Abstract
Alginates are widely used as gelling agents in textile print pastes, medical industries, impression material in dentistry, and anticoagulant material in toothpaste. In the present study, the content and spectroscopic characterization (1H NMR and FT-IR) of the sodium alginates were investigated in the eight brown seaweeds Sargassum muticum, Fucus vesiculosus f. volubilis, Carpodesmia tamariscifolia, Bifurcaria bifurcata, Laminaria ochroleuca, Cystoseira humilis, Saccorhiza polyschides, and Fucus guiryi harvested from the NW Atlantic coast of Morocco. The results proved that the most studied algae depicted alginate yields higher than 18% dry weight. The FT-IR analysis showed that the spectra of the extracted alginates exhibited significant similarities to the commercial alginate from Sigma-Aldrich. The 1H NMR spectroscopy indicated that the extracted alginates have a high content of β-d-mannuronic (M) than α-l-guluronic acid (G) with M/G ratio values ranging from 1.04 to 4.41. The homopolymeric fractions FMM are remarkably high compared to the FGG and heteropolymeric fractions (FGM = FMG) especially for F. guiryi, C humilis, C. tamariscifolia, L. ochroleuca, and S. polyschides. Nevertheless, the heteropolymeric fractions (FGM/FMG) are quite abundant in the alginates of S. muticum, F. vesiculosus f. volubilis, and B. bifurcata accounting for more than 52% of the polymer diads. Based on these results, the investigated algal species (except Fucus guiryi and Bifurcaria bifurcata) could be regarded as potential sources of alginates for industrial uses.
Keywords: brown seaweeds, alginates, spectroscopic characterization, Morocco
1. Introduction
Macroalgae are documented mainly as a source of critical chemical components with applications in human food, phycocolloids, cosmeceuticals, and pharmaceutical industries, as well as the agricultural sector for incorporation into animal feeds and plant stimulants [1]. Three types of phycocolloids are commonly involved in the seaweed chemical industry such as alginates, carrageenans, and agars. Alginate is the common name given to a family of linear phycocolloid β-d-mannuronic (1,4-linked) and α-l-guluronic acids arranged in a non-regular, blockwise order along the chain [2]. This polysaccharide is found in the matrix and the cell wall of the brown algae. It plays a vital role in cell binding and makes available some mechanical properties to the algae, e.g., flexibility [3]. The viscosity of alginate hydrocolloids depends on the average molecular weight, whereas the gelling properties are affected by the amounts and distribution of the three types of blocks (The homopolymeric fractions of β-d-mannuronic (FM) and α-l-guluronic (FG) acids, and the heteropolymeric fractions (FMG) [1,3]). In general, alginates with a low M/G ratio and a large proportion of guluronic blocks form a robust and rigid gel. Those with a low number of guluronic blocks and a high M/G ratio produce soft and elastic gels [4]. Alginates are widely used as stabilizers in various food industries, textile print pastes, gelling agents in medical industries, impression material in dentistry, and anticoagulant material in toothpaste [1,5]. Several brown algal species are cultivated to produce the alginate required for the industry such as Ascophyllum nodosum, Durvillaea antarctica, Laminaria hyperborea, Saccharina latissima, Ecklonia maxima, Macrocystis pyrifera, Lessonia nigrescens, and Lessonia trabeculata [6,7].
The Atlantic coast of Moroccan is a favorable habitat for diverse algal species and constitutes a reserve of species of considerable economic, social, and ecologic potentials. Nevertheless, the phycocolloid industry is limited to the exploitation of Gelidium corneum (Gelidiales, Rhodophyceae), which is commercially harvested for agar extraction. Indeed, several other red and brown algae are abundant on this coast, but the data on the composition and physicochemical properties of their polysaccharides are rare. In particular, there is no alginate industry in Morocco, and almost no information is available on potential alginophytes species and the quantity and quality of alginates. In this context, the present study explores the extraction yield, as well as the spectroscopic characterization (1H NMR and FT-IR) of the sodium alginates from eight brown algal species (Sargassum muticum, Fucus vesiculosus f. volubilis, Carpodesmia tamariscifolia, Bifurcaria bifurcata, Laminaria ochroleuca, Cystoseira humilis, Saccorhiza polyschides, and Fucus guiryi) harvested from the Atlantic coast of Morocco.
2. Results and Discussion
2.1. Alginates’ Yield
The alginate yields of the eight brown algal species collected from the Northwestern Atlantic coast of Morocco ranged from 2.7% to 27.5% dw (Table 1). The highest percent of alginates was obtained from Laminaria ochroleuca, and the minimum alginate content was found in Bifurcaria bifurcata. The alginates’ yield recorded in S. muticum, C. tamariscifolia, C. humilis and F. vesiculosus exceeded 18% dw. These contents are within the range of those reported from some well-known worldwide alginophytes such as Saccharina japonica (20–26% dw [8,9]), Ascophyllum nodosum (24% [10]), and Saccharina longicruris (20% dw [10]). However, these values are still lower in comparison to the special alginate contents of Durvillaea antarctica (from 37 to 52% [11]) and Ecklonia cava from (35 to 38% dw [9]). The alginate content observed in F. guiryi (13.6% dw) is still higher than that previously reported from others Fucales species (10–12% dw [12,13]). The kelp species L. ochroleuca and S. polyschides contained 27.5% and 25% alginates, respectively (Table 1). Similar values have been reported for Laminaria digitata (22–34% dw [8,14]), Laminaria hyperborea (21–33% dw [14,15]), and Macrocystis pyrifera (29–38% dw [11]).
Table 1.
Alginate contents of the investigated algal species compared to various brown seaweeds.
| Algal Species | Alginates’ Yield (% dw) | References |
|---|---|---|
| Sargassum muticum | 25.6 | This study |
| Laminaria ochroleuca | 27.5 | |
| Saccorhiza polyschides | 25.0 | |
| Cystoseira humilis | 19.1 | |
| Carpodesmia tamariscifolia | 17.22 | |
| Fucus vesiculosus f. volubilis | 18.3 | |
| Fucus guiryi | 13.6 | |
| Ascophyllum nodosum | 24 | [10] |
| Durvillaea antarctica | 37–52 | [11] |
| Ecklonia maxima | 35 | [8] |
| Laminaria japonica | 20–26 | [8] |
| Laminaria digitata | 22–34 | [8] |
| Laminaria hyperborea | 21–33 | [15] |
| Lessonia nigrescens | 34–41 | [16] |
| Macrocystis pyrifera | 29–38 | [11] |
| Saccharina longicruris | 20 | [10] |
| Sargassum asperifolium | 12 | [12] |
| Sargassum filipendula | 17 | [17] |
| Sargassum fluitans | 21 | [18] |
| Sargassum vulgare | 17 | [19] |
| Sargassum muticum | 18 | [20] |
| Sargassum oligocystum | 19 | [18] |
| Sargassum thunbergii | 13 | [20] |
| Sargassum polycystum | 17–28 | [20] |
| Sargassum turbinarioides | 10 | [13] |
| Fucus vesiculosus | 16.2 | [10] |
| Fucus serratus | 20–29 | [21] |
| Fucus ceranoides | 21–29 | [22] |
2.2. FT-IR Spectroscopy Analysis
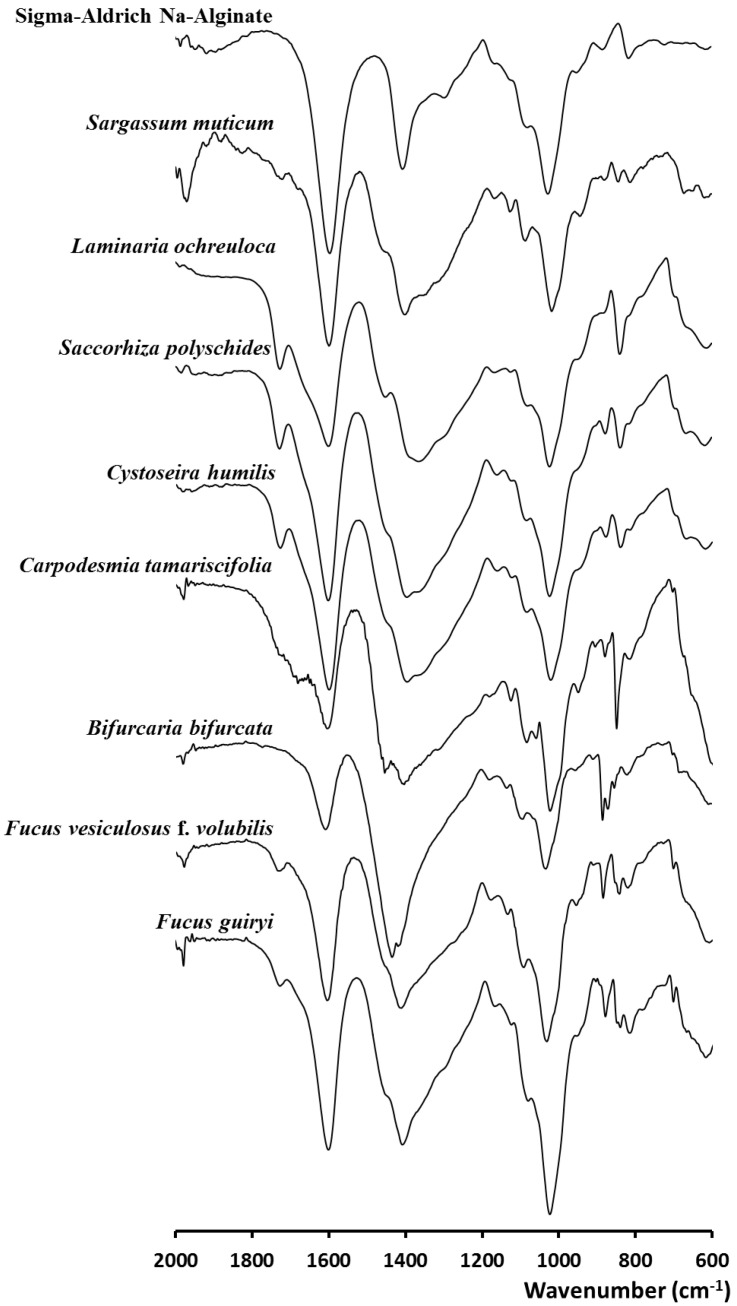
The infrared spectra in the range 2000 to 600 cm−1 of alginates extracted from the investigated brown algae with reference to commercial sodium alginate (Sigma-Aldrich, Gillingham, UK) are given in Figure 1. The spectra of extracted alginates are very similar to those of the commercial standard showing similar positions of the characteristic bands. Therefore, sodium alginate is the main polysaccharide found in the tested brown seaweeds, despite the small-signal appearing between 1710 cm−1 and 1730 cm−1, particularly in the spectra of Fucus guiryi, S. muticum, S. polyschides, L. ochroleuca, and F. vesiculosus, corresponding to the carbonyl group as the carboxylic acid ester form (C=O). The abroad bands at 1600–1610 cm−1 were suggested as the O-C-O carboxylate asymmetric stretching [23,24]. The bands located at 1400–1428 cm−1 were assigned to C-OH deformation vibration with the involvement of the symmetric stretching vibration of O-C-O [13,25]. According to previous reports, the bands at 1025–1030 cm−1 can be attributed to the C-O group [18]. The anomeric region (950 to 750 cm−1) is the most discussed in carbohydrates [23,26]. Indeed, the C-O stretching vibration of uronic acid residues is generally linked to the bands centered around 930–950 cm−1, and those recorded between 871 and 883 cm−1 were attributed to the C1-H deformation vibration of β-mannuronic acid residues [27]. The signals around 815–833 cm−1 were attributed to mannuronic acid residues [27].
Figure 1.
FT-IR spectra of sodium alginates extracted from the investigated brown seaweeds and the sodium alginate standard (Sigma-Aldrich, Gillingham, UK).
2.3. 1H NMR Spectroscopy Analysis
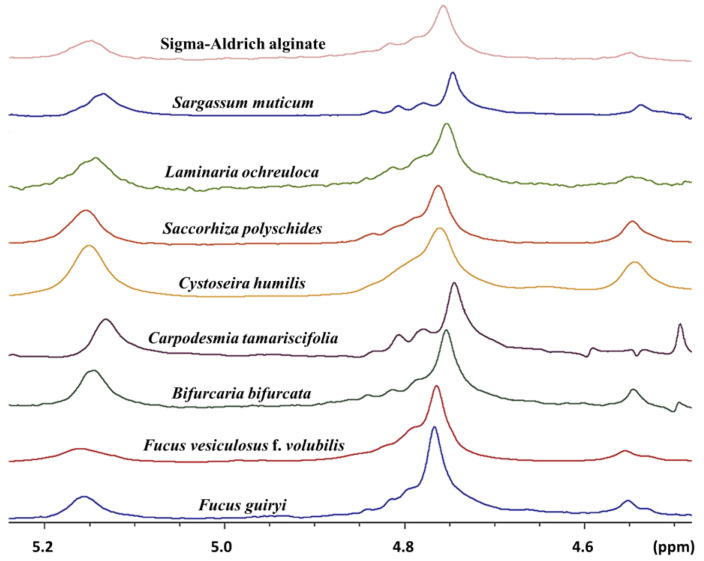
1H NMR spectroscopy is a consistent method for the complete characterization of the composition and the block structure of alginate [11,12]. The phycocolloids extracted from the studied brown algae revealed typical 400 MHz-1H NMR spectra (Figure 2) similar to the commercial sodium alginate with three key signals attributed to the anomeric hydrogen of guluronic acid (G) at 5.1–5.2 ppm (pic I), the anomeric hydrogens of mannuronic acid (M1), and the H-5 of alternating blocks (GM-5) overlapping at 4.7–4.9 ppm (pic II), and the H-5 of guluronic acid residues in the homopolymeric G blocks, at 4.5–4.6 ppm (pic III) (Figure S1 in the Supplementary Material).
Figure 2.
1H NMR spectra of the studied sodium alginates using D2O as a solvent.
The M/G ratio, the molar fractions of the monads (FG, FM), and the diad (FGG, FMM, FMG, FGM) sequences were calculated from the area of 1H NMR signals (Figure 2) employing the formula given by Grasdalen et al. [28]:
| FG = AI/(AII + AIII) |
| FM = 1 − FG |
| FGG = AIII/(AII + AIII) |
| FGM = FMG = FG − FGG |
| FMM = FM − FMG |
| M/G = (1 − FG)/FG |
The M/G ratios of sodium alginates in all tested brown algae were greater than one (Table 2), revealing a dominance of mannuronic acid over guluronic acid, in particular in L. ochroleuca and F. guiryi, showing the M/G ratios of 2.3 and 4.4, respectively. Such dominance has been reported for alginates in Durvillaea antarctica [11] and for the analyzed commercial alginate (Sigma-Aldrich, Gillingham, UK). According to Murillo-Álvarez and Hernández-Carmona [29], alginates with a low M/G ratio would provide strong, brittle gels, making them suitable for cell encapsulation for biomedical or environmental applications, whereas alginates with a high M/G ratio would give more elastic gels, desirable for food, cosmetic, or pharmaceutical products. The M/G ratio is not the only factor that controls the alginate gelling properties. It has been reported that the percentages of homopolymeric block structures (FMM, FGG) and alternating blocks (FMG, FGM) influence the physical properties of alginates [19]. Based on the 1H NMR results (Table 2), the homopolymeric regions (FMM) are remarkably high compared to the guluronic blocks (FGG) and heteropolymeric fractions (FGM = FMG) for F. guiryi, C humilis, C. tamariscifolia, L. ochroleuca, and S. polyschides and the tested commercial alginate. Similar compositions have previously been found for Durvillaea antarctica, Saccharina japonica, Laminaria digitata, and Macrocystis pyrifera [8,11]. The heteropolymeric fractions (FGM/FMG) are quite abundant in S. muticum, F. vesiculosus, and B. bifurcata, accounting for more than 52% of the polymer diads.
Table 2.
Composition data of alginates extracted from studied seaweeds compared to other brown seaweed species.
| Species | M/G | FM | FG | FMM | FGG | FGM | FMG | η | References |
|---|---|---|---|---|---|---|---|---|---|
| Sargassum muticum | 1.04 | 0.51 | 0.49 | 0.17 | 0.15 | 0.34 | 0.34 | 1.35 | |
| Laminaria ochroleuca | 2.52 | 0.72 | 0.28 | 0.50 | 0.06 | 0.22 | 0.22 | 1.09 | |
| Fucus guiryi | 4.41 | 0.82 | 0.18 | 0.78 | 0.15 | 0.04 | 0.04 | 0.27 | |
| Cystoseira humilis | 1.46 | 0.59 | 0.41 | 0.40 | 0.21 | 0.20 | 0.20 | 0.83 | |
| Carpodesmia tamariscifolia | 1.31 | 0.57 | 0.43 | 0.42 | 0.28 | 0.15 | 0.15 | 0.61 | This study |
| Saccorhiza polyschides | 1.73 | 0.63 | 0.37 | 0.38 | 0.11 | 0.25 | 0.25 | 1.09 | |
| Bifurcaria bifurcata | 1.88 | 0.65 | 0.35 | 0.33 | 0.02 | 0.32 | 0.32 | 1.43 | |
| Fucus vesiculosus f. volubilis | 1.84 | 0.65 | 0.35 | 0.39 | 0.09 | 0.26 | 0.26 | 1.13 | |
| Sigma-Aldrich Na-Alginate | 3.42 | 0.77 | 0.23 | 0.68 | 0.14 | 0.09 | 0.09 | 0.51 | |
| Ascophyllum nodosum | 0.85 | 0.46 | 0.54 | 0.28 | 0.36 | 0.18 | 0.18 | 0.72 | [10] |
| Durvillaea antarctica | 4.00 | 0.8 | 0.2 | 0.64 | 0.04 | 0.16 | 0.16 | 1.00 | [11] |
| Ecklonia maxima | 1.22 | 0.55 | 0.45 | 0.32 | 0.22 | 0.23 | 0.23 | 0.93 | [8] |
| Laminaria japonica | 1.86 | 0.65 | 0.35 | 0.48 | 0.18 | 0.17 | 0.17 | 0.75 | [8] |
| Laminaria digitata | 1.44 | 0.59 | 0.41 | 0.43 | 0.25 | 0.16 | 0.16 | 0.66 | [8] |
| Laminaria hyperborea | 0.82 | 0.45 | 0.55 | 0.28 | 0.38 | 0.17 | 0.17 | 0.69 | [15] |
| Lessonia nigrescens | 1.44 | 0.59 | 0.41 | 0.4 | 0.22 | 0.19 | 0.19 | 0.79 | [12] |
| Macrocystis pyrifera | 1.7 | 0.63 | 0.37 | 0.42 | 0.16 | 0.21 | 0.21 | 0.90 | [11] |
| Saccharina longicruris | 0.69 | 0.41 | 0.59 | 0.07 | 0.25 | 0.34 | 0.34 | 1.41 | [10] |
| Sargassum asperifolium | 0.69 | 0.41 | 0.59 | 0.3 | 0.48 | 0.22 | 0.22 | 0.91 | [12] |
| Sargassum filipendula | 0.78 | 0.44 | 0.56 | 0.33 | 0.45 | 0.11 | 0.11 | 0.45 | [17] |
| Sargassum muticum | 0.31 | 0.24 | 0.76 | 0.07 | 0.59 | 0.17 | 0.17 | 0.93 | [20] |
| Sargassum oligocystum | 0.62 | 0.38 | 0.62 | 0.31 | 0.55 | 0.14 | 0.14 | 0.59 | [18] |
| Sargassum thunbergii | 0.53 | 0.34 | 0.66 | 0.17 | 0.48 | 0.34 | 0.34 | 1.52 | [20] |
| Sargassum polycystum | 0.21 | 0.18 | 0.82 | 0.12 | 0.77 | 0.1 | 0.1 | 0.68 | [20] |
| Sargassum vulgare | 1.27 | 0.56 | 0.44 | 0.02 | 0.55 | 0.43 | 0.43 | 1.75 | [19] |
| Fucus vesiculosus | 1.44 | 0.59 | 0.41 | 0.39 | 0.22 | 0.19 | 0.19 | 0.78 | [10] |
The description of the alginate sequence could be completed via the parameter η = FMG/(FM × FG), which evaluates and reveals the distribution of sequences. Indeed, η values <1 correspond to the abundance of homopolymeric blocks MM and GG, η = 1 for completely random cases and 1 < η < 2 for alternate-like cases MG and GM [28].
The η values exceeded one for the alginates extracted from S. muticum, B. bifurcata, and F. vesiculosus (Table 2), proving the dominance of the heteropolymeric fractions MG and GM. The η values were less than one for C. tamariscifolia, C. humilis, and F. guiryi, reflecting the abundance of homopolymeric fractions MM and GG. Nonetheless, L. ochroleuca and S. polyschides showed η values equal to one with a codominance of homopolymeric and heteropolymeric blocks.
3. Materials and Methods
The studied brown algal species (Figure S2 in the Supplementary Material) were sampled from the El Jadida coastline (33°14′43.7″ N 8°32′35.2″ W), except F. vesiculosus f. volubilis, which was harvested from Oualidia lagoon (32°44′52.7″ N 9°01′26.8″ W). In the laboratory, the samples were washed with running tap water and then with deionized water to remove debris sticking to their surface. Algal biomasses were dried at 60 °C until constant weight.
The alginates’ extraction was carried out according to the slightly modified procedure of Calumpong et al. [30]. Dried biomasses of the investigated algal species was covered with 2% formaldehyde at room temperature for 24 h, washed with water, and then added to 0.2 M HCl and left for another 24 h. Subsequently, the algal biomasses were rinsed with distilled water and flooded in 2% sodium carbonate for 24 h. The soluble part was collected by filtration through three layers of cheesecloth, and the filtrate was collected by centrifugation. The extract was discarded, and the procedure was repeated for the solid residue. The whole filtrates were precipitated by three volumes of ethanol. The sodium alginate recovered was washed with acetone and placed in an oven at 50 °C. The yield of alginate is expressed as a percentage of the initial dry weight of seaweed (% dw).
FTIR spectral measurements of the dried sodium alginate samples (50 °C for 3h) were performed using a Thermo Scientific Nicolet Impact 400D FT-IR Spectrometer (Nicolet Instrument Co., Madison, USA). The spectra were scanned between 4000 and 600 cm−1 in attenuated total reflectance (ATR) mode. A total of 32 scans were averaged for each sample at a 4 cm−1 resolution, and subsequently, the IR spectra were processed using the OMNIC software (Nicolet, Madison, USA).
The 1H NMR spectra of the sodium alginate solutions in D2O were recorded on the spectrometer AV II 400 MHz, 9.4T (Proton Larmor frequency of 400.33 MHz, Bruker Corporation, Billerica, MA, USA), using A 5 mm Triple Resonance Broadband Inverse probe (Bruker Corporation, Billerica, MA, USA), at 343 K. The spectra of 16 K in size of Free Induction Decay were recorded with a sweep width of 4800 Hz. Presaturation was applied during the relaxation delay and mixing time. The raw data were apodized in one dimension with 0.5 for line broadening prior to Fourier transformation. The number of scans was 32 transients. A commercial sodium alginate (CAS No. 9005-38-3, Lot MKBQ4519V, Sigma-Aldrich, Gillingham, UK) was used as the standard.
4. Conclusions
The present study investigates the yield and spectroscopic (FT-IR and 1H NMR) characterization of alginates extracted from eight brown seaweeds from the Moroccan Atlantic coastline. The alginates contents were higher than 18% dw for most of the algae except F. guiryi and B. bifurcata. FT-IR spectroscopy analysis showed an interesting similarity between the alginate spectra of the studied algae and that of the commercial alginate (Sigma-Aldrich, Gillingham, UK). 1H NMR spectroscopy revealed that the extracted alginates are richer in mannuronic acid than guluronic acid (M/G ratio >1), thus providing elastic hydrogels. The quasi-dominance of mannuronic (FMM) and guluronic (FGG) homopolymers over heteropolymeric fractions (FGM and FMG) was detected in F. guiryi, C. humilis, and C. tamariscifolia. However, the heteropolymeric fractions dominated the diads of the alginates extracted from S. muticum, B. bifurcata, and F. vesiculosus. The noteworthy yield associated with the physical and chemical proprieties of the extracted alginates makes these species potential alginophytes for commercial purposes. Nonetheless, future research must place emphasis on the seasonal variation of yield and the composition of the extracted phycocolloids.
Supplementary Materials
The following are available online. Figure S1: The anomeric region in the 400 MHz—1H NMR spectrum of the Sigma-Aldrich sodium alginate using D2O as solvent. Figure S2: Morphological traits of the studied macroalgae collected on the Northwestern Atlantic coast of Morocco.
Author Contributions
B.S., F.B., and V.V. conceived of and designed the experiments. Z.B., S.K., S.E.A., and C.K. performed the field sampling, alginates’ analyses, and original draft writing. B.S., C.J., and F.B. collaborated in the sample analyses. V.V., B.S., and C.J. contributed to the funding, materials, and analysis tools. A.R., B.S., and F.B. provided laboratory facilities and collaborated in the sample analyses. All co-authors contributed to proof reading of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project H2020 RISE project EMERTOX—Emergent Marine Toxins in the North Atlantic and Mediterranean: New Approaches to Assess their Occurrence and Future Scenarios in the Framework of Global Environmental Changes—Grant Agreement No. 778069, and FCT Projects UIDB/04423/2020 and UIDP/04423/2020. This research was also funded by the FCT-Portugal/CNRST-Morocco Cooperation Convention under References 1006/13/CNR, 1795/14/CNR, and 0504/19/CNR.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; nor in the decision to publish the results.
Footnotes
Sample Availability: Algal species samples and the studied sodium alginates are available from the authors.
References
- 1.Stengel B.D., Connan S. Marine Algae: A Source of Biomass for Biotechnological Applications. In: Stengel B.D., Connan S., editors. Natural Products From Marine Algae: Methods and Protocols. Springer Science and Business Media; New York, NY, USA: 2015. pp. 1–38. [DOI] [PubMed] [Google Scholar]
- 2.Andrade L., Salgado L.T., Farina M., Pereira M.S., Mourão P.A., Filho G.M.A. Ultrastructure of acidic polysaccharides from the cell walls of brown algae. J. Struct. Boil. 2004;145:216–225. doi: 10.1016/j.jsb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Kloareg B., Quatrano R. Structure of the cell walls of marine algae and ecophysiological function of the matrix polysaccharides. Oceanogr. Mar. Biol. 1988;26:259–315. [Google Scholar]
- 4.Bourgougnon N., Stiger-Pouvreau V. Handbook of Marine Macroalgae. Wiley; Hoboken, NJ, USA: 2011. Chemodiversity and Bioactivity within Red and Brown Macroalgae Along the French coasts, Metropole and Overseas Departements and Territories; pp. 58–105. [Google Scholar]
- 5.Hanzhi L., Song Q., Peng J. Handbook of Marine Macroalgae. Wiley; Hoboken, NJ, USA: 2011. Biotechnology of Seaweeds: Facing the Coming Decade; pp. 424–430. [Google Scholar]
- 6.Helgerud T., Gaserød O., Fjæreide T., Andersen P.O., Larsen C.K. Alginates. In: Imeson A., editor. Food Stabilizers, Thickeners and Gelling Agents. Willey-Blackwell; Iowa, IA, USA: 2009. pp. 50–56. [Google Scholar]
- 7.Rioux L.-E., Turgeon S.L. Seaweed Sustainability. Elsevier BV; Amsterdam, The Netherlands: 2015. Seaweed carbohydrates; pp. 141–192. [Google Scholar]
- 8.Smidsrød O., Draget K.I. Alginates: Chemistry and physical properties. Carbohydr. Eur. 1996;14:6–13. [Google Scholar]
- 9.Pérez R. Les extraits des végétaux marins: Les phycocolloïdes. In: Pérez R., editor. Ces algues qui nous entourent: Conception actuelle, rôle dans la biosphère, utilisations, culture. IFREMER; Nantes, France: 1997. pp. 102–168. [Google Scholar]
- 10.Rioux L.-E., Turgeon S.L., Beaulieu M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007;69:530–537. doi: 10.1016/j.carbpol.2007.01.009. [DOI] [Google Scholar]
- 11.Panikkar R., Brasch D.J. Composition and block structure of alginates from New Zealand brown seaweeds. Carbohydr. Res. 1996;293:119–132. doi: 10.1016/0008-6215(96)00193-0. [DOI] [Google Scholar]
- 12.Larsen B., Salem D.M., Sallam M.A., Mishrikey M.M., Beltagy A.I. Characterization of the alginates from algae harvested at the Egyptian Red Sea coast. Carbohydr. Res. 2003;338:2325–2336. doi: 10.1016/S0008-6215(03)00378-1. [DOI] [PubMed] [Google Scholar]
- 13.Fenoradosoa T.A., Ali G., Delattre C., Laroche C., Petit E., Wadouachi A., Michaud P. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. Environ. Boil. Fishes. 2009;22:131–137. doi: 10.1007/s10811-009-9432-y. [DOI] [Google Scholar]
- 14.Schiener P., Black K.D., Stanley M.S., Green D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. Environ. Boil. Fishes. 2014;27:363–373. doi: 10.1007/s10811-014-0327-1. [DOI] [Google Scholar]
- 15.Martinsen A., Skjåk-BraeK G., Smidsrød O. Alginate as immobilization material: I. Correlation between chemical and physical properties of alginate gel beads. Biotechnol. Bioeng. 1989;33:79–89. doi: 10.1002/bit.260330111. [DOI] [PubMed] [Google Scholar]
- 16.Donati I., Paoletti S. Material Properties of Alginates. Springer Science and Business Media LLC; Berlin, Germany: 2009. pp. 1–53. [Google Scholar]
- 17.Bertagnolli C., Espindola A.P.D., Kleinübing S.J., Tasic L., Da Silva M.G.C. Sargassum filipendula alginate from Brazil: Seasonal influence and characteristics. Carbohydr. Polym. 2014;111:619–623. doi: 10.1016/j.carbpol.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Davis T.A., Ramirez M., Mucci A., Larsen B. Extraction, isolation and cadmium binding of alginate from Sargassum spp. Environ. Boil. Fishes. 2004;16:275–284. doi: 10.1023/B:JAPH.0000047779.31105.ec. [DOI] [Google Scholar]
- 19.Torres M.R., Sousa A.P., Filho E.A.S., Melo D.F., Feitosa J.P., De Paula R.C., Lima M.G. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr. Res. 2007;342:2067–2074. doi: 10.1016/j.carres.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Davis T.A., Llanes F., Volesky B., Mucci A. Metal Selectivity of Sargassum spp. and Their Alginates in Relation to Their α-l-Guluronic Acid Content and Conformation. Environ. Sci. Technol. 2003;37:261–267. doi: 10.1021/es025781d. [DOI] [PubMed] [Google Scholar]
- 21.Haug A. Composition and Properties of Alginates. Norwegian Institute of Seaweed Research; Trondheim, Norway: 1964. [Google Scholar]
- 22.Hoppe H.A., Schmid O.J. Meeresalgen als moderne Industrie produkte. Bot. Mar. 1962;3:16–66. doi: 10.1515/botm.1962.3.s1.16. [DOI] [Google Scholar]
- 23.Mathlouthi M., Koenig J.L. Vibrational Spectra of Carbohydrates. Adv. Carbohydr. Chem. Biochem. 1987;44:7–89. doi: 10.1016/s0065-2318(08)60077-3. [DOI] [PubMed] [Google Scholar]
- 24.Bi F., Mahmood S.J., Arman M., Taj N., Iqbal S. Physicochemical characterization and ionic studies of sodium alginate fromSargassum terrarium(brown algae) Phys. Chem. Liq. 2007;45:453–461. doi: 10.1080/00319100600745198. [DOI] [Google Scholar]
- 25.Papageorgiou S.K., Kouvelos E.P., Favvas E.P., Sapalidis A., Romanos G.E., Katsaros F. Metal-carboxylate interactions in metal-alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010;345:469–473. doi: 10.1016/j.carres.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Leal D., Matsuhiro B., Rossi M., Caruso F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008;343:308–316. doi: 10.1016/j.carres.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Gómez-Ordóñez E., Jiménez-Escrig A., Rupérez P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010;43:2289–2294. doi: 10.1016/j.foodres.2010.08.005. [DOI] [Google Scholar]
- 28.Grasdalen H., Larsen B., Smidsrød O. A p.m.r. study of the composition and sequence of uronate residues in alginates. Carbohydr. Res. 1979;68:23–31. doi: 10.1016/S0008-6215(00)84051-3. [DOI] [Google Scholar]
- 29.Murillo-Alvarez J.I., Hernández-Carmona G. Monomer composition and sequence of sodium alginate extracted at pilot plant scale from three commercially important seaweeds from Mexico. Environ. Boil. Fishes. 2007;19:545–548. doi: 10.1007/s10811-007-9168-5. [DOI] [Google Scholar]
- 30.Calumpong H.P., Maypa A.P., Magbanua M. Population and alginate yield and quality assessment of four Sargassum species in Negros Island, central Philippines. Hydrobiologia. 1999;398:211–215. doi: 10.1023/A:1017015824822. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.