Abstract
Background
Ankylosing spondylitis (AS) is a disease that causes pathological changes in the spine and sacroiliac joints. Numerous studies have shown that the characteristics of AS differ between males and females. The purpose of this study was to discover the key molecules that contribute to sex-associated differences in AS, which may provide a new molecular target for personalized treatment.
Material/Methods
The gene expression profile of GSE39340 was downloaded from the Gene Expression Comprehensive database, and 2 groups (AS vs. No-AS groups and male AS vs. female AS groups) of differentially expressed genes (EDGs) were obtained by GEO2R. The DAVID database was used for DEGs function and enrichment analysis. Based on data in the STRING online database, a protein–protein interaction (PPI) network was constructed in Cytoscape. Hub genes were selected from CytoHubba. With the intersection of the top 30 hub genes of 2 sets of EDGs, genes coexisting with the KEGG-related pathway were found.
Results
We screened 560 genes between the AS and No-AS groups, and screened 710 genes that were differentially expressed between the male and female AS groups. GO analysis showed that DEGs were mainly co-enriched in molecular functions, including structural constituent of muscle. The KEGG pathway mainly included the structural constituent of muscle. Seven hub genes were obtained. Troponin C2 and fast skeletal type (TNNC2) were the key genes participating in the calcium signaling pathway.
Conclusions
This study contributes to understanding the molecular biological mechanism underlying sex-associated differences in AS. TNNC2 and calcium signaling pathway may be new targets for the individualized treatment of AS.
MeSH Keywords: Neuroimmunomodulation, Sex Characteristics, Signal Transduction
Background
Ankylosing spondylitis (AS) is a chronic, inflammatory, immunological disease that mainly affects the axial joints, including the sacroiliac joints and spine. The disease can lead to chronic pain and progressive damage to the joints. In severe cases, joint stiffness can develop, which ultimately affects patients’ quality of life [1–4]. There are many studies on the overall pathogenesis of AS involving heredity, environment, intestinal diseases, and intracellular environmental barriers [2,5–8]. Genetic factors are generally considered to be the most influential factors, including major histocompatibility complex class I allele human leucocyte antigen B27 (HLA-B27), endoplasmic reticulum aminopeptidase 1 (ERAP1), and the interleukin (IL)-17/IL-23 pathway [2,7,9–11]. Some studies have shown that 90–95% of AS patients are HLA-B27-positive, but only 1%–2% of HLA-B27-positive people have AS [9,12]. The involvement of the ERAP1 and IL-17/IL-23 pathways has also been suggested [13,14]. AS appears to be caused by the abnormal expression of various genes, but the underlying mechanism has not yet been fully elucidated.
Some researchers have shown that OA is most common in young males [2,15]. Qian et al. found that the male-female ratio of patients with AS was 2.7: 1, and men were more likely to be hla-b27 carriers than women, with a higher level of c-reactive protein (CRP). Other researchers have suggested that the incidence of spinal cord changes is higher in male patients with AS, and their radiological progression is faster, while women may have more peripheral arthritis [16–19]. In addition, the effect of drugs on the treatment of AS is sex-dependent [19–21]. The differences in clinical characteristics and therapeutic effects of AS in males and females have been confirmed [15,17–21]. Moreover, there is no obvious evidence of sex hormones as a trigger [20,22–24].
To the best of our knowledge, there has been no published research on differentially expressed genes (DEGs) in males and females with AS. Investigation of key genes contributed to the exploration of the different characteristics of AS between the sexes. We used multiple bioinformatics methods to analyze the data in GSE39340. The purpose of the study was to discover the key molecules that cause sex-associated differences in patients with AS, which may improve understanding of the biological characteristics of AS and provide new molecular targets for personalized treatment.
Material and Methods
Microarray data
Data (GSE39340) were obtained from GPL10558 (Illumina humanht-12 V4.0 expression beadchip), including 5 AS samples (3 males, 2 females), 7 osteoarthritis samples, and 10 rheumatoid arthritis samples. All samples were divided into 2 groups: an AS group and a No-AS group. AS patients were further divided into 2 groups: a male AS (M-AS) group and a female AS (F-AS) group. The data were analyzed and compared as described below. This research was based on the data in an open database; therefore, ethics requirements and patient consent were not required.
Data processing
GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/) is an online analysis tool used to identify DEGs [25]. We used GEO2R to identify DEGs between the AS and No-AS groups, and DEGs between M-AS patients and F-AS patients. The results were saved in the file format, and the truncation condition of DEGs was set as: adj. P<0.05, and |log (fold change) |>2.
GO and KEGG enrichment analysis
The DEGs of the 2 groups were analyzed in the DAVID database (DAVID version: 6.8) for GO and KEGG enrichment [26]. The first 10 analysis results were illustrated using WPS Office (version: 2009 11.1.0.9339).
PPI network construction
We integrated the DEGs into the PPI network separately, and used STRING [27] (version 11.0) to evaluate the interaction between DEGs. A composite score of >0.4 was considered to be a statistically significant interaction. The results of PPI network analysis were loaded into Cytoscape (version: 3.6.1) software for visual adjustment.
Identification of hub genes
Cellhubba [28] (version 0.1) is a plug-in in Cytoscape software used to identify hub genes from PPI networks. The first 30 hub genes of the 2 PPI networks were intersected using a Venn diagram to obtain the key genes, and the pathways related to the key genes were searched and analyzed.
Results
Identifying and assessing differentially expressed genes
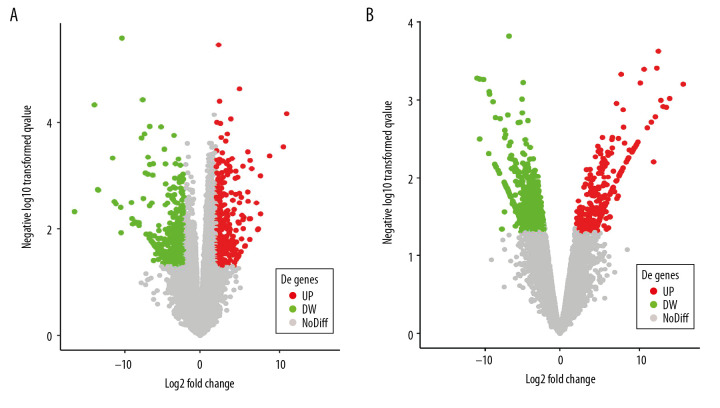
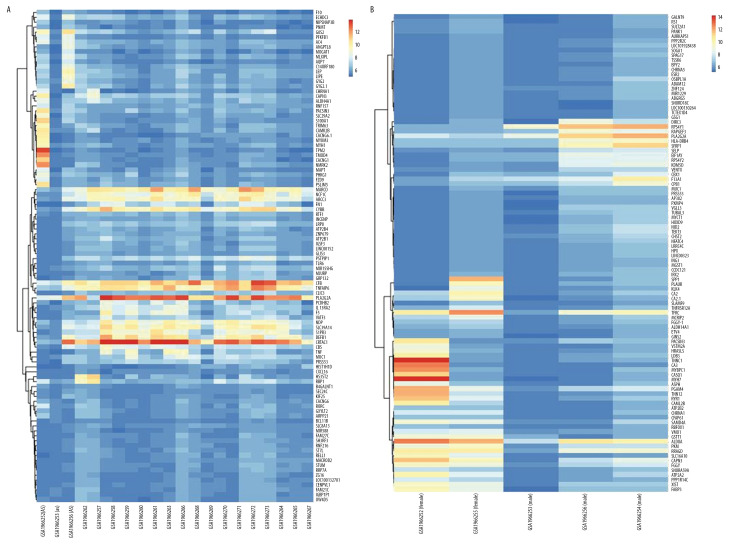
According to GEO2R screening analysis, there were 699 DEGs (332 upregulated genes and 367 downregulated genes) between the AS group and the No-AS group, and 710 DEGs (264 upregulated genes and 446 downregulated genes) between the M-AS group and the F-AS group. Volcano maps and heat maps showed significant genetic differences between the groups (Figures 1A, 1B, 2A, 2B).
Figure 1.
Active volcano map of DEGs, screening criteria: P<0.05 and |logFC|>2. The red dots represent upregulated genes and the green dots represent downregulated genes. (A) Volcano map of DEGs between AS and No-AS groups; (B) Volcano map of DEGs between male and female AS groups.
Figure 2.
Heat map of the top 100 DEGs (50 upregulated and 50 downregulated). Red is the upregulated gene, and blue is the downregulated gene. (A) DEGs heat map between AS and No-AS groups. (B) DEGs heat map between male and female AS groups.
GO functional enrichment analysis
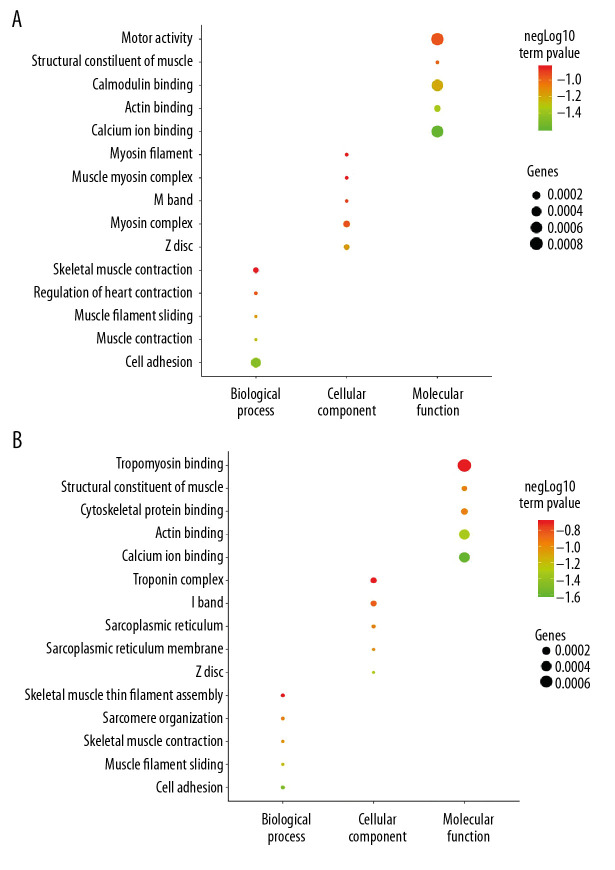
The DAVID database was used to carry out the GO enrichment analysis of DEGs. Two groups of DEGs were enriched with GO function, as shown in Figure 3. The results showed that 2 groups of DEGs had some commonality in GO enrichment. In the biological process, DEGs of the 2 groups mainly concentrated on the following processes: muscle filament sliding, skeletal muscle contraction, and cell adhesion. In terms of cell components, the 2 groups mainly share Z disc. In terms of molecular function, the 2 groups mainly share structural constituent of muscle, actin binding, and calcium ion binding. Figure 3 shows that EDGs of the 2 groups tended to converge on the molecular functions (Figure 3).
Figure 3.
GO enrichment analysis of DEGs between AS and No-AS groups. The function of DEGs in tissues is described according to its GO characteristics (biological process, molecular function, cell components). The top 5 items for each category are shown in the figure. The color of the dot represents the -log10 (p-value) of the item. The size of the dot represents the number of genes with the given GO annotation. (A) AS and No-AS groups, (B) M-AS and F-AS groups.
KEGG pathway enrichment analysis
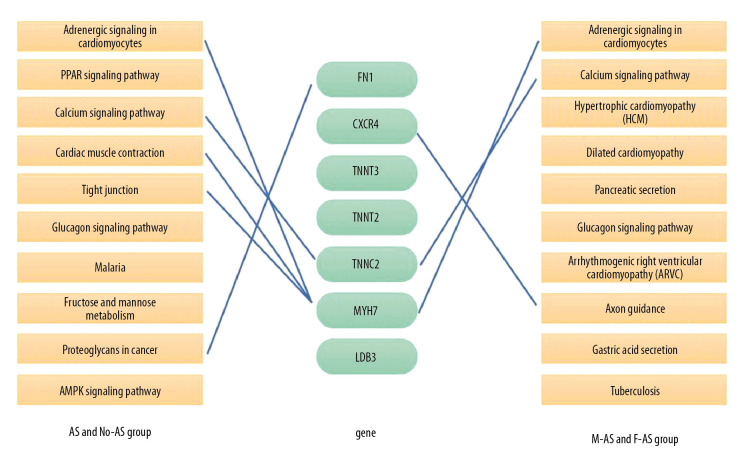
KEGG analysis was performed separately and the analysis of pathways of the top 10 KEGG genes in the 2 groups were visually displayed (P<0.05) (Figure 4A, 4B). The mutual enrichment pathways in the 2 groups were: hypertrophic cardiomyopathy (HCM), adrenergic signaling in cardiomyocytes, glucagon signaling pathway, dilated cardiomyopathy, and calcium signaling pathway.
Figure 4.
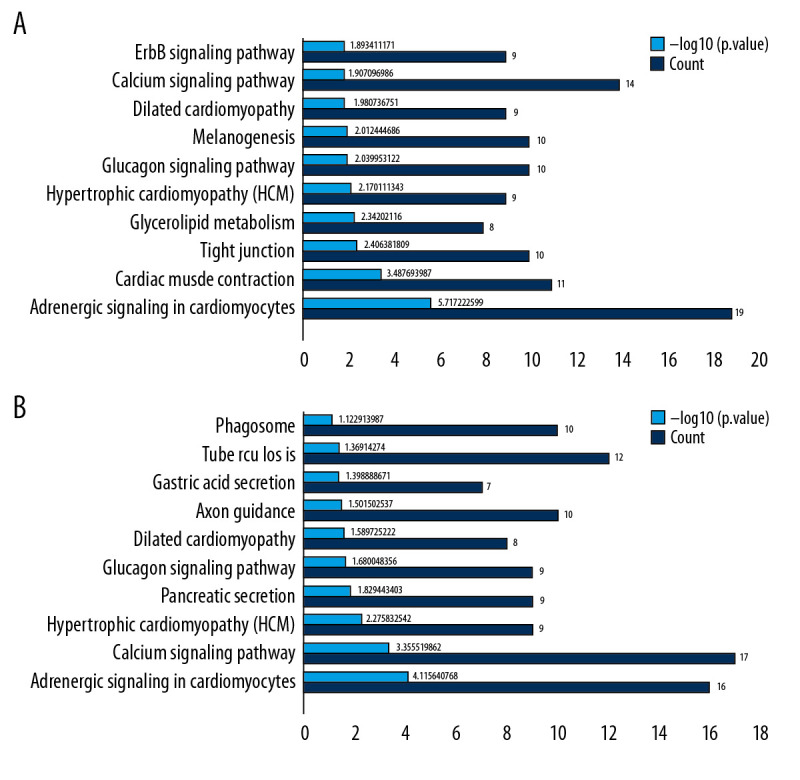
KEGG pathway analysis of DEGs. Ten most important pathways are shown, with yellow column representing -log10 (p-value) and green representing the number of proteins involved in the pathway. (A) AS group and No-AS group; (B) M-AS group and F-AS group.
PPI network construction
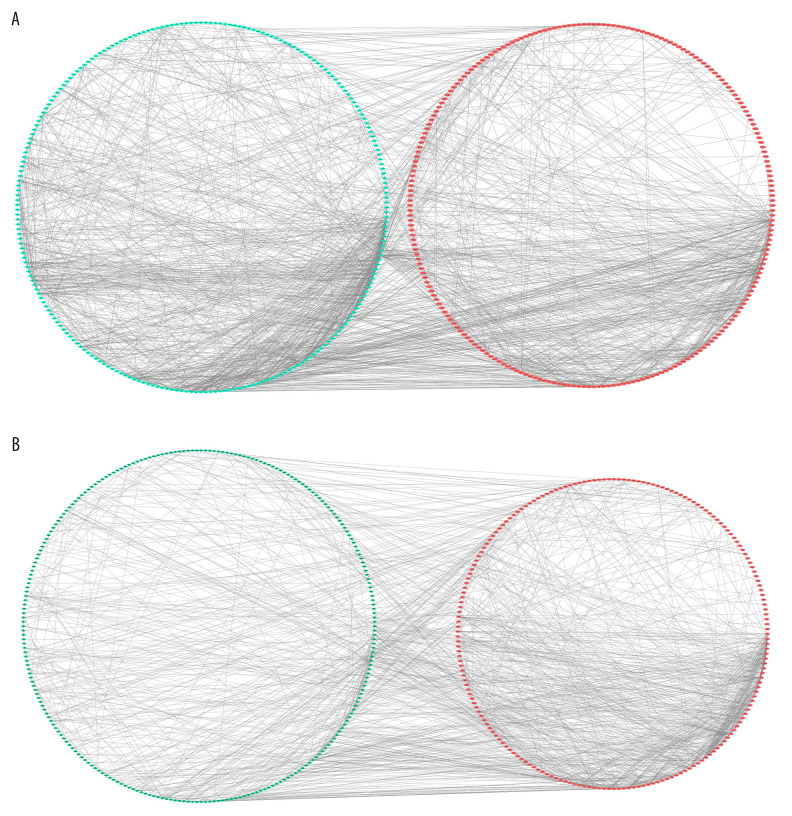
The STRING database was used to evaluate the interaction between the 2 groups of DEGs, and the PPI network structure was constructed. Data were entered into Cytoscape for visual adjustment. The DEGs visualization between the AS and No-AS groups had 596 nodes and 1670 edges (Figure 5A). The DEGs visualization between the M-AS and F-AS groups has 600 nodes and 1258 edges (Figure 5B).
Figure 5.
All DEGs PPI networks are visualized in Cytoscape. Red balls represent the upregulated DEGs and green diamonds represent the downregulated DEGs. (A) AS group and No-AS group; (B) M-AS group and F-AS group.
Identification of hub genes
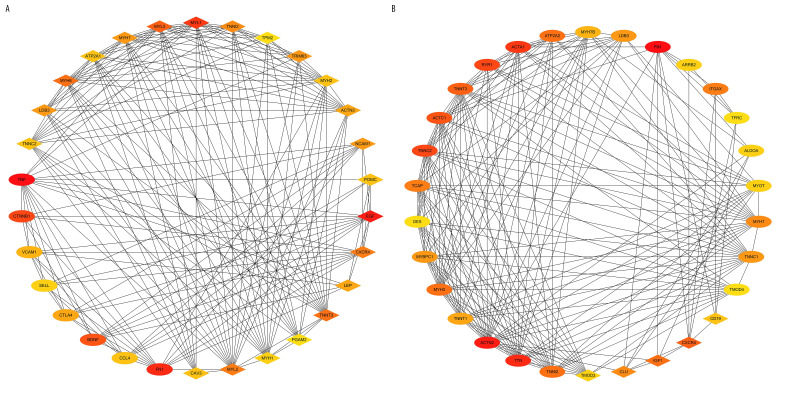
The top 30 hub genes were obtained using CytoHubba (Figure 6A, 6B). The intersection of 30 genes of the 2 groups resulted in 7 genes: FN1, CXCR4, TNNT3, TNNI2, TNNC2, MYH7, and LDB3 (Figure 7A, 7B). The upregulated and downregulated expressions of these 7 genes were different in different groups (Table 1). TNNT3 and FN1 were among the top 10 hub genes. TNNC2 and MYH7 genes were involved in the top 10 KEGG pathways in the 2 groups. The key KEGG pathways were calcium signaling pathway and adrenergic signaling in cardiomyocytes (Figure 8).
Figure 6.
Top 30 genes in Degree score from CytoHubba. (A) AS group and No-AS group; (B) M-AS group and F-AS group. Balls represent the upregulated DEGs and diamonds represent the downregulated DEGs.
Figure 7.
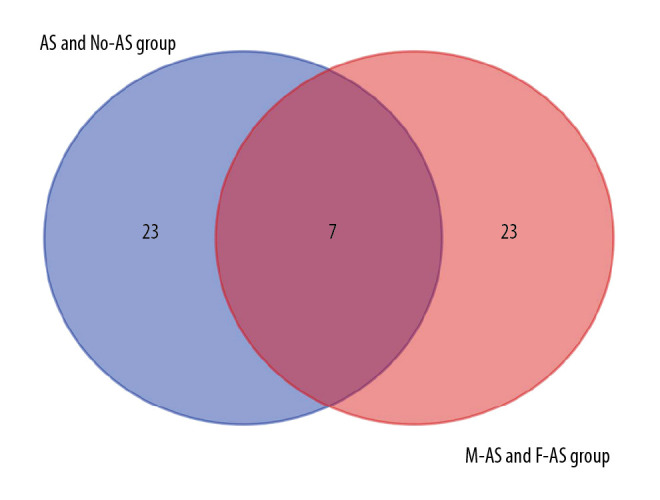
Intersection of the top 30 key genes of DEGs in 2 groups.
Table 1.
Expression of 7 hub key genes in different subjects in different groups.
| Genes | AS and No-AS group | F-AS and M-AS group | ||
|---|---|---|---|---|
| Expression | Rank | Expression | Rank | |
| FN1 | Up | 3 | Up | 1 |
| CXCR4 | Down/Up | 11 | Down | 9 |
| TNNT3 | Down | 8 | Up | 8 |
| TNNI2 | Down | 12 | Up | 10 |
| TNNC2 | Down | 21 | Up | 5 |
| MYH7 | Down | 13 | Up | 16 |
| LDB3 | Down | 16 | Up | 19 |
Figure 8.
Connections between 2 groups of KEGG pathways and 7 hub genes.
Discussion
The clinical manifestations and therapeutic effects of AS are obviously different in males and females. Grubisi et al. [29] indicated that the distribution of clinical manifestations and specific radiological characteristics of AS in the sacroiliac joint and axial bone was sex-dependent, and that male sex is one of the risk factors associated with poor prognosis of AS. Jung et al. [30] found that, on average, males were younger at the time of onset of symptoms, had a higher positive rate of HLA-b27, and had a greater degree of involvement of the spinal cord on imaging, whereas females tended to have less spinal involvement and better mobility, but had a higher incidence of plantar fasciitis. Jiménez-Balderas et al. [31] suggested that male patients were more likely than females to have uveitis, bamboo spine, and hip arthroplasty. Calin et al. [32] found that female patients with AS tended to have a longer delay in diagnosis and thus can miss the optimal treatment time. The above studies show that male patients with ankylosing spondylitis tend to have earlier symptoms, more rapid progression, and more severe radiologic findings, while female patients tend to have milder symptoms and to account for a smaller proportion of cases. These characteristics may be why many female patients with AS are not diagnosed, which also inevitably affects the accuracy of clinical research. Autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus are more common in women [33]. Some studies have shown that estrogen plays an important role in autoimmune diseases [34,35] Jeong et al. [36] reported that estrogen may also be associated with the radiological progression of AS. Aydin et al. [37] suggested that bone loss in male AS patients might be related to the level of dehydroepiandrosterone sulfate. Although AS is also considered to be an autoimmune disease, it often occurs in males [15,16] Giltay et al. [22] found that serum testosterone levels of AS patients often did not increase. Studies have also found that the relevant sex steroid hormones cannot directly explain the male predominance in AS, nor its sex-associated characteristics [23,38]. Therefore, we conducted bioinformatics analysis of DEGs in combination with sex differences of patients (AS vs. No-AS group, M-AS vs. F-AS group), and found that the 2 groups of DEGs had common characteristics in GO function and KEGG pathway. In the GO functional analysis, the EDGs of the 2 groups mainly involved molecular function, including: structural constituent of muscle, actin binding, and calcium ion binding. The common characteristics of the KEGG pathway mainly included: adrenergic signaling in cardiomyocytes and calcium signaling pathway. Seven hub genes were obtained: FN1, CXCR4, TNNT3, TNNI2, TNNC2, MYH7, and LDB3. TNNC2, as the key gene, was involved in the calcium signaling pathway, which may be the main mechanism underlying sex-associated differences in AS (Figure 9).
Figure 9.
Role of TNNC2 in calcium signaling pathway.
Compared with other tissues, these hub genes are highly expressed in the prostate of normal people [39]. TNNT3, TNNI2, and TNNC2 are involved in coding skeletal muscle proteins for rapid twitching. Changes in Ca2+ concentration affect the structure of their coding proteins and regulate rapid skeletal muscle contraction [40,41] Robinson et al. [41] reported that mutations in these genes can result in distal arthrogryposes, a disease characterized by congenital distal limb contracture, with no apparent neurological or muscular disease. Tomasselli et al. [42] reported that TNNC2 is one of the substrates of human immunodeficiency virus protease. These hub genes seem to share a common trait: they are highly expressed in prostate tissue and participate in skeletal muscle activity, although there is still little research on TNNC2. Further studies are needed to determine whether the male prostate and skeletal muscle are involved in AS. We also found that the expression of these genes was not significantly different between males and females in the No-AS groups (P>0.05). This further confirms that there are significant sex-associated differences in the expression of these genes only in patients with AS.
Ca2+ is an intracellular messenger that has an important role in cellular physiological activities. It involves stabilization of the intracellular environment and cell contraction. The calcium signaling pathway is an important pathway in which Ca2+ plays a role [43]. Tu et al. [44] indicated that the calcium signaling pathway plays a key role in the development, maintenance, and regeneration of skeletal muscle. Feske [45] found that abnormalities of the calcium signaling pathway could induce severe immunodeficiency diseases. As a participant in the calcium signaling pathway, TNNC2 also plays an important role in maintaining the normal physiology of the human skeletal muscle system. Abnormalities of TNNC2 can lead to abnormalities of physiological functions of skeletal muscles, ligaments, and bone cells through the calcium signaling pathway, and induce the sex-associated differences in clinical characteristics of AS.
Conclusions
The topic of sex-based differences in AS warrants further discussion. TNNC2 appears to affect skeletal muscle contraction through the calcium signaling pathway, which contributes to the sex-associated differences in patients with AS. FN1, CXCR4, TNNT3, TNNI2, MYH7, and LDB3 were all involved. These findings contribute to understanding of the pathogenesis and therapeutic targets of AS.
Limitations
This study has some limitations and further research is needed to verify the role of these genes and their expression in disease processes, including cellular or animal experiments.
Abbreviations
- AS
ankylosing spondylitis
- HLA-B27
major histocompatibility complex class I allele human leucocyte antigen B27
- ERAP1
endoplasmic reticulum aminopeptidase 1
- IL
interleukin
- CRP
c-reactive protein
- DEGs
differentially expressed genes
- FC
fold change
- GEO
gene expression omnibus
- GO
gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- PPI
protein–protein interaction
- STRING
search tool for the retrieval of interacting genes
- FN1
fibronectin 1
- CXCR4
C-X-C motif chemokine receptor 4
- TNNT3
troponin T3 fast skeletal type
- TNNI2
troponin I2, fast skeletal type
- TNNC2
troponin C2, fast skeletal type
- MYH7
myosin heavy chain 7
- LDB3
LIM domain binding 3
Footnotes
Conflict of interest
None.
Source of support: National Natural science Foundation of China (grants no. 81560359 and 81860393)
References
- 1.Dean LE, Jones GT, MacDonald AG, et al. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 2014;53:650–57. doi: 10.1093/rheumatology/ket387. [DOI] [PubMed] [Google Scholar]
- 2.Zhu W, He X, Cheng K, et al. Ankylosing spondylitis: Etiology, pathogenesis, and treatments. Bone Res. 2019;7:22. doi: 10.1038/s41413-019-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramonda R, Marchesoni A, Carletto A, et al. Patient-reported impact of spondyloarthritis on work disability and working life: The ATLANTIS survey. Arthritis Res Ther. 2016;18:78. doi: 10.1186/s13075-016-0977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean LE, Macfarlane GJ. Five potentially modifiable factors predict poor quality of life in ankylosing spondylitis: Results from the Scotland Registry for Ankylosing Spondylitis. J Rheumatol. 2018;45:62–69. doi: 10.3899/jrheum.160411. [DOI] [PubMed] [Google Scholar]
- 5.Smith JA. Update on ankylosing spondylitis: Current concepts in pathogenesis. Curr Allergy Asthma Rep. 2015;15:489. doi: 10.1007/s11882-014-0489-6. [DOI] [PubMed] [Google Scholar]
- 6.Ding N, Hu Y, Zeng Z, et al. Case-only designs for exploring the interaction between FCRL4 gene and suspected environmental factors in patients with ankylosing spondylitis. Inflammation. 2015;38:632–36. doi: 10.1007/s10753-014-9970-6. [DOI] [PubMed] [Google Scholar]
- 7.Babaie F, Hasankhani M, Mohammadi H, et al. The role of gut microbiota and IL-23/IL-17 pathway in ankylosing spondylitis immunopathogenesis: New insights and updates. Immunol Lett. 2018;196:52–62. doi: 10.1016/j.imlet.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Zhang X, Ma Y, et al. Serum levels of leptin, adiponectin and resistin in patients with ankylosing spondylitis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;52:310–17. doi: 10.1016/j.intimp.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Akkoç N, Yarkan H, Kenar G. Ankylosing spondylitis: HLA-B*27-positive versus HLA-B*27-negative disease. Curr Rheumatol Rep. 2017;19:26. doi: 10.1007/s11926-017-0654-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee YH, Choi SJ, Ji JD. Associations between ERAP1 polymorphisms and ankylosing spondylitis susceptibility: A meta-analysis. Inflamm Res. 2011;60:999–1003. doi: 10.1007/s00011-011-0374-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhong L, Wang W. Complex role of IL-23R polymorphisms on ankylosing spondylitis: A meta-analysis. Expert Rev Clin Immunol. 2018;14:635–43. doi: 10.1080/1744666X.2018.1491308. [DOI] [PubMed] [Google Scholar]
- 12.Reveille JD. An update on the contribution of the MHC to AS susceptibility. Clin Rheumatol. 2014;33:749–57. doi: 10.1007/s10067-014-2662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su W, Du L, Liu S, et al. ERAP1/ERAP2 and RUNX3 polymorphisms are not associated with ankylosing spondylitis susceptibility in Chinese Han. Clin Exp Immunol. 2018;193:95–102. doi: 10.1111/cei.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jethwa H. The interleukin (IL)-23/IL-17 axis in ankylosing spondylitis: New advances and potentials for treatment. Clin Exp Immunol. 2016;183:30–36. doi: 10.1111/cei.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Winter JJ, van Mens LJ, van der Heijde D, et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: A meta-analysis. Arthritis Res Ther. 2016;18:196. doi: 10.1186/s13075-016-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian Q, Xu X, He H, et al. Clinical patterns and characteristics of ankylosing spondylitis in China. Clin Rheumatol. 2017;36:1561–68. doi: 10.1007/s10067-017-3660-3. [DOI] [PubMed] [Google Scholar]
- 17.Deminger A, Klingberg E, Geijer M, et al. A five-year prospective study of spinal radiographic progression and its predictors in men and women with ankylosing spondylitis. Arthritis Res Ther. 2018;20:162. doi: 10.1186/s13075-018-1665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee W, Reveille JD, Davis JC, et al. Are there gender differences in severity of ankylosing spondylitis? Results from the PSOAS cohort. Ann Rheum Dis. 2007;66:633–38. doi: 10.1136/ard.2006.060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in ankylosing spondylitis: A 12-year prospective follow-up of the OASIS study. Ann Rheum Dis. 2015;74:52–59. doi: 10.1136/annrheumdis-2013-204055. [DOI] [PubMed] [Google Scholar]
- 20.Rusman T, van Vollenhoven RF. Gender differences in axial spondyloarthritis: women are not so lucky. Curr Rheumatol Rep. 2018;20:35. doi: 10.1007/s11926-018-0744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubrano E, Perrotta FM, Manara M, et al. The sex influence on response to tumor necrosis factor-α inhibitors and remission in axial spondyloarthritis. J Rheumatol. 2018;45:195–201. doi: 10.3899/jrheum.170666. [DOI] [PubMed] [Google Scholar]
- 22.Giltay EJ, Popp-Snijders C, van Schaardenburg D, et al. Serum testosterone levels are not elevated in patients with ankylosing spondylitis. J Rheumatol. 1998;25:2389–94. [PubMed] [Google Scholar]
- 23.Straub RH, Struhárová S, Schölmerich J. No alterations of serum levels of adrenal and gonadal hormones in patients with ankylosing spondylitis. Clin Exp Rheumatol. 2002;20:S52–59. [PubMed] [Google Scholar]
- 24.Odeh S, Odeh M, Slobodin G, et al. [Spinal syndesmophyte score does not correlate with serum testosterone level in male patients with ankylosing spondylitis]. Harefuah. 2019;158:568–70. [in Hebrew] [PubMed] [Google Scholar]
- 25.Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: Achive for functional genomics data sets – update. Nucleic Acids Res. 2013;41:D991–95. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang da W, Sherman BT. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 27.von Mering C, Huynen M, Jaeggi D, et al. STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 2003;31:258–61. doi: 10.1093/nar/gkg034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin CH, Chen SH, Wu HH, et al. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grubisić F, Jajić Z, Alegić-Karin A, et al. Advanced clinical and radiological features of ankylosing spondylitis: Relation to gender, onset of first symptoms and disease duration. Coll Antropol. 2015;39:927–34. [PubMed] [Google Scholar]
- 30.Jung YO, Kim I, Kim S, et al. Clinical and radiographic features of adult-onset ankylosing spondylitis in Korean patients: Comparisons between males and females. J Korean Med Sci. 2010;25:532–35. doi: 10.3346/jkms.2010.25.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiménez-Balderas FJ. Ankylosing spondylitis: Clinical course in women and men. J Rheumatol. 1993;20:2069–72. [PubMed] [Google Scholar]
- 32.Calin A, Elswood J, Rigg S. Ankylosing spondylitis – an analytical review of 1500 patients: The changing pattern of disease. J Rheumatol. 1988;15:1234–38. [PubMed] [Google Scholar]
- 33.Zandman-Goddard G, Peeva E. Gender and autoimmunity. Autoimmun Rev. 2007;6:366–72. doi: 10.1016/j.autrev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Ackerman LS. Sex hormones and the genesis of autoimmunity. Arch Dermatol. 2006;142:371–76. doi: 10.1001/archderm.142.3.371. [DOI] [PubMed] [Google Scholar]
- 35.Holroyd CR. The effects of hormone replacement therapy on autoimmune disease: Rheumatoid arthritis and systemic lupus erythematosus. Climacteric. 2009;12:378–86. doi: 10.1080/13697130903025449. [DOI] [PubMed] [Google Scholar]
- 36.Jeong H, Bea EK, Lee J, et al. Body mass index and estrogen predict radiographic progression in the spine in ankylosing spondylitis. Joint Bone Spine. 2015;82:473–74. doi: 10.1016/j.jbspin.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Aydin T, Karacan I, Demir SE. Bone loss in males with ankylosing spondylitis: Its relation to sex hormone levels. Clin Endocrinol (Oxf) 2005;63:467–69. doi: 10.1111/j.1365-2265.2005.02369.x. [DOI] [PubMed] [Google Scholar]
- 38.Gooren LJ, Giltay EJ, van Schaardenburg D. Gonadal and adrenal sex steroids in ankylosing spondylitis. Rheum Dis Clin North Am. 2000;26:969–87. doi: 10.1016/s0889-857x(05)70179-4. [DOI] [PubMed] [Google Scholar]
- 39.Fagerberg L, Hallström BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troponin T. Genetics, properties and function. J Muscle Res Cell Motil. 1998;19:575–602. doi: 10.1023/a:1005397501968. [DOI] [PubMed] [Google Scholar]
- 41.Robinson P, Lipscomb S, Preston LC, et al. Mutations in fast skeletal troponin I, troponin T, and beta-tropomyosin that cause distal arthrogryposis all increase contractile function. FASEB J. 2007;21:896–905. doi: 10.1096/fj.06-6899com. [DOI] [PubMed] [Google Scholar]
- 42.Tomasselli AG, Hui JO, Adams L, et al. Actin, troponin C, Alzheimer amyloid precursor protein and pro-interleukin 1 beta as substrates of the protease from human immunodeficiency virus. J Biol Chem. 1991;266:14548–53. [PubMed] [Google Scholar]
- 43.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 44.Tu MK, Levin JB, Hamilton AM, Borodinsky LN. Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell Calcium. 2016;59(2–3):91–97. doi: 10.1016/j.ceca.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feske S. Immunodeficiency due to defects in store-operated calcium entry. Ann NY Acad Sci. 2011;1238:74–90. doi: 10.1111/j.1749-6632.2011.06240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]