Abstract
The release of reactive oxygen species (ROS) and oxidative stress is associated with the development of many ailments, including cardiovascular diseases, diabetes and cancer. The causal link between oxidative stress and cancer is well established and antioxidants are suggested as a protective mechanism against cancer development. Recently, an increase in the consumption of antioxidant supplements was observed globally. The main sources of these antioxidants include fruits, vegetables, and beverage. Herbal infusions are highly popular beverages consumed daily for different reasons. Studies showed the potent antioxidant effects of plants used in the preparation of some herbal infusions. Such herbal infusions represent an important source of antioxidants and can be used as a dietary protection against cancer. However, uncontrolled consumption of herbal infusions may cause toxicity and reduced antioxidant activity. In this review, eleven widely consumed herbal infusions were evaluated for their antioxidant capacities, anticancer potential and possible toxicity. These herbal infusions are highly popular and consumed as daily drinks in different countries. Studies discussed in this review will provide a solid ground for researchers to have better understanding of the use of herbal infusions to reduce oxidative stress and as protective supplements against cancer development.
Keywords: antioxidant, natural products, anti-tumor, dietary agents
1. Introduction
The intimate link between nutrition and health is well documented [1] and people in many countries have strong beliefs that foods provide more benefits than just being a source of energy [2]. Herbs and plant-derived natural products are considered the oldest medications in the world [3]. Plants were traditionally used to treat different ailments, including cancer, which is the second leading cause of death after cardiovascular diseases [4]. More than 30,000 plants were evaluated for their anticancer effects by the National Cancer Institute [5] and several studies were conducted to prove the anticancer potential of plants or their natural products [6,7,8,9]. However, the medical field shows very limited use of plants and dietary agents in cancer prevention and treatment.
The consumption of herbal infusions is very common in the Mediterranean region and globally. In a study conducted on 1260 cancer patients in Palestine, 60.9% were consuming herbs, mostly in the form of decoctions [10]. These drinks are mainly prepared from aromatic plants belonging to the following families: Lauracae, Umbelliferae, Lamiaceae, Myrtacae and Compositae [3]. The plants used in the preparation of herbal infusions were subjected to several studies and some of these plants exhibited potent antioxidant and anticancer properties. However, overconsumption of these herbal infusions may result in contradictory and side effects. This review summarizes the antioxidant capacities, anticancer potential, and possible toxicity of eleven widely-consumed herbal infusions.
Oxidative Stress and Cancer
Oxidative stress is the unbalance between production and elimination of free radicals and reactive species, like reactive oxygen species (ROS) and reactive nitrogen species (RNS) [11,12]. Oxidative stress is responsible for causing damage in cells and vital biomolecules. It is associated with induction of chronic inflammation and subsequently the development of many diseases including cancer, diabetes, cardiovascular, neurological and pulmonary diseases [12]. Oxidative stress is produced by external sources, like UV radiation, toxic chemicals and drugs, by physiological changes such as aging and inflammation and by internal sources via enzymatic and non-enzymatic reactions [13,14]. Many enzymes play an essential role in oxidative stress formation. These enzymes include xanthine oxidase (XO), P450 complex, NADPH oxidase (NOX), uncoupled endothelial nitric oxide synthase (eNOS), arachidonic acid (AA), lipoxygenase, peroxisomes and cyclooxygenase (COX) [11,13]. On the other hand, superoxide radicals are non-enzymatically generated by mitochondrial respiration chain complex I (NADPH–ubiquinone oxidoreductase) and complex III (the ubiquinol–cytochrome c oxidoreductase) [11,13]. Complex III mediates ROS production which has significant effect in cancer development and progression [11]. Clearly, ROS interact and oxidize many cellular constituents involving proteins, lipids and nucleic acids followed reversibly or irreversibly with changes in the structure and the function of these molecules [15]. On the other side, inducible NOS produces significant amounts of RNS which play critical role in the induction of lipid peroxidation and consequently the production of other reactive species, like reactive aldehydes-malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) [12].
In our body, redox homeostasis balances the oxidative stress via enzymatic and non-enzymatic antioxidants. Several types of antioxidants have critical roles in ROS elimination, including dietary natural antioxidants, like tocopherol, selenium, β-carotene, ascorbic acid, polyphenol metabolites, and synthetic antioxidants (e.g., N-acetylcysteine). On the contrary, many endogenous antioxidant molecules contribute in this role, such as glutathione, α-lipoic acid, coenzyme Q, ferritin, uric acid, bilirubin, metallothionein, L-carnitine, and melatonin. Endogenous antioxidant enzymes are also involved in balancing the oxidative stress. These enzymes include superoxide dismutase (SOD), glutathione reductase (Gr), thioredoxin reductase (TRX), catalase (CAT), glutathione peroxidases (GPXs) and peroxiredoxins (PRXs) [13].
All cancer phases entailing initiation, promotion and progression are affected by oxidative stress. Oxidative stress has the ability to activate several transcription factors including nuclear factor (NF)-κB, hypoxia inducible factor (HIF)-1α, activator protein (AP)-1, p53, peroxisome proliferator-activated receptor (PPAR)-γ,β-catenin/Wnt, and nuclear factor erythroid 2-related factor 2 (Nrf2). These factors regulate the expression of diverse genes included in immune modulation, inflammatory response, carcinogenesis, metastasis, tissue remodeling and fibrosis [12,16]. Besides that, ROS activate signaling pathways associated with cell growth, e.g., p38MAPK, p70S6K, p90Rsk, JAK/STAT, JNK, ERK, RAS, AKT and phospholipase D [13]. Moreover, ROS can oxidize cysteine residues in tyrosine phosphatases, for example PTEN and PTP-1B, and decrease their activities. Such changes promote hyper-activation of the PI3K and AKT pathways [17]. Additionally, pro-angiogenic factors, like HIF-1, actuate the transcription of angiogenic factors, such as VEGF, leading to neovascularization [18]. NOX1-derived RO stimulates angiogenic switch in fibroblasts and matrix remodeling [19]. In addition, iron-induced oxidative stress by ferric nitrilotriacetate (Fe-NTA) assesses in p16/p15 tumor suppressor genes deletion which results in carcinogenesis [20].
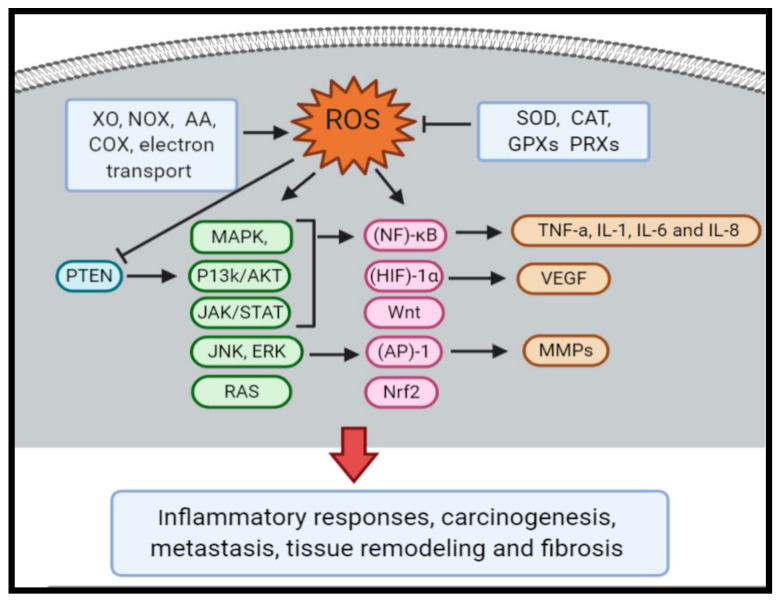
Antioxidants in diet and supplements are widely used to protect cells from the damage induced by ROS. However, some clinical trials have shown conflicting results that do not support this concept. Based on recent studies, antioxidants may increase melanoma metastasis in mice [21,22] and accelerate tumor progression in later stages of lung cancer [23]. One explanation of the antioxidant activity in promoting tumor growth is the disruption of the ROS-p53 axis, which is related to the somatic mutation in p53 that occurred in the late stage of tumor progression [23]. In fact, mutant p53 isoforms cannot apply antioxidant activities, and rather induce intracellular ROS and promote a pro-tumorigenic survival [24]. Interestingly, the administration of mitochondria and non-mitochondria-targeted antioxidants resulted in two distinguished outcomes of liver cancer prevention by altering DNA repair [25]. Figure 1 describes the role of oxidative stress in cancer.
Figure 1.
The role of the oxidative stress in cancer. ROS are generated by enzymes such as xanthine oxidase (XO), NADPH oxidase (NOX), nitric oxide synthases (NOS), arachidonic acid (AA) and cyclooxygenase (COX) and by mitochondrial respiration chain, this production is countered by endogenous antioxidant enzymes (e.g., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GPXs) and peroxiredoxins (PRXs). However, over production of ROS activate several transcription factors including nuclear factor (NF)-κB, hypoxia inducible factor (HIF)-1α, activator protein (AP)-1, p53, Wnt, and Nuclear factor erythroid 2-related factor 2 (Nrf2), which regulate the expression of genes included in inflammatory responses, carcinogenesis and metastasis, tissue remodeling and fibrosis. Furthermore, ROS activate signaling pathways associated with cell growth, e.g., JAK/STAT, JNK, ERK, RAS and AKT. Moreover, ROS oxidize cysteine residues in phosphatase and tensin homolog (PTEN) and decrease their activities, hence, these changes reveal activation of the PI3K/AKT pathways. Also, HIF-1 actuate transcription of angiogenic factors, such as Vascular endothelial growth factor (VEGF), leading to neovascularization. In addition, NOX1-derived ROS upregulate VEGF, VEGF receptors and matrix metalloproteinases (MMPs).
2. Herbal Infusions Antioxidant and Anticancer Capacities
Several plants with high antioxidant abilities and total phenolic contents have been screened out to be used as a rich source of natural antioxidants. These plants could be developed into herbal infusions, functional food or pharmaceuticals for the inhibition and treatment of diseases caused by oxidative stress [26]. Medicinal plants with potent anticancer activities might be potential sources of vigorous natural antioxidants and beneficial chemopreventive agents [27]. In this section, eleven popular herbal infusions are evaluated for their antioxidant and anticancer beneficial effects.
2.1. Lemon and Ginger Combination
Lemon (Citrus limonum) belongs to the Rutaceae family. GC-MS/MS analysis showed the presence of high concentrations of limonene with percentages in the lemon water extract of 23.271% [6]. Limonene protected cells against the oxidative stress induced by the exogenous addition of H2O2. Limonene also defended normal lymphocytes from diseases related to oxidative stress, including cancer [28]. Moreover, (+)-limonene epoxide enhanced the activity of antioxidant enzymes like catalase and superoxide dismutase in mice [29]. Lemon peel essential oil exhibited 55.09% inhibition of 2,2-diphenyl-1picrylhydrazy l (DPPH), while ascorbic acid (positive control) showed a 5.18% activity, demonstrating its potent antioxidant effects [30]. It was found that peels of citrus fruits are a significant source of various antioxidants, and such by-products of the juice extraction industry could be utilized as natural antioxidants. Using the whole extract instead of individual antioxidants allows taking advantage of additive and synergistic impacts of diverse phenolics, flavonoids, ascorbic acid, carotenoids, and reducing sugars present in the samples [31].
Many indolofuroquinoxaline derivatives in lemon citrus have displayed promising growth prevention effect against the K562, MDA-MB 231, and MCF7 cell lines, though, no significant effects have been seen on the HEK293 cell line (normal cells), suggesting a selectivity of these derivatives towards cancer cells [32]. Studies have shown that lemon and grapefruit peel essential oils showed moderated to weak cytotoxicity against the human prostate (PC-3), lung (A549), and breast (MCF-7) cancer cell lines [33]. In lemon juice, the presence of limonene together with other components like alkaloids, phenols, flavonoids, and terpenoids was shown to be responsible for inducing apoptosis and inhibiting angiogenesis in cancer cells [6].
Ginger (Zingiber officinale Roscoe) belongs to the Zingiberaceae family. Herbal teas prepared from ginger are used as a folk remedy to treat coughs, colds, and flu. It is also applied as a paste for external applications to treat headaches [34]. Gingerols and shogaols are considered significant ingredients in ginger, as both of these ingredients exhibit biological activities, including anticancer, antioxidant, antimicrobial, anti-inflammatory, and anti-allergic properties [35]. Ginger has antioxidant properties and there is a positive relationship between antioxidant activities and total phenolic contents in ginger [36]. The antioxidant capacity of ginger infusion was measured by using spectroscopic methods and the result were 16.0 μmol gallic acid equivalent per gram of ginger extract [37]. Ginger can improve hepatic changes after an administration of a high dose of acetaminophen in vivo. This hepatic protection is caused by reduction of oxidative stress and increase in antioxidant capacity [38].
Oleoresin, extracted from the ginger’s rhizomes contains [6]-gingerol which suppresses cell adhesion, invasion, motility, and activities of MMP-2 and MMP-9 in the MDA-MB-231 human breast cancer cell line [39]. The cancer preventive properties of ginger have also been linked to the presence of flavonoids and polyphenolic components, particularly quercetin [40]. The fresh, dried, and steamed ginger has an antiproliferative effect against human Hela cancer cells. Interesitingly, the antiproliferative effect of steamed ginger at 120 °C for 4 h was found to be approximately 1.5- and 2-fold higher than that of dried and fresh ginger [41].
2.2. Wild Thyme (Thymus Serpyllum)
Thymus serpyllum belongs to the Lamiaceae family. It is a perennial shrub that has a woody base [42]. Rosmarinic acid is the principal ingredient identified in aqueous tea infusion (93.13 mg/g) of wild thyme. Rosmarinic acid possesses a variety of biological features, including anti-oxidant, anti-inflammatory, anti-viral, and anti-bacterial effects [43].The strong protective impact of wild thyme infusions is proposed to be the consequence of large amounts of rosmarinic acid and flavonoids (quercetin, eriocitrin, luteolin-7-O-glucoside, apigenin-7-O-glucoside, luteolin, apigenin) [44]. Wild thyme is a good source of compounds essential to prevent oxidation of low-density lipoproteins in vivo [43]. By using the pFRAP method, wild thyme infusion extracted for 30 min showed significantly the highest average antioxidant activity (268.01 mg GAE/100 g), while extraction for 60 min showed lowest antioxidant activity (111.56 mg GAE/100 g). It is concluded that the antioxidant activity of the tea of wild thyme depends on extraction time [45]. Another study conducted by Zhang et al. showed that rosmarinic acid in thyme increases the activity of superoxide dismutase, catalase, and glutathione peroxidase with a reduction in malondialdehyde [46]. Moreover, wild thyme essential oils demonstrated better overall antioxidant activity compared to other thymus species, due to the presence of thymol in its essential oil [47].
In osteosarcoma cells, rosmarinic acid showed anticancer effects by suppressing DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway [48]. Previous study confirmed that rosmarinic acid reverses non-small cell lung cancer cisplatin resistance through the activation of the MAPK signaling pathway [49]. Moreover, rosmarinic acid inhibited lung metastasis of murine colon carcinoma cells by activating AMP-activated protein kinase [50]. The hexane extract of Thymus serpyllum was cytotoxic to six different cancer cell lines.The highest anticancer activity was found in HepG2 (Liver Carcinoma Cell Line), followed by HCT 116 (a colon cancer cell line), MCF7 (breast cancer cell line), MDA-MB-231 (breast cancer cell line), PC3 (prostate cancer cell line), and A549 (lung carcinoma cell line) [51].
2.3. Marjoram (Organum Majorana)
Organum majorana belongs to the Lamiaceae family. Marjoram is a shrub and a perennial plant native in Asia and the Mediterranean area. It has been used traditionally in the folk medicine as an antifungal, antiviral, and antiparasitic remedy [52]. Using the active oxygen method [53] and ferric reducing antooxidant properties [54], methanolic extracts of marjoram have shown potent antioxidant activity that was attributed to the presence of polyphenolic compounds in the plant. Up to date, 31 polyphenols were identified in marjoram [55]. Recently, LC–ESI-MS/MS analysis detected rosmarinic acid as the most potent antioxidant polyphenol in marjoram’s methanolic extract [56], while gas chromatography-mass spectroscopy analysis of essential oil of both the stem and aerial parts revealed the presence of linalool and estragole as main components [57]. The marjoram water extract plays an important role in the initiation of apoptosis by inducing DNA damage in human colon cancer HT-29 cells and down-regulation of survivin (inhibitor of apoptosis) and the activation of caspases, in human breast cancer MDA-MB-231 cells [58]. Supporting this finding, the essential oil of marjoram showed cytotoxic anti-cancer effect against the HT29 and Caco-2 colon cancer cell lines, partially through the down-regulation of survivin [59]. The pure essential oil of marjoram was also shown to cause a concentration- and time-dependent reduction in the proliferation of the lung cancer cells (A549 and LNM35) and the growth of their relevant colonies in vitro. Likewise, treatment with marjoram significantly reduced the growth of LNM35 and A549 xenografts in the chick embryo and in nude mice models in vivo without notable side effects [60]. Moreover, the highest phenolic contents and antioxidant actions of the marjoram water extract lead to the upregulation of cyclin-dependent kinase inhibitor 1 (p21), leading to apoptosis and suppression of the cell cycle in the breast cancer MCF-7 cell line [61].
2.4. Palestinian Herbal Mix
The Palestinian herbal infusion contains green tea, lemon verbena, sage, and citrus lemon. Green tea (Camellia Sinensis) belongs to the Theaceae family and it is widely consumed in Asian countries [62]. Fresh tea leaves contain caffeine, theobromine, theophylline, and other methylxanthines, lignin, organic acids, chloro-phylland, theanine, and free amino acids [63]. Moreover, other components exist, including, flavones, phenolic acids, and depsides, carbohydrates, alkaloids, minerals, vitamins, and enzymes [64]. Tea polyphenols, essentially flavonoids, are well-known for their antioxidant capacities. Various studies have confirmed that polyphenols and tea catechins are exceptional electron donors and efficient scavengers of physiologically-relevant ROS in vitro, including superoxide anions [65]. The most critical bioactive agent in green tea is epigallocatechin-3-gallate [66], which is listed as an antioxidant. Epigallocatechin 3-gallate exerts its beneficial biological actions directly by interacting with proteins and phospholipids in the plasma membrane and regulating signal transduction pathways, transcription factors, DNA methylation, mitochondrial function, and autophagy [67]. Green tea, which is rich in polyphenols, has been found to increase the inhibitory effect of tamoxifen on the proliferation of the ER (estrogen receptor)-positive MCF-7, ZR75, and T47D human breast cancer cells in vitro [68]. The dietary green tea polyphenol has a potentiating impact on cisplatin anti-tumor activity and a protective influence against cisplatin-induced renal dysfunction. It is suggested that green tea polyphenol may be used with cisplatin as a modulator in anticancer treatment [69]. Interestingly, the grape extracts work synergistically with decaffeinated green tea extracts in the prevention of the activity of tumor-associated NADH oxidase (tNOX) and the prevention of cancer cell growth. Intra-tumoral injections of 25:1 mixture of the green tea extract and ground freeze-dried pomace were effective in repressing the growth of 4T1 mammary tumors in mice [70]. Combining (-)-epigallocatechin gallate and quercetin synergistically prevented stem cell characteristics of human prostate cancer cells [71].
Lemon verbena (Lippia citriodora) belongs to the Verbenaceae family. It is a shrub with scented leaves that grows in both tropical and subtropical regions [72]. Chemical composition of lemon verbena showed that it is composed of large amounts of polyphenolic compounds including verbascoside (400 mg/L) and luteolin 7-diglucuronide (100 mg/L). It also contains 42 mg/L of essential oil with much more citral (77% of the essential oil) [73]. Infusion of Lippia citriodora protected against lipid peroxidation and protein carbonylation. Also, the decoction showed higher antioxidant capacity compared to the infusion [74]. Leaf infusion of lemon verbena worked as a free radical scavenger and exhibited an antigenotoxic activity by increasing the antioxidant status [75]. In a clinical trial including 43 healthy subjects, lemon verbena leaves induced oxidant/antioxidant balance by causing a reduction in the lipid peroxidation and an increase in the total antioxidant ability [76]. Lemon verbena essential oil showed high antiproliferative activity against a panel of human cancer cell lines (A375 > Caco2 > HepG2 > MCF-7 > THP-1). Citral or geranial is the main component in the essential oil of lemon verbena, and while it has strong anticancer and antimicrobial properties, it has weak direct antioxidant activities [77]. Lemon verbena is also rich with luteolin, which is a flavone bioflavonoid and has anticancer properties. It induces apoptotic cell death, inhibits the proliferation of cancer cells, and inhibits tumor angiogenesis [78].
Sage (Salvia officinalis) belong to the Lamiaceae family. Sage is a perennial shrub, an aromatic and remedial plant endemic in the Mediterranean region [79]. According to Gas Chromatography-Mass Spectrometry (GC-MS) analysis, the main detected compounds were oxygenated monoterpenes followed monohydrocarbone, squiterpenes, and others. The main essential oil constituents were α-terpineol (33.07%), camphor (11.57%), α-pinene (8.96%) camphene (5.09%) β-cymene (5.40%) caryphyllene (3.76%) β-myrcene (3.65%) β-menthen-1-ol (3.45%) and bomeol (3.38%) [80]. Several in vivo and in vitro studies have investigated the activity of polyphenols as sage tea active ingredients that may prevent lipid peroxidation and augment antioxidant defense mechanisms [81]. Daily drinking of sage in both mice and rat causes significant increase in the liver antioxidant enzyme glutathione-S-trans- ferase (GST) activity [82]. Similar effect was observed when rat hepatocytes (isolated from the livers of sage drinking rats) showed an increase in glutathione (GSH) level and GST activity. Also, the treatment of rats with water extracts of sage for five weeks protected rat hepatocytes against azathioprine toxicity [83]. It is important to note that the method of extraction affects the antioxidant activity. The highest antioxidant activities of sage were discovered in the methanolic extract, followed by water infusion and decoction [84]. The hydroalcoholic extract of sage exhibited high antioxidant activity [85]. For example, earlier study has shown that the hydroalcoholic extract of sage has hepatoprotective action against isoniazid-induced hepatic damage in rats. This activity may be attributed to free radical-scavenging, and antioxidant activities of flavonoids in the extract [86]. Furthermore, many diterpenes, isolated from plants of several species of the genus Salvia, have been demonstrated to have interesting antitumor activity [87]. S. officinalis essential oil inhibited human HNSCC cell by activating different anticancer mechanisms [88].
2.5. Lebanese Herbal Mix
This infusion contains green tea, lemon verbena, cinnamon, damask rose, chamomile flowers, primrose, and ginger. Cinnamon (Cinnamomum zeylanicum) belongs to the Lauraceaeis family. It is a small evergreen tree with a height of 5–7 m. The plant is charecterized by the aromatic odor and pleasant smell. The biological activities of cinnamon are due to the presence of tannins [89]. The bark of C. zeylanicum essential oil was analyzed by GC–MS which revealed the presence of (E)-cinnamaldehyde (68.95%), as the major component, in addition to benzaldehyde (9.94%), and (E)-cinnamyl acetate (7.44%) [90]. Saponins, tannins, phenols, terpenoids, and phytosterols were observed in the cinnamon plant whether dried or fresh [91]. Cinnamon’s essential oil prevents the hepatic 3-hydroxy-3-methylglutaryl CoA reductase activity in rats which leads to lower hepatic cholesterol content and decrease the lipid peroxidation via enhancement of the hepatic antioxidant enzyme activities [92]. Differences in antioxidant activities of cinnamon maybe related to different parts of the plant. For example, cinnamon leaf oils have high antioxidant activities, whereas cinnamon bark oils have low antioxidant activities [93]. A cinnamon water extract contained the highest amount of phenolics and had the highest antioxidative activity [94]. Earlier study has investigated the hepatoprotective activity of both aqueous and ethanolic extracts of cinnamon against carbon tetrachloride (CCl4) that induced lipid peroxidation and hepatic injury in rats. The raised serum AST and ALT enzymatic activities induced by CCl4 were significantly decreased by oral administration of 200 mg/kg of each extract once daily for seven days, as compared to the untreated rats [95].
A review of the literature showed that cinnamon has various cytotoxic activities against different cancer cell lines, namely basal cell carcinoma, human epithelioid cervix carcinoma (HeLa), human cancer promyelocytic leukemia (HL-60), human colorectal carcinoma (HCT 116, HT 29, and SW 480), epidermoid carcinoma (A431), and human cervical carcinoma (SiHa) [96]. The alcoholic and aqueous extracts of the stem bark of Cinnamomum malabatrum possess protective effects against Dalton’s Ascitic lymphoma-induced cancer in mice. Such activity is due to the presence of flavonoids, essential oils, amino acids, tannins, and phytosterols [97]. At a concentration of 1.28 mg/mL (including 10.24 µM cinnamaldehyde), aqueous cinnamon extract treatment resulted in 35–85% growth prevention of the majority of the cancerous cells. Similarly, a concentration of 10 µM cinnamaldehyde treatment resulted in a 30% growth prevention of only SK-N-MC cells with no effect on other cancer cell lines. These results suggest that aqueous cinnamon extract had a significant inhibitory effect on the majority of cancer cells [98].
Damask rose (Rosa damascena Mil.) belongs to the Rosacea family. It is a deciduous shrub and is the most significant aromatic medicinal plant. The damask rose is mainly used in the perfume industry and as a flavoring agent in food products [99]. In R. damascena essential oil, a total of 22 compounds were detected by GC-MS analysis. Both citronellol (23.43%) and geraniol (34.91%) were the main scent compounds of the fresh rose flowers [100]. The entire flavonoid content of aqueous and ethanolic extracts of R. damascena flower petals were found to be 12.73% and 32%, respectively. These flavonoids work as potent antioxidant agents [101]. Moreover, the main volatile component of rose water is geraniol (3.3–6.6%); meanwhile, in the essential oil, the geraniol component represents 8.3–30.2% [102]. The rose oil showed a remarkable inhibition against acetylcholinesterase (60.86 ± 1.99%) and butyrylcholinesterase (51.08 ± 1.70%) at 1000 μg/mL and moderate activity in DPPH radical scavenging and ferric reducing antioxidant power tests [103]. Leaf methanolic extracts (hot and cold) of R. damascena displayed anti-free radical activity at a concentration of 50 μg/mL. The leaf cold extraction had the most potent antioxidant activity measured with the FRAP assay at the concentration of 100 μg/mL, compared with the hot methanolic extraction [104]. Fresh and spent R. damascena flower extracts showed 74.51% and 75.94% antiradical activities, while the antioxidant activity of fresh flower extract (372.26 mg/g) was higher than that of spent flower extract (351.36 mg/g) [105]. The damask rose essential oil was found to have significant cytotoxic impacts against the cancer cell line (A549) in comparison with the normal cell line (NIH3T3) [106].
Chamomile (Matricaria chamomilla L.) belongs to the Asteraceae family. It is a slow growing aromatic annual plant with branched stems, double feathery shared leaves, and tiny, soft, hollow, lettuce head flowers [107]. The foremost components of chamomile include the terpenoids α-bisabolol and its oxides and azulenes, including chamazulene [108]. GC-MS and GC-FID analysis discovered the qualitative and quantitative composition of the chamomile flowers essential oil. The achieved results revealed the presence of 52 components, and the essential contents were β-farnesene (29.8%), α-farnesene (9.3%), α-bisabolol and its oxide (15.7%) and chamazulene (6.4%).
The antioxidant activity of M. chamomilla was investigated using DPPH assay [107]. The antioxidant effect of water and alcohol extracts of chamomile flowers on long-term storage of anhydrous butter fat was measured by peroxide value and free fatty acids. The results of this study revealed a moderate effect in controlling hydrolytic rancidity. However, the antioxidant effect of the water extract was described to be significantly higher than the alcohol extract [109]. The antioxidant activity and stability were investigated with three methods, DPPH free radical scavenging system, determination of the peroxide, in addition to thiobarbituric acid numbers. The antioxidant activity of the essential oil was evaluated in 0.2, 0.4, 0.6, 0.8, and 1 mg/mL concentrations by measuring peroxide and thiobarbituric acid numbers in crude sunflower oil as a greasy food. The antioxidant activities of the extracts were increased by increasing the extract concentrations [110]. The highest total phenolic content and maximum antioxidant capacity of aqueous extract of chamomile tested at temperatures 25, 80, and 100 °C were achieved at 80 °C. For the aqueous herbal extract, total phenolic content was significantly correlated with antioxidant activity [111]. In a single-blind randomized controlled clinical trial conducted on 64 subjects (males and females; age between 30 to 60 years), the antioxidant capacity, superoxide dismutase, glutathione peroxidase, and catalase activities were significantly (p < 0.05) increased by 6.81%, 26.16%, 36.71%, and 45.06%, respectively in chamomile group compared with patients in the control group [112].
Primrose (Primula vulgaris) belongs to the Primulaceae family. The primrose flower is funnel-shaped, with orange spots at the base of its lobes [113]. p-coumaric acid and rutin have been recognized as the main phenolics in primrose water extract [114]. Primrose reduced H2O2-induced DNA damage in a concentration-dependent manner in fibroblast cells compared to the positive controls (only 20 μM H2O2 treatment) [114]. Using MTT assay, dimethyl sulfoxide extract of primrose flowers showed a cytotoxic effect on lung (A549), liver (HepG2), breast (MCF-7), and prostate (PC-3) cancer cells [115]. Further, the extract exhibited selective cytotoxic impacts against human cervical cancer cells (HeLa cells) by arresting their cell cycle at the S phase [116].
A recent study on five herbal infusions including lemon and ginger combination, wild thyme, marjoram, Palestinian and Lebanese herbal mix was conducted to evaluate their antcancer activities. The water extract of lemon and ginger combination was found to be the most potent against MDA-MB231, MCF-7, and A549 cell lines. Both lemon and ginger combination and wild thyme separately, showed the highest apoptosis induction and angiogenesis suppression abilities on the MDA-MB231 cell line at concentrations of 3.5 and 4.4 mg/mL, respectively. Furthermore, lemon and ginger combination, wild thyme, marjoram, and the Lebanese herbal drink were the most active extracts in stimulating pinocytosis, respectively while Palestinian herbal drink had a moderate effect [117].
2.6. Roselle (Hibiscus sabdariffa L.f)
Roselle (Hibiscus sabdariffa L.) is a well-known species that belongs to the Malvaceae family. The plant is an annual or perennial herb found in tropical and subtropical regions of the world. It is used to produce phloem fibers and as an infusion (herbal tea) [118,119]. A preliminary phytochemical analysis has shown the presence of alkaloids, tannins, saponins, glycosides, phenols, and flavonoids in different solvent extracts of H. sabdariffa [120]. Studies have also shown that calyces of H. sabdariffa contained alkaloids, flavonoids, saponins, tannins, and a high anthocyanin content with the lowest flavonoids and phenolic acid [121]. Gas chromatography/mass spectrometry (GC-MS) analysis has identified 18 volatile components in the calyx of H. sabdariffa, most of which were fatty acids and ester compounds [122]. Ethanimidic acid and its ethyl ester (31%) were the major phytocompounds detected in the methanol extract of hibiscus flowers, which are reported to possess antioxidant and cancer-preventive properties [123]. A previous study has reported the presence of anthocyanins in H. sabdariffa calyces in two major forms; delphinidine-3-sambubioside and cyanidine-3-sambubioside [124]. The relation between total anthocyanin content (TAC) and antioxidant activity of hibiscus infusion has been investigated and revealed that under optimum conditions (10 g/mL, 88.7 °C and 15.5 min), TAC was 132.7 ± 7.8 mg and antioxidant activity was high according to DPPH and ABTS assays [125]. The antioxidant effect of anthocyanins is due to their phenolic structure that has the ability to scavenge ROS [126]. Extraction conditions, like temperature, extraction time, and solid to solvent ratio, affect the antioxidant activities of H. sabdariffa, which tends to be high when the extraction temperature is in the range of 70–80 °C and the extraction time is from 120 to 150 min. At the same time, it decreases as the solvent to solid ratio is increased [127]. Furthermore, bound soluble phenolic compounds in H. sabdariffa extracts and their impact on antioxidant activity have been investigated and have shown that the highest total phenolic content (TPC) resulted in high antioxidant scavenging capacity [128]. In a later study, H. sabdariffa aqueous extract exhibited a higher ability to scavenge peroxyl radicals in the water environment than in the lipophilic system and exhibited a more potent metal-reducing activity than olive leaf extract [129].
Previous studies have shown that H. sadbariffa is a promising anticancer plant against different cancer types. The aqueous extract of H. sadbariffa was able to reduce cell viability and induced apoptosis of human adenocarcinoma cell line (MCF-7) [130]. In this regard, a study has indicated that anthocyanin-rich extract from H. sadbariffa calyx was able to inhibit tumor growth, lung metastasis, and tumor angiogenesis. All these effects could be mediated via inhibition of tumor Ras, NF-κB, CD31, and VEGF/VEGF-R-induced angiogenesis [131]. In a recent study, cytotoxic and antitumor activities of polyphenolic leaf extract of H. sabdariffa were investigated. It has shown the ability of the extract to reduce the growth of breast cancer cell lines (MDA-MB-231) and estrogen receptor-expressing breast cancer cell lines (MCF-7 and T-47-D) [132]. H. sabdariffa aqueous flower extract was able to induce apoptosis in a human gastric carcinoma cell line (AGS) via the JNK/p38 signaling cascade [133].
2.7. Pomegranate (Punica granatum)
Pomegranate (Punica granatum L.) is a member of the Punicaceae family and is considered to be of Middle East origin. The plant has also been used in the traditional medicine for ages [134]. P. granatum is a good source of phenolic compounds and anthocyanins, as demonstrated in a previous study [135]. A preliminary phytochemical screening has revealed the presence of different phytochemicals from ethanolic, aqueous, and chloroform extracts of pomegranate peel, whole fruit, and seeds. The extracts of the entire fruit contained the majority of the phytocompounds represented by triterpenoids, steroids, glycosides, saponins, alkaloids, flavonoids, tannins, carbohydrates, and vitamin C. [136]. P. granatum peels ethanolic extract has been analyzed using GC-MS chromatography and showed various constituents with antioxidant activity such as decahydro-1-pentadactyl- naphthalene, 5-hydroxymethyl furfural and 1, 3-cyclohexadiene [137]. According to a recent study, two extracts of P. granatum—hydroalcoholic and infusion—exhibited high efficiency in inhibiting DPPH radical and had significant reducing power of the Fe3+/ferricyanide complex. The antioxidant activity of fruit peel extracts has been justified by the presence of a high level of phenolic compounds like ellagic acid and its derivatives, as identified by UPLC-PDA-MS analysis [138]. Moreover, a study carried on arils juice and peel decoction of fifteen varieties of P. granatum and has shown that the TPC and total flavonoids content (TFC) of arils juices were about 20- and 300-fold inferior to decoctions. Regardless of variety, each decoction revealed better antioxidant and chelating activity compared to the juices [139]. The antioxidant activity of P. granatum peel was assessed using three aqueous extraction techniques; continuous shaking extraction, maceration, and hot water infusion. It was found that infusion method resulted in significant (p < 0.05) level of antioxidant activity compared to other extraction techniques [140]. Since P. granatum has an impact on ROS, an in vivo study was carried out to test the effect of methanolic extracts of pomegranate seeds and peel on oxidative stress induced by methotrexate. The results have demonstrated a significant reduction in GPX and SOD, and an improvement in MDA values after methotrexate treatment [141].
The use of P. granatum preparations has a long ethnomedical history and preclinical studies have described different pharmacological abilities, including chemopreventive, chemosensitization, and chemotherapeutic activities [142]. It was reported that P. granatum has the ability to down-regulate various signaling pathways like NF-ᴋB, P13K/AKT/mTOR and Wnt, as well as reduece the expression of genes that are associated with cancer development, such as anti-apoptotic genes, MMPs, VEGF, c-met, pro-inflammatory cytokines, cyclines, and Cdks [143]. In this regard, the antitumor effect of punicalagin, a pomegranate polyphenol, has been investigated in a human prostate cancer cell line (PC-3) and LNCaP cells. It was found that punicalagin inhibited cell viability of both cell lines in a dose-dependent manner and induced the expression of caspase-3 and -8 in PC-3 [144]. Moreover, an in vivo study has shown that aqueous extract of pomegranate fruit has anticancer activity against Ehrlich-ascites-carcinoma (EAC)-bearing Swiss albino mice. After intraperitoneal injection of mice with pomegranate aqueous extract, a significant reduction has occurred in tumor volume and weight, as well as, decreased viable cell count and improvement in the life span of EAC bearing mice [145]. In another study, pomegranate fruit juice and two of its components (ellagic acid and luteolin) have been shown to reduce cell viability of ovarian cancer cell line (A2780), inhibit metastasis via down-regulation of matrix metalloproteinases MMP2 and MMP9. Also, all three treatments inhibited tumor growth of ovarian cancer cell line (ES-2) in nude mice experiments [146].
2.8. Anise Seeds (Pimpinella anisum L.)
Pimpinella anisum L. is an annual herbaceous plant that belongs to the family Apiaceae [147]. It is widely used as a flavoring agent and as a primary ingredient in herbal infusions [148]. A recent study was conducted to evaluate the antioxidant potentials of Portuguese P. anisum seeds infusion. The results of this study have detected high content of flavonoids, phenols, and anthocyanins. These bioactive components reflected the high antioxidant activity of P. anisum against free radicals [149]. Use of a combination of anion-exchange, gel filtration, and hydrophobic interaction column chromatographies facilitated the isolation of three lignin-carbohydrate protein complexes from a hot water extract of the seeds of P. anisum [150]. The results of GS-MS analysis of P. anisum infusion have shown the presence of fatty acids (linoleic, oleic, and palmitic acids), triterpenoids (lupeol, β-amyrin and betulinic acids), and sterols (β-sitosterol and stigmasterol) [149]. Anethole is the major constituent of anise seed oil [151].
A previous study has demonstrated the correlation between total phenolic content and antioxidant activity of both anise and cumin infusions (ground form). It was found that phenolic compounds in these infusions are the main contributors to their free radical-scavenging activity and oxidant reduction potency [152]. Another study has investigated two extracts—water and alcohol—of chamomile flowers, anise seeds and dill seeds. The extracts exhibited significant antioxidant activity in both linoleic acid and liposome model systems, however the water extract showed higher activity comparing to alcohol extracts [148]. Moreover, anise aqueous extract was assessed in streptozotocin-induced diabetic rat model and showed pancreatic damage reduction via modulation of insulin secretion, oxidative stress, autophagy and down-regulation of caspase 3 [153].
Consumption of anise seed has many medicinal benefits, such as anticancer, hepato-protective and antioxidant abilities. In this regard, a comparative study with cisplatin has conducted to evaluate the cytotoxic effect of (PA) aqueous extract on oral squamous cell carcinoma (KB cell line). It was reported that anise seed extract exhibited anticancer activity by being able to reduce cell viability in dose dependent manner [154]. Moreover, P. anisum extracts and essential oil have shown antiproliferative effect on gastric cancer cells (AGS), and anti-angiogenesis activity in HUVEC cells [155]. Another study has investigated the cytotoxic effect of anise seed ethanolic extract on human prostate cancer cell line (PC-3). The treatment with P. anisum extract showed significant anticancer activity comparing to the normal cell line [156].
2.9. Cumin (Cuminum cyminum)
Cumin (Cuminum cyminum L.) is a small annual herbaceous plant that belongs to the Apiaceae family [157]. It is originated from Egypt, Turkistan, Iran, and Eastern Mediterranean [158]. The bioactive phytochemicals found in cumin seeds are associated with their various industrial applications that range from food to pharmaceutical products [157]. Phytochemical analysis of C. cyminum has revealed the presence of alkaloids, anthraquinones, coumarins, flavonoids, glycosides, resins, saponins, tannins and steroids [159]. Cumin seed essential oil has been analyzed using GC-MS analysis and the presence of 18 compounds demonstrated, with 3-caren-10-al and cuminal being the main constituents [160]. A previous study has investigated the antioxidant activity of alcoholic and aqueous extracts of cumin seeds. Results have shown that the alcoholic extract had higher activity comparing to the aqueous extract [161]. It was found that alcoholic extracts of C. cyminum have a higher phenolic content and antioxidant capacity comparing to coriander extracts [162]. Cumin seed aqueous extract was able to protect WRL-68 cells from hexavalent chromium-induced oxidative injury via reducing ROS in dose-dependent manner. The antioxidant potential of the cumin seed extract is positively correlated with the high content of phenolic acid [163].
The antioxidant activity of cumin seeds plays a role in its cytotoxic ability against the human cervical carcinoma HeLa cell line. It was reported that at concentration of 0.1 μl/mL, essential oil of cumin reduced HeLa cells by 79% [164]. Another study investigated the activity of ethanolic extract of C. cyminum L. against seven human cancer cell lines and showed 61% maximum cytotoxic activity present in Colon 502713 cell line [165]. Various cumin seed extracts exhibited anticancer and neuro-protective effects against IMR32 human neuroblastoma cell lines [166]. Different flavonoids have been purified and identified from C. cyminum and showed anticancer potency against breast cancer MCF-7 cell line [167].
2.10. Lemon Balm (Mellissa officinalis L.)
Mellissa officinalis L. is popularly known as lemon balm and belongs to Lamiaceae family. It is an edible perennial herb that has been used for ages in the form of decoctions, infusions or a natural flavoring in food [168,169]. Although distributed worldwide, lemon balm is originated from Asia and Europe [170]. The chemical composition of M. officinalis oil has been analyzed using the GC-FID technique. The major components were geranial (34%) and neral (26%) [171,172,173]. Several studies have demonstrated a high content of phenolic compounds in lemon balm aqueous extract [172,174,175]. The most abundant phenolic compound found in lemon balm was rosmarinic acid (derived from caffeic acid), as well as some flavonoids such as luteolin-7-O-glucoside [172,176]. M. officinalis exhibited strong antioxidant activity, which were 10 times stronger than the antioxidant effects of vitamin C and vitamin B [177]. An in vivo study has shown the efficacy of M. officinalis aqueous extract in reducing Mn-induced brain oxidative stress in mice [178]. Furthermore, antioxidant properties of four Lamiaceae species have shown that M. officinalis has the highest total phenolic content and antioxidant activity compared to the other species [173]. In this regard, lemon balm extract and its major constituent, rosmarinic acid, effectively attenuated the oxidative stress by inducing antioxidant enzymes and alleviating liver damage in an animal model of nonalcoholic steatohepatitis [179]. Another study has also reported that M. officinalis extracts have strong antioxidant capacity and DPPH radical scavenging activities compared to butylated hydroxytoluene (BHT) [180].
The anticancer potency of lemon balm has been previously studied using different types of extracts on various tumor cell lines [181,182]. Aqueous extract of M. officinalis has shown chemo-preventive effect against hepatocellular carcinoma (HCC) in rats and exhibited antioxidant activity via increasing GSH concentration and inhibiting lipid peroxidation in the liver tissues of HCC rats [183]. Lemon balm extracts inhibited cancer progression and angiogenesis in the ovo CAM model with high cell inhibitory against MCF-7 breast cancer cell line [184]. Moreover, five different extracts of M. officinalis have shown cytotoxic activity against three human tumor cell lines: NCI-H460 (non-small cell lung cancer), MCF-7 (breast adenocarcinoma), and AGS (gastric adenocarcinoma) [174].
2.11. Rosemary (Rosmarinus officinalis L.)
Rosemary (Rosmarinus officinalis L.) belongs to the mint family Lamiaceae which is widely distributed in the Mediterranean region [185,186]. This plant has phenolic diterpenes and triterpenes as the main active constituents, namely caffeic acid, rosmarinic acid (RA), ursolic acid, carnosic acid, and carnosol, all of which are reported for their antioxidant activities [187,188]. Essential oil of rosemary contains α-pinene (45.7%), camphene (18.3%), eucalyptol (16.9%) and p-cymene (6.4%), berbonone, in addition to camphor bornyl acetate [187]. Steam distillated rosemary oils contain mainly 1,8-cineole (46.4%), camphor (11.4%) and α-pinene (11.0%), camphor (37.6%), 1,8-cineole (10.0%), p-cymene-7-ol (7.8%) and borneol (5.4%). Moreover, R. officinalis L. (RO) is rich in flavonoids that have antioxidant activities such as 30-O-β-d-glucuronide, 7-O-glucoside, hispidulin, diosmin, genkwanin, hesperidin and isoscutellarein 7-O-glucoside which are found in flowers, leaves, roots and stems of R. officinalis [188]. Along with the antioxidant activities, R. officinalis has anti-inflammatory, hepatoprotective, antidiabetic and antimicrobial activity which depend on the content of the phenolic compounds mainly rosmarinic acid, caffeic acid and carnosic acid [189,190,191,192].
In view of the antioxidant and antiproliferative activities of R. officinalis, many in vitro studies confirmed these effects depending on the polyphenolic content. For example, Ðilas et al. investigated the effect of a number of oil-soluble rosemary extracts with varying content of carnosic acid, carnosol and methylcarnosol. Results of this study revealed that the extract with the highest content of carnosic acid had the most powerful scavenging ability to hydroxyl, superoxide and 2,2-diphenyl-1-picrylhydrazyl (DPPH)-free radicals. Tested rosemary extracts also exhibited significant antiproliferative effect indifferent cell lines. In both breast adenocarcinoma (MCF7) and cervix epitheloid carcinoma (HeLa) celll lines, the extracts yielded low IC50 (9–10 μgmL−1) [193]. Supporting this finding, rosemary extract inhibited MDA-MB-231 breast cancer cell proliferation, prevented the phosphorylation/activation of Akt and mTOR and enhanced the cleavage of PARP, a marker of apoptosis, indicating that rosemary extract modulates key signaling molecules involved in cell proliferation and survival [194]. Pro-apoptotic effect of rosemary crude extract (ursolic acid, carnosol and carnosic acid) was also indicated in 184-B5/HER cells via increased sub-G0 cell population along with suppression of G1-S phase transition and reduction of cyclin D1 expression. As a result, the colonies of 184-B5/HER cells were reduced significantly [195].
The antiproliferative effect of rosemary extract and its active ingradients carnosol, carnosic acid and rosmarinic acid on human ovarian cancer cells was investigated by Tai et al. Rosmary extract was shown to have significant antiproliferative effect in human ovarian cancer cells (A2780) and cisplatin resistance daughter cell line A2780CP70 with effective IC50 at 1/1000 and 1/400 dilutions, respectively. Moreover, the extract and its active ingradients enhanced the antiproliferative effect (synergistic effect) of cisplatin in both A2780 and A2780CP70 cells. The effect of rosemary extract was due to the suppression of the expression of Bcl-2, Bcl-x, cIAP-1, HIF-, and HO-1 (anti-apoptosis proteins) and Bax, Fas and FADD (pro-apoptosis molecules). Furthermore, A2780 cells were more sensitive to carnosol, carnosic acid and rosmarinic acid than A2780CP70 cells. Interestingly, rosmary extract upregulated a heme-containing protein, cytochrome C, that was emitted from the mitochondria in response to pro-apoptotic stimuli [196]. Alternatively, rosemary extract inhibited cell proliferation and enhanced apoptosis of human NSCLC adenocarcinoma A549 cells in a dose-dependent manner. This effect was associated with the reduction in phosphorylated/activated Akt, mTOR and p70S6K levels [197]. Additionally, aqueous extract of the fruits of rosemary showed anti-cancer and cytotoxic effect against gastrointestinal cell lines, KYSE30 (human esophageal squamous cell carcinoma) and AGS (human gastric carcinoma). Using MTT assay, IC50 value was 150 mg/mL after 72 h of exposure in KYSE30 cell lines, however, in AGS cell lines, IC50 value was 1.3 mg/mL after 72 h of exposure [198]. In prostate cancer, rosmarinic acid minimized cell proliferation through the downregulation of p53 expression [199].
In vivo, rosemary polyphenols have antiproliferative effects against colon cancer cells in animal models. For example, when male nude mice were grafted with human colon cancer cells (HT-29) treated with fluid extract of rosemary, the extract exhibited a clear reduction in the tumor size. This outcome was strongly correlated with the role of rosemary extract in sharp increase of intracellular ROS that stimulated necrotic cell death. In addition, Nrf2 gene silencing increased rosemary cytotoxic effects [200]. Another study revealed that administration of 200 mg/kg of rosemary extract per day in mice leads to the reduction in the progression of colorectal cancer HT-29 cells. This is attributed to the alteration in RNA post-transcriptional modification, protein synthesis and the amino acid metabolism which are responsible for tumor reduction. For example, rosemary extract altered nucleic acid binding capacity, followed by enzyme modulator proteins, hydrolases, cytoskeletal proteins, oxidoreductases and transferases [201]. In another nude mouse model grafted with HCT116 cells and treated with rosemary extract and carnosic acid, the extract was also able to inhibit the HCT116 xenograft tumor initiation [202]. Table 1, summarizes the all discussed herbs and their active ingredients, mechanism of action to reduce oxidative stress and the types of cancers that are tested.
Table 1.
Antioxidant and anticancer activities of the phytochemical components in the eleven herbal infusions.
| Name of the Herbal Infusion/Ref. | Extracts/Oils | Active Ingredients | Antioxidant and Anti-Tumor Mechanisms | Type of Cancer Treated | Cell Lines Used (In Vitro) |
|---|---|---|---|---|---|
| Lemon [203,204] |
water extract, volatile oils, lemon juice | limonene, ascorbic acid, phenolics, flavonoids, carotenoids, reducing sugars, indolofuroquinoxaline, alkaloids, terpenoids, geranial, neral | reduced exogenous H2O2 effect, enhance the activity of catalase and SOD, inhibit DPPH, decrease the expression of BcL-2 and the proliferative marker Ki-67, downregulate of caspase 3 | myeloid leukemia, prostate, lung and breast, gastric cancer | K562 MDA-MB 231 MCF7 PC-3 A549 AGS BGC-823 SGC-7901 |
| Ginger [205,206] |
aqueous extract, oil/water soluble extract | gingerols and shogaols, gallic acid, quercetin | reduce oxidative stress and raise total antioxidant capacity, represse activities of MMP-2 and MMP-9, increase p53, CASP2 and DEDD, high expression levels of ABCA2 or ABCA3 transporter genes | breast and cervical cancer, ovarian, leukemia | Hela MDA-MB-231 SKOV-3 CCRF-CEM Nalm-6 |
| Wild thyme (Thymus serpyllum) [207,208] |
aqueous extract, essential oils, hexane extract | rosmarinic acid, eriocitrin, luteolin, apigenin, quercetin, luteolin7-O-glucoside, apigenin-7-O-glucoside, luteolin, apigenin, thymol, p-cymene, caryophyllene camphene eucalyptol and β-pinene | prevent oxidation of low-density lipoproteins, increases the activity of SOD, catalase, and GPXs, reduce malondialdehyde, reduce DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway, activate MAPK signaling pathway and AMP-activated protein kinase, decrease of cells in the S phase | liver carcinoma, colon, breast, prostate and lung, pancreatic cancer, osteosarcoma, melanoma | MDA-MB-231 MCF-7 HepG2 HCT-116 PC3 A549 PANC-1 U2OS A375 B164A5 |
| Marjoram (Organum Majorana) [209,210] |
methanolic extracts, water extract, essential oil, ethanolic extract aqueous extract | rosmarinic, linalool, estragole | reduce ferric reducing ability, down-regulation of survivin, upregulation of cyclin-dependent kinase inhibitor 1 (p21), activate caspase-dependent extrinsic apoptotic pathway and TNFα pathway, suppress NF-kB | breast and lung cancer, colon, liver cancer | Caco-LNM35 A549 MDA-MB231 MCF-7 HT29 HepG2 |
| Green tea [211] |
caffeine, theobromine, theophylline, lignin, organic acids, chloro-phylland, theanine, free amino acids, depsides, carbohydrates, alkaloids, minerals, vitamins, enzymes, polyphenols, tea catechins, epigallocatechin-3gallate, polyphenols, quercetin, epigallocatechin gallate | electron donors and efficient scavengers, interact with proteins and phospholipids in the plasma membrane and regulates signal transduction pathways, transcription factors, DNA methylation, mitochondrial function, and autophagy, prevents of tNOX activity, modulate Bax/blc-2 ratio and trigger G2/M cell cycle arrest | breast cancer, non-small lung cancer | MCF-7 ZR75 T47D A-549 |
|
| Lemon verbena [212,213] |
crude extract | verbascoside, luteolin 7-diglucuronide, citral or geranial, luteolin, verbascoside, gardoside | protected against lipid peroxidation and protein carbonylation, free radical scavenger, increase in the total antioxidant ability, modulate AMPK activity, decrease NF-κB, increase GST and GPx | human melanoma, human leukemia, colon, liver, brest cancer | A375 Caco2 HepG2 MCF-7 THP-1 |
| Sage [214,215] |
water extracts, essential oil, methanolic extract, hydroalcoholic extract, n-hexane soluble extract | were α-terpineol, camphor, α-pinene, camphene, β-cymen, caryphyllene, β-myrcene, β-menth1-en-b-ol, bomeol, flavonoids, diterpenes, manool | prevent lipid peroxidation, increase in the liver antioxidant enzyme GST activity, increase in glutathione (GSH) level and free radical-scavenging | head and neck squamous cell carcinoma, Hodgkin lymphoma, melanoma, human breast cervical, human hepatocellular carcinoma, MO59J, U343 and human glioblastoma, lung. | HNSCC L-540 HD-MyZ HepG2 MO59J U343 U251 NCI-H187 |
| Cinnamon [216,217] |
essential oil water extract aqueous and ethanolic extracts, distillate oil | (E)-cinnamaldehyde, benzaldehyde, (E)-cinnamyl acetate, saponins, tannins, phenols, terpenoids, and phytosterols, flavonoids and amino acids, coumarin, melatonin | decrease the lipid peroxidation via enhancement of the hepatic antioxidant enzyme activities | basal cell carcinoma, cervix carcinomacancer, leukemia, colorectal carcinoma, epidermoid carcinoma, brain cancer, breast cancer | HeLa HL-60 HCT-116 HT-29 SW-480 A431 SiHa SK-N-MC MCF-7, MDA-MB-231, BT-549 |
| Damask rose [218] |
essential oil, aqueous and ethanolic extracts, methanolic extracts | flavonoid, citronellol, n-nonadecane, n-heneicosane, 1-nonadecene, geraniol | inhibits acetylcholinesterase and butyrylcholinesterase, radical scavenging and ferric reducing antioxidant | lung | A549 |
| Chamomile [219,220] |
water and alcohol extracts, methanol extract, hydroalcoholic Extract | terpenoids α-bisabolol and its oxides and azulenes, including chamazulene, β-farnesene, α-farnesene, α-bisabolol, and its oxide and chamazulene, bisabololoxide A | free radical scavenging, increase SOD, GPXs, and catalase activities, reduce lipid peroxidation | Leukemia, colon | K562 HT29 |
| Primrose [221,222] |
water extract, dimethyl sulfoxide Extract, oil, crude aqueous ethanolic extract | ρ-coumaric acid and rutin, decane, campesterol, caryophyllene, sitosterol, flavanol (proanthocyanidins) | reduces H2O2-induced DNA damage, increases malondialdehyde, and TNF-α, decrease NF-kB, cyclooxygenase-2, and MMP -9 | lung, liver, breast, and prostate and cervix cancer cells, colon cancer | A549 HepG2 MCF-7 PC-3 HeLa SW-480 |
|
Hibiscus sabdariffa L. [223,224] |
methanol extract, aqueous extract, ethanolic extract, n-hexane extract, ethyl acetate extract | alkaloids, tannins, saponins, glycosides, flavonoids (anthocyanin), alkaloids, phenolic acid, ethanimidic acid and ethyl ester | scavenge ROS and free radicals, potent metal-reducing activity, inhibits tumor Ras, NF-κB, CD31, and VEGF/VEGF-R-induced angiogenesis, JNK/p38 signaling cascade -induced apoptosis, increase activation of p21, p53, and caspase-3 | adenocarcinoma, breast cancer, estrogen receptor-expressing breast cancer, human gastric carcinoma, lung cancer | MCF-7 MDA-MB-231 T-47-D A549 |
| Pomegranate [225,226] |
alcoholic, aqueous, chloroform extracts, juice | anthocyanins, triterpenoids, steroids, glycosides, saponins, alkaloids, flavonoids, tannins, carbohydrates, and vitamin C, naphthalene, decahydro-1-pentadactyl, 5 hydroxymethyl furfurals, and 1, 3-cyclohexadiene, ellagic acid and luteolin, polyphenols | scavenger for free radical and significant reducing power of the Fe3+/ferricyanide complex, down-regulate various signaling pathways like NF-κB, P13K/AKT/mTOR, and Wnt, reduces MMPs, VEGF, c-met, pro-inflammatory cytokines, cyclines, and Cdks, induces the expression of caspase-3 and -8, reduce phosphorylation levels of Akt, S6K1, inhibit IGF-I/Akt/mTOR pahway | prostate cancer, Ehrlich-ascites-carcinoma and ovarian cancer, thyroid cancer | PC-3 LNCaP A2780 ES-2 DU145 BCPAP |
| Anise seeds (Pimpinella anisum L.) [227,228] |
water extract, alcohol extract, ethanolic extract, aqueous-n-butanolic extract, essential oils | flavonoids, phenols, and anthocyanins, lignin-carbohydrate protein, fatty acids (linoleic, oleic, and palmitic acids), triterpenoids (lupeol, β-amyrin and betulinic acids), and sterols (β-sitosterol and stigmasterol), anethole, gallic Acid, catechins, estragole, naringin, chloroginic acid, rosmarinic acid | scavenge DPPH free radicals, reduce oxidant potency, down-regulate of caspase 3 | oral squamous cell carcinoma, gastric cancer, human prostate cancer, breast cancer | AG HUVEC PC-3 MCF-7 |
| Cumin (Cuminum cyminum L.) [163,229] |
essential oil, alcoholic and aqueous extracts | alkaloids, anthraquinones, coumarins, flavonoids, glycosides, resins, saponins, tannins, steroids, 3-caren-10-al and cuminal | reducing ROS, diminish the expressions of mTOR and survivin and elevate BECN1 expression | cervical, colon cancer, neuroblastoma, breast cancer | Hela 502713 IMR32 MCF-7 AU565 |
|
Mellissa officinalis L. [182,230] |
essential oil, aqueous extract, infusion extracts, hydromethanolic, hydroethanolic, methanolic and alcoholic extracts, dichloromethane extract | geranial and neral, luteolin-7-O-glucoside, caffeic acid, 3,4-dihydroxyphenyl lactic acid, 3,4-dihydroxybenzoic acid, lithospermic acid, luteolin-7-O-glucoside, methyl caffeate and rosmarinic acid. | inducing antioxidant enzymes and alleviated liver damage, free radical scavenging activities, increasing GSH concentration and inhibiting lipid peroxidation in the liver tissues, reduce pro-caspase 3 levels | liver, non-small cell lung cancer, breast and gastric cancer | HCC MDA-MB-231 MCF-7 NCI-H460 AGS LNCAP, PC3 MDA-MB-468 |
| Rosemary [191,202] |
oil-soluble extracts, aqueous extract, essintial oils, crude extract | caffeic acid, rosmarinic acid, ursolic acid, carnosic acid, and carnosol, α-pinene, camphene, eucalyptol, p-cymene, camphor bornyl acetate, berbonone, 1,8-cineole | scavenge DPPH, prevent Akt and mTOR, reduce cyclin D1, suppress expression of Bcl-2, Bcl-x, cIAP-1, HIF-, and HO-1, Bax, Fas and FADD | breast, cervical, colon, ovarian, lung, esophageal and prostate cancer | MCF7 HeLa A2780 A2780CP7 A549 MDA-MB-231 KYSE30 184-B5/HER HT-29 HCT116 HDAC2 |
3. Herbal Infusions in Human Clinical Trials
A short-term intake of chamomile tea (3 g/150 mL hot water, three times daily) has beneficial effects on glycemic control and antioxidant status in patients with type 2 diabetes mellitus [112]. Consumption of green tea extract for six weeks combined with CrossFit training resulted in a significant increase in the blood antioxidant capacity and a marginal effect on aerobic capacity and serum brain-derived neurotrophic factor in trained men [231]. Moreover, a randomized double-blinded, placebo-controlled phase II clinical trial was conducted to evaluate the effect of green tea extract (GTE) on mammographic density (MD). Results showed a reduction in percent MD in younger women (50–55 years), but with no significant effect on MD measures in all women [232].
A randomized controlled trial suggested that aromatherapy (1 mL of lemon and 0.5 mL of ginger essential oil) has efficacy in preventing treatment-related salivary gland disorder [233]. Interestingly, a pilot study’s outcomes have shown that administering 2 g of ginger daily in patients with high risk for colorectal cancer may improve cell-cycle biomarkers in the normal-appearing colonic mucosa [234].
Cumin essential oil supplementation enhanced some antioxidative indices, as superoxide dismutase and total antioxidant capacity in patients with metabolic syndrome [235]. Based on a randomized crossover trial, pomegranate juice consumption for eight weeks in hemodialysis patients resulted in beneficial effects on blood pressure, oxidative stress, inflammation, and serum lipoprotein cholesterol [236]. Moreover, pomegranate extract (900 mg daily) intake by colorectal cancer patients has moderate modulation of specific tissue microRNAs (colorectal cancer biomarkers) [237]. Studies have shown that supplementation with pomegranate juice significantly affects oxidative stress by improving antioxidant response [238,239]. The oral administration of Melissa officinalis infusion markedly improved oxidative stress conditions and DNA damage that arises from radiation exposure among radiology staff [240].
4. Herbal Infusion Contradictory Effects
Altough herbal infusions are widely consumed, some studies have reported a lack of effect or even contraductory responses. For example, one of the common uses of lemon juice was the treatment of high blood pressure. Quercetin, a flavonoid present in citrus lemon, was found to lower lower blood pressure in several rat models of hypertension [241]. Interestingly, in 2012, a study showed that lemon juice has no beneficial effect on raised blood pressure, despite the common usage of lemon juice by hypertensive patients. In addition, lemon juice has potential risks for hypertensive patients like drug interaction and noncompliance with prescribed treatments. For example, it has been demonstrated that lime juice inhibits activity of cytochrome P450 3A4 [242].
In contrast to a previous study which showed that ginger interferes with iron absorption [243], a recent study concluded that ginger improves iron absorption, and therefore, it is beneficial as a supplement in the therapy of anemia [244].
H. sabdariffa has been used in the traditional medicine as an anti-hyperlipidemic agent [245]. Randomized conrolleed trials have demonstrated that H. sabdariffa has no significant role to lowering serum lipids when compared with placebo, black tea, or diet [246]. Alternatively, recent study of randomized clinical trials assessed the effect of H. sabdariffa on fasting plasma glucose (FPG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoproteins (LDL), and triglyceride (TG). It was shown that H. sabdariffa has no significant effect on blood TC, HDL, and TG, however, H. sabdariffa was able to lower both FPG and LDL [247].
Despite the potential activity of phytochemicals as therapeutics for chemoprevention, they have certain limitations that are related to the complex mixture of metabolites, biphasic effects (hormesis), and their bioavailability [248]. Hormesis is known as a biphasic dose-response phenomenon such that a chemical has a stimulatory effect at low doses but is toxic at high doses. It is charecterized by either a U-shaped or an inverted U-shaped dose-response curve, based on the end-point measured [249]. Many phytochemicals are described as hormetins due to their hermetic response [250]. For example, P. granatum seed oil exhibited a hormetic effect when applied to mouse mammary organ culture. This was evident by the high inhibitory effect on on tumor at low tested concentration [251]. Moreover, the inactivity of green tea polyphenols against superoxide free radicals may be due to high doses used, which resulted in a pro-oxidant toxic effect [252]. The main constituent of green tea, (−)-epigallocatechin-3-gallate, revealed biphasic dose-response on a broad range of cell types (non-tumor and tumor cell lines) [253].
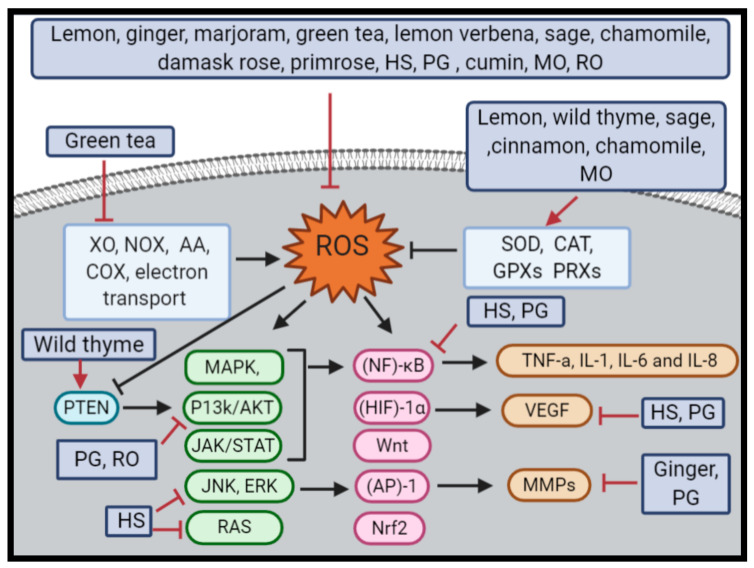
In addition to the abovementioned effects, thymol exhibited a hormetic effect in terms of antioxidant and pro-antioxidant activity, cell viability and DNA genotoxicity [254]. Thyme essential oil improved the metabolic activity of MCF-7 cancer cell line only at low concentrations [255]. In MCF-7 and HeLa cell lines, both lemon balm Kombucha and tea exhibited biphasic response at different concentrations [256]. Figure 2 shows the role of each herb in fighting cancer through oxidative stress pathways.
Figure 2.
The role of each herb in fighting cancer through oxidative stress pathways. ROS and free radicals are reduced by lemon, ginger, marjoram, green tea, lemon verbena, sage, chamomile, damask rose, primrose, Hibiscus sabdariffa L. (HS), Punica granatum (PG), cumin, Mellissa officinalis L. (MO) and rosemary (RO). Moreover, antioxidant enzymes are increased via lemon, wild thyme, sage, cinnamon, chamomile and MO, on the other hand, green tea reduce ROS-inducing enzymes. Clearly, HS and PG reduce the activity of (NF)-κB, which induce the proinflammatory cytokines (e.g., TNF-a, IL-1, IL-6 and IL-8), and they repress VEGF, besides PG inhibition activity on P13k/AKT pathway and HS inhibition activity on JNK and RAS pathways. As observed, ginger and PG paly important role on MMPs inhibition. Wild thyme increases the activity of PTEN, which consequently reduce P13k/AKT pathway. Finally, RO have the ability to scavenge free radicals and have a role in prevention of Akt.
5. Conclusions
Herbal infusions are important sources of antioxidant agents and can be used to reduce oxidative stress and protect against cancer. Diverse phytochemicals are present in these infusions with the ability to activate different mechanisms involved in lowering oxidative stress and enhancing anticancer effects. Extraction temperature and time are important variables for some herbal infusions in order to obtain the highest antioxidant effect. However, uncontrolled consumption of herbal infusions may cause toxicity and reduced antioxidant activity.
Acknowledgments
The authors are grateful to the Applied Science Private University, Amman, Jordan, for the full financial support granted to this research (Grant No. DRGS-2014-2015-166).
Abbreviations
| 4-HNE | 4-Hydroxynonenal |
| 4T1 | Breast cancer (mammary gland) cell line |
| A2780 | Human ovarian cancer cell line |
| A375 | Human melanoma cell line |
| A431 | Epidermoid carcinoma |
| A549 | Adenocarcinomic human alveolar basal epithelial cell line |
| AA | Arachidonic Acid |
| ABTS assay | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AGS | Human gastric carcinoma cell line |
| AKT | Protein kinase B |
| ALT | Alanine transaminase |
| AMP | Adenosine monophosphate-activated protein kinase |
| AP-1 | Activator Protein -1 |
| AST | Aspartate transaminase |
| BHT | Butylated hydroxytoluene |
| Caco-2 | Colon cancer cell line |
| CAT | Catalase |
| CC1(4)) | Carbon tetrachloride |
| CD31 | Cluster of Differentiation 31 |
| Cdks | Cyclin-dependent kinase complex |
| c-met | Tyrosine-protein kinase Met |
| CoA | Coenzyme A reductase |
| COX | Cyclooxygenase |
| DJ-1 | Protein deglycase |
| DNA | Deoxyriboncleic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EAC | Ehrlich-ascites-carcinoma |
| eNOS | Endothelial Nitric Oxide Synthase |
| ERK | Extracellular signal-regulated kinases |
| ES-2 | Human ovarian cancer cell line |
| Fe-NTA | Ferric nitrilotriacetate |
| FPG | Fasting plasma glucose |
| FRAP | Ferric ion reducing antioxidant power |
| GC-FID | Gas Chromatography with Flame Ionization Detection |
| GC-MS analysis | Gas chromatography–mass spectrometry analysis |
| GPX | Glutathione peroxidase |
| Gr | Glutathione reductase |
| GSH | Glutathione |
| GST | Glutathione-S-transferase |
| H2O2 | Hydrogen peroxide |
| HCC | Hepatocellular carcinoma |
| HCT-116 | Human Colon Tumour Cell line 116 |
| HDL | High-density lipoprotein |
| HEK293 | Human embryonic kidney 293 |
| HeLa | Human epithelioid cervix carcinoma |
| HepG2 | Hepatocellular cancer cell lines |
| HIF-1α | Hypoxia Inducible Factor -1α |
| HL-60 | Human cancer promyelocytic leukemia |
| HS | Hibiscus sabdariffa L. |
| HT-29 | Human colon cancer cell line |
| HUVEC | Human umbilical vein endothelial cell |
| IMR32 | Human neuroblastoma cell lines |
| JAK/STAT | Janus kinase/signal transducer and activator of Transcription proteins |
| JNK | Mitogen-activated protein kinase 8 |
| KB | Oral squamous cell carcinoma |
| LC–ESI-MS/MS analysis | Liquid chromatography positive ion electrospray ionization tandem mass spectrometry |
| LDL | Low-density lipoproteins |
| LNCaP | Androgen-sensitive human prostate adenocarcinoma cells |
| LNM35 | Human lung cancer cells |
| MAPK | Mitogen-activated protein kinase |
| MCF7 | Michigan Cancer Foundation-7 (breast cancer cells) |
| MDA | Malondialdehyde |
| MDA-MB 231 | Epithelial, human breast cancer cell line |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP9 | Matrix metallopeptidase 9 |
| MMPs | Matrix metalloproteinases |
| mTOR | The mammalian target of rapamycin |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| PH oxidase (NOX) | Nicotinamide Adenine Dinucleotide Phosphate oxidase |
| NCI-H460 | Human Lung Carcinoma |
| NF | Nuclear Factor |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIH3T3 | Mouse embryonic fibroblast cells |
| NOS | Nitric oxide synthases |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| ovo CAM model | In Ovo Chick Chorioallantoic Membrane |
| p15 | Tumor suppressor protein |
| p16 | Tumor suppressor protein |
| p21 | Cyclin-dependent kinase inhibitor 1 |
| P38 | P38 mitogen-activated protein kinases |
| p38MAPK | P38 mitogen-activated protein kinases |
| P450 complex | Cytochromes P450 |
| P53 | Tumor suppressor protein |
| p70S6K | Ribosomal protein S6 kinase beta-1 |
| p90Rsk | 90 kDa ribosomal s6 kinases |
| PC3 | Prostate cancer cell line |
| Pfrap | Potassium ferricyanide reducing power |
| PI3K | Phosphoinositide 3-kinases |
| PN | Punicalagin |
| PPAR-γ | Peroxisome proliferator-activated receptor |
| PRXs | Peroxiredoxins |
| PTEN | Phosphatase and tensin homolog |
| PTP-1B | Protein-tyrosine phosphatase 1B |
| RA | Rosmarinic acid |
| RAS | Rat sarcoma |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| S phase | Synthesis Phase |
| SiHa | Human cervical carcinoma |
| SK-N-MC | Human Neuroblastoma Cell Line |
| SOD | Superoxide dismutase |
| SW480 | Human colorectal carcinoma |
| T47D | Human breast cancer cell line |
| T-47-D | Human breast cancer cell line |
| TAC | Total anthocyanin content |
| TC | Total cholesterol |
| TG | Triglyceride |
| THP-1 | Human monocytic cell line |
| tNOX | Tumor-associated NADH oxidase |
| TPC | Total phenolic content |
| TFC | Total flavonoids concentration |
| TRX | Thioredoxin reductase |
| UPLC-PDA-MS | Ultra-performance liquid chromatography (UPLC) coupled to photodiode array detection (PDA) and electrospray ionization (ESI) tandem mass spectrometry (MS) |
| UV radiation | Ultraviolet radiation |
| VEGF | Vascular endothelial growth factor |
| WRL-68 | Human hepatic cell line |
| XO | Xanthine oxidase |
| ZR75 | Human breast cancer cell line |
| β-catenin/Wnt | β-Catenin/Wingless-related integration |
Author Contributions
Conceptualization, W.H.T. and I.A.A.-a.; methodology, S.J., I.A.A.-a. and A.I.M.; software, S.J. and I.H.A.-Y.; validation, L.T.A., W.H.T. and I.H.A.-Y.; formal analysis, L.T.A.K.; investigation, A.I.M. and S.J.; resources, S.J., I.A.A.-a. and A.I.M.; data curation, S.J., I.A.A.-a. and A.I.M.; writing—original draft preparation, I.A.A.-a. and and A.I.M.; writing—review and editing, L.T.A.K.; visualization, S.J.; supervision, W.H.T.; project administration, W.H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kennedy E. Nutrition policy in the US: 50 years in review. Asia Pac. J. Clin. Nutr. 2008;17:340–342. [PubMed] [Google Scholar]
- 2.Benzie I.F., Wachtel-Galor S. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. CRC Press; Boca Raton, FL, USA: 2011. [PubMed] [Google Scholar]
- 3.Kaliora A., Kogiannou D.A., Kefalas P., Papassideri I.S., Kalogeropoulos N. Phenolic profiles and antioxidant and anticarcinogenic activities of Greek herbal infusions; balancing delight and chemoprevention? Food Chem. 2014;142:233–241. doi: 10.1016/j.foodchem.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 4.Al Obaydi M.F., Hamed W.M., Al Kury L.T., Talib W.H. Terfezia boudieri: A Desert Truffle With Anticancer and Immunomodulatory Activities. Front. Nutr. 2020;7:7. doi: 10.3389/fnut.2020.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ipek E., Zeytinoğlu H., Okay S., Tuylu B.A., Kurkcuoglu M., Başer K.H.C. Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem. 2005;93:551–556. doi: 10.1016/j.foodchem.2004.12.034. [DOI] [Google Scholar]
- 6.Talib W.H. Consumption of garlic and lemon aqueous extracts combination reduces tumor burden by angiogenesis inhibition, apoptosis induction, and immune system modulation. Nutrition. 2017;43:89–97. doi: 10.1016/j.nut.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Talib W.H. Regressions of Breast Carcinoma Syngraft Following Treatment with Piperine in Combination with Thymoquinone. Sci. Pharm. 2017;85:27. doi: 10.3390/scipharm85030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talib W.H., Al Kury L.T. Parthenolide inhibits tumor-promoting effects of nicotine in lung cancer by inducing P53-dependent apoptosis and inhibiting VEGF expression. Biomed. Pharmacother. 2018;107:1488–1495. doi: 10.1016/j.biopha.2018.08.139. [DOI] [PubMed] [Google Scholar]
- 9.Talib W.H., Al-Hadid S.A., Ali M.B.W., Al-Yasari I.H., Ali M.R.A. Role of curcumin in regulating p53 in breast cancer: An overview of the mechanism of action. Breast Cancer (Dove. Med. Press) 2018;10:207–217. doi: 10.2147/BCTT.S167812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali-Shtayeh M.S., Jamous R.M., Jamous R.M. Herbal preparation use by patients suffering from cancer in Palestine. Complement. Ther. Clin. Pr. 2011;17:235–240. doi: 10.1016/j.ctcp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadi A., Shadboorestan A. Oxidative stress and cancer; the role of hesperidin, a citrus natural bioflavonoid, as a cancer chemoprotective agent. Nutr. Cancer. 2015;68:29–39. doi: 10.1080/01635581.2015.1078822. [DOI] [PubMed] [Google Scholar]
- 12.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Boil. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht F., Pessoa C.F., Gentile L.B., Rosenthal D., De Carvalho D.P., Fortunato R.S. The role of oxidative stress on breast cancer development and therapy. Tumor Boil. 2016;37:4281–4291. doi: 10.1007/s13277-016-4873-9. [DOI] [PubMed] [Google Scholar]
- 14.Morry J., Ngamcherdtrakul W., Yantasee W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Boil. 2017;11:240–253. doi: 10.1016/j.redox.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liochev S.I. Reactive oxygen species and the free radical theory of aging. Free. Radic. Boil. Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Saed G.M., Diamond M.P., Fletcher N.M. Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol. Oncol. 2017;145:595–602. doi: 10.1016/j.ygyno.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 17.Gebremedhin D., Terashvili M., Wickramasekera N., Zhang D.X., Rau N., Miura H., Harder D.R. Redox Signaling via Oxidative Inactivation of PTEN Modulates Pressure-Dependent Myogenic Tone in Rat Middle Cerebral Arteries. PLoS ONE. 2013;8:e68498. doi: 10.1371/journal.pone.0068498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niecknig H., Tug S., Reyes B.D., Kirsch M., Fandrey J., Berchner-Pfannschmidt U. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free. Radic. Res. 2012;46:705–717. doi: 10.3109/10715762.2012.669041. [DOI] [PubMed] [Google Scholar]
- 19.Coso S., Harrison I., Harrison C.B., Vinh A., Sobey C.G., Drummond G.R., Williams E.D., Selemidis S. NADPH Oxidases as Regulators of Tumor Angiogenesis: Current and Emerging Concepts. Antioxid. Redox Signal. 2012;16:1229–1247. doi: 10.1089/ars.2011.4489. [DOI] [PubMed] [Google Scholar]
- 20.Toyokuni S. Oxidative stress as an iceberg in carcinogenesis and cancer biology. Arch. Biochem. Biophys. 2016;595:46–49. doi: 10.1016/j.abb.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Bagati A., Moparthy S., Fink E.E., Bianchi-Smiraglia A., Yun D.H., Kolesnikova M., Udartseva O., Wolff D.W., Roll M.V., Lipchick B.C., et al. KLF9-dependent ROS regulate melanoma progression in stage-specific manner. Oncogene. 2019;38:3585–3597. doi: 10.1038/s41388-019-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Gal K., Ibrahim M.X., Wiel C., Sayin V.I., Akula M.K., Karlsson C., Dalin M., Akyürek L.M., Lindahl P., Nilsson J., et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015;7:308re8. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 23.Wiel C., Le Gal K., Ibrahim M.X., Jahangir C.A., Kashif M., Yao H., Ziegler D.V., Xu X., Ghosh T., Mondal T., et al. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell. 2019;178:330–345.e22. doi: 10.1016/j.cell.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Cordani M., Butera G., Pacchiana R., Masetto F., Mullappilly N., Riganti C., Donadelli M. Mutant p53-Associated Molecular Mechanisms of ROS Regulation in Cancer Cells. Biomolecules. 2020;10:361. doi: 10.3390/biom10030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B., Fu J., Yu T., Xu A., Qin W., Yang Z., Chen Y., Wang H. Contradictory effects of mitochondria- and non-mitochondria-targeted antioxidants on hepatocarcinogenesis by altering DNA repair in mice. Hepatology. 2018;67:623–635. doi: 10.1002/hep.29518. [DOI] [PubMed] [Google Scholar]
- 26.Li S., Li S.-K., Gan R.-Y., Song F.-L., Kuang L., Li H.-B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crop. Prod. 2013;51:289–298. doi: 10.1016/j.indcrop.2013.09.017. [DOI] [Google Scholar]
- 27.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberto D., Micucci P., Sebastian T., Graciela F., Anesini C. Antioxidant Activity of Limonene on Normal Murine Lymphocytes: Relation to H2O2Modulation and Cell Proliferation. Basic Clin. Pharmacol. Toxicol. 2009;106:38–44. doi: 10.1111/j.1742-7843.2009.00467.x. [DOI] [PubMed] [Google Scholar]
- 29.De Almeida A.A.C., De Carvalho R.B.F., Silva O.A., De Sousa D.P., De Freitas R.M. Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol. Biochem. Behav. 2014;118:69–78. doi: 10.1016/j.pbb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Moosavy M., Hassanzadeh P., Mohammadzadeh E., Mahmoudi R., Khatibi S., Mardani K. Antioxidant and antimicrobial activities of essential oil of Lemon (Citrus limon) peel in vitro and in a food model. J. Food Qual. Hazards Control. 2017;4:42–48. [Google Scholar]
- 31.Guimarães R., Barros L., Barreira J.C.M., Sousa M.J., Carvalho A.M., Ferreira I.C. Targeting excessive free radicals with peels and juices of citrus fruits: Grapefruit, lemon, lime and orange. Food Chem. Toxicol. 2010;48:99–106. doi: 10.1016/j.fct.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Prasanna G.L., Rao B.V.D., Reddy A.G., Rao M.V.B., Pal M. Lemon Juice Mediated Reaction under Ultrasound Irradiation: Synthesis of Indolofuroquinoxalines as Potential Anticancer Agents. Mini Rev. Med. Chem. 2019;19:671–678. doi: 10.2174/1389557518666181029100044. [DOI] [PubMed] [Google Scholar]
- 33.Zu Y.-G., Yu H., Liang L., Fu Y.-J., Efferth T., Liu X., Wu N. Activities of Ten Essential Oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 Cancer Cells. Molecules. 2010;15:3200–3210. doi: 10.3390/molecules15053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar N.V., Srinivas P., Bettadaiah B. New scalable and eco-friendly synthesis of gingerols. Tetrahedron Lett. 2012;53:2993–2995. doi: 10.1016/j.tetlet.2012.03.092. [DOI] [Google Scholar]
- 35.Semwal R.B., Semwal D.K., Combrinck S., Viljoen A. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry. 2015;117:554–568. doi: 10.1016/j.phytochem.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Shirin A.P.R., Jamuna P., Pilerood S.A., Prakash J. Chemical composition and antioxidant properties of ginger root (Zingiber officinale) J. Med. Plants Res. 2010;4:2674–2679. doi: 10.5897/JMPR09.464. [DOI] [Google Scholar]
- 37.Idris N.A., Yasin H.M., Usman A. Voltammetric and spectroscopic determination of polyphenols and antioxidants in ginger (Zingiber officinale Roscoe) Heliyon. 2019;5:e01717. doi: 10.1016/j.heliyon.2019.e01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azeem A.A., Hegazy A., Ibrahim K.S., Farrag A.-R.H., El-Sayed E.M. Hepatoprotective, Antioxidant, and Ameliorative Effects of Ginger (Zingiber officinale Roscoe) and Vitamin E in Acetaminophen Treated Rats. J. Diet. Suppl. 2013;10:195–209. doi: 10.3109/19390211.2013.822450. [DOI] [PubMed] [Google Scholar]
- 39.Lee H.S., Seo E.Y., E Kang N., Kim W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008;19:313–319. doi: 10.1016/j.jnutbio.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Shukla Y., Prasad S., Tripathi C., Singh M., George J., Kalra N. In vitro andin vivomodulation of testosterone mediated alterations in apoptosis related proteins by [6]-gingerol. Mol. Nutr. Food Res. 2007;51:1492–1502. doi: 10.1002/mnfr.200700197. [DOI] [PubMed] [Google Scholar]
- 41.Cheng X.-L., Liu Q., Peng Y., Li P., Li P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011;129:1785–1792. doi: 10.1016/j.foodchem.2011.06.026. [DOI] [Google Scholar]
- 42.Sáez F., Stahl-Biskup E. Thyme—The genus Thymus. CRC Press; London, UK: 2002. Essential oil polymorphism in the genus Thymus; pp. 125–143. [Google Scholar]
- 43.Bilušić T., Krisko A., Dragović-Uzelac V., Milos M., Pifat G. The effects of essential oils and aqueous tea infusions of oregano (Origanum vulgare L. spp. hirtum), thyme (Thymus vulgaris L.) and wild thyme (Thymus serpyllum L.) on the copper-induced oxidation of human low-density lipoproteins. Int. J. Food Sci. Nutr. 2007;58:87–93. doi: 10.1080/09637480601108307. [DOI] [PubMed] [Google Scholar]
- 44.Petersen M., Simmonds M.S. Rosmarinic acid. Phytochemistry. 2003;62:121–125. doi: 10.1016/S0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 45.Asimovic Z., Tufek N. Determination of total phenols and antioxidative activity in teas of wild thyme and mint depending on duration of the extraction; Proceedings of the 25th Scientific-Experts Congress on Agriculture and Food Industry; Izmir, Turkey. 25–27 September 2014; pp. 85–88. [Google Scholar]
- 46.Zhang Y., Chen X., Yang L., Zu Y., Lu Q. Effects of rosmarinic acid on liver and kidney antioxidant enzymes, lipid peroxidation and tissue ultrastructure in aging mice. Food Funct. 2015;6:927–931. doi: 10.1039/C4FO01051E. [DOI] [PubMed] [Google Scholar]
- 47.Jaric S., Mitrović M., Pavlović P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evid. Based Complement. Altern. Med. 2015;2015:1–10. doi: 10.1155/2015/101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Z., Yang J., Yang Y., Wang X.-X., Chen G., Shi A., Lu Y., Jia S., Kang X., Lu L. Rosmarinic acid exerts an anticancer effect on osteosarcoma cells by inhibiting DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway. Phytomedicine. 2020;68:153186. doi: 10.1016/j.phymed.2020.153186. [DOI] [PubMed] [Google Scholar]
- 49.Liao X.-Z., Gao Y., Sun L.-L., Liu J.-H., Chen H.-R., Yu L., Chen Z.-Z., Chen W.-H., Lin L.-Z. Rosmarinic acid reverses non-small cell lung cancer cisplatin resistance by activating the MAPK signaling pathway. Phytotherapy Res. 2020;34:1142–1153. doi: 10.1002/ptr.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han Y.-H., Kee J.-Y., Hong S.-H. Rosmarinic Acid Activates AMPK to Inhibit Metastasis of Colorectal Cancer. Front. Pharmacol. 2018;9:68. doi: 10.3389/fphar.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baig S., Ahma B., Azizan A., Ali S., Rouhollahi E., Abdulla M. Hexane extract of Thymus serpyllum L.: GC-MS profile, antioxidant potential and anticancer impact on HepG2 (liver carcinoma) cell line. Int. Sci. Index. 2014;8:1518–1525. [Google Scholar]
- 52.El Babili F., Bouajila J., Souchard J.P., Bertrand C., Bellvert F., Fouraste I., Moulis C., Valentin A. Oregano: Chemical Analysis and Evaluation of Its Antimalarial, Antioxidant, and Cytotoxic Activities. J. Food Sci. 2011;76:C512–C518. doi: 10.1111/j.1750-3841.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- 53.Zandi P., Ahmadi L. Antioxidant effect of plant extracts of labiatae family. J. Food Sci. Technol. 2000;37:436–439. [Google Scholar]
- 54.Hossain M., Brunton N., Barry-Ryan C., Martin-Diana A.B., Wilkinson M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan J. Chem. 2008;1:751–756. [Google Scholar]
- 55.Hossain M.B., Rai D.K., Brunton N.P., Martin-Diana A.B., Barry-Ryan C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010;58:10576–10581. doi: 10.1021/jf102042g. [DOI] [PubMed] [Google Scholar]
- 56.Hossain M.B., Camphuis G., Aguiló-Aguayo I., Gangopadhyay N., Rai D.K. Antioxidant activity guided separation of major polyphenols of marjoram (Origanum majorana L.) using flash chromatography and their identification by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2014;37:3205–3213. doi: 10.1002/jssc.201400597. [DOI] [PubMed] [Google Scholar]
- 57.Vasudeva N. Comparative Study of Volatile Oil of Stem and Aerial Parts of Origanum majorana Linn. J. Essent. Oil Bear. Plants. 2016;19:2091–2099. doi: 10.1080/0972060X.2016.1264276. [DOI] [Google Scholar]
- 58.Makrane H., El Messaoudi M., Melhaoui A., El Mzibri M., Benbacer L., Aziz M. Cytotoxicity of the Aqueous Extract and Organic Fractions from Origanum majorana on Human Breast Cell Line MDA-MB-231 and Human Colon Cell Line HT-29. Adv. Pharmacol. Sci. 2018;2018:1–9. doi: 10.1155/2018/3297193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alrashedi A.N.R. Master Thesis. United Arab Emirates University; Abu Dhabi, UAE: Apr, 2018. Anti-Colon Cancer Effect of Origanum Majorana Essential Oil. [Google Scholar]
- 60.Attoub S., Shahrazad S., Kholoud A. PO-419 Use of origanum majorana oil in lung cancer therapy. ESMO Open. 2018;3:A187. doi: 10.1136/esmoopen-2018-EACR25.445. [DOI] [Google Scholar]
- 61.Elansary H.O., Mahmoud E.A. Egyptian herbal tea infusions’ antioxidants and their antiproliferative and cytotoxic activities against cancer cells. Nat. Prod. Res. 2014;29:474–479. doi: 10.1080/14786419.2014.951354. [DOI] [PubMed] [Google Scholar]
- 62.Du G.-J., Zhang Z., Wen X.-D., Yu C., Calway T., Yuan C.-S., Wang C.-Z. Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients. 2012;4:1679–1691. doi: 10.3390/nu4111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graham H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-F. [DOI] [PubMed] [Google Scholar]
- 64.Chaturvedula V.S.P., Prakash I. The aroma, taste, color and bioactive constituents of tea. J. Med. Plant Res. 2011;5:2110–2124. doi: 10.5897/JMPR.9001187. [DOI] [Google Scholar]
- 65.Babu P.A., Liu N., Velayutham A.B.P., Babu A. Green Tea Catechins and Cardiovascular Health: An Update. Curr. Med. Chem. 2008;15:1840–1850. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hügel H.M., Jackson N. Redox chemistry of green tea polyphenols: Therapeutic benefits in neurodegenerative diseases. Mini Rev. Med. Chem. 2012;12:380–387. doi: 10.2174/138955712800493906. [DOI] [PubMed] [Google Scholar]
- 67.Kim H.-S., Quon M.J., Kim J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Boil. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sartippour M.R., Pietras R.J., Márquez-Garbán D.C., Chen H.-W., Heber D., Henning S.M., Sartippour G., Zhang L., Lu M., Weinberg O., et al. The combination of green tea and tamoxifen is effective against breast cancer. Carcinogenesis. 2006;27:2424–2433. doi: 10.1093/carcin/bgl066. [DOI] [PubMed] [Google Scholar]
- 69.Lee B.-R., Cho J.-I., Park P.-S. Effects of Dietary Tea Polyphenol on Tumor Growth Inhibition by Cisplatin in EMT6 Breast Tumor-bearing Mice. J. Korean Soc. Food Sci. Nutr. 2014;43:47–54. doi: 10.3746/jkfn.2014.43.1.047. [DOI] [Google Scholar]
- 70.Morré D.M., Morré D.J. Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer Lett. 2006;238:202–209. doi: 10.1016/j.canlet.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Tang S.-N., Singh C., Nall D., Meeker D., Shankar S., Srivastava R.N. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J. Mol. Signal. 2010;5:14. doi: 10.1186/1750-2187-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ebadi M., Azizi M., Sefidkon F., Ahmadi N. Influence of different drying methods on drying period, essential oil content and composition of Lippia citriodora Kunth. J. Appl. Res. Med. Aromat. Plants. 2015;2:182–187. doi: 10.1016/j.jarmap.2015.06.001. [DOI] [Google Scholar]
- 73.Carnat A., Fraisse D., Lamaison J. The aromatic and polyphenolic composition of lemon verbena tea. Fitoterapia. 1999;70:44–49. doi: 10.1016/S0367-326X(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 74.Portmann E., Nigro M.M.L., Reides C.G., Llesuy S., Ricco R.A., Wagner M.L., Gurni A.A., Carballo M.A. Aqueous Extracts of Lippia turbinata and Aloysia citriodora (Verbenaceae): Assessment of Antioxidant Capacity and DNA damage. Int. J. Toxicol. 2012;31:192–202. doi: 10.1177/1091581812436726. [DOI] [PubMed] [Google Scholar]
- 75.Zamorano-Ponce E., Fernández J., Vargas G., Rivera P., A Carballo M. Protective activity of cedron (Aloysia triphylla) infusion over genetic damage induced by cisplatin evaluated by the comet assay technique. Toxicol. Lett. 2004;152:85–90. doi: 10.1016/j.toxlet.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Abdollahi M., Malekirad A.A., Hosseini N., Bayrami M., Hashemi T., Rahzani K. Benefit of Lemon Verbena in Healthy Subjects; Targeting Diseases Associated with Oxidative Stress. Asian J. Anim. Veter. Adv. 2011;6:953–957. doi: 10.3923/ajava.2011.953.957. [DOI] [Google Scholar]
- 77.Fitsiou E., Mitropoulou G., Spyridopoulou K., Vamvakias M., Bardouki H., Galanis A., Chlichlia K., Kourkoutas Y., I Panayiotidis M., Pappa A. Chemical Composition and Evaluation of the Biological Properties of the Essential Oil of the Dietary Phytochemical Lippia citriodora. Molecules. 2018;23:123. doi: 10.3390/molecules23010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fotsis T., Pepper M.S., Aktas E., Breit S., Rasku S., Adlercreutz H., Wähälä K., Montesano R., Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 79.Zervoudakis G., Salahas G., Kaspiris G., Konstantopoulou E. Influence of light intensity on growth and physiological characteristics of common sage (Salvia officinalis L.) Braz. Arch. Boil. Technol. 2012;55:89–95. doi: 10.1590/S1516-89132012000100011. [DOI] [Google Scholar]
- 80.Mohamed A.Y., Mustafa A.A. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Essential Oil Salvia Officinalis in Sudan. J. Multidis. Res. Rev. 2019;1:43–45. doi: 10.20944/preprints201902.0218.v1. [DOI] [Google Scholar]
- 81.Zimmermann B.F., Walch S.G., Tinzoh L.N., Stühlinger W., Lachenmeier D.W. Rapid UHPLC determination of polyphenols in aqueous infusions of Salvia officinalis L. (sage tea) J. Chromatogr. B. 2011;879:2459–2464. doi: 10.1016/j.jchromb.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 82.Lima C.F., Andrade P.B., Seabra R.M., Fernandes-Ferreira M., Pereira-Wilson C. The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats. J. Ethnopharmacol. 2005;97:383–389. doi: 10.1016/j.jep.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 83.Amin A., Hamza A.A. Hepatoprotective effects of Hibiscus, Rosmarinus and Salvia on azathioprine-induced toxicity in rats. Life Sci. 2005;77:266–278. doi: 10.1016/j.lfs.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 84.Albayrak S., Aksoy A., Albayrak S., Sagdic O. In vitro antioxidant and antimicrobial activity of some Lamiaceae species. Iran J. Sci. Technol. A. 2013;37:1–9. [Google Scholar]
- 85.Shahrzad K., Mahya N., Fatemeh T.B., Maryam K., Mohammadreza F.B., Jahromy M.H. Hepatoprotective and Antioxidant Effects of Salvia officinalis L. Hydroalcoholic Extract in Male Rats. Chin. Med. 2014;5:130–136. doi: 10.4236/cm.2014.52016. [DOI] [Google Scholar]
- 86.Veličković D.T., Karabegović I.T., Stojičević S.S., Lazić M.L., Marinković V.D., Veljković V. Comparison of antioxidant and antimicrobial activities of extracts obtained from Salvia glutinosa L. and Salvia officinalis L. Chem. Ind. 2011;65:599–605. doi: 10.2298/HEMIND110412034V. [DOI] [Google Scholar]
- 87.Liu J., Shen H.-M., Ong C.N. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG(2) cells. Cancer Lett. 2000;153:85–93. doi: 10.1016/S0304-3835(00)00391-8. [DOI] [PubMed] [Google Scholar]
- 88.Sertel S., Eichhorn T., Plinkert P., Efferth T. Anticancer activity of Salvia officinalis essential oil against HNSCC cell line (UMSCC1) HNO. 2011;59:1203–1208. doi: 10.1007/s00106-011-2274-3. [DOI] [PubMed] [Google Scholar]
- 89.Ebadi M. Pharmacodynamic Basis of Herbal Medicine. 2nd ed. CRC press; Boca Raton, FL, USA: 2006. [Google Scholar]
- 90.Unlu M., Ergene E., Unlu G.V., Zeytinoglu H.S., Vural N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae) Food Chem. Toxicol. 2010;48:3274–3280. doi: 10.1016/j.fct.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Bernard D., Kwabena A.I., Osei O.D., Daniel G.A., Elom S.A., Sandra A. The Effect of Different Drying Methods on the Phytochemicals and Radical Scavenging Activity of Ceylon Cinnamon (Cinnamomum zeylanicum) Plant Parts. Eur. J. Med. Plants. 2014;4:1324–1335. doi: 10.9734/EJMP/2014/11990. [DOI] [Google Scholar]
- 92.Lee J.-S., Jeon S.-M., Park E.-M., Huh T.-L., Kwon O.-S., Lee M.-K., Choi M.-S. Cinnamate Supplementation Enhances Hepatic Lipid Metabolism and Antioxidant Defense Systems in High Cholesterol-Fed Rats. J. Med. Food. 2003;6:183–191. doi: 10.1089/10966200360716599. [DOI] [PubMed] [Google Scholar]
- 93.Juliani H.R., Simon J.E., Ramboatiana M.M.R., Behra O., Garvey A., Raskin I. Malagasy aromatic plants: Essential oils, antioxidant and antimicrobial activities. Acta Hortic. 2004;629:77–81. doi: 10.17660/ActaHortic.2004.629.9. [DOI] [Google Scholar]
- 94.Jayaprakasha G., Ohnishi-Kameyama M., Ono H., Yoshida M., Jaganmohan Rao L. Phenolic constituents in the fruits of Cinnamomum zeylanicum and their antioxidant activity. J. Agri. Food Chem. 2006;54:1672–1679. doi: 10.1021/jf052736r. [DOI] [PubMed] [Google Scholar]
- 95.Moselhy S.S., Ali H.K.H. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Boil. Res. 2009;42:93–98. doi: 10.4067/S0716-97602009000100009. [DOI] [PubMed] [Google Scholar]
- 96.Herdwiani W., Soemardji A., Elfahmi I., Tan M. A review of cinnamon as a potent anticancer drug. Asian J. Pharm. Clin. Res. 2016;9:8–13. [Google Scholar]
- 97.Packiaraj R. Antimicrobial and cytotoxic activities of endophytic fungus Colletotrichum gloeosporioides isolated from endemic tree Cinnamomum malabatrum. Stud. Fungi. 2016;1:104–113. doi: 10.5943/sif/1/1/10. [DOI] [Google Scholar]
- 98.Singh R., Koppikar S.J., Paul P., Gilda S., Paradkar A., Kaul-Ghanekar R. Comparative analysis of cytotoxic effect of aqueous cinnamon extract from Cinnamomum zeylanicum bark with commercial cinnamaldehyde on various cell lines. Pharm. Boil. 2009;47:1174–1179. doi: 10.3109/13880200903019242. [DOI] [Google Scholar]
- 99.Mirzaei M., Sefidkon F., Ahmadi N., Shojaeiyan A., Hosseini H. Damask rose (Rosa damascena Mill.) essential oil is affected by short-and long-term handling. Ind. Crop. Prod. 2016;79:219–224. doi: 10.1016/j.indcrop.2015.11.011. [DOI] [Google Scholar]
- 100.Salman S.Y., Erbas S. Contact and repellency effects of Rosa damascena Mill. essential oil and its two major constituents against Tetranychus urticae Koch (Acari: Tetranychidae) Türk. Entomol. Derg. 2014;38:365–376. doi: 10.16970/ted.17492. [DOI] [Google Scholar]
- 101.Himesh S., Nanda S., Singhai A., Jitender M. Radical scavenging activities and natural indicator activity of aqueous and ethanolic extract of Rosa damascena. Int. J. Pharm. Pharm. Sci. 2012;4:581–586. [Google Scholar]
- 102.Verma R.S., Padalia R.C., Chauhan A., Singh A., Yadav A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascene Mill.) cultivars from North Indian hills. Nat. Prod. Res. 2011;25:1577–1584. doi: 10.1080/14786419.2010.520162. [DOI] [PubMed] [Google Scholar]
- 103.Senol F.S., Orhan I.E., Kurkcuoglu M., Khan M.T.H., Altintas A., Sener B., Başer K.H.C. A mechanistic investigation on anticholinesterase and antioxidant effects of rose (Rosa damascena Mill.) Food Res. Int. 2013;53:502–509. doi: 10.1016/j.foodres.2013.05.031. [DOI] [Google Scholar]
- 104.Baydar N.G., Baydar H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crop. Prod. 2013;41:375–380. doi: 10.1016/j.indcrop.2012.04.045. [DOI] [Google Scholar]
- 105.Ozkan G., Sagdic O., Baydar N.G., Baydar H. Note: Antioxidant and Antibacterial Activities of Rosa damascena Flower Extracts. Food Sci. Technol. Int. 2004;10:277–281. doi: 10.1177/1082013204045882. [DOI] [Google Scholar]
- 106.Shokrzadeh M., Habibi E., Modanloo M. Cytotoxic and genotoxic studies of essential oil from Rosa damascene Mill., Kashan, Iran. Med. Glas. (Zenica) 2017;14:152–157. doi: 10.17392/901-17. [DOI] [PubMed] [Google Scholar]
- 107.Stanojević L., Marjanovic-Balaban Z.R., Kalaba V.D., Stanojević J.S., Cvetkovic D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.) J. Essent. Oil Bear. Plants. 2016;19:2017–2028. doi: 10.1080/0972060X.2016.1224689. [DOI] [Google Scholar]
- 108.Gupta V., Mittal P., Bansal P., Khokra S.L., Kaushik D. Pharmacological potential of Matricaria recutita—A review. Int. J. Pharm. Sci. Drug Res. 2010;2:12–16. [Google Scholar]
- 109.Al-Ismail K., Talal A. A study of the effect of water and alcohol extracts of some plants as antioxidants and antimicrobial on long-term storage of anhydrous butter fat. Dirasat. Agric. Sci. 2003;30:330–337. [Google Scholar]
- 110.Sazegar M., Banakar A., Bahrami N., Bahrami A., Baghbani M., Nematolahi P., Mottaghi M. Determination of the antioxidant activity and stability of Chamomile (Matricaria chamomilla L.) extract in sunflower oil. World App. Sci. J. 2011;12:1500–1504. [Google Scholar]
- 111.Sotiropoulou N.S., Megremi S.F., Tarantilis P.A. Evaluation of Antioxidant Activity, Toxicity, and Phenolic Profile of Aqueous Extracts of Chamomile (Matricaria chamomilla L.) and Sage (Salvia officinalis L.) Prepared at Different Temperatures. Appl. Sci. 2020;10:2270. doi: 10.3390/app10072270. [DOI] [Google Scholar]
- 112.Zemestani M., Rafraf M., Jafarabadi M.A., Information P.E.K.F.C. Chamomile tea improves glycemic indices and antioxidants status in patients with type 2 diabetes mellitus. Nutrients. 2016;32:66–72. doi: 10.1016/j.nut.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 113.Demir N., Gungor A.A., Nadaroglu H., Demir Y. The antioxidant and radical scavenging activities of Primrose (Primula vulgaris) Eur. J. Exp. Biol. 2014;4:395–401. [Google Scholar]
- 114.Ozkan M.T., Aliyazicioglu R., Demir S., Misir S., Turan I., Yildirmis S., Aliyazicioglu Y. Phenolic characterisation and antioxidant activity of Primula vulgaris and its antigenotoxic effect on fibroblast cells. Jundishapur J. Nat. Pharm. Prod. 2017;12:395–401. doi: 10.5812/jjnpp.40073. [DOI] [Google Scholar]
- 115.Demir S., Turan I., Aliyazicioğlu Y. Antioxidant properties of Primula vulgaris flower extract and its cytotoxic effect on human cancer cell lines. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Dergisi. 2019;22:78–84. [Google Scholar]
- 116.Demir S., Turan I., Aliyazicioğlu R., Yaman S.O., Aliyazicioglu Y. Primula vulgaris extract induces cell cycle arrest and apoptosis in human cervix cancer cells. J. Pharm. Anal. 2018;8:307–311. doi: 10.1016/j.jpha.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adnan I., Talib W. Do herbal drinks augment the cytotoxic effect against different cancer cells? Are they potent stimulators of innate and acquired immunity? J. Clin. Oncol. 2020;38:e21657. doi: 10.1200/JCO.2020.38.15_suppl.e21657. [DOI] [Google Scholar]
- 118.Adegunloye B.J., O Omoniyi J., A Owolabi O., Ajagbonna O.P., Sofola O.A., A Coker H. Mechanisms of the blood pressure lowering effect of the calyx extract of Hibiscus sabdariffa in rats. Afr. J. Med. Med. Sci. 1996;25:235–238. [PubMed] [Google Scholar]
- 119.Gaya I., Mohammed O., Suleiman A., Maje M., Adekunle A. Toxicology and lactogenic studies on the seed of Hibiscus sabdariffa extract on serum prolactin levels of albino wistar rats. Internet J. Endocrinal. 2009;5:2–3. [Google Scholar]
- 120.Nkumah O.C. Phytochemical analysis and medicinal uses of Hibiscus sabdariffa. Int. J. Herb. Med. 2015;2:16–19. [Google Scholar]
- 121.Obouayeba A.P., Diarrassouba M., Soumahin E.F., Kouakou T.H. Phytochemical analysis, purification and identification of Hibiscus anthocyanins. J. Pharm. Chem. Biol. Sci. 2015;3:156–168. [Google Scholar]
- 122.Shen C.-Y., Zhang T.-T., Jiang J.-G. Anti-inflammatory activities of essential oil isolated from the calyx of Hibiscus sabdariffa L. Food Funct. 2016;7:4451–4459. doi: 10.1039/C6FO00795C. [DOI] [PubMed] [Google Scholar]
- 123.Rassem H., Nour A.H., Yunus R.M. GC-MS analysis of bioactive constituents of Hibiscus flower. Aust. J. Basic Appl. Sci. 2017;11:91–97. [Google Scholar]
- 124.Cisse M., Vaillant F., Pallet D., Dornier M. Selecting ultrafiltration and nanofiltration membranes to concentrate anthocyanins from roselle extract (Hibiscus sabdariffa L.) Food Res. Int. 2011;44:2607–2614. doi: 10.1016/j.foodres.2011.04.046. [DOI] [Google Scholar]
- 125.Salmerón-Ruiz M.L., Domínguez-Avila J.A., Ayala-Zavala J.F., Alvarez-Parrilla E., Villegas-Ochoa M.A., Sáyago-Ayerdi S.G., Valenzuela-Melendez M., González-Aguilar G.A. Optimization of total anthocyanin content and antioxidant activity of a Hibiscus sabdariffa infusion using response surface methodology. BIOtecnia. 2019;21:114–122. doi: 10.18633/biotecnia.v21i2.937. [DOI] [Google Scholar]
- 126.Saed-Moucheshi A., Shekoofa A., Pessarakli M. Reactive Oxygen Species (ROS) Generation and Detoxifying in Plants. J. Plant Nutr. 2014;37:1573–1585. doi: 10.1080/01904167.2013.868483. [DOI] [Google Scholar]
- 127.Nguyen Q.D., Pham T.N., Binh M.L.T., Thuan M., Van N.T.T., Lam T.D., Nguyen P.T.N. Effects of Extraction Conditions on Antioxidant Activities of Roselle (Hibiscus sabdariffa L.) Extracts. Mater. Sci. 2020;977:201–206. doi: 10.4028/www.scientific.net/MSF.977.201. [DOI] [Google Scholar]
- 128.Haron N., Jusoh H.M., Abul N.S.B., Rahman A. The Total Phenolic Content and Antioxidant Activity of Roselle (Hibiscus sabdariffa) Extract. Int. J. Allied Heal. Sci. 2019;3:725–733. [Google Scholar]
- 129.Fernández-Arroyo S., Rodríguez-Medina I.C., Beltrán-Debón R., Pasini F., Joven J., Micol V., Segura-Carretero A., Fernández-Gutiérrez A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res. Int. 2011;44:1490–1495. doi: 10.1016/j.foodres.2011.03.040. [DOI] [Google Scholar]
- 130.Khaghani S., Razi F., Yajloo M.M., Paknejad M., Shariftabrizi A., Pasalar P. Selective Cytotoxicity and Apoptogenic Activity of Hibiscus Sabdariffa Aqueous Extract Against MCF-7 Human Breast Cancer Cell Line. J. Cancer Ther. 2011;2:394–400. doi: 10.4236/jct.2011.23054. [DOI] [Google Scholar]
- 131.Su C.-C., Wang C.-J., Huang K.-H., Lee Y.-J., Chan W.-M., Chang Y.-C. Anthocyanins from Hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods. 2018;48:614–631. doi: 10.1016/j.jff.2018.07.032. [DOI] [Google Scholar]
- 132.Ahirwar B., Ahirwar D. In vivo and in vitro investigation of cytotoxic and antitumor activities of polyphenolic leaf extract of Hibiscus sabdariffa against breast cancer cell lines. Res. J. Pharm. Technol. 2020;13:615. doi: 10.5958/0974-360X.2020.00116.X. [DOI] [Google Scholar]
- 133.Lin H.-H., Chen J.-H., Kuo W.-H., Wang C.-J. Chemopreventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem. Biol. Interact. 2007;165:59–75. doi: 10.1016/j.cbi.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 134.Longtin R. The Pomegranate: Nature’s Power Fruit? J. Natl. Cancer Inst. 2003;95:346–348. doi: 10.1093/jnci/95.5.346. [DOI] [PubMed] [Google Scholar]
- 135.Di Stefano V., Pitonzo R., Novara M.E., Bongiorno D., Indelicato S., Gentile C., Avellone G., Bognanni R., Scandurra S., Melilli M.G. Antioxidant activity and phenolic composition in pomegranate (Punica granatum L.) genotypes from south Italy by UHPLC-Orbitrap-MS approach. J. Sci. Food Agric. 2018;99:1038–1045. doi: 10.1002/jsfa.9270. [DOI] [PubMed] [Google Scholar]
- 136.Bhandary S.K., Bhat V.S., Sharmila K.P., Bekal M.P. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. NITTE Univ. J. Heal. Sci. 2012;2:34–38. doi: 10.1055/s-0040-1703609. [DOI] [Google Scholar]
- 137.Attia E. Antimicrobial Activity and Bio-active compounds analysis in Ethanolic plant extracts of Punica Grantanum (Pomegranate) using GC-MS. Egypt J. Exp. Boil. 2019;15:325. doi: 10.5455/egyjebb.20190707112923. [DOI] [Google Scholar]
- 138.Kachkoul R., Houssaini T.S., Mohim M., El Habbani R., Lahrichi A. Chemical Compounds Identification and Antioxidant and Calcium Oxalate Anticrystallization Activities of Punica granatum L. Evid. Based Complement. Altern. Med. 2020;2020:9424510. doi: 10.1155/2020/9424510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Balli D., Cecchi L., Khatib M., Bellumori M., Cairone F., Carradori S., Zengin G., Cesa S., Innocenti M., Mulinacci N. Characterization of Arils Juice and Peel Decoction of Fifteen Varieties of Punica granatum L.: A Focus on Anthocyanins, Ellagitannins and Polysaccharides. Antioxidants. 2020;9:238. doi: 10.3390/antiox9030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ghosh S., Chatterjee J.K., Chalkroborty B., Hazra A.K. Comparison of different aqueous extraction methods for optimum extraction of polyphenols and in-vitro anti-oxidant activity from pomegranate peel. J. Pharmacogn. Phytochem. 2019;8:342–347. [Google Scholar]
- 141.Doostan F., Vafafar R., Zakeri-Milani P., Pouri A., Afshar R.A., Abbasi M.M. Effects of Pomegranate (Punica Granatum L.) Seed and Peel Methanolic Extracts on Oxidative Stress and Lipid Profile Changes Induced by Methotrexate in Rats. Adv. Pharm. Bull. 2017;7:269–274. doi: 10.15171/apb.2017.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.González J.C.R., Hernández R.D., Berghe W.V. Punica granatum L. Constituents for Cancer Prevention, Chemosensitisation and Therapeutic Treatment. In: Pezzuto J.M., Vang O., editors. Natural Products for Cancer Chemoprevention: Single Compounds and Combinations. Springer International Publishing; Cham, Switzerland: 2020. pp. 401–468. [Google Scholar]
- 143.Khwairakpam A.D., Bordoloi D., Thakur K.K., Monisha J., Arfuso F., Sethi G., Mishra S., Kumar A.P., Kunnumakkara A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018;133:53–64. doi: 10.1016/j.phrs.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 144.Adaramoye O.A., Erguen B., Nitzsche B., Höpfner M., Jung K., Rabien A. Punicalagin, a polyphenol from pomegranate fruit, induces growth inhibition and apoptosis in human PC-3 and LNCaP cells. Chem. Interactions. 2017;274:100–106. doi: 10.1016/j.cbi.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 145.Uddandrao V.V.S., Parim B., Nivedha P.R., Swapna K., Rameshreddy P., Vadivukkarasi S., Begum M.S., Ganapathy S. Anticancer activity of pomegranate extract: Effect on hematological and antioxidant profile against ehrlich-ascites-carcinoma in Swiss albino mice. Orient. Pharm. Exp. Med. 2018;19:243–250. doi: 10.1007/s13596-018-0348-4. [DOI] [Google Scholar]
- 146.Liu H., Zeng Z., Wang S., Li T., Mastriani E., Li Q.-H., Bao H.-X., Zhou Y.-J., Wang X., Liu Y., et al. Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating MMP2 and MMP9. Cancer Boil. Ther. 2017;18:990–999. doi: 10.1080/15384047.2017.1394542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Özcan M.M., Chalchat J.C. Chemical composition and antifungal effect of anise (Pimpinella anisum L.) fruit oil at ripening stage. Ann. Microbiol. 2006;56:353–358. doi: 10.1007/BF03175031. [DOI] [Google Scholar]
- 148.Al-Ismail K., Aburjai T. Antioxidant activity of water and alcohol extracts of chamomile flowers, anise seeds and dill seeds. J. Sci. Food Agric. 2004;84:173–178. doi: 10.1002/jsfa.1625. [DOI] [Google Scholar]
- 149.Farzaneh V., Gominho J., Pereira H., Carvalho I.S. Screening of the Antioxidant and Enzyme Inhibition Potentials of Portuguese Pimpinella anisum L. Seeds by GC-MS. Food Anal. Methods. 2018;11:2645–2656. doi: 10.1007/s12161-018-1250-x. [DOI] [Google Scholar]
- 150.Lee J.-B., Yamagishi C., Hayashi K., Hayashi T. Antiviral and Immunostimulating Effects of Lignin-Carbohydrate-Protein Complexes from Pimpinella anisum. Biosci. Biotechnol. Biochem. 2011;75:459–465. doi: 10.1271/bbb.100645. [DOI] [PubMed] [Google Scholar]
- 151.Andallu B., Rajeshwari C.U. Chapter 20—Aniseeds (Pimpinella anisum L.) in Health and Disease. In: Preedy V.R., Watson R.R., Patel V.B., editors. Nuts and Seeds in Health and Disease Prevention. Academic Press; San Diego, CA, USA: 2011. pp. 175–181. [Google Scholar]
- 152.Kharobi K. Effect of Grinding the Herb and Boiling the Infusion on Total Phenolic Content and Antioxidant Capacity of Herbal Infusions. J. Pharm. Nutr. Sci. 2019;9:66–70. doi: 10.29169/1927-5951.2019.09.02.2. [DOI] [Google Scholar]
- 153.Faried M.A., El-Mehi A.E.-S. Aqueous anise extract alleviated the pancreatic changes in streptozotocin-induced diabetic rat model via modulation of hyperglycemia, oxidative stress, apoptosis and autophagy: A biochemical, histological and immunohistochemical study. Folia Morphol. 2019 doi: 10.5603/FM.a2019.0117. [DOI] [PubMed] [Google Scholar]
- 154.Mukunda A., Pynadath M.K., Kadar N., Mohan A. Cytotoxic effect of anise seed (Pimpinella anisum) extract on KB cell line—A comparative study with CISPLATIN. Oral Maxillofac. Pathol. J. 2020;11:1–10. [Google Scholar]
- 155.Asadi H., Rahamooz-Haghighi S. Anti-proliferative effect of the extracts and essential oil of Pimpinella anisum on gastric cancer cells. J. HerbMed Pharm. 2016;5:157–161. [Google Scholar]
- 156.Kadan S. Anticancer Activity of Anise (Pimpinella anisum L.) Seed Extract. Open Nutraceuticals J. 2013;6:1–5. doi: 10.2174/1876396001306010001. [DOI] [Google Scholar]
- 157.Chaudhry Z., Khera R.A., Hanif M.A., Ayub M.A., Sumrra S.H. Chapter 13—Cumin. In: Hanif M.A., Nawaz H., Khan M.M., Byrne H.J., editors. Medicinal Plants of South Asia. Elsevier; Amsterdam, The Netherlands: 2020. pp. 165–178. [Google Scholar]
- 158.Moghaddam M., Miran S.N.K., Pirbalouti A.G., Mehdizadeh L., Ghaderi Y. Variation in essential oil composition and antioxidant activity of cumin (Cuminum cyminum L.) fruits during stages of maturity. Ind. Crop. Prod. 2015;70:163–169. doi: 10.1016/j.indcrop.2015.03.031. [DOI] [Google Scholar]
- 159.Rai N., Yadav S., Verma A., Tiwari L., Sharma R.K. A monographic profile on quality specifications for a herbal drug and spice of commerce—Cuminum cyminum L. Int. J. Adv. Sci. 2012;1:1–12. [Google Scholar]
- 160.Ghasemi G., Fattahi M., Alirezalu A., Ghosta Y. Antioxidant and antifungal activities of a new chemovar of cumin (Cuminum cyminum L.) Food Sci. Biotechnol. 2018;28:669–677. doi: 10.1007/s10068-018-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Al-Shawi S., Al-Younis Z., Al-Kareem N. Study of cumin antibacterial and antioxidant activity of alcoholic and aqueous extracts. Pakistan J. Biotechnol. 2017;14:227–231. [Google Scholar]
- 162.Demir S., Korukluoglu M. A comparative study about antioxidant activity and phenolic composition of cumin (Cuminum cyminum L.) and coriander (Coriandrum sativum L.) Indian J. Tradit. Know. 2020;19:383–393. [Google Scholar]
- 163.Mahalakshmi R., Priyanga J., Hari B.N.V., Bhakta-Guha D., Guha G. Hexavalent chromium-induced autophagic death of WRL-68 cells is mitigated by aqueous extract of Cuminum cyminum L. seeds. 3 Biotech. 2020;10:191. doi: 10.1007/s13205-020-02184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Allahghadri T., Rasooli I., Owlia P., Nadooshan M.J., Ghazanfari T., Taghizadeh M., Astaneh S.D.A. Antimicrobial Property, Antioxidant Capacity, and Cytotoxicity of Essential Oil from Cumin Produced in Iran. J. Food Sci. 2010;75:H54–H61. doi: 10.1111/j.1750-3841.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 165.Prakash E., Gupta D.K. Cytotoxic activity of ethanolic extract of Cuminum cyminum Linn against seven human cancer cell line. Univers. J. Agric. Res. 2014;2:27–32. doi: 10.13189/ujar.2014.020104. [DOI] [Google Scholar]
- 166.Kumar S., Nair R., Gupta S., Abdullah A., Talwar P., Ravanan P. Anti-Cancer and Neuro-Protective effect of Cuminum cyminum extracts on IMR32 Human Neuroblastoma Cell Lines. Res. J. Pharm. Technol. 2018;11:1547. doi: 10.5958/0974-360X.2018.00288.3. [DOI] [Google Scholar]
- 167.Goodarzi S., Tabatabaei M.J., Jafari R.M., Shemirani F., Tavakoli S., Mofasseri M., Tofighi Z. Cuminum cyminum fruits as source of luteolin-7-O-glucoside, potent cytotoxic flavonoid against breast cancer cell lines. Nat. Prod. Res. 2018;34:1602–1606. doi: 10.1080/14786419.2018.1519824. [DOI] [PubMed] [Google Scholar]
- 168.Barros L., Dueñas M., Dias M.I., Sousa M.J., Santos-Buelga C., Ferreira I.C., Dueñas M. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013;136:1–8. doi: 10.1016/j.foodchem.2012.07.107. [DOI] [PubMed] [Google Scholar]
- 169.Carocho M., Barros L., Calhelha R.C., Ćirić A., Soković M., Santos-Buelga C., Morales P., Ferreira I.C. Melissa officinalis L. decoctions as functional beverages: A bioactive approach and chemical characterization. Food Funct. 2015;6:2240–2248. doi: 10.1039/C5FO00309A. [DOI] [PubMed] [Google Scholar]
- 170.Shakeri A., Sahebkar A., Javadi B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016;188:204–228. doi: 10.1016/j.jep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 171.Ismael M.F., Pedro R.E.R., Selvin A.S.M., Vany P.F., Ricardo S.A., Jhunior A.M.F., Luis A.B.A. Chemical composition of essential oil of Melissa officinalis L. and antioxidant activity from Boa Vista-RR, Brazil. Afr. J. Pharm. Pharmacol. 2020;14:41–45. doi: 10.5897/AJPP2020.5121. [DOI] [Google Scholar]
- 172.Skotti E., Sotiropoulou N.S., Lappa I., Kaiafa M., Tsitsigiannis D.I., Tarantilis P.A. Screening of Lemon Balm Extracts for Anti-Aflatoxigenic, Antioxidant and Other Biological Activities. Preprints. 2019:2019070005. doi: 10.20944/preprints201907.0005.v1. [DOI] [Google Scholar]
- 173.Yaldiz G., Arici K., Yilmaz G. Phytochemical analysis, antioxidant and antibacterial activities of four Lamiaceae species cultivated in barnyard manure. J. Agric. Sci. 2017;23:95–108. [Google Scholar]
- 174.Papoti V.T., Totomis N., Atmatzidou A., Zinoviadou K., Androulaki A., Petridis D., Ritzoulis C. Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption. Processes. 2019;7:88. doi: 10.3390/pr7020088. [DOI] [Google Scholar]
- 175.Žlabur J.Š., Voća S., Dobričević N., Pliestić S., Galić A., Boričević A., Borić N. Ultrasound-assisted extraction of bioactive compounds from lemon balm and peppermint leaves. Int. Agrophys. 2016;30:95–104. doi: 10.1515/intag-2015-0077. [DOI] [Google Scholar]
- 176.Ghazizadeh J., Hamedeyazdan S., Torbati M., Farajdokht F., Fakhari A., Mahmoudi J., Araj-khodaei M., Sadigh-Eteghad S. Melissa officinalis L. hydro-alcoholic extract inhibits anxiety and depression through prevention of central oxidative stress and apoptosis. Exp. Physiol. 2020;105:707–720. doi: 10.1113/EP088254. [DOI] [PubMed] [Google Scholar]
- 177.Popova A., Dalemska Z., Mihaylova D., Hristova I., Alexieva I. Melissa officinalis L.—GC profile and antioxidant activity. Int. J. Pharmacogn. Phytochem. Res. 2016;8:634–638. [Google Scholar]
- 178.Martins E.N., Pessano N.T., Leal L., Roos D.H., Folmer V., Puntel G.O., Da Rocha J.B.T., Aschner M., Ávila D.S., Puntel R.L. Protective effect of Melissa officinalis aqueous extract against Mn-induced oxidative stress in chronically exposed mice. Brain Res. Bull. 2012;87:74–79. doi: 10.1016/j.brainresbull.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 179.Kim M., Yoo G., Randy A., Son Y.-J., Hong C.R., Kim S.M., Nho C.W. Lemon Balm and Its Constituent, Rosmarinic Acid, Alleviate Liver Damage in an Animal Model of Nonalcoholic Steatohepatitis. Nutrients. 2020;12:1166. doi: 10.3390/nu12041166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hassan R.A., Abotaleb S.T., Hamed H.B., Eldeen M.S. Antioxidant and Antimicrobial Activities of Melissa officinalis L. (Lemon Balm) Extracts. J. Agric. Chem. Biotechnol. 2019;10:183–187. doi: 10.21608/jacb.2019.56823. [DOI] [Google Scholar]
- 181.Encalada M.A., Hoyos K.M., Rehecho S., Berasategi I., De Ciriano M.G.-I., Ansorena D., Astiasarán I., Navarro-Blasco I., Cavero R.Y., Calvo M.I. Anti-proliferative Effect of Melissa officinalis on Human Colon Cancer Cell Line. Plant Foods Hum. Nutr. 2011;66:328–334. doi: 10.1007/s11130-011-0256-y. [DOI] [PubMed] [Google Scholar]
- 182.Magalhaes D.B., Castro I., Lopes-Rodrigues V., Pereira J.M., Barros L., Ferreira I.C., Xavier C.P.R., Vasconcelos M.H. Melissa officinalis L. ethanolic extract inhibits the growth of a lung cancer cell line by interfering with the cell cycle and inducing apoptosis. Food Funct. 2018;9:3134–3142. doi: 10.1039/C8FO00446C. [DOI] [PubMed] [Google Scholar]
- 183.Shamseini M., Mohammadi M., Shirazi F.H., Andalib S., Gholami S., Hosseini S.H., Noubarani M., Kamalinejad M., Eskandari M.R. Prevention of liver cancer by standardized extract of Melissa officinalis L. in a rat model of hepatocellular carcinoma: Its potential role as a chemopreventive agent. Int. Pharm. Acta. 2019;2:2e8-1–2e8-7. doi: 10.22037/ipa.v2i1.23970. [DOI] [Google Scholar]
- 184.Ghiulai R., Avram S., Stoian D., Pavel I.Z., Coricovac D., Oprean C., Vlase L., Farcas C., Mioc M., Minda D., et al. Lemon Balm Extracts Prevent Breast Cancer Progression In Vitro and In Ovo on Chorioallantoic Membrane Assay. Evid. Based Complement. Altern. Med. 2020;2020:6489159. doi: 10.1155/2020/6489159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moore J., Yousef M., Tsiani E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients. 2016;8:731. doi: 10.3390/nu8110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ngo S.N.T., Williams D.B., Head R. Rosemary and Cancer Prevention: Preclinical Perspectives. Crit. Rev. Food Sci. Nutr. 2011;51:946–954. doi: 10.1080/10408398.2010.490883. [DOI] [PubMed] [Google Scholar]
- 187.Nie J.-Y., Li R., Jiang Z.-T., Wang Y., Tan J., Tang S.-H., Zhang Y. Antioxidant activity screening and chemical constituents of the essential oil from rosemary by ultra-fast GC electronic nose coupled with chemical methodology. J. Sci. Food Agric. 2020;100:3481–3487. doi: 10.1002/jsfa.10388. [DOI] [PubMed] [Google Scholar]
- 188.Nieto G., Ros G., Sánchez J.C. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines. 2018;5:98. doi: 10.3390/medicines5030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Christina A., Joseph D., Packialakshmi M., Kothai R., Robert S.H., Chidambaranathan N., Ramasamy M. Anticarcinogenic activity of Withania somnifera Dunal against Dalton’s Ascitic Lymphoma. J. Ethnopharmacol. 2004;93:359–361. doi: 10.1016/j.jep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 190.Al-Attar A.M., Shawush N.A. Influence of olive and rosemary leaves extracts on chemically induced liver cirrhosis in male rats. Saudi J. Boil. Sci. 2014;22:157–163. doi: 10.1016/j.sjbs.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Al Laham S.A., Al Fadel F.M. Antibacterial efficacy of variety plants against the resistant Streptococcus which cause clinical mastitis in cows. Asian J. Pharm. Res. Health Care. 2013;5:32–41. [Google Scholar]
- 192.Arranz E., Mes J., Wichers H., Jaime L., Mendiola J.A., Reglero G., Santoyo S. Anti-inflammatory activity of the basolateral fraction of Caco-2 cells exposed to a rosemary supercritical extract. J. Funct. Foods. 2015;13:384–390. doi: 10.1016/j.jff.2015.01.015. [DOI] [Google Scholar]
- 193.Đilas S., Knez Ž., Četojević-Simin D., Šaponjac V.T., Škerget M., Čanadanović-Brunet J., Ćetković G. In vitro antioxidant and antiproliferative activity of three rosemary (Rosmarinus officinalis L.) extract formulations. Int. J. Food Sci. Technol. 2012;47:2052–2062. doi: 10.1111/j.1365-2621.2012.03069.x. [DOI] [Google Scholar]
- 194.Jaglanian A., Tsiani E. Rosemary Extract Inhibits Proliferation, Survival, Akt, and mTOR Signaling in Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2020;21:810. doi: 10.3390/ijms21030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Telang N. Anti-proliferative and pro-apoptotic effects of rosemary and constituent terpenoids in a model for the HER-2-enriched molecular subtype of clinical breast cancer. Oncol. Lett. 2018;16:5489–5497. doi: 10.3892/ol.2018.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tai J., Cheung S., Wu M., Hasman D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine. 2012;19:436–443. doi: 10.1016/j.phymed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 197.Zhu D.-Y., Li X.-N., Qi Y., Liu D.-L., Yang Y., Zhao J., Zhang C.-Y., Wu K., Zhao S. MiR-454 promotes the progression of human non-small cell lung cancer and directly targets PTEN. Biomed. Pharmacother. 2016;81:79–85. doi: 10.1016/j.biopha.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 198.Karimi N., Rashedi J., Poor B.M., Arabi S., Ghorbani M., Tahmasebpour N., Asgharzadeh M. Cytotoxic effect of rosemary extract on gastric adenocarcinoma (AGS) and esophageal squamous cell carcinoma (KYSE30) cell lines. Gastroenterol. Hepatol. Bed Bench. 2017;10:102–107. [PMC free article] [PubMed] [Google Scholar]
- 199.Jang Y.-G., Hwang K.-A., Choi K.-C. Rosmarinic Acid, a component of rosemary tea, induced the cell cycle arrest and apoptosis through modulation of HDAC2 expression in prostate cancer cell lines. Nutrients. 2018;10:1784. doi: 10.3390/nu10111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pérez-Sánchez A., Barrajón-Catalán E., Ruiz-Torres V., Agulló-Chazarra L., Herranz-López M., Valdés A., Cifuentes A., Micol V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-018-37173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Valdés A., García-Cañas V., Pérez-Sánchez A., Barrajón-Catalán E., Ruiz-Torres V., Artemenko K.A., Micol V., Bergquist J., Cifuentes A. Shotgun proteomic analysis to study the decrease of xenograft tumor growth after rosemary extract treatment. J. Chromatogr. A. 2017;1499:90–100. doi: 10.1016/j.chroma.2017.03.072. [DOI] [PubMed] [Google Scholar]
- 202.Yan M., Li G., Petiwala S.M., Householter E., Johnson J.J. Standardized rosemary (Rosmarinus officinalis) extract induces Nrf2/sestrin-2 pathway in colon cancer cells. J. Funct. Foods. 2015;13:137–147. doi: 10.1016/j.jff.2014.12.038. [DOI] [Google Scholar]
- 203.Ashmawy A., Mostafa N., Eldahshan O.A. GC/MS Analysis and Molecular Profiling of Lemon Volatile Oil against Breast Cancer. J. Essent. Oil Bear. Plants. 2019;22:903–916. doi: 10.1080/0972060X.2019.1667877. [DOI] [Google Scholar]
- 204.Yang M., Liu X., Luo Q., Xu L., Chen F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020;18:1–12. doi: 10.1186/s12951-020-00656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Babasheikhali S.R., Rahgozar S., Mohammadi M. Ginger extract has anti-leukemia and anti-drug resistant effects on malignant cells. J. Cancer Res. Clin. Oncol. 2019;145:1987–1998. doi: 10.1007/s00432-019-02949-5. [DOI] [PubMed] [Google Scholar]
- 206.Pashaei-Asl R., Pashaei-Asl F., Gharabaghi P.M., Khodadadi K., Ebrahimi M., Ebrahimie E., Pashaiasl M. The Inhibitory Effect of Ginger Extract on Ovarian Cancer Cell Line; Application of Systems Biology. Adv. Pharm. Bull. 2017;7:241–249. doi: 10.15171/apb.2017.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alexa E., Sumalan R.-M., Danciu C., Obistioiu D.M., Negrea M., Poiana M.-A., Rus C., Radulov I., Pop G., Dehelean C.A. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules. 2018;23:185. doi: 10.3390/molecules23010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Catauro M., Bollino F., Tranquillo E., Sapio L., Illiano M., Caiafa I., Naviglio S. Chemical analysis and anti-proliferative activity of Campania Thymus Vulgaris essential oil. J. Essent. Oil Res. 2017;29:461–470. doi: 10.1080/10412905.2017.1351405. [DOI] [Google Scholar]
- 209.Benhalilou N., Alsamri H., Alneyadi A., Athamneh K., Alrashedi A., Altamimi N., Al Dhaheri Y., Eid A.H., Iratni R. Origanum majorana Ethanolic Extract Promotes Colorectal Cancer Cell Death by Triggering Abortive Autophagy and Activation of the Extrinsic Apoptotic Pathway. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fathy S.A., Emam M.A., Agwa S.H.A., Abu Zahra F.A., Youssef F.S., Sami R.M. The antiproliferative effect of Origanum majorana on human hepatocarcinoma cell line: Suppression of NF-κB. Cell. Mol. Boil. 2016;62:80–84. [PubMed] [Google Scholar]
- 211.Huang J., Chen S., Shi Y., Li C.-H., Wang X.-J., Li F.-J., Wang C.-H., Meng Q.-H., Zhong J.-N., Liu M. Epigallocatechin gallate from green tea exhibits potent an-ticancer effects in A-549 non-small lung cancer cells by inducing apoptosis, cell cycle arrest and inhibition of cell migration. J. BUON. 2017;22:1422–1427. [PubMed] [Google Scholar]
- 212.Cádiz-Gurrea M.D.L.L., Olivares-Vicente M., Herranz-López M., Arráez-Román D., Fernández-Arroyo S., Micol V., Segura-Carretero A. Bioassay-guided purification of Lippia citriodora polyphenols with AMPK modulatory activity. J. Funct. Foods. 2018;46:514–520. doi: 10.1016/j.jff.2018.05.026. [DOI] [Google Scholar]
- 213.Hoseinifar S.H., Shakouri M., Van Doan H., Shafiei S., Yousefi M., Raeisi M., Yousefi S., Harikrishnan R., Reverter M. Dietary supplementation of lemon verbena (Aloysia citrodora) improved immunity, immune-related genes expression and antioxidant enzymes in rainbow trout (Oncorrhyncus mykiss) Fish Shellfish. Immunol. 2020;99:379–385. doi: 10.1016/j.fsi.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 214.De Oliveira P.F., Munari C.C., Nicolella H.D., Veneziani R.C.S., Tavares D.C. Manool, a Salvia officinalis diterpene, induces selective cytotoxicity in cancer cells. Cytotechnology. 2015;68:2139–2143. doi: 10.1007/s10616-015-9927-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nguyen C.V. Exploring the Efficacy of Long Pepper and Sage Ethanolic Extracts for Inducing Selective Cell Death in Hodgkin Lymphoma Cell Lines; Presented at the Undergraduate Research Conference; Windsor, ON, Canada. 29 March 2016. [Google Scholar]
- 216.Hasan M., Genovese S., Fiorito S., Epifano F., Witt-Enderby P.A. Oxyprenylated Phenylpropanoids Bind to MT1 Melatonin Receptors and Inhibit Breast Cancer Cell Proliferation and Migration. J. Nat. Prod. 2017;80:3324–3329. doi: 10.1021/acs.jnatprod.7b00853. [DOI] [PubMed] [Google Scholar]
- 217.Larasati Y.A., Putri D.P., Utomo R.Y., Hermawan A., Meiyanto E. Combination of cisplatin and cinnamon essential oil inhibits HeLa cells proliferation through cell cycle arrest. J. Appl. Pharm. Sci. 2014;4:14–19. doi: 10.7324/JAPS.2014.41203. [DOI] [Google Scholar]
- 218.Golmakani M.T., Barani S., Alavi N., Tahsiri Z. Oxidative stability of UV irradiated and X-rayed soybean oil incorporated with rose oil. Grasas y Aceites. 2019;70:286. doi: 10.3989/gya.0349181. [DOI] [Google Scholar]
- 219.Menghini L., Ferrante C., Leporini L., Recinella L., Chiavaroli A., Leone S., Pintore G., Vacca M., Orlando G., Brunetti L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytotherapy Res. 2016;30:1513–1518. doi: 10.1002/ptr.5655. [DOI] [PubMed] [Google Scholar]
- 220.Ogata I., Seo H., Kawanai T., Hashimoto E., Oyama Y. Cytotoxic action of bisabololoxide A of German chamomile on human leukemia K562 cells in combination with 5-fluorouracil. Phytomedicine. 2011;18:362–365. doi: 10.1016/j.phymed.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 221.Khodeer D.M., Mehanna E.T., Abushouk A., Abdel-Daim M. Protective Effects of Evening Primrose Oil against Cyclophosphamide-Induced Biochemical, Histopathological, and Genotoxic Alterations in Mice. Pathogens. 2020;9:98. doi: 10.3390/pathogens9020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Owczarek K., Hrabec E., Fichna J., Sosnowska D., Koziolkiewicz M., Szymański J., Lewandowska U. Inhibition of nuclear factor-kappaB, cyclooxygenase-2, and metalloproteinase-9 expression by flavanols from evening primrose (Oenothera paradoxa) in human colon cancer SW-480 cells. J. Funct. Foods. 2017;37:553–563. doi: 10.1016/j.jff.2017.08.029. [DOI] [Google Scholar]
- 223.Amran N., Rani A.N.A., Mahmud R., Yin K.B. Antioxidant and Cytotoxic Effect of Barringtonia racemosa and Hibiscus sabdariffa Fruit Extracts in MCF-7 Human Breast Cancer Cell Line. Pharmacogn. Res. 2016;8:66–70. doi: 10.4103/0974-8490.171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fithrotunnisa Q., Arsianti A., Kurniawan G., Qorina F., Tejaputri N.A., Azizah N.N. In vitro Cytotoxicity of Hibiscus sabdariffa Linn Extracts on A549 Lung Cancer Cell Line. Pharmacogn. J. 2020;12:14–19. doi: 10.5530/pj.2020.12.3. [DOI] [Google Scholar]
- 225.Chaves F.M., Pavan I.C.B., Da Silva L.G.S., De Freitas L.B., Rostagno M.A., Antunes A.E.C., Bezerra R.M.N., Simabuco F.M. Pomegranate Juice and Peel Extracts are Able to Inhibit Proliferation, Migration and Colony Formation of Prostate Cancer Cell Lines and Modulate the Akt/mTOR/S6K Signaling Pathway. Plant Foods Hum. Nutr. 2019;75:54–62. doi: 10.1007/s11130-019-00776-0. [DOI] [PubMed] [Google Scholar]
- 226.Li Y., Hu M., Liang L., Zeng A., Ye T., Yang F., He H., Yao Y.-Q., Xie Y., An Z. Punica granatum (pomegranate) peel extract exerts potent antitumor and anti-metastasis activity in thyroid cancer. RSC Adv. 2016;6:84523–84535. doi: 10.1039/C6RA13167K. [DOI] [Google Scholar]
- 227.Díaz-Castillo C. Junk DNA Contribution to Evolutionary Capacitance Can Drive Species Dynamics. Evol. Boil. 2016;44:190–205. doi: 10.1007/s11692-016-9404-5. [DOI] [Google Scholar]
- 228.Khateef R., Khadri H., Almatroudi A., A Alsuhaibani S., Mobeen S.A., A Khan R. Potential in-vitro anti-breast cancer activity of green-synthesized silver nanoparticles preparation against human MCF-7 cell-lines. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019;10:045012. doi: 10.1088/2043-6254/ab47ff. [DOI] [Google Scholar]
- 229.Dinparvar S., Bagirova M., Allahverdiyev A.M., Abamor E.S., Safarov T., Aydogdu M., Aktas D. A nanotechnology-based new approach in the treatment of breast cancer: Biosynthesized silver nanoparticles using Cuminum cyminum L. seed extract. J. Photochem. Photobiol. B Boil. 2020;208:111902. doi: 10.1016/j.jphotobiol.2020.111902. [DOI] [PubMed] [Google Scholar]
- 230.Khallouki F., Breuer A., Akdad M., Laassri F.E., Attaleb M., Elmoualij B., Mzibri M., Benbacer L., Owen R.W. Cytotoxic activity of Moroccan Melissa officinalis leaf extracts and HPLC-ESI-MS analysis of its phytoconstituents. Future J. Pharm. Sci. 2020;6:1–12. doi: 10.1186/s43094-020-00037-x. [DOI] [Google Scholar]
- 231.Sadowska-Krępa E., Domaszewski P., Pokora I., Żebrowska A., Gdańska A., Podgórski T. Effects of medium-term green tea extract supplementation combined with CrossFit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: A pilot study. J. Int. Soc. Sports Nutr. 2019;16:13. doi: 10.1186/s12970-019-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Samavat H., Ursin G., Emory T.H., Lee E., Wang R., Torkelson C.J., Dostal A.M., Swenson K., Le C.T., Yang C.S., et al. A Randomized Controlled Trial of Green Tea Extract Supplementation and Mammographic Density in Postmenopausal Women at Increased Risk of Breast Cancer. Cancer Prev. Res. 2017;10:710–718. doi: 10.1158/1940-6207.CAPR-17-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nakayama M., Okizaki A., Takahashi K. A Randomized Controlled Trial for the Effectiveness of Aromatherapy in Decreasing Salivary Gland Damage following Radioactive Iodine Therapy for Differentiated Thyroid Cancer. BioMed Res. Int. 2016;2016:9509810. doi: 10.1155/2016/9509810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Citronberg J., Bostick R., Ahearn T., Turgeon D.K., Ruffin I.M.T., Djuric Z., Sen A., Brenner D.E., Zick S.M. Effects of Ginger Supplementation on Cell Cycle Biomarkers in the Normal-Appearing Colonic Mucosa of Patients at Increased Risk for Colorectal Cancer: Results from a Pilot, Randomized, Controlled Trial. Cancer Prev. Res. 2013;6:271–281. doi: 10.1158/1940-6207.CAPR-12-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Morovati A., Gargari B.P., Sarbakhsh P., Azari H., Lotfi-Dizaji L. The effect of cumin supplementation on metabolic profiles in patients with metabolic syndrome: A randomized, triple blind, placebo-controlled clinical trial. Phytother. Res. 2019;33:1182–1190. doi: 10.1002/ptr.6313. [DOI] [PubMed] [Google Scholar]
- 236.Boldaji R.B., Akhlaghi M., Sagheb M.M., Esmaeilinezhad Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: A randomized crossover trial. J. Sci. Food Agric. 2019;100:846–854. doi: 10.1002/jsfa.10096. [DOI] [PubMed] [Google Scholar]
- 237.Nuñez-Sánchez M.A., Dávalos A., González-Sarrías A., Casas-Agustench P., Visioli F., Monedero-Saiz T., García-Talavera N.V., Gómez-Sánchez M.B., Sánchez-Álvarez C., García-Albert A.M., et al. MicroRNAs expression in normal and malignant colon tissues as biomarkers of colorectal cancer and in response to pomegranate extracts consumption: Critical issues to discern between modulatory effects and potential artefacts. Mol. Nutr. Food Res. 2015;59:1973–1986. doi: 10.1002/mnfr.201500357. [DOI] [PubMed] [Google Scholar]
- 238.Ammar A., Turki M., Hammouda O., Chtourou H., Trabelsi K., Bouaziz M., Abdelkarim O., Hoekelmann A., Ayadi F., Souissi N., et al. Effects of Pomegranate Juice Supplementation on Oxidative Stress Biomarkers Following Weightlifting Exercise. Nutrients. 2017;9:819. doi: 10.3390/nu9080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Urbaniak A., Basta P., Ast K., Wołoszyn A., Wołoszyn J.K., Latour E., Skarpanska-Stejnborn A. The impact of supplementation with pomegranate fruit (Punica granatum L.) juice on selected antioxidant parameters and markers of iron metabolism in rowers. J. Int. Soc. Sports Nutr. 2018;15:35. doi: 10.1186/s12970-018-0241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zeraatpishe A., Oryan S., Bagheri M.H., Pilevarian A.A., Malekirad A.A., Baeeri M., Abdollahi M. Effects of Melissa officinalis L. on oxidative status and DNA damage in subjects exposed to long-term low-dose ionizing radiation. Toxicol. Ind. Heal. 2010;27:205–212. doi: 10.1177/0748233710383889. [DOI] [PubMed] [Google Scholar]
- 241.Perez-Vizcaino F., Duarte J., Jiménez R., Santos-Buelga C., Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacol. Rep. 2009;61:67–75. doi: 10.1016/S1734-1140(09)70008-8. [DOI] [PubMed] [Google Scholar]
- 242.Sari A., Selim N., Dilek M. Effect of lemon juice on blood pressure. J. Exp. Clin. Med. 2012;29:38–41. doi: 10.5835/jecm.omu.29.01.010. [DOI] [Google Scholar]
- 243.Langmead L., Rampton D. Herbal treatment in gastrointestinal and liver disease—Benefits and dangers. Aliment. Pharmacol. Ther. 2001;15:1239–1252. doi: 10.1046/j.1365-2036.2001.01053.x. [DOI] [PubMed] [Google Scholar]
- 244.Kulkarni R., Deshpande A., Saxena K., Varma M., Sinha A.R. Ginger supplementary therapy for iron absorption in iron deficiency anemia. Indian J. Tradit. Know. 2012;11:78–80. [Google Scholar]
- 245.Hopkins A.L., Lamm M.G., Funk J.L., Ritenbaugh C. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: A comprehensive review of animal and human studies. Fitoterapia. 2013;85:84–94. doi: 10.1016/j.fitote.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Aziz Z., Wong S.Y., Chong N.J. Effects of Hibiscus sabdariffa L. on serum lipids: A systematic review and meta-analysis. J. Ethnopharmacol. 2013;150:442–450. doi: 10.1016/j.jep.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 247.Bule M.H., Albelbeisi A.H., Nikfar S., Amini M., Abdollahi M. The antidiabetic and antilipidemic effects of Hibiscus sabdariffa: A systematic review and meta-analysis of randomized clinical trials. Food Res. Int. 2020;130:108980. doi: 10.1016/j.foodres.2020.108980. [DOI] [PubMed] [Google Scholar]
- 248.Kumar M., Sharma A., Kumar P. Pharmacotherapeutic Botanicals for Cancer Chemoprevention. Springer; Singapore: 2020. [Google Scholar]
- 249.Calabrese E.J., Baldwin L.A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol. Sci. 2001;22:285–291. doi: 10.1016/S0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- 250.Lakshmi P., Kumar S., Pawar S., Kuriakose B.B., Sudheesh M., Pawar R.S. Targeting metabolic syndrome with phytochemicals: Focus on the role of molecular chaperones and hormesis in drug discovery. Pharmacol. Res. 2020;159:104925. doi: 10.1016/j.phrs.2020.104925. [DOI] [PubMed] [Google Scholar]
- 251.Mehta R., Lansky E.P. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eur. J. Cancer Prev. 2004;13:345–348. doi: 10.1097/01.cej.0000136571.70998.5a. [DOI] [PubMed] [Google Scholar]
- 252.Forester S.C., Lambert J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011;55:844–854. doi: 10.1002/mnfr.201000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Calabrese E.J., Tsatsakis A., Agathokleous E., Giordano J., Calabrese V. Does Green Tea Induce Hormesis? Dose Response. 2020;18:1559325820936170. doi: 10.1177/1559325820936170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Günes-Bayir A., Kocyigit A., Guler E.M., Dadak A. In Vitro Hormetic Effect Investigation of Thymol on Human Fibroblast and Gastric Adenocarcinoma Cells. Molecules. 2020;25:3270. doi: 10.3390/molecules25143270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kubatka P., Uramova S., Kello M., Kajo K., Samec M., Jašek K., Vybohova D., Líšková A., Mojzis J., Adamkov M., et al. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro. Int. J. Mol. Sci. 2019;20:1749. doi: 10.3390/ijms20071749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Četojević-Simin D.D., Velićanski A.S., Cvetković D.D., Markov S.L., Mrđanović J.Ž., Bogdanović V.V., Šolajić S.V. Bioactivity of Lemon Balm Kombucha. Food Bioprocess Technol. 2010;5:1756–1765. doi: 10.1007/s11947-010-0458-6. [DOI] [Google Scholar]