-Heptanol | 70 [59] | fruity, herbaceous [46] | 2.85 | 24.47 | 7.66 a ± 4.56 | 0.40 | 10.07 | 6.66 a ± 4.89 | 6.51 | 6.74 | 6.63 a ± 0.09 | |
| trans-2-Hexene-1-ol | 100 [59] | herbaceous, green [46] | 0.59 | 10.31 | 2.96 b ± 2.86 | 1.86 | 15.25 | 8.53 a ± 5.49 | 1.14 | 2.15 | 1.65 b ± 0.41 | |
| cis-2-Hexene-1-ol | green [46] | 9.21 | 29.58 | 15.74 b ± 5.94 | 4.18 | 17.95 | 11.19 b ± 4.47 | 20.8 | 32.56 | 26.68 a ± 4.80 | ||
| 2-Methyl-1-butanol | 30,000 [60] | whiskey, burnt, nail polish [61] | 9245 | 13,171 | 10835 b ± 1180 | 6898. | 22720 | 17390 a ± 6395 | 8031 | 9147 | 8589 b ± 455 | |
| 2-Pentadecanol | n.d. | 4.50 | 0.80 b ± 1.31 | n.d. | 3.52 | 1.90 a ± 1.39 | - | - | n.d. | |||
| 2-Pentene-1-ol | 1.84 | 18.92 | 4.75 b ± 3.97 | 4.63 | 28.41 | 18.61 a ± 9.04 | 3.38 | 3.78 | 3.58 b ± 0.16 | |||
| cis-3-Hexene-1-ol | 400 [51] | grass, green [51] | 29.46 | 678.41 | 102.16 a ± 132.05 | 52.76 | 343.47 | 146.75 a ± 88.32 | 77.12 | 240.48 | 158.80 a ± 66.69 | |
| trans-3-Hexene-1-ol | 1000 [51] | grass, resinous, cream [51] | 23.69 | 212.65 | 81.96 a ± 56.82 | 1.24 | 116.61 | 53.84 a ± 30.47 | 48.97 | 53.12 | 51.05 a ± 1.69 | |
| 3-Octanol | n.d. | 2.45 | 1.26 a ± 0.98 | n.d. | 4.97 | 2.22 a ± 1.64 | 0.10 | 2.32 | 1.21 a ± 0.91 | |||
| Isoamyl alcohol | 30,000 [60] | alcohol, nail polish [58] | >1 | 16317 | 29862 | 23888 b ± 2628 | 25039 | 53991 | 39580 a ± 10019 | 20291 | 20724 | 20507 b ± 176 |
| Isobutanol | 40,000 [39] | alcohol, nail polish [58] | 1467 | 5273 | 2686 a ± 1013 | n.d. | 3042 | 937 b ± 1330 | 2403 | 2467 | 2435 ab ± 26 | |
| Phenylethyl alcohol | 14,000 [62] | floral, rose, honey [58] | >1 | 2950 | 12347 | 9734 ab ± 2077 | 5315 | 26107 | 15759 a ± 7467 | 7349 | 8731 | 8040 b ± 564 |
| Volatile phenols | ||||||||||||
| 4-Ethylguaiacol | 33 [39] | toasted bread, smoky, clove [51] | 2.37 | 23.44 | 7.38 b ± 5.36 | 5.35 | 56.60 | 26.68 a ± 18.97 | 7.93 | 14.49 | 11.21 ab ± 2.68 | |
| 4-Ethylphenol | 35 [63] | phenol, stable [51] | n.d. | 146.86 | 13.35 a ± 37.64 | - | - | n.d. | 0.11 | 88.68 | 44.40 a ± 36.16 | |
| 4-Vinylguaiacol | 40 [63] | clove, curry [51] | >1 | 40.57 | 207.87 | 114.75 b ± 40.41 | 0.06 | 166.26 | 28.44 c ± 57.37 | 135.81 | 377.52 | 256.67 a ± 98.68 |
| 4-Vinylphenol | 180 [62] | phenolic, medicinal [51] | n.d. | 0.42 | 0.08 b ± 0.09 | n.d. | 0.19 | 0.10 b ± 0.11 | n.d. | 1.35 | 0.68 a ± 0.55 | |
| Eugenol | 6 [54] | cinnamon, clove [51] | 0.04 | 8.13 | 1.79 a ± 1.91 | 0.03 | 0.32 | 0.18 a ± 0.14 | 0.09 | 3.80 | 1.95 a ± 1.51 | |
| Guaiacol | 9.5 [55] | smoky, hospital [55] | 0.07 | 5.76 | 0.97 a ± 1.32 | n.d. | 0.32 | 0.20 a ± 0.31 | 0.13 | 1.03 | 0.58 a ± 0.37 | |
| Homovanillyl alcohol | - | - | n.d. | n.d. | 4.18 | 1.69 ± 1.34 | - | - | n.d. | |||
| Vanillin | 200 [60] | vanilla [51] | 1.06 | 278.59 | 32.77 a ± 57.53 | 3.38 | 71.33 | 16.98 a ± 21.89 | 60.34 | 63.09 | 61.72 a ± 1.12 | |
| Tyrosol | 33 [39] | toasted bread, smoky, clove [39] | 1.45 | 15.39 | 7.50 a ± 4.30 | 1.35 | 30.38 | 11.07 a ± 8.82 | 3.73 | 4.49 | 4.11 a ± 0.31 | |
| Terpenes | ||||||||||||
| 1,8-Terpin | 0.22 | 9.93 | 2.74 a ± 3.01 | 0.65 | 3.88 | 1.68 a ± 15.05 | 0.54 | 2.52 | 1.53 a ± 0.81 | |||
| 6,7-Dihydro-7-hydroxylinalool | 0.11 | 80.02 | 24.31 a ± 27.38 | 10.33 | 56.2 | 39.41 a ± 250.41 | 0.43 | 55.03 | 27.73 a ± 22.29 | |||
| 8-Hidroxylinalool | 0.91 | 74.75 | 23.71 a ± 19.55 | 1.54 | 20.52 | 11.85 a ± 11.01 | 7.31 | 13.44 | 10.38 a ± 2.50 | |||
| α-Terpineol | 330 [64] | lilac, floral, sweet [51] | 6.52 | 51.93 | 21.04 a ± 12.52 | 9.32 | 44.34 | 26.05 a ± 165.20 | 24.41 | 52.36 | 38.39 a ± 11.41 | |
| β-Farnesen | 87 [65] | 0.07 | 8.50 | 2.15 a ± 2.78 | n.d. | 0.51 | 0.27 a ± 0.16 | 0.44 | 0.59 | 0.52 ab ± 0.06 | ||
| β-Ocimene | 0.17 | 2.00 | 0.87 b ± 0.63 | n.d. | 0.76 | 0.23 b ± 0.46 | 1.17 | 1.68 | 1.43 a ± 0.21 | |||
| cis-Linalool oxide, furan | 6000 [55] | flower [55] | 0.10 | 20.13 | 10.85 a ± 6.87 | 2.28 | 23.15 | 12.04 a ± 38.62 | 17.69 | 30.44 | 24.07 a ± 5.21 | |
| Citronelol | 40 [64] | rose [66] | 1.86 | 19.15 | 4.59 a ± 3.81 | n.d. | 5.48 | 1.46 a ± 2.13 | 6.4 | 13.36 | 9.88 a ± 2.84 | |
| δ-Carene | n.d. | 1.73 | 0.58 a ± 0.49 | n.d. | 3.54 | 2.51 a ± 1.70 | 0.35 | 2.53 | 1.44 a ± 0.89 | |||
| Dihydroactinidiolide | n.d. | 22.57 | 7.69 a ± 5.79 | n.d. | 6.91 | 2.87 a ± 2.22 | n.d. | n.d. | n.d. | |||
| Farnesol | 20 [67] | floral, clove [46] | 0.77 | 14.25 | 5.73 a ± 4.65 | 0.14 | 9.22 | 3.06 a ± 2.68 | 7.98 | 8.08 | 8.03 a ± 0.04 | |
| γ-Terpinene | n.d. | 90.92 | 7.79 b ± 22.99 | 0.40 | 240.91 | 131.94 a ± 88.91 | 0.32 | 0.41 | 0.37 b ± 0.04 | |||
| Geraniol | 20 [62] | citrus, citric fruit [51] | >1 | 0.35 | 16.52 | 2.21 b ± 3.38 | 0.58 | 152.65 | 88.33 a ± 52.92 | 1.57 | 14.28 | 7.93 b ± 5.19 |
| Geranyl acetate | 9 [68] | flowery [68] | 0.11 | 17.91 | 3.65 a ± 3.84 | n.d. | 11.19 | 5.97 a ± 18.23 | 2.65 | 6.58 | 4.62 a ± 1.60 | |
| Hotrienol | 110 [52] | fresh, floral, fruity [52] | >1 | 7.69 | 121.11 | 31.94 a ± 28.55 | 1.68 | 142.97 | 29.30 a ± 607.88 | 128.35 | 268.59 | 198.47 a ± 57.25 |
| Linalool | 25 [62] | citrus, floral, sweet [51] | >1 | 0.69 | 103.38 | 11.31 b ± 23.72 | 1.38 | 84.8 | 45.03 a ± 30.83 | 1.3 | 144.91 | 73.11 a ± 58.63 |
| Linalool oxide, pyran | 3000 [55] | flower [51] | 2.18 | 13.23 | 7.17 a ± 3.37 | 0.62 | 19.77 | 6.54 a ± 133.08 | 22.65 | 26.44 | 24.55 a ± 1.55 | |
| Neric acid | 1.87 | 149.88 | 26.84 a ± 40.12 | 1.93 | 14.99 | 7.42 a ± 4.88 | 32.44 | 40.05 | 36.25 a ± 3.11 | |||
| Nerol | 300 [64] | rose, fruity, floral [51] | 0.12 | 6.13 | 0.91 b ± 1.23 | 0.17 | 60.41 | 33.19 a ± 20.17 | 0.77 | 3.09 | 1.93 b ± 0.95 | |
| Nerolidol | 250 [50] | rose, apple, green, waxy, woody [46] | 0.11 | 3.22 | 0.57 a ± 0.64 | 0.10 | 3.58 | 1.28 a ± 1.44 | 0.01 | 0.67 | 0.34 a ± 0.27 | |
| Pseudoionon | n.d. | 0.14 | 0.06 b ± 0.03 | n.d. | 1.49 | 0.09 b ± 0.49 | 0.08 | 1.53 | 0.81 a ± 0.59 | |||
| Terpendiol I | n.d. | 1.89 | 0.74 a ± 0.53 | n.d. | 0.98 | 0.87 a ± 0.80 | 0.8 | 1.18 | 0.99 a ± 0.16 | |||
| Terpendiol II | 2.40 | 40.50 | 11.30 c ± 9.32 | 0.81 | 48.83 | 31.69 b ± 39.30 | 53.43 | 114.59 | 84.0 a ± 24.97 | |||
| Terpinene-4-ol | 0.03 | 3.97 | 2.25 b ± 0.85 | 1.79 | 91.4 | 36.23 a ± 29.88 | 1.73 | 3.5 | 2.62 b ± 0.72 | |||
| Tetrahydrolinalool | 0.1 | 1015 | 183.23 a ± 226.07 | 68.33 | 436.64 | 198.41 a ± 125.28 | 1.26 | 53.42 | 27.34 b ± 21.29 | |||
| trans-Linalool oxide, furan | 6000 [55] | flower [55] | 0.25 | 105.39 | 13.87 a ± 21.89 | 1.15 | 50.99 | 20.81 a ± 54.31 | 18.23 | 49.27 | 33.75 a ± 12.67 | |
| trans-Rose oxide | 80–160 [69] | floral, green [69] | 0.11 | 0.39 | 0.20 a ± 0.12 | 0.10 | 0.24 | 0.24 a ± 0.45 | 0.24 | 0.48 | 0.36 a ± 0.10 | |
| Linalyl formate | n.d. | 4.69 | 0.81 b ± 1.04 | 0.41 | 5.99 | 2.61 a ± 2.06 | 0.27 | 0.64 | 0.46 b ± 0.15 | |||
| Ethyl linalyl acetal | 0.64 | 5.74 | 2.62 b ± 1.41 | 1.01 | 28.94 | 9.90 a ± 8.92 | 1.92 | 2.45 | 2.19 b ± 0.22 | |||
| 2,6-Dimethyl-3,7-octadiene-2,6-diol | 0.07 | 12.17 | 3.77 b ± 3.29 | 0.20 | 14.03 | 5.80 b ± 25.31 | 20.2 | 30.78 | 25.49 a ± 4.32 | |||
| 2,6-Dimethyl-7-octene-2,6-diol | 25.16 | 80.60 | 46.12 a ± 15.09 | 8.06 | 40.19 | 16.79 a ± 14.38 | 48.81 | 54.76 | 51.79 a ± 2.43 | |||
| Menthol | 0.93 | 57.06 | 9.97 a ± 17.41 | n.d. | 1.61 | 0.55 a ± 1.67 | 2.21 | 3.71 | 2.96 a ± 0.61 | |||
| Ocimenol | n.d. | 2.47 | 0.95 a ± 0.65 | n.d. | 5.59 | 3.96 a ± 7.46 | 0.59 | 1.42 | 1.01 a ± 0.34 | |||
| C13- norisoprenoids | ||||||||||||
| α-Ionol | 0.01 | 0.78 | 0.24 a ± 0.21 | n.d. | 0.09 | 0.07 b ± 0.13 | 0.05 | 0.18 | 0.12 ab ± 0.05 | |||
| α-Ionon | 10.5 [70] | floral [70] | n.d. | 0.05 | 0.03 b ± 0.01 | 0.18 | 0.56 | 0.37 a ± 0.18 | 0.02 | 0.06 | 0.04 b ± 0.02 | |
| β-Damascenone | 0.05 [60] | sweet, fruity, floral, honey [62] | >1 | 0.05 | 9.95 | 1.91 a ± 2.95 | 0.03 | 6.54 | 3.68 a ± 2.14 | n.d. | 0.28 | 0.14 a ± 0.11 |
| TDN | 2 [71] | petrol, kerosene [55] | >1 | 0.35 | 8.86 | 2.55 a ± 2.26 | 0.1 | 7.45 | 3.89 a ± 2.72 | 0.1 | 1.1 | 0.55 a ± 0.45 |
| Lactones | ||||||||||||
| cis-Whiskey lactone | 67 [39] | nutty, coconut [61] | 0.46 | 5.31 | 1.59 a ± 1.19 | 2.54 | 4.64 | 2.13 a ± 1.25 | 0.74 | 0.81 | 0.78 a ± 0.03 | |
| δ-Nonalactone | 5.51 | 13.07 | 8.71 a ± 2.04 | n.d. | 8.73 | 2.86 b ± 2.92 | 7.59 | 8.11 | 7.85 a ± 0.21 | |||
| γ-Butyrolactone | 10,000 [58] | coconut, caramel [51] | n.d. | 695.54 | 131.27 a ± 208.88 | n.d. | 439.29 | 85.94 a ± 227.18 | - | - | n.d. | |
| γ-Decalactone | 1000 [58] | peach, fruity [51] | 0.55 | 7.09 | 1.93 b ± 1.45 | n.d. | 7.34 | 5.12 b ± 2.37 | 1.93 | 112.45 | 57.19 a ± 45.12 | |
| γ-Hexalactone | 1600 [72] | sweet, cake, peach [51] | 0.19 | 28.81 | 5.80 a ± 5.71 | n.d. | 6.22 | 3.33 a ± 2.37 | n.d. | 13.82 | 6.91 a ± 5.64 | |
| γ-Nonalacton | 25 [48] | coconut, peach [51] | >1 | 2.67 | 23.85 | 7.58 b ± 5.27 | 5.06 | 49.11 | 25.44 a ± 17.36 | 7.95 | 14.66 | 11.31 ab ± 2.74 |
| γ-Octalacton | 7 [48] | n.d. | 8.48 | 1.06 a ± 1.78 | n.d. | 2.45 | 1.77 a ± 0.80 | 1.74 | 1.85 | 1.80 a ± 0.04 | ||
| γ-Undecalactone | 60 [48] | apricot, peach [46] | 0.15 | 1.84 | 0.54 b ± 0.44 | n.d. | 4.66 | 2.21 b ± 1.68 | 2.01 | 28.55 | 15.28 a ± 10.84 | |
| trans-Whiskey lactone | 790 [39] | nutty, coconut [61] | 0.10 | 26.93 | 2.21 a ± 5.58 | 2.37 | 2.65 | 1.53 a ± 0.86 | 0.07 | 0.14 | 0.11 a ± 0.03 | |
| Esters | ||||||||||||
| 2-Phenylethyl acetate | 250 [73] | rose, honey, tobacco [58] | 7.04 | 115.22 | 23.40 a ± 22.55 | 0.16 | 41.73 | 24.05 a ± 14.97 | 10.14 | 47.31 | 28.73 a ± 15.17 | |
| Diethyl glutarate | n.d. | 28.02 | 15.39 a ± 8.21 | 5.32 | 35.62 | 19.93 a ± 10.19 | 5.73 | 18.29 | 12.01 a ± 5.13 | |||
| Diethyl hydroxysuccinate | n.d. | 5.42 | 1.26 b ± 1.96 | 0.89 | 69609 | 32046 a ± 27325.14 | n.d. | 11.81 | 5.91 b ± 4.82 | |||
| Diethyl malonate | sweet, fruity, apple [46] | 0.93 | 13.03 | 7.08 b ± 2.94 | 4.09 | 10.95 | 8.55 b ± 2.34 | 13.23 | 46.31 | 29.77 a ± 13.50 | ||
| Diethyl oxalate | n.d. | 1.31 | 0.34 a ± 0.35 | n.d. | 0.96 | 0.48 a ± 0.30 | - | - | n.d. | |||
| Diethyl succinate | 200,000 [39] | overripe, aged [55] | 1600 | 10272 | 5401 a ± 2141 | 3141 | 11498 | 7430 a ± 2545 | 2700 | 5134 | 3917 b ± 993 | |
| Dimethyl malate | 0.10 | 10.03 | 3.82 b ± 3.08 | - | - | n.d. | n.d. | 15.61 | 7.81 a ± 6.37 | |||
| Ethyl benzeneacetate | 6.76 | 77.85 | 34.36 a ± 23.91 | 0.97 | 71.60 | 11.80 b ± 25.34 | 16.4 | 40.00 | 28.20 ab ± 9.63 | |||
| Ethyl butanoate | 20 [54] | pineapple, apple, peach [58] | >1 | 117.13 | 404.93 | 270.94 b ± 82.85 | 204.06 | 580.88 | 417.08 a ± 119.30 | 352.95 | 372.23 | 362.59 ab ± 7.87 |
| Ethyl decanoate | 200 [63] | floral, grape, fruty [61] | 0.10 | 150.72 | 9.32 b ± 32.79 | 0.16 | 239.31 | 136.02 a ± 85.11 | 0.18 | 0.20 | 0.19 b ± 0.01 | |
| Ethyl furoate | 16,000 [54] | 6.85 | 81.43 | 43.46 a ± 18.89 | 2.51 | 111.91 | 21.80 a ± 34.42 | 32.66 | 46.97 | 39.82 a ± 5.84 | ||
| Ethyl hexadecanoate | >2000 [74] | 0.17 | 2.50 | 0.94 b ± 0.73 | 0.30 | 16.27 | 6.54 a ± 6.04 | 0.24 | 1.46 | 0.85 b ± 0.50 | ||
| Ethyl hexanoate | 14 [62] | fruity, green apple, banana [61] | >1 | 111.89 | 578.37 | 334.98 b ± 129.46 | 181.96 | 803.90 | 520.55 a ± 190.77 | 423.93 | 447.10 | 435.52 ab ± 9.46 |
| Ethyl hydrogensuccinate | 4096 | 9546 | 6671 a ± 1754 | 0.35 | 7160 | 2346.13 b ± 314.10 | 2692 | 6870 | 4781 ab ± 1705 | |||
| Ethyl lactate | 154,000 [54] | butter [58] | 2131 | 14871 | 6664 a ± 3755 | 2645 | 7325 | 4025 b ± 2275 | 8315 | 9128 | 8721 a ± 331 | |
| Ethyl linoleate | 450 [65] | 0.19 | 1.91 | 0.68 b ± 0.41 | 0.54 | 3.00 | 2.00 a ± 0.79 | 0.44 | 1.01 | 0.73 b ± 0.23 | ||
| Ethyl octanoate | 580 [63] | sweet, floral, fruity, pear [58] | >1 | 74.15 | 715.98 | 393.31 b ± 179.89 | 199.76 | 890.39 | 631.90 a ± 230.08 | 365.04 | 499.41 | 432.23 ab ± 54.86 |
| Ethyl vanillate | 3000 [63] | creamy, vanilla [61] | 29.10 | 747.97 | 160.67 a ± 178.33 | 0.32 | 151.67 | 28.54 a ± 50.99 | 60.33 | 135.82 | 98.08 a ± 30.82 | |
| Ethyl-2-hydroxy-3-methylbutanoate | 9.07 | 440.28 | 99.98 b ± 88.60 | 67.73 | 653.62 | 431.39 a ± 217.56 | 23.25 | 45.36 | 34.31 b ± 9.03 | |||
| Ethyl-2-hydroxybutanoate | 4.24 | 81.79 | 26.00 a ± 23.45 | 0.98 | 33.32 | 14.09 a ± 13.99 | 14.62 | 26.73 | 20.68 a ± 4.94 | |||
| Ethyl-2-methylbutanoate | 18 [54] | apple, strawberry [61] | >1 | 2.91 | 106.06 | 30.43 a ± 21.68 | 0.14 | 29.06 | 9.42 b ± 9.82 | 5.54 | 17.86 | 11.70 ab ± 5.03 |
| Ethyl-2-oxopropanoate | 38.2 | 253.61 | 126.68 a ± 71.24 | 0.14 | 217.10 | 55.96 a ± 96.89 | 64.31 | 116.59 | 90.45 a ± 21.34 | |||
| Ethyl-3-ethoxypropanoate | 0.02 | 2.64 | 0.54 a ± 0.68 | 0.05 | 0.14 | 0.13 a ± 0.46 | 0.02 | 0.10 | 0.06 a ± 0.03 | |||
| Ethyl-3-hydroxybutanoate | 20,000 [66] | grape, fruity, caramel, toasted [75] | 0.11 | 75.28 | 21.75 a ± 28.47 | 0.25 | 1.61 | 0.93 b ± 10.12 | 0.96 | 76.04 | 38.50 a ± 30.65 | |
| Ethyl-3-hydroxyhexanoate | 45 [58] | rubber [46] | 0.20 | 12.35 | 7.19 a ± 3.04 | 0.26 | 10.43 | 4.99 a ± 2.96 | 7.33 | 7.91 | 7.62 a ± 0.24 | |
| Ethyl-3-methylbutanoate | 3 [54] | fruity, pineapple [46] | >1 | 6.71 | 150.40 | 51.39 a ± 30.19 | 28.95 | 92.38 | 61.76 a ± 20.19 | 8.71 | 33.35 | 21.03 a ± 10.06 |
| Ethyl-hydroxyhexanoate | 0.15 | 376.69 | 120.86 a ± 92.70 | 0.50 | 161.62 | 20.64 b ± 69.54 | 68.68 | 92.95 | 80.82 ab ± 9.91 | |||
| Ethylmethyl succinate | 12.98 | 64.42 | 32.58 a ± 13.00 | 0.02 | 40.21 | 8.00 b ± 13.89 | 22.09 | 43.41 | 32.75 a ± 8.70 | |||
| Hexyl acetate | 670 [57] | fruity, green, sweet [61] | n.d. | 183.29 | 51.91 a ± 60.85 | 0.13 | 161.50 | 56.90 a ± 7.88 | 49.15 | 83.69 | 66.42 a ± 14.10 | |
| Isoamyl acetate | 30 [62] | banana [58] | >1 | 117.76 | 2825.79 | 436.95 a ± 552.20 | 76.89 | 726.31 | 473.72 a ± 240.34 | 599.36 | 610.28 | 604.82 a ± 4.46 |
| Isoamyl lactate | 1.6 [60] | fruity, apple, banana [46] | >1 | 0.17 | 602.50 | 144.88 ab ± 158.71 | 1.10 | 47.61 | 17.46 b ± 15.35 | 173.59 | 218.43 | 196.01 a ± 18.31 |
| Isobutyl acetate | 6140 [58] | apple, banana [61] | 11.36 | 77.84 | 23.30 b ± 15.09 | 10.38 | 38.33 | 21.38 b ± 9.35 | 39.99 | 42.06 | 41.03 a ± 0.85 | |
| Isobutyl lactate | 340,000 [58] | 3.36 | 77.54 | 28.01 b ± 21.70 | 27.72 | 346.96 | 112.05 a ± 109.45 | 22.89 | 26.33 | 24.61 b ± 1.40 | ||
| Methyl hexadecanoate | >2000 [74] | 0.41 | 1.18 | 0.78 a ± 0.36 | n.d. | 1.61 | 0.84 a ± 0.54 | - | - | n.d. | ||
| Methyl hydroxyisovalerate | 2.14 | 323.55 | 52.30 a ± 69.63 | 0.67 | 57.49 | 18.95 a ± 22.50 | 4.92 | 50.53 | 27.73 a ± 18.62 | |||
| Methyl hexanoate | 84 [76] | n.d. | 1.42 | 0.41 b ± 0.39 | n.d. | 0.75 | 0.43 b ± 0.24 | 1.5 | 1.84 | 1.67 a ± 0.14 | ||
| Methyl octadecanoate | - | - | n.d. | - | - | n.d. | n.d. | 1.04 | 0.52 ± 0.42 | |||
| Methyl-2-furoate | 0.05 | 1.24 | 0.56 b ± 0.36 | 0.09 | 24.54 | 5.00 a ± 7.36 | 0.52 | 0.90 | 0.71 b ± 0.16 | |||
| Methyl-3-hydroxyoctanoate | 0.06 | 0.47 | 0.17 b ± 0.10 | 0.10 | 29.40 | 10.08 a ± 9.45 | 0.11 | 0.18 | 0.15 b ± 0.03 | |||
| Methyl geranoate | 0.33 | 1.84 | 1.10 a ± 0.64 | 0.46 | 2.04 | 0.88 a ± 1.03 | 0.64 | 0.73 | 0.69 a ± 0.04 | |||
| o-Methylbenzyl acetate | 2.59 | 91.59 | 30.20 a ± 23.69 | 2.70 | 208.21 | 71.44 a ± 74.59 | 5.75 | 18.63 | 12.19 a ± 5.26 | |||
| Phenyl acetate | 250 [60] | 0.25 | 789.13 | 87.35 a ± 226.77 | 0.15 | 2.03 | 1.25 a ± 1.72 | 0.11 | 0.36 | 0.24 a ± 0.10 | ||
| Fatty acids | ||||||||||||
| 2-Methylpropionic acid | 230 [55] | rancid, cheesy [55] | 0.92 | 9.11 | 3.31 b ± 2.06 | 4.82 | 16.96 | 8.67 a ± 4.22 | 3.94 | 4.53 | 4.24 b ± 0.24 | |
| Butanoic acid | 400 [55] | rancid, cheese [51] | 0.20 | 1.91 | 1.08 a ± 0.47 | 0.12 | 0.97 | 0.84 b ± 0.36 | 0.96 | 1.09 | 1.03 a ± 0.05 | |
| Decanoic acid | 1000 [73] | rancid, waxy [51] | >1 | 851 | 5665 | 2456 a ± 1324 | 0.94 | 2100 | 798.88 b ± 1155.30 | 997.14 | 2434 | 1715.72 ab ± 586.71 |
| Heptanoic acid | 3000 [53] | rancid, cheesy [51] | 0.96 | 2.19 | 1.39 b ± 0.35 | 0.11 | 13.87 | 5.65 a ± 4.18 | 1.13 | 1.85 | 1.49 b ± 0.29 | |
| Hexanoic acid | 420 [53] | cheese, oily [58] | >1 | 137 | 10848 | 7006 a ± 2541 | 12.62 | 739.12 | 318.30 b ± 250.82 | 195.35 | 303.17 | 249.26 b ± 44.02 |
| Isovaleric acid | 33 [62] | sweat, rancid [51] | >1 | 0.78 | 43.93 | 7.01 ab ± 10.17 | 0.32 | 4.71 | 3.03 b ± 1.58 | 13.99 | 17.14 | 15.57 a ± 1.29 |
| Octanoic acid | 500 [54] | rancid, oily [61] | >1 | 2097 | 9123 | 6207 a ± 1937 | 4.81 | 6453 | 1252.90 b ± 2500.16 | 5700 | 7592 | 6646 a ± 772 |
| Propanoic acid | 8100 [57] | rancid, oily [46] | 0.07 | 16.58 | 4.15 b ± 3.65 | 2.34 | 38.37 | 24.27 a ± 14.05 | 3.06 | 9.12 | 6.09 b ± 2.47 | |
| Other alcohols | ||||||||||||
| 1,4-Butandiol | 0.04 | 14.49 | 7.37 a ± 5.27 | 0.5 | 2.95 | 2.29 a ± 2.05 | 3.47 | 12.07 | 7.77 a ± 3.51 | |||
| 2,6-Dimethyl-4-heptanol | 57.74 | 657.53 | 246.34 a ± 125.92 | 4.55 | 283.81 | 91.41 b ± 110.96 | 119.63 | 163.54 | 141.59 ab ± 17.93 | |||
| 2-Butoxy-ethanol | 2.69 | 75.65 | 31.87 a ± 22.62 | 0.20 | 20.43 | 10.31 b ± 6.15 | 12.01 | 45.16 | 28.59 ab ± 13.53 | |||
| 2-Ethyl-1-hexanol | 8.01 | 34.46 | 16.47 a ± 7.14 | 0.48 | 40.55 | 12.17 a ± 19.41 | 26.71 | 28.84 | 27.78 a ± 0.87 | |||
| 3,4-Dimethyl-2-hexanol | 9.80 | 1579.33 | 169.17 a ± 396.65 | 0.56 | 71.53 | 29.10 a ± 25.57 | 98.14 | 135.69 | 116.92 a ± 15.33 | |||
| 3-Ethoxy-1-propanol | 50,000 [58] | overripe, pear [75] | 3.60 | 148.12 | 61.84 a ± 44.46 | 1.29 | 41.05 | 7.87 b ± 12.88 | 23.25 | 90.11 | 56.68 ab ± 27.30 | |
| 3-Methyl-1-pentanol | 50,000 [49] | 73.67 | 225.57 | 137.92 a ± 38.41 | 55.52 | 113.69 | 89.29 b ± 22.35 | 87.19 | 94.30 | 90.75 b ± 2.90 | ||
| 3-Methyl-3-buten-1-ol | 10.54 | 31.23 | 16.35 a ± 5.10 | 0.45 | 23.48 | 6.30 b ± 4.70 | 16.11 | 18.34 | 17.23 a ± 0.91 | |||
| 4-Ethylcyclohexanol | 0.15 | 6.6 | 1.61 a ± 1.66 | 0.17 | 3.20 | 3.14 a ± 0.11 | 2.44 | 2.73 | 2.59 a ± 0.12 | |||
| 4-Methyl-1-pentanol | 50,000 [58] | almond, toasted [46] | 35.03 | 147.72 | 70.08 a ± 28.06 | 25.93 | 60.72 | 38.90 b ± 15.49 | 34.91 | 39.79 | 37.35 b ± 1.99 | |
| Benzylalcohol | 10,000 [64] | roasted, toasted, sweet, fruity [51] | 1.52 | 19.09 | 6.69 a ± 5.13 | 2.08 | 6.79 | 3.64 a ± 1.70 | 3.18 | 3.71 | 3.45 a ± 0.22 | |
| Cyclohexanol | 160,000 [65] | 0.03 | 4.22 | 1.39 a ± 1.40 | 0.20 | 6.24 | 2.30 a ± 2.25 | 0.15 | 3.35 | 1.75 a ± 1.31 | ||
| Furfuril alkohol | 15,000 [58] | sweet, nutty [61] | 0.29 | 45.10 | 13.32 a ± 12.84 | 0.21 | 12.00 | 6.15 b ± 6.80 | 10.53 | 11.85 | 11.19 ab ± 0.54 | |
| 2,3-Butanediol | 668,000 [23] | buttery, creamy [58] | 0.34 | 564.66 | 158.57 a ± 135.96 | 13.97 | 157.11 | 45.37 b ± 110.71 | 138.48 | 180.36 | 159.42 a ± 17.10 | |
| Sulfur compounds | ||||||||||||
| 4-(Methylthio)-1-butanol | 1000 [40] | metallic-bitter, garlic, earthy [46] | 0.02 | 28.12 | 13.23 b ± 8.57 | 8.15 | 932.95 | 548.45 a ± 352.40 | 11.97 | 19.61 | 15.79 b ± 3.12 | |
| 3-(Methylthio)-1-propanol | 1000 [62] | cooked potato [61] | 0.42 | 186.93 | 89.71 a ± 41.91 | n.d. | 123.66 | 23.61 b ± 17.69 | 18.26 | 45.94 | 32.10 b ± 11.30 | |
| Other compounds | ||||||||||||
| 2,5-Hexadione | 0.38 | 4.53 | 2.89 a ± 1.15 | n.d. | 1.07 | 0.97 b ± 0.76 | 2.45 | 2.69 | 2.57 a ± 0.10 | |||
| 2,7-Octanedione | 0.11 | 81.61 | 11.43 a ± 16.45 | n.d. | 42.76 | 22.16 a ± 16.45 | n.d. | 3.48 | 1.74 a ± 1.42 | |||
| 2 H-Pyran-2,6(3H)-dione | 0.19 | 48.76 | 24.08 a ± 18.89 | n.d. | 70.58 | 10.39 a ± 24.30 | 0.49 | 0.50 | 0.50 a ± 0.00 | |||
| 2-Pentylfuran | 2000 [65] | 3.78 | 9.73 | 6.63 b ± 1.80 | 3.87 | 14.25 | 9.81 a ± 2.78 | 8.48 | 8.94 | 8.71 ab ± 0.19 | ||
| 3-Penten-2-on | 0.52 | 1021.26 | 79.69 a ± 215.90 | n.d. | 3.89 | 3.81 a ± 1.71 | 1.19 | 3.99 | 2.59 a ± 1.14 | |||
| 4-Hydroxy-4-methyl-2-pentanone | n.d. | 14.52 | 5.85 a ± 2.79 | n.d. | 5.33 | 1.64 b ± 1.32 | 2.63 | 3.84 | 3.24 ab ± 0.49 | |||
| 4-Methyl-2-penten-2-one | n.d. | 4.14 | 0.93 b ± 1.25 | 1.36 | 54.51 | 30.18 a ± 20.50 | n.d. | 0.32 | 0.16 b ± 0.13 | |||
| 5-Ethyl-4-methyl-3-heptanone | 0.26 | 89.89 | 16.29 a ± 22.79 | 0.11 | 55.89 | 12.56 a ± 16.90 | 0.60 | 1.44 | 1.02 a ± 0.34 | |||
| Acetoin | 150,000 [54] | buttery, creamy [58] | 17.30 | 2904.72 | 310.34 a ± 729.87 | 0.96 | 131.26 | 34.45 a ± 50.24 | 176.86 | 247.6 | 212.23 a ± 28.88 | |
| Acetylfurane | 3.81 | 31.36 | 15.60 a ± 6.09 | 0.13 | 17.53 | 14.33 a ± 7.14 | 12.41 | 19.12 | 15.77 a ± 2.74 | |||
| Benzofurane | 350 [50] | 2.60 | 178.34 | 34.30 a ± 38.71 | 0.31 | 22.68 | 5.32 b ± 7.13 | 8.64 | 20.46 | 14.55 ab ± 4.83 | ||
| Dihydro-2-methyl-3(2H)-furanone | n.d. | 8.48 | 2.55 a ± 1.95 | 0.21 | 9.79 | 3.75 a ± 2.81 | 1.12 | 1.50 | 1.31 a ± 0.16 | |||
| N-(2-phenylethyl)acetamide | 0.40 | 2.91 | 1.53 b ± 0.51 | 1.14 | 25.63 | 8.59 a ± 8.63 | 0.98 | 1.36 | 1.17 b ± 0.16 | |||
| N-Ethylacetamide | 15.33 | 56.90 | 31.59 a ± 11.28 | 2.41 | 52.00 | 19.32 a ± 17.69 | 30.24 | 40.38 | 35.31 a ± 4.14 | |||
ODT- odour detection treshold, OAV-odour active value, MIN-minimum value, MAX-maximum value, SD-standard deviation; Means mean± S.D. are calculated only from samples in which analytes were quantified; Means with different superscript letters in the same row differ significantly (p ≤ 0.05).
2.3. Multivariate Analysis
Discriminant analysis using forward stepwise model for all volatile compounds showed that 21 volatile compounds were selected and ranked based on their discrimination efficiency of three vine-growing regions (Table 4), while other compounds were not included by the model as the threshold to enter was set to 0.05.
Table 4.
Summary of the variables selection and ranking in Discriminant analysis using forward stepwise model for discrimination among sparkling wines samples from three vine growing region.
| Rank | Variable Included | Partial R² | F | p | Wilks’ Lambda a | p |
|---|---|---|---|---|---|---|
| 1 | Methyl hexanoate | 0.86 | 88.5 | <0.1 × 10−4 | 1.45 × 10−1 | <0.1 × 10−4 |
| 2 | Hexanoic acid | 0.81 | 60.0 | <0.1 × 10−4 | 2.82 × 10−2 | <0.1 × 10−4 |
| 3 | 4-Hydroxy-4-methyl-2-pentanone | 0.48 | 13.0 | 1.02 × 10−4 | 1.46 × 10−2 | <0.1 × 10−4 |
| 4 | Heptanal | 0.57 | 17.9 | <0.1 × 10−4 | 6.29 × 10−3 | <0.1 × 10−4 |
| 5 | Octanoic acid | 0.58 | 17.7 | <0.1 × 10−4 | 2.66 × 10−3 | <0.1 × 10−4 |
| 6 | 3-Penten-2-on | 0.42 | 8.9 | 1.21 × 10−3 | 1.55 × 10−3 | <0.1 × 10−4 |
| 7 | Isobutyl acetate | 0.40 | 7.8 | 2.41 × 10−3 | 9.41 × 10−4 | <0.1 × 10−4 |
| 8 | 2-Methylpropionic acid | 0.43 | 8.8 | 1.47 × 10−3 | 5.34 × 10−4 | <0.1 × 10−4 |
| 9 | 1,4-Butandiol | 0.40 | 7.4 | 3.40 × 10−3 | 3.18 × 10−4 | <0.1 × 10−4 |
| 10 | Acetylfurane | 0.42 | 7.5 | 3.43 × 10−3 | 1.85 × 10−4 | <0.1 × 10−4 |
| 11 | Isobutyl lactate | 0.41 | 6.8 | 5.54 × 10−3 | 1.10 × 10−4 | <0.1 × 10−4 |
| 12 | Ethyl linoleate | 0.58 | 13.0 | 2.78 × 10−4 | 4.66 × 10−5 | <0.1 × 10−4 |
| 13 | Propanoic acid | 0.46 | 7.8 | 3.63 × 10−3 | 2.50 × 10−5 | <0.1 × 10−4 |
| 14 | 2H-Pyran-2,6(3H)-dione | 0.53 | 9.4 | 1.79 × 10−3 | 1.19 × 10−5 | <0.1 × 10−4 |
| 15 | TDN | 0.52 | 8.6 | 2.86 × 10−3 | 5.70 × 10−6 | <0.1 × 10−4 |
| 16 | Ethyl linalyl acetal | 0.48 | 7.0 | 7.30 × 10−3 | 2.96 × 10−6 | <0.1 × 10−4 |
| 17 | Ethyl-2-oxopropanoate | 0.60 | 10.7 | 1.53 × 10−3 | 1.17 × 10−6 | <0.1 × 10−4 |
| 18 | Benzaacetaldehyde | 0.51 | 6.7 | 9.94 × 10−3 | 5.76 × 10−7 | <0.1 × 10−4 |
| 19 | Hexanal | 0.51 | 6.2 | 1.42 × 10−3 | 2.84 × 10−7 | <0.1 × 10−4 |
| 20 | β-Farnesen | 0.42 | 4.0 | 4.91 × 10−2 | 1.64 × 10−7 | <0.1 × 10−4 |
| 21 | Benzaldehyde | 0.58 | 6.9 | 1.33 × 10−2 | 6.92 × 10−8 | <0.1 × 10−4 |
a Wilks’ Lambda test of the assumption of equality of the mean vectors of classes.
Three vine-growing regions can be clearly separated, and that Fisher distances between all of them are significant (Table 5).
Table 5.
Fisher distance among samples of sparkling wines from three vine-growing regions based on 3 volatile compounds.
| Krašić | Plešivica | Zelina | |
|---|---|---|---|
| Krašić | 0 | 26.3 *** | 14.0 *** |
| Plešivica | 57.3 *** | ||
| Zelina | 0 |
p-values for Fisher distances: *** p < 0.001.
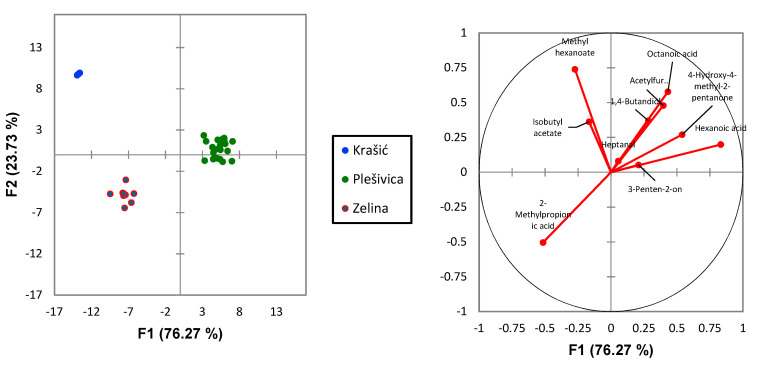
Scatter plot presented in Figure 1 obtained on the basis of the discriminant analysis showed the distribution of the sparkling wines in the space defined with two discrimination factors. Based on the vector diagram of ten highest ranked volatile compounds selected using forward stepwise model in discriminant analysis we can conclude that due to the position of the plot, samples from Plešivica vine-growing region are specific for high content of hexanoic acid, octanoic acid, 4-hydroxy−4-methyl-2-pentanon, 1,4-butandiol, and acetylfurane, samples from Zelina are specific for a higher content of 2-methylpropionic acid, while Krašić samples have higher level of methyl hexanoate and isobutyl acetate. After the series of discriminant analyses were performed starting with two variables with the highest rank based on stepwise discriminant analysis, followed by the introduction of one new variable in each new analysis, we have discovered that using first two variables (methyl hexanoate and hexanoic acid) 100% correct classification was achieved for Krašić and Zelina while 95% correct classification was achieved for Plešivica samples. After one additional variable (4-hydroxy-4-methyl-2-pentanone) was included, all samples were classified within the corresponding vine-growing region. Significant Fisher distances were detected (Table 5) among all three groups using these three variables (methyl hexanoate, hexanoic acid, and 4-hydroxy-4-methyl-2-pentanone).
Figure 1.
Discriminant analysis of 30 sparkling wines (left) based on the concentrations of ten volatile compounds (right)) with highest rank after forward stepwise model applied on total number of volatile compounds detected. The vector diagram indicates the direction and intensity of the effect of nine variables on the distribution of wine samples in the space defined by two discrimination factors (F1 and F2).
In the past years’ classification of musts and/or wines by multivariate analysis were carried on on the basis of their geographical origin or viticultural region [14], based on their chemical attributes [74] and aroma profile [66]. In the work by Arozarena et al. [76] discriminant selection process showed that correct classification by grape cultivar was achieved in the 94% of the training wines and 85% of the test wines. These percentages were very similar when the separation model was used to test the differences between regions, achieving 89% in training sample set and 92% in test wines samples. Similar results were achieved by Marais et al. [77] where stepwise discriminant analysis was applied for the separation based on the aroma compounds data of dry white table wine. The highest discriminatory value components were isoamyl and hexyl acetate and isobutanol, in the Colombar wines while in the Chenin blanc wines they were 2-phenyl ethanol and hexanol.
2.4. Odour Active Values (OAV) and Relative Odour Contribution (ROC)
To evaluate the influence of individual volatile compounds on overall aroma of sparkling wines from three vine-growing regions, OAV values and ROC indexes were calculated and presented in Table 6. From the total of 174 compounds only 26 exceeded the treshold values (OAV > 1). Between them, the most abundand were esters with seven individual compounds and aldehydes with five compounds. The highest OAV was noted in Krašić samples where isoamly lactate OAV value was notably higher compared to other two vine-growing regions. Among others hexanal and β-damascenone stood up in Zelina samples while in the sparkling wines from Plešivica vine-growing region hexanoic acid OAV was the highest. ROC index pointed out pronounced influence of volatile compounds belonging to esters and aldehydes in all sparkling wine samples.
Table 6.
Odour activity values (OAV) and relative odour contribution (ROC) in sparkling wines.
| Parameters | Vine-Growing Regions | |||||
|---|---|---|---|---|---|---|
| Plašivica (n = 19) | Zelina (n = 8) | Krašić (n = 3) | ||||
| OAV | ROC(%) | OAV | ROC(%) | OAV | ROC(%) | |
| Aldehydes | ||||||
| 2,4-Nonadienal | 18.88 | 5.1 | 7.77 | 2.25 | 21 | 6.08 |
| 2-Heptenal | 46.76 | 14.25 | 3.71 | 1.15 | 33.13 | 9.61 |
| Decanal | 12.85 | 3.5 | 27.61 | 8.28 | 13 | 3.76 |
| Hexanal | 40.73 | 11.06 | 86.74 | 26.38 | 27.33 | 7.92 |
| Nonanal | 10.04 | 2.7 | 3.65 | 1.11 | 17.47 | 5.06 |
| ∑ | 36.61 | 39.17 | 32.43 | |||
| Higher alcohols | ||||||
| 1-Hexanol | 0.74 | 0.2 | 0.64 | 0.19 | 1.18 | 0.34 |
| Isoamyl alcohol | 0.79 | 0.21 | 1.32 | 0.4 | 0.68 | 0.19 |
| Phenyethyl alcohol | 0.69 | 0.18 | 1.12 | 0.34 | 0.57 | 0.16 |
| ∑ | 0.59 | 0.93 | 0.69 | |||
| Volatile phenols | ||||||
| 4-Vinylguaicol | 2.85 | 0.77 | 0.71 | 0.21 | 6.4 | 1.85 |
| Terpenes | ||||||
| Geraniol | 0.11 | 0.02 | 4.4 | 1.34 | 0.39 | 0.11 |
| Hotrienol | 0.28 | 0.07 | 0.26 | 0.07 | 1.8 | 0.52 |
| Linalool | 0.45 | 0.12 | 1.8 | 0.55 | 2.92 | 0.84 |
| ∑ | 0.21 | 1.96 | 1.47 | |||
| C13-norisoprenoides | ||||||
| β-Damascenone | 38.2 | 10.4 | 73.6 | 22.57 | 2.8 | 0.81 |
| TDN | 1.27 | 0.3 | 1.94 | 0.59 | 0.27 | 0.07 |
| ∑ | 10.7 | 23.16 | 0.88 | |||
| Lactones | ||||||
| γ-Nonalacton | 0.3 | 0.08 | 1 | 0.31 | 0.45 | 0.13 |
| Esters | ||||||
| Ethyl butanoate | 13.5 | 3.4 | 20.85 | 6.39 | 18.1 | 5.24 |
| Ethyl hexanoate | 23.85 | 6.39 | 37.14 | 11.54 | 31.07 | 9.16 |
| Ethyl octanoate | 0.67 | 0.18 | 1.08 | 0.33 | 0.74 | 0.21 |
| Ethyl-2-methylbutanoate | 1.69 | 0.46 | 0.52 | 0.15 | 0.65 | 0.18 |
| Ethyl-3-methylbutanoate | 17.13 | 4.66 | 20.33 | 6.43 | 6.67 | 1.93 |
| Isoamyl aceatet | 14.53 | 2.95 | 15.76 | 4.83 | 20.13 | 5.83 |
| Isoamyl lactate | 90 | 24.52 | 10.9 | 3.34 | 122.5 | 35.36 |
| ∑ | 42.56 | 33.01 | 57.91 | |||
| Fatty acids | ||||||
| Decanoic acid | 2.4 | 0.62 | 0.79 | 0.24 | 1.7 | 0.49 |
| Hexanoic acid | 16.66 | 4.43 | 0.75 | 0.23 | 0.59 | 0.17 |
| Isovaleric acid | 0.21 | 0.05 | 0.09 | 0.02 | 0.45 | 0.13 |
| Octanoic acid | 12.41 | 3.38 | 2.5 | 0.76 | 13.29 | 3.85 |
| ∑ | 8.48 | 1.25 | 4.64 | |||
3. Materials and Methods
3.1. Samples
Commercial Croatian sparkling wines samples (n = 30), were obtained from the wineries located in Plešivica, Zelina and Krašić vine-growing regions, all within Zagreb County. In Plešivica vine-growing region the dominant grape varieties were Chardonnay, Pinot noir and Portugizer, in Zelina beside Pinot noir and Chardonnay the presence of Kraljevina (autohotnous Croatian variety) was notable while in Krašić for all the sparkling wines Manzoni bianco grape variety was used. Grapes used for the sparkling wines production were manually harvested while second fermentation was conducted in bottles for the period of 9 months. The first step, before chemical analysis, was to degas sparkling wines by use of Sonorex Ultrasonic bath (Bandelin ElectronicGmbH & Co. KG, Berlin, Germany). To eliminate carbon dioxide, approximately 50 mL of sparkling wines sample was put in a centrifuge tube and degassed for around 2 min.
3.2. Volatile Compounds Determination
Wine sample volatile compounds analysis was performed according to the described method [78]. Isolation of analytes was performed by solid phase extraction on LiChrolut EN cartridges (200 mg/3 mL, Merck, Darmstadt, Germany). In the column which was previously conditioned by successive washing with 3 mL dichloromethane (UHPLC gradient grade J.T.Baker, Deventar, The Netherland), methanol (UHPLC gradient grade J.T.Baker, Deventar, The Netherland) and a 13% aqueous ethanol (LiChrosolv, Merck, Darmstadt, Germany) solution 50 mL of sample was loaded. After the passage of the sample through column, residual sugars and other polar compounds were washed out by 3 mL of water. The column was dried by passing the air. The eluation of analytes was done by 1 mL of dichloromethane. As a quality control, 50 mL of water was injected to the SPE column instead of the sample. Quantitative and qualitative analysis was performed on an Agilent 6890 system coupled with 5973N mass spectrometer with the column ZB-WAX (60 m × 0.32 mm i.d., with 0.5 µm film thickness, Phenomenex, Torrance, USA). The temperature program was as follow 40 °C for 15 min, from 40 to 250 °C with increments of 2 °C per minute and 250 °C for 15 min. Transfer line was set to 250 °C, the flow rate of helium was 1 mL min−1. The MS was operated in electron ionization (EI) mode at 70 eV with a total ion current (TIC) monitoring. Identification was done by comparing retention times and mass spectra with those of standards. List of used standards, linear retention indices and other parameters for identification and quantification is presented in Table 7. Quantification was done by calibration curves. The curves (based on quantification ions) were constructed with software Enhanced ChemStation (Agilent Technologies, Santa Clara, CA, USA, SAD). For all available standards (172) six different concentrations were prepared. For two compounds (Terpendiol I and II) semi-quantitative analysis was performed. Their concentrations were expressed in equivalents of similar compounds with assumption that a response factor was equal to one.
Table 7.
Identification and quantification parameters for GC-MS analysis.
| Parameters | RT/min | LRI | Qion | ID | Chemical Standard |
|---|---|---|---|---|---|
| Aldehydes | |||||
| 2,4-Decadienal | 67.87 | 1837 | 81 | S, MS, RI | Sigma |
| 2,4-Heptadienal (E,E) | 49,36 | 1518 | 81 | S, MS, RI | Sigma |
| 2,4-Heptadienal (Z,Z) | 47.49 | 1488 | 81 | S, MS, RI | Sigma |
| 2,4-Nonadienal | 61.83 | 1728 | 81 | S, MS, RI | Sigma |
| 2-Heptenal | 38.01 | 1344 | 41 | S, MS, RI | Sigma |
| 5-Hydroxymethylfurfural | 100.53 | 2525 | 97 | S, MS, RI | Sigma |
| 5-Methyl-furfural | 54.47 | 1601 | 110 | S, MS, RI | Sigma |
| Benzeneacetaldehyde | 58.90 | 1676 | 91 | S, MS, RI | Sigma |
| Benzaldehyde | 51.54 | 1553 | 106 | S, MS, RI | Sigma |
| Decanal | 49.32 | 1518 | 43 | S, MS, RI | Sigma |
| Furfural | 47.70 | 1492 | 96 | S, MS, RI | Sigma |
| Heptanal | 28.29 | 1201 | 44 | S, MS, RI | Sigma |
| Hexanal | 21.27 | 1097 | 44 | S, MS, RI | Sigma |
| Nonanal | 42.53 | 1412 | 57 | S, MS, RI | Sigma |
| Higher alcohols | |||||
| 1-Butanol | 25.20 | 1555 | 56 | S, MS, RI | Sigma |
| 1-Decanol | 64.67 | 1779 | 55 | S, MS, RI | Sigma |
| 1-Heptanol | 46.33 | 1471 | 70 | S, MS, RI | Sigma |
| 1-Hexanol | 39.61 | 1368 | 41 | S, MS, RI | Sigma |
| 1-Methoxy-2-propanol | 24.03 | 1138 | 45 | S, MS, RI | Sigma |
| 1-Octadecanol | 104.93 | 2604 | 83 | S, MS, RI | Sigma |
| 1-Octanol | 52.77 | 1573 | 56 | S, MS, RI | Sigma |
| 1-Pentanol | 32.50 | 1263 | 42 | S, MS, RI | Sigma |
| 2-Heptanol | 37.20 | 1332 | 45 | S, MS, RI | Sigma |
| trans-2-Hexene-1-ol | 43.28 | 1423 | 57 | S, MS, RI | Sigma |
| cis-2-Hexene-1-ol | 41.76 | 1446 | 57 | S, MS, RI | Sigma |
| 2-Methyl-1-butanol | 29.55 | 1220 | 57 | S, MS, RI | Sigma |
| 2-Pentadecanol | 75.47 | 1983 | 45 | S, MS, RI | Sigma |
| 2-Pentene-1-ol | 37.40 | 1335 | 57 | S, MS, RI | Sigma |
| cis-3-Hexene-1-ol | 41.76 | 1405 | 41 | S, MS, RI | Sigma |
| trans-3-Hexene-1-ol | 40.42 | 1380 | 41 | S, MS, RI | Sigma |
| 3-Octanol | 42.19 | 1407 | 59 | S, MS, RI | Sigma |
| Isoamyl alcohol | 29.80 | 1223 | 55 | S, MS, RI | Sigma |
| Isobutanol | 21.66 | 1102 | 43 | S, MS, RI | Sigma |
| Phenylethyl alcohol | 71.53 | 1907 | 91 | S, MS, RI | Sigma |
| Volatile phenols | |||||
| 4-Ethylguaiacol | 79.53 | 2066 | 85 | S, MS, RI | Sigma |
| 4-Ethylphenol | 86.58 | 2216 | 107 | S, MS, RI | Sigma |
| 4-Vinylguaiacol | 87.46 | 2236 | 150 | S, MS, RI | Sigma |
| 4-Vinylphenol | 96.41 | 2439 | 120 | S, MS, RI. | Sigma |
| Eugenol | 86.07 | 2205 | 164 | S, MS, RI | Sigma |
| Guaiacol | 70.93 | 1895 | 124 | S, MS, RI | Sigma |
| Homovanillyl alcohol | 116.11 | 2817 | 137 | - S, MS, RI | Sigma |
| Vanillin | 103.41 | 2577 | 151 | S, MS, RI | Sigma |
| Tyrosol | 97.96 | 2473 | 107 | S, MS, RI | Sigma |
| Terpenes | |||||
| 1,8-Terpin | 82.19 | 2122 | 81 | S, MS, RI | Sigma |
| 6,7-Dihydro-7-hydroxylinalool | 75.92 | 1992 | 71 | S, MS, RI | Boc Science |
| 8-Hidroxylinalool | 92.01 | 2339 | 43 | S, MS, RI | Aurora Fine Chemicals |
| α-Terpineol | 61.39 | 1720 | 59 | S, MS, RI | Extrasynthese |
| β-Farnesen | 59.30 | 1683 | 69 | S, MS, RI | Extrasynthese |
| β-Ocimene | 62.74 | 1744 | 93 | S, MS, RI | Extrasynthese |
| cis-Linalool oxide, furan | 47.54 | 1489 | 59 | S, MS, RI | Extrasynthese |
| Citronelol | 65.03 | 1785 | 69 | S, MS, RI | Extrasynthese |
| δ-Carene | 37.71 | 1340 | 93 | S, MS, RI | Extrasynthese |
| Dihydroactinidiolide | 94.54 | 2398 | 111 | S, MS, RI | Boc Science |
| Farnesol | 93.73 | 2380 | 69 | S, MS, RI | Extrasynthese |
| γ-Terpinene | 32.29 | 1260 | 93 | S, MS, RI | Extrasynthese |
| Geraniol | 69.49 | 1868 | 69 | S, MS, RI | Extrasynthese |
| Geranyl acetate | 91.81 | 2334 | 69 | S, MS, RI | Boc Science |
| Hotrienol | 56.14 | 1629 | 71 | S, MS, RI | Boc Science |
| Linalool | 52.17 | 1563 | 71 | S, MS, RI | Extrasynthese |
| Linalool oxide, pyran | 63.76 | 1762 | 68 | S, MS, RI | Boc Science |
| Neric acid | 93.83 | 2381 | 69 | S, MS, RI | Extrasynthese |
| Nerol | 67.04 | 1822 | 69 | S, MS, RI | Extrasynthese |
| Nerolidol | 79.20 | 2059 | 69 | S, MS, RI | Boc Science |
| Pseudoionon | 83.10 | 2141 | 58 | S, MS, RI | Boc Science |
| Terpendiol I | 62.31 | 1736 | 82 | MS | |
| Terpendiol II | 74.54 | 1965 | 67 | MS | |
| Terpinene-4-ol | 55.87 | 1624 | 71 | S, MS, RI | Extrasynthese |
| Tetrahydrolinalool | 44.62 | 1444 | 73 | S, MS, RI | Boc Science |
| trans-Linalool oxide, furan | 45.67 | 1460 | 59 | S, MS, RI | Extrasynthese |
| trans-Rose oxide | 39.64 | 1368 | 139 | S, MS, RI | Sigma |
| Linalyl formate | 47.38 | 1487 | 69 | S, MS, RI | Boc Science |
| Ethyl linalyl acetal | 46.95 | 1480 | 73 | S, MS, RI | Boc Science |
| 2,6-Dimethyl-3,7-octadiene-2,6-diol | 61.25 | 1718 | 82 | S, MS, RI | Boc Science |
| 2,6-Dimethyl-7-octene-2,6-diol | 76.07 | 1995 | 71 | S, MS, RI | Boc Science |
| Menthol | 57.94 | 1660 | 71 | S, MS, RI | Sigma |
| Ocimenol | 58.50 | 1669 | 93 | S, MS, RI | Extrasynthese |
| C13- norisoprenoids | |||||
| α-Ionol | 72.23 | 1920 | 95 | S, MS, RI | Sigma |
| α-Ionon | 70.00 | 1877 | 121 | S, MS, RI | Sigma |
| β-Damascenone | 68.46 | 1848 | 69 | S, MS, RI | Sigma |
| TDN | 64.43 | 1774 | 157 | S, MS, RI | Boc Science |
| Lactones | |||||
| cis-Whiskey lactone | 75.95 | 1993 | 91 | S, MS, RI | Sigma |
| δ-Nonalactone | 87.43 | 2235 | 99 | S, MS, RI | Sigma |
| γ-Butyrolactone | 58.30 | 1666 | 42 | S, MS, RI | Sigma |
| γ-Decalactone | 85.06 | 2183 | 85 | S, MS, RI | Sigma |
| γ-Hexalactone | 62.40 | 1738 | 85 | S, MS, RI | Sigma |
| γ-Nonalacton | 79.55 | 2067 | 85 | S, MS, RI | Sigma |
| γ-Octalacton | 73.94 | 1953 | 85 | S, MS, RI | Sigma |
| γ-Undecalactone | 90.28 | 2299 | 85 | S, MS, RI | Sigma |
| trans-Whiskey lactone | 72.28 | 1921 | 99 | S, MS, RI | Sigma |
| Esters | |||||
| 2-Phenylethyl acetate | 68.28 | 1845 | 105 | S, MS, RI | Sigma |
| Diethyl glutarate | 65.93 | 1801 | 143 | S, MS, RI | Sigma |
| Methyl hydroxyisovalerate | 70.10 | 1879 | 131 | S, MS, RI | Sigma |
| Diethyl hydroxysuccinate | 97.00 | 2454 | 101 | S, MS, RI | Boc Science |
| Diethyl malonate | 54.35 | 1598 | 115 | S, MS, RI | Boc Science |
| Diethyl oxalate | 43.67 | 1429 | 59 | S, MS, RI | Sigma |
| Diethyl succinate | 60.09 | 1697 | 101 | S, MS, RI | Sigma |
| Dimethyl malate | 78.27 | 2040 | 103 | S, MS, RI | Sigma |
| Ethyl benzeneacetate | 66.64 | 1814 | 91 | S, MS, RI | Sigma |
| Ethyl butanoate | 18.32 | 1053 | 71 | S, MS, RI | Sigma |
| Ethyl decanoate | 57.48 | 1652 | 88 | S, MS, RI | Sigma |
| Ethyl furoate | 57.28 | 1648 | 95 | S, MS, RI | Sigma |
| Ethyl hexadecanoate | 88.91 | 2268 | 88 | S, MS, RI | Sigma |
| Ethyl hexanoate | 31.40 | 1247 | 88 | S, MS, RI | Sigma |
| Ethyl hydrogensuccinate | 96.70 | 2445 | 101 | S, MS, RI | Sigma |
| Ethyl lactate | 39.28 | 1363 | 45 | S, MS, RI | Sigma |
| Ethyl linoleate | 101.66 | 2645 | 67 | S, MS, RI | Sigma |
| Ethyl octanoate | 45.05 | 1447 | 88 | S, MS, RI | Sigma |
| Ethyl vanillate | 107.17 | 1425 | 151 | S, MS, RI | Sigma |
| Ethyl-2-hydroxy-3-methylbutanoate | 44.82 | 1067 | 73 | S, MS, RI | Sigma |
| Ethyl-2-hydroxybutanoate | 43.41 | 1290 | 59 | S, MS, RI | Boc Science |
| Ethyl-2-methylbutanoate | 19.29 | 1350 | 57 | S, MS, RI | Boc Science |
| Ethyl-2-oxopropanoate | 34.34 | 1541 | 43 | S, MS, RI | Sigma |
| Ethyl-3-ethoxypropanoate | 38.39 | 1350 | 59 | S, MS, RI | Boc Science |
| Ethyl-3-hydroxybutanoate | 50.79 | 1541 | 43 | S, MS, RI | Boc Science |
| Ethyl-3-hydroxyhexanoate | 72.00 | 1915 | 117 | S, MS, RI | Boc Science |
| Ethyl-3-methylbutanoate | 20.30 | 1082 | 88 | S, MS, RI | Sigma |
| Ethyl-hydroxyhexanoate | 52.19 | 1563 | 69 | S, MS, RI | Sigma |
| Ethylmethyl succinate | 57.80 | 1657 | 115 | S, MS, RI | Mol Port |
| Hexyl acetate | 34.16 | 1287 | 43 | S, MS, RI | Sigma |
| Isoamyl acetate | 23.81 | 1135 | 43 | S, MS, RI | Sigma |
| Isoamyl lactate | 53.70 | 1588 | 45 | S, MS, RI | Sigma |
| Isobutyl acetate | 16.97 | 1032 | 43 | S, MS, RI | Sigma |
| Isobutyl lactate | 46.90 | 1479 | 45 | S, MS, RI | Sigma |
| Methyl hexadecanoate | 87.32 | 2233 | 74 | S, MS, RI | Sigma |
| Methyl hexanoate | 28.33 | 1202 | 74 | S, MS, RI | Sigma |
| Methyl octadecanoate | 96.36 | 2438 | 74 | S, MS, RI | Sigma |
| Methyl-2-furoate | 54.67 | 1603 | 95 | S, MS, RI | Sigma |
| Methyl-3-hydroxyoctanoate | 77.70 | 2029 | 103 | S, MS, RI | Boc Science |
| Methyl geranoate | 61.25 | 1718 | 69 | S, MS, RI | Boc Science |
| o-Methylbenzyl acetate | 92.95 | 2361 | 104 | S, MS, RI | Sigma |
| Phenyl acetate | 57.31 | 1649 | 94 | S, MS, RI | Sigma |
| Fatty acids | |||||
| 2-Methylpropionic acid | 54.73 | 1604 | 43 | S, MS, RI | MolPort |
| Butanoic acid | 58,56 | 1671 | 60 | S, MS, RI | Sigma |
| Decanoic acid | 90.90 | 2313 | 60 | S, MS, RI | Sigma |
| Heptanoic acid | 76.17 | 1997 | 60 | S, MS, RI | Sigma |
| Hexanoic acid | 70.25 | 1882 | 60 | S, MS, RI | Sigma |
| Isovaleric acid | 60.88 | 1711 | 60 | S, MS, RI | Sigma |
| Octanoic acid | 81.05 | 2097 | 60 | S, MS, RI | Sigma |
| Propanoic acid | 53.16 | 1579 | 74 | S, MS, RI | Sigma |
| Other alcohols | |||||
| 1,4-Butandiol | 73.70 | 1949 | 42 | S, MS, RI | Sigma |
| 2,6-Dimethyl-4-heptanol | 52.26 | 1565 | 69 | S, MS, RI | Sigma |
| 2-Butoxy-ethanol | 41.34 | 1394 | 57 | S, MS, RI | Sigma |
| 2-Ethyl-1-hexanol | 48.54 | 1505 | 57 | S, MS, RI | Sigma |
| 3,4-Dimethyl-2-hexanol | 35.48 | 1306 | 45 | S, MS, RI | Sigma |
| 3-Ethoxy-1-propanol | 41.34 | 1394 | 59 | S, MS, RI | Sigma |
| 3-Methyl-1-pentanol | 37.86 | 1342 | 56 | S, MS, RI | Sigma |
| 3-Methyl-3-buten-1-ol | 32.63 | 1265 | 41 | S, MS, RI | Sigma |
| 4-Ethylcyclohexanol | 49.37 | 1518 | 81 | S, MS, RI | Sigma |
| 4-Methyl-1-pentanol | 37.00 | 1329 | 56 | S, MS, RI | Sigma |
| Benzylalcohol | 71.50 | 1906 | 78 | S, MS, RI | Sigma |
| Cyclohexanol | 41.95 | 1403 | 43 | S, MS, RI | Sigma |
| Furfuril alkohol | 59.56 | 1688 | 98 | S, MS, RI | Sigma |
| 2,3-Butanediol | 51.90 | 1559 | 45 | S, MS, RI | Sigma |
| Sulfur compounds | |||||
| 4-(Methylthio)-1-butanol | 69.56 | 1869 | 61 | S, MS, RI | Sigma |
| 3-(Methylthio)-1-propanol | 62.80 | 1745 | 106 | S, MS, RI | Sigma |
| Other compounds | |||||
| 2,5-Hexadione | 50.09 | 1530 | 43 | S, MS, RI | Sigma |
| 2,7-Octanedione | 41.95 | 1403 | 43 | S, MS, RI | Aurora Fine Chemicals |
| 2H-Pyran-2,6(3H)-dione | 78.28 | 2040 | 112 | S, MS, RI | MolPort |
| 2-Pentylfuran | 31.40 | 1247 | 81 | S, MS, RI | Sigma |
| 3-Penten-2-on | 24.21 | 1141 | 69 | S, MS, RI | Sigma |
| 4-Hydroxy-4-methyl-2-pentanone | 40.46 | 1381 | 43 | S, MS, RI | Sigma |
| 4-Methyl-2-penten-2-one | 24.65 | 1147 | 55 | S, MS, RI | Sigma |
| 5-Ethyl-4-methyl-3-heptanone | 26.19 | 1170 | 57 | S, MS, RI | Aurora Fine Chemicals |
| Acetoin | 35.55 | 1307 | 45 | S, MS, RI | Sigma |
| Acetylfurane | 50.27 | 1533 | 95 | S, MS, RI | Sigma |
| Benzofurane | 66.16 | 1805 | 118 | S, MS, RI | Sigma |
| Dihydro-2-methyl-3(2H)-furanone | 63.07 | 1750 | 43 | S, MS, RI | Sigma |
| N-(2-phenylethyl)acetamide | 106.27 | 2629 | 104 | S, MS, RI | Sigma |
| N-Ethylacetamide | 57.77 | 1657 | 43 | S, MS, RI | Sigma |
RT-retention time; LRI-linear retention indices; Qion-ion qualifier; ID: S-retention time and mass spectrum consistent with standard, RI—retention index consistent with those find in the literature, MS—mass spectra consistent with those find in NIST02 electronic library.
3.3. Determination of Organic Acids
Analysis of individual acids (tartaric, malic and citric acid) were done by HPLC system Agilent Series 1100 equipped with Diode Array Detector (Agilent, Palo Alto, CA, USA). In brief, the determination was performed isocratically with a flow rate set to 0.6 mL min−1 with 0.065 % phosphoric acid (p.a. Merck, Darmstadt, Germany) as a mobile phase. Column Aminex HPX-87H 300 × 7.8 mm i.d (Bio-Rad Laboratories, Hercules, CA, USA) was heated at 65 °C, while the detector was set to 210 nm.
3.4. Determination of Odour Activity Values and Relative Odour Contributions
Each chemical substance can have specific influence on the wine aroma. It can be presented by the odour activity value (OAV) and relative odour contributions (ROC). So they can be used as a markers in determining the role of a specific compound in the sample aroma composition. OAV is calculated as the quotients of their concentration (c) and the corresponding odour detection threshold (t) reported in the literature [79]. Volatile aroma substances with an OAV ≥ 1 can have direct impact on aroma and they are usually marked as one of the most significant volatile substances or the most active odours [80]. The volatiles with an OAV < 1 can also positively influence the wine aroma complexity and aromatic intensity of other compounds through synergistic effects. The ROC of each aroma compound is calculated as the ratio of the OAV of the respective compound to the total OAV of each wine [81].
3.5. Statistical Analysis
The analysis of variance was used for the statistical assessment of the data and Duncan’s multiple range test was used to determine significant differences (p < 0.05) among means. Multivariate analysis was carried out with XLSTAT software v.2020.3.1. (Addinsoft, New York, NY, USA). The forward stepwise model was used to select and rank the variables based on contribution to the discrimination of the groups. The selection process starts by using the variable with the largest contribution to the model and then the following variable is added with an enter probability greater than the threshold value. When the third and all the following variables are being added, model then evaluate the impact of removing each previously present variable in contrast to the removal threshold. To test the minimal number of dependent variables required to achieve the 100% correct classification using cross-validation of the samples within the belonging group, i.e., vine-growing region, a series of discriminant analyses were performed using all of the samples, starting with two variables with the highest rank based on stepwise discriminant analyses, and in each new analysis, one new variable was added as a differentiating factor among cultivars. This determined the cumulative efficiency of the parameters applied in the correct classification of wine samples in the corresponding vine-growing region.
4. Conclusions
Even though in the current work little was known about the enological steps used in the production of the wines studied, differences were clearly demonstrated and wines classified according to the vine-growing regions, indicating that future studies using greater control over enological factors are likely to demonstrate an even stronger role of the site in the sparkling wine composition. As can be seen from the results, in all sparkling wines, esters had an important role, among them especially the once with the OAV > 1 as ethyl butanoate, hexanoate, octanoate, ethyl-2-methylbutanoate, ethyl-3-methylbutanoate, isoamyl acetate, and isoamyl lactate. The presence of diethyl succinate as well as diethyl glutarate, compounds whose presence can be used as an ageing marker was detected. Another compound that could be used as discriminate marker is TDN whose concentrations were notably higher in sparkling wines from Zelina vine-growing region. Such data could lead to a better understanding of what defines sparkling wines of a specific vine-growing region. However, this work provides a basis for the future research variations of volatile aroma compounds within Croatian sparkling wines from Zagreb County and for the development of models that better explain these variations due to the geographic origin that is associated with similar climatic conditions or soil.
Author Contributions
Conceptualization, A.-M.J.K. and A.J.; methodology, I.T.; formal analysis, I.T.; data curation, D.P.; writing—original draft preparation, AM.J.K.; writing—review and editing, AM.J.K. and D.P.; supervision, A.J. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this work is attributed to the project, KK.01.1.1.04.0031, New Start for Croatian Grapevine Varieties (CroVitiRestart) funded by European Structural and Investment Funds and Croatian Ministry of Science and Education.
Conflicts of Interest
Authors declare no conflict of interest
Footnotes
Sample Availability: Samples from the described study are not available from the authors.
References
- 1.OIV Focus the Global Sparkling. [(accessed on 1 September 2020)]; Available online: http://www.oiv.int/en/technical-standards-and-documents/statistical-analysis/thematic-focus.
- 2.Kemp B., Alexandre H., Robillard B., Marchal R. Effect of production phase on bottle-fermented sparkling wine quality. J. Agric. Food Chem. 2015;63:19–38. doi: 10.1021/jf504268u. [DOI] [PubMed] [Google Scholar]
- 3.Pérez-Magariño S., Ortega-Heras M., Martínez-Lapuente L., Guadalupe Z., Ayestarán B. Multivariate analysis for the differentiation of sparkling wines elaborated from autochthonous Spanish grape varieties: Volatile compounds, amino acids and biogenic amines. Eur. Food Res. Technol. 2013;236:827–841. doi: 10.1007/s00217-013-1934-9. [DOI] [Google Scholar]
- 4.Campo E., Cacho J., Ferreira V. The chemical characterization of the aroma of dessert and sparkling white wines (Pedro Ximénez, Fino, Sauternes, and Cava) by gas chromatography-olfactometry and chemical quantitative analysis. J. Agric. Food Chem. 2008;56:2477–2484. doi: 10.1021/jf072968l. [DOI] [PubMed] [Google Scholar]
- 5.Pozo-Bayón M.Á., Martínez-Rodríguez A., Pueyo E., Moreno-Arribas M.V. Chemical and biochemical features involved in sparkling wine production: From a traditional to an improved winemaking technology. Trends Food Sci. Technol. 2009;20:289–299. doi: 10.1016/j.tifs.2009.03.011. [DOI] [Google Scholar]
- 6.Kemp B., Hogan C., Xu S., Dowling L., Inglis D. The Impact of Wine Style and Sugar Addition in liqueur d’expedition (dosage) Solutions on Traditional Method Sparkling Wine Composition. Beverages. 2017;3:7. doi: 10.3390/beverages3010007. [DOI] [Google Scholar]
- 7.Francioli S., Torrens J., Riu-Aumatell M., López-Tamames E., Buxaderas S. Volatile compounds by SPME-GC as age markers of sparkling wines. Am. J. Enol. Vitic. 2003;54:158–162. [Google Scholar]
- 8.Hidalgo P., Pueyo E., Pozo-Bayón M.A., Martínez-Rodríguez A.J., Martín-Álvarez P., Polo M.C. Sensory and analytical study of rosé sparkling wines manufactured by second fermentation in the bottle. J. Agric. Food Chem. 2004;52:6640–6645. doi: 10.1021/jf040151b. [DOI] [PubMed] [Google Scholar]
- 9.Torrens J., Rlu-Aumatell M., Vichi S., López-Tamames E., Buxaderas S. Assessment of volatlle and sensory profiles between base and sparkling wines. J. Agric. Food Chem. 2010;58:2455–2461. doi: 10.1021/jf9035518. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz-Redondo J.M., Cuevas F.J., León J.M., Ramírez P., Moreno-Rojas J.M., Ruiz-Moreno M.J. Quantitative Profiling of Ester Compounds Using HS-SPME-GC-MS and Chemometrics for Assessing Volatile Markers of the Second Fermentation in Bottle. J. Agric. Food Chem. 2017;65:2768–2775. doi: 10.1021/acs.jafc.6b05265. [DOI] [PubMed] [Google Scholar]
- 11.Riu-Aumatell M., Bosch-Fusté J., López-Tamames E., Buxaderas S. Development of volatile compounds of cava (Spanish sparkling wine) during long ageing time in contact with lees. Food Chem. 2006;95:237–242. doi: 10.1016/j.foodchem.2005.01.029. [DOI] [Google Scholar]
- 12.Gallardo-Chacón J.J., Vichi S., López-Tamames E., Buxaderas S. Changes in the sorption of diverse volatiles by saccharomyces cerevisiae lees during sparkling wine aging. J. Agric. Food Chem. 2010;58:12426–12430. doi: 10.1021/jf103086e. [DOI] [PubMed] [Google Scholar]
- 13.Loyaux D., Roger S., Adda J. The evolution of champagne volatiles during ageing. J. Sci. Food Agric. 1981;32:1254–1258. doi: 10.1002/jsfa.2740321219. [DOI] [Google Scholar]
- 14.Cynkar W., Dambergs R., Smith P., Cozzolino D. Classification of Tempranillo wines according to geographic origin: Combination of mass spectrometry based electronic nose and chemometrics. Anal. Chim. Acta. 2010;660:227–231. doi: 10.1016/j.aca.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 15.Roullier-Gall C., Boutegrabet L., Gougeon R.D., Schmitt-Kopplin P. A grape and wine chemodiversity comparison of different appellations in Burgundy: Vintage vs terroir effects. Food Chem. 2014;152:100–107. doi: 10.1016/j.foodchem.2013.11.056. [DOI] [PubMed] [Google Scholar]
- 16.Goldner C.M., Zamora C.M. Sensory Characterization of Vitis Vinifera cv. Malbec Wines from Seven Viticulture Regions of Argentina. J. Sens. Stud. 2007;22:520–532. doi: 10.1111/j.1745-459X.2007.00123.x. [DOI] [Google Scholar]
- 17.Vilanova M., Vilariño F. Influence of geographic origin on aromatic descriptors of Spanish Albariño wine. Flavour Fragr. J. 2006;21:373–378. doi: 10.1002/ffj.1677. [DOI] [Google Scholar]
- 18.Robinson A.L., Adams D.O., Boss P.K., Heymann H., Solomon P.S., Trengove R.D. Influence of geographic origin on the sensory characteristics and wine composition of Vitis vinifera cv. Cabernet Sauvignon wines from Australia. Am. J. Enol. Vitic. 2012;63:467–476. doi: 10.5344/ajev.2012.12023. [DOI] [Google Scholar]
- 19.Voce S., Škrab D., Vrhovsek U., Battistutta F., Comuzzo P., Sivilotti P. Compositional characterization of commercial sparkling wines from cv. Ribolla Gialla produced in Friuli Venezia Giulia. Eur. Food Res. Technol. 2019;245:2279–2292. doi: 10.1007/s00217-019-03334-9. [DOI] [Google Scholar]
- 20.Focea M.C., Luchian C.E., Moroşanu A.M., Niculaua M., Cotea V.V., Odăgeriu G., Zamfir C.I. Content of metals and organic acids from experimental sparkling white wine. BIO Web Conf. 2017;9:02007. doi: 10.1051/bioconf/20170902007. [DOI] [Google Scholar]
- 21.Caliari V., Panceri C.P., Rosier J.P., Bordignon-Luiz M.T. Effect of the traditional, charmat and asti method production on the volatile composition of moscato giallo sparkling wines. LWT-Food Sci. Technol. 2015;61:393–400. doi: 10.1016/j.lwt.2014.11.039. [DOI] [Google Scholar]
- 22.Gallardo-Chacón J.J., Vichi S., Urpí P., López-Tamames E., Buxaderas S. Antioxidant activity of lees cell surface during sparkling wine sur lie aging. Int. J. Food Microbiol. 2010;143:48–53. doi: 10.1016/j.ijfoodmicro.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Sartor S., Toaldo I.M., Panceri C.P., Caliari V., Luna A.S., de Gois J.S., Bordignon-Luiz M.T. Changes in organic acids, polyphenolic and elemental composition of rosé sparkling wines treated with mannoproteins during over-lees aging. Food Res. Int. 2019;124:34–42. doi: 10.1016/j.foodres.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Ribéreau-Gayon P., Glories Y., Maujean A., Dubourdieu D. Handbook of Enology: The Chemistry of Wine Stabilization and Treatments, 2nd ed. Volume 2 John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2006. [Google Scholar]
- 25.Caliari V., Burin V.M., Rosier J.P., BordignonLuiz M.T. Aromatic profile of Brazilian sparkling wines produced with classical and innovative grape varieties. Food Res. Int. 2014;62:965–973. doi: 10.1016/j.foodres.2014.05.013. [DOI] [Google Scholar]
- 26.Torchio F., Segade S.R., Gerbi V., Cagnasso E., Giordano M., Giacosa S., Rolle L. Changes in varietal volatile composition during shelf-life of two types of aromatic red sweet Brachetto sparkling wines. Food Res. Int. 2012;48:491–498. doi: 10.1016/j.foodres.2012.04.014. [DOI] [Google Scholar]
- 27.Rapp A., Mandery H. Wine aroma. Experientia. 1986;42:873–884. doi: 10.1007/BF01941764. [DOI] [Google Scholar]
- 28.Slegers A., Angers P., Ouellet É., Truchon T., Pedneault K. Volatile compounds from grape skin, juice and wine from five interspecific hybrid grape cultivars grown in Québec (Canada) for wine production. Molecules. 2015;20:10980–11016. doi: 10.3390/molecules200610980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexandre H., Guilloux-Benatier M. Yeast autolysis in sparkling wine-A review. Aust. J. Grape Wine Res. 2006;12:119–127. doi: 10.1111/j.1755-0238.2006.tb00051.x. [DOI] [Google Scholar]
- 30.Benucci I., Cerreti M., Maresca D., Mauriello G., Esti M. Yeast cells in double layer calcium alginate–chitosan microcapsules for sparkling wine production. Food Chem. 2019;300:125174. doi: 10.1016/j.foodchem.2019.125174. [DOI] [PubMed] [Google Scholar]
- 31.Genovese A., Lamorte S.A., Gambuti A., Moio L. Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 2013;53:15–23. doi: 10.1016/j.foodres.2013.03.051. [DOI] [Google Scholar]
- 32.Martínez-García R., García-Martínez T., Puig-Pujol A., Mauricio J.C., Moreno J. Changes in sparkling wine aroma during the second fermentation under CO2 pressure in sealed bottle. Food Chem. 2017;237:1030–1040. doi: 10.1016/j.foodchem.2017.06.066. [DOI] [PubMed] [Google Scholar]
- 33.Welke J.E., Manfroi V., Zanus M., Lazzarotto M., Zini C.A. Differentiation of wines according to grape variety using multivariate analysis of comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection data. Food Chem. 2013;141:3897–3905. doi: 10.1016/j.foodchem.2013.06.100. [DOI] [PubMed] [Google Scholar]
- 34.Carlin S., Vrhovsek U., Franceschi P., Lotti C., Bontempo L., Camin F., Toubiana D., Zottele F., Toller G., Fait A., et al. Regional features of northern Italian sparkling wines, identified using solid-phase micro extraction and comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. Food Chem. 2016;208:68–80. doi: 10.1016/j.foodchem.2016.03.112. [DOI] [PubMed] [Google Scholar]
- 35.Bordiga M., Rinaldi M., Locatelli M., Piana G., Travaglia F., Coïsson J.D., Arlorio M. Characterization of Muscat wines aroma evolution using comprehensive gas chromatography followed by a post-analytic approach to 2D contour plots comparison. Food Chem. 2013;140:57–67. doi: 10.1016/j.foodchem.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 36.Soares R.D., Welke J.E., Nicolli K.P., Zanus M., Caramão E.B., Manfroi V., Zini C.A. Monitoring the evolution of volatile compounds using gas chromatography during the stages of production of Moscatel sparkling wine. Food Chem. 2015;183:291–304. doi: 10.1016/j.foodchem.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira J.M., Oliveira P., Baumes R.L., Maia O. Changes in aromatic characteristics of Loureiro and Alvarinho wines during maturation. J. Food Compos. Anal. 2008;21:695–707. doi: 10.1016/j.jfca.2008.08.002. [DOI] [Google Scholar]
- 38.Marais J., van Wyk C.J., Rapp A. Effect of Sunlight and Shade on Norisoprenoid Levels in Maturing Weisser Riesling and Chenin blanc Grapes and Weisser Riesling Wines. S. Afr. J. Enol. Vitic. 1992;13:23–32. doi: 10.21548/13-1-2191. [DOI] [Google Scholar]
- 39.Shinohara T. Gas chromatographic analysis of volatile fatty acids in wines. Agric. Biol. Chem. 1985;49:2211–2212. doi: 10.1080/00021369.1985.10867054. [DOI] [Google Scholar]
- 40.Etieven P.X. Wine. In: Maarse H., editor. Volatile Compounds in Foods and Beverages. Marcel Dekker; New York, NY, USA: 1991. pp. 483–546. [Google Scholar]
- 41.Fedrizzi B., Magno F., Finato F., Versini G. Variation of some fermentative sulfur compounds in Italian “millesimè” classic sparkling wines during aging and storage on lees. J. Agric. Food Chem. 2010;58:9716–9722. doi: 10.1021/jf101478w. [DOI] [PubMed] [Google Scholar]
- 42.Rapp A., Güntert M., Almy J. Identification and Significance of Several Sulfur-Containing Compounds in Wine. Am. J. Enol. Vitic. 1985;36:219–221. [Google Scholar]
- 43.Pérez-Olivero S.J., Pérez-Pont M.L., Conde J.E., Pérez-Trujillo J.P. Determination of lactones in wines by headspace solid-phase microextraction and gas chromatography coupled with mass spectrometry. J. Anal. Methods Chem. 2014;2014 doi: 10.1155/2014/863019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosmerl T., Cegnar S. Aroma compounds in sparkling wines. Sodob. Kmet. 2003;35:476–482. [Google Scholar]
- 45.Zea L., Moyano L., Ruiz M.J., Medina M. Chromatography-Olfactometry Study of the Aroma of Fino Sherry Wines. Int. J. Anal. Chem. 2010;2010:626298. doi: 10.1155/2010/626298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The Good Scent Company. [(accessed on 5 September 2020)]; Available online: www.thegoodscentcompany.com.
- 47.Pino J.A., Mesa J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006;21:207–213. doi: 10.1002/ffj.1703. [DOI] [Google Scholar]
- 48.Noguerol-Pato R., González-Álvarez M., González-Barreiro C., Cancho-Grande B., Simal-Gándara J. Evolution of the aromatic profile in Garnacha Tintorera grapes during raisining and comparison with that of the naturally sweet wine obtained. Food Chem. 2013;139:1052–1061. doi: 10.1016/j.foodchem.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 49.Sacks G.L., Gates M.J., Ferry F.X., Lavin E.H., Kurtz A.J., Acree T.E. Sensory threshold of 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) and concentrations in young Riesling and non-Riesling wines. J. Agric. Food Chem. 2012;60:2998–3004. doi: 10.1021/jf205203b. [DOI] [PubMed] [Google Scholar]
- 50.Ferreira V., López R., Cacho J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000;80:1659–1667. doi: 10.1002/1097-0010(20000901)80:11<1659::AID-JSFA693>3.0.CO;2-6. [DOI] [Google Scholar]
- 51.Ferreira V., Lopez R. The actual and potential aroma of winemaking grapes. Biomolecules. 2019;9:818. doi: 10.3390/biom9120818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engel K.H., Flath R.A., Buttery R.G., Mon T.R., Teranishi R., Ramming D.W. Investigation of volatile constituents in nectarines. 1. Analytical and sensory characterization of aroma components in some nectarine cultivars. J. Agric. Food Chem. 1988;36:549–553. doi: 10.1021/jf00081a036. [DOI] [Google Scholar]
- 53.Sonni F., Moore E.G., Chinnici F., Riponi C., Smyth H.E. Characterisation of Australian Verdelho wines from the Queensland Granite Belt region. Food Chem. 2016;196:1163–1171. doi: 10.1016/j.foodchem.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 54.Fariña L., Villar V., Ares G., Carrau F., Dellacassa E., Boido E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015;69:244–255. doi: 10.1016/j.foodres.2014.12.029. [DOI] [Google Scholar]
- 55.Ferreira V., Ortín N., Escudero A., López R., Cacho J. Chemical characterization of the aroma of Grenache rosé wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002;50:4048–4054. doi: 10.1021/jf0115645. [DOI] [PubMed] [Google Scholar]
- 56.Qian M.C., Wang Y. Seasonal variation of volatile composition and odor activity value of “Marion” (Rubus spp. hyb) and “Thornless Evergreen” (R. laciniatus L.) blackberries. J. Food Sci. 2005;70:13–20. doi: 10.1111/j.1365-2621.2005.tb09013.x. [DOI] [Google Scholar]
- 57.Guth H. Quantification and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997;45:3027–3032. doi: 10.1021/jf970280a. [DOI] [Google Scholar]
- 58.Odor & Flavor Detection Thresholds in Water (In Parts per Billion) [(accessed on 12 September 2020)]; Available online: http://www.leffingwell.com/odorthre.htm.
- 59.Herrero P., Sáenz-Navajas P., Culleré L., Ferreira V., Chatin A., Chaperon V., Litoux-Desrues F., Escudero A. Chemosensory characterization of Chardonnay and Pinot Noir base wines of Champagne. Two very different varieties for a common product. Food Chem. 2016;207:239–250. doi: 10.1016/j.foodchem.2016.03.068. [DOI] [PubMed] [Google Scholar]
- 60.Fenoll J., Manso A., Hellín P., Ruiz L., Flores P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009;114:420–428. doi: 10.1016/j.foodchem.2008.09.060. [DOI] [Google Scholar]
- 61.Zhao P., Gao J., Qian M., Li H. Characterization of the key aroma compounds in Chinese syrah wine by gas chromatography-Olfactometry-Mass spectrometry and Aroma reconstitution studies. Molecules. 2017;22:1045. doi: 10.3390/molecules22071045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Gemert L.J. Compilation of Odour Treshold Values in Air and Water. Oliemans Punter & Partners BV; Zeist, The Netherlands: 2011. [Google Scholar]
- 63.Ohloff G. Importance of minor components in flavors and fragrances. Perfum. Flavor. 1978;3:11–22. [Google Scholar]
- 64.Moyano L., Chaves M., Zea L. A Technical Alternative to Aging. J. Agric. Sci. Appl. 2012;1:116–121. [Google Scholar]
- 65.Castro-Vázquez L., Díaz-Maroto M.C., Pérez-Coello M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007;103:601–606. doi: 10.1016/j.foodchem.2006.08.031. [DOI] [Google Scholar]
- 66.Sáenz C., Cedrón T., Cabredo S. Classification of wines from five Spanish origin denominations by aromatic compound analysis. J. AOAC Int. 2010;93:1916–1922. doi: 10.1093/jaoac/93.6.1916. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto T., Matsuda H., Utsumi Y., Hagiwara T., Kanisawa T. Synthesis and odor of optically active rose oxide. Tetrahedron Lett. 2002;43:9077–9080. doi: 10.1016/S0040-4039(02)02311-0. [DOI] [Google Scholar]
- 68.Pardo E., Rico J., Gil J.V., Orejas M. De novo production of six key grape aroma monoterpenes by a geraniol synthase-engineered S. cerevisiae wine strain. Microb. Cell Fact. 2015;14:1–8. doi: 10.1186/s12934-015-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Escudero A., Gogorza B., Melús M.A., Ortín N., Cacho J., Ferreira V. Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity values. J. Agric. Food Chem. 2004;52:3516–3524. doi: 10.1021/jf035341l. [DOI] [PubMed] [Google Scholar]
- 70.Langen J., Wegmann-Herr P., Schmarr H.G. Quantitative determination of α-ionone, β-ionone, and β-damascenone and enantiodifferentiation of α-ionone in wine for authenticity control using multidimensional gas chromatography with tandem mass spectrometric detection. Anal. Bioanal. Chem. 2016;408:6483–6496. doi: 10.1007/s00216-016-9767-6. [DOI] [PubMed] [Google Scholar]
- 71.Buttery R.G., Turnbaugh J.G., Ling L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988;36:1006–1009. doi: 10.1021/jf00083a025. [DOI] [Google Scholar]
- 72.González Álvarez M., González-Barreiro C., Cancho-Grande B., Simal-Gándara J. Relationships between Godello white wine sensory properties and its aromatic fingerprinting obtained by GC-MS. Food Chem. 2011;129:890–898. doi: 10.1016/j.foodchem.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 73.Takeoka G.R., Flath R.A., Mon T.R., Teranishi R., Guentert M. Volatile Constituents of Apricot (Prunus Armeniaca) J. Agric. Food Chem. 1990;38:471–477. doi: 10.1021/jf00092a031. [DOI] [Google Scholar]
- 74.Azcarate S.M., Cantarelli M.A., Marchevsky E.J., Camiña J.M. Evaluation of Geographic Origin of Torrontés Wines by Chemometrics. J. Food Res. 2013;2:48. doi: 10.5539/jfr.v2n5p48. [DOI] [Google Scholar]
- 75.Mestre M.V., Maturano Y.P., Gallardo C., Combina M., Mercado L., Toro M.E., Carrau F., Vazquez F., Dellacassa E. Impact on sensory and aromatic profile of low ethanol malbec wines fermented by sequential culture of Hanseniaspora uvarum and Saccharomyces cerevisiae native yeasts. Fermentation. 2019;5:65. doi: 10.3390/fermentation5030065. [DOI] [Google Scholar]
- 76.Arozarena I., Casp A., Marín R., Navarro M. Multivariate differentiation of Spanish red wines according to region and variety. J. Sci. Food Agric. 2000;80:1909–1917. doi: 10.1002/1097-0010(200010)80:13<1909::AID-JSFA728>3.0.CO;2-U. [DOI] [Google Scholar]
- 77.Marais J., Van Rooyen P.C., Du Plessis C.S. Classification of White Cultivar Wines by Origin using Volatile Aroma Components. South. African J. Enol. Vitic. 2017;2 doi: 10.21548/2-2-2395. [DOI] [Google Scholar]
- 78.Jagatić Korenika A.-M., Biloš J., Kozina B., Tomaz I., Preiner D., Jeromel A. Effect of Different Reducing Agents on Aromatic Compounds, Antioxidant and Chromatic Properties of Sauvignon Blanc Wine. Foods. 2020;9:996. doi: 10.3390/foods9080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Falqué E., Fernández E., Dubourdieu D. Differentiation of white wines by their aromatic index. Talanta. 2001;54:271–281. doi: 10.1016/S0039-9140(00)00641-X. [DOI] [PubMed] [Google Scholar]
- 80.Allen M.S., Lacey M.J., Boyd S. Determination of Methoxypyrazines in RedWines by Stable Isotope Dilution Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 1994;42:1734–1738. doi: 10.1021/jf00044a030. [DOI] [Google Scholar]
- 81.Ohloff G. The Fashion of Odors and Their Chemical Perspectives: Scent and Fragrances. Springer; Berlin/Heidelberg, Germany: 1994. [Google Scholar]