Abstract
Elaeagnus angustifolia (EA) is a medicinal plant used for treating several human diseases in the Middle East. Meanwhile, the outcome of EA extract on HER2-positive breast cancer remains nascent. Thus, we herein investigated the effects of the aqueous EA extract obtained from the flowers of EA on two HER2-positive breast cancer cell lines, SKBR3 and ZR75-1. Our data revealed that EA extract inhibits cell proliferation and deregulates cell-cycle progression of these two cancer cell lines. EA extract also prevents the progression of epithelial-mesenchymal transition (EMT), an important event for cancer invasion and metastasis; this is accompanied by upregulations of E-cadherin and β-catenin, in addition to downregulations of vimentin and fascin, which are major markers of EMT. Thus, EA extract causes a drastic decrease in cell invasion ability of SKBR3 and ZR75-1 cancer cells. Additionally, we found that EA extract inhibits colony formation of both cell lines in comparison with their matched control. The molecular pathway analysis of HER2 and JNK1/2/3 of EA extract exposed cells revealed that it can block HER2 and JNK1/2/3 activities, which could be the major molecular pathway behind these events. Our findings implicate that EA extract may possess chemo-preventive effects against HER2-positive breast cancer via HER2 inactivation and specifically JNK1/2/3 signaling pathways.
Keywords: Elaeagnus angustifolia, breast cancer, EMT, chemoprevention, apoptosis
1. Introduction
Breast cancer (BC) commonly affects women worldwide, comprising 25% of cancer cases [1]. There are several risk factors, both environmental and genetic, associated with the onset of breast cancer [2]. Gene-expression-profiling studies classified breast cancer into five molecular subtypes: Luminal (A and B), HER2, basal-like, and normal-like, using hierarchical cluster analysis [3]. Among all subtypes, HER2-positive breast cancer accounts for 20–25% and is associated with aggressive phenotype, poor prognosis, and survival rate, in addition to increased recurrence [4]. Systemic management modalities for HER2-positive breast cancer include chemotherapy, radiation, and targeted anti-HER2 treatment modalities [4,5,6,7]. Although the treatment is generally effective in the early stages of therapy, nevertheless, ~90% of primary and half of metastatic breast cancer cases resistance to therapy leads to treatment failure and mortality [8]. Thus, it is important to identify novel potent therapeutic agents that can inhibit cell proliferation of HER2+ breast cancer with minimal side-effects. An alternate to conventional therapy can be found in naturally present phytochemicals in foods such as vegetables, fruits, spices, and plant roots [9,10]. Traditionally, Elaeagnus angustifolia (EA) plant has been used extensively for centuries in the treatment of various diseases due to its antioxidant, anti-inflammatory, and antimicrobial properties [11], along with bioactive compounds (flavonoids and coumarins) [12] that regulate key events associated with cancer development, such as cell-signaling pathways, including Wnt-signaling, cell proliferation, cell-cycle progression, apoptosis, and epithelial-to-mesenchymal transition (EMT) [13,14].
Elaeagnus angustifolia (EA), commonly known as wild olive, oleaster, silver berry, or Russian olive [11,15], is a deciduous tree belonging to the family of Elaeagnacea (Araliaceae) widely distributed in the Middle East, as well as Mediterranean regions [12,16]. EA fruit is highly nutritious and contains vitamins (vitamin C, tocopherol, thiamine B1, and carotene), sugar, proteins, and several minerals, like potassium, iron, magnesium, and calcium [17,18,19]; the leaves and flower extract are rich in secondary metabolites such as coumarins, phenolcarboxylic acids, flavonoids, saponins, and tannins [16,20,21]. Previous studies have shown that EA exhibits anticancer effects as a result of its essential oils (ethyl cinnamate, 2-phenyl-ethyl benzoate, 2-phenyl-ethyl isovalerate, nerolidole, squalene, and acetaphenone), flavonoids (quercetin), and pro anthocyanosides [22,23]. In cancer, flavonoids are shown to enhance p53 expression and cause cell-cycle arrest in the G2/M phase [24]. Moreover, they are known to inhibit Ras protein expression and regulate heat-shock proteins in various cancers, mainly in leukemia and colorectal cancer [24]. One of the key flavonoid components of EA is Quercetin, which is an anti-proliferative agent [24]. Furthermore, quercetin also promotes TRAIL-induced apoptosis by enhancing the expression of Bax and inhibiting Bcl-2 protein [25,26,27]. Additionally, ethyl acetate has been shown to significantly reduce proliferation of Hela cells in vitro [22]. Apart from this, volatile oils present in the plant have medicinal properties and are also used in perfume industries [23,28]. EA possesses numerous therapeutic and pharmacological properties, including antifungal, antibacterial, antimutagenic, anti-inflammation, antioxidant, and gastroprotective effects [11,29,30,31,32]. Traditionally, EA is also used to cure other diseases, including osteoporosis, amoebic dysentery, jaundice, asthma, flu, cough, cold, nausea, diarrhea, sore throat, fever, tetanus, and female aphrodisiac [12,15,33,34]. However, there are limited studies regarding the role of EA extract on cancer. In this context, our group recently demonstrated that EA extract can reduce the progression of human oral cancer by the inhibition of angiogenesis and cell invasion via Erk1/Erk2 signaling pathways [12].
A previous study showed that hydroalcoholic extracts of EA flower significantly inhibit angiogenesis, one of the known hallmarks of cancer [35]. Nevertheless, there are no studies reported on the anticancer activity of EA in breast cancer, especially in HER2-positive type, and its mechanism of cancer inhibition. To investigate the potent therapeutic and antitumor properties of EA extract in human breast cancer and its underlying mechanism, we explored the effect of aqueous extract of EA flower on cell proliferation and cell-cycle progression, cell invasion, and colony formation in two HER2-positive human breast cancer cell lines (SKBR3 and ZR75-1).
2. Results
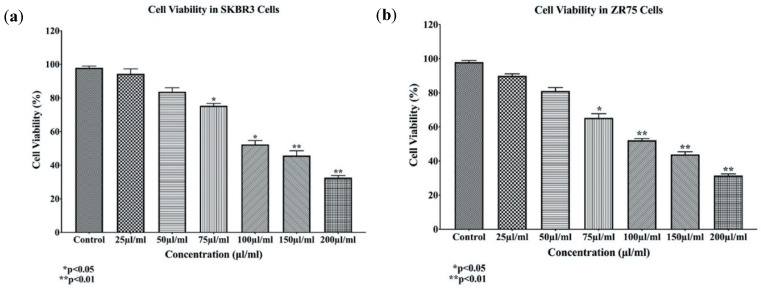
In order to determine the effects of EA extract on HER2-positive cell lines SKBR3 and ZR75-1, cells were treated with varying concentrations of EA extract (25, 50, 75, 100, 150, and 200 µL/mL) for 48 h. Treatment with EA extract reduced the number of proliferating HER2-positive breast cancer cells in a dose-dependent manner (Figure 1); notably, concentrations of 100 and 200 µL/mL showed a substantial decrease in cell viability of SKBR3 and ZR75-1 by 50% and 75%, respectively.
Figure 1.
(a,b) The effects of different concentrations of Elaeagnus angustifolia (EA) plant extract on cell proliferation of HER2-positive breast cancer cell lines SKBR3 (a) and ZR75-1 (b) at 48 h. Data indicate an inverse relation between concentrations of EA extract and cell proliferation in both SKBR3 and ZR75-1 cell lines. Data are expressed as percent of growth ± SEM.
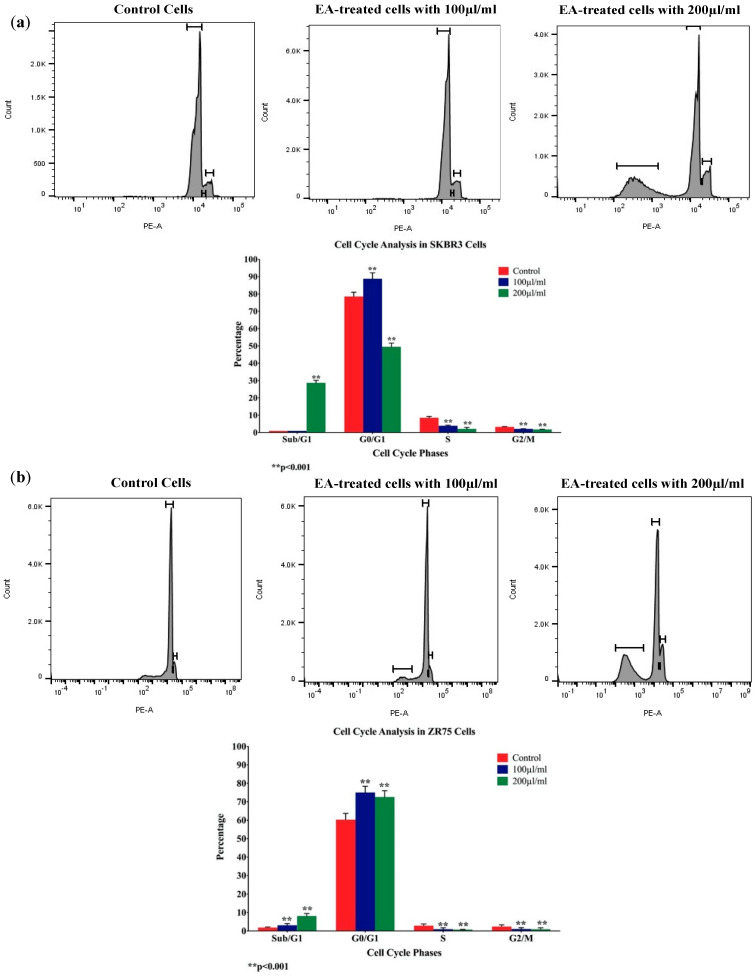
Meanwhile, and to examine whether the antiproliferative effect of the EA flower extract on SKBR3 and ZR75-1 cells is associated with cell-cycle deregulation, we analyzed cell-cycle phase distributions of EA-treated cells, using flow cytometric analysis. Our results showed that exposure to EA extract (100 and 200 µL/mL) for 48 h enhanced the G0/G1 phase, with a simultaneous decrease in S and G2/M phases of both the breast cancer cell lines, thus indicating EA-induced cell-cycle inhibition (Figure 2). Furthermore, we observed a significant increase in the sub/G1 phase of both cell lines, indicating that cells undergo apoptosis when treated with EA extract (Figure 2).
Figure 2.
(a,b) Flow cytometry data analysis of SKBR3 and ZR75 cells after EA-treatment. Data demonstrate an increase in G0/G1 phase with simultaneous reduction in S and G2/M phases in both cell lines. Meanwhile, there is a significant increase in cell apoptosis (Sub/G1 phase) of SKBR3 cells treated with EA, and a small increase in cell apoptosis of treated ZR75 cells.
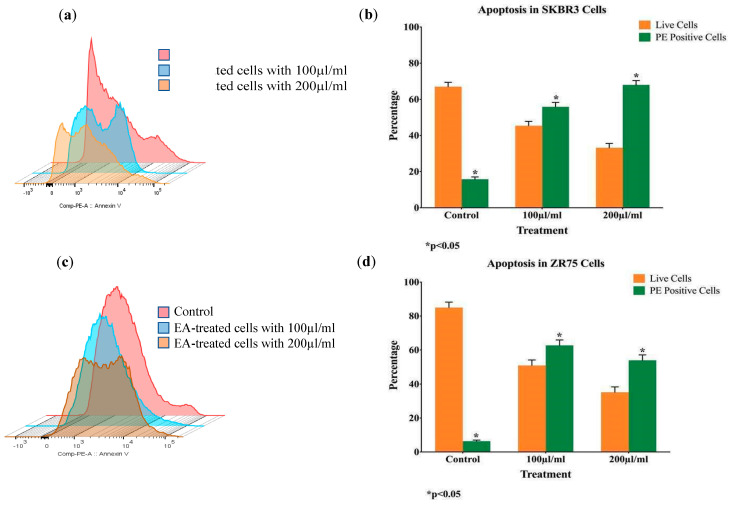
To confirm EA-induced apoptosis, Annexin V-FITC and 7-AAD staining by flow cytometry were performed. Therefore, the presence of apoptosis is clearly demonstrated in both cell lines (Figure 3).
Figure 3.
(a,b) Induction of apoptosis by EA extract in SKBR3 (a, b) and ZR75 (c, d) cells, as determined by Annexin V-FITC and 7-AAD apoptosis assay.
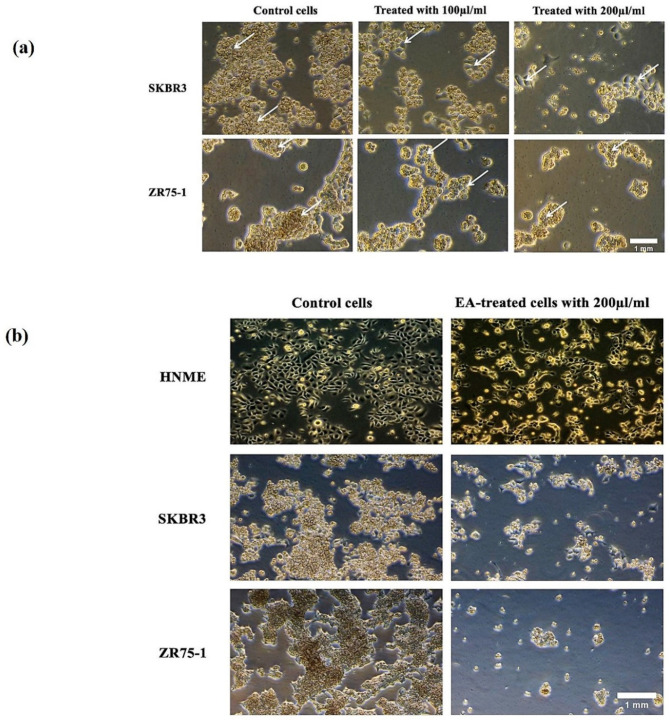
Next, we examined the cell morphology of SKBR3 and ZR75-1 in addition to HNME-E6/E7 cell lines, using phase-contrast microscopy, under the effect of 100 and 200 μL/mL of EA extract. In the absence of treatment, SKBR3 and ZR75-1 cells displayed a round morphology and disorganized multilayered cells. In contrast, and as indicated in Figure 4a, treatment for 48 h with 100 and 200 μL/mL of EA plant extract led to a phenotypic conversion from round cells to epithelial-like phenotype. Clearly, cells became more flattened in appearance and showed an increase in cell-cell adhesion, in comparison with untreated cells (Figure 4a). However, at three days of treatment with 200 μL/mL of EA plant extract, cells started detaching from the surface of the tissue culture dish, indicating cell death in SKBR3 and ZR75-1 cells; however, this was not observed in the human normal immortalized mammary epithelial cell line (HNME-E6/E7), as shown in Figure 4b. Nevertheless, it is evident that EA extract inhibits cell proliferation HNME-E6/E7 cells, with a slight effect on their epithelial morphology (Figure 4b).
Figure 4.
(a,b) EA plant extract induces morphological changes in HER2-positive cell lines, SKBR3 and ZR75-1. (a) We observe that treatment for 48 h with 100 and 200 μL/mL of EA extract induces epithelial transition and the formation of a monolayer of cells in both cell lines, in comparison with untreated (control) cells which display a round phenotype and form multilayers; arrows indicate epithelial morphology with clear cell-cell adhesion. (b) At three days of treatment of SKBR3, ZR75-1, and HNME-E6/E7 cell lines with 200 μL/mL of EA plant extract, the two cancer cell lines start detaching from the surface of the tissue culture dish, indicating cell death; this observation was not noted in the HNME-E6/E7 cells (images a and b at ×20 magnification).
These results imply that moderate concentrations of 100 μL/mL of EA plant extract induce cell differentiation after 24 and/or 48 h, while higher concentrations (200 μL/mL of EA plant extract) can provoke apoptosis after 48 h of exposure.
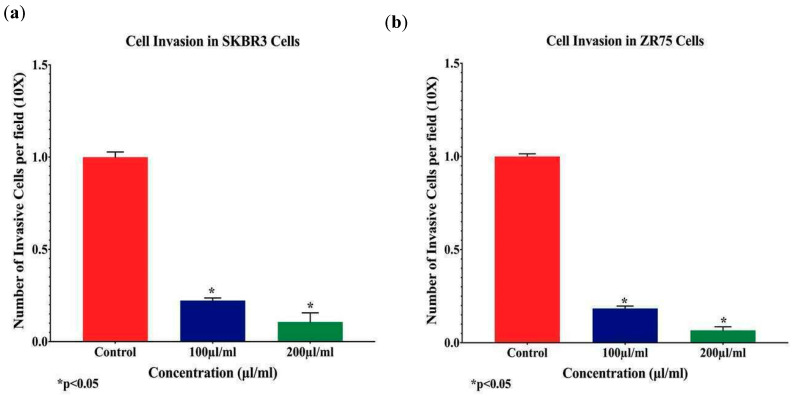
Subsequently, and to analyze the anti-invasion effects of EA on HER2-positive breast cancer cells, Matrigel invasion assay was performed, using SKBR3 and ZR75-1 cells, upon EA treatment with 100 and 200 μL/mL concentrations; our data revealed that EA extract significantly inhibits cell invasion ability of both cell lines by ~70% to 88%, respectively, in comparison with control cells (Figure 5, p < 0.05). This suggests that EA plant extract can considerably downgrade cell invasion and metastasis of HER2-positive breast cancer.
Figure 5.
(a,b): The effects of EA flower extract on cell invasion of human HER2-positive breast cancer cells. EA extract inhibits cell invasion ability of SKBR3 (a) and ZR75-1 (b) cell lines by approximately 70% in comparison with their matched control cells (unexposed) (p < 0.05). Boyden chambers were used to assess cell-invasion ability of SKBR3 and ZR75-1 cell lines. Cancer cells treated for 24 h with 100 and 200 µL/mL EA plant extract showed a significant inhibition of cell invasion in both cell lines, when compared with their matched control (p < 0.05). Data are quantified by normalizing the number of invasive cells by their total number.
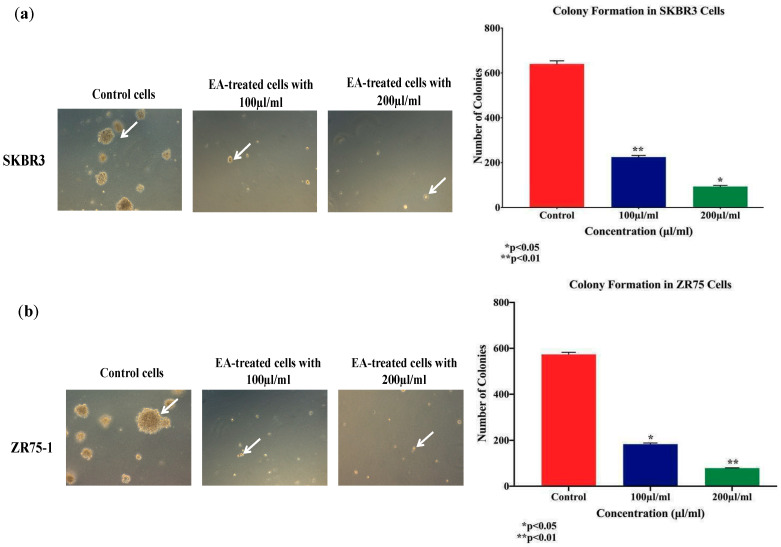
On the other hand, we assessed the colony formation of SKBR3 and ZR75-1 cells, in soft agar, under the effect of EA plant extract at 100 and 200 µL/mL, for two weeks; we observed a significant decrease in the number of colonies for both cell lines treated with EA plant extract, compared with their matched control, as shown in Figure 6. SKBR3 sustained significant inhibition of colony formation by 60% (p < 0.01) and 80% (p < 0.05) when exposed to 100 and 200 µL/mL EA plant extract, in comparison to the control, respectively (Figure 6a). In parallel, ZR75-1 cell line also displayed a similar pattern after two weeks of treatment; the number of colonies decreased by 70% (p < 0.05) and 85% (p < 0.01) at 100 and 200 µL/mL concentrations, respectively (Figure 6b). This indicates that EA plant extract suppresses colony formation and probably tumor growth in vivo.
Figure 6.
(a,b) Effect of EA flower extract on colony formation, in soft agar, in human HER2-positive cancer cell lines, SKBR3 (a) and ZR75-1 (b). EA extract inhibits colony formation of SKBR3 and ZR75-1, in comparison with their matched control cells (images of figure a,b at ×10 magnification). Colony formation in soft agar is a solid indicator of tumor formation in vivo. The colonies were counted manually and expressed as percentage of treatment relative to the control (mean ± SEM).
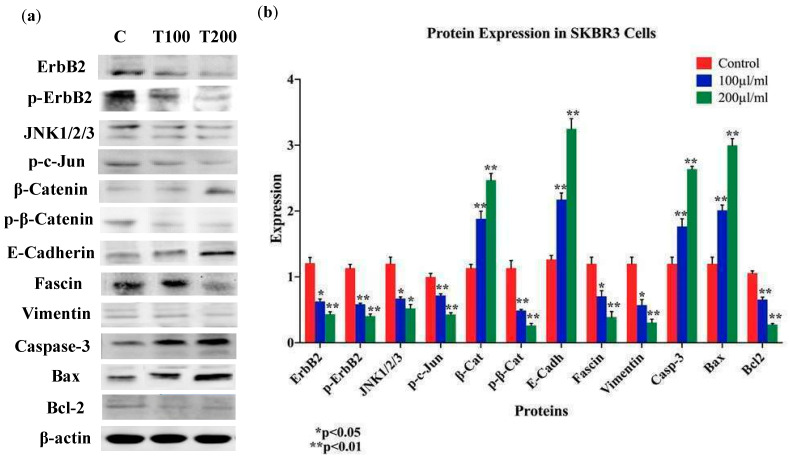
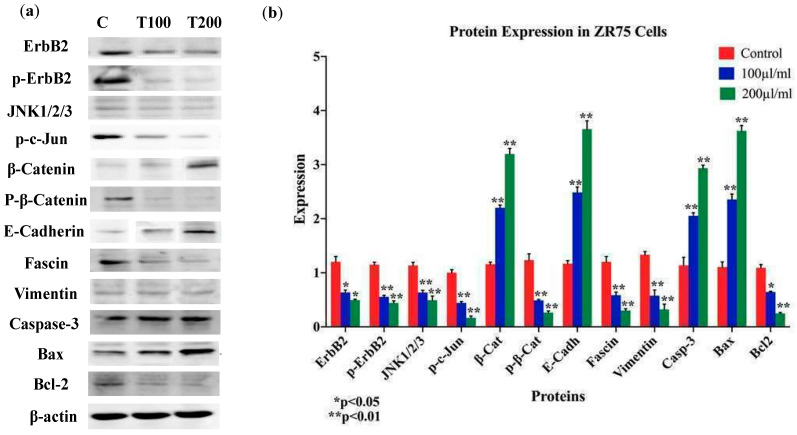
Based on the above data, we explored the expression patterns of key markers of EMT and cancer progression: E-cadherin, β-catenin, vimentin, and fascin; our data pointed out that EA extract enhances the expression of E-cadherin and β-catenin in SKBR3 and ZR75-1 cell lines, while the expression of vimentin and fascin are decreased in comparison to their control cells (Figure 7 and Figure 8). In parallel, we examined the outcome of EA on pro-apoptotic proteins (caspase-3 and Bax) and anti-apoptotic protein (Bcl-2) with 100 and 200 μL/mL of EA plant extract after 24 and 48 h of exposure. We found enhanced expression of both pro-apoptotic proteins (Bax and caspase-3) in SKBR3 and ZR75-1 in EA-treated cells, compared to their control (Figure 7 and Figure 8). In contrast, the expression of Bcl-2 was lost in SKBR3 and ZR75-1 (Figure 7 and Figure 8). Our data suggest that high concentrations of EA induce apoptosis in HER2-positive cancer cells, which might be associated with the Bcl-2/Bax/caspase-3 signaling pathway.
Figure 7.
(a,b) Protein expression and molecular mechanisms of EA inhibitory actions in SKBR3 cell line. This plant extract induces an overexpression of E-cadherin, β-catenin, and downregulation of vimentin and fascin, while upregulating pro-apoptotic markers (Bax and Caspase-3), in comparison with their control and inhibiting anti-apoptotic markers (Bcl-2). Furthermore, EA plant extract inhibits the phosphorylation of ErbB2 and β-catenin, as well as the expression of JNK1/2/3. β-actin was used as a control for the proteins amount in this assay. Cells were treated with 100 and 200 μL/mL of EA extract for 48 h, as explained in the materials and methods and the results sections. (a) Blot image and (b) quantification of bands.
Figure 8.
(a,b) Protein expression and molecular mechanisms of EA inhibitory actions in ZR75 cell line. This plant extract induces an overexpression of E-cadherin, β-catenin, and downregulation of vimentin and fascin; in addition, pro-apoptotic markers Bax and Caspase-3 are upregulated in comparison with their control, while anti-apoptotic marker Bcl-2 is inhibited. Furthermore, EA plant extract inhibits the phosphorylation of ErbB2 and β-catenin, as well as JNK1/2/3 expression. β-actin served as a control in this assay. Cells were treated with 100 and 200 μL/mL of EA extract for 48 h, as explained in the materials and Methods section. (a) Blot image and (b) quantification of bands.
Vis-à-vis the underlying molecular pathways of EA extract on cell proliferation, EMT progression, cell invasion, and colony formation of HER2-positive breast cancer cells, we assumed that HER2 activation, as well as c-Jun N-terminal kinase (JNK), could have major roles in regulating these events [36,37,38,39]; therefore, the expression patterns of HER2 and JNK1/2 were explored. We found that EA extract inhibits the phosphorylation of HER2 (with slight change in its expression level) and β-catenin, while it provokes a downregulation of JNK1/2 in SKBR3 and ZR75-1 upon treatment with EA plant extract after 24 and 48 h of exposure (Figure 7 and Figure 8).
3. Discussion
In this study, we investigated the effect of EA extract in HER2-positive human breast cancer cell lines (SKBR3 and ZR75-1) with regard to certain parameters related to cell proliferation, cell cycle, morphological changes (round to epithelial-like transition: RELT), cell invasion, and colony formation. Additionally, we explored the molecular pathways behind these events. We report that EA plant extract can suppress cell proliferation, as well as dysregulate cell-cycle progression of SKBR3 and ZR75-1 cells, along with induction of RELT and inhibition of colony formation in both cell lines. EA plant is known for its antioxidant characteristics and has been used conventionally for the treatment of several diseases and inflammation [16,20,21]. Moreover, EA consists of bioactive compounds (flavonoids and neoclerodane diterpenoids), which can play a role in promoting apoptosis and cell-cycle progression, as well as inhibiting angiogenesis and EMT events, thus potentially preventing cancer development and progression [12,13,16,40]. Meanwhile, we herein demonstrate that EA plant extract inhibits cell proliferation and dysregulate cell-cycle and EMT progression of HER2-positive breast cancer cells.
Indeed, EMT is a crucial phenomenon in cancer progression, characterized by disruption of intracellular tight junctions and the loss of cell-cell contact and epithelial cell features, along with the gain of mesenchymal morphology [41]. On the other hand, it is well-known that cancer progression is characterized by loss of differentiation in human carcinomas, together with downregulation of E-cadherin, which is associated with the degree of tumor malignancy [33]. Moreover, previous studies on different types of human carcinomas have shown that loss of E-cadherin and β-catenin, in addition to enhanced expression of vimentin and fascin, can promote EMT, which is associated with cancer progression [41,42,43,44]. In this investigation, we analyzed the effect of EA on E-cadherin, β-catenin, vimentin, and fascin expression patterns in HER2-positive breast cancer cell lines. We observed two different major events that are provoked by EA treatment, namely induction of RELT “MET” and apoptosis. More specifically, we found that, upon EA-treatment for 24 and 48 h at both low and high concentrations (100 and 200 μL/mL), E-cadherin and β-catenin are upregulated, while vimentin, p-β-catenin, and fascin expressions are downregulated, which are important elements of mesenchymal-epithelial transition (MET), the opposite event of EMT, and RELT. Thus, EA plant extract can induce differentiation to an epithelial phenotype and consequently block cell invasion of the two HER2-positive human breast cancer cell lines. On the other hand, we found that EA extract inhibits colony formation of SKBR3 and ZR75-1 cell lines, which could be considered as an in vivo tumor formation.
On the other hand, regarding the molecular pathways of EA extract on our cell line models, we herein reported that, upon EA-treatment after 24 h at both low and high concentration, EA extract can inactivate HER2 receptor, as well as deregulate the expression patterns of JNK1/2, which can lead to increased expression of E-cadherin and β-catenin and decreased expression of vimentin and fascin, thus indicating restoration of cell-cell adhesion, especially E-cadherin/catenins complex. Furthermore, in accordance with our data, a study by Wang et al. showed overexpression of JNK to be linked with breast cancer cell migration and invasion, as well as EMT [45]. Therefore, in this present investigation, we show that EA plant extract can regulate the RELT/EMT event and inhibit cell invasion of the two human HER2-positive breast cancer cell lines. Moreover, these present data are concurrent with our recently published work regarding the outcome of EA plant extract on human oral cancer cells, where we have demonstrated that EA induces differentiation to an epithelial phenotype; and therefore, it causes a dramatic decrease in cell invasion and motility of human oral cancer cells, along with an upregulation of E-cadherin expression [12]. Additionally, our study of EA extract on oral cancer cells revealed that EA can inhibit the phosphorylation of Erk1/Erk2 and β-catenin, which could be behind the initiation of RELT/MET event and the overexpression of E-cadherin [12]. In the present work, we demonstrated that JNK1/2 pathway is one of the main molecular pathways of EA in HER2-positive human breast cancer cells.
JNK substrate proteins encompass several nuclear proteins, including transcription factors, as well as nuclear hormone receptors involved in maintaining various cellular activities comprising cell proliferation, differentiation, cell death, and cell survival [46]. JNKs phosphorylate and stimulate both, nuclear and non-nuclear proteins and form the transcription factor activator protein-1 (AP-1) by dimerization of the Jun proteins (c-Jun, JunB, and JunD) with the Fos proteins (c-Fos, FosB, Fra-1, and Fra-2); other downstream molecules include activating transcription factor 2 (ATF-2), c-Myc, p53, STAT1/3, Pax family of proteins, Elk1, NFAT, and Bcl-2 family (Bcl-2, Bcl-xl, Bad, Bim, and Bax) [47]. Of these nuclear substrates, c-jun is the most vital nuclear substrate; JNKs enhance c-jun transcription by binding and phosphorylating c-jun at Ser73 and Ser63 via Ha-Ras, c-Raf, and v-Src [48,49,50]. c-Jun, the downstream target of the JNK pathway, is necessary for Ras-induced carcinogenesis [51,52]. In vivo studies have indicated an oncogenic role of c-Jun in the liver [53,54,55], as well as intestinal cancers [54], thus indicating a pro-oncogenic role for the JNK/c-Jun axis. The activity of c-jun is essential for the Ha-Ras mediating carcinogenesis transformation. Our data indicate that EA-treatment reduced p-c-Jun expression, indicating an EA-tumor-suppressive role in cancer.
In parallel, it is evident that β-catenin signaling pathways are also involved in these events; this is based on the fact that β-catenin acts as a transcription regulator, as well as cell-cell adhesion molecule, which was elegantly reported by Kandouz et al., under the effect Teucrium polium on human prostate cancer cells [12,56]. Thus, it is possible that EA plant extract can have a similar effect on β-catenin pathways, especially since our data showed that EA extract inhibit β-catenin phosphorylation, consequently allowing it to translocate from the nucleus to undercoat membrane to act as a cell-cell adhesion protein leading to the inhibition of cell-invasion ability of SKBR3 and ZR75-1 cell lines. We surmise that EA exhibits anticancer activity due to the high levels of flavonoids, coumarins, and antioxidants [12,14,57].
Vis-à-vis the interaction between the activation of HER2 receptor and its downstream pathways, including JNK, it is well-established that HER2 overexpression causes homo- or heterodimerization, leading to phosphorylation of this receptor, which in turn triggers downstream signaling pathways responsible for important cellular functions, including cell proliferation, invasion, migration, angiogenesis, chemoresistance, and apoptosis [38,39]. We investigated the downstream target of HER2 stimuli, JNK, as JNK-dependent gene regulatory circuitry underlying cell-fate changes from epithelial to mesenchymal state. Our study demonstrates that EA slightly suppresses the expression of HER2 receptor, while mostly affecting its phosphorylation, as well as one of its main downstream targets JNK. More specifically, HER2 downregulation is associated with inhibition of proliferation and invasion of HER2-positive human breast cancer cells [58]; this correlates with our results of EA-induced decreased cell proliferation, cell invasion, and colony formation.
Regarding the outcome of high-concentration treatment of EA (200 μL/mL) after 48 h, we observed induction of apoptosis in EA-treated cells by analyzing the mitochondrial apoptosis regulators of Bcl-2 family (Bcl-2 and Bax), as well as Caspase-3 [59]. Bcl-2 homodimers have been shown to inhibit apoptosis; however, Bax homodimers initiates cell death [60]. Heterodimerization between Bax and Bcl-2 and its ratio of Bax to Bcl-2 determine the susceptibility of cells to apoptosis, whereas caspase-3 is known to act as a downstream target of Bax/Bcl-2 control and play a key role in the execution of apoptosis [60]. We herein report that EA can reduce the growth and provoke apoptosis of human HER2-positive breast cancer cells. This effect is associated with caspase-3 activation and reduced Bcl-2 expression. Moreover, mitochondrial Bax translocation and the expression of Bcl-2 slightly decreased upon EA-extract treatment, indicating that caspase-dependent pathways are involved in EA-induced apoptosis and Bcl2/Bax/Caspase-3-regulated cell death through JNK inactivation.
The JNK pathway is predominantly involved in the stimulation of the intrinsic apoptotic pathway facilitated by mitochondria [61]. However, the JNK pathway is also involved in TRAIL-induced apoptosis, autophagy, mitotic catastrophe, and immunogenic cell death [62,63,64,65,66]. Our data show upregulation of Bax and capsase-3 expression and downregulation of Bcl-2, indicating that apoptosis occurs via the extrinsic pathway as well [65]; a loss of JNK could primarily trigger extrinsic apoptosis. Moreover, Bax/Bcl-2/caspase-3 is also involved in other types of regulated cell death, including immunogenic cell death, mitotic catastrophe, and mitochondrial permeability transition (MPT)-driven necrosis [67], thus suggesting JNK inhibition to mediate Bax/Bcl-2/caspase-3 apoptosis. We herein showed loss of JNK, which is in concordance with a study by Wang et al., in breast cancer where overexpression of JNK did not cause apoptosis and correlated with poor prognosis [45]. Moreover, while activation of JNK results in loss of Bcl-2 expression [68,69,70], the mechanism is controversial as Bcl-2 phosphorylation enhances cell survival signaling [68,71,72,73,74], thus making the role of Bcl-2 phosphorylation in JNK-stimulated apoptosis nascent. Moreover, studies have demonstrated that JNK activation does not result in Bcl-2 phosphorylation [59,75], thus indicating that JNK might regulate another kinase or phosphatase resulting in Bcl-2 phosphorylation.
Although the roles of the Bcl-2 family of proteins in JNK-dependent apoptosis remain nascent, the results of the current study indicate that the proapoptotic Bax subfamily of Bcl-2-related proteins is not essential for JNK-dependent apoptosis. These data demonstrate a dual role of JNK in carcinogenesis which can be both oncogenic and tumor suppressive, as indicated previously. Alternatively, JNK activity can be tissue-specific and cell-type-dependent, differing based on tumor stage and status, as well as the presence of activated upstream and downstream molecules and stress signals [76,77,78,79,80]. Nevertheless, further work is needed to unravel the complexity of the interaction of JNK pathway and its molecules, to help pave the way for the development of anticancer therapeutic strategies. Moreover, in our laboratory, we are aiming to derive the active compounds of EA that can plausibly be involved in the inhibition of cancer progression.
4. Materials and Methods
4.1. Plant Collection and Extract Preparation
EA flowers were obtained during the second week of June, from Montreal, Quebec, Canada, and were dried and stored in a dark place, at room temperature, as previously described [12]. The extract was prepared by boiling 3 g of finely grounded dry EA flowers per 100 mL of autoclaved distilled water, at 150 °C, on a hot plate, for 20 min, with continuous stirring. The flower extract solution was then filtered, using a 0.45 μm filter unit, and stored at 4 °C until use. Dilutions were prepared in cell culture media for various applications. For each experiment, the extract was freshly prepared.
4.2. Cell Culture
Two different human HER2-positive breast cancer cell lines (SKBR3 and ZR75-1) derived from females were obtained from American Type Culture Collection (ATCC) (Rockville, MD, USA). Cell lines were grown and expanded in RPMI-1640 (Gibco, Life Technologies) supplemented with 10% fetal bovine serum (Gibco, Life Technologies, Massachusetts, MA, USA), 2 mM l-glutamine, 1% PenStrep antibiotic (Invitrogen, Life Technologies, Carlsbad, CA, USA) at 37 °C, and 5% CO2 humidified atmosphere. Human normal mammary epithelial cells immortalized by E6/E7 of HPV type 16 (HNME-E6/E7) were used to assess plant extract toxicity [81]. Cells were maintained in Gibco® Keratinocyte-SFM (1X) media (Gibco, Life Technologies). All the experiments were carried out when cells were ~70–80% confluent.
4.3. Cell Viability Assay
HER-2-positive breast cancer cell lines, SKBR3 and ZR75-1, were seeded on clear bottom 96-well plates (10,000 cells/well) and cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (100 µL/well).
Elaeagnus angustifolia (EA) solution was used to treat cells at different concentrations (25, 50, 75, 100, 150, and 200 µL/mL) for a period of 48 h. Control wells received 100 μL of media (control). The inhibition of cell viability was determined, using Alamar Blue Cell viability reagent (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s protocol. The shift in fluorescence was measured at 570 nm (excitation) and 600 nm (emission), in a fluorescent plate reader (Infinite M200, Tecan, Grödig, Austria), after 4 h of incubation with the dye. Relative cell proliferation was determined based on the fluorescence of EA-treated cells relative to that of control cells.
4.4. Cell Cycle and Apoptosis Assay
SKBR3 and ZR75-1 cells (1 × 106 cells/dish) were plated in 100 mm Petri dishes, with overnight incubation. The cells were then starved with serum-free RPMI-1640 medium for a period of 6–12 h to synchronize the cells into the G0 phase of the cell cycle. Synchronized cells were then treated with EA extract (100 and 200 µL/mL) for 48 h. Cells were harvested, washed twice with PBS, fixed overnight in 70% ice-cold ethanol, and, subsequently, their DNA was stained with 50 μg/mL FXCycle PI/RNase staining solution (Invitrogen, Thermo Fisher Scientific) after RNase A treatment (50 μg/mL) (Thermo Fisher Scientific), at 37 °C, for 30 min, according to standard protocol [12]. Cell-cycle analysis was performed by flow-cytometry (BD Accuri C6, BD Biosciences, USA), and cells in G0/G1, S, G2/M and the sub-G0/G1 (apoptotic) phases were quantified by using FlowJo software.
Furthermore, for apoptosis assay, the Annexin V-fluorescein isothiocyanate (FITC)/7-amino-actinomycin D (7-AAD) Apoptosis Kit-559763 (BD Biosciences, USA) was used as per the manufacturer’s instructions. Briefly, cells (1 × 106 cells/dish) were seeded into 100 mm culture dishes and were maintained overnight in a medium containing 10% fetal bovine serum. The cells were collected by trypsinization and washed with phosphate buffered saline (PBS). Then, cells were resuspended in 200 µL of binding buffer. Annexin V staining was accomplished following product instructions (Clontech, Palo Alto, CA). In brief, 5 µL Annexin V-FITC and 5 µL 7-AAD were added to the samples for 15 min in the dark. However, for controls (unstained cells), they were stained with PE Annexin V (no 7-AAD) as well as with 7-AAD (no PE Annexin V). The cells were analyzed by flow cytometry (BD Accuri C6, BD Biosciences, San Jose, CA, USA). Data were presented as density plots of Annexin V-FITC and 7-AAD staining.
4.5. Cell Invasion Assay
Cell invasion assay was carried out in 24-well Biocoat Matrigel invasion chambers (pore size of 8 µm, Corning, USA) as per manufacturer’s protocol. In brief, the bottom chamber was filled with RPMI-1640 medium, and the upper chamber was seeded with untreated, as well as treated, cells (5 × 104 cells), and then incubated at 37 °C. After 24 h incubation, non-invasive cells were scraped with a cotton swab, and cells that migrated to the lower surface of the membrane were fixed with methanol and stained with 0.4% crystal violet. For quantification, cells were counted under the Leica DMi1 inverted microscope (Leica Microsystems, Wetzlar, Germany) in five predetermined fields, as previously described [81]. Percentage inhibition of invasive cells was calculated with respect to untreated cells. Each experiment was carried out in triplicates.
4.6. Soft Agar Colony Formation Assay
Next, we determined the number of colonies formed prior and post-treatment, using soft agar growth assay. A total of 2 × 103 cells of SKBR3 and ZR75-1 were placed in their medium containing 0.2% agar with/without 100 and 200 µL/mL of EA extract (treated and control cells, respectively) and plated in a 6-well plate covered with a layer of 0.4% agar prepared in RPMI-1640 medium. Colony formation was examined every 2 days for a period of 2 weeks. Colonies in each well were counted, using the Leica SP8 UV/Visible Laser confocal microscope (Leica Microsystems, Wetzlar, Germany).
4.7. Western Blot Analysis
We analyzed the expression levels of proteins involved in the molecular pathways, such as apoptosis by Western blot analysis, as previously described by our group [81]. Briefly, SKBR3 and ZR75-1 cells (1 × 106 cells) were seeded and treated with EA extract (100 and 200 µL/mL) for 48 h. Cell lysates were collected, and equal amounts of protein (30 μg) were resolved on 10% polyacrylamide gels and electroblotted onto PVDF membranes. The PVDF membranes were probed with the following primary antibodies: anti-mouse E-cadherin (AbcamID#: ab1416), anti-rabbit β-catenin (CST 9562), anti-rabbit phosphorylated β-catenin (CST 4176), anti-rabbit Vimentin (Abcam: abID# 92547), anti-rabbit Fascin (AbcamID#: ab183891), anti-mouse Bax (ThermoFisher Scientific: MA5-14003), anti-mouse Bcl-2 (Abcam: abID# 692), anti-rabbit Caspase-3 (Abcam: abID# 13847), anti-mouse ErbB2 (Abcam: abID# 16901), anti-rabbit phosphorylated ErbB2 (Abcam: abID# 47262), anti-rabbit JNK1/JNK2/JNK3 (Abcam: abID# 179461), and anti-rabbit phosphorylated-c-Jun (Ser73) (Cell Signal Technologies, ID# 9164). To ensure equal loading of protein samples, the membranes were re-probed with anti-mouse β-actin (Abcam: abID# 6276).
Immunoreactivity was detected by using ECL Western blotting substrate (Pierce Biotechnology, Rockford, IL, USA), as described by the manufacturer.
In order to obtain a relative quantification of protein expressions, images acquired from Western blotting were analyzed, using ImageJ software. The intensity of the bands relative to the β-actin bands was used to calculate a relative expression of proteins in each cell line.
4.8. Statistical Analysis
The data were presented as mean ± SEM from three independent experiments performed in triplicates, and a t-test was used to compare the difference between treated and untreated cells. To evaluate significance for cell cycle, a Chi-square test was performed to compare significance between the different phases. Data were analyzed by using GraphPad Prism software (version 8.4.3), and differences with p < 0.05 were considered significant.
5. Conclusions
To the best of our knowledge, this is the first report, on the effect of EA in HER2-positive breast cancer and its underlying mechanism. Furthermore, this study brings about novel therapeutic potential by demonstrating the induced inhibition of HER2 and JNK activation by EA plant extract in human breast cancer cells. Our study points out that the downregulation of JNK can be one of the molecular pathways responsible of increasing E-cadherin and β-catenin and decreasing the expressions of vimentin and fascin. This is an interesting finding, since it can be potentially used as a target to inhibit cell invasion of HER2-positive breast cancer cells by reversing EMT or inducing RELT. In parallel, our data also demonstrate that high consecrations of EA trigger apoptosis, particularly in breast cancer cells, which is associated with Bcl-2/Bax/caspase-3 signaling pathway in HER2-positive cancer cells. We believe that EA might act as a candidate therapeutic agent based on its anticancer activity which can pave the way for potential more advanced therapeutic approaches in breast cancer management, especially HER2-positive cases.
Acknowledgments
The authors would like to thank A. Kassab for her critical reading of the manuscript.
Author Contributions
Conceptualization, A.-E.A.M. and H.F.A.F.; methodology, A.J., A.S., and H.K.; validation, A.J., A.S., and I.G.; resources, A.-E.A.M. and H.F.A.F.; data curation, A.J. and I.G.; writing—original draft preparation, A.S., I.G., and A.J.; writing—review and editing, I.G., S.V., and A.-E.A.M.; supervision, A.-E.A.M. and H.F.A.F.; funding acquisition, A.-E.A.M. and H.F.A.F. A.J., A.S., and I.G. contributed equally to this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Our lab is supported by grants from Qatar University: # QUCP-CMED-2019-1, QUHI-CMED-19/20-1, and QUCG-CMED-20/21-2.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Sample Availability: Samples of the aqueous EA extract compounds are available from the corresponding author per reasonable request.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Gupta I., Burney I., Al-Moundhri M.S., Tamimi Y. Molecular genetics complexity impeding research progress in breast and ovarian cancers. Mol. Clin. Oncol. 2017;7:3–14. doi: 10.3892/mco.2017.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perou C.M., Sørlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Sareyeldin R.M., Gupta I., Al-Hashimi I., Al-Thawadi H.A., Al Farsi H.F., Vranic S., Al Moustafa A.-E. Gene Expression and miRNAs Profiling: Function and Regulation in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer. Cancers. 2019;11:646. doi: 10.3390/cancers11050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordano S.H., Temin S., Chandarlapaty S., Crews J.R., Esteva F.J., Kirshner J.J., Krop I.E., Levinson J., Lin N.U., Modi S., et al. Systemic Therapy for Patients with Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018;36:2736–2740. doi: 10.1200/JCO.2018.79.2697. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishna N., Temin S., Chandarlapaty S., Crews J.R., Davidson N.E., Esteva F.J., Giordano S.H., Kirshner J.J., Krop I.E., Levinson J., et al. Recommendations on Disease Management for Patients with Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer and Brain Metastases: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018;36:2804–2807. doi: 10.1200/JCO.2018.79.2713. [DOI] [PubMed] [Google Scholar]
- 7.Vranic S., Beslija S., Gatalica Z. Targeting HER2 expression in cancer: New drugs and new indications. Bosn. J. Basic Med. Sci. 2020 doi: 10.17305/bjbms.2020.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Angulo A.M., Morales-Vasquez F., Hortobagyi G.N. Overview of Resistance to Systemic Therapy in Patients with Breast Cancer. In: Yu D., Hung M.-C., editors. Breast Cancer Chemosensitivity. Springer; New York, NY, USA: 2007. pp. 1–22. [DOI] [PubMed] [Google Scholar]
- 9.De Melo F.H.M., Oliveira J.S., Sartorelli V.O.B., Montor W.R. Cancer Chemoprevention: Classic and Epigenetic Mechanisms Inhibiting Tumorigenesis. What Have We Learned So Far? Front. Oncol. 2018;8 doi: 10.3389/fonc.2018.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gullett N.P., Amin A.R., Bayraktar S., Pezzuto J.M., Shin D.M., Khuri F.R., Aggarwal B.B., Surh Y.-J., Kucuk O. Cancer Prevention with Natural Compounds. Semin. Oncol. 2010;37:258–281. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Hamidpour R., Hamidpour S., Hamidpour M., Shahlari M., Sohraby M., Shahlari N., Hamidpour R. Russian olive (Elaeagnus angustifolia L.): From a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-mutagenic and analgesic agent. J. Tradit. Complement. Med. 2016;7:24–29. doi: 10.1016/j.jtcme.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleh A.I., Mohamed I., Mohamed A.A., Abdelkader M., Yalcin H.C., Aboulkassim T., Batist G., Yasmeen A., Al Moustafa A.-E. Elaeagnus angustifolia Plant Extract Inhibits Angiogenesis and Downgrades Cell Invasion of Human Oral Cancer Cells via Erk1/Erk2 Inactivation. Nutr. Cancer. 2018;70:297–305. doi: 10.1080/01635581.2018.1412472. [DOI] [PubMed] [Google Scholar]
- 13.Pudenz M., Roth K., Gerhauser C. Impact of Soy Isoflavones on the Epigenome in Cancer Prevention. Nutrients. 2014;6:4218–4272. doi: 10.3390/nu6104218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X.-J., Jia S.-S. Fisetin inhibits laryngeal carcinoma through regulation of AKT/NF-κB/mTOR and ERK1/2 signaling pathways. Biomed. Pharmacother. 2016;83:1164–1174. doi: 10.1016/j.biopha.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Amereh Z., Hatami N., Shirazi F.H., Gholami S., Hosseini S.H., Noubarani M., Kamalinejad M., Andalib S., Keyhanfar F., Eskandari M.R. Cancer chemoprevention by oleaster (Elaeagnus angustifoli L.) fruit extract in a model of hepatocellular carcinoma induced by diethylnitrosamine in rats. EXCLI J. 2017;16:1046–1056. doi: 10.17179/excli2017-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saboonchian F., Jamei R., Sarghein S.H. Phenolic and flavonoid content of Elaeagnus angustifolia L. (leaf and flower) Avicenna J. phytomedicine. 2014;4:231–238. [PMC free article] [PubMed] [Google Scholar]
- 17.Boudraa S., Hambaba L., Zidani S., Boudraa H. Mineral and vitamin composition of fruits of five underexploited species in Algeria: Celtis australis L., Crataegus azarolus L., Crataegus monogyna Jacq., Elaeagnus angustifolia L. and Zizyphus lotus L. Fruits (Paris) 2010;65:75–84. doi: 10.1051/fruits/20010003. [DOI] [Google Scholar]
- 18.Fonia A., White I.R., White J.M.L. Allergic contact dermatitis toElaeagnusplant (Oleaster) Contact Dermat. 2009;60:178–179. doi: 10.1111/j.1600-0536.2008.01485.x. [DOI] [PubMed] [Google Scholar]
- 19.Taheri J.B., Anbari F., Maleki Z., Boostani S., Zarghi A., Pouralibaba F. Efficacy of Elaeagnus angustifolia Topical Gel in the Treatment of Symptomatic Oral Lichen Planus. J. Dent. Res. Dent. Clin. Dent. Prospect. 2010;4:29–32. doi: 10.5681/joddd.2010.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farzaei M.H., Bahramsoltani R., Abbasabadi Z., Rahimi R. A comprehensive review on phytochemical and pharmacological aspects of E laeagnus angustifolia L. J. Pharm. Pharmacol. 2015;67:1467–1480. doi: 10.1111/jphp.12442. [DOI] [PubMed] [Google Scholar]
- 21.Niknam F., Azadi A., Barzegar A., Faridi P., Tanideh N., Zarshenas M.M. Phytochemistry and Phytotherapeutic Aspects of Elaeagnus angustifolia L. Curr. Drug Discov. Technol. 2016;13:199–210. doi: 10.2174/1570163813666160905115325. [DOI] [PubMed] [Google Scholar]
- 22.Torbati M., Asnaashari S., Afshar F.H. Essential Oil from Flowers and Leaves of Elaeagnus Angustifolia (Elaeagnaceae): Composition, Radical Scavenging and General Toxicity Activities. Adv. Pharm. Bull. 2016;6:163–169. doi: 10.15171/apb.2016.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ya W., Shang-Zhen Z., Chun-Meng Z., Tao G., Jian-Ping M., Ping Z., Qiu-xiu R. Antioxidant and Antitumor Effect of Different Fractions of Ethyl Acetate Part from Elaeagnus angustifolia L. Adv. J. Food Sci. Technol. 2014;6:707–710. doi: 10.19026/ajfst.6.98. [DOI] [Google Scholar]
- 24.Kurdali F., Al-Shamma’A M. Natural abundances of15N and13C in leaves of some N2-fixing and non-N2-fixing trees and shrubs in Syria. Isot. Environ. Heal. Stud. 2009;45:198–207. doi: 10.1080/10256010903084126. [DOI] [PubMed] [Google Scholar]
- 25.Murakami A., Ashida H., Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269:315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H., Zhang M., Yu L., Zhao Y., He N., Yang X. Antitumor activities of quercetin and quercetin-5′,8-disulfonate in human colon and breast cancer cell lines. Food Chem. Toxicol. 2012;50:1589–1599. doi: 10.1016/j.fct.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Duo J., Ying G.G., Wang G.W., Zhang L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 2012;5:1453–1456. doi: 10.3892/mmr.2012.845. [DOI] [PubMed] [Google Scholar]
- 28.Kiseleva T.I., Chindyaeva L.N. Biology of oleaster (Elaeagnus angustifolia L.) at the northeastern limit of its range. Contemp. Probl. Ecol. 2011;4:218–222. doi: 10.1134/S1995425511020147. [DOI] [Google Scholar]
- 29.Faramarz S., Dehghan G., Jahanban-Esfahlan A. Antioxidants in different parts of oleaster as a function of genotype. BioImpacts. 2015;5:79–85. doi: 10.15171/bi.2015.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panahi Y., Alishiri G., Bayat N., Hosseini S.M., Sahebkar A. Efficacy of Elaeagnus Angustifolia extract in the treatment of knee osteoarthritis: A randomized controlled trial. EXCLI J. 2016;15:203–210. doi: 10.17179/excli2015-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahan Y., Dundar A.N., Aydın E., Kilci A., Dulger D., Kaplan F.B., Gocmen D., Celik G. Characteristics of Cookies Supplemented with Oleaster (Elaeagnus angustifolia L.) Flour. I Physicochemical, Sensorial and Textural Properties. J. Agric. Sci. 2013;5:160. doi: 10.5539/jas.v5n2p160. [DOI] [Google Scholar]
- 32.Tehranizadeh Z.A., Baratian A., Hosseinzadeh H. Russian olive (Elaeagnus angustifolia) as a herbal healer. BioImpacts. 2016;6:155–167. doi: 10.15171/bi.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asadiar L.S., Rahmani F., Siami A. Assessment of genetic diversity in the Russian olive (Elaeagnus angustifolia) based on ISSR genetic markers. Revista Ciência Agronômica. 2013;44:310–316. doi: 10.1590/S1806-66902013000200013. [DOI] [Google Scholar]
- 34.Natanzi M.M., Pasalar P., Kamalinejad M., Dehpour A.R., Tavangar S.M., Sharifi R., Ghanadian N., Balaei M.R., Gerayesh-Nejad S. Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta medica Iran. 2012;50:589–596. [PubMed] [Google Scholar]
- 35.Badrhadad A., Kh P., Mansouri K. In vitro anti-angiogenic activity fractions from hydroalcoholic extract of Elaeagnus angustifolia L. flower and Nepeta crispa L. arial part. J. Med. Plants Res. 2012;6:4633–4639. doi: 10.5897/jmpr11.1573. [DOI] [Google Scholar]
- 36.Choi Y., Ko Y.S., Park J.J., Choi Y., Kim Y., Pyo J.-S., Jang B.G., Hwang D.H., Kim W.H., Lee B.L. HER2-induced metastasis is mediated by AKT/JNK/EMT signaling pathway in gastric cancer. World J. Gastroenterol. 2016;22:9141–9153. doi: 10.3748/wjg.v22.i41.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han J.S., Crowe D.L. Jun amino-terminal kinase 1 activation promotes cell survival in ErbB2-positive breast cancer. Anticancer. Res. 2010;30:3407–3412. [PubMed] [Google Scholar]
- 38.Nahta R. Molecular Mechanisms of Trastuzumab-Based Treatment in HER2-Overexpressing Breast Cancer. ISRN Oncol. 2012;2012:1–16. doi: 10.5402/2012/428062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf-Yadlin A., Kumar N., Zhang Y., Hautaniemi S., Zaman M., Kim H.-D., Grantcharova V., Lauffenburger D.A., White F.M. Effects of HER2 overexpression on cell signaling networks governing proliferation and migration. Mol. Syst. Biol. 2006;2:54. doi: 10.1038/msb4100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maggioni D., Biffi L., Nicolini G., Garavello W. Flavonoids in oral cancer prevention and therapy. Eur. J. Cancer Prev. 2015;24:517–528. doi: 10.1097/CEJ.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y., Sarkissyan M., Vadgama J.V. Epithelial-Mesenchymal Transition and Breast Cancer. J. Clin. Med. 2016;5:13. doi: 10.3390/jcm5020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao X., Duan X., Jiang B. Fascin Induces Epithelial-Mesenchymal Transition of Cholangiocarcinoma Cells by Regulating Wnt/β-Catenin Signaling. Med. Sci. Monit. 2016;22:3479–3485. doi: 10.12659/MSM.897258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satelli A., Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 2011;68:3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao C., Wu C.-H., Hu H.-Z. LncRNA UCA1 promotes epithelial-mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2016;20:2819–2824. [PubMed] [Google Scholar]
- 45.Wang J., Kuiatse I., Lee A.V., Pan J., Giuliano A., Cui X. Sustained c-Jun-NH2-kinase activity promotes epithelial-mesenchymal transition, invasion, and survival of breast cancer cells by regulating extracellular signal-regulated kinase activation. Mol. Cancer Res. 2010;8:266–277. doi: 10.1158/1541-7786.MCR-09-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bubici C., Papa S. JNK signalling in cancer: In need of new, smarter therapeutic targets. Br. J. Pharmacol. 2014;171:24–37. doi: 10.1111/bph.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogoyevitch M.A., Kobe B. Uses for JNK: The Many and Varied Substrates of the c-Jun N-Terminal Kinases. Microbiol. Mol. Biol. Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leppä S., Saffrich R., Ansorge W., Bohmann D. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J. 1998;17:4404–4413. doi: 10.1093/emboj/17.15.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L., Porter A.G., Feng Z. JNK-dependent Phosphorylation of c-Jun on Serine 63 Mediates Nitric Oxide-induced Apoptosis of Neuroblastoma Cells. J. Biol. Chem. 2004;279:4058–4065. doi: 10.1074/jbc.M310415200. [DOI] [PubMed] [Google Scholar]
- 50.Tournier C. The 2 Faces of JNK Signaling in Cancer. Genes Cancer. 2013;4:397–400. doi: 10.1177/1947601913486349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lloyd A., Yancheva N., Wasylyk B. Transformation suppressor activity of a Jun transcription factor lacking its activation domain. Nature. 1991;352:635–638. doi: 10.1038/352635a0. [DOI] [PubMed] [Google Scholar]
- 52.Eferl R., Wagner E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 53.Min L., Ji Y., Bakiri L., Qiu Z., Cen J., Chen X., Chen L., Scheuch H., Zheng H., Qin L., et al. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat. Cell Biol. 2012;14:1203–1211. doi: 10.1038/ncb2590. [DOI] [PubMed] [Google Scholar]
- 54.Nateri A.S., Spencer-Dene B., Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- 55.Maeda S., Karin M. Oncogene at last—c-Jun promotes liver cancer in mice. Cancer Cell. 2003;3:102–104. doi: 10.1016/S1535-6108(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 56.Kandouz M., Alachkar A., Zhang L., Dekhil H., Chehna F., Yasmeen A., Al Moustafa A.-E. Teucrium polium plant extract inhibits cell invasion and motility of human prostate cancer cells via the restoration of the E-cadherin/catenin complex. J. Ethnopharmacol. 2010;129:410–415. doi: 10.1016/j.jep.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 57.Koirala N., Thuan N.H., Ghimire G.P., Van Thang D., Sohng J.K. Methylation of flavonoids: Chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzym. Microb. Technol. 2016;86:103–116. doi: 10.1016/j.enzmictec.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Roh H., Pippin J., Drebin J.A. Down-regulation of HER2/neu expression induces apoptosis in human cancer cells that overexpress HER2/neu. Cancer Res. 2000;60:560–565. [PubMed] [Google Scholar]
- 59.Lei K., Nimnual A., Zong W.-X., Kennedy N.J., Flavell R.A., Thompson C.B., Bar-Sagi D., Davis R.J. The Bax Subfamily of Bcl2-Related Proteins Is Essential for Apoptotic Signal Transduction by c-Jun NH2-Terminal Kinase. Mol. Cell. Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gross A., McDonnell J.M., Korsmeyer S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 61.Davis R.J. Signal Transduction by the JNK Group of MAP Kinases. Cell. 2000;103:239–252. doi: 10.1016/S0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 62.Corazza N., Jakob S., Schaer C., Frese S., Keogh A., Stroka D., Kassahn D., Torgler R., Mueller C., Schneider P., et al. TRAIL receptor–mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J. Clin. Investig. 2006;116:2493–2499. doi: 10.1172/JCI27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim S.-C., Jeon H.J., Kee K.-H., Lee M.J., Hong R., Han S.I. Involvement of DR4/JNK pathway-mediated autophagy in acquired TRAIL resistance in HepG2 cells. Int. J. Oncol. 2016;49:1983–1990. doi: 10.3892/ijo.2016.3699. [DOI] [PubMed] [Google Scholar]
- 64.Puduvalli V.K., Sampath D., Bruner J.M., Nangia J., Xu R., Kyritsis A.P. TRAIL-induced apoptosis in gliomas is enhanced by Akt-inhibition and is independent of JNK activation. Apoptosis. 2005;10:233–243. doi: 10.1007/s10495-005-6078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Recio-Boiles A., Ilmer M., Rhea P.R., Kettlun C., Heinemann M.L., Ruetering J., Vykoukal J., Alt E. JNK pathway inhibition selectively primes pancreatic cancer stem cells to TRAIL-induced apoptosis without affecting the physiology of normal tissue resident stem cells. Oncotarget. 2016;7:9890–9906. doi: 10.18632/oncotarget.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reilly E.O., Tirincsi A., Logue S.E., Szegezdi E. The Janus Face of Death Receptor Signaling during Tumor Immunoediting. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng X., Xiao L., Lang W., Gao F., Ruvolo P., May W.S., Jr. Novel Role for JNK as a Stress-activated Bcl2 Kinase. J. Biol. Chem. 2001;276:23681–23688. doi: 10.1074/jbc.M100279200. [DOI] [PubMed] [Google Scholar]
- 69.Maundrell K., Antonsson B., Magnenat E., Camps M., Muda M., Chabert C., Gillieron C., Boschert U., Vial-Knecht E., Martinou J.-C., et al. Bcl-2 Undergoes Phosphorylation by c-Jun N-terminal Kinase/Stress-activated Protein Kinases in the Presence of the Constitutively Active GTP-binding Protein Rac1. J. Biol. Chem. 1997;272:25238–25242. doi: 10.1074/jbc.272.40.25238. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto K., Ichijo H., Korsmeyer S.J. BCL-2 Is Phosphorylated and Inactivated by an ASK1/Jun N-Terminal Protein Kinase Pathway Normally Activated at G2/M. Mol. Cell. Biol. 1999;19:8469–8478. doi: 10.1128/MCB.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Breitschopf K., Haendeler J., Malchow P., Zeiher A.M., Dimmeler S. Posttranslational Modification of Bcl-2 Facilitates Its Proteasome-Dependent Degradation: Molecular Characterization of the Involved Signaling Pathway. Mol. Cell. Biol. 2000;20:1886–1896. doi: 10.1128/MCB.20.5.1886-1896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dimmeler S., Breitschopf K., Haendeler J., Zeiher A.M. Dephosphorylation Targets Bcl-2 for Ubiquitin-dependent Degradation: A Link between the Apoptosome and the Proteasome Pathway. J. Exp. Med. 1999;189:1815–1822. doi: 10.1084/jem.189.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ito T., Deng X., Carr B., May W.S. Bcl-2 Phosphorylation Required for Anti-apoptosis Function. J. Biol. Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 74.Ruvolo P.P., Deng X., May W.S. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001;15:515–522. doi: 10.1038/sj.leu.2402090. [DOI] [PubMed] [Google Scholar]
- 75.Tournier C., Dong C., Turner T.K., Jones S.N., Flavell R.A., Davis R.J. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cho Y.-L., Tan H.W.S., Saquib Q., Ren Y., Ahmad J., Wahab R., He W., Bay B., Shen H.-M. Dual role of oxidative stress-JNK activation in autophagy and apoptosis induced by nickel oxide nanoparticles in human cancer cells. Free. Radic. Biol. Med. 2020;153:173–186. doi: 10.1016/j.freeradbiomed.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 77.Dhanasekaran D.N., Reddy E.P. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dou Y., Jiang X., Xie H., He J., Xiao S.-S. The Jun N-terminal kinases signaling pathway plays a "seesaw" role in ovarian carcinoma: A molecular aspect. J. Ovarian Res. 2019;12:99. doi: 10.1186/s13048-019-0573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gkouveris I., Nikitakis N.G. Role of JNK signaling in oral cancer: A mini review. Tumor Biol. 2017;39 doi: 10.1177/1010428317711659. [DOI] [PubMed] [Google Scholar]
- 80.Liu J., Lin A. Role of JNK activation in apoptosis: A double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 81.Yasmeen A., Alachkar A., Dekhil H., Gambacorti-Passerini C., Al Moustafa A.-E. Locking Src/Abl Tyrosine Kinase Activities Regulate Cell Differentiation and Invasion of Human Cervical Cancer Cells Expressing E6/E7 Oncoproteins of High-Risk HPV. J. Oncol. 2010;2010:1–10. doi: 10.1155/2010/530130. [DOI] [PMC free article] [PubMed] [Google Scholar]