Abstract
Objectives:
Anti-Ro52 autoantibodies are associated with more severe interstitial lung disease (ILD) in adult myositis patients with anti-aminoacyl tRNA synthetase autoantibodies. However, few studies have examined anti-Ro52 autoantibodies in juvenile myositis. The purpose of this study was to define the prevalence and clinical features associated with anti-Ro52 autoantibodies in a large cohort of patients with juvenile myositis.
Methods:
We screened sera from 302 patients with juvenile dermatomyositis (JDM), 25 patients with juvenile polymyositis (JPM), and 44 patients with juvenile connective tissue disease-myositis overlap (JCTM) for anti-Ro52 autoantibodies by ELISA. Clinical characteristics were compared between myositis patients with and without anti-Ro52 autoantibodies.
Results:
Anti-Ro52 autoantibodies were found in 14% of JDM, 12% of JPM, and 18% of JCTM patients. Anti-Ro52 autoantibodies were more frequent in patients with anti-aminoacyl tRNA synthetase (64%, p<0.001) and anti-MDA5 (31%, p<0.05) autoantibodies. After controlling for the presence of myositis-specific autoantibodies, anti-Ro52 autoantibodies were associated with the presence of ILD (36% vs 4%, p<0.001). Disease course was more frequently chronic, remission was less common, and an increased number of medications was received in anti-Ro52 positive patients.
Conclusions:
Anti-Ro52 autoantibodies are present in 14% of juvenile myositis patients and are strongly associated with anti-MDA5 and anti-aminoacyl tRNA synthetase autoantibodies. In all juvenile myositis patients, those with anti-Ro52 autoantibodies were more likely to have ILD. Furthermore, patients with anti-Ro52 autoantibodies have more severe disease and a poorer prognosis.
Keywords: myositis, juvenile idiopathic inflammatory myopathies, anti-Ro52 autoantibodies, myositis associated autoantibodies, interstitial lung disease
INTRODUCTION
Idiopathic inflammatory myopathies (IIM) are a heterogeneous group of systemic autoimmune diseases characterized by weakness, chronic inflammation of skeletal muscles, and elevated serum muscle enzyme levels.1 Many patients also have extramuscular manifestations, including involvement of the skin, lungs, and/or joints. Most IIM patients have a myositis-specific autoantibody (MSA), defined as an autoantibody found only in IIM patients, which are typically mutually exclusive.2 In contrast, myositis-associated autoantibodies (MAAs) are found in IIM, but may also be present in patients with other autoimmune diseases and may be seen in association with an MSA or other MAAs.
MSAs are associated with specific phenotypes.2,3 For instance, anti-melanoma differentiation-associated gene 5 (MDA5) autoantibodies are associated with cutaneous ulceration and palmar papules, minimal muscle involvement, arthritis, interstitial lung disease (ILD), and a high fatality rate.4–7 In contrast, patients with autoantibodies recognizing histidyl-tRNA synthetase (i.e., Jo1), have anti-synthetase syndrome, a unique multisystem autoimmune disease characterized by a combination of myositis, ILD, arthritis, Raynaud’s phenomenon, fever, and/or mechanic’s hands.8 Of note, while many phenotypic features are similar between juvenile and adult IIM with the same MSAs, there are some important differences. For example, adults with anti-p155/140 (TIF-1) autoantibodies have an increased risk of malignancy, whereas anti-p155/140 (TIF-1) autoantibody positive children do not.2,9
In adult IIM patients, the most common MAA is anti-Ro52.10 Interestingly, anti-Ro52 autoantibodies often co-occur with anti-Jo1 autoantibodies11 and adult patients with both autoantibodies have more severe ILD and more frequently develop lung fibrosis than those with anti-Jo1 autoantibodies alone.12,13 In addition, higher anti-Ro52 autoantibody titers are associated with the development of more severe ILD14, myositis, and joint impairment in anti-Jo1-positive adult patients.15 Patients with both anti-Jo1 and anti-Ro52 autoantibodies have a poorer response to various immunosuppressive drugs and a decrease in survival.13,15
A recent analysis of 22 children with myositis revealed that 23% had anti-Ro52 autoantibodies, although specific clinical associations were not examined.16 The purpose of this study was to define the prevalence of and clinical features associated with anti-Ro52 autoantibodies in a large cohort of patients with juvenile myositis.
PATIENTS AND METHODS
Patients and serum samples
Of the 543 patients from the Childhood Myositis Heterogeneity Collaborative Study who were enrolled between 1989 and 2016 with probable or definite myositis by Bohan and Peter criteria,17 those with a serum sample available for autoantibody testing at the time of enrollment were included in the study. Among the 317 juvenile myositis patients included, 302 (81.4%) had juvenile dermatomyositis (JDM), 25 (6.7%) had juvenile polymyositis (JPM) and 44 (11.9%) had juvenile connective tissue disease–myositis (JCTM) overlap. The JCTM subgroup included patients meeting criteria for myositis and another autoimmune disease, including 13 with juvenile systemic lupus erythematosus, 11 with juvenile systemic sclerosis, 7 patients with juvenile idiopathic arthritis, and 13 with other autoimmune conditions including autoimmune hepatitis, eosinophilic fasciitis, diabetes mellitus, lichen sclerosis, linear morphea, psoriasis, Sjögren’s syndrome, and ulcerative colitis. Sera from 90 healthy control children enrolled in the same studies were available.
All subjects were enrolled in institutional review board-approved natural history studies as previously described,18 and all provided informed consent. A standardized physician questionnaire captured demographics, clinical and laboratory features, environmental exposures at illness onset or diagnosis, as well as therapeutic usage and responses.18 Seven organ system symptom scores at diagnosis, defined as the number of symptoms present divided by the number of symptoms assessed, and an overall clinical symptom score as the average of the seven individual organ symptom scores, were calculated as previously described.19–21 In 7 of 33 patients, the presence of ILD was diagnosed by high resolution computed tomography (HRCT) and lung biopsy. In 11 of 33 patients, ILD was diagnosed by HRCT alone and in 5 patients, ILD was diagnosed by biopsy alone. Seven patients were diagnosed with ILD by chest radiographic imaging combined with pulmonary function testing and did not undergo HRCT or lung biopsy. Three patients did not have imaging records available and the diagnosis of ILD was based on physician documentation in the medical record. Complete clinical response and remission were defined as at least 6-months of inactive disease on or off therapy, respectively.20 A course of treatment was defined as a single episode from beginning of administration of a given medication to the termination of treatment with that medication, or combination of medications, in each patient. Medical record review, conducted in >75% of patients, verified the clinical, demographic, laboratory and therapeutic data contained in the physician questionnaires. Follow up visits occurred in 55% of patients, with an average time from enrollment date to final evaluation of 4.3 years. Patient characteristics in our cohort are comparable with other registry-based JDM cohorts in terms of demographics and disease manifestations.22–25
Autoantibody assays
Anti-Ro52 autoantibodies were detected using an enhanced performance Ro52 enzyme-linked immunosorbent assay (ELISA) [SS-A 52 ELISA, Quanta Lite, INOVA Diagnostics, San Diego, CA] according to the manufacturer’s instructions. Other myositis autoantibodies were detected as previously described.18,26
Analysis
Dichotomous variables were expressed as percentages and absolute frequencies, and continuous features were reported as means and SD. Pairwise comparisons for categorical variables between groups were made using χ2 test or Fisher’s exact test, as appropriate, while continuous variables were compared using Student’s t-test. Logistic and linear regression were used to adjust the comparisons for possible confounding variables, including the year of diagnosis, length of follow-up and MSAs. Creatine kinase, a highly positively skewed variable, was expressed as median, first and third quartile for descriptive purposes and transformed through a base-10 logarithm for analysis. All statistical analyses were performed using Stata/MP V.14.1 (StataCorp LLC, College Station, Texas). As this was an exploratory study, a two-sided P value of ≤0.05 was considered statistically significant.
RESULTS
Prevalence and demographics of patients with anti-Ro52 autoantibodies
Anti-Ro52 autoantibodies were more prevalent in patients with juvenile IIM (JIIM) than in healthy control children (14% vs 1%). Sera from 14% of patients with JDM, 12% with JPM, and 18% with JCTM had anti-Ro52 autoantibodies (Figure 1, Table 1). There were no significant differences in gender, race, age at diagnosis, or delay to diagnosis between juvenile myositis patients with and without anti-Ro52 autoantibodies (Table 2).
Figure 1. Swarm plot of anti-Ro52 autoantibody ELISA results for juvenile healthy controls and JIIM patients divided into JDM, JPM, and JCTM.
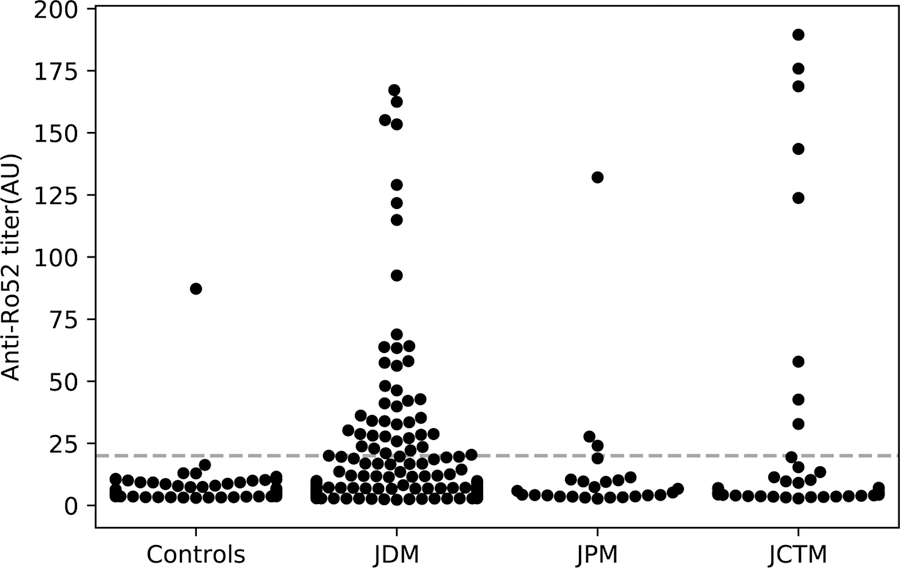
The dashed line of 20 units indicates the cut-off value for anti-Ro52 autoantibody positivity. Out of 371 JIIM patients, 53 (14%) were positive for anti-Ro52 autoantibodies by ELISA. Of these patients, 42 had JDM, 3 had JPM, and 8 had JCTM. Out of 90 juvenile healthy controls, one patient (1.1%) was positive for anti-Ro52 autoantibodies by ELISA.
Table 1.
Prevalence of anti-Ro52 autoantibodies among patients with juvenile myositis.
| Clinical subgroup | Anti-Ro52 autoantibody positive % (n/N) |
|---|---|
| Juvenile myositis (N=371) | 14% (n=53) *** |
| Juvenile dermatomyositis (N=302) | 14% (n=42) *** |
| Juvenile polymyositis (N=25) | 12% (n=3) * |
| Juvenile connective tissue-disease myositis (N=44): | 18% (n=8) *** |
| Juvenile lupus erythematosus (N=13) | 23% (n=3) ** |
| Juvenile systemic sclerosis (N=11) | 0% (n=0) |
| Juvenile idiopathic arthritis (N=7) | 29% (n=2) * |
| Other autoimmune diseasesa (N=13) | 23% (n=3) ** |
| Myositis specific autoantibody subgroup | |
| Anti-p155/140 (TIF-1) (N=119) | 11% (n=13) |
| Anti-NXP2 (N=77) | 14% (n=11) |
| Anti-MDA5 (N=32) | 31% (n=10) * |
| Anti-aminoacyl tRNA synthetase (N=14) | 64% (n=9) *** |
| Anti-SRP (N=7) | 0% (n=0) |
| Anti-Mi2 (N=13) | 15% (n=2) |
| Anti-HMGCR (N=4) | 50% (n=2) |
| MSA negative (N=96) | 5% (n=5) ** |
| Juvenile healthy controls (N=90) | 1% (n=1) |
p<0.05
p<0.01
p<0.001
Chi-squared or Fisheŕs exact tests were used to compare the percentage of positive patients compared with the percentage of negative patients within each myositis clinical and autoantibody subgroup.
Abbreviations: TIF-1: transcription intermediary factor 1, NXP2: nuclear matrix protein-2, MDA5: melanoma differentiation associated protein-5, SRP: signal recognition particle, HMGCR: 3-Hydroxy-3-Methylglutaryl-CoA Reductase, MSA: myositis specific autoantibody
autoimmune hepatitis, eosinophilic fasciitis, fasciitis, juvenile diabetes mellitus, lichen sclerosis, linear morphea, psoriasis, Sjögren’s syndrome, ulcerative colitis.
Table 2.
General features of juvenile myositis patients with and without anti-Ro52 autoantibodies.
| Total (N=371) % (n/N) or Mean (SD) | Anti-Ro52 autoantibody positive (N=53) % (n/N) or Mean (SD) | Anti-Ro52 autoantibody negative (N=318) % (n/N) or Mean (SD) | p-value | |
|---|---|---|---|---|
| Age at diagnosis | 9.0 (4.4) | 9.5 (4.7) | 8.9 (4.3) | 0.3 |
| Age at enrollment | 12.5 (7.1) | 12.6 (7.7) | 12.5 (7.0) | 1.0 |
| Delay to diagnosis (years) | 0.7 (1.2) | 0.55 (0.56) | 0.75 (1.27) | 0.3 |
| Follow-up (years) | 5.8 (6.4) | 4.3 (6.4) | 6.0 (6.4) | 0.09 |
| Female | 71% (263/371) | 74% (39/53) | 70% (224/318) | 0.6 |
| Race | ||||
| White | 65% (240/371) | 57% (30/53) | 66% (210/318) | 0.2 |
| Black | 16% (59/371) | 21% (11/53) | 15% (48/318) | 0.3 |
| Hispanic | 6% (24/371) | 6% (3/53) | 7% (21/318) | 1.0 |
| Other races a | 13% (48/371) | 17% (9/53) | 12% (39/318) | 0.3 |
| Myositis-specific autoantibodies | ||||
| Anti-p155/140 (TIF-1) | 33% (119/359) | 26% (13/50)b | 34% (106/309) c | 0.2 |
| Anti-NXP2 | 21% (77/366) | 21% (11/52) b | 21% (66/314) c | 1.0 |
| Anti-MDA5 | 9% (32/368) | 19% (10/53) | 7% (22/315) c | 0.01 |
| Anti-aminoacyl tRNA synthetase | 4% (14/360) | 18% (9/49) b | 2% (5/311) c | < 0.001 |
| Anti-SRP | 2% (7/360) | 0% (0/49) b | 2% (7/311) c | 0.6 |
| Anti-Mi2 | 4% (13/354) | 4% (2/49) b | 4% (11/305) c | 0.7 |
| Anti-HMGCR | 1% (4/371) | 4% (2/53) | 1% (2/318) | 0.10 |
| MSA negative | 27% (96/362) | 9% (5/53) | 29% (91/309) c | 0.002 |
Dichotomous variables were represented as percentage (count/total) and continuous variables as mean (SD). Chi-squared or Fisheŕs exact tests were used to compare dichotomous variables, as appropriate, while continuous variables were compared using Studentś t-test.
Abbreviations: TIF-1: transcription intermediary factor 1, NXP2: nuclear matrix protein-2, MDA5: melanoma differentiation associated protein-5, SRP: signal recognition particle, HMGCR: 3-Hydroxy-3-Methylglutaryl-CoA Reductase, MSA: myositis specific autoantibody.
Asian (Korean, Japanese, Chinese, Indian, Filipino), Pacific Islands, American Indian.
N ≠ 53 due to missing data.
N ≠ 318 due to missing data.
Prevalence of anti-Ro52 autoantibodies among myositis-specific autoantibody subgroups
Of those patients positive for anti-Ro52 autoantibodies, 26% had co-existing anti-p155/140 (TIF-1) autoantibodies, 21% had anti-NXP-2 autoantibodies, 19% had anti-MDA5 autoantibodies, 18% had anti-aminoacyl tRNA synthetase autoantibodies, 4% had anti-Mi2 autoantibodies, 4% had anti-HMGCR autoantibodies, and 9% were MSA negative (Table 2). Anti Ro-52 autoantibodies were significantly increased in the anti-MDA5 and anti-aminoacyl tRNA synthetase autoantibody subgroups than in other MSA subgroups (Table 1). For instance, anti-Ro52 autoantibodies co-existed in 31% of juvenile IIM sera with anti-MDA5 autoantibodies and 64% of those with anti-aminoacyl tRNA synthetase autoantibodies (Table 1). Similarly, anti-MDA5 autoantibodies co-existed in 19% of anti-Ro52 autoantibody positive sera and 7% of anti-Ro52 autoantibody negative sera. Anti-aminoacyl tRNA synthetase autoantibodies co-existed in 18% of anti-Ro52 autoantibody positive sera and 2% of anti-Ro52 autoantibody negative sera (Table 2). Less than 15% of those with anti-p155/140 (TIF1), anti-nuclear matrix protein-2 (NXP2), anti-signal recognition particle (SRP), or anti-Mi2 autoantibodies, and only 5% of those without an MSA were anti-Ro52 positive (Table 1).
Pulmonary manifestations among patients with anti-Ro52 autoantibodies
After controlling for the presence of MSAs (including anti-aminoacyl tRNA synthetase and anti-MDA5 autoantibodies) a multivariate analysis showed anti-Ro52 autoantibodies were highly associated with pulmonary involvement. Overall, patients with anti-Ro52 autoantibodies more often had ILD (36% vs 4%), dyspnea on exertion (59% vs 25%), and a higher early pulmonary score (mean 0.18 vs 0.08) than those without these autoantibodies (Table 3). Within the anti-MDA5 autoantibody positive subgroup, Ro52 reactivity was even more strongly associated with ILD: 70% of those with co-existing anti-Ro52 autoantibodies had ILD compared to only 9% of those who were anti-Ro52 negative (Table 4). Similarly, among the anti-aminoacyl tRNA synthetase autoantibody subgroup, 100% of anti-Ro52 autoantibody positive and 40% of anti-Ro52 negative patients had ILD (Table 4). Other pulmonary manifestations were also associated with Ro52 reactivity within the anti-MDA5 and anti-aminoacyl tRNA synthetase autoantibody subgroups. Specifically, among those patients with anti-MDA5 autoantibodies, patients who also were positive for anti-Ro52 autoantibodies more often had dyspnea on exertion (90% vs 27%) and higher early pulmonary scores than those who were anti-Ro52 autoantibody negative. Only 1 of 33 patients with ILD in our JIIM cohort had rapidly progressive ILD, and this patient was positive for both anti-MDA5 and anti-Ro52 autoantibodies. In patients with anti-aminoacyl tRNA synthetase autoantibodies, anti-Ro52 autoantibody positive patients had increased frequency of dyspnea on exertion (89% vs 40%), although this did not reach statistical significance. Patients with co-existing anti-p155/140 (TIF-1) and anti-Ro52 autoantibodies also had an increased frequency of ILD (15% vs 1%) and dyspnea on exertion (50% vs 16%) compared to anti-p155/140 (TIF-1) autoantibody positive patients who were anti-Ro52 autoantibody negative (Table 4). Of note, in the MSA negative subgroup, none of 5 anti-Ro52 autoantibody positive patients had ILD (Table 4). The association of anti-Ro52 autoantibodies with ILD was significant within the JDM clinical subgroup: 33% of JDM patients with anti-Ro52 autoantibodies had ILD compared to 1% of anti-Ro52 negative JDM patients (Table 4).
Table 3.
Clinical features of juvenile myositis patients with and without anti-Ro52 autoantibodies.
| Signs/symptoms ever present | Total (N=371) % (n/N) or Mean (SD) | Anti-Ro52 autoantibody positive (N=53) % (n/N) or Mean (SD) | Anti-Ro52 autoantibody negative (N=318) % (n/N) or Mean (SD) | Univariate p-value | Multivariate p-value |
|---|---|---|---|---|---|
| Muscle involvement | |||||
| Proximal weakness | 99% (369/371) | 98% (52/53) | 100% (317/318) | 0.3 | 0.3 |
| Myalgia | 64% (234/363) | 62% (32/52) a | 65% (202/311) b | 0.6 | 0.1 |
| Distal weakness | 47% (170/363) | 46% (24/52) a | 47% (146/311) b | 0.9 | 0.9 |
| Muscle atrophy | 37% (136/367) | 44% (23/52) a | 36% (113/315) b | 0.2 | 0.3 |
| Falling episodes | 45% (164/367) | 44% (23/52) a | 45% (141/315) b | 0.9 | 1.0 |
| Lung involvement | |||||
| Dyspnea on exertion | 30% (109/366) | 59% (30/51) a | 25% (79/315) b | < 0.001 | < 0.001 |
| Interstitial lung disease | 9% (33/369) | 36% (19/53) | 4% (14/316) b | < 0.001 | < 0.001 |
| Dysphonia | 32% (118/367) | 32% (17/53) | 32% (101/314) b | 1.0 | 0.7 |
| Joint involvement | |||||
| Arthralgia | 64% (236/369) | 70% (37/53) | 63% (199/316) b | 0.3 | 0.4 |
| Joint contractures | 61% (224/370) | 63% (33/52) a | 60% (191/318) | 0.6 | 0.7 |
| Arthritis | 51% (189/370) | 60% (31/52) a | 50% (158/318) | 0.2 | 0.7 |
| Skin involvement | |||||
| Heliotrope | 79% (293/369) | 87% (46/53) | 78% (247/316) b | 0.2 | 0.2 |
| Gottrońs papules | 82% (305/370) | 77% (41/53) | 83% (264/317) b | 0.3 | 0.3 |
| Malar rash | 70% (259/371) | 68% (36/53) | 70% (223/318) | 0.7 | 0.6 |
| Photosensitivity | 48% (172/362) | 49% (25/51) a | 47% (147/311) b | 0.8 | 0.9 |
| V or Shawl sign rash | 31% (113/369) | 42% (22/53) | 29% (91/316) b | 0.06 | 0.07 |
| Linear extensor erythema | 36% (130/363) | 31% (16/52) a | 37% (114/311)b | 0.4 | 0.3 |
| Calcinosis | 29% (109/371) | 28% (15/53) | 30% (94/318) | 0.9 | 0.1 |
| Raynaudś phenomenon | 15% (55/369) | 23% (12/53) | 14% (43/316) b | 0.09 | 0.04 |
| Mechanićs hands | 7% (27/366) | 9% (5/53) | 7% (22/313) b | 0.6 | 0.5 |
| Gastrointestinal involvement | |||||
| Dysphagia | 41% (151/370) | 38% (20/53) | 41% (131/317) b | 0.6 | 1.0 |
| Regurgitation | 21% (77/370) | 26% (14/53) | 20% (63/317) b | 0.3 | 0.5 |
| Systemic involvement | |||||
| Weight loss | 42% (155/369) | 52% (27/52) a | 40% (128/317) b | 0.1 | 0.8 |
| Fever | 31% (112/358) | 41% (21/51) a | 30% (91/307) b | 0.10 | 0.8 |
| Muscle Enzymes | |||||
| Peak creatine kinase, IU/L | 781 (252–5142) | 1121 (225–3971) | 750 (256–5249) | 0.7 | 0.9 |
| Peak aldolase, IU/L | 20.0 (34.5) | 18.0 (22.5) | 20.3 (36.1) | 0.6 | 0.3 |
| Severity at onset | 2.2 (1.1) | 2.2 (1.7) | 2.2 (0.9) | 0.9 | 0.4 |
| Early total symptom score | 0.2 (0.1) | 0.27 (0.14) | 0.23 (0.11) | 0.03 | 0.8 |
| Early muscle score | 0.4 (0.2) | 0.37 (0.18) | 0.38 (0.20) | 0.7 | 0.5 |
| Early joint score | 0.5 (0.4) | 0.48 (0.38) | 0.45 (0.43) | 0.6 | 0.1 |
| Early cutaneous score | 0.3 (0.1) | 0.26 (0.15) | 0.25 (0.14) | 0.6 | 0.4 |
| Early gastrointestinal score | 0.1 (0.1) | 0.08 (0.11) | 0.07 (0.11) | 0.6 | 1.0 |
| Early pulmonary score | 0.1 (0.2) | 0.18 (0.23) | 0.08 (0.14) | < 0.001 | 0.002 |
| Early cardiac score | 0.0 (0.1) | 0.05 (0.12) | 0.02 (0.07) | 0.04 | 0.05 |
| Early constitutional symptoms score | 0.4 (0.3) | 0.48 (0.34) | 0.38 (0.26) | 0.02 | 1.0 |
Dichotomous variables were represented as percentage (count/total), continuous variables as mean (SD) and the creatine kinase was presented as median (Q1-Q3). For the univariate analysis, dichotomous variables were compared using chi-squared or Fisheŕs exact tests, as appropriate while continuous variables were compared using Studentś t-test. Multivariate analysis used linear or logistic regression adjusted for length of follow-up, year of onset and autoantibodies. Creatine kinase was log-transformed prior to statistical analysis.
N ≠ 53 due to missing data.
N ≠ 318 due to missing data.
Table 4:
Pulmonary features of juvenile myositis patients with and without anti-Ro52 autoantibodies within juvenile myositis clinical and autoantibody subgroups
| Anti-Ro52 autoantibody positive % (n/N) or Mean (SD) | Anti-Ro52 autoantibody negative % (n/N) or Mean (SD) | p-value | |
|---|---|---|---|
| JDM subgroup (N=302) | |||
| Interstitial lung disease | 33% (14/42) | 1% (3/258) a | < 0.001 |
| Dyspnea on exertion | 62% (26/42) | 19% (50/258) a | < 0.001 |
| Early pulmonary score | 0.20 (0.22) | 0.07 (0.13) | < 0.001 |
| JPM subgroup (N=25) | |||
| Interstitial lung disease | 33% (1/3) | 18% (4/22) | 0.5 |
| Dyspnea on exertion | 50% (1/2) a | 67% (14/21) a | 1.0 |
| Early pulmonary score | 0.17 (0.29) | 0.19 (0.20) | 0.8 |
| JCTM subgroup (N=44) | |||
| Interstitial lung disease | 50% (4/8) | 19% (7/36) | 0.09 |
| Dyspnea on exertion | 43% (3/7) b | 42% (15/36) | 1.0 |
| Early pulmonary score | 0.12 (0.25) | 0.09 (0.16) | 0.7 |
| Anti-MDA5 autoantibody subgroup (N=32) | |||
| Interstitial lung disease | 70% (7/10) | 9% (2/22) | 0.001 |
| Dyspnea on exertion | 90% (9/10) | 27% (6/22) | 0.002 |
| Early pulmonary score | 0.29 (0.19) | 0.02 (0.06) | < 0.001 |
| Anti-aminoacyl tRNA synthetase autoantibody subgroup (N=14) | |||
| Interstitial lung disease | 100% (9/9) | 40% (2/5) | 0.03 |
| Dyspnea on exertion | 89% (8/9) | 40% (2/5) | 0.09 |
| Early pulmonary score | 0.31 (0.31) | 0.27 (0.30) | 0.8 |
| Anti-p155/140 (TIF-1) autoantibody subgroup (N=119) | |||
| Interstitial lung disease | 15% (2/13) | 1% (1/106) | 0.03 |
| Dyspnea on exertion | 50% (6/12) a | 16% (17/105) a | 0.01 |
| Early pulmonary score | 0.16 (0.24) | 0.06 (0.12) | 0.01 |
| Anti-NXP2 autoantibody subgroup (N=76) | |||
| Interstitial lung disease | 9% (1/11) | 0% (0/65) b | 0.1 |
| Dyspnea on exertion | 45% (5/11) | 27% (18/66) | 0.3 |
| Early pulmonary score | 0.16 (0.19) | 0.10 (0.14) | 0.2 |
| MSA negative subgroup (N=96) | |||
| Interstitial lung disease | 0% (0/5) | 10% (9/90) | 1.0 |
| Dyspnea on exertion | 25% (1/4) | 33% (30/90) | 1.0 |
| Early pulmonary score | 0.04 (0.09) | 0.08 (0.15) | 0.5 |
Dichotomous variables were represented as percentage (count/total), continuous variables as mean (SD). For the univariate analysis, dichotomous variables were compared using chi-squared or Fisheŕs exact tests, as appropriate while continuous variables were compared using Studentś t-test.
Abbreviations: JDM: juvenile dermatomyositis, JPM: juvenile polymyositis, JCTM: juvenile connective tissue myositis; MDA5: melanoma differentiation associated protein-5, TIF-1: transcription intermediary factor 1, NXP2: nuclear matrix protein-2, SRP: signal recognition particle.
Data missing for two patients within juvenile myositis clinical or autoantibody subgroup.
Data missing for one patient within juvenile myositis clinical or autoantibody subgroup.
Other clinical manifestations among patients with anti-Ro52 autoantibodies
Independent of MSA status, anti-Ro52 autoantibodies were also associated with Raynaud’s phenomenon (23% vs 14%) (Table 3). Furthermore, within the anti-NXP2 subgroup, Ro52 reactivity was associated with more cutaneous involvement: patients with both anti-NXP2 and anti-Ro52 autoantibodies had a higher prevalence of V- or Shawl-sign rashes (55% vs 17%) and linear extensor erythema (64% vs 20%) than anti-NXP2 autoantibody positive patients without anti-Ro52 autoantibodies. Those with both anti-NXP2 and anti-Ro52 autoantibodies also had more frequent gastroesophageal regurgitation (55% vs 17%). Within the anti-MDA5 subgroup, however, anti-Ro52 autoantibodies were associated with less frequent linear extensor erythema (11% vs 50%). Patients with anti-Ro52 autoantibodies also had a higher mean early cardiac score, defined by the presence of cardiac symptoms at diagnosis.19 There were no other significant differences in the prevalence of the muscle, lung, joint, cutaneous, gastrointestinal, or constitutional manifestations between patients with and without anti-Ro52 autoantibodies in univariate or multivariate analysis, or in examining these features in anti-Ro52 autoantibody positive patients in the presence of another MSA.
Disease severity among patients with anti-Ro52 autoantibodies
Several other differences in outcomes and medications received between patients positive and negative for anti-Ro52 autoantibodies suggested that anti-Ro52 autoantibodies are associated with more severe disease (Table 5). The disease course in patients with anti-Ro52 autoantibodies was more often chronic continuous (78% vs 52%) and less often monocyclic (3% vs 25%). Anti-Ro52 positive patients were more often American College of Rheumatology (ACR) functional class 4 (11% vs 4%) at the last clinical evaluation and had a higher mean ACR functional class score at that assessment. Anti-Ro52 autoantibodies were also associated with an increased total number of medications received (mean 4.8 vs 3.8). Anti-Ro52 autoantibody positive patients more often received intravenous pulse steroids (79% vs 52%). Anti-Ro52 autoantibody positive patients less often achieved clinical remission (5% vs 27%). Lastly, on univariate analysis, but not multivariable analysis, patients with anti-Ro52 autoantibodies less often experienced a complete clinical response (17% vs 32%) and had more medication treatment trials per year (mean 3.5 vs 2.2).
Table 5.
Disease outcomes and medications used in juvenile myositis patients with and without anti-Ro52 autoantibodies
| Total (N=371) % (n/N) or Mean (SD) | Anti-Ro52 autoantibody positive (N=53) % (n/N) or Mean (SD) | Anti-Ro52 autoantibody negative (N=318) % (n/N) or Mean (SD) | Univariate p-value | Multivariate p-value | |
|---|---|---|---|---|---|
| Disease | |||||
| Monocyclic course | 22% (65/297) | 3% (1/37) b | 25% (64/260) c | 0.003 | 0.02 |
| Polycyclic course | 23% (68/297) | 19% (7/37) b | 23% (61/260) c | 0.5 | 0.9 |
| Chronic continuous course | 55% (164/297) | 78% (29/37) b | 52% (135/260) c | 0.002 | 0.05 |
| Steinbrocker functional class at final assessment | |||||
| Mean functional class | 1.4 (0.8) | 1.7 (1.0) | 1.4 (0.8) | 0.007 | 0.007 |
| Functional class 1 | 70% (257/367) | 53% (28/53) | 73% (229/314) c | 0.003 | 0.09 |
| Functional class 2 | 21% (77/367) | 34% (18/53) | 19% (59/314) c | 0.01 | 0.3 |
| Functional class 3 | 4% (13/367) | 2% (1/53) | 4% (12/314) c | 0.7 | 0.2 |
| Functional class 4 | 5% (20/367) | 11% (6/53) | 4% (14/314) c | 0.05 | 0.008 |
| Mortality | 4% (13/371) | 6% (3/53) | 3% (10/318) | 0.4 | 0.4 |
| Hospitalized | 58% (206/355) | 66% (35/53) | 57% (171/302) c | 0.2 | 0.4 |
| Mean number of hospitalizations | 1.3 (1.9) | 1.3 (1.4) | 1.3 (2.0) | 0.9 | 0.8 |
| Wheelchair use | 19% (68/360) | 24% (12/50) b | 18% (56/310) c | 0.3 | 0.2 |
| Response to treatment | |||||
| Complete clinical response | 30% (91/304) | 17% (7/42) | 32% (84/262) c | 0.04 | 0.4 |
| Remission | 24% (74/312) | 5% (2/43) | 27% (72/269) c | 0.002 | 0.05 |
| Total number of medications used | 3.9 (2.1) | 4.8 (2.5) | 3.8 (2.0) | 0.003 | 0.05 |
| Treatment trials per year | 2.3 (2.8) | 3.5 (3.0) | 2.2 (2.7) | 0.004 | 0.1 |
| Medications received | |||||
| Oral steroids | 99% (309/312) | 100% (43/43) b | 99% (266/269) c | 1.0 | . |
| Intravenous pulsed steroids | 56% (174/312) | 79% (34/43) b | 52% (140/269) c | < 0.001 | 0.03 |
| Methotrexate | 74% (230/312) | 86% (37/43) b | 72% (193/269) c | 0.05 | 0.4 |
| Intravenous immunoglobulin | 36% (112/312) | 49% (21/43) b | 34% (91/269) c | 0.06 | 0.08 |
| Other DMARDs | 23% (73/312) | 35% (15/43) b | 22% (58/269) c | 0.06 | 0.3 |
Dichotomous variables were represented as percentage (count/total), continuous variables as mean (SD). For the univariate analysis, dichotomous variables were compared using chi-squared or Fisheŕs exact tests, as appropriate while continuous variables were compared using Studentś t-test. Multivariate analysis used linear or logistic regression adjusted for length of follow-up, year of onset and autoantibodies.
Abbreviations: ACR: American College of Rheumatology, DMARDs: disease modifying anti-rheumatic agents
Azathioprine, Chlorambucil, Chloroquine, Colchicine, Cyclophosphamide, Cyclosporine, Dapsone, Hydroxychloroquine, Intravenous Immunoglobulin, Lefluonmide, Methotrexate, Mycophenolate mofetil, Sodium thiosulfate, Quinacrine
N ≠ 53 due to missing data
N ≠ 318 due to missing data
Those with both anti-NXP2 and anti-Ro52 autoantibodies also more often had a severe (class IV) ACR functional class (27% vs 3%) and more frequent wheelchair use (60% vs 20%) as compared to patients positive for anti-NXP2 who were anti-Ro52 autoantibody negative. There was no other association of co-existing MSAs and anti-Ro52 autoantibodies on clinical outcomes or medications received.
Anti-Ro52 autoantibody titers
Anti-Ro52 autoantibody titers did not significantly differ between JDM, JPM, and JCTM groups. Overall, we found that higher anti-Ro52 titers are associated with shorter follow-up time, more treatment trials per year, higher early total symptom score, more total number of medications used, higher total functional class, higher severity at onset, higher early pulmonary score, higher early constitutional symptoms score, and higher total functional class in patients with juvenile IIM (all p<0.05; data not shown). However, as the Spearman correlation coefficients were ≤ 0.2 for each association, the clinical significance of high autoantibody titers is modest.
DISCUSSION
Here, we utilized a large cohort of juvenile myositis patients to study the prevalence and clinical significance of anti-Ro52 autoantibodies in children with IIM. We found anti-Ro52 autoantibodies to be strongly associated with ILD and other pulmonary manifestations in juvenile myositis patients. We also found that children with anti-Ro52 autoantibodies have more severe disease, underwent more intense treatment regimens, and had lower rates of disease remission than those without anti-Ro52 autoantibodies. In children with myositis, anti-Ro52 autoantibodies were associated with anti-aminoacyl tRNA synthetase autoantibodies, as previously described in adults.11 We also found that anti-Ro52 autoantibodies were associated with anti-MDA5 autoantibodies in pediatric myositis patients, which has not been reported previously.
Importantly, our analyses indicate that the presence of anti-Ro52 autoantibodies is strongly associated with ILD, even after adjusting for the presence of MSAs such as anti-MDA5 and anti-aminoacyl tRNA synthetase autoantibodies. Indeed, the association of Ro52 reactivity with ILD is not limited to the anti-MDA5 and anti-aminoacyl tRNA synthetase autoantibody subgroups, but extends to other MSA subgroups that are not classically associated with ILD, such as children with anti-p155/140 (TIF-1) autoantibodies. However, none of the 5 anti-Ro52 autoantibody positive MSA-negative patients had ILD. Current practice encourages screening juvenile myositis patients for MSAs such as anti-MDA5 and anti-aminoacyl tRNA synthetase autoantibodies, as these autoantibodies confer risk for developing ILD and their presence is a determinant of clinical management and patient prognosis. In light of the current findings demonstrating that anti-Ro52 autoantibodies are an independent predictor of ILD, screening juvenile myositis patients for these autoantibodies may also be prudent.
In adult patients with IIM, anti-Ro52 autoantibodies have been associated with poorer response to immunosuppressive drugs and decreased survival.13,15 Similarly, in our juvenile cohort, anti-Ro52 autoantibodies are associated with more severe disease and poorer outcomes. Of note, the presence of anti-Ro52 autoantibodies was associated with a higher early cardiac score which is a measure of patient reported cardiac symptoms including palpitations, chest pain, and syncope. However, among the 9 anti-Ro52-positive patients with one or more of these symptoms, only 3 had EKG changes or ECHO abnormalities. As the severity of other clinical manifestations, including muscle, joint, skin, gastrointestinal, and systemic features were not associated with Ro52 reactivity, it seems likely that disease severity seen in the anti-Ro52 positive patients is a consequence of pulmonary disease. Additional studies are required to clarify this point. Nonetheless, our findings highlight the potential utility of anti-Ro52 autoantibodies as a predictor of disease severity and poor prognosis in juvenile myositis, which underscores the potential utility of screening juvenile IIM patients for anti-Ro52 autoantibodies.
Of particular significance is the novel association of anti-Ro52 autoantibodies and anti-MDA5 autoantibodies in our JIIM cohort. In adult IIM patients, anti-Ro52 autoantibodies often co-occur with anti-Jo1 autoantibodies, and in adult anti-Jo1 positive patients, Ro52 reactivity is associated with more severe ILD. A small case series reported co-existing anti-Ro52 autoantibodies in 6 of 13 anti-MDA5 autoantibody positive patients, 5 of whom had rapidly progressive ILD.27 Interestingly, only 1 of 33 patients with ILD in our JIIM cohort had rapidly progressive ILD and this patient was positive for both anti-MDA5 and anti-Ro52 autoantibodies.
Although we have now established an association between anti-aminoacyl tRNA synthetase and anti-Ro52 autoantibodies not only in adults, but also in children, it remains unclear why these autoantibodies co-occur. It has been proposed that local autoantibody production induced by type I IFN28 could be a driving force behind the production of both anti-Jo1 and anti-Ro52 autoantibodies, given the increase in B-cell activating factor (BAFF) receptors in the sera of IIM patients with these autoantibodies.29 In the current study of juvenile IIM, we now also demonstrate an association between anti-MDA5 and anti-Ro52 autoantibodies. Interestingly, both MDA5 and Ro52 are cytosolic, interferon (IFN)-induced proteins; perhaps concurrent over-expression of these proteins in juvenile IIM patients leads to the development of autoimmunity against both. However, we do not have adequate type I IFN measurements to further examine this hypothesis.
This current study has several limitations. First, this cohort of patients with juvenile myositis had some data collected retrospectively, resulting in some missing data, and was collected over more than 20-years, with potential chronology bias. However, we adjusted the variables of this study for the year of diagnosis and tested the distribution of missing values across groups and did not find evidence of a significant bias. Second, although imaging studies were available to confirm the diagnosis of ILD in more than 90% of patients who had ILD, pulmonary function testing data were not available for many of the patients, as a number of the children were of young age when such testing is unreliable in children. Thus, we were not able to study whether ILD patients with anti-Ro52 autoantibodies had more severe pulmonary dysfunction than those without these autoantibodies. Also, we cannot confirm the absence of ILD as many of the children without clinical suspicion of ILD did not have imaging and/or pulmonary function testing. This however, is a limitation of standard clinical care in pediatric patients who have challenges to undergo such testing.
Overall, this study shows that anti-Ro52 autoantibodies are present in 14% of patients with juvenile myositis and are strongly associated with ILD, more severe illness, and poorer outcomes, even when correcting for the co-existence of MSAs. In juvenile myositis patients, anti-Ro52 autoantibodies are associated not only with the presence of anti-synthetase autoantibodies, as previously reported in adult myositis patients, but also with anti-MDA5 autoantibodies, and the co-existence of these MSAs increases the likelihood of ILD and poor outcome. The current standard of care in patients with juvenile myositis who have reactivity to MSAs associated with pulmonary manifestations (such as anti-MDA5 and anti-aminoacyl tRNA synthetase autoantibodies) is to have a high index of suspicion for the development of ILD and modify management accordingly. Our data suggest that testing for anti-Ro52 autoantibodies may also have a role in disease monitoring, management, and patient prognosis in juvenile myositis patients.
KEY MESSAGES.
What is already known about this subject?
The clinical features and prognosis of juvenile myositis patients with anti-Ro52 autoantibodies was poorly defined.
What does this study add?
Approximately 15% of a large North American cohort of juvenile myositis patients have anti-Ro52 autoantibodies.
Juvenile myositis patients with anti-Ro52 autoantibodies are more likely to develop interstitial lung disease.
Anti-Ro52 autoantibodies are more common in juvenile myositis patients with anti-MDA5 and anti-synthetase autoantibodies.
Juvenile myositis patients with anti-Ro52 autoantibodies more often have a chronic disease course and require more medications.
How might this impact on clinical practice?
Anti-Ro52 autoantibodies are useful prognostic markers for ILD and severe disease in juvenile myositis patients.
ACKNOWLEDGEMENTS:
This work was presented in abstract form at ACR 2018.
FUNDING INFO: This research was supported by the Intramural Research Programs of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS; ZIA AR041203) and the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH).
Footnotes
COMPETING INTERESTS: The authors have no competing interests to report.
PATIENT AND PUBLIC INVOLVEMENT: Patients were involved in the research from the time they provided consent to join this natural history study. The research questions were not explicitly developed nor informed by their priorities, experience, and preferences. The patients/public were not involved in the design of this study. Patients were not involved in the recruitment to and conduct of the study. Patients were not asked to assess the burden of the intervention and time required to participate in the research. Patients have not and will not be involved in choosing the methods and agreeing plans for dissemination of the study results to participants and wider relevant communities.
ETHICAL APPROVAL INFORMATION: All subjects were enrolled in natural history study approved by the National Institutes of Health Institutional IRB and all patients provided informed consent.
DATA SHARING STATEMENT: All data relevant to the study are included in the article or uploaded as supplementary information. No unpublished data from this study is available.
Contributor Information
Sara Sabbagh, Muscle Disease Unit, Laboratory of Muscle Stem Cells and Gene Regulation, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (NIH), Bethesda, MD..
Iago Pinal-Fernandez, Muscle Disease Unit, Laboratory of Muscle Stem Cells and Gene Regulation, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (NIH), Bethesda, MD.; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD.; Faculty of Health Sciences, Universitat Oberta de Catalunya, Barcelona, Spain..
Takayuki Kishi, Environmental Autoimmunity Group, National Institute of Environmental Health Sciences, NIH, Bethesda, MD..
Ira N. Targoff, VA Medical Center and Oklahoma Medical Research Foundation, Oklahoma City, OK.
Frederick W. Miller, Environmental Autoimmunity Group, National Institute of Environmental Health Sciences, NIH, Bethesda, MD.
Lisa G. Rider, Environmental Autoimmunity Group, National Institute of Environmental Health Sciences, NIH, Bethesda, MD..
Andrew L. Mammen, Muscle Disease Unit, Laboratory of Muscle Stem Cells and Gene Regulation, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (NIH), Bethesda, MD.; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD.; Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD..
REFERENCES:
- 1.Pachman LM, Khojah AM. Advances in Juvenile Dermatomyositis: Myositis Specific Antibodies Aid in Understanding Disease Heterogeneity. J Pediatr 2018;195:16–27. doi: 10.1016/j.jpeds.2017.12.053 [published Online First: 2018/03/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rider LG, Shah M, Mamyrova G, et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 2013;92(4):223–43. doi: 10.1097/MD.0b013e31829d08f9 [published Online First: 2013/07/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rider LG, Nistala K. The juvenile idiopathic inflammatory myopathies: pathogenesis, clinical and autoantibody phenotypes, and outcomes. J Intern Med 2016;280(1):24–38. doi: 10.1111/joim.12444 [published Online First: 2016/03/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakurai N, Nagai K, Tsutsumi H, et al. Anti-CADM-140 antibody-positive juvenile dermatomyositis with rapidly progressive interstitial lung disease and cardiac involvement. J Rheumatol 2011;38(5):963–4. doi: 10.3899/jrheum.101220 [published Online First: 2011/05/03] [DOI] [PubMed] [Google Scholar]
- 5.Tansley SL, Betteridge ZE, Gunawardena H, et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther 2014;16(4):R138. doi: 10.1186/ar4600 [published Online First: 2014/07/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: A concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol 2018;78(4):776–85. doi: 10.1016/j.jaad.2017.12.010 [published Online First: 2017/12/13] [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi N, Takezaki S, Kobayashi I, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology (Oxford) 2015;54(5):784–91. doi: 10.1093/rheumatology/keu385 [published Online First: 2014/10/08] [DOI] [PubMed] [Google Scholar]
- 8.Miller FW, Love LA, Barbieri SA, et al. Lymphocyte activation markers in idiopathic myositis: changes with disease activity and differences among clinical and autoantibody subgroups. Clin Exp Immunol 1990;81(3):373–9. [published Online First: 1990/09/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum 2006;54(11):3682–9. doi: 10.1002/art.22164 [published Online First: 2006/11/01] [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi I, Okura Y, Yamada M, et al. Anti-melanoma differentiation-associated gene 5 antibody is a diagnostic and predictive marker for interstitial lung diseases associated with juvenile dermatomyositis. J Pediatr 2011;158(4):675–7. doi: 10.1016/j.jpeds.2010.11.033 [published Online First: 2011/01/15] [DOI] [PubMed] [Google Scholar]
- 11.Pinal-Fernandez I, Casal-Dominguez M, Huapaya JA, et al. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology (Oxford) 2017;56(6):999–1007. doi: 10.1093/rheumatology/kex021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Corte R, Lo Mo Naco A, Locaputo A, et al. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 2006;39(3):249–53. doi: 10.1080/08916930600623791 [published Online First: 2006/06/14] [DOI] [PubMed] [Google Scholar]
- 13.Vancsa A, Csipo I, Nemeth J, et al. Characteristics of interstitial lung disease in SS-A positive/Jo-1 positive inflammatory myopathy patients. Rheumatol Int 2009;29(9):989–94. doi: 10.1007/s00296-009-0884-9 [published Online First: 2009/03/07] [DOI] [PubMed] [Google Scholar]
- 14.Bauhammer J, Blank N, Max R, et al. Rituximab in the Treatment of Jo1 Antibody-associated Antisynthetase Syndrome: Anti-Ro52 Positivity as a Marker for Severity and Treatment Response. J Rheumatol 2016;43(8):1566–74. doi: 10.3899/jrheum.150844 [published Online First: 2016/06/03] [DOI] [PubMed] [Google Scholar]
- 15.Marie I, Hatron PY, Dominique S, et al. Short-term and long-term outcome of anti-Jo1-positive patients with anti-Ro52 antibody. Semin Arthritis Rheum 2012;41(6):890–9. doi: 10.1016/j.semarthrit.2011.09.008 [published Online First: 2011/11/15] [DOI] [PubMed] [Google Scholar]
- 16.Srivastava P, Dwivedi S, Misra R. Myositis-specific and myositis-associated autoantibodies in Indian patients with inflammatory myositis. Rheumatol Int 2016;36(7):935–43. doi: 10.1007/s00296-016-3494-3 [published Online First: 2016/05/20] [DOI] [PubMed] [Google Scholar]
- 17.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292(8):403–7. doi: 10.1056/NEJM197502202920807 [published Online First: 1975/02/20] [DOI] [PubMed] [Google Scholar]
- 18.Shah M, Mamyrova G, Targoff IN, et al. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 2013;92(1):25–41. doi: 10.1097/MD.0b013e31827f264d [published Online First: 2012/12/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habers GE, Huber AM, Mamyrova G, et al. Brief Report: Association of Myositis Autoantibodies, Clinical Features, and Environmental Exposures at Illness Onset With Disease Course in Juvenile Myositis. Arthritis Rheumatol 2016;68(3):761–8. doi: 10.1002/art.39466 [published Online First: 2015/10/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oddis CV, Rider LG, Reed AM, et al. International consensus guidelines for trials of therapies in the idiopathic inflammatory myopathies. Arthritis Rheum 2005;52(9):2607–15. doi: 10.1002/art.21291 [published Online First: 2005/09/06] [DOI] [PubMed] [Google Scholar]
- 21.Kishi T, Bayat N, Ward MM, et al. Medications received by patients with juvenile dermatomyositis. Semin Arthritis Rheum 2018. doi: 10.1016/j.semarthrit.2018.03.016 [published Online First: 2018/05/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCann LJ, Juggins AD, Maillard SM, et al. The Juvenile Dermatomyositis National Registry and Repository (UK and Ireland)--clinical characteristics of children recruited within the first 5 yr. Rheumatology (Oxford) 2006;45(10):1255–60. doi: 10.1093/rheumatology/kel099 [published Online First: 2006/03/29] [DOI] [PubMed] [Google Scholar]
- 23.Pachman LM, Abbott K, Sinacore JM, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr 2006;148(2):247–53. doi: 10.1016/j.jpeds.2005.10.032 [published Online First: 2006/02/24] [DOI] [PubMed] [Google Scholar]
- 24.Pachman LM, Hayford JR, Chung A, et al. Juvenile dermatomyositis at diagnosis: clinical characteristics of 79 children. J Rheumatol 1998;25(6):1198–204. [published Online First: 1998/06/19] [PubMed] [Google Scholar]
- 25.Constantin T, Ponyi A, Orban I, et al. National registry of patients with juvenile idiopathic inflammatory myopathies in Hungary--clinical characteristics and disease course of 44 patients with juvenile dermatomyositis. Autoimmunity 2006;39(3):223–32. doi: 10.1080/08916930600622819 [published Online First: 2006/06/14] [DOI] [PubMed] [Google Scholar]
- 26.Kishi T, Rider LG, Pak K, et al. Association of Anti-3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Autoantibodies With DRB1*07:01 and Severe Myositis in Juvenile Myositis Patients. Arthritis Care Res (Hoboken) 2017;69(7):1088–94. doi: 10.1002/acr.23113 [published Online First: 2017/01/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang K, Shojania K, Yeung J, et al. FRI0448 Mda5 antibody positive clinical amyopathic dermatomyositis (CADM): a single tertiary centre case series of 13 patients. Annals of the Rheumatic Diseases 2018;77(Suppl 2):753–53. doi: 10.1136/annrheumdis-2018-eular.1887 [DOI] [Google Scholar]
- 28.Ittah M, Miceli-Richard C, Eric Gottenberg J, et al. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren’s syndrome. Arthritis Res Ther 2006;8(2):R51. doi: 10.1186/ar1912 [published Online First: 2006/03/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krystufkova O, Barbasso Helmers S, Venalis P, et al. Expression of BAFF receptors in muscle tissue of myositis patients with anti-Jo-1 or anti-Ro52/anti-Ro60 autoantibodies. Arthritis Res Ther 2014;16(5):454. doi: 10.1186/s13075-014-0454-8 [published Online First: 2014/10/11] [DOI] [PMC free article] [PubMed] [Google Scholar]


