Abstract
The prevalence of diabetes mellitus (DM), considered one of the most common metabolic disorders, has dramatically increased and resulted in higher rates of morbidity and mortality around the world in the past decade. It is well known that insulin resistance in target tissues and a deficiency in insulin secretion from pancreatic β-cells are the main characteristics of type 2 diabetes. The aim of this study was the bio-evaluation of compounds isolated from three selected plant species: namely, Salvia africana-lutea, Leonotis ocymifolia, and Plectranthus madagascariensis, for their glucose-uptake ability. Methanolic extracts were produced from the aerial parts of each plant. Compounds were identified using different spectroscopic techniques. The glucose-uptake ability of each compound was then evaluated in mammalian cells using 2-deoxyglucose-6-phosphate. The cytotoxicity of each compound was established via the MTT assay. Chromatographic purification of the three plant species yielded sixteen pure terpenoids. Compounds 1 (p = 0.0031), 8 (p = 0.0053), and 6 (p = 0.0086) showed a marked increase in glucose uptake, respectively. Additionally, 1, 4, and 6 exhibited cytotoxicity toward mammalian tissue with a decrease in cell viability of ~70%, ~68%, and ~67%, respectively. The results suggested that several compounds demonstrated a marked increase in glucose uptake, while two of the compounds exhibited signs of cytotoxicity. It may, therefore, be suggested that these compounds be considered as potential candidates for novel plant-derived alternative therapies in the treatment of type 2 diabetes.
Keywords: diabetes mellitus, Lamiaceae, terpenoids, abietane diterpenes, ursolic acid, glucose uptake, toxicity, insulin resistance
1. Introduction
Diabetes mellitus (DM) is considered as one of the most common metabolic disorders with high rates of morbidity and mortality around the world [1]. It is well known that insulin is vital in glucose homeostasis, as it stimulates the transport of blood glucose within the skeletal muscle [2]. However, insulin resistance in target tissues and deficiency of insulin secretion from pancreatic β-cells are the main characteristics of type 2 diabetes. In addition, characterization of DM also includes a decrease in peripheral glucose uptake into muscle, adipose, or liver cells as well as an increase in endogenous glucose production, causing the increase in blood glucose concentration [3,4,5]. Therefore, agents with a capacity to stimulate glucose uptake in these tissues can be used to improve insulin resistance and consequently treat diabetes [6]. A huge number of synthetic antidiabetic agents, such as acarbose, miglitol, sulfonylurea, metformin, and thiozolidinedione, are readily available in the market. However, their effectiveness is limited due to high cost and adverse side effects [7,8], with the exception of the widespread use of metformin due to its relatively low cost. Therefore, there is a great need to develope potent natural antidiabetic products of high-safety margin. Furthermore, selected species from the Lamiaceae family have long been used to treat a plethora of ailments. These include Leonotis ocymifolia, traditionally used in Ethiopia for the treatment of diabetes [9]; Plectranthus madagascariensis, used for scabies and small wounds as well as in the treatment of colds, asthma, cough, and chest complaints [10], also reported to be an effective inhibitor of alpha-glucosidase [11], and Salvia africana-lutea, which is traditionally used for the treatment of different kinds of ailments and/or diseases, such as coughs, sexual debility, mental, and nervous conditions, throat inflammation, chronic bronchitis, tuberculosis, influenza, stomach-ache, diarrhea, and urticaria [12]. It has been reported to be a rich source of terpenoids with potent alpha-glucosidase and alpha-amylase inhibitory activities [8]. Numerous terpenoids isolated from Lamiaceae, such as ursolic acid, (8) have been reported to stimulate glucose uptake in adipocytes through the phosphatidylinositol (PI) 3-kinase (PI3K) pathway and enhance glucose transporter 4 (GLUT4) translocation and expression [13]. In addition, Oleanolic acid (7) and its derivatives have also been reported to up-regulate the expression of GLUT4, which increases the glucose-uptake activity in adipose and muscle cell lines [14,15]. Carnosic acid and carnosol also activate Akt and AMPKα signaling and then enhance glucose uptake in L6 myotubes as well as stimulate glucose uptake in L6 myotubes [16,17,18].
The present study primarily examines the glucose-uptake activity as well as the cytotoxicity of different phytochemical constituents isolated from three different plant species of Lamiaceae family: S. africana-lutea, P. madagascariensis, and L. ocymifolia.
2. Results
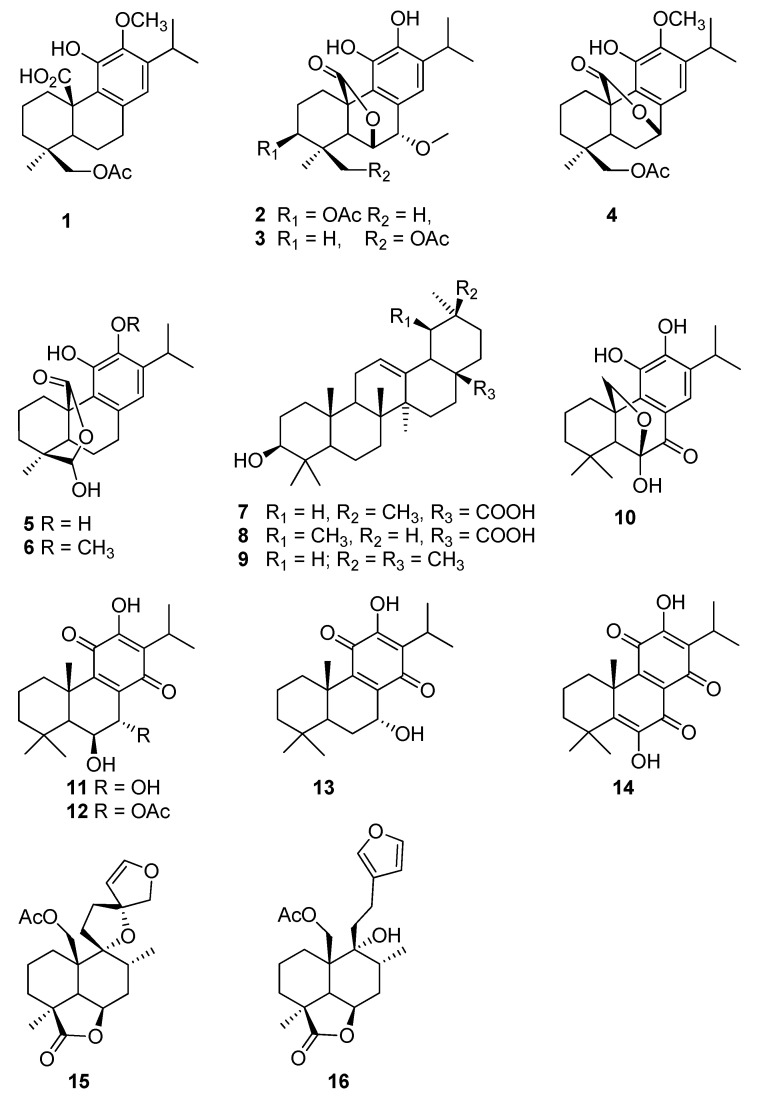
Sixteen terpenoids (Figure 1), including thirteen diterpenes and three triterpenes, were purified from three Lamiaceae species, S. africana-lutea, P. madagascariensis, and L. ocymifolia and tested for their ability to regulate the glucose intake in HEK293 kidney cells line.
Figure 1.
Chemical structures of the isolated compounds.
From S. africana lutea, six abietane diterpenes were isolated and identified as 19-acetoxy-12-methoxycarnosic acid (1), 3β-acetoxy-7α-methoxyrosmanol (2), 19-acetoxy-7α-methoxyrosmanol (3), 19-acetoxy-12-methoxy carnosol (4), clinopodiolides A (5), and B (6), in addition to three triterpenes, oleanolic and ursolic acids (7, 8) and β-amyrin (9) [19,20,21,22,23,24].
The phytochemical analysis of P. madagascariensis total extract resulted in the isolation of five known compounds namely carnosolon (10), 6β,7α-dihydroxyroyleanone (11), 7α-acetoxy-6β-hydroxyroyleanone (12), horminone (13), and coleon U quinone (14). The NMR data of the isolated abietane diterpenoids were compared to that of previously isolated constituents from the plant and other species of the genus Plectranthus [25,26,27,28].
From L. ocymofolia, two labdane diterpenes were isolated and identified as Leonurun (15) and 20-acetoxy-marrubiin (16) [29,30].
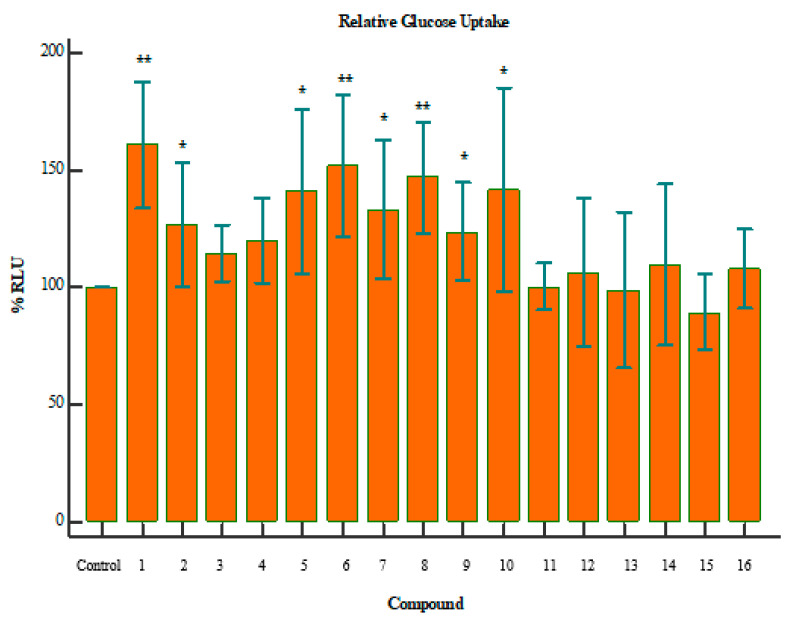
The results demonstrated that when cells are exposed to 2-deoxyglucose (2DG), there are transported across the membrane and rapidly phosphorylated in the same manner as glucose. However, enzymes that further modify glucose-6-phosphate (G6P) cannot modify 2DG6P, and thus, a membrane-impermeable analyte accumulates in the cell. After a brief period of incubation, the acidic Stop Buffer is added to lyse cells, terminates uptake and destroys any NADPH. A high-pH buffer solution (neutralization buffer) is then added to neutralize the acid. A detection reagent is added to the sample wells. Glucose-6-phosphate dehydrogenase oxidizes the deoxyglucose to 6-phosphodeoxygluconate and simultaneously reduces NADP+ to NADPH. The reductase uses NADPH to convert the proluciferin to luciferin, which is then used by Ultra-Glo™ recombinant luciferase to produce a luminescent signal that is proportional to the concentration of 2DG6P. Figure 2 indicates the relative glucose uptake for a given compound, compared to the control. The p-values in these graphs were calculated using independent two-tailed T-test, where 0.05 is the threshold for significance.
Figure 2.
Relative glucose uptake of compounds 1–16; where the p-value is indicative of the statistical significance versus the control, using an independent two-tailed T-test. * p < 0.05, ** p < 0.01. Compounds 1 (p = 0.0031), 8 (p = 0.0053), and 6 (p = 0.0086) showed a marked increase in glucose uptake.
3. Discussion
With the global rise in the cost of medicines, many are turning to alternative forms of treatment. Herbal medicine is traditionally used in many cultures globally as a more cost-effective method of treatment. The data presented herein aid in confirming the usefulness of specific plant-derived compounds (phytochemicals) in the treatment of diabetes mellitus [31,32].
Plectranthus madagascariensis has been reported to be an effective inhibitor of alpha-glucosidase and a promising source of secondary metabolites with significant alpha-glucosidase inhibitory activity. Three abietane diterpenoids, such as 6β,7α-dihydroxyroyleanone (11), 7α-acetoxy-6β-hydroxyroyleanone (12), coleon U quinone (14), in addition to rosmarinic acid, were isolated from methanol extract of P. madagascariensis and exhibited alpha-glucosidase inhibitory activity with IC50 values of 274.9 ± 12.3, 108.2 ± 1.3, 142.7 ± 1.4 μmol/L, and 33.0 ± 4.6 μmol/L, respectively [10].
Etsassala et al. [8] have reported on the in vitro bio-evaluation of terpenes isolated from Salvia africana lutea against alpha-glucosidase and alpha-amylase. The results showed strong inhibitory activities for 8, 10, and 7 with IC50 values of 11.3 ± 1.0, 17.1 ± 1.0, and 22.9 ± 2.0 µg/mL, respectively. Compound 7 demonstrated the strongest in vitro alpha-amylase inhibitory activity among the tested compounds with IC50 of 12.5 ± 0.7 µg/mL, followed by compounds 8 and 10 with IC50 values of 66.1 ± 2.0 µg/mL and 76.6 ± 2.1 µg/mL, respectively. [8]. Other studies also confirmed the bioactivity demonstrated by 7 and 8 [33]. Leonurun (15) and 20-acetoxy-9α,l3-dihydroxy-15(16)-epoxylabd-14-en-6β(19)-lactone (16) were not active against alpha-glucosidase and amylase comparing with other compounds when tested up to 50 µg/mL.
Compounds 1, 8, and 6 showed a marked increase in glucose uptake (Figure 2). The exact mechanism of this action is yet to be derived, but it is suspected that these compounds increase glucose sensitivity through stimulation of glucose metabolism. In addition, it may be concluded that these compounds could potentially aid in the treatment of diabetes mellitus. Compound 8 has been reported to stimulate glucose uptake in 3T3-L1 adipocytes through the PI3K pathway. Additionally, 8 has also been reported to lower blood glucose and improve insulin resistance and diabetes which corroborate with our findings [13,33,34]. 7 has been reported to up-regulate the expression of GLUT4, which increases the glucose-uptake activity in adipose and muscle cell lines [14,35].
However, it is important to note that the raw compound may not be effectively taken up by the cells [36]. This could be influenced by a number of factors including the number of surviving cells after treatment, the solubility of the compound, binding affinity between the compound and the cell membrane, and the length of treatment incubation [36].
Furthermore, although a marginal increase in glucose uptake was seen in the remaining compounds, the statistical significance of those findings is lacking. It may be suggested that they are more effective in combination with other compounds, or that they simply have an even weaker cellular uptake efficiency. Nonetheless, further investigation will be required to define the capabilities of these compounds.
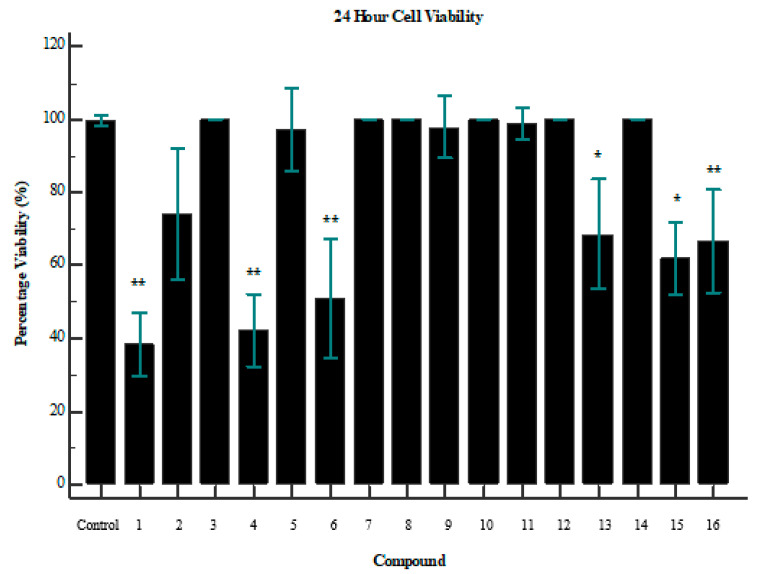
Interestingly, an overlap between glucose uptake and cytotoxicity exists for some of the compounds. Compounds 1 and 6 both improved glucose uptake and showed signs of cytotoxicity (Figure 3). Compound 1 reduced cell viability by ~70% (p = 0.0056), while 6 reduced cell viability by ~52% (p = 0.0060). However, it is important to note the difference in dosage between the assays. Cell-viability assays were performed with a concentration of 250 µg/mL, while glucose-uptake assays were performed with a maximum dose of 100 µg/mL. It is, therefore, confirmed that beyond a dosage of 100 µg/mL the compound becomes toxic to the cells. This is a crucial consideration when evaluating compounds as potential drug targets. Further investigation of the minimum usable dosage for improving glucose uptake is required, as well as the minimum dosage that causes toxicity in a larger collection of cell types.
Figure 3.
Cell viability of tested compounds after 24 h; where the p-value is indicative of the statistical significance versus the control, using an independent two-tailed T-test (n = 3). * p < 0.05, ** p < 0.01. Compounds 1 (p = 0.0056), 4 (p = 0.0017), and 6 (p = 0.0060) showed the greatest impact on cell viability.
4. Materials and Methods
4.1. Chemicals and Reagents
Organic solvents, such as methanol (HPLC grade), ethanol, ethyl acetate, and hexane, were supplied by Merck (Cape Town, South Africa). Thin layer chromatography (TLC) was performed on normal-phase (Merck) Silica gel 60 PF254 pre-coated aluminum plates. Column chromatography was conducted on silica gel 60 H (0.040–0.063 mm particle size, Merck, Cape Town, South Africa) and Sephadex LH-20 (Sigma-Aldrich, Cape Town, South Africa).
NMR spectra were recorded on an Avance 400 MHz NMR spectrometer (Bruker, Rheinstetten, Germany)/Varian 200 MHz Mercury, in deuterated chloroform and acetone, using the solvent signals as the internal reference. HRMS analysis was conducted on an Ultimate 3000 LC (Dionex, Sunnyvale, CA, USA) coupled to a Bruker QTOF with an electrospray ionization (ESI) interface working in the positive ion mode. Preparative HPLC was used for further isolation of pure compounds using HPLC methanol and distilled water.
Renocytes (HEK293 kidney cells) were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM containing essential amino acids, sodium pyruvate, and l-glutamine. Cell seeding was done on 24-well plates (50,000 cells/well) for the 2-deoxy-[3H]-D-glucose assay.
4.2. Plant Material
Salvia africana-lutea and Leonotis ocymifolia aerial parts were collected in May, 2018, from Cape Flats Nature Reserve, University of the Western Cape, and Plectranthus madagascariensis was collected in February 2019 from Cape Peninsula University of Technology, Bellville campus. The identification of the plants was carried out by Prof. Christopher Cupido (Department of Botany, Fort Hare University, South Africa), with herbarium numbers NBG1465544-0, NBG1465551-0, and NBG1465552-0 respectively.
4.3. Extraction and Purification of Chemical Constituents
Compounds 1–9 were available in the lab from previous study and isolated from S. africana lutea [8]. Compounds 10–14 were isolated from P. madagascariensis as follows: The aerial parts of P. madagascariensis were extracted with DCM-MeOH (3:1) and the total extract (13.0 g) was subjected to silica gel column chromatography using Hex/EtOAc gradient of increasing polarity to yield 16 main fractions. The main fraction III (90.8 mg) was subjected to isocratic column chromatography using Hex/EtOAc gradient (90:10) to yield 13 (13.5 mg). Main fraction VI (200 mg) was subjected to sephadex LH-20 using 95% methanol (MeOH) and 5% deionized water (DW), then isocratic silica gel column chromatography using Hex/EtOAc gradient (98:2) to yield 12. (61.9 mg). The main fraction VIII (97 mg) was applied to a sephadex LH-20 (5% aqueous MeOH) to produce 11 (25.7 mg). Main fraction XI (62.21 mg) was subjected to sequential Sephadex LH-20 (5% aqueous MeOH), then HPLC using gradient of acetonitrile/DW (60% to 80% in 30 min, then 100% acetonitrile for 15 min) to produce 14 (Rt 39.5 min, 6 mg). Main fraction XIII and XIV (400 mg) were combined and chromatographed to Sephadex LH-20 using MeOH/DW (95:5) to produce 10 (26.7 mg).
Compounds 15 and 16 were isolated from Leonotis ocymifolia var. raineriana as follows: The fresh plant materials of (1.0 kg) were blended and extracted with methanol (4.5 L), and after filtration and solvent evaporation, the extract (42.0 g) was loaded on silica gel column and eluted using gradient of Hex/EtOAc in order of increasing polarity. Fraction 12 yielded crystals, which was identified as compound 15. Fraction 20 after fractionation using silica gel column Hex: EtOAc (80: 20 to 70:30) gave compound 16.
4.4. Spectroscopic Data of Compounds 10–16
Compound 10.1H-NMR (400 MHz, CDCl3), δH 7.65 (s, H-14), 4.29 (d, J = 7.5 Hz, Hβ-20), 3.37 (d, J = 7.5 Hz, Hα-20), 3.02, (sept, J = 7.1 Hz, H-15), 1.31 (s, Me-19), 1.17 (d, J = 7.1 Hz, H-16), 1.16 (d, J = 7.1 Hz, H-17), 1.04 (s, Me-18). 13C-NMR (100 MHz, CDCl3), δC 192.8 (C-7), 148.3 (C-12), 140.5 (C-11), 137.7 (C-9), 133.3 (C-13), 120.1 (C-14), 121.4 (C-8), 105.2 (C-6), 72.0 (C-20), 58.2 (C-5), 51.47 (C-10), 41.33 (C-3), 33.7 (C-18), 32.4 (C-4), 29.6 (C-1), 27.1 (C-15), 22.5 (C-17), 22.4 (C-16), 22.2 (C-19), 18.5 (C-2).
Compound 11.1H-NMR (400 MHz, CDCl3), δH 4.53 (d, J = 1.5 Hz, H-7), 4.46 (br s, H-6), 3.18 (sept, J = 7.1 Hz, H-15), 1.62 (s, Me-20), 1.27 (s, Me-19), 1.23 (d, J = 7.1 Hz, H-16), 1.23 (d, J = 7.1 Hz, H-17), 1.06 (s, Me-18). 13C-NMR (100 MHz, CDCl3), δC 189.5 (C-14), 183.1 (C-11), 151.2 (C-12), 147.6 (C-9), 140.9 (C-8), 124.3 (C-13), 69.3 (C-6), 69.1 (C-7), 49.5 (C-5), 42.3 (C-3), 38.6 (C-10), 38.4 (C-1), 33.7 (C-4), 33.5 (C-18), 24.3 (C-19), 24.0 (C-15), 21.6 (C-20), 19.9 (C-17), 19.8 (C-16), 19.0 (C-2).
Compound 12.1H-NMR (400 MHz, CDCl3), δH 5.60 (d, J = 1.8 Hz, H-7), 4.24 (s, H-6), 3.09 (sept, J = 7.1 Hz, H-15), 1.98 (s, CH3CO), 1.55 (s, Me-20), 1.16 (s, Me-19), 1.13 (d, J = 7.1 Hz, H-16), 1.11 (d, J = 7.1 Hz, H-17), 0.92 (s, Me-18). 13C-NMR, δC (100 MHz, CDCl3) 186.0 (C-14), 183.1 (C-11), 151.2 (C-12), 150.1 (C-9), 137.0 (C-8), 124.3 (C-13), 69.0 (C-7), 66.4 (C-6), 49.7 (C-5), 42.33 (C-3) 38.6 (C-4) 38.3 (C-1), 33.6 (C-10), 33.5 (C-18), 18.9 (C-2), 24.1 (C-15), 23.6 (C-19), 21.3 (C-20), 19.8 (C-17), 19.6 (C-16), 21.0/170.1 (CH3CO).
Compound 13.1H-NMR (400 MHz, CDCl3), δH 4.73 (d, J = 1.5 Hz, H-7), 3.16 (sept, J = 7.1 Hz, H-15), 1.22 (s, Me-20), 1.21 (d, J = 7.1Hz, H-16), 1.20 (d, J = 7.1 Hz, H-17), 0.98 (s, Me-18), 0.90 (s, Me-19). 13C-NMR (100 MHz, CDCl3) δC 189.2 (C-14), 183.9 (C-11), 151.1 (C-12), 147.8 (C-9), 143.3 (C-8), 124.2 (C-13), 63.2 (C-7), 45.8 (C-5), 41.13 (C-3), 39.8 (C-10), 35.8 (C-1), 33.2 (C-4), 33.1 (C-18), 25.8 (C-6), 24.0 (C-15), 21.7 (C-19), 19.9 (C-16), 19.8 (C-17), 19.0 (C-2), 18.4 (C-20).
Compound 14.1H-NMR (400 MHz, CDCl3), δH 3.22 (sept, J = 7.0 Hz, H-15), 1.64 (s, Me-20), 1.43 (s, Me-18), 1.42 (s, Me-19), 1.25 (d, J = 7.1 Hz, H-16), 1.24 (d, J = 7.0 Hz, H-17). 13C-NMR (100 MHz, CDCl3), δC 184.3 (C-14), 183.6 (C-11), 177.5 (C-7), 155.1 (C-9), 150.7 (C-12), 146.8 (C-6), 143.3 (C-5), 126.8 (C-8), 126.0 (C-13), 41.4 (C-10), 36.4 (C-4), 36.3 (C-3), 30.8 (C-1), 29.1 (C-19), 27.5 (C-20), 27.2 (C-18), 24.4 (C-15), 19.8 (C-16), 19.8 (C-17), 17.7 (C-2).
Compound 15.1H-NMR (200 MHz, CDCl3), δH 6.34 (br d, J= 2.6 Hz, H-15), 5.04 (br d, J= 2.6 Hz, H-14), 4.59 (br t, J= 4.0 Hz, H-6), 4.26 and 4.17 (d each, J= 12.5 Hz, CH2-20), 4.28 and 3.95 (d, each, J = 10.6 Hz, H-16a, b), 1.12 (s, Me-18), 0.73 (d, J = 6.4 Hz, Me-17). 13C-NMR (50 MHz CDCl3) δC 182.3 (C-19), 147.9 (C-15), 107.0 (C-14), 91.3 (C-13), 88.7 (C-9), 79.7 (C-16), 75.5 (C-6), 65.6 (C-20), 46.4 (C-5), 43.2 (C-4), 42.0 (C-10), 37.0 (C-12), 32.4 (C-8), 31.5 (C-7), 31.4 (C-11), 28.0 (C-3), 23.4 (C-18), 22.2 (C-1), 17.6 (C-2), 17.4 (C-17), 20.7/169.8 (COCH3).
Compound 16.1H-NMR (200 MHz, CDCl3) δH 7.34 (br s, H15), 7.21 (br s, H-16), 6.24 (br s, H-14), 5.13 (br s, H-6), 4.27/4.62 (br d each J= 12.3 Hz, CH2-20), 1.01 (s, Me-18), 0.96 (d, J = 6.3 Hz, Me-17). 13C-NMR (50 MHz, CDCl3) δC 175.9 (C-19), 143.4 (C-15), 138.4 (C-16), 124.2 (C-13), 110.3 (C-14), 75.8 (C-20), 74.7 (C-9), 69.3 (C-6), 46.5 (C-5), 43.9 (C-11), 41.0 (C-10), 40.8 (C-4), 39.6 (C-3), 33.3 (C-8), 30.1 (C-1), 32.8 (C-7), 22.3 (C-18), 22.4 (C-2), 20.3 (C-12), 15.6 (C-17), 20.9/170.2 (COCH3).
4.5. Glucose-Uptake Assay
The method for measuring glucose uptake in mammalian cells was based on the detection of 2-deoxyglucose-6-phosphate and was performed according to the manufacturer’s guidelines listed in the Table 1. When cells are exposed to 2-deoxyglucose (2DG), there are transported across the membrane and rapidly phosphorylated in the same manner as glucose. However, enzymes that further modify glucose-6-phosphate (G6P) cannot modify 2DG6P, and thus, a membrane-impermeable analyte accumulates in the cell. After a brief period of incubation, the acidic stop buffer is added to lyse cells, terminates uptake and destroys any NADPH. A high-pH buffer solution (neutralization buffer) is then added to neutralize the acid. A detection reagent is added to the sample wells. Glucose-6-phosphate dehydrogenase oxidizes the deoxyglucose to 6-phosphodeoxygluconate and simultaneously reduces NADP+ to NADPH. The reductase uses NADPH to convert the proluciferin to luciferin, which is then used by Ultra-Glo™ recombinant luciferase to produce a luminescent signal that is proportional to the concentration of 2DG6P.
Table 1.
Constituents of glucose-uptake assay.
| Reagent | 1 Reaction (µL) | 50 Reactions (µL) |
|---|---|---|
| Luciferase reagent | 100 | 5000 |
| NADP+ | 1 | 50 |
| G6PDH | 2.5 | 125 |
| Reductase | 0.5 | 25 |
| Reductase substrate | 0.0625 | 3 |
The reaction mixtures were incubated at room temperature for 1 h. After 1 h, the cells were incubated with various concentrations of each compound, and the cells were washed with 100µl PBS. A volume of 50 µL 1 mM 2DG was added to each well and allowed to incubate for 10 min. A volume of 25 µL stop buffer was added to each well and shaken briefly. Thereafter, 25 µL of neutralization buffer was added to each well and shaken briefly. Finally, a volume of 100 µL of reductase substrate mix was added and the plate shaken briefly. The plate was then incubated at room temperature for 30 min and read on a plate reader at 15 min intervals for 2 h.
4.6. Cytotoxicity Assay (MTT)
The cytotoxic effect of each compound on human embryonic kidney (HEK293) cells was assessed following the well-established MTT protocol. Cells were cultured in DMEM containing essential amino acids, sodium pyruvate, and L-glutamine at 37 °C in 96-well microtiter plates (10,000 cells/well). The plates were exposed to a dose of 250 µg of each compound for 24 h. Untreated cells served as control. After treatment, the medium was separated, and cells were incubated with 200 µL of MTT in fresh medium at 37 °C for 4 h. The resultant formazan crystals from the mitochondrial reduction in MTT were solubilized in DMSO. The absorbance of each sample was determined using a microplate absorbance reader at 570 nm, and the percentage of cell viability was calculated using the following equation: cell viability (%) = (absorbance of test – absorbance of background/absorbance of control – absorbance of background) × 100, according to manufacturer instructions (Dojindo, Maryland, MD, USA).
5. Conclusions
This present work is the first scientific report on the investigation of the glucose-uptake activity and cytotoxicity of abietane and labdane diterpenes and triterpenes isolated from selected plants species from Lamiaceae family, and the results suggested that 1, 8, and 6 showed a marked increase in glucose uptake, while 1 and 6 exhibited signs of cytotoxicity. Oleanolic (7) and ursolic (8) acids have been reported to increase the glucose uptake using several bioassays [13,14,15]; however, in this report, ursolic acid (8) exhibited more significant activity over oleanolic acid (7). It may, therefore, be suggested that ursolic acid be considered as a potential candidate for novel plant-derived alternative therapies in the treatment of type 2 diabetes.
Author Contributions
Designing and manuscript drafting, B.P.; N.G.E.R.E.; performing of phytochemical isolation K.O.N.; T.J.M.; N.G.E.R.E.; experimental design (glucose assay), experimental work, data analysis, draft writing, B.P.; K.P.; project supervision and manuscript review, E.I.I.; A.A.H.; M.B. All authors have read and agreed to the published version of the manuscript.
Funding
The work reported herein was made possible through funding by the South African Research Foundation and the Medical Research Council through its Division of Research Capacity Development under funding received from the South African National Treasury. The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the SAMRC or the funders. In addition, partial funding from the National Research Foundation of South African and the University of the Western Cape was used for this study.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available.
References
- 1.Tripathy J.P. Burden and risk factors of diabetes and hyperglycemia in India: Findings from the Global Burden of Disease Study 2016. Diabetes Metab. Syndr. Obes. Targets Ther. 2018;11:381–387. doi: 10.2147/DMSO.S157376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeFronzo R.A., Tripathy D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care. 2009;32:S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilcox G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 4.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zúñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Röder P.V., Wu B., Liu Y., Han W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016;48:e219. doi: 10.1038/emm.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czech M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017;23:804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dal S., Sigrist S. The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications. Diseases. 2016;4:24. doi: 10.3390/diseases4030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etsassala N.G., Cupido C.N., Iwuoha E., Hussein A.A. Review: Abietane diterpenes as potential candidates for the management of type 2 diabetes. Curr. Pharm. Des. 2020;26:1–13. doi: 10.2174/1381612826666200331082917. [DOI] [PubMed] [Google Scholar]
- 9.Alemu A., Tamiru W., Nedi T., Shibeshi W. Analgesic and Anti-Inflammatory Effects of 80% Methanol Extract of Leonotis ocymifolia (Burm.f.) Iwarsson Leaves in Rodent Models. Evidence-Based Complement. Altern. Med. 2018;2018:1–8. doi: 10.1155/2018/1614793. [DOI] [Google Scholar]
- 10.Kubinova R., Pořízková R., Navrátilová A., Farsa O., Hanaková Z., Bačinská A., Cizek A., Valentová M. Antimicrobial and enzyme inhibitory activities of the constituents of Plectranthus madagascariensis (Pers.) Benth. J. Enzym. Inhib. Med. Chem. 2014;29:749–752. doi: 10.3109/14756366.2013.848204. [DOI] [PubMed] [Google Scholar]
- 11.Yin Z., Zhang W., Feng F., Zhang Y., Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness. 2014;3:136–174. doi: 10.1016/j.fshw.2014.11.003. [DOI] [Google Scholar]
- 12.Manning J., Goldblatt P. Plants of the Greater Cape Floristic Region 1: The Core Cape Flora. South African National Biodiversity Institute; Pretoria, South Africa: 2012. [Google Scholar]
- 13.He Y.-H., Li W., Li Y., Zhang S., Wang Y., Sun C. Ursolic Acid Increases Glucose Uptake through the PI3K Signaling Pathway in Adipocytes. PLoS ONE. 2014;9:e110711. doi: 10.1371/journal.pone.0110711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamede M., Mabuza L.P., Ngubane P., Khathi A. The Effects of Plant-Derived Oleanolic Acid on Selected Parameters of Glucose Homeostasis in a Diet-Induced Pre-Diabetic Rat Model. Molecules. 2018;23:794. doi: 10.3390/molecules23040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teoh S.L., Das S. Phytochemicals and their effective role in the treatment of diabetes mellitus: A short review. Phytochem. Rev. 2018;17:1111–1128. doi: 10.1007/s11101-018-9575-z. [DOI] [Google Scholar]
- 16.Lipina C., Hundal H.S. Carnosic acid stimulates glucose uptake in skeletal muscle cells via a PME-1/PP2A/PKB signalling axis. Cell. Signal. 2014;26:2343–2349. doi: 10.1016/j.cellsig.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Samarghandian S., Borji A., Farkhondeh T. Evaluation of Antidiabetic Activity of Carnosol (Phenolic Diterpene in Rosemary) in Streptozotocin-Induced Diabetic Rats. Cardiovasc. Hematol. Disord. Targets. 2017;17:11–17. doi: 10.2174/1871529X16666161229154910. [DOI] [PubMed] [Google Scholar]
- 18.Vlavcheski F., Baron D., Vlachogiannis I.A., MacPherson R.E.K., Tsiani E. Carnosol Increases Skeletal Muscle Cell Glucose Uptake via AMPK-Dependent GLUT4 Glucose Transporter Translocation. Int. J. Mol. Sci. 2018;19:1321. doi: 10.3390/ijms19051321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etsassala N.G., Badmus J., Waryo T., Marnewick J.L., Cupido C.N., Hussein A.A., Iwuoha E.I. Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities of Novel Abietane Diterpenes from Salvia africana-lutea. Antioxidants. 2019;8:421. doi: 10.3390/antiox8100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J.-B., Yang S.-J., Yan Z.-R., Zhang X.-J., Pu D.-B., Wang L.-X., Li X.-L., Zhang R.-H., Xiao W.-L. Isolation, Characterization, and Structure–Activity Relationship Analysis of Abietane Diterpenoids from Callicarpa bodinieri as Spleen Tyrosine Kinase Inhibitors. J. Nat. Prod. 2018;81:998–1006. doi: 10.1021/acs.jnatprod.7b01082. [DOI] [PubMed] [Google Scholar]
- 21.Bajpai V.K., Kang S.C. Tyrosinase and α-Glucosidase Inhibitory Effects of an Abietane Type Diterpenoid Taxodone from Metasequoia glyptostroboides. Natl. Acad. Sci. Lett. 2015;38:399–402. doi: 10.1007/s40009-015-0383-3. [DOI] [Google Scholar]
- 22.Batista O., Simões M.F., Nascimento J., Riberio S., Duarte A., Rodríguez B., De La Torre M.C. A rearranged abietane diterpenoid from Plectranthus hereroensis. Phytochemistry. 1996;41:571–573. doi: 10.1016/0031-9422(95)00646-X. [DOI] [PubMed] [Google Scholar]
- 23.Hussein A.A., Meyer J.J.M., Jimeno M.L., Rodríguez B. Bioactive Diterpenes from Orthosiphon labiatus and Salvia africana-lutea. J. Nat. Prod. 2007;70:293–295. doi: 10.1021/np0680376. [DOI] [PubMed] [Google Scholar]
- 24.Bustos-Brito C., Joseph-Nathan P., Burgueño-Tapia E., Martínez-Otero D., Nieto-Camacho A., Calzada F., Yépez-Mulia L., Esquivel B., Quijano L. Structure and Absolute Configuration of Abietane Diterpenoids from Salvia clinopodioides: Antioxidant, Antiprotozoal, and Antipropulsive Activities. J. Nat. Prod. 2019;82:1207–1216. doi: 10.1021/acs.jnatprod.8b00952. [DOI] [PubMed] [Google Scholar]
- 25.Rasikari H. Ph.D. Thesis. Southern Cross University; Lismore, Australia: 2007. Phytochemistry and Arthropod Bioactivity of Australian Lamiaceae. [Google Scholar]
- 26.Naman C.B. Ph.D. Thesis. Ohio State University; Ohio, OH, USA: 2015. Phytochemical Investigation of the Medicinal Plant Taxodium distichum and Library Screening of Thalictrum Alkaloids for New Antileishmanial Drug Leads. [Google Scholar]
- 27.Matloubi-Moghadam F., Rüedi P., Eugster C.H. Drüsefarbstoffe aus Labiaten: Identifizierung von 17 Abietanoiden ausPlectranthus sanguineusBRITTEN. Helvetica Chim. Acta. 1987;70:975–983. doi: 10.1002/hlca.19870700407. [DOI] [Google Scholar]
- 28.Horvath T., Linden A., Yoshizaki F., Eugster C.H., Ruedi P. Abietanes and a Novel 20-Norabietanoid from Plectranthus cyaneus (Lamiaceae) Helvetica Chim. Acta. 2004;87:2346–2353. doi: 10.1002/hlca.200490210. [DOI] [Google Scholar]
- 29.McKenzie J.M., Green I.R., Mugabo P. Leonurun. A novel labdane diterpenoid from Leonotis leonurus. S. Afr. J. Chem. 2006;59:114–116. [Google Scholar]
- 30.Habtemariam S., Gray A.I., Waterman P.G. Diterpenes from the Leaves of Leonotis ocymifolia var. raineriana. J. Nat. Prod. 1994;57:1570–1574. doi: 10.1021/np50113a017. [DOI] [Google Scholar]
- 31.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahomoodally M.F. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evidence-Based Complement. Altern. Med. 2013;2013:617459. doi: 10.1155/2013/617459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castro A.J.G., Frederico M.J.S., Cazarolli L.H., Mendes C.P., Bretanha L.C., Schmidt E.C., Bouzon Z.L., Pinto V.A.D.M., Ramos C.D.F., Pizzolatti M.G., et al. The mechanism of action of ursolic acid as insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015;1850:51–61. doi: 10.1016/j.bbagen.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Birgani G.A., Ahangarpour A., Kahorsandi L., Moghaddam H.F. Anti-diabetic effect of betulinic acid on streptozotocin-nicotinamide induced diabetic male mouse model. Braz. J. Pharm. Sci. 2018;54 doi: 10.1590/s2175-97902018000217171. [DOI] [Google Scholar]
- 35.Sayem A.S.M., Arya A., Karimian H., Krishnasamy N., Hasamnis A.A., Hossain C.F. Action of Phytochemicals on Insulin Signaling Pathways Accelerating Glucose Transporter (GLUT4) Protein Translocation. Molecules. 2018;23:258. doi: 10.3390/molecules23020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aung T.N., Qu Z., Kortschak R.D., Adelson D.L. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. Int. J. Mol. Sci. 2017;18:656. doi: 10.3390/ijms18030656. [DOI] [PMC free article] [PubMed] [Google Scholar]