Functional neuroendocrine tumors (NETs) of the GI tract are characterized by hormone secretion resulting in a specific clinical syndrome.1 With insulinomas, one of the most common types of pancreatic NET (pNETs), hypoglycemia can be profound and debilitating.1,2 Surgical resection has been the longstanding treatment, but in patients unfit for or unwilling to undergo surgery, a nonoperative approach with EUS-guided radiofrequency ablation (EUS-RFA) has been described as an alternative to restoring euglycemia.2,3 The Habib system (EMcision/Boston Scientific, Marlborough, Mass, USA), the initial device for EUS-RFA, is no longer commercially available.4 More recently, the endoscopic ultrasound-guided radiofrequency ablation electrode device (EUSRA; TaeWoong Medical, Gyeonggi-do, South Korea) has been investigated in the management of pancreatic adenocarcinoma and pNETs in a few patients in Asia and Europe2,5,6 and has recently been released in the United States. The EUSRA device is composed of a 19-gauge radiofrequency (RF) monopolar electrode with a sharp tip for EUS-guided puncture. The RF electrode undergoes cooling using chilled saline solution maintained at 32°F to minimize risk of injury to surrounding structures. The device is passed through a therapeutic linear echoendoscope to the lesion of interest.
In this report, we present a patient with a symptomatic insulinoma successfully treated with the new EUSRA device (Video 1, available online at www.VideoGIE.org). Briefly, a 57-year-old woman with multiple comorbidities, including obesity (body mass index 40 kg/m2), cirrhosis with portal hypertension, and aortic dissection, was admitted with refractory hypoglycemia (blood glucose as low as 40 mg/dL) requiring a continuous dextrose-10% infusion. A CT scan demonstrated an enhancing 2.2- × 1.8-cm pancreatic neck mass suggestive of an insulinoma. Serum insulin level obtained during a hypoglycemic episode was elevated at 37 μIU/mL (normal 2-23). She was deemed to be a high-risk surgical candidate, and the decision was made to proceed with EUS-RFA of the insulinoma.
Procedure
Using EUS, we identified a lobular, hypoechoic oval mass in the pancreatic neck, correlating with the CT scan findings (Fig. 1). Color Doppler was used to confirm the absence of intervening vascular structures, and fine-needle biopsy specimens were first obtained, with preliminary onsite cytology confirming NET. The 19-gauge EUSRA device was advanced into the lesion through a linear echoendoscope (Olympus America, Center Valley, Pa, USA) via a transgastric approach. Ablation was performed at 30W for 20 seconds, with appearance of hyperechoic bubbles. The needle was withdrawn and reoriented in a different plane within the lesion. Ablation was subsequently performed in a similar fashion in a fanning motion, for a total of 5 treatments.
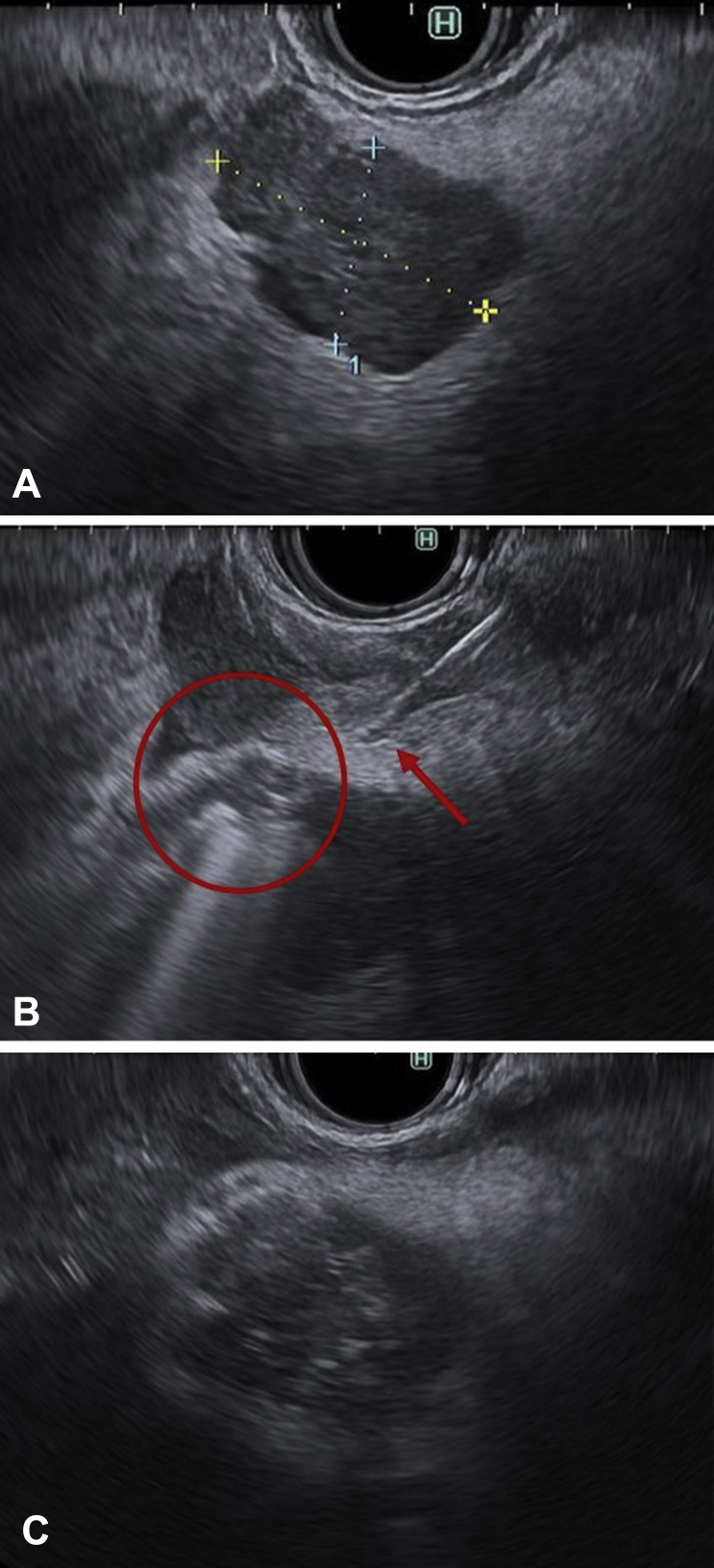
Figure 1.
EUS-guided radiofrequency ablation (RFA) of insulinoma. A, A hypoechoic lesion is seen in the neck of the pancreas. B, The EUS-guided radiofrequency ablation electrode device is advanced inside the lesion, with hyperechoic bubbles seen during ablation. C, Immediate posttreatment appearance showing hyperechoic changes after RFA.
Outcome
The patient did well after ablation, without evidence of abdominal pain, pancreatitis, or pancreatic leak. Hypoglycemia resolved, with discontinuation of the dextrose infusion within 24 hours postprocedure (Fig. 2). The patient did not exhibit any signs of pancreatitis secondary to EUS-RFA and was discharged home 2 days later. A CT scan 7 weeks after RFA demonstrated a significant decrease in the size of the insulinoma with new areas of hypoenhancement within the lesion secondary to ablation (Fig. 3). She has since remained asymptomatic at 6-month follow-up without signs of recurrent hypoglycemia.
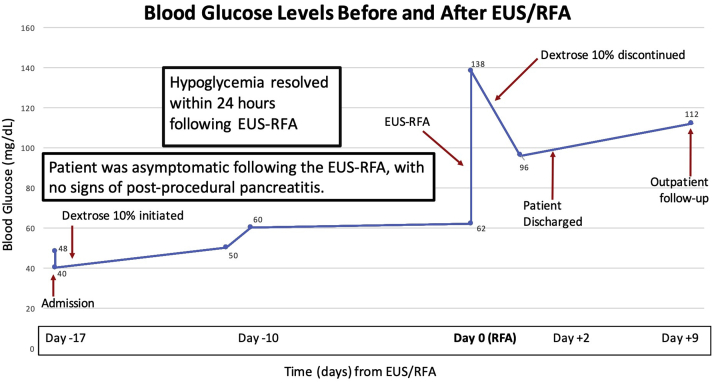
Figure 2.
Patient’s blood glucose trend throughout her hospital course is displayed in relation to timing of EUS–radiofrequency ablation (RFA) (date of procedure designated as day 0). Euglycemia was restored within 24 hours postprocedure, and dextrose-10% infusion was discontinued the evening after the RFA. She did not exhibit any signs of pancreatitis secondary to EUS-RFA and was discharged home 2 days later.
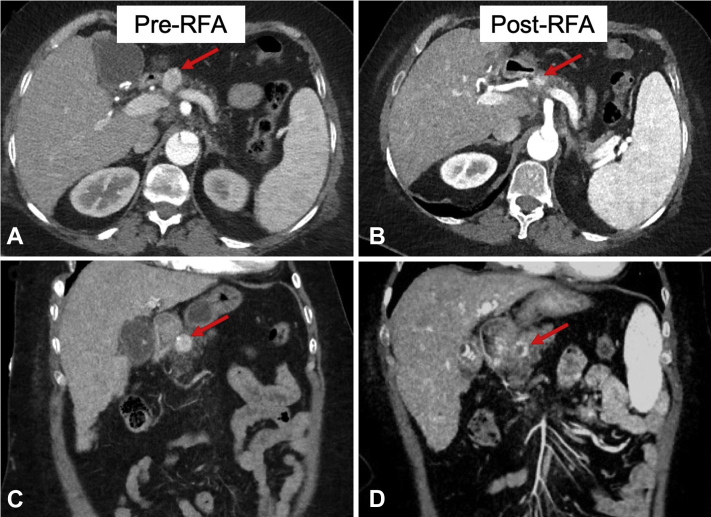
Figure 3.
Abdominal CT scan before (A and C) and after (B and D) EUS–radiofrequency ablation (RFA). A, B, Transverse plane. C, D, Coronal plane. An enhancing lesion is seen in the pancreas neck, measuring 2.2 × 1.8 cm and suggestive of an insulinoma. Adjacent images of follow-up CT scan 7 weeks after RFA reveal a significant decrease in enhancement and size of the lesion.
Conclusion
EUS-RFA represents a less invasive alternative to operative resection of pancreatic insulinomas in nonsurgical candidates. We report the successful treatment of a symptomatic insulinoma with the EUSRA device newly available in the United States. The long-term efficacy of EUSRA for treatment of solid and cystic pancreatic lesions and the risk of adverse events, such as pancreatitis and pancreatic leak, need to be assessed in future studies.
Disclosure
Dr Wagh is a consultant for Boston Scientific, Medtronic, Olympus, and Incyte. All other authors disclosed no financial relationships.
Supplementary data
Details pertaining to the patient’s clinical presentation of a symptomatic insulinoma are first summarized in the video. Subsequently, the video features intraprocedural EUS footage demonstrating the lesion preintervention, followed by treatment using the endoscopic ultrasound-guided radiofrequency ablation electrode device, with clearly visible bubbles seen during ablation. Finally, the video shows the post–radiofrequency ablation (RFA) appearance. Follow-up CT imaging 7 weeks after RFA is shown adjacent to the index CT, with interval significant decrease in size and enhancement of the insulinoma.
References
- 1.Ito T., Lee L., Jensen R.T. Treatment of symptomatic neuroendocrine tumor syndromes: recent advances and controversies. Expert Opin Pharmacother. 2016;17:2191–2205. doi: 10.1080/14656566.2016.1236916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakhtakia S., Ramchandani M., Galasso D. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos) Gastrointest Endosc. 2016;83:234–239. doi: 10.1016/j.gie.2015.08.085. [DOI] [PubMed] [Google Scholar]
- 3.Waung J., Todd J.F., Keane M.G. Successful management of sporadic pancreatic insulinoma by endoscopic ultrasound-guided radiofrequency ablation. Endoscopy. 2016;48:E144–E145. doi: 10.1055/s-0042-104650. [DOI] [PubMed] [Google Scholar]
- 4.Pai M., Habib N., Senturk H. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52–59. doi: 10.4240/wjgs.v7.i4.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song T.J., Seo D.W., Lakhtakia S. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc. 2016;83:440–443. doi: 10.1016/j.gie.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 6.Barthet M., Giovannini M., Lesavre N. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836–842. doi: 10.1055/a-0824-7067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details pertaining to the patient’s clinical presentation of a symptomatic insulinoma are first summarized in the video. Subsequently, the video features intraprocedural EUS footage demonstrating the lesion preintervention, followed by treatment using the endoscopic ultrasound-guided radiofrequency ablation electrode device, with clearly visible bubbles seen during ablation. Finally, the video shows the post–radiofrequency ablation (RFA) appearance. Follow-up CT imaging 7 weeks after RFA is shown adjacent to the index CT, with interval significant decrease in size and enhancement of the insulinoma.