Abstract
Photodynamic therapy (PDT) is emerging as a significant complementary or alternative approach for cancer treatment. PDT drugs act as photosensitisers, which upon using appropriate wavelength light and in the presence of molecular oxygen, can lead to cell death. Herein, we reviewed the general characteristics of the different generation of photosensitisers. We also outlined the emergence of rhenium (Re) and more specifically, Re(I) tricarbonyl complexes as a new generation of metal-based photosensitisers for photodynamic therapy that are of great interest in multidisciplinary research. The photophysical properties and structures of Re(I) complexes discussed in this review are summarised to determine basic features and similarities among the structures that are important for their phototoxic activity and future investigations. We further examined the in vitro and in vivo efficacies of the Re(I) complexes that have been synthesised for anticancer purposes. We also discussed Re(I) complexes in conjunction with the advancement of two-photon PDT, drug combination study, nanomedicine, and photothermal therapy to overcome the limitation of such complexes, which generally absorb short wavelengths.
Keywords: cancer, medicinal inorganic chemistry, metals in medicine, photodynamic therapy, photosensitisers, rhenium(I) tricarbonyl complexes
1. Introduction
Cancer has caused approximately 10 million deaths around the world [1]. Despite the advancement in cancer diagnosis and therapy, it is still the second leading cause of death even in the United States [2]. Conventional cancer therapies include surgery, radiotherapy, and chemotherapy [3,4]. Surgery is the mainstay of treatment for most of the solid tumours. Unfortunately, not all tumours are resectable [5,6]. Cancer patients who can undergo radiotherapy would have better long-term survival, tumour cure, and local tumour regression. However, radiotherapy may induce metastatic spread, tissue complications, and high rates of loco-regional failure [7,8,9,10]. For patients who cannot receive surgery or radiotherapy, chemotherapy has been the mainstay of treatment. Cisplatin, gemcitabine, and doxorubicin are some of the commonly prescribed chemotherapies for most cancer patients. However, their poor cellular penetration and adverse side effects may limit their therapeutic efficacy [11]. Although the recently emerging immunotherapy has promising therapeutic efficacy in selected solid tumours, its efficacy is often unpredictable due to the variability in the host’s immune system and the complicated cancer-immune crosstalk [12,13]. The cancerous cells might develop resistance towards both chemotherapy and immunotherapy when its efficacy drops to the sub-therapeutic zone. Hence, there is an urgency to develop more alternative approaches to prevent and to overcome drug resistance [14]. One of such alternatives would be to utilise photodynamic therapy (PDT).
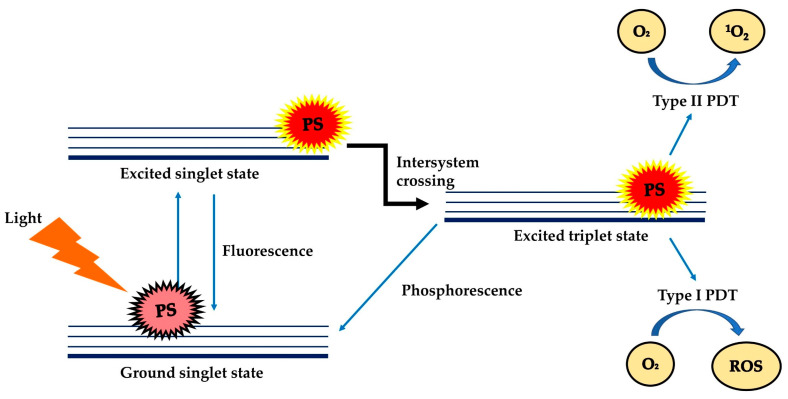
PDT is a treatment modality that uses harmless visible light to activate non-toxic light-excitable molecules (i.e., the photosensitisers), for the generation of cytotoxic reactive oxygen species (ROS, type I photoactivation) and singlet oxygen (type II photoactivation) from molecular oxygen (Figure 1) [15,16,17,18,19,20,21] within or at near vicinity to cancer cells, to cause cell death and tissue necrosis [22]. PDT was claimed to be relatively selective and safe compared to classical anticancer therapies due to the use of harmless therapeutic agents during the process, liberty of activating only the photosensitisers (PSs) at the tumour region via selective exposure of the tumour to light, and increased tendency of the targeted photosensitisers to accumulate at the tumour site [21,23]. PDT can be used repetitively without causing resistance to tumour or hypersensitivity to normal tissue, as a single agent or in conjunction with other anticancer therapies such as chemotherapy and radiotherapy [24,25,26,27,28].
Figure 1.
Schematic illustration of the mechanism of PDT which involves Type I and Type II mechanisms. PS: photosensitiser; O2: oxygen; 1O2: singlet oxygen; ROS: reactive oxygen species.
At present, a number of PSs have received approval from the FDA for the treatment of cancer (e.g., skin, pancreatic and other cancers—refer to Table 1) [22] and non-cancer disorders (e.g., verteporfin for age-related macular degeneration treatment and 5-aminolevulinic acid for moderate to severe acne vulgaris) [29]. Despite the advantages, the extensive clinical use of PDT was limited by the inherent weaknesses of common photosensitisers, such as water insolubility, aggregation tendency in the physiological environment, and requirement of a rich oxygen environment for the production of singlet oxygen/ROS [30,31,32,33,34]. In recent years, Re(I) complexes have been increasingly explored as a new alternative choice of photosensitisers for PDT [19,35,36]. This review gives a brief account of the development of anticancer PDT and assesses the potential of Re(I) complexes as potent photosensitisers for PDT.
Table 1.
Application of clinically approved photosensitisers for treatment of cancer.
| Photosensitisers | Application | References |
|---|---|---|
| NPe6 (Talaporfin sodium) | Non-small cell lung carcinoma | [37] |
| Motexafin lutetium | Prostate cancer | [38] |
| Temoporfin | Head, neck, prostate and pancreatic cancers | [39,40,41] |
| Porfimer sodium | Obstructive oesophageal, lung, bladder and cervical cancers | [28,42,43] |
| 2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide-a | Head, lung and neck cancers, basal cell carcinoma | [44,45,46] |
| Hexaminolevulinate | Bladder cancer | [47] |
| Methyl aminolevulinate | Basal cell carcinoma | [40,48,49] |
| Aluminium phthalocyanine tetrasulfonate | Lung, breast, skin and stomach cancers | [50] |
| Padeliporfin | Early-stage of prostate cancer | [51] |
| Verteporfin | Basal cell carcinoma | [52,53] |
2. Photosensitisers for Photodynamic Therapy—A Brief History
Photosensitisers (PS) were first introduced as an anticancer treatment on a commercial scale by Thomas Dougherty and his co-workers in the 1970s. Since this pioneering work, three generations of photosensitisers have been developed [48,54].
2.1. First and Second Generation Photosensitisers
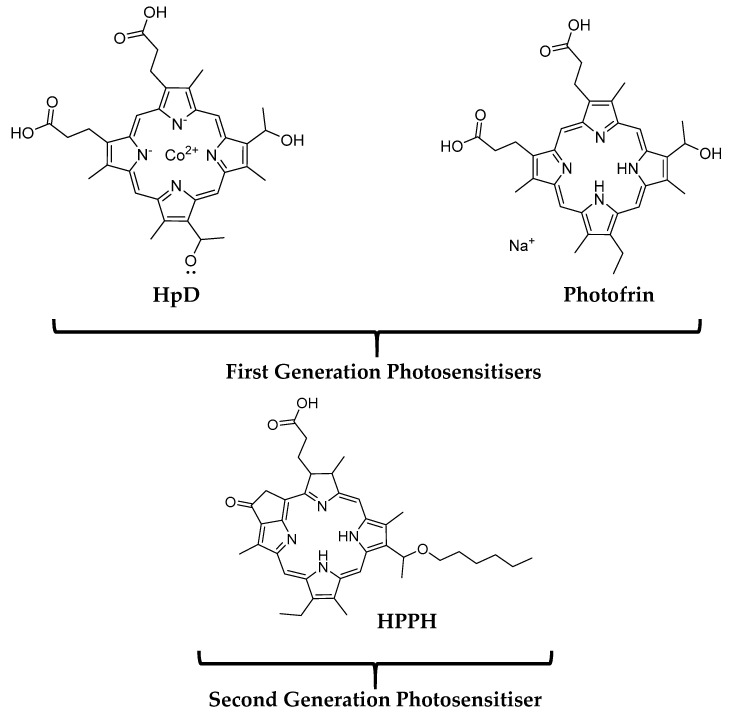
The first generation of PDT PSs was mainly porphyrin-based, notable examples include hematoporphyrin derivatives (HpD) and Photofrin (porfimer sodium) for the treatment of skin cancer (shown in Figure 2) [55]. The use of first-generation PSs for anticancer purposes was not well received due to their poor tissue penetration, low chemical purity and long half-life, which results in high accumulation in the skin thus causing skin hypersensitivity [56]. In an attempt to address these weaknesses, the development of second-generation photosensitisers was initiated in the early 1980s [57,58,59]. The examples of second-generation photosensitisers include chlorin, benzoporphyrin derivatives and hematoporphyrin derivatives [60]. Compared to their predecessors, second-generation photosensitisers can be synthesised at a higher purity, absorb light that better penetrates the body tissues (range of wavelength: 650–800 nm), produce a higher amount of singlet oxygen and cause fewer side effects. For instance, highly lipophilic 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH), as known as Photochlor (shown in Figure 2), has been evaluated extensively and approved in the USA to treat head, lung and neck cancers. Photochlor demonstrates enhanced pharmacokinetic properties and only result in mild skin photosensitivity which undergoes a rapid reduction after a few days of administration of Photochlor in comparison with the first generation of PSs [44,45,46]. Nevertheless, the second generation photosensitisers are still poorly water-soluble. This limits their intravenous (IV) use in clinical settings [32].
Figure 2.
Structures of a few examples of common photosensitisers.
2.2. Third Generation Photosensitisers
The third generation of PSs was developed to enhance their bioavailability to the targeted tissue [61]. They were either novel compounds that possess higher selectivity and affinity to cells of the targeted tissue (e.g., cancer) than normal tissues or nano-complexes formed by combining selected second-generation photosensitisers with suitable antibodies [62,63] or active/passive disease-tissue-targeting nano-drug-carriers, such as peptides, polymers and polymeric nanoparticles. One example of such nano-carrier-photosensitiser complexes was chlorin E6 (Ce6) complexes incorporated into ursodeoxycholic acid-conjugated chitosan nanoparticles that showed a promising anticancer effect towards HuCC-T1 (human cholangiocarcinoma cells) [64]. Similarly, conjugation of protein (Shiga-like toxin B subunit) to Ce6 to specifically target the overexpressed receptors in ovarian cancers, glycosphingolipid receptor Gb3 has caused an improved photodynamic anticancer effect on the in vitro ovarian cancer cells by a factor of 10 in comparison with Ce6 alone [65,66]. Of particular interest in the second and third generation of PDT photosensitisers is the use of metal-based drugs, especially ruthenium [67,68,69,70,71,72]. As a significant highlight, the ruthenium polypyridyl complex TLD1433 of the McFarland group is currently in phase II clinical trials against bladder cancer [73,74].
2.3. Characteristics of an Ideal Class of Photosensitisers for PDT
Considering the numerous stringent requirements that need to be met in pharmaceutical production and clinical use, an ideal photosensitiser should possess the following characteristics (i) able to generate singlet oxygen even in a hypoxia state and high quantum yield of 1O2, (ii) chemically pure and stable at room temperature, (iii) able to be synthesised through an easy, straightforward, and inexpensive method which allows consistent and reproducible product, (iv) possess high photochemical activity, molar absorption coefficient and absorbs tissue penetrating light (around 650 nm to 800 nm) for the production of reactive oxygen species, (v) possess good photochemical reactivity with long lifetimes of triplet state and high triplet state yields (for the efficient production of singlet oxygen or reactive oxygen species upon irradiation), (vi) the absorption band of the PS must not overlap with the absorption band of the substances located in the body such as hemoglobin, melatonin, (vii) selectively phototoxic at a defined wavelength, (viii) solubilise easily in the body tissues to keep appropriate lipophilic ability to cross phospholipid cell membrane and avoid tendency to self-aggregate in biological environment, (ix) can be easily cleared from normal tissue and do not cause dark toxicity, (x) selectively accumulate at the targeted tissue and (xi) be a pain-free and reliable PDT treatment [28,75,76,77,78,79]. With reference to the above, Re(I) tricarbonyl complexes meet most of the requirements as metal-based phosphorescent PSs except for the excitation wavelength in which this class of compounds generally absorbs short wavelengths below 400 nm.
3. Rhenium(I) Tricarbonyl Complexes: General Overview
3.1. Background of Rhenium (Re)
Re, a neighbour of Ru in the periodic table is a rare element in the earth. It possesses a mean concentration of around 0.5 to 1 ppb in the earth’s crust, which is lesser than the other third-row transition metals [80,81,82]. Generally, Re occurs in little amounts in minerals and ores but not in its elemental form in nature. Re exists as two natural isotopes, 185Re (37.4%) and 187Re (62.6%). Both isotopes are potential candidates for medical applications. 187Re is a beta emitter that possesses a long-decay half-life but is proposed to be safe for medical use owing to its relatively weak emission of radiation [83,84,85,86,87,88]. It has been evaluated as an anticancer drug candidate in clinical trials previously in the form of organic-metal complexes [89,90,91,92,93].
3.2. Re(I) Complexes: Brief History and Suitability as a PDT PS
The synthesis of Re(I) carbonyl Re(I)(CO)3+ complexes was first performed by Walter and co-workers in 1941. In general, the core of Re(I)(CO)3+ complexes (shown in Figure 3) is hard Lewis acid-based and stable in aqueous solutions, even in dilute hydrochloric acid and coordinating solvents [94,95]. Re(I) tricarbonyl complexes possess more photophysical and chemical features that meet the requirements of ideal photosensitisers when compared to other metal complexes and some conventional PSs. For instance, Re(I) tricarbonyl complexes exhibited polarised emission, large Stokes shifts, high photostability, and long lifetimes [96,97,98]. They are also more biocompatible due to the presence of a low spin d6 electronic configuration at the Re(I)’s outermost shell. Such configuration makes the Re(I) tricarbonyl complexes kinetically inert, and thus devoid heavy metal related toxicity [99]. However, in terms of photoreactivity, native Re(I) tricarbonyl complexes were activated by light of short wavelength (350 nm) to generate ROS [100]. This naturally limits their use as PDT PSs due to the low tissue penetration characteristics of short-wavelength light. Nevertheless, ongoing efforts of structural modifications and the recent advancement using two-photon PDT technology, photothermal therapy, drug combination study, as well as the incorporation of rhenium(I) complex into nanoparticles are potential strategies to overcome this main drawback.
Figure 3.
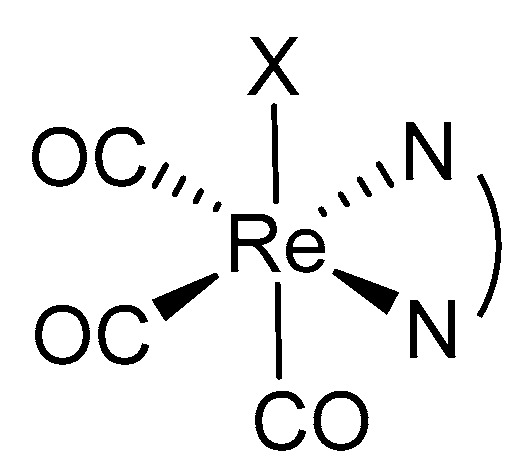
General structure of Re(I) tricarbonyl core with variable X depending on the ligands incorporated to form different Re(I) tricarbonyl complexes with different phototoxic activity on cancer cell lines.
4. Phototoxic Effect of Rhenium(I) Tricarbonyl Complexes
4.1. Rhenium(I) Tricarbonyl Complexes in PDT: Modifications and Advancements
Despite being natively activated by low-tissue-penetrating lights, the relatively reduced heavy metal toxicity and interesting photophysical and photochemical properties of Re(CO)3 complexes when compared to other metal-based PSs have attracted ongoing interests and efforts to improve this class of compounds for PDT [101]. The attempted approaches were described as follows.
4.2. Structural Modification for the Enhancement of Re(I) Complexes’ Phototoxicity, Tissue Selectivity and Photo-Stability
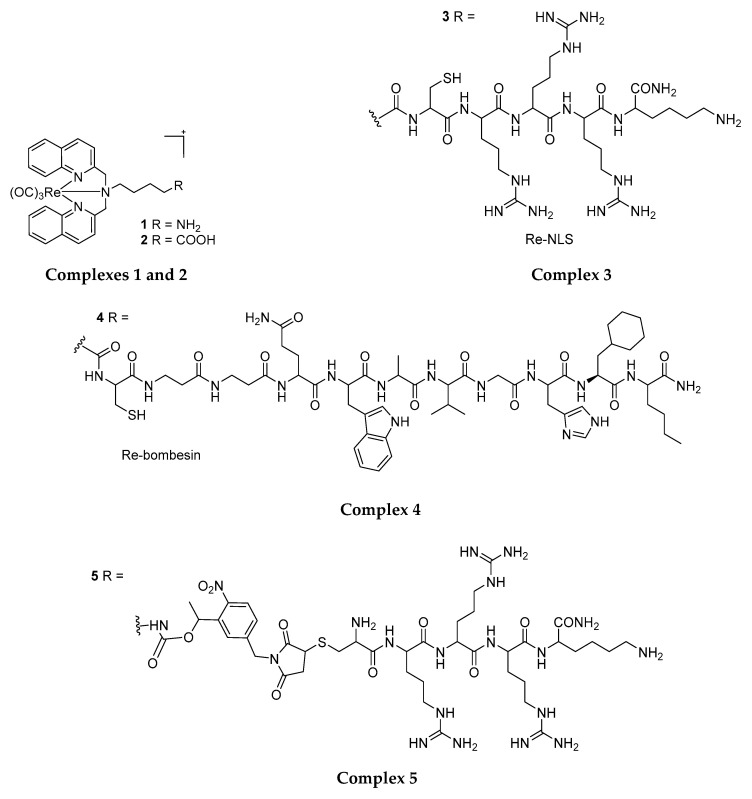
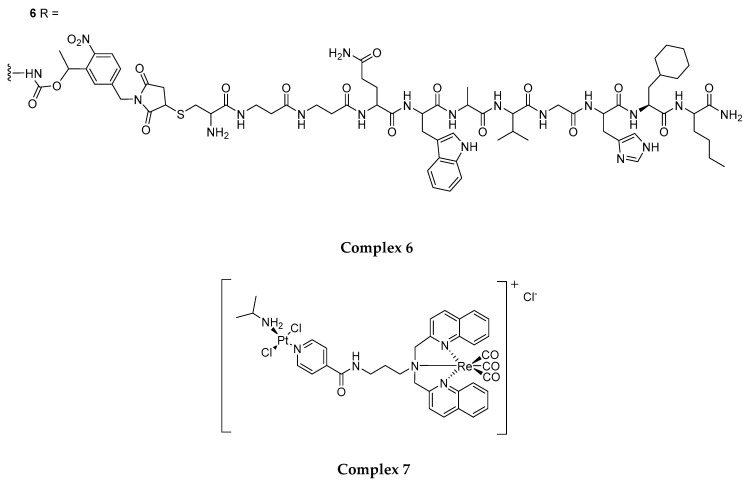
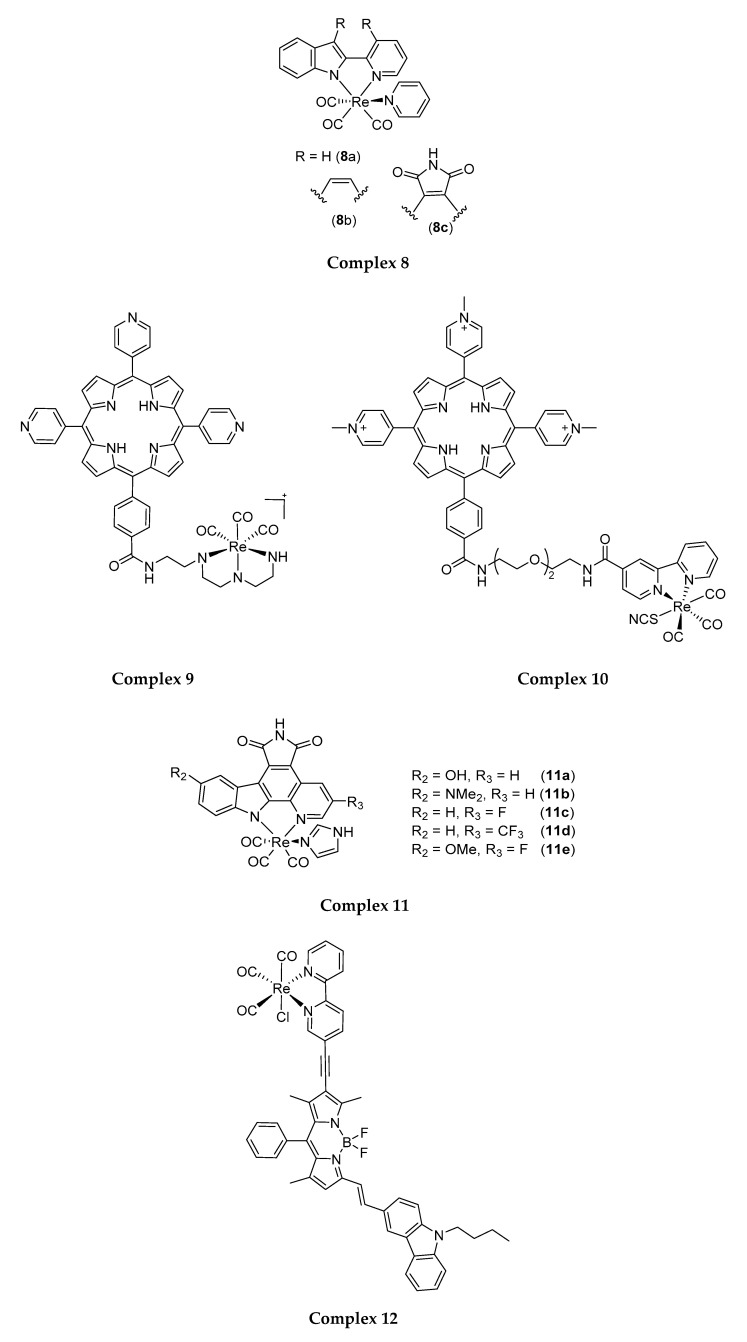
Gasser et al. (2014) attempted to enhance the phototoxicity, tissue selectivity and photo-stability of Re(CO)3 complexes through structural modification and targeting ligands/protecting groups conjugation. The first products of the group, i.e., amino and carboxylate functionalised N,N-bisquinoline Re(I) tricarbonyl complexes (1 and 2 in Figure 4) [35] showed phototoxicity toward HeLa cells (IC50: 17.3 µM and 9.3 µM respectively) and no dark toxicity (IC50: > 100 µM) upon UVA light irradiation at 350 nm (fluence rate of 2.58 J cm−2). Conjugation of 1 and 2 respectively with an NLS peptide (nuclear localisation signal, to yield 3) to target the nucleus and bring the complex in close proximity of DNA and bombesin (to yield 4), a neuropeptide has known to selectively target cancer cells over healthy cells further improved the phototoxicity of these compounds against HeLa cells (IC50: 18.3 µM and 5.3 µM respectively). This exhibits the possibility and flexibility to improve the cell selectivity and phototoxic effects of these PSs using cell-targeting ligands [35]. Gasser et al. further explored the use of a photolabile protecting group (PLPG) to enable the release of 1 at low irradiation energy (1.2 J cm−2). Such a strategy combining targeting with peptides and irradiation-dependent release of the Re complex (yield 5 and 6) has again increased the phototoxicity of these compounds against cancer cells (HeLa and PC-3, IC50: 9.3 µM and 9.7 µM respectively) while, at the same time, reduced the phototoxic effects towards non-cancerous cells (MRC-5)(20.5 µM and 23.3 µM respectively) [19].
Figure 4.
A series of different ligands complexed to the Re(I) tricarbonyl core forming different Re(I) tricarbonyl complexes with different phototoxic activity on cancer cell lines.
Meanwhile, Quental et al. (2017) designed a heterobimetallic complex consisting of a Re(I) tricarbonyl core and a platinum-iminedipyridyl group. Addition of platinum into the Re(I) complex (complex 7) was found to be capable of improving the toxicity of Re(I) by three folds towards HeLa cell lines (IC50 from 42.8 µM to 13.5 µM, upon 10 min light irradiation at 350 nm and 2.58 J cm−2), achieving a similar photo-index value (PI) to that of the Photofrin (PI: average of 5) [35,102,103]. This suggests the potential for improving Re(I)’s phototoxicity via attachment of a Pt(II) cytotoxic complex.
4.3. Structural Modification for the Improvement of Re(I) Complexes’ Photo-Absorption Profiles
The phototoxicity and PDT compatibility of the Re(I) complexes can be enhanced through improving the light absorption profiles of these compounds.
Following this approach, Meggers and co-workers (2013) synthesised Re(I) tricarbonyl indolato complexes (8 in Figure 5) that can be activated by light of λ > 505 nm. The most potent derivative 8c was found to be capable of inducing phototoxicity on HeLa cells (IC50: 0.1 µM) and was localised at the edges of melanoma spheroids to induce loss of spheroid integrity and decrease spheroid size [104,105]. Interestingly, the addition of a pentene ring with nitrogen and oxygen substitution to complex 8c made it more lipophilic, easier to penetrate through cell membranes, and thus result in better phototoxic activity towards HeLa cells.
Figure 5.
A number of different ligands complexed to the Re(I) tricarbonyl core forming different Re(I) tricarbonyl complexes with different photo-absorption profiles.
Gianferraraand co-workers (2014) synthesised water-soluble porphyrin-Re conjugates bearing with a diethylenetriamine 9 or a bipyridine ligand 10 that can be excited at 590–700 nm (fluence rate of 9 mW cm−2). These porphyrin-Re conjugates 9 and 10 were found to be phototoxic against HeLa cancer cells (IC50: 1.9 µM and 4.0 µM, respectively). Nevertheless, the phototoxic activity of these complexes was later attributed to the porphyrin moiety, as the Re complex does not produce any singlet oxygen upon 590–700 nm irradiation [106].
In another attempt, Ludewig et al. (2014) produced Re(I) pyridocarbazole complexes that induce cancer cell apoptosis post-irradiation with light of long visible wavelengths (600–850 nm) [104]. Modification of pyridocarbazole heterocycle with different functional groups was also attempted to achieve redshifts in the light absorption profile of the PSs (11a–11e). This includes the introduction of a dimethylamino group and π-donating hydroxyl group onto the indole moiety at the 5th position; trifluoromethyl and σ-accepting fluoride group onto the pyridine at the 3rd position; and π-donating methoxyl group to the indole combine with a σ-accepting fluoride in pyridine moiety [107]. Among these new complexes, 11c and 11d generated singlet oxygen at λ ≥ 620 nm and showed enhanced phototoxicity upon irradiation for 60 min with 7W LED light. Complex 11c with fluorine at the 3rd position of the pyridine moiety showed the most significant red light-induced phototoxic effect on HeLa cells with an EC50 of 0.3 µM [108]. The current study suggested the feasibility of performing structural modification using Re(I) tricarbonyl-pyridocarbazole complexes as a scaffold to improve Re(I) complexes’ photo-absorption profiles, and pointed a potential direction for the development of tissue-penetrating near-infrared light-absorbing Re(I) complexes, for effective clinical PDT.
Zhong and co-workers (2015) published another Re(I) tricarbonyl complex linked with bipyridine (bpy) and boron dipyrromethene (bodipy). Bpy is a polypyridyl ligand while bodipy is a visible light-harvesting chromophore whose molecular structure can be readily derivatised [109,110,111,112]. Attachment of bodipy to the Re(I) tricarbonyl core (compound 12) increased the λmax of the Re(I) complexes from 399 nm to around 635 nm, and at the same time, confers to the Re(I) complexes the ability to produce long-lived triplet state (life-time of 448.9 µs) compared to that of the Re(I) complex conjugated only to bipyridine (2.22 µs) [113]. Such characteristics are important for the efficient production of singlet oxygen in an environment of low dissolved oxygen concentration such as cancer interstitium. Complex 12 was subsequently tested on LCC (lung cancer cells) upon irradiation at 635 nm using an LED light irradiation for 4 h, but was found to only produce minimal phototoxic effects [112]. Although unable to exhibit good phototoxicity, it may be worthwhile to revisit the current design to identify the pitfalls and produce a functional molecule, in viewing at the feasibility of the concept.
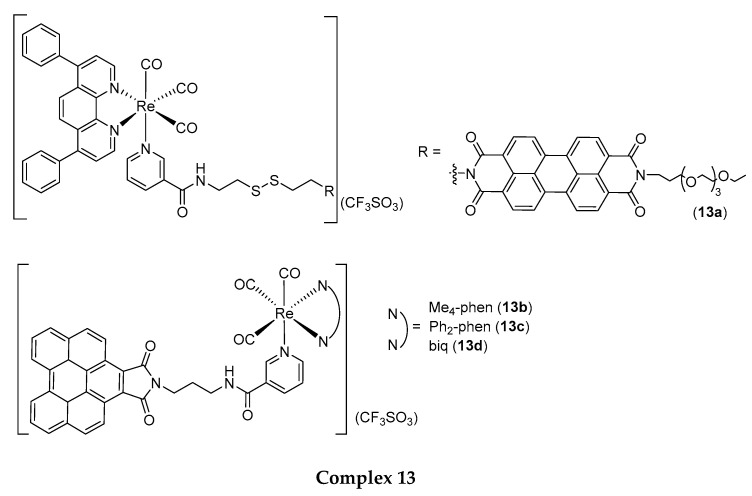
Another recent study by Alex et al. (2019) reported that luminescent Re(I) tricarbonyl complexes bearing either a perylene diimide (complex 13a) or benzoperylene monoamide moiety (BPMI) (complex 13b, 13c, 13d) showed significant phototoxic effect on HeLa cells after 15 min of 6 W UV-A irradiation at 365 nm (IC50: 13a: 18.21 µM; 13b: 0.27 µM; 13c: 2.21 µM; 13d: 1.51 µM). Among the four Re(I) complexes, complex 13b appeared to be the most efficacious with an IC50 of 0.27 µM. This has been attributed to its longer lifetime, which is likely caused by the presence of the Re(I) core, enhancing intersystem crossing to populate excited triplet state of BPMI [114]. Therefore, the Re(I) tricarbonyl core with phenanthroline (phen) ligand complexed to it is important for its luminescent long lifetime properties, thus causing a strong phototoxic effect.
5. Re(I) Tricarbonyl Complexes: In Vivo Studies
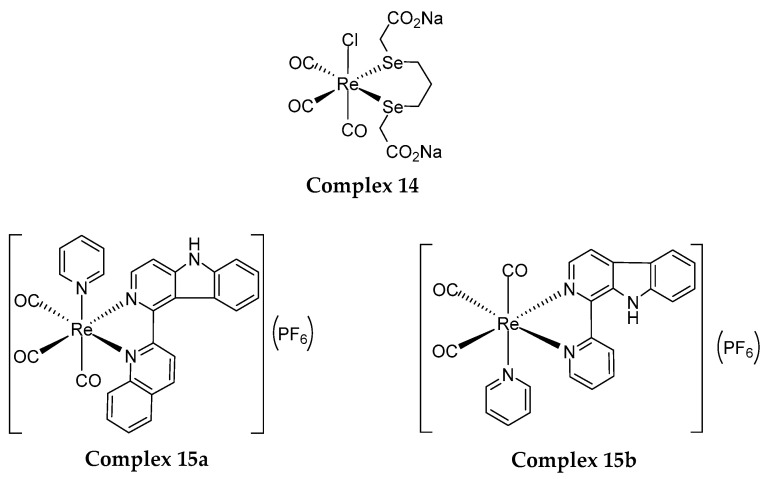
Despite reports on Re(I) tricarbonyl complexes tested in vitro, there were only a few Re(I) complexes that went through in vivo testing. Collery et al. (2015) first reported a water-soluble, stable and lipophilic Re(I) tricarbonyl complex, which coordinates to two selenium (Se) atoms (Complex 14 as shown in Figure 6). Complex 14 showed good cytotoxic effect towards breast cancer cells and mice bearing with MDA-MB-231 Luc+ human breast cancer cells. Complex 14 was previously reported with an anticancer effect (IC50: 10 µM) towards MDA-MB-23l cells [115] and (IC50: 4.75 µM) MCF-7 breast cancer cells [116] before it proceeded to in vivo testing. Complex 14, orally administered was then tested on mice that were transplanted with breast cancer cells as mentioned and it was found that a 10 mg/kg dose was safe, non-toxic, tolerable, and able to lessen tumour growth for at least four weeks of treatment [117,118]. This concludes the addition of Se to the Re(I) tricarbonyl core can efficiently treat breast cancer by targeting the cancer cell and its microenvironment.
Figure 6.
Different ligands complexed to a Re(I) tricarbonyl core forming different Re(I) tricarbonyl complexes with different cytotoxic activity on cancer cell lines.
In a more recent study performed by He and co-workers (2019), stable Re(I) tricarbonyl complexes that bind with β-carboline and pyridine ligands, complexes 15a and 15b, were synthesised and shown to kill A549 (human lung cancer cells) and A549R (cisplatin-resistant human lung cancer cells). Higher anticancer activity was achieved by 15a, which displayed an IC50 of around 2 µM towards both cell lines mentioned as compared to 15b (IC50: 4 µM). Then, they proceeded with in vivo testing of the most cytotoxic complex, complex 15a on mice which were transplanted with A549 cancer cells. It is reported that a 5 mg/kg dose of complex 15a is required to cause lysosomal dysfunction and cell death through autophagy over a three-week treatment [119]. The coordination of pyridine and β-carboline ligands did enhanced the anticancer activity of the Re(I) complex.
However, to our best knowledge, there is still no in vivo testing of the Re(I) tricarbonyl complex through PDT. This could be due, among others, to a wavelength of absorption that is too short.
6. Future Perspective—How to Improve Anticancer Activity of Re(I) Tricarbonyl Complexes in PDT?
Although there have been unsuccessful attempts, examples illustrated in the current review have also pointed out the possibility of improving the various photophysical and photochemical properties of Re(I) complexes, to address its inherent weakness (activation by short-wavelength light) and to improve its phototoxicity, tissue selectivity and photo-stability. Coupled with its reduced heavy metal toxicity [101], Re(CO)3 complexes may be an attractive candidate to be further investigated upon, for the generation of new effective PSs for PDT. The introduction of more ring structures or fluorine or methyl groups as displayed by reported complexes will be the structural strategy for improved lipophilicity and phototoxic effect of new Re(I) tricarbonyl complexes. Moreover, Re(I) complexes can be included in formulations with various nanoparticles such as liposomes, liquid crystalline nanoparticles or micelles to enhance its solubility and delivery to the cancer cells or be paired with synergistic agents like current chemotherapeutic agents to improve cellular targeting effect and further enhance treatment outcomes. Advancements in combination therapies discovery may unlock a new platform to strengthen the antitumor effects and enhance clinical outcomes. From our review, only a few Re(I) complexes advanced into the in vivo or clinical phase [120,121,122]. The in vivo studies mainly focused on Re(I) tricarbonyl complexes tested on the cytotoxic activity but not on PDT. This might be due to the short UV wavelength produced. With the emergence of two-photon laser technology, Re(I) complex can be further investigated to produce a deeper tissue penetration through a two times higher wavelength. Besides, the Re(I) complex may also be studied further in PTT to improve its anticancer activity through heat energy. As Re(I) complexes have been most frequently studied in cell culture, more preclinical and clinical studies are expected to be carried out in the future.
6.1. Two-Photon Photodynamic Therapy
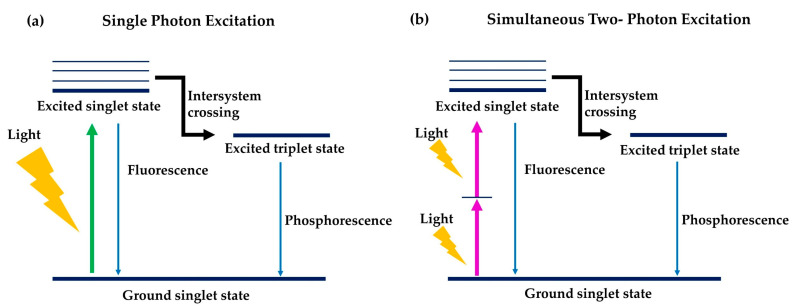
Two-photon absorption (TPA) is a nonlinear optical process that can be used to supply light energy to short wavelength-light-absorbing PSs for their photoactivation. In this process, two photons of lower energy i.e., at a longer wavelength (2× that of the short-wavelength photon normally absorbed by the PS) are supplied simultaneously to a short wavelength-light-absorbing PSs. These photons will be absorbed by the PSs and their energy combined to promote the PSs to an excited state. Such combined energy would be similar to the energy supplied by one short wavelength-photon for excitation of PSs (Figure 7). One important factor that needs to be considered in TPA is the ability of PS to absorb two photons concurrently, which is quantified by the TPA cross-section (δ), expressed as Goeppert–Mayer units (GM) [123,124,125]. Table 2 shows the similarities and differences between traditional one-photon PDT and two-photon PDT.
Figure 7.
Comparison mechanism of PDT of single-photon excitation (a) and simultaneous two-photon excitation (b).
Table 2.
Comparison between traditional one-photon photodynamic therapy and two-photon photodynamic therapy.
| Photodynamic Therapy | |||
| One-Photon Photodynamic Therapy | Two-Photon Photodynamic Therapy | ||
| Similarities | |||
| The general mechanism is the same, with the presence of light and oxygen, the photosensitisers are excited to its excited triplet state which leads to the production of reactive oxygen species (ROS) and thus, causing cell death. | |||
| Differences | |||
| One-photon photosensitiser is used |
Photosensitisers | Two-photon photosensitiser is used |
|
| 600–800 nm | Ideal range of wavelength (nm) of the photosensitisers | Wide range, not fixed, can go as low as 300 nm | |
| A laser within the UV-visible range | Activation of photosensitisers | Two low energy photons of near-infrared region of light absorbed simultaneously | |
| Less | Precision of cancer treatment | Higher | |
| Shallower | Depth of tissue penetration | Deeper | |
| - | Determination of ability of photosensitiser to absorb 2 photons simultaneously | Quantified by two-photon cross-sections, δ, which is expressed in Goeppert-Mayer (GM), best to > 50 GM |
|
To apply TPA techniques for PDT, a two-photon laser that emits photons at a wavelength that are (i) 2× longer than the PS’s absorbing wavelength, and (ii) falls within the optimal range for tissue penetration (650–800 nm) is employed. Due to their lower energy, the photons involved in TPA have deeper tissue penetration and induce less photo-bleaching of the PS than the shorter wavelength photons [126,127,128]. Also, TPA enables focusing the delivery of these photons (in the form of laser) and thus the activation of PS only at the laser focal point. This gives superior spatial control on the PS activation at the cancer site and prevents photo-induced damage to neighbouring healthy tissues [124]. To date, several attempts have been made to utilise short wavelength-light-activated PSs for PDT cancer treatment using a two-photon light source [125,129,130,131]. For example, a study done by Kobuke and co-researchers (2007) proved that zinc porphyrin derivatives which connect two porphyrins through a bis-acetylene or monoacetylene bond and form a porphyrin dimer through imidazolyl coordination to zinc are able to degrade the HeLa cell membrane under 2 mW light irradiation for 5 min at 780–890 nm [132]. Besides, Anderson et al. (2009) synthesised a series of zinc porphyrin dimers with high singlet oxygen quantum yields. These compounds showed an anticancer and phototoxic effect towards human ovarian adenocarcinoma cell lines at 650–800 nm via TPA activation [133,134,135]. Also, Chao and Gasser (2020) have demonstrated that mice bearing extremely difficult to treat tumours could be eradicated with a Ru(II) polypyridyl complex using two-photon irradiation [136].
However, more mature optical technology for the delivery of two-photon light for clinical PDT is not yet available. On top of that, in the lab, the current achievable two-photon excitation volumes are still relatively small. Thus, this recent advancement of pulsed fibre-optic lasers such as that used as the light delivery method for multiphoton microscopy utilising static infrared beam through in vivo studies especially on the skin of human volunteers may assist in addressing this need [137].
To date, several groups designed a series of transitional metal complexes including ruthenium (Ru) complexes and Re complexes in TPA studies. The published complexes showed a significant phototoxic effect on the tested cell lines. The group of Qiu et al. (2019) reported four Ru(II) polypyridyl complexes having a morpholine moiety and increasingly bipyridine ligand, which possess marked phototoxic effect towards A549 cells. The Ru(II) complex with the most bipyridine ligand showed a better phototoxic effect (0.4 µM) and efficiency (274 GM) in TPA among other synthesised Ru(II) complexes towards A549 cells [138]. Gassser and co-workers (2017) showed that [Ru(phen)2dppz]2+ derivatives (phen = 1,10-phenanthroline, dppz = dipyrido[3,2-a:2′,3′-c]phenazine) with functional groups on the dppz ligand [dppz-7,8-(OMe)2] had a large value of TPA cross-section (245 GM) and better phototoxic effect with TPA on 3D multicellular spheroid in vitro (a better cell model to assess TPA studies)(9.5 µM) as compared to traditional one-photon PDT (32.5 µM). After light irradiation, accumulation of this synthesised Ru(II) complex in the nucleus signifies that the oxidative damage that occurred is severe enough to cause cell necrosis [139,140].
As far as we know, there is no Re(I) complex used in PDT via TPA excitation. However, Lokawicz and co-workers (1999) have described the Re(I) tricarbonyl complex with bipyridine ligand excitable with TPA since the 1990s [141]. A more recent study done by Ferri et al. (2010) proved that the photostable dinuclear Re(I) complex containing a carboxyl group on the diazine ligand conjugated with a peptide nucleic acid conjugate can be excited at 750 nm via TPA (power: 80 mW) [142]. Consequently, it would be worth investigating the Re(I) complex as a potential PS candidate for TPA application in PDT.
6.2. Anticancer Combinatorial Therapy
The combination of PDT with chemotherapy has received increased attention as a novel promising approach for cancer treatment. Presently, cisplatin, gemcitabine or doxorubicin are the common chemotherapeutic agents used to treat most cancer which includes pancreatic, breast and bladder cancer. Nevertheless, chemotherapy can cause toxicity in normal cells, lowering the bioavailability of the drug making it less available to the cells and thus, causes poor treatment efficacy or even the possibility of developing multidrug resistance (MDR) [143,144,145,146,147]. In view of this, combined therapy has advantages over a single therapeutic agent, notably, by reducing the possibility of multidrug resistance, enhancing cancer therapeutic efficacy, lowering drug doses needed for treatment and subsequently, and lowering the side effects of the drugs [148]. The synergistic effects of PDT and chemotherapeutic agents allow the selective killing of cancer cells as PDT photosensitisers possess the benefit to selectively kill cancer cells with the generation of ROS following light activation. As PDT derived ROS-based intracellular damage is severe and multi-targeted, DNA damage and repair are expected. In such instances, the efficacies of antimetabolites such as gemcitabine may very much possibly be amplified, as more gemcitabine will be adopted into the DNA repair machinery to induce masked chain termination and cell death.
A recent study by Amin et al. 2019 proved that with a low dose of laser (5 J cm−2), higher synergism between doxorubicin and zinc phthalocyanine (ZnPc) were observed when compared to the high dose of laser (20 J cm−2) on human melanoma cells (SK-MEL-3). A significant synergy effect was seen with increased sensitivity towards the tested cell line and hence, a lower dose of doxorubicin is required. This results in a lower risk of serious side effects as the resistance of SK-MEL-3 cells are reduced significantly. Besides, it was shown that combination therapy of ZnPc and doxorubicin trigger some anticancer biological processes including increased cell apoptosis, decreased cell migration ability, and autophagy activation [149]. Transition metal complexes can be synergistically paired with current chemotherapeutic agents for anticancer combination therapy. For instance, NAMI-A (imidazolium-trans-tetrachloro(dimethylsulfoxide) imidazoleruthenium(III)) was combined with gemcitabine to treat non-small cell lung cancer patients (phase I/II clinical trial). Although patients were found to tolerate the combination treatment moderately, additional studies with larger groups of patients are necessary to draw a decisive conclusion on the anticancer effect of the Re complexes [150].
Re(I) complexes have the potential to be developed in combination therapy. Nevertheless, to our best knowledge, none have tried utilising the potential Re(I) complex in combination therapy with other chemotherapeutic drugs. Hence, more studies can be done to determine the combined therapy effectiveness between luminescent Re(I) complex and currently approved drugs.
6.3. Advancement in Nanoparticles with Photosensitisers Utilising Drug Combination Study
Nowadays, the introduction of nanomedicine opens a new avenue towards cancer therapeutic approaches. Nanomedicine brings a solution towards the issue shown by conventional chemotherapeutic agents such as low bioavailability, low lipophilicity, and non-targeted chemotherapy which kills both cancer and healthy cells [151]. Nanoparticle-mediated therapy is an advanced therapeutic approach, which can improve drug tumour selectivity, bioavailability and lower the possibility of drug-causing side effects due to the killing effect towards healthy cells previously [152]. Nanoparticles are able to target tumours selectively, which enables the drugs to accumulate in cancer cells either through active or passive targeting, without being eliminated by the body [152]. Thus, this increases drug concentration in the tumour and substantially enhances the drug cytotoxic effect, which results in lower drug doses required for cancer therapies. As such, the possibility of overcoming multiple drug resistance also increases [152,153]. For instance, paclitaxel, an antimitotic drug, has been approved to be formulated in different types of nano-formulation, which includes albumin-based nanoparticles (Abraxane®), liposomes (Lipusu®), polymeric micelles (Nanoxel®, Genexol®, Paclical®) to treat pancreatic, breast and non-small-cell lung cancers [154,155]. Nano-drug-delivery systems may improve the efficacy and reduce the adverse effects of anticancer combinatorial therapy through selective and simultaneous delivery of two or more therapeutic drugs to the tumour at a synergistic ratio [151].
In relation to PDT, co-delivery of PS-chemotherapeutic drugs combination has been recently attempted. For instance, Wang and co-workers (2019) have synthesised doxorubicin-loaded PEGylated BODIPY nanoparticles (PEG: polyethylene glycol; BODIPY: distyryl boron dipyrromethene) in which doxorubicin is a chemotherapeutic agent while BODIPY act as photosensitive agents. This synthesised formulation demonstrated synergistic effect and better anticancer activity towards HeLa cells (IC50 of 10 nM) when compared to BODIPY alone (IC50 of 25 nM) under LED light irradiation for 30 min (20 mW cm−2) [148]. Besides, Gaio et al. (2019) have also reported a strong synergistic effect between docetaxel (drug) and Chlorin e6 (Ce-6, photosensitiser) towards HeLa cells. Both mentioned drugs are encapsulated in keratin-based nanoparticles and tested with HeLa parental and resistant cell lines. It demonstrated improvement in the killing effect towards both cell lines after encapsulating docetaxel and Ce-6 into keratin nanoparticles (after formulation of both agents: (IC50 of HeLa parental: 0.70 µM; IC50 of HeLa resistant: 2.57 µM); docetaxel nanoparticles only: (IC50 of HeLa parental: 0.72 µM; IC50 of HeLa resistant: 4.16 µM); Ce-6 nanoparticles only: (IC50 of HeLa parental: 5.87 µM; IC50 of HeLa resistant: 6.27 µM)) [156]. Therefore, utilisation the advancement of combination therapy and nanomedicine to develop a better anticancer agent can be further studied to improve treatment outcomes and relieve patient suffering from the side effects of therapies.
The aforementioned examples have demonstrated enhancements in anticancer combinatorial effects following the co-integration of PS and chemotherapeutic drugs within the nano-drug-carriers. In light of this, co-integration of Re(I) complexes along with other anticancer drugs at a synergistic ratio may be a feasible approach to attempt, for the enhancement of its anticancer efficacy.
6.4. Photothermal Therapy
Photothermal therapy (PTT) is another effective, non-invasive way to treat cancers whereby it utilises a photosensitive agent to turn photonic energy into heat energy and induce thermal ablation upon light irradiation [157]. It works by elevating the ground-state electrons to an excited state through photonic energy absorption and then the kinetic energy release and overheat the cells, which results in cell and tissue damage [158]. In PTT, hyperthermia (with an intracellular temperature of cells of around 50 °C) can cause the cell membrane to lyse and thus, cause necrotic or apoptotic cell death [159,160,161]. In comparison with PDT, PTT does not require oxygen to kill the cancer cells but it generates the cytotoxic effect through an increase in the temperature.
Saw and co-researchers (2017) have reported that citric acid-coated confeito-like gold nanoparticles with 30 nm has the potential to become a PTT agent. The synthesised nanoparticles with 30 nm show the best in vitro PTT efficacy on MDA-MB-231 cells with laser irradiation at 532 nm for one minute as compared to the other size of nanoparticles (60, 80, 100 nm). It also displayed higher localisation at endoplasmic reticulum which further enhances endocytosis towards the cancerous cells [162]. For instance, Zhao et al. (2018) have published one of the transition metal complexes, ruthenium nanoparticles with good biocompatibility and was easily metabolised to the PTT field. With 10 min light irradiation at 808 nm on A549 cells, the ruthenium nanoparticles induce significant cell death, cell ablation and destruction in in vitro and in vivo experiments [163]. Thus, it is possible for the Re(I) complex to be tested on cancer cells through PTT as it possesses similar properties to ruthenium since they both are neighbours in the periodic table. PTT might be another option for the Re(I) complex to exhibit its unique characteristics to kill the cancer cells.
7. Conclusions
The development of Re(I) tricarbonyl complexes has unlocked a new avenue for the treatment of cancer via photodynamic therapy to improve the overall survival rate of cancer patients. To achieve this, researchers and clinicians have to improvise their understandings of tumour immunology, novel complexes, and optimise the timing of photodynamic therapy. We believed that these novel complexes represent a promising alternative in all types of cancer, thus progressing from bench to bedside at a swift pace. Re(I) complexes as photosensitisers in photodynamic therapy will bring a bright future ahead for patients suffering from this aggressive and challenging killer.
Acknowledgments
H.S.L. and M.L.L. would like to thank the School of Postgraduate Studies and the Institute for Research, Development and Innovation (IRDI) of the International Medical University for the support given. The authors also like to acknowledge the help and constructive feedback extended by Gilles Gasser from Chimie ParisTech in this review paper.
Author Contributions
Conceptualisation and supervision, M.L.L. and L.V.K.; writing and original draft preparation, H.S.L.; writing, review and editing, C.-W.M., M.Z., T.M., L.V.K., N.D. and M.L.L. All authors have read and agreed to the published version of the manuscript.
Funding
The review was a collective effort of researchers receiving a Pancreatic Cancer Challenge Grant from the International Medical University, Kuala Lumpur, Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cancer. [(accessed on 24 November 2019)];2018 Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Cheng Z., Al Zaki A., Hui J.Z., Muzykantov V.R., Tsourkas A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin S.Y., Zhang A.Q., Cheng S.X., Rong L., Zhang X.Z. Drug self-delivery systems for cancer therapy. Biomaterials. 2017;112:234–247. doi: 10.1016/j.biomaterials.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Strobel O., Neoptolemos J., Jaeger D., Buechler M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019;16:11–26. doi: 10.1038/s41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 6.Hartwig W., Werner J., Jäger D., Debus J., Büchler M.W. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14:e476–e485. doi: 10.1016/S1470-2045(13)70172-4. [DOI] [PubMed] [Google Scholar]
- 7.Auperin A., Le Pechoux C., Rolland E., Curran W.J., Furuse K., Fournel P., Belderbos J., Clamon G., Ulutin H.C., Paulus R., et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 8.Crabtree T.D., Denlinger C.E., Meyers B.F., El Naqa I., Zoole J., Krupnick A.S., Kreisel D., Patterson G.A., Bradley J.D. Stereotactic body radiation therapy versus surgical resection for stage I non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2010;140:377–386. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 9.Grills I.S., Mangona V.S., Welsh R., Chmielewski G., McInerney E., Martin S., Wloch J., Ye H., Kestin L.L. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non–small-cell lung cancer. J. Clin. Oncol. 2010;28:928–935. doi: 10.1200/JCO.2009.25.0928. [DOI] [PubMed] [Google Scholar]
- 10.Onishi H., Shirato H., Nagata Y., Hiraoka M., Fujino M., Gomi K., Karasawa K., Hayakawa K., Niibe Y., Takai Y., et al. Stereotactic body radiotherapy (SBRT) for operable stage I non–small-cell lung cancer: Can SBRT be comparable to surgery? Int. J. Radiat. Oncol. Biol. Phys. 2011;81:1352–1358. doi: 10.1016/j.ijrobp.2009.07.1751. [DOI] [PubMed] [Google Scholar]
- 11.Sun C., Ansari D., Andersson R., Wu D. Does gemcitabine-based combination therapy improve the prognosis of unresectable pancreatic cancer? World J. Gastroenterol. 2012;18:4944. doi: 10.3748/wjg.v18.i35.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiriva-Internati M., Bot A. A new era in cancer immunotherapy: Discovering novel targets and reprogramming the immune system. Int. Rev. Immunol. 2015 doi: 10.3109/08830185.2015.1015888. [DOI] [PubMed] [Google Scholar]
- 13.Ventola C.L. Cancer immunotherapy, part 3: Challenges and future trends. Pharm. Ther. 2017;42:514–521. [PMC free article] [PubMed] [Google Scholar]
- 14.Suda K., Mitsudomi T. Successes and limitations of targeted cancer therapy in lung cancer. Prog. Tumor Res. 2014;41:62–77. doi: 10.1159/000355902. [DOI] [PubMed] [Google Scholar]
- 15.Bhuvaneswari R., Gan Y.Y., Soo K.C., Olivo M. The effect of photodynamic therapy on tumor angiogenesis. Cell. Mol. Life Sci. 2009;66:2275–2283. doi: 10.1007/s00018-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oleinick N.L., Evans H.H. The photobiology of photodynamic therapy: Cellular targets and mechanisms. Radiat. Res. 1998;150:S146–S156. doi: 10.2307/3579816. [DOI] [PubMed] [Google Scholar]
- 17.Castano A.P., Mroz P., Hamblin M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnett R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995;24:19–33. doi: 10.1039/cs9952400019. [DOI] [Google Scholar]
- 19.Leonidova A., Pierroz V., Rubbiani R., Lan Y., Schmitz A.G., Kaech A., Sigel R.K.O., Ferrari S., Gasser G. Photo-induced uncaging of a specific Re(I) organometallic complex in living cells. Chem. Sci. 2014;5:4044–4056. doi: 10.1039/C3SC53550A. [DOI] [Google Scholar]
- 20.Zuluaga M., Lange N. Combination of photodynamic therapy with anti-cancer agents. Curr. Med. Chem. 2008;15:1655–1673. doi: 10.2174/092986708784872401. [DOI] [PubMed] [Google Scholar]
- 21.Robertson C.A., Evans D.H., Abrahamse H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009;96:1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Bown S.G., Rogowska A.Z., Whitelaw D.E., Lees W.R., Lovat L.B., Ripley P., Jones L., Wyld P., Gillams A., Hatfield A.W.R. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549–557. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin. Dermatol. 2006;24:16–25. doi: 10.1016/j.clindermatol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Wilson B.C., Patterson M.S., Lilge L. Implicit and Explicit Dosimetry in Photodynamic Therapy: A New Paradigm. Springer; London, UK: 1997. [DOI] [PubMed] [Google Scholar]
- 25.Robinson D.J., de Bruijn H.S., van der Veen N., Stringer M.R., Brown S.B., Star W.M. Fluorescence photobleaching of ALA-induced protoporphyrin IX during photodynamic therapy of normal hairless mouse skin: The effect of light dose and irradiance and the resulting biological effect. Photochem. Photobiol. 1998;67:140–149. doi: 10.1111/j.1751-1097.1998.tb05177.x. [DOI] [PubMed] [Google Scholar]
- 26.Boere I.A., Robinson D.J., de Bruijn H.S., van den Boogert J., Tilanus H.W., Sterenborg H.J.C.M., De Bruin R.W.F. Monitoring in situ dosimetry and protoporphyrin IX fluorescence photobleaching in the normal rat esophagus during 5-aminolevulinic acid photodynamic therapy. Photochem. Photobiol. 2003;78:271. doi: 10.1562/0031-8655(2003)078<0271:MISDAP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Ackroyd R., Kelty C., Brown N., Reed M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001;74:656. doi: 10.1562/0031-8655(2001)074<0656:THOPAP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Allison R.R., Sibata C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagnosis Photodyn. Ther. 2010;7:61–75. doi: 10.1016/j.pdpdt.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Wu L., Murphy R.P. Photodynamic therapy: A new approach to the treatment of choroidal neovascularization secondary to age-related macular degeneration. Curr. Opin. Ophthalmol. 1999;10:217–220. doi: 10.1097/00055735-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Brown J.M. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007;435:295–321. doi: 10.1016/S0076-6879(07)35015-5. [DOI] [PubMed] [Google Scholar]
- 31.Bozzini G., Colin P., Betrouni N., Maurage C.A., Leroy X., Simonin S., Martin-Schmitt C., Villers A., Mordon S. Efficiency of 5-ALA mediated photodynamic therapy on hypoxic prostate cancer: A preclinical study on the dunning R3327-AT2 rat tumor model. Photodiagnosis Photodyn. Ther. 2013;10:296–303. doi: 10.1016/j.pdpdt.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Nowak-Stepniowska A., Pergoł P., Padzik-Graczyk A. Photodynamic method of cancer diagnosis and therapy--mechanisms and applications. Postep. Biochem. 2013;59:53–63. [PubMed] [Google Scholar]
- 33.Juzeniene A., Moan J. The history of PDT in Norway. part one: Identification of basic mechanisms of general PDT. Photodiagn. Photodyn. Ther. 2007;4:3–11. doi: 10.1016/j.pdpdt.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Luksiene Z. Photodynamic therapy: Mechanism of action and ways to improve the efficiency of treatment. Medicina (Kaunaslithuania) 2003;39:1137–1150. [PubMed] [Google Scholar]
- 35.Leonidova A., Pierroz V., Rubbiani R., Heier J., Ferrari S., Gasser G. Towards cancer cell-specific phototoxic organometallic rhenium(I) complexes. Dalton Trans. 2014;43:4287–4294. doi: 10.1039/C3DT51817E. [DOI] [PubMed] [Google Scholar]
- 36.Kitanovic I., Can S., Alborzinia H., Kitanovic A., Pierroz V., Leonidova A., Pinto A., Spingler B., Ferrari S., Molteni R., et al. A deadly organometallic luminescent probe: Anticancer activity of a Re(I) bisquinoline complex. Chem.—A Eur. J. 2014;20:2496–2507. doi: 10.1002/chem.201304012. [DOI] [PubMed] [Google Scholar]
- 37.Kimura M., Miyajima K., Kojika M., Kono T., Kato H. Photodynamic therapy (PDT) with chemotherapy for advanced lung cancer with airway stenosis. Int. J. Mol. Sci. 2015;16:25466–25475. doi: 10.3390/ijms161025466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du K., Mick R., Busch T., Zhu T., Finlay J., Yu G., Yodh A.G., Malkowicz S.B., Smith D., Whittington R., et al. Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2006;38:427–434. doi: 10.1002/lsm.20341. [DOI] [PubMed] [Google Scholar]
- 39.Triesscheijn M., Ruevekamp M., Aalders M., Baas P., Stewart F.A. Outcome of mTHPC mediated photodynamic therapy is primarily determined by the vascular response. Photochem. Photobiol. 2005;81:1161–1167. doi: 10.1562/2005-04-04-RA-474. [DOI] [PubMed] [Google Scholar]
- 40.Brown S.B., Brown E.A., Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 41.Berenbaum M., Bonnett R., Chevretton E., Akande-Adebakin S., Ruston M. Selectivity ofmeso-tetra (hydroxyphenyl) porphyrins and chlorins and of photofrin II in causing photodamage in tumour, skin, muscle and bladder. The comcept of cost-benefit in analysing the results. Lasers Med. Sci. 1993;8:235–243. doi: 10.1007/BF02547845. [DOI] [Google Scholar]
- 42.Breskey J.D., Lacey S.E., Vesper B.J., Paradise W.A., Radosevich J.A., Colvard M.D. Photodynamic therapy: Occupational hazards and preventative recommendations for clinical administration by healthcare providers. Photomed. Laser Surg. 2013;31:398–407. doi: 10.1089/pho.2013.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds T. Photodynamic therapy expands its horizons. J. Natl. Cancer Inst. 1997 doi: 10.1093/jnci/89.2.112. [DOI] [PubMed] [Google Scholar]
- 44.Bellnier D.A., Greco W.R., Nava H., Loewen G.M., Oseroff A.R., Dougherty T.J. Mild skin photosensitivity in cancer patients following injection of photochlor (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a; HPPH) for photodynamic therapy. Cancer Chemother. Pharmacol. 2006;57:40–45. doi: 10.1007/s00280-005-0015-6. [DOI] [PubMed] [Google Scholar]
- 45.Bellnier D.A., Greco W.R., Loewen G.M., Nava H., Oseroff A.R., Pandey R.K., Tsuchida T., Dougherty T.J. Population pharmacokinetics of the photodynamic therapy agent 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a in cancer patients. Cancer Res. 2003;63:1806–1813. [PubMed] [Google Scholar]
- 46.Mody T.D. Pharmaceutical development and medical applications of porphyrin-type macrocycles. J. Porphyr. Phthalocyanines. 2000;4:362–367. doi: 10.1002/(SICI)1099-1409(200006/07)4:4<362::AID-JPP250>3.0.CO;2-Z. [DOI] [Google Scholar]
- 47.Hillemanns P., Petry K., Soergel P., Collinet P., Ardaens K., Gallwas J., Luyten A., Dannecker C. Efficacy and safety of hexaminolevulinate photodynamic therapy in patients with low-grade cervical intraepithelial neoplasia. Lasers Surg. Med. 2014;46:456–461. doi: 10.1002/lsm.22255. [DOI] [PubMed] [Google Scholar]
- 48.O’Connor A.E., Gallagher W.M., Byrne A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009;85:1053–1074. doi: 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 49.Zeitouni N.C., Oseroff A., Najarian D.J. Skin Cancer Management. Springer; New York, NY, USA: 2009. Photodynamic therapy; pp. 41–56. [Google Scholar]
- 50.Arnold R. Dyes and pigments: New research. Nova Science; Hauppauge, NY, USA: 2009. [Google Scholar]
- 51.Ashur I., Goldschmidt R., Pinkas I., Salomon Y., Szewczyk G., Sarna T., Scherz A. Photocatalytic generation of oxygen radicals by the water-soluble bacteriochlorophyll derivative WST11, noncovalently bound to serum albumin. J. Phys. Chem. A. 2009;113:8027–8037. doi: 10.1021/jp900580e. [DOI] [PubMed] [Google Scholar]
- 52.Sickenberg M., Schmidt-Erfurth U., Miller J.W., Pournaras C.J., Zografos L., Piguet B., Donati G., Laqua H., Barbazetto I., Gragoudas E.S., et al. A preliminary study of photodynamic therapy using verteporfin for choroidal neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid streaks, and idiopathic causes. Arch. Ophthalmol. 2000;118:327–336. doi: 10.1001/archopht.118.3.327. [DOI] [PubMed] [Google Scholar]
- 53.Mellish K.J., Brown S.B. Verteporfin: A milestone in opthalmology and photodynamic therapy. Expert Opin. Pharmacother. 2001;2:351–361. doi: 10.1517/14656566.2.2.351. [DOI] [PubMed] [Google Scholar]
- 54.Gold M.H. History of Photodynamic Therapy. Springer; New York, NY, USA: 2011. pp. 1–4. [Google Scholar]
- 55.Abrahamse H., Hamblin M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016;473:347–364. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baas P., van Mansom I., van Tinteren H., Stewart F.A., van Zandwijk N. Effect of N-acetylcysteïne on photofrin-induced skin photosensitivity in patients. Lasers Surg. Med. 1995;16:359–367. doi: 10.1002/lsm.1900160407. [DOI] [PubMed] [Google Scholar]
- 57.Chatterjee D.K., Fong L.S., Zhang Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008;60:1627–1637. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J., Jiang C., Figueiró Longo J.P., Azevedo R.B., Zhang H., Muehlmann L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B. 2018;8:137–146. doi: 10.1016/j.apsb.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kessel D., Thompson P. Purification and analysis of hematoporphyrin and hematoporphyrin derivative by gel exclusion and reverse-phase chromatography. Photochem. Photobiol. 1987;46:1023–1025. doi: 10.1111/j.1751-1097.1987.tb04888.x. [DOI] [PubMed] [Google Scholar]
- 60.Yoon I., Li J.Z., Shim Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013;46:7–23. doi: 10.5946/ce.2013.46.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Josefsen L.B., Boyle R.W. Photodynamic therapy: Novel third-generation photosensitizers one step closer? Br. J. Pharmacol. 2008;154:1–3. doi: 10.1038/bjp.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kataoka H., Nishie H., Hayashi N., Tanaka M., Nomoto A., Yano S., Joh T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017;5:183. doi: 10.21037/atm.2017.03.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savellano M.D., Hasan T. Targeting cells that overexpress the epidermal growth factor receptor with polyethylene glycolated BPD verteporfin photosensitizer immunoconjugates. Photochem. Photobiol. 2003;77:431. doi: 10.1562/0031-8655(2003)077<0431:TCTOTE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 64.Lee H.M., Jeong Y., Kim D.H., Kwak T.W., Chung C., Kim C.H., Kang D.H. Ursodeoxycholic acid-conjugated chitosan for photodynamic treatment of HuCC-T1 human cholangiocarcinoma cells. Int. J. Pharm. 2013;454:74–81. doi: 10.1016/j.ijpharm.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 65.Tarragó-Trani M.T., Jiang S., Harich K.C., Storrie B. Shiga-like toxin subunit B (SLTB)-enhanced delivery of chlorin e6 (Ce6) improves cell killing. Photochem. Photobiol. 2006;82:527–537. doi: 10.1562/2005-06-20-RA-583. [DOI] [PubMed] [Google Scholar]
- 66.Gariépy J. The use of shiga-like toxin 1 in cancer therapy. Crit. Rev. Oncol. 2001;39:99–106. doi: 10.1016/S1040-8428(01)00126-3. [DOI] [PubMed] [Google Scholar]
- 67.Mari C., Pierroz V., Ferrari S., Gasser G. Combination of Ru(II) complexes and light: New frontiers in cancer therapy. Chem. Sci. 2015;6:2660–2686. doi: 10.1039/C4SC03759F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jakubaszek M., Goud B., Ferrari S., Gasser G. Mechanisms of action of Ru(II) polypyridyl complexes in living cells upon light irradiation. Chem. Commun. 2018;54:13040–13059. doi: 10.1039/C8CC05928D. [DOI] [PubMed] [Google Scholar]
- 69.McKenzie L.K., Bryant H.E., Weinstein J.A. Transition metal complexes as photosensitisers in one-and two-photon photodynamic therapy. Coord. Chem. Rev. 2019;379:2–29. doi: 10.1016/j.ccr.2018.03.020. [DOI] [Google Scholar]
- 70.Zeng L., Gupta P., Chen Y., Wang E., Ji L., Chao H., Chen Z.S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017;46:5771–5804. doi: 10.1039/C7CS00195A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soliman N., McKenzie L.K., Karges J., Bertrand E., Tharaud M., Jakubaszek M., Guérineau V., Goud B., Hollenstein M., Gasser G., et al. Ruthenium-initiated polymerization of lactide: A route to remarkable cellular uptake for photodynamic therapy of cancer. Chem. Sci. 2020;11:2657–2663. doi: 10.1039/C9SC05976H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knoll J.D., Turro C. Control and utilization of ruthenium and rhodium metal complex excited states for photoactivated cancer therapy. Coord. Chem. Rev. 2015;282:110–126. doi: 10.1016/j.ccr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monro S., Colon K.L., Yin H., Roque I.I.I.J., Konda P., Gujar S., Thummel R.P., Lilge L., Cameron C.G., McFarland S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2018;119:797–828. doi: 10.1021/acs.chemrev.8b00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McFarland S.A., Mandel A., Dumoulin-White R., Gasser G. Metal-based photosensitizers for photodynamic therapy: The future of multimodal oncology? Curr. Opin. Chem. Biol. 2020;56:23–27. doi: 10.1016/j.cbpa.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandis A.S., Salomon Y., Scherz A. Chlorophylls and Bacteriochlorophylls. Springer; Dordrecht, The Netherlands: Chlorophyll sensitizers in photodynamic therapy; pp. 461–483. Advances in Photosynthesis and Respiration. [Google Scholar]
- 76.Sternberg E.D., Dolphin D., Brückner C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron. 1998;54:4151–4202. doi: 10.1016/S0040-4020(98)00015-5. [DOI] [Google Scholar]
- 77.Castano A.P., Demidova T.N., Hamblin M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Celli J.P., Spring B.Q., Rizvi I., Evans C.L., Samkoe K.S., Verma S., Pogue B.W., Hasan T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010;110:2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwiatkowski S., Knap B., Przystupski D., Saczko J., Kędzierska E., Knap-Czop K., Kotlińska J., Michel O., Kotowski K., Kulbacka J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 80.Bleam W.F. Soil and Environmental Chemistry. Academic Press; Cambridge, MA, USA: 2016. [Google Scholar]
- 81.Greenwood Chemistry of Elements. Sykepleien. 1968;55:412. [Google Scholar]
- 82.Emsley J. Nature’s Building Blocks: Everything You Need to Know about the Elements. Oxford University Press; Oxford, UK: 2011. [Google Scholar]
- 83.Machlan L.A., Gramlich J.W., Powell L.J., Lamhert G.M. Absolute isotopic abundance ratio and atomic weight of a reference sample of gallium. J. Res. Natl. Bur. Stand. 1986;91:323–331. doi: 10.6028/jres.091.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brodzinski R.L., Conway D.C. Decay of rhenium-187. Phys. Rev. 1965;138:B1368. doi: 10.1103/PhysRev.138.B1368. [DOI] [Google Scholar]
- 85.Phillips W.T., Goins B., Bao A., Vargas D., Guttierez J.E., Trevino A., Miller J.R., Henry J., Zuniga R., Vecil G., et al. Rhenium-186 liposomes as convection-enhanced nanoparticle brachytherapy for treatment of glioblastoma. Neuro-oncology. 2012;14:416–425. doi: 10.1093/neuonc/nos060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia C.V., Parrilha G.L., Rodrigues B.L., Teixeira S.F., De Azevedo R.A., Ferreira A.K., Beraldo H. Tricarbonylrhenium(I) complexes with 2-acetylpyridine-derived hydrazones are cytotoxic to NCI-H460 human large cell lung cancer. New J. Chem. 2016;40:7379–7387. doi: 10.1039/C6NJ00050A. [DOI] [Google Scholar]
- 87.Jeong J.M., Knapp F.F. Use of the oak ridge national laboratory tungsten-188/rhenium-188 generator for preparation of the rhenium-188 HDD/lipiodol complex for trans-arterial liver cancer therapy. Semin. Nucl. Med. 2008;38:S19–S29. doi: 10.1053/j.semnuclmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 88.Haase A.A., Bauer E.B., Kühn F.E., Crans D.C. Speciation and toxicity of rhenium salts, organometallics and coordination complexes. Coord. Chem. Rev. 2019;394:135–161. doi: 10.1016/j.ccr.2019.05.012. [DOI] [Google Scholar]
- 89.Bauer E.B., Haase A.A., Reich R.M., Crans D.C., Kühn F.E. Organometallic and coordination rhenium compounds and their potential in cancer therapy. Coord. Chem. Rev. 2019;393:79–117. doi: 10.1016/j.ccr.2019.04.014. [DOI] [Google Scholar]
- 90.Find Trials. [(accessed on 20 April 2020)]; Available online: https://clinicaltrials.gov/
- 91.Maximum Tolerated Dose, Safety, and Efficacy of Rhenium Nanoliposomes in Recurrent Glioma—Full Text View. [(accessed on 21 April 2020)]; Available online: https://clinicaltrials.gov/
- 92.Treatment of Non-responding to Conventional Therapy Inoperable Liver Cancers by in situ introduction of imdendrim—Full Text View. [(accessed on 21 April 2020)]; Available online: https://clinicaltrials.gov/
- 93.Lipiodol as an Imaging Biomarker in Patients with Primary and Metastatic Liver Cancer—Full Text View. [(accessed on 21 April 2020)]; Available online: https://clinicaltrials.gov/
- 94.Hieber W., Fuchs H. About metal carbonyls. XXXIX. amine substituted rhenium carbonyls. J. Inorg. Gen. Chem. 1941;248:269–275. [Google Scholar]
- 95.Pearson R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963;85:3533–3539. doi: 10.1021/ja00905a001. [DOI] [Google Scholar]
- 96.Zhao Q., Huang C., Li F. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011;40:2508–2524. doi: 10.1039/c0cs00114g. [DOI] [PubMed] [Google Scholar]
- 97.Fernández-Moreira V., Thorp-Greenwood F.L., Coogan M.P. Application of d6 transition metal complexes in fluorescence cell imaging. Chem. Commun. 2010;46:186–202. doi: 10.1039/B917757D. [DOI] [PubMed] [Google Scholar]
- 98.Konkankit C.C., Marker S.C., Knopf K.M., Wilson J.J. Anticancer activity of complexes of the third-row transition metals, rhenium, osmium, and iridium. Dalton Trans. 2018;47:9934–9974. doi: 10.1039/C8DT01858H. [DOI] [PubMed] [Google Scholar]
- 99.Amoroso A.J., Coogan M.P., Dunne J.E., Fernández-Moreira V., Hess J.B., Hayes A.J., Lloyd D., Millet C., Pope S.J.A., Williams C. Rhenium fac tricarbonyl bisimine complexes: Biologically useful fluorochromes for cell imaging applications. Chem. Commun. 2007:3066–3068. doi: 10.1039/B706657K. [DOI] [PubMed] [Google Scholar]
- 100.Joshi T., Gasser G. Towards tris(diimine)–ruthenium(II) and bis(quinoline)–Re(I)(CO)3 complexes as photoactivated anticancer drug candidates. Synlett. 2015;26:275–284. doi: 10.1055/s-0034-1379426. [DOI] [Google Scholar]
- 101.Leonidova A., Gasser G. Underestimated potential of organometallic rhenium complexes as anticancer agents. Acs Chem. Biol. 2014;9:2180–2193. doi: 10.1021/cb500528c. [DOI] [PubMed] [Google Scholar]
- 102.Quental L., Raposinho P., Mendes F., Santos I., Navarro-Ranninger C., Alvarez-Valdes A., Huang H., Chao H., Rubbiani R., Gasser G., et al. Combining imaging and anticancer properties with new heterobimetallic Pt(II)/M(I) (M = Re, 99mTc) complexes. Dalton Trans. 2017;46:14523–14536. doi: 10.1039/C7DT00043J. [DOI] [PubMed] [Google Scholar]
- 103.Mari C., Pierroz V., Rubbiani R., Patra M., Hess J., Spingler B., Oehninger L., Schur J., Ott L., Salassa L., et al. DNA intercalating Ru(II) polypyridyl complexes as effective photosensitizers in photodynamic therapy. Chem.—A Eur. J. 2014;20:14421–14436. doi: 10.1002/chem.201402796. [DOI] [PubMed] [Google Scholar]
- 104.Kastl A., Dieckmann S., Wähler K., Völker T., Kastl L., Merkel A.L., Vultur A., Shannan B., Harms K., Ocker M., et al. Rhenium complexes with visible-light-induced anticancer activity. ChemMedChem. 2013;8:924–927. doi: 10.1002/cmdc.201300060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dougherty T.J., Gomer C.J., Henderson B.W., Jori G., Kessel D., Korbelik M., Moan J., Peng Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gianferrara T., Spagnul C., Alberto R., Gasser G., Ferrari S., Pierroz V., Bergamo A., Alessio E. Towards matched pairs of porphyrin-ReI/99mTc I conjugates that combine photodynamic activity with fluorescence and radio imaging. ChemMedChem. 2014;9:1231–1237. doi: 10.1002/cmdc.201300501. [DOI] [PubMed] [Google Scholar]
- 107.Kraljić I., Mohsni S.E. A new method for the detection of singlet oxygen in aqueous solutions. Photochem. Photobiol. 1978;28:577–581. doi: 10.1111/j.1751-1097.1978.tb06972.x. [DOI] [Google Scholar]
- 108.Wähler K., Ludewig A., Szabo P., Harms K., Meggers E. Rhenium complexes with red-light-induced anticancer activity. Eur. J. Inorg. Chem. 2014;2014:807–811. doi: 10.1002/ejic.201301474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niu L.Y., Guan Y.S., Chen Y.Z., Wu L.Z., Tung C.H., Yang Q.Z. A turn-on fluorescent sensor for the discrimination of cystein from homocystein and glutathione. Chem. Commun. 2013;49:1294–1296. doi: 10.1039/c2cc38429a. [DOI] [PubMed] [Google Scholar]
- 110.Lu H., Mac K.J., Yang Y., Shen Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014;43:4778–4823. doi: 10.1039/C4CS00030G. [DOI] [PubMed] [Google Scholar]
- 111.Yu X., Jia X., Yang X., Liu W., Qin W. Synthesis and photochemical properties of BODIPY-functionalized silica nanoparticles for imaging Cu2+ in living cells. Rsc Adv. 2014;4:23571–23579. doi: 10.1039/C4RA03183K. [DOI] [Google Scholar]
- 112.Zhong F., Yuan X., Zhao J., Wang Q. Visible light-harvesting tricarbonyl Re(I) complex: Synthesis and application in intracellular photodynamic effect and luminescence imaging. Sci. China Chem. 2016;59:70–77. doi: 10.1007/s11426-015-5491-x. [DOI] [Google Scholar]
- 113.Zhao L., Odaka H., Ono H., Kajimoto S., Hatanaka K., Hobley J., Fukumura H. Dynamics of Re(2,2′-bipyridine)(CO)3C1 MLCT formation and decay after picosecond pulsed X-ray excitation and femtosecond UV excitation. Photochem. Photobiol. Sci. 2005;4:113–117. doi: 10.1039/B409936B. [DOI] [PubMed] [Google Scholar]
- 114.Yip A.M., Shum J., Liu H., Zhou H., Jia M., Niu N., Li Y., Yu C., Lo K.K.W. Luminescent rhenium(I)–polypyridine complexes appended with a perylene diimide or benzoperylene monoimide moiety: Photophysics, intracellular sensing, and photocytotoxic activity. Chem. –A Eur. J. 2019;25:8970–8974. doi: 10.1002/chem.201900345. [DOI] [PubMed] [Google Scholar]
- 115.Collery P., Veena V., Harikrishnan A., Desmaele D. The rhenium(I)-diselenoether anticancer drug targets ROS, TGF-β1, VEGF-A, and IGF-1 in an in vitro experimental model of triple-negative breast cancers. Investig. New Drugs. 2019;37:973–983. doi: 10.1007/s10637-019-00727-1. [DOI] [PubMed] [Google Scholar]
- 116.Kermagoret A., Morgant G., d’Angelo J., Tomas A., Roussel P., Bastian G., Collery P., Desmaële D. Synthesis, structural characterization and biological activity against several human tumor cell lines of four rhenium(I) diseleno-ethers complexes: Re (CO)3Cl (PhSe (CH2) 2SePh), Re (CO)3Cl (PhSe (CH2) 3SePh), Re(CO)3Cl (HO2C–CH2Se (CH2) 2SeCH2–CO2H) and Re (CO)3Cl (HO2C–CH2Se (CH2)3SeCH2–CO2H) Polyhedron. 2011;30:347–353. [Google Scholar]
- 117.Collery P., Santoni F., Mohsen A., Mignard C., Desmaele D. Negative impact of total body irradiation on the antitumor activity of rhenium-(I)-diselenoether. Anticancer Res. 2016;36:5813–5819. doi: 10.21873/anticanres.11165. [DOI] [PubMed] [Google Scholar]
- 118.Capper M.S., Rehkämper M., Packman H. Rhenium based complexes and in vivo testing: A brief history. ChemBioChem. 2020 doi: 10.1002/cbic.202000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.He L., Pan Z., Qin W., Li Y., Tan C., Mao Z. Impairment of the autophagy-related lysosomal degradation pathway by an anticancer rhenium(I) complex. Dalton Trans. 2019;48:4398–4404. doi: 10.1039/C9DT00322C. [DOI] [PubMed] [Google Scholar]
- 120.Knopf K.M., Murphy B.L., Macmillan S.N., Baskin J.M., Barr M.P., Boros E., Wilson J.J. In vitro anticancer activity and in vivo biodistribution of rhenium(I) tricarbonyl aqua complexes. J. Am. Chem. Soc. 2017;139:14302–14314. doi: 10.1021/jacs.7b08640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Collery P., Mohsen A., Kermagoret A., Corre S., Bastian G., Tomas A., Wei M., Santoni F., Guerra N., Desmaële D., et al. Antitumor activity of a rhenium(I)-diselenoether complex in experimental models of human breast cancer. Investig. New Drugs. 2015;33:848–860. doi: 10.1007/s10637-015-0265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Collery P., Santoni F., Ciccolini J., Tran T.N.N., Mohsen A., Desmaele D. Dose effect of rhenium(I)-diselenoether as anticancer drug in resistant breast tumor-bearing mice after repeated administrations. Anticancer Res. 2016;36:6051–6057. doi: 10.21873/anticanres.11194. [DOI] [PubMed] [Google Scholar]
- 123.Heinemann F., Karges J., Gasser G. Critical overview of the use of Ru(II) polypyridyl complexes as photosensitizers in one-photon and two-photon photodynamic therapy. Acc. Chem. Res. 2017;50:2727–2736. doi: 10.1021/acs.accounts.7b00180. [DOI] [PubMed] [Google Scholar]
- 124.Shen Y., Shuhendler A.J., Ye D., Xu J., Chen H. Two-photon excitation nanoparticles for photodynamic therapy. Chem. Soc. Rev. 2016;45:6725–6741. doi: 10.1039/C6CS00442C. [DOI] [PubMed] [Google Scholar]
- 125.Sun B., Wang L., Li Q., He P., Liu H., Wang H., Yang Y., Li J. Bis (pyrene)-doped cationic dipeptide nanoparticles for two-photon-activated photodynamic therapy. Biomacromolecules. 2017;18:3506–3513. doi: 10.1021/acs.biomac.7b00780. [DOI] [PubMed] [Google Scholar]
- 126.Baggaley E., Weinstein J.A., Williams J.G. Lighting the way to see inside the live cell with luminescent transition metal complexes. Coord. Chem. Rev. 2012;256:1762–1785. doi: 10.1016/j.ccr.2012.03.018. [DOI] [Google Scholar]
- 127.Bolze F., Jenni S., Sour A., Heitz V. Molecular photosensitisers for two-photon photodynamic therapy. Chem. Commun. 2017;53:12857–12877. doi: 10.1039/C7CC06133A. [DOI] [PubMed] [Google Scholar]
- 128.Ogawa K., Kobuke Y. Recent advances in two-photon photodynamic therapy. Anti-Cancer Agents Med. Chem. 2008;8:269–279. doi: 10.2174/187152008783961860. [DOI] [PubMed] [Google Scholar]
- 129.Sun J., Xin Q., Yang Y., Shah H., Cao H., Qi Y., Gong J.R., Li J.B. Nitrogen-doped graphene quantum dots coupled with photosensitizers for one-/two-photon activated photodynamic therapy based on a FRET mechanism. Chem. Commun. 2018;54:715–718. doi: 10.1039/C7CC08820E. [DOI] [PubMed] [Google Scholar]
- 130.Yang Y., Liu H., Han M., Sun B., Li J. Multilayer microcapsules for FRET analysis and two-photon-activated photodynamic therapy. Angew. Chem. Int. Ed. 2016;55:13538–13543. doi: 10.1002/anie.201605905. [DOI] [PubMed] [Google Scholar]
- 131.Liu H., Yang Y., Wang A., Han M., Cui W., Li J. Hyperbranched polyglycerol-doped mesoporous silica nanoparticles for one-and two-photon activated photodynamic therapy. Adv. Funct. Mater. 2016;26:2561–2570. doi: 10.1002/adfm.201504939. [DOI] [Google Scholar]
- 132.Ogawa K., Hasegawa H., Inaba Y., Kobuke Y., Inouye H., Kanemitsu Y., Kohno E., Hirano T., Ogura S.I., Okura I. Water-soluble bis (imidazolylporphyrin) self-assemblies with large two-photon absorption cross sections as potential agents for photodynamic therapy. J. Med. Chem. 2006;49:2276–2283. doi: 10.1021/jm051072+. [DOI] [PubMed] [Google Scholar]
- 133.Dahlstedt E., Collins H.A., Balaz M., Kuimova M.K., Khurana M., Wilson B.C., Philips D., Anderson H.L. One-and two-photon activated phototoxicity of conjugated porphyrin dimers with high two-photon absorption cross sections. Org. Biomol. Chem. 2009;7:897–904. doi: 10.1039/b814792b. [DOI] [PubMed] [Google Scholar]
- 134.Collins H.A., Khurana M., Moriyama E.H., Mariampillai A., Dahlstedt E., Balaz M., Kuimova M.K., Drobizhev M., Yang V.X.D., Philips D., et al. Blood-vessel closure using photosensitizers engineered for two-photon excitation. Nat. Photonics. 2008;2:420–424. doi: 10.1038/nphoton.2008.100. [DOI] [Google Scholar]
- 135.Kuimova M.K., Collins H.A., Balaz M., Dahlstedt E., Levitt J.A., Sergent N., Suhling K., Drobizhev M., Makarov N.S., Rebane A., et al. Photophysical properties and intracellular imaging of water-soluble porphyrin dimers for two-photon excited photodynamic therapy. Org. Biomol. Chem. 2009;7:889–896. doi: 10.1039/b814791d. [DOI] [PubMed] [Google Scholar]
- 136.Kargesa J., Kuangb S., Maschiettoc F., Blacqued O., Ciofinic I., Chao H., Gasser G. Ruthenium complexes for 1-and 2-photon photodynamic therapy: From in silico prediction to in vivo applications. Chemrxiv. 2020 doi: 10.26434/chemrxiv.11767995.v1. [DOI] [Google Scholar]
- 137.Sherlock B., Warren S.C., Alexandrov Y., Yu F., Stone J., Knight J., Neil M.A.A., Paterson C., French P.M.W., Dunsby C. In vivo multiphoton microscopy using a handheld scanner with lateral and axial motion compensation. J. Biophotonics. 2018;11:e201700131. doi: 10.1002/jbio.201700131. [DOI] [PubMed] [Google Scholar]
- 138.Qiu K., Wen Y., Ouyang C., Liao X., Liu C., Rees T.W., Zhang Q., Ji L., Chao H. The stepwise photodamage of organelles by two-photon luminescent ruthenium (ii) photosensitizers. Chem. Commun. 2019;55:11235–11238. doi: 10.1039/C9CC05962H. [DOI] [PubMed] [Google Scholar]
- 139.Hess J., Huang H., Kaiser A., Pierroz V., Blacque O., Chao H., Gasser G. Evaluation of the medicinal potential of two ruthenium(II) polypyridine complexes as one-and two-photon photodynamic yherapy photosensitizers. Chem. –A Eur. J. 2017;23:9888–9896. doi: 10.1002/chem.201701392. [DOI] [PubMed] [Google Scholar]
- 140.Huang H., Yu B., Zhang P., Huang J., Chen Y., Gasser G., Ji L., Chao H. Highly charged ruthenium(II) polypyridyl complexes as lysosome localized photosensitizers for two-photon photodynamic therapy. Angew. Chem. Int. Ed. 2015;54:14049–14052. doi: 10.1002/anie.201507800. [DOI] [PubMed] [Google Scholar]
- 141.Lakowicz J.R., Castellano F.N., Gryczynski I., Gryczynski Z., Dattelbaum J.D. Two-photon excitation of rhenium metal–ligand complexes. J. Photochem. Photobiol. A. 1999;122:95–101. doi: 10.1016/S1010-6030(99)00013-1. [DOI] [Google Scholar]
- 142.Ferri E., Donghi D., Panigati M., Prencipe G., D’Alfonso L., Zanoni I., Baldoli C., Maiorana S., D’Alfonso G., Licandro E. Luminescent conjugates between dinuclear rhenium (I) complexes and peptide nucleic acids (PNA) for cell imaging and DNA targeting. Chem. Commun. 2010;46:6255–6257. doi: 10.1039/c0cc00450b. [DOI] [PubMed] [Google Scholar]
- 143.Chen C., Law W., Aalinkeel R., Yu Y., Nair B., Wu J., Mahajan S., Reynolds J.L., Li Y., Lai C.K., et al. Biodegradable cationic polymeric nanocapsules for overcoming multidrug resistance and enabling drug–gene co-delivery to cancer cells. Nanoscale. 2014;6:1567–1572. doi: 10.1039/C3NR04804G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Duan J., Mansour H.M., Zhang Y., Deng X., Chen Y., Wang J., Pan Y., Zhao J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly (butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2012;426:193–201. doi: 10.1016/j.ijpharm.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 145.Vivek R., Thangam R., NipunBabu V., Rejeeth C., Sivasubramanian S., Gunasekaran P., Muthuchelian K., Kannan S. Multifunctional HER2-antibody conjugated polymeric nanocarrier-based drug delivery system for multi-drug-resistant breast cancer therapy. Acs Appl. Mater. Interfaces. 2014;6:6469–6480. doi: 10.1021/am406012g. [DOI] [PubMed] [Google Scholar]
- 146.Duan X., Xiao J., Yin Q., Zhang Z., Yu H., Mao S., Li Y. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. Acs Nano. 2013;7:5858–5869. doi: 10.1021/nn4010796. [DOI] [PubMed] [Google Scholar]
- 147.Xiong X., Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. Acs Nano. 2011;5:5202–5213. doi: 10.1021/nn2013707. [DOI] [PubMed] [Google Scholar]
- 148.Wang S., Li J., Ye Z., Li J., Wang A., Hu J., Bai S., Yin J. Self-assembly of photosensitive and chemotherapeutic drugs for combined photodynamic-chemo cancer therapy with real-time tracing property. Colloids Surf. A: Physicochem. Eng. Asp. 2019;574:44–51. doi: 10.1016/j.colsurfa.2019.04.060. [DOI] [Google Scholar]
- 149.Doustvandi M.A., Mohammadnejad F., Mansoori B., Tajalli H., Mohammadi A., Mokhtarzadeh A., Baghbani E., Khaze V., Hajiasgharzadeh K., Moghaddam M.M., et al. Photodynamic therapy using zinc phthalocyanine with low dose of diode laser combined with doxorubicin is a synergistic combination therapy for human SK-MEL-3 melanoma cells. Photodiagnosis Photodyn. Ther. 2019;28:88–97. doi: 10.1016/j.pdpdt.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 150.Leijen S., Burgers S.A., Baas P., Pluim D., Tibben M., van Werkhoven E., Alessio E., Sava G., Beijnen J.H., Schellens J.H.M. Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy. Investig. New Drugs. 2015;33:201–214. doi: 10.1007/s10637-014-0179-1. [DOI] [PubMed] [Google Scholar]
- 151.Mignani S., Bryszewska M., Klajnert-Maculewicz B., Zablocka M., Majoral J. Advances in combination therapies based on nanoparticles for efficacious cancer treatment: An analytical report. Biomacromolecules. 2015;16:1–27. doi: 10.1021/bm501285t. [DOI] [PubMed] [Google Scholar]
- 152.Hu C.J., Zhang L. Therapeutic nanoparticles to combat cancer drug resistance. Curr. Drug Metab. 2009;10:836–841. doi: 10.2174/138920009790274540. [DOI] [PubMed] [Google Scholar]
- 153.Yuan Y.G., Peng Q.L., Gurunathan S. Silver nanoparticles enhance the apoptotic potential of gemcitabine in human ovarian cancer cells: Combination therapy for effective cancer treatment. Int. J. Nanomed. 2017;12:6487–6502. doi: 10.2147/IJN.S135482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bernabeu E., Cagel M., Lagomarsino E., Moretton M., Chiappetta D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017;526:474–495. doi: 10.1016/j.ijpharm.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 155.Kundranda M.N., Niu J. Albumin-bound paclitaxel in solid tumors: Clinical development and future directions. Drug Des. Dev. Ther. 2015;9:3767–3777. doi: 10.2147/DDDT.S88023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gaio E., Guerrini A., Ballestri M., Varchi G., Ferroni C., Martella E., Columbaro M., Moret F., Reddi E. Keratin nanoparticles co-delivering docetaxel and chlorin e6 promote synergic interaction between chemo-and photo-dynamic therapies. J. Photochem. Photobiol. B Biol. 2019;199:111598. doi: 10.1016/j.jphotobiol.2019.111598. [DOI] [PubMed] [Google Scholar]
- 157.Huang X., El-Sayed M.A. Plasmonic photo-thermal therapy (PPTT) Alex. J. Med. 2011;47:1–9. doi: 10.1016/j.ajme.2011.01.001. [DOI] [Google Scholar]
- 158.Huang X., Jain P.K., El-Sayed I.H., El-Sayed M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008;23:217. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 159.Spyratou E., Makropoulou M., Efstathopoulos E.P., Georgakilas A.G., Sihver L. Recent advances in cancer therapy based on dual mode gold nanoparticles. Cancers. 2017;9:173. doi: 10.3390/cancers9120173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mouratidis P.X., Rivens I., ter Haar G. A study of thermal dose-induced autophagy, apoptosis and necroptosis in colon cancer cells. Int. J. Hyperth. 2015;31:476–488. doi: 10.3109/02656736.2015.1029995. [DOI] [PubMed] [Google Scholar]
- 161.Cherukuri P., Glazer E.S., Curley S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010;62:339–345. doi: 10.1016/j.addr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saw W.S., Ujihara M., Chong W.Y., Voon S.H., Imae T., Kiew L.V., Lee H.B., Sim K.S., Chung L.Y. Size-dependent effect of cystine/citric acid-capped confeito-like gold nanoparticles on cellular uptake and photothermal cancer therapy. Colloids Surf. B Biointerfaces. 2018;161:365–374. doi: 10.1016/j.colsurfb.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 163.Zhao S., Zhu X., Cao C., Sun J., Liu J. Transferrin modified ruthenium nanoparticles with good biocompatibility for photothermal tumor therapy. J. Colloid Interface Sci. 2018;511:325–334. doi: 10.1016/j.jcis.2017.10.023. [DOI] [PubMed] [Google Scholar]