Abstract
Objective:
To identify potential predictors for invasive and non-invasive mechanical ventilation in coronavirus disease 2019 (COVID-19) patients.
Methods:
This study retrospectively analyzes data of 516 patients with confirmed COVID-19, who were categorized into three groups based on which mechanical ventilation method was used during the hospitalization period.
Results:
Among 516 confirmed cases with COVID-19, 446 patients did not receive mechanical ventilation, 38 patients received invasive mechanical ventilation (IMV) and 32 received non-invasive mechanical ventilation (NIMV). The median age of the included patients was 61 years old (interquartile range, 52–69). A total of 432 patients had one or more coexisting illnesses. The main clinical symptoms included fever (79.46%), dry cough (66.47%) and shortness of breath (46.90%). IMV and NIMV patients included more men, more coexisting illnesses and received more medication. Patients in the IMV group and NIMV had higher leukocyte and neutrophil count, lower lymphocyte count, higher aspartate aminotransferase (AST), lactate dehydrogenase (LDH), C-reactive protein (CRP), procalcitonin (PCT) and D-dimer levels and lower albumin (ALB) level. The univariate and multiple logistic regression analysis showed that the use of glucocorticoid, increased neutrophil count and LDH had a predictive role as indicators for IMV, and the use of glucocorticoid, increased neutrophil count and PCT had a predictive role as indicators for NIMV. The area under the curve (AUC) of use of glucocorticoid, increased neutrophil count and LDH was 0.885 (95% confidence interval (CI) 0.838–0.933, p < 0.0001), which provided the specificity and sensitivity 77.7% and 90.9%, respectively. AUC of the use of glucocorticoid, increased neutrophil count and PCT for NIMV was 0.888 (95% CI 0.825–0.952, p < 0.0001), which provided the specificity and sensitivity 70.3% and 96.4%, respectively.
Conclusion:
Glucocorticoid, increased neutrophil and LDH were predictive indicators for IMV, whereas glucocorticoid, increased neutrophil and PCT were predictive indicators for NIMV. In addition, the above-mentioned mediators had the most predictive meaning for mechanical ventilation when combined.
The reviews of this paper are available via the supplemental material section.
Keywords: coronavirus disease 2019 (COVID-19), mechanical ventilation, predictors, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Introduction
In December 2019, an outbreak of pneumonia caused by a novel coronavirus (officially named as coronavirus disease 2019 (COVID-19)) occurred in Wuhan, Hubei province, China. After the initial outbreak, it has rapidly spread through the world in the following months, creating more than millions of cases and hundreds of thousands of deaths by 25 June 2020. A previously unknown coronavirus (officially named as SARS-CoV-2) was identified from bronchoalveolar lavage fluid (BALF) and reported as the pathogen of the pneumonia on 7 January 2020.1 SARS-CoV-2 belongs to betacoronavirus genus lineage B, which includes severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV).2 SARS-CoV-2 is mainly transmitted through the respiratory tract, close contact and aerosol, while fecal–oral transmission may be a potential way as well.3 The reproductive number of person to person transmission is estimated to be 3.58 in the early stage, revealing it is a highly contagious disease.4
The typical clinical manifestations include fever, cough, fatigue, myalgia and dyspnea. Natural clinical histories have ranged from febrile respiratory symptoms, without hypoxemia, to mechanical ventilation, even organ dysfunction and death. In recent studies, almost 5% of patients with COVID-19 were admitted to the intensive care unit (ICU), and 2.3% of patients needed invasive mechanical ventilation (IMV).5 In another study, 63.2% of patients who were admitted to the ICU required IMV;6 2.3–12.3% of patients required IMV, and even extracorporeal membrane oxygenation (ECMO) afterwards.7 For critical respiratory diseases, mechanical ventilation is an essential and effective treatment method used to create an artificial airway, thus maintaining effective ventilation and helping in the control of broncho-pulmonary infections. Identifying the early predictors for mechanical ventilation on admission becomes critically significant as it could help to decrease the rate of mechanical ventilation and improve patients’ prognosis. Therefore, we conducted retrospective research to predict early indicators for invasive and non-invasive mechanical ventilation (NIMV) among patients with COVID-19.
Methods
Study design and participants
This was a retrospective cohort study among patients with COVID-19 recruited in several isolation wards at the West Court of Union Hospital of Huazhong University from 29 January to 14 March 2020. Diagnosis of COVID-19 was confirmed by positive results on real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR) or genome sequencing of nasopharyngeal swab specimens. Only laboratory-confirmed cases were included in this study. This study was approved by the Ethics Committee of Xiangya Hospital Central South University (Changsha, China, no. 202003049) and Wuhan Union Hospital (Wuhan, China). Written informed consent was waived by the ethics commission of the designated hospital for emerging infectious diseases.
The patient would receive oxygen inhalation with common nasal catheter if one of the following conditions occurred: (a) respiratory frequency ⩾30 times per minute; (b) blood oxygen saturation ⩽93%; (c) ratio of partial arterial oxygen pressure (PaO2)/fractional inspired oxygen (FIO2) ⩽300 mmHg; (d) the radiological image showed the lesion progressed within 24–48 h. If conventional oxygen inhalation failed to improve hypoxemia, the clinical doctors would provide non-invasive or invasive mechanical ventilation. These patients were categorized into three groups according to the oxygen methods during their hospitalization: invasive mechanical ventilation group (IMV group), non-invasive mechanical ventilation group (NIMV group) and non-mechanical ventilation group.
Measurements
The demographic characteristics and clinical features including age, gender, coexisting illnesses, symptoms, treatments and radiological features on admission were collected. The patients’ chest tomography (CT) image were scored by three independent chest radiologists on admission. CT score was assigned on the basis of the area involved (a score of 0–5 for each lobe, with a total possible score of 0–25) as previous studies reported.8,9 The main laboratory parameters on admission included the numbers of leukocyte, lymphocyte, neutrophil and platelet, serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), globulin (GLB), creatinine kinase (CK), lactate dehydrogenase (LDH), C-reactive protein (CRP), procalcitonin (PCT) and D-dimer. Treatment strategies, including antivirus treatment, uses of antibiotics and glucocorticoid, were obtained as well.
Statistical analysis
We compared differences in the demographic information, clinical symptoms, laboratory findings and treatments between patients who received invasive or non-invasive mechanical ventilation and their counterparts without mechanical ventilation. Continuous data were expressed with a median and interquartile range (IQR) and compared by the Mann–Whitney U test as most laboratory data depicted a skewed distribution. Categorical data were expressed as counts and proportions and analyzed with the chi-square test. For calculation of the odds ratio of mechanical ventilation in the logistical regression analysis, each variable was dichotomized using a normal value as ‘cut-off’ (for example, CRP upper limit is 8 mg/L). We used a multivariate stepwise logistic regression model to assess the early predictive models of IMV and NIMV. The receiver operating characteristic curve (ROC curve) was carried out to determine the role of potential predictors for mechanical ventilation among patients with COVID-19. A p-value less than 0.05 (two-tailed) was considered statistically significant. All analysis was carried out using the Statistical Package for the Social Science version 24. The ROC curve was drawn by GraphPad Prism 8.0.1.
Results
Baseline characteristics of the patients with COVID-19
A total of 516 patients with laboratory-confirmed COVID-19 between 29 January and 14 March 2020 were included in the study. Thirty-eight patients received invasive mechanical ventilation (IMV), 32 patients received non-invasive mechanical ventilation (NIMV) and 446 patients did not receive mechanical ventilation. The demographic and clinical characteristics are shown in Table 1. The median age was 61 years old (IQR 52–69). The most common symptoms at the onset of illness were fever (79.46%), dry cough (66.47%), shortness of breath (46.9%) and fatigue (40.11%). Four hundred and thirty-two patients (83.72%) had at least one coexisting illness, including hypertension (33.53%), diabetes (19.38%), cardiovascular disease (13.18%), chronic pulmonary disease (7.56%), cerebral vascular disease (3.88%), chronic liver disease (3.1%) and chronic renal insufficiency (3.1%). Major treatment strategies included the use of arbidol [450 (87.21%)], ribavirin [127 (24.61%)], interferon alpha [100 (19.38%)] and lopinavir/litonavir [97 (18.8%)], antibiotics [383 (74.22%)], glucocorticoid [176 (34.11%)].
Table 1.
Demographic and baseline characteristics of COVID-19 patients.
| Invasive mechanical ventilation (n = 38) | Non-invasive mechanical ventilation (n = 32) | Non-mechanical ventilation group (n = 446) | Total (n = 516) | p*-valuea | p*-valueb | |
|---|---|---|---|---|---|---|
| Age (yr); mean (range) | 67 (59.75–72.50) | 64 (55–72.25) | 61 (51.75–68) | 61 (52–69) | 0.112 | 0.003 |
| >60 yr, n (%) | 23 (60.53) | 24 (75) | 228 (51.12) | 275 (53.29) | 0.265 | 0.009 |
| Male gender, n (%) | 30 (78.95) | 21 (65.63) | 211 (47.31) | 262 (50.78) | <0.0001 | 0.045 |
| Any comorbidity, n (%) | ||||||
| Hypertension | 17 (44.74) | 17 (53.13) | 139 (31.17) | 173 (33.53) | 0.086 | 0.010 |
| Diabetes | 6 (15.79) | 9 (28.13) | 85 (19.06) | 100 (19.38) | 0.621 | 0.213 |
| Chronic pulmonary disease | 6 (15.79) | 5 (15.63) | 28 (6.28) | 39 (7.56) | 0.028 | 0.044 |
| Cardiovascular disease | 8 (21.05) | 3 (9.38) | 57 (12.78) | 68 (13.18) | 0.151 | 0.574 |
| Cerebral vascular disease | 4 (10.53) | 4 (12.5) | 12 (2.69) | 20 (3.88) | 0.010 | 0.003 |
| Chronic liver disease | 2 (5.26) | 1 (3.13) | 13 (2.91) | 16 (3.10) | 0.423 | 0.946 |
| Chronic renal dysfunction | 0 (0) | 2 (6.25) | 16 (3.59) | 16 (3.10) | 0.235 | 0.445 |
| Symptoms, n (%) | ||||||
| Fever | 31 (81.58) | 30 (93.75) | 349 (78.25) | 410 (79.46) | 0.632 | 0.037 |
| Dry cough | 23 (60.53) | 26 (81.25) | 294 (65.92) | 343 (66.47) | 0.502 | 0.075 |
| Sputum | 10 (26.32) | 14 (43.75) | 115 (25.78) | 139 (26.94) | 0.943 | 0.027 |
| Shortness of breath | 16 (42.11) | 17 (53.13) | 209 (46.86) | 242 (46.90) | 0.573 | 0.493 |
| Fatigue | 17 (44.74) | 13 (40.63) | 177 (39.69) | 207 (40.11) | 0.542 | 0.917 |
| Myalgia | 5 (13.16) | 4 (12.5) | 72 (16.14) | 81 (15.70) | 0.629 | 0.586 |
| Chest pain | 0 (0) | 0 (0) | 17 (3.81) | 17 (3.29) | 0.220 | 0.261 |
| Chest tightness | 6 (15.79) | 4 (12.50)_ | 33 (7.40) | 43 (8.33) | 0.068 | 0.297 |
| Diarrhea | 5 (13.16) | 5 (15.63) | 90 (20.18) | 100 (19.38) | 0.296 | 0.533 |
| Headache | 2 (5.26) | 2 (6.25) | 41 (9.19) | 45 (8.72) | 0.414 | 0.574 |
| Sore throat | 4 (10.53) | 1 (3.130 | 14 (3.14) | 19 (3.68) | 0.021 | 0.996 |
| Running nose | 1 (2.26) | 1 (3.13) | 12 (2.69) | 14 (2.71) | 0.983 | 0.884 |
| Treatment, n (%) | ||||||
| Interferon alpha | 12 (31.58) | 10 (31.25) | 78 (17.49) | 100 (19.38) | 0.021 | 0.112 |
| Lopinavir/ritonavir | 11 (28.95) | 10 (31.25) | 76 (17.04) | 97 (18.80) | <0.0001 | 0.071 |
| Ribavirin | 13 (34.21) | 11 (34.38) | 103 (23.09) | 127 (24.61) | <0.0001 | 0.342 |
| Arbidol | 30 (78.95) | 28 (87.50) | 392 (87.89) | 450 (87.21) | 0.042 | 0.847 |
| Other antibiotics | 36 (94.74) | 30 (93.75) | 317 (71.08) | 383 (74.22) | <0.0001 | 0.021 |
| Glucocorticoid | 29 (76.32) | 26 (81.25) | 121 (27.13) | 176 (34.11) | <0.0001 | <0.0001 |
| CT score | 11 (9–13) | 11 (9–13.5) | 10 (8–13) | 10 (9–13) | 0.440 | 0.494 |
IQR, interquartile range; n, numbers.
p-valuesa and p-valuesb comparing invasive mechanical ventilation or non-invasive mechanical ventilation with non-mechanical ventilation group are from χ² test, or Mann–Whitney U test. Continuous variables are expressed as median (IQR) and compared with the Mann–Whitney U test; categorical variables are expressed as number (%) and compared by χ² test between invasive mechanical ventilation and non-mechanical ventilation groups or non-invasive mechanical ventilation with non-mechanical ventilation group.
Compared with the non-mechanical ventilation group, the IMV group were male-predominant (78.95% versus 47.31%, p < 0.0001). However, the average age was not significantly different between these two groups. Patients in the IMV group had a higher ratio of chronic pulmonary disease and cerebral vascular disease. A higher proportion of patients in the IMV group had sore throats than in the non-mechanical ventilation group. The IMV group received more therapeutic medicines including interferon alpha, lopinavir/litonavir, ribavirin, antibiotis and glucocorticoid. However, the CT score between the two groups had no significant difference.
Compared with the non-mechanical ventilation group, the median age of patients in the NIMV group were older (64 versus 61, p = 0.003) and male-predominant as well (65.63% versus 47.31%, p = 0.045).Patients in NIMV had more preexisting illnesses, including hypertension, chronic pulmonary disease and cerebral vascular disease. More patients had symptoms of fever and sputum. Patients in the NIMV group received more antibiotics and glucocorticoid treatment compared to the non-mechanical ventilation group. However, there was no significant difference for CT score on admission between these two groups as well.
Laboratory findings in patients of COVID-19 on admission
The laboratory data on admission are summarized in Table 2. Routine blood examination revealed: lymphopenia (lymphocyte count <1.1 × 109/L) in 51.55%, increased leukocyte (leukocyte count >9.5 × 109/L) in 14.73%, increased neutrophil (neutrophil count >6.3 × 109/L) in 25.78% and thrombocytopenia (platelet count <100 × 109/L) in 5.81%. Biochemical tests showed 60.27% of patients had decreased albumin (<33 g/L). Two hundred and eighty-six patients (55.43%) had elevated C-reactive protein (CRP >8 mg/L). The following enzymes were elevated: ALT and AST were elevated in 171 (33.14%) and 133 patients (25.77%), respectively. CK was elevated in 63 patients (12.2%, CK >200 U/L), and LDH was elevated in 238 patients (46.12%, LDH >250 U/L).
Table 2.
Laboratory findings in 516 patients with COVID-19.*
| At admission, mean (IQR) | Invasive mechanical ventilation (n = 38) | Noninvasive mechanical ventilation (n = 32) | Non-mechanical ventilation group (n = 446) | Total (n = 516) | p-valueaƗ | p-valuebƗ |
|---|---|---|---|---|---|---|
| Leukocyte count, ×109 cells/L | 8.43 (5.2–11.4) | 8.99 (4.76–10.26) | 5.72 (4.45–7.45) | 5.98 (4.54–7.91) | <0.0001 | 0.003 |
| >9.5 × 109 cells/L, n (%) | 16 (42.10) | 12 (37.5) | 48 (10.76) | 76 (14.73) | <0.0001 | <0.0001 |
| RBC, ×1012 cells/L | 4.14 (3.88–4.5) | 4.13 (3.83–4.35) | 4.09 (3.69–4.44) | 4.10 (3.7–4.44) | 0.291 | 0.695 |
| Hemoglobin level, g/L | 130 (117–143) | 129 (117.75–139.25) | 125 (112–136) | 125 (113–136) | 0.071 | 0.103 |
| Neutrophil count, ×109 cells/L | 7.32 (4.49–9.86) | 7.45 (3.76–9.09) | 3.95 (2.88–5.82) | 4.08 (2.91–6.45) | <0.0001 | <0.0001 |
| >6.3 × 109 cells/L, n (%) | 24 (63.16) | 19 (59.38) | 90 (20.18) | 133 (25.78) | <0.0001 | <0.0001 |
| Lymphocyte count, ×109 cells/L | 0.56 (0.4–0.71) | 0.77 (0.51–1.19) | 1.14 (0.81–1.57) | 1.08 (0.72–1.5) | <0.0001 | <0.0001 |
| <1.1 × 109 cells/L, n (%) | 32 (84.21) | 23 (71.88) | 211 (47.31) | 266 (51.55) | <0.0001 | 0.010 |
| HCT | 38.3 (35.8–42.3) | 38 (34.93–40.63) | 37 (33.5–40.1) | 37.2 (33.9–40.3) | 0.075 | 0.196 |
| LYMPHO-P% | 7.6 (5.3–12) | 9.7 (5.63–15.38) | 20.3 (13.6–28.6) | 18.9 (11.5–27.53) | <0.0001 | <0.0001 |
| <20%, n (%) | 34 (89.47) | 26 (81.25) | 213 (47.76) | 273 (52.91) | <0.0001 | <0.0001 |
| NEUT-P% | 87.9 (82.9–90.9) | 82.5 (77.13–88.78) | 70.2 (61.4–79.6) | 71.8 (62.4–82.2) | <0.0001 | <0.0001 |
| >75%, n (%) | 33 (86.84) | 25 (78.13) | 155 (34.75) | 213 (41.28) | <0.0001 | <0.0001 |
| Platelet count, ×109 cells/L | 172 (123–205) | 218.5 (159.5–277.5) | 221 (169–293) | 218 (164–286.25) | <0.0001 | 0.579 |
| <100 × 109 cells/L, n (%) | 5 (13.16) | 2 (6.25) | 23 (5.16) | 30 (5.81) | 0.024 | 0.805 |
| ALB, g/l | 27.6 (26.4–29.2) | 30.7 (24.65–32.1) | 31.4 (27.9–34.8) | 30.95 (27.58–34.4) | <0.0001 | 0.031 |
| <33 g/l, n (%) | 32 (84.21) | 24 (75) | 255 (57.17) | 311 (60.27) | <0.0001 | 0.023 |
| GLB, g/l | 31 (27.2–37.2) | 33.5 (30.2–39.05) | 31.3 (28.5–34.8) | 31.4 (28.48–35.03) | 0.286 | 0.010 |
| >35 g/l, n (%) | 13 (34.21) | 12 (37.5) | 96 (21.52) | 121 (23.45) | 0.018 | 0.027 |
| ALT, U/L | 34 (25–47) | 34.5 (24.5–54) | 30 (20–52) | 31 (21–51.25) | 0.168 | 0.043 |
| >40 U/L, n (%) | 14 (36.84) | 14 (43.75) | 143 (32.06) | 171 (33.14) | 0.406 | 0.129 |
| AST, U/L | 42 (30–51) | 30.5 (25–41) | 28 (21–40) | 29 (22–42) | <0.0001 | 0.010 |
| >40 U/L n (%) | 18 (47.37) | 10 (31.25) | 105 (23.54) | 133 (25.77) | <0.0001 | 0.256 |
| CK, U/L | 130 (55–294) | 79.5 (44–132) | 69 (45–124) | 72 (46–137) | 0.012 | 0.786 |
| >200 U/L, n (%) | 11 (28.95) | 3 (9.38) | 49 (10.98) | 63 (12.2) | <0.0001 | 0.940 |
| LDH, U/L | 488 (256–613) | 363.5 (256–613) | 238 (188–329) | 252.5 (192–359) | <0.0001 | <0.0001 |
| >250 U/L, n (%) | 34 (89.47) | 24 (75) | 180 (40.36) | 238 (46.12) | <0.0001 | <0.0001 |
| CRP, mg/L | 98.82 (58.69–123.32) | 66.05 (17.48–122.16) | 17.24 (3.28–53.31) | 22.97 (3.74–63.65) | <0.0001 | <0.0001 |
| >20 mg/L, n (%) | 30 (78.95) | 23 (71.88) | 180 (40.36) | 233 (45.15) | <0.0001 | <0.0001 |
| PCT, ng/ml | 0.24 (0.15–0.63) | 0.125 (0.07–0.255) | 0.06 (0.05–0.12) | 0.07 (0.05–0.15) | <0.0001 | <0.0001 |
| >0.05 ng/ml, n (%) | 34 (89.47) | 26 (81.25) | 224 (50.22) | 284 (55.04) | <0.0001 | 0.001 |
| D-dimer, μg/ml | 2.29 (0.77–8) | 2.07 (0.41–7.28) | 0.54 (0.25–1.47) | 0.6 (0.27–1.83) | <0.0001 | <0.0001 |
| >0.5 μg/ml, n (%) | 26 (68.42) | 22 (68.75) | 201 (45.07) | 249 (48.26) | <0.0001 | 0.081 |
| SpO2, % | 87 (80–92) | 87 (80–93) | 94 (91–97) | 93 (90–97) | 0.03 | 0.019 |
| <93%, n (%) | 30 (78.94) | 23 (71.88) | 222 (49.78) | 275 (53.29) | <0.0001 | 0.016 |
ALB, albumin; ALT, alanine transaminase; AST, aspartate transaminase; CK, creatine kinase; CRP, C-reactive protein; GLB, globulin; HCT, hematocrit; LDH, lactate dehydrogenase; PaO2, partial arterial oxygen pressure; PCT, procalitonin; RBC, red blood cell.
Data are expressed as the median (range).
Mann–Whitney U test.
Compared with the non-mechanical ventilation group, patients in the IMV group had higher leukocyte and neutrophil counts, lower lymphocyte and platelet counts, higher AST, CK, LDH, CRP, PCT, D-dimer and lower ALB. And in the IMV group, oxygen saturation (SpO2) on admission in 78.94% of patients was lower than 93%. Whereas those in the NIMV group had higher leukocyte and neutrophil counts, lower lymphocyte, higher GLB, ALT and AST, higher LDH, CRP, PCT, D-dimer and lower ALB. SpO2 on admission in 71.88% of patients was lower than 93%.
Predictive factors for mechanical ventilation
The univariate and multivariate analysis for predictive indicators for mechanical ventilation among patients with COVID-19 are summarized in Table 3. A logistic regression model was conducted to reveal the potential indicators for invasive and non-invasive mechanical ventilation in COVID-19 patients. In the univariate analysis, the following risk factors, including male gender, chronic pulmonary disease, cerebral vascular disease, sore throat, interferon alpha, lopinavir/litonavir, the use of antibiotics, glucocorticoid, increased leukocyte count, neutrophil count, GLB, AST, CK, LDH, CRP, PCT, D-dimer and decreased lymphocyte count, platelet count, ALB, SpO2, were found to be associated with invasive mechanical ventilation. In the NIMV group, the following risk factors, including age >60 years, male gender, hypertension, cerebral vascular disease, sputum, lopinavir/litonavir, the use of antibiotics, glucocorticoid, increased leukocyte count, neutrophil count, GLB, LDH, CRP, PCT and decreased lymphocyte count, ALB, SpO2, were found to be associated with non-invasive mechanical ventilation.
Table 3.
Predictors of mechanical ventilation in COVID-19 patients.£
| Univariate analysis | Invasive mechanical ventilation |
Univariate analysis |
Non-invasive mechanical ventilation |
||||
|---|---|---|---|---|---|---|---|
| OR | p-value | Odds ratio (95% CI) | OR | p-value | Odds ratio (95% CI) | ||
| Age >60 yr | 1.466 | 0.268 | 0.745–2.884 | Age >60 yr | 2.868 | 0.012 | 1.262–6.522 |
| Male | 4.177 | <0.0001 | 1.873–9.311 | Male | 2.126 | 0.050 | 1.002–4.514 |
| Hypertension | 1.788 | 0.089 | 0.915–3.494 | Hypertension | 2.503 | 0.013 | 1.215–5.156 |
| Chronic pulmonary disease | 2.799 | 0.034 | 1.080–7.254 | Chronic pulmonary disease | 2.765 | 0.053 | 0.989–7.729 |
| Cerebral vascular disease | 4.255 | 0.017 | 1.302–13.905 | Cerebral vascular disease | 5.167 | 0.007 | 1.565–17.058 |
| Fever | 1.231 | 0.632 | 0.526–2.881 | Fever | 4.169 | 0.053 | 0.979–17.754 |
| Sputum | 1.028 | 0.943 | 0.484–2.182 | Sputum | 2.239 | 0.030 | 1.079–4.645 |
| Sore throat | 3.630 | 0.030 | 1.133–11.635 | Sore throat | 0.995 | 0.996 | 0.127–7.819 |
| Fatigue | 1.230 | 0.543 | 0.631–2.397 | Fatigue | 1.040 | 0.917 | 0.501–2.159 |
| Interferon alpha | 2.314 | 0.025 | 1.110–4.826 | Interferon alpha | 2.204 | 0.050 | 0.998–4.865 |
| Lopinavir/ritonavir | 2.297 | 0.032 | 1.074–4.911 | Lopinavir/litonavir | 2.287 | 0.041 | 1.035–5.052 |
| Ribavirin | 1.962 | 0.067 | 0.955–4.032 | Ribavirin | 1.739 | 0.155 | 0.812–3.727 |
| Arbidol | 0.638 | 0.340 | 0.253–1.608 | Abidol | 0.893 | 0.838 | 0.301–2.651 |
| Other antibiotics | 7.211 | 0.007 | 1.711–30.397 | Other antibiotics | 6.009 | 0.015 | 1.415–25.519 |
| Glucocorticoid | 12.702 | <0.0001 | 12.702–5.146 | Glucocorticoid | 11.388 | <0.0001 | 4.575–28.350 |
| Leukocyte count >9.5 × 109 cells/L | 6.860 | <0.0001 | 3.307–14.227 | Leukocyte count >9.5 × 109 cells/L | 4.888 | <0.0001 | 2.250–10.619 |
| Lymphocyte count <1.1 × 109 cells/L | 8.569 | <0.0001 | 2.980–24.639 | Lymphocyte count <1.1 × 109 cells/L | 2.737 | 0.013 | 1.238–6.050 |
| Neutrophil count >6.3 × 109 cells/L | 9.280 | <0.0001 | 4.283–20.108 | Neutrophil count >6.3 × 109 cells/L | 5.651 | <0.0001 | 2.689–11.875 |
| LYMPHO-P% <20% | 35.756 | <0.0001 | 4.852–263.517 | LYMPHO-P% <20% | 4.557 | 0.001 | 1.839–11.291 |
| NEUT-P% >75% | 30.126 | <0.0001 | 7.133–127.236 | NEUT-P% >75% | 6.521 | <0.0001 | 2.757–15.420 |
| Platelet count <100 × 109 cells/L | 3.118 | 0.032 | 1.105–8.803 | Platelet count <100 × 109 cells/L | 1.206 | 0.806 | 0.271–5.359 |
| ALB <33 g/l | 7.404 | 0.001 | 2.233–24.554 | ALB <33 g/l | 2.776 | 0.029 | 1.112–6.932 |
| GLB >35 g/l | 2.388 | 0.021 | 1.138–5.010 | GLB >35 g/l | 2.326 | 0.030 | 1.083–4.999 |
| AST >40 U/L | 3.546 | <0.0001 | 1.747–7.201 | AST >40 U/L | 1.576 | 0.259 | 0.715–3.474 |
| CK >200 U/L | 4.894 | <0.0001 | 2.126–11.268 | CK >200 U/L | 0.953 | 0.940 | 0.274–3.316 |
| LDH >250 U/L | 16.685 | <0.0001 | 5.048–55.150 | LDH >250 U/L | 4.417 | <0.0001 | 1.941–10.051 |
| CRP >20 mg/L | 35.500 | <0.0001 | 4.794–262.894 | CRP >20 mg/L | 4.536 | 0.001 | 1.807–11.384 |
| PCT >0.05 ng/ml | 10.777 | 0.001 | 2.549–45.554 | PCT >0.05 ng/ml | 8.241 | 0.004 | 1.926–35.256 |
| D-dimer >0.5 μg/ml | 5.821 | 0.001 | 1.993–16.999 | D-dimer >0.5 μg/ml | 1.970 | 0.086 | 0.908–4.272 |
| SpO2 <93% | 2.579 | 0.019 | 1.167–5.697 | SpO2 <93% | 4.324 | 0.001 | 1.861–10.051 |
| Multivariate analysis | |||||||
| Glucocorticoid | 5.600 | <0.0001 | 2.133–14.704 | Glucocorticoid | 14.982 | <0.0001 | 4.317–51.991 |
| Neutrophil count >6.3 × 109 cells/L | 4.429 | 0.001 | 1.909–10.275 | Neutrophil count >6.3 × 109 cells/L | 4.251 | 0.001 | 1.754–10.304 |
| LDH >250 U/L | 7.343 | 0.002 | 2.114–25.506 | PCT > 0.05ng/ml | 7.220 | 0.010 | 1.596–32.661 |
Used the logistic regression analysis.
CI, confidence interquartile; OR: odds ratio.
Then, the multivariate stepwise logistic regression model was carried out to avoid potential mediators and reveal the early predictors for mechanical ventilation in COVID-19 patients. The results showed that the use of glucocorticoid, neutrophil count >6.3 × 109 cells/L and LDH >250 U/L were the early predictive indicators of invasive mechanical ventilation. Similarly, glucocorticoid, neutrophil count >6.3 × 109 cells/L and PCT >0.05 ng/ml were found to be potential indicators for non-invasive mechanical ventilation.
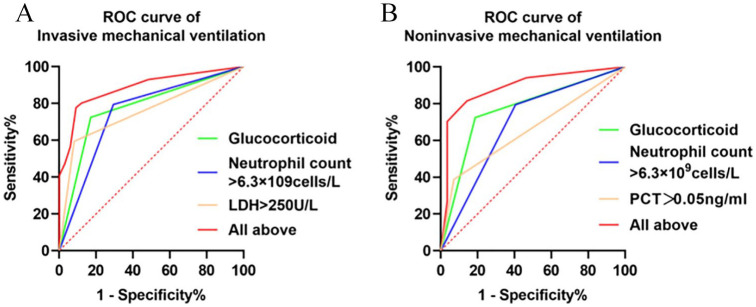
In order further to confirm the role of the afore-mentioned covariates for the predictive ability of mechanical ventilation among patients with COVID-19, ROC curve analysis was conducted (Table 4, Figure 1). The area under the curve (AUC) of glucocorticoid, neutrophil count >6.3 × 109 cells/L and LDH >250 U/L for IMV was 0.885 (95% CI 0.838–0.933, p < 0.0001), which provided the specificity and sensitivity of 77.7% and 90.9%, respectively. The AUC of glucocorticoid, neutrophil count >6.3 × 109 cells/L and PCT >0.05 ng/ml for NIMV was 0.888 (95% CI 0.825–0.952, p < 0.0001), which provided the specificity and sensitivity of 70.3% and 96.4%, respectively.
Table 4.
The receiver operating characteristic curve (ROC curve) data.
| Invasive mechanical ventilation |
Non-invasive mechanical ventilation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | AUC | 95 CI% | p-value | Sensitivity, % | Specificity, % | Factors | AUC | 95 CI% | p-value | Sensitivity, % | Specificity, % |
| Glucocorticoid | 0.776 | 0.700–0.853 | <0.0001 | 82.9 | 72.4 | Glucocorticoid | 0.768 | 0.686–0.851 | <0.0001 | 81.3 | 72.4 |
| Neutrophil count >6.3 × 109 cells/L | 0.750 | 0.659–0.842 | <0.0001 | 70.6 | 79.5 | Neutrophil count >6.3 × 109 cells/L | 0.694 | 0.591–0.797 | <0.0001 | 59.4 | 79.5 |
| LDH >250 U/L | 0.757 | 0.693–0.823 | <0.0001 | 91.9 | 59.6 | PCT >0.05 ng/ml | 0.658 | 0.572–0.745 | 0.005 | 92.9 | 38.8 |
| All above | 0.885 | 0.838–0.933 | <0.0001 | 90.9 | 77.7 | All above | 0.888 | 0.825–0.952 | <0.0001 | 96.4 | 70.3 |
AUC, area under the curve; CI, confidence interquartile; LDH, lactate dehydrogenase; OR: odds ratio; PCT, procalitonin.
Figure 1.
The receiver operating characteristic curve (ROC curve).
Discussion
In this retrospective study, the demographic, clinical and laboratory characteristics were compared between patients with invasive mechanical ventilation or non-invasive mechanical ventilation and non-mechanical ventilation among patients with COVID-19. A multivariate stepwise logistic regression model was conducted and the results showed that the use of glucocorticoid, increased neutrophil count and LDH level were effective predictors for invasive mechanical ventilation. Similarly, the use of glucocorticoid, increased neutrophil count and PCT level were predictive indicators for non-invasive mechanical ventilation among patients with COVID-19.
In the current study, the demographic and clinical manifestations are similar to previous studies.5,10,11 Age was associated with severity and prognosis among patients with COVID-19. A recent study that enrolled 72,314 patients in China revealed that patients aged 70–79 years had an 8% case fatality rate, while those aged 80 years or older had a 14.8% fatality rate.12 In our study, patients who received mechanical ventilation (IMV and NIMV) were older than those patients without mechanical ventilation. In addition, the results also showed that men might be more susceptible to receive IMV and NIMV than women. Sex may influence the infectious severity of SARS-CoV-2, as the X-chromosome contains a higher density of immune-related genes and regulatory elements that refer to inherent and adaptive immunity.13 Sex hormones and sex-associated immune activity could influence immunity,14 which may be one of the possible reasons that women seemed to be less susceptible to viral infection or the infection was milder than it was in men, if infected. A higher smoking rate in men may be another factor, based on a previous study showing that cigarette smoke caused a dose-dependent upregulation of angiotensin-converting enzyme 2 (ACE2), the SARS-CoV-2 receptor, in rodent and human lungs.15 However, this piece of information was not available in our study. Chronic co-existing comorbidities were reported to be associated with the severity and prognosis of COVID-19. A study revealed that two or more comorbidities led to a five-fold times increase of the death rate.16 Our previous study found that diabetes was independently associated with severity and prognosis of COVID-19.17 Here, we also found that patients with hypertension, chronic pulmonary disease and cerebralvascular disease are more likely to require mechanical ventilation during hospitalization. Physicians should pay more attention to the patients combined with those comorbidities. Moreover, some evaluation system of illness severity such as acute physiology and chronic health evaluation scoring systems (APACHE II score) and the high sequential organ failure assessment (SOFA) score were demonstrated to be significantly higher in severe COVID-19 patients than non-severe patients.18 The SOFA score at admission was reported to be an independent predictor of developing severe SARS-CoV-2 infection.18 Another study reported that the quick SOFA score was significantly different between the mechanical ventilation group and the non-mechanical ventilation group.19 It is a limitation that these evaluation data were not available in this study.
For the laboratory findings, a total of 51.55% of patients in the study had lymphopenia, which was similar to previous studies as well.5,10,11 There was a study showing that lymphopenia was a predictor of severe COVID-19.20 SARS-CoV particles targeted lymphocytes and destroyed its cytoplasmic components, thus causing a reduction of T cells.21 Another study revealed CD8+T cells decreased more significantly than CD4+T cells among patients with COVID-19.22 However, the specific mechanism of lymphopenia still remained unclear. Patients who required mechanical ventilation had higher ALT and AST in the study, suggesting more severe liver damage among COVID-19 patients with mechanical ventilation. It was estimated that SARS-CoV-2 could directly attack ACE2 positive biliary epithelial cells, and cause liver injury.23 In addition, hypoxia, micro-thrombus and drugs might be also be possible contributors to liver damage. A study showed that CRP was an independent predictor of severe COVID-19,24 while in this study, it was dropped out during stepwise regression analysis. There was no significant difference in the CT scores on admission between the patients who required mechanical ventilation and those who did not in this study, which may be explained by the following reasons: most of the patients included in the study were divided into severe to critical COVID-19 patients, and their CT images on admission were presented with infection in multiple lobes. CT scoring can only evaluate the area and size of lesions involved, but the evaluation of the density of lesions is flawed, which is a limitation of this scoring system. In mechanical ventilation groups, there was a higher proportion of patients with SpO2 lower than 93%, demonstrating that patients requiring mechanical ventilation had worse oxygenation. A study showed that the oxygen saturation was significantly lower in severe patients than non-severe patients.25
In the patients with IMV and NIMV, higher leukocyte, neutrophil counts but lower lymphocyte counts were detected. Previous studies showed patients infected with the virus commonly had normal or decreased leukocyte and neutrophil counts. However, many patients infected with SARS-CoV-2 had increased leukocyte and neutrophil counts, which were even demonstrated to be associated with severe or critical COVID-19.26–28 It was estimated to be evolved with the susceptibility of severe or critical patients infected with other pathogens such as bacteria and fungi. ACE2 was reported to be the entry receptor of SARS-CoV-2 and expressed on epithelial cells in lungs, kidneys, heart and intestines.29 Neutrophil is the first line of innate immunity, against exogenous microbial agents, and the dynamic variation of pulmonary ACE2 is associated with neutrophil infiltration.30,31 ACE2 is reduced along with bacterial infection, which could help to recruit neutrophils into lung lobes. The infiltration of neutrophils, degranulation and release of neutrophil extracellular traps (NETs) could induce accumulation of cytokines and chemokines, resulting in cytokine storm and acute respiratory distress syndrome.30,31 Recovery of ACE2 could restrict neutrophil infiltration and activity by limiting interleukin 17 (IL-17) signaling through reducing the activity of the STAT3 pathway.30,31 In the study, the patients that required mechanical ventilation had a higher neutrophil count than those patients without mechanical ventilation. Moreover, increased neutrophil count was predicted as a potential indicator in the models of IMV and NIMV by multivariate stepwise logistic regression analysis. The specific mechanism of the association between ACE2 and neutrophils in SARS-CoV-2 infection would require further investigation.
LDH is a cytoplasmic glycolytic enzyme expressed in all tissues, and its elevation suggests tissue damage, especially liver and heart damage. Increased LDH was observed in patients with SARS-CoV infection,32 and predicted as an indicator for severity and prognosis in COVID-19.33 In the study, the elevated LDH was an effective predictor of invasive mechanical ventilation among patients with COVID-19. PCT is released by bacterial infectious tissue under the irritation of pro-inflammatory cytokines. Secondary infection of bacterium following viral infection could lead to the elevation of PCT.34 Higher PCT implies a more severe condition of co-infection in COVID-19 patients. In this study, we found patients who received mechanical ventilation had higher PCT. Moreover, it was an independent predictor of non-invasive mechanical ventilation.
In addition, the application of glucocorticoid also differed between the COVID-19 patients with mechanical ventilation (IMV and NIMV) and those patients without non-mechanical ventilation. According to the diagnostic and treatment guideline (version 6) by the National Health Committee of China,35 glucocorticoid can be administered in a short time for patients with progressive deterioration of oxygenation indexes, rapid imaging progress and excessive activation of inflammatory response. The use of glucocorticoid among patients with COVID-19 is controversial. Russell and colleagues36 believed that glucocorticoid could not only suppress inflammatory procedure but also inhibit immune activity and viral clearance. Furthermore, while other research about SARS revealed that the combined use of interferon alfacon-1 and corticosteroids could be associated with reduced disease-associated impaired oxygen saturation, more rapid resolution of radiographic lung abnormalities, and lower levels of creatine kinase.37 Whether glucocorticoid has had benefits in the treatment of a SARS-CoV-2 infection needs to be further investigated.
Limitations
Several limitations cannot be ignored within the current study. Firstly, due to its nature as a retrospective study and the inclusion of mostly severe and critical subtype patients, a Berkson bias might be introduced because asymptomatic patients and those with mild symptoms were less likely to be enrolled. Secondly, the sample sizes in invasive mechanical ventilation and non-invasive mechanical ventilation were relatively small, thus the validity of predictors derived from our cohort requires further verification in a future study with larger sample size. Thirdly, as the data were collected based on electronic medical records, some severity scores such as APACHE, SOFA, PaO2/FiO2 ratio, arterial blood gas analysis results were not available to be analyzed and support a better conclusion.
Conclusion
In conclusion, the use of glucocorticoid, increased neutrophil and LDH were effective predictive indicators for invasive mechanical ventilation, whereas glucocorticoid, increased neutrophil and PCT were predictive indicators for non-invasive mechanical ventilation. In addition, the combined above mediators had the most predictive meaning for mechanical ventilation. Clinical physicians should pay more attention to those patients with high risks of mechanical ventilation and allocate medical sources reasonably, thus reducing the rate of mechanical ventilation and mortality during hospitalization.
Supplemental Material
Supplemental material, Author_Response for Early predictors for mechanical ventilation in COVID-19 patients by Wen Li, Fengyu Lin, Minhui Dai, Lingli Chen, Duoduo Han, Yanhui Cui and Pinhua Pan in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Early predictors for mechanical ventilation in COVID-19 patients by Wen Li, Fengyu Lin, Minhui Dai, Lingli Chen, Duoduo Han, Yanhui Cui and Pinhua Pan in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Early predictors for mechanical ventilation in COVID-19 patients by Wen Li, Fengyu Lin, Minhui Dai, Lingli Chen, Duoduo Han, Yanhui Cui and Pinhua Pan in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors thank all the patients involved in the study. They thank all the medical staff who are fighting against this public crisis. They thank Sha Li, Ben Liu, Hongyi Tan for analyzing the radiological image data. They also thank Abira Afzal Choudhry and Yan Zhang for proofreading the paper.
Footnotes
Author contribution(s): Wen Li: Conceptualization; Data curation; Methodology; Project administration; Writing-original draft.
Fengyu Lin: Conceptualization; Data curation; Formal analysis; Project administration; Software; Writing-review & editing.
Minhui Dai: Data curation; Methodology; Resources; Writing-review & editing.
Lingli Chen: Data curation; Methodology; Writing-review & editing.
Duoduo Han: Data curation; Methodology; Writing-review & editing.
Yanhui Cui: Funding acquisition; Methodology; Resources; Writing-review & editing.
Pinhua Pan: Funding acquisition; Methodology; Resources; Writing-review & editing.
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the Emergency Project of Prevention and Control for COVID-19 of Central South University (grant no. 160260004); the Project of Special Program on COVID-19 of Changsha Technology Hall (no. kq2001049); the National Key R&D Program of China (2018YFC1311900).
Research ethics and patient consent: This study was approved by the ethics committee of Xiangya Hospital Central South University (Changsha, China, no. 202003049) and Wuhan Union Hospital (Wuhan, China). Written informed consent was waived by the ethics commission of the designated hospital for emerging infectious diseases.
ORCID iD: Wen Li  https://orcid.org/0000-0002-5985-8285
https://orcid.org/0000-0002-5985-8285
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Wen Li, The Department of Respiratory and Critical Care Medicine, Xiangya Hospital Central South University, Changsha, Hunan, China.
Fengyu Lin, The Department of Respiratory and Critical Care Medicine, Xiangya Hospital Central South University, Changsha, Hunan, China.
Minhui Dai, The Department of Respiratory and Critical Care Medicine, Xiangya Hospital Central South University, Changsha, Hunan, China.
Lingli Chen, The Department of Respiratory and Critical Care Medicine, Xiangya Hospital Central South University, Changsha, Hunan, China.
Duoduo Han, The Department of Respiratory and Critical Care Medicine, Xiangya Hospital Central South University, Changsha, Hunan, China.
Yanhui Cui, The Department of Respiratory and Critical Care Medicine, Xiangya Hospital Central South University, Changsha, Hunan 410008, China.
Pinhua Pan, The Department of Respiratory and Critical Care Medicine, Xiangya Hospital Central South University, Changsha, Hunan 410008, China.
References
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen L, Liu W, Zhang Q, et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect 2020; 9: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen TM, Rui J, Wang QP, et al. A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect Dis Poverty 2020; 9: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitra AR, Fergusson NA, Lloyd-Smith E, et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series. CMAJ 2020; 192: E694–E701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang YC, Yu CJ, Chang SC, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology 2005; 236: 1067–1075. [DOI] [PubMed] [Google Scholar]
- 9. Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. Epub ahead of print 13 February 2020. DOI: 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med 2020; 382: 2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu ZY, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020; 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 13. Schurz H, Salie M, Tromp G, et al. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics 2019; 13: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vemuri R, Sylvia KE, Klein SL, et al. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol 2019; 41: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith JC, Sausville EL, Girish V, et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev Cell 2020; 53: 514–529.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369: m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Y, Cui YH, Shen MX, et al. Association of diabetes mellitus with disease severity and prognosis in COVID-19: a retrospective cohort study. Diabetes Res Clin Pract 2020; 165: 108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao QC, Wang P, Wang XG, et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med 2020; 130: 390–399. [DOI] [PubMed] [Google Scholar]
- 19. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020; 146: 136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med 2005; 202: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang M, Guo Y, Luo Q, et al. T cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of COVID-19. J Infect Dis 2020; 222: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morgan K, Samuel K, Vandeputte M, et al. SARS-CoV-2 infection and the liver. Pathogens 2020; 9: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo XM, Zhou W, Yan XJ, et al. Prognostic value of C-reactive protein in patients with COVID-19. Clin Infect Dis. Epub ahead of print 23 May 2020. DOI: 10.1093/cid/ciaa641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cao ZH, Li TZ, Liang LC, et al. Clinical characteristics of Coronavirus Disease 2019 patients in Beijing, China. PLoS One 2020; 15: e0234764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burian E, Jungmann F, Kaissis GA, et al. Intensive care risk estimation in COVID-19 pneumonia based on clinical and imaging parameters: experiences from the Munich cohort. J Clin Med 2020; 9: 1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang JJY, Lee KS, Ang LW, et al. Risk factors of severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis and meta-regression analysis. Clin Infect Dis. Epub ahead of print 14 May 2020. DOI: 10.1093/cid/ciaa576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi R, Shan C, Duan XM, et al. A human neutralizing antibody targets the receptor binding site of SARS-CoV-2. Nature 2020; 584: 120–124. [DOI] [PubMed] [Google Scholar]
- 30. Sodhi CP, Nguyen J, Yamaguchi Y, et al. A dynamic variation of pulmonary ACE2 is required to modulate neutrophilic inflammation in response to Pseudomonas aeruginosa lung infection in mice. J Immunol 2019; 203: 3000–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tomar B, Anders HJ, Desai J, et al. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells 2020; 9: 1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003; 289: 2801–2809. [DOI] [PubMed] [Google Scholar]
- 33. Poggiali E, Zaino D, Immovilli P, et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clin Chim Acta 2020; 509: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. An PJ, Zhu YZ, Yang LP. Biochemical indicators of coronavirus disease 2019 exacerbation and the clinical implications. Pharmacol Res 2020; 159: 104946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Health Commission, National Administration of Traditional Chinese Medicine.Novel coronavirus pneumonia diagnosis and treatment plan (trial version sixth)[J/OL].http://libdb.csu.edu.cn:80/rwt/CNKI/https/NNYHGLUDN3WXTLUPMW4A/kcms/detail/detail.aspx?FileName=ZRYX202002001&DbName=DKFX2020 (published 2nd March 2020). [Google Scholar]
- 36. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020; 395: 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loutfy MR, Blatt LM, Siminovitch KA, et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA 2003; 290: 3222–3228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response for Early predictors for mechanical ventilation in COVID-19 patients by Wen Li, Fengyu Lin, Minhui Dai, Lingli Chen, Duoduo Han, Yanhui Cui and Pinhua Pan in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Early predictors for mechanical ventilation in COVID-19 patients by Wen Li, Fengyu Lin, Minhui Dai, Lingli Chen, Duoduo Han, Yanhui Cui and Pinhua Pan in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Early predictors for mechanical ventilation in COVID-19 patients by Wen Li, Fengyu Lin, Minhui Dai, Lingli Chen, Duoduo Han, Yanhui Cui and Pinhua Pan in Therapeutic Advances in Respiratory Disease