Abstract
Adult stem cells maintain tissue homeostasis by continuously replenishing damaged, aged and dead cells in any organism. Five types of region and organ-specific multipotent adult stem cells have been identified in the Drosophila digestive system: intestinal stem cells (ISCs) in the posterior midgut; hindgut intestinal stem cells (HISCs) at the midgut/hindgut junction; renal and nephric stem cells (RNSCs) in the Malpighian Tubules; type I gastric stem cells (GaSCs) at foregut/midgut junction; and type II gastric stem cells (GSSCs) at the middle of the midgut. Despite the fact that each type of stem cell is unique to a particular organ, they share common molecular markers and some regulatory signaling pathways. Due to the simpler tissue structure, ease of performing genetic analysis, and availability of abundant mutants, Drosophila serves as an elegant and powerful model system to study complex stem cell biology. The recent discoveries, particularly in the Drosophila ISC system, have greatly advanced our understanding of stem cell self-renewal, differentiation, and the role of stem cells play in tissue homeostasis/regeneration and adaptive tissue growth.
Keywords: Drosophila, Gastric stem cells, Intestinal stem cells, Midgut, Renal and nephric stem cells
5.1. Introduction
Stem cells (SCs) are defined as cells with clonogenic and self-renewing capabilities that can differentiate into multiple cell lineages [1]. Based on the stages of development, the SCs can be divided into two major categories: (1) Embryonic stem (ES) cells and (2) Adult SCs. ES cells are pluripotent cells found at the embryonic stage that have the ability to generate any type of differentiated cells. Adult SCs on the other hand are multipotent and can only generate tissue-specific cells. During every stage of development, the resident adult SCs in any tissue maintain tissue homeostasis by replacing damaged, aged or dead cells. As expected adult SC self-renewal and differentiation are tightly regulated processes and an imbalance in adult SC homeostasis can result in complications like tumor formation, degenerative diseases, etc. Moreover, precise regulation of adult SC behavior is necessary for them to promptly respond to tissue damage and stress.
Amongst various tissues, the digestive system is exposed to most antigens by way of food consumption and is renewed the fastest in almost all animals. Drosophila midgut is the second largest organ and serves as the entry site for not only nutrients like food and water, but also for pathogens like harmful bacteria, viruses, and toxins [2]. As a result, the midgut epithelium is constantly exposed to environmental assault and undergoes rapid turnover. The integrity of the epithelium is maintained by the replenishment of lost cells by Intestinal SCs (ISCs). In particular, Drosophila posterior midgut is maintained by around 1,000 ISCs that are dispersed among roughly 10,000 posterior midgut cells [3]. These ISCs lie adjacent to the basement membrane and divide almost once a day to give rise to a new ISC and an enteroblast (EB). The EBs can then differentiate into either absorptive enterocytes (EC) or secretory enteroendocrine (EE) cells [3, 4].
Due to the simple tissue structure, ease to perform genetic analysis, and availability of abundant mutants, Drosophila serves as a powerful and popular model system to study stem cell regulation [5, 6]. In particular, the simple cell lineage and extremely fast turnover of midgut epithelium, makes midgut ISCs an exciting model to study adult stem cell mediated tissue homeostasis and regeneration. This chapter briefly discusses the types of adult SCs identified in the Drosophila digestive system and highlights the recent advances in our understanding of the signaling pathways regulating the self-renewal, differentiation, proliferation and the fate of adult SCs in Drosophila with focus on midgut ISCs. In addition, the chapter also elaborates maintenance of homeostasis in normal midgut and provides a comparison of this scenario with an injury model.
5.2. The Origin of Adult ISCs
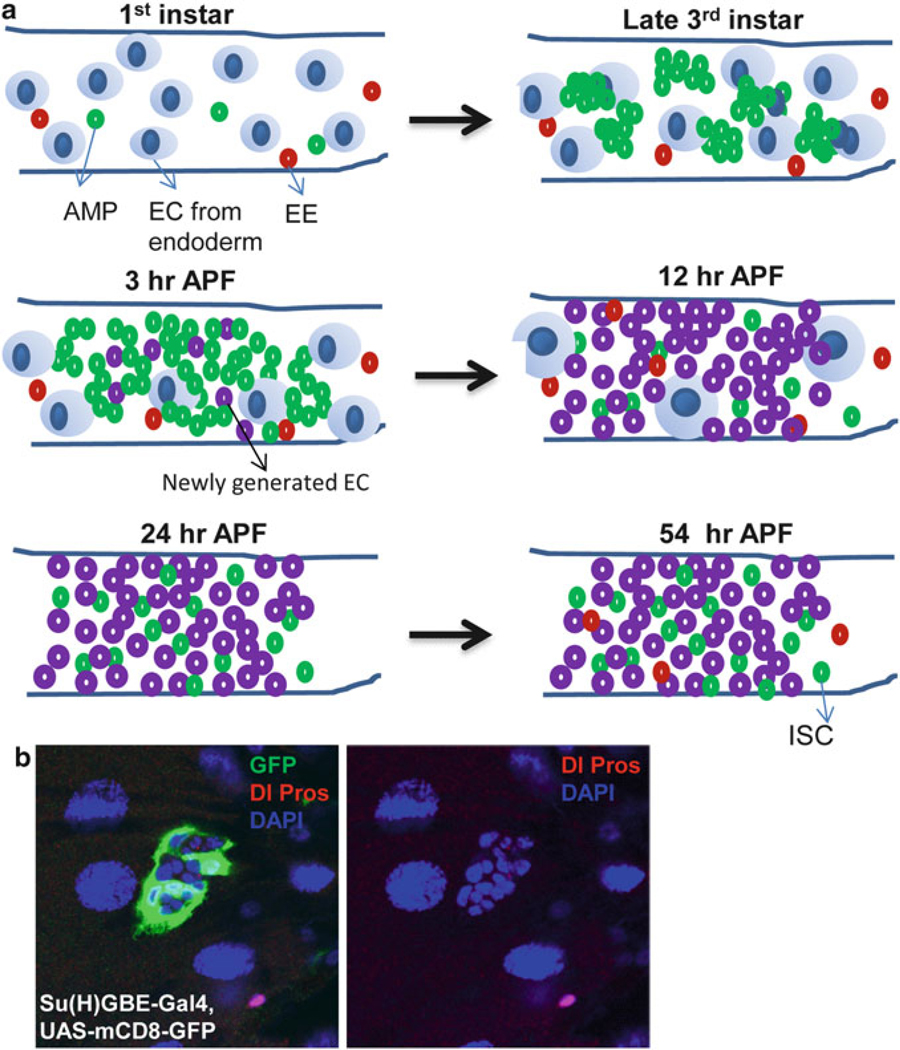
The adult Drosophila midgut epithelium is derived from adult midgut progenitor (AMP) cells found in the embryonic, larval and pupal stages [7–10] (Fig. 5.1a). The AMPs proliferate and disperse throughout the midgut during the first two larval instars (L1 and L2), and by the third instar (L3), they form clusters known as midgut imaginal islands. At the onset of the metamorphosis, the AMP islands start to release the AMPs and merge with each other to form a continuous epithelial layer; some of AMPs differentiate into Pdm-1 positive ECs. As metamorphosis further proceeds, most AMPs differentiate into Pdm-1 positive ECs, while few AMPs remain at undifferentiating status [8, 11, 12] (Fig. 5.1a). These undifferentiated AMPs further divide and develop into ISCs of the adult midgut (Fig. 5.1a).
Fig. 5.1. AMP proliferation and differentiation during Drosophila development.
(a) The adult midgut progenitors (AMP) divide symmetrically to increase their number and remain dispersed as individual cells throughout the midgut during the first two instars. Then each dispersed AMP further divides symmetrically for several rounds to form AMP clusters at the third instar stage. At 3 h after puparium formation (APF), the AMP clusters start fusing together and some of AMPs differentiate into EC. As metamorphosis continues, most of AMPs differentiate into EC and only a few of AMPs remain. These undifferentiated AMPs further divide symmetrically to increase their number and develop into pupal and adult ISC. (b) The Su(H)GBE-Gal4,UAS-mCD8-GFP(green) labeled peripheral cell (PC) extend their process to wrap around the AMP clusters to regulate their proliferation and repress their differentiation. Dl (cytoplasmic red) and Pros (nuclear red) label the AMP and EE respectively
The epidermal growth factor receptor (EGFR)/Ras/mitogen-activated protein kinase (MAPK) signaling has been reported to be necessary and sufficient to induce AMP proliferation [7]. Blocking EGFR signaling results in a decrease in both the number and the size of the AMP clusters. Conversely, over-activation of EGFR/Ras/MAPK signaling by expressing constitutively active forms of Egfr, Ras, and Raf in AMPs leads to a dramatic increase in the AMP number. In addition, ecdysone hormone signaling also plays an important role in regulating the proliferation of AMPs during development [9]. Also, Notch (N) signaling determines AMP fate: high N activity is required for larval absorptive enterocytes (EC) and limits secretory endocrine cells, while low level of N activity leads to increase in AMP number and endocrine cells in the midgut at the expense of differentiated larval ECs [11, 13, 14].
Toward late L2, each AMP divides asymmetrically to produce one new AMP and a peripheral cell (PC) that can be identified by the expression of Su(H)GBE-LacZ, a transcriptional reporter of the N pathway (Fig. 5.1b). The newly generated PCs exit the cell cycle, undergo morphological changes, and extend their processes to tightly wrap the dividing AMP island. It has been demonstrated that N signaling is required to generate the PCs. N mutant clones induced in early-L1 larvae lack discernible PCs in late L3, whereas ectopically activating N directs AMPs to become PC-like cells [8, 15]. The PCs act as a transient niche to maintain the AMPs in an undifferentiated state until the onset of metamorphosis. Ablation of the PCs in early L3 induces the premature differentiation of the AMPs into polyploid EC-like cells. In addition, without the PCs, AMP islands tend to merge. Therefore, PCs not only prevent AMP differentiation but also inhibit AMP islands from prematurely fusing before metamorphosis [8, 15].
PCs maintain the AMPs in an undifferentiated state through DPP signaling. That is, PCs express the ligand DPP, which activates the DPP signal-transduction pathway in the AMPs to maintain the AMPs. However, the repression of DPP signaling is not responsible for the AMPs’ differentiation into Pdm1-positive ECs; other signals in PCs may also repress the AMPs’ differentiation [8]. During metamorphosis, the PCs may either undergo programmed cell death and disappear from the pupal midgut [8] or spread out and form a transient pupal epithelium surrounding the degenerating larval midgut [11]. The released AMPs respond to N signaling and differentiate into ECs. However, one AMP per island, on average, becomes an ISC. The mechanism that maintains the undifferentiated state of this one AMP after the PCs’ death remains to be determined. In addition, it has long been known that two lipophilic hormones coordinately control the entry to metamorphosis in Drosophila larvae. At the end of the third larval instar (L3), juvenile hormone (JH) declines and a strong 20-hydroxyecdysone (20E) pulse trigger the larval-pupal transition [16–18]. It is currently unclear how the hormone signals are connected to AMP differentiation.
5.3. ISC Identification and Regulation
5.3.1. ISCs in the Adult Drosophila Midgut
In the adult Drosophila posterior midgut, the differentiated mature cells are constantly replaced by new cells generated from intestinal stem cells (ISCs) [3, 4]. ISCs divide asymmetrically to produce one new ISC (self-renewal) and one immature daughter cell, enteroblast (EB), which further differentiates into an EC or a secretory enteroendocrine (EE) cell [3, 4]. The dividing ISCs reside immediately adjacent to the basement membrane and the visceral musculature surrounding the midgut [3, 19, 20].
These different cell types in midgut can be identified morphologically as well as by their expression of marker genes. ISCs are diploid, have a small nucleus, and express Delta (Dl) and Sanpodo (Spdo) [21, 22]. Dl is a ligand for the N receptor signal-transduction pathway and Spdo encodes a transmembrane protein that regulates N signal transduction pathway. EBs are diploid, have a small nucleus, and express a transcriptional reporter of the N pathway, Su(H)GBE-lacZ. ECs are polyploid with a large nucleus and express the transcriptional factor Pdm1. EE cells are diploid, have a small nucleus, and express the transcription factor Prospero (Pros). Approximately 90 % of the EBs differentiate into ECs, and 10 % become EEs [21]. As in the mammalian intestinal epithelium, the ECs and EEs continually migrate from the basal location to the gut lumen to replace the damaged cells on the surface of the epithelium.
5.3.2. N Signaling in ISC Regulation
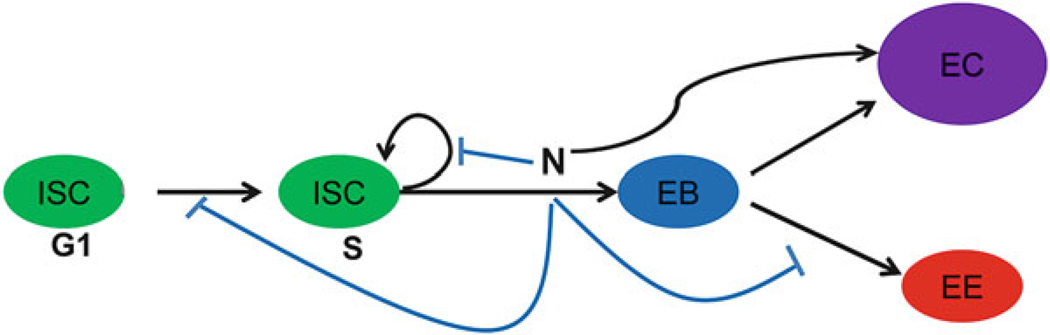
N signaling plays a major role in regulating ISC self-renewal and differentiation [21]. As mentioned in the previous section, N ligand Dl is specifically expressed on newly emerged ISCs. The ISC-expressed Dl binds to the N receptor on the newly formed neighboring EB [21] to activate the N in EB resulting in several outcomes [3, 4, 21] (Fig. 5.2). First, the N activity blocks ISC self-renewal and a high N activity promotes ISC commitment to EB fate. Low N activity on the other hand, such as in GDP-mannose-4,6-dehydratase (Gmd) mutant fly, can result in partially committed cells that co-express the ISC marker Dl and the EB marker Su(H)GBE-lacZ [22]. Second, N activity suppresses ISC proliferation as knockdown of N activity results in ISC overexpansion (tumor) phenotype. Third, N activity blocks EB to EE differentiation, since knockdown of N activity results in EE over-production (tumor) phenotype. N signaling regulates EE differentiation through the achaete-scute complex (AS-C). Over-expression of AS-C in ISCs and EBs results in increase of Pros expressing EEs and AS-C mutant clones are reported to be devoid of Pros expressing EEs [23]. Finally, the N activity promotes EB differentiation into EC, which may only require low N activity since EC differentiation is normal in Gmd mutant flies that have low N activity [22]. The regulations of above four processes may require different levels of N activity and are through unique downstream targets since some mutations only affect one of the four processes. For example, Gmd mutation only affect ISC commitment to EB fate and does not affect EB differentiation into EC; AS-C activity is only required for EE fate determination and does not regulate ISC proliferation or EC commitment. Further, expression level of Dl in ISCs and the N activity in EBs may also be quite dynamic. It was observed that newly formed EE cells are always accompanied by adjacent parental ISCs with low-level expression of Dl and newly formed ECs are accompanied by parental ISCs with high-level expression of Dl. Daughters of ISCs with high Dl levels receive a strong N signal and become ECs, while daughters of ISC with low-level or undetectable Dl receive a much weaker N signal and become EE cells [21].
Fig. 5.2. N signaling in ISC regulation.
Dl, specifically expressed in ISC, activates N signaling (N on) at the neighboring EB. Different level of N activity controls the ISC self-renewal and EC versus EE fates. N signaling also controls ISC proliferation
5.3.3. The Enhancer of Split Complex E(spl)-C in ISC Regulation
It has recently been demonstrated that several transcription factors function downstream of N in regulating ISC fates [23]. In the ISCs, the expression of Daughterless (Da), a basic helix loop helix (bHLH) transcriptional activator, is required to maintain ISC identity. N signal antagonist Hairless (H), which represses N target genes, prevents the expression of the enhancer of split complex [E(spl)-C] genes. Since the E(spl)-C proteins suppress Da-dependent bHLH activity, their inhibition by Hairless maintains Da expression in the ISCs, and hence the ISC identity. The opposite chain of events occurs in the EBs: Dl expressed on an ISC activates N in the adjacent EB, triggering N proteolysis and releasing N intracellular domain (NICD), which competes with H to Suppressor of Hairless [Su(H)]. The NICD-bound Su(H) turns on the E(spl)-C genes, which commits the EBs to a non-ISC fate and promotes their differentiation.
However, the phenotypes of E(spl)-C mutation does not entirely mimic phenotypes of N mutation. Loss of E(spl)-C genes leads to an increase of Dl expressing ISC-like cells, a normal density of EC-like cells and a reduction of Pros expressing EE-like cells. It is possible that the E(spl)-C mutation may only partially disrupt N activity since the mutant phenotypes are somehow similar to the phenotypes of Gmd mutation [22, 23]. Therefore, other mechanisms besides E(spl)-C may be responsible for asymmetric ISC division to EB.
5.3.4. Possible Mechanisms for the Regulation of Asymmetric ISC Division
The asymmetric division of ISCs is in some ways akin to the asymmetric divisions of Drosophila sensory organ precursor (SOP) cells [23, 24], both systems use asymmetric N signaling to direct SC/progenitor asymmetric division.
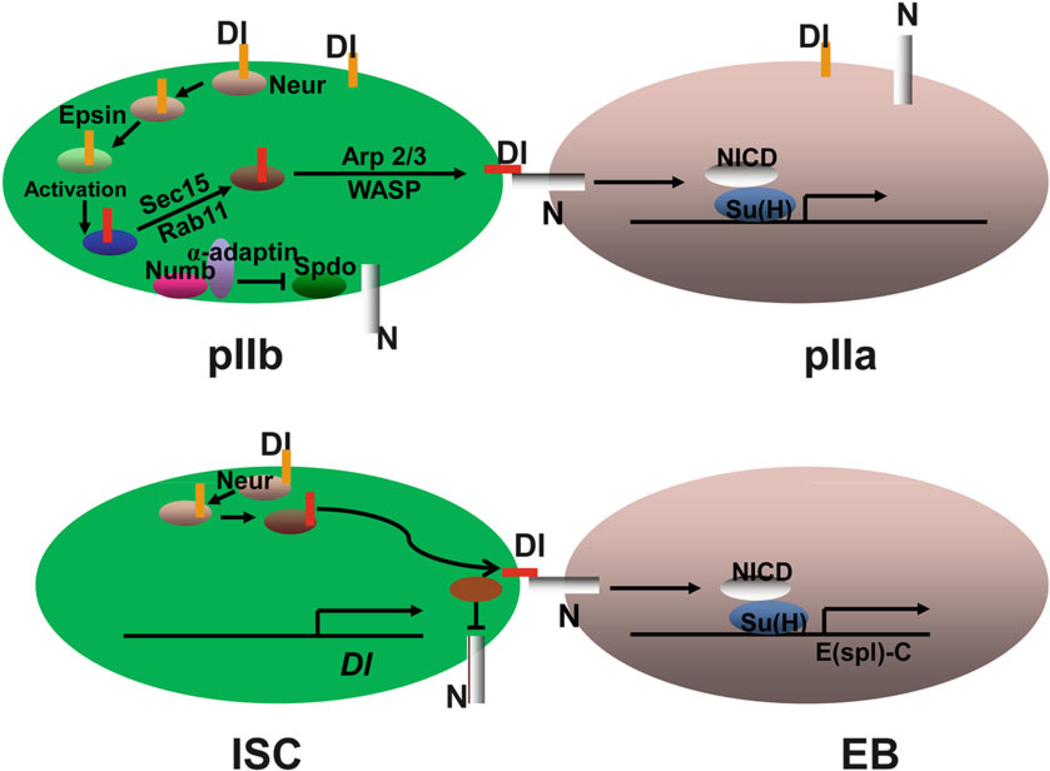
Drosophila SOP is a well-studied example of intrinsically asymmetric cell division (Fig. 5.3). SOP first divides into an anterior pIIb cell and a posterior pIIa cell. The pIIb cell can then divide to form a neuron, a sheath cell, and a glial cell, whereas the pIIa cell divides to produce a posterior socket cell and an anterior hair cell. The asymmetric division is directed by asymmetric N signaling. N signaling is only activated in the posterior pIIa cell. During SOP differentiation, asymmetric N signaling is established twice by two different mechanisms. First, N signaling is downregulated in the pIIb cell by the asymmetric segregation of Numb/α-Adaptin at the time of SOP division, which blocks N activation by regulating the endocytosis of N activator Sanpodo [25]. Second, Dl is only activated in pIIb through a long endocytosis process, which involves several steps [24, 26–28]. Like Numb, the E3 ubiquitin ligase, Neuralized (Neur), is asymmetrically segregated to the anterior pIIb cell, where it endocytoses Dl through ubiquitination. The endocytosed Dl is further trafficked by a protein, Epsin, to an endocytic compartment, where it undergoes activation. The activated Dl is then recycled back to the membrane through a compartment that is positive for Rab11 and the exocyst complex member Sec15. Finally, Wiskott-Aldrich syndrome family protein (WASP)-dependent Arp2/3 actin polymerization is required to transport the endocytosed vesicles containing activated Dl to a prominent actin-rich microvilli at the apical membrane of the pIIb cell, where activated Dl can bind and further activate N on the surface of pIIa. The activated N then promotes Su(H)-dependent transcription to specify pIIa cell fate (Fig. 5.3).
Fig. 5.3. Comparison of asymmetric divisions between SOP and ISC.
Models of asymmetric divisions of SOP and ISC. See text for detail
The asymmetric N signaling in the SOP system is determined by the asymmetric segregation of Numb and Neur into the pIIb cell, whereas both Dl and N are expressed in both pIIb and pIIa cells. However, so far there has been no evidence for asymmetric segregation of either Numb or Neur in dividing ISCs. Additionally, Numb is not important in regulating ISC fate [23]. The data so far suggests that the asymmetric division of ISC is regulated by asymmetric segregation of Dl. During ISC division, asymmetric Dl may be established by two coordinated mechanisms. First, Dl is only transcribed in ISC since a Dl-lacZ insertion reporter driven by Dl promoter is only expressed in ISCs [7, 29, 30]. Second, the low amount of the Dl protein in EBs inherited from ISCs during ISC division is quickly degraded [21]. N is expressed in both ISCs and EBs, but is only activated in EBs. Since Numb is not important in regulating ISC fate, N may be inactivated in ISCs by an unknown factor or may be inactive because no activated Dl is available in neighboring cells to activate it. Neur mutant ISCs form ISC and EE tumors at the expense of differentiated ECs, which are similar to the phenotypes of Dl mutant ISCs [21], suggesting that Neur-mediated Dl activation through endocytosis is required for asymmetric N signaling during ISC division (Fig. 5.3). However, the other steps of Dl endocytosis that occurred in pIIb and involved Epsin, Sec15, Rab11, Arp2/3, and WASP, may not be important in regulating ISC fate, since RNAi-mediated knockdowns of these genes do not affect ISC differentiation (X. Z. and S. H., unpublished).
In summary, the mechanism that regulates Dl transcription in ISCs and the mechanism that limits N activation in EBs may together determine the asymmetric ISC to EB signaling and asymmetric ISC division.
5.4. External Signals
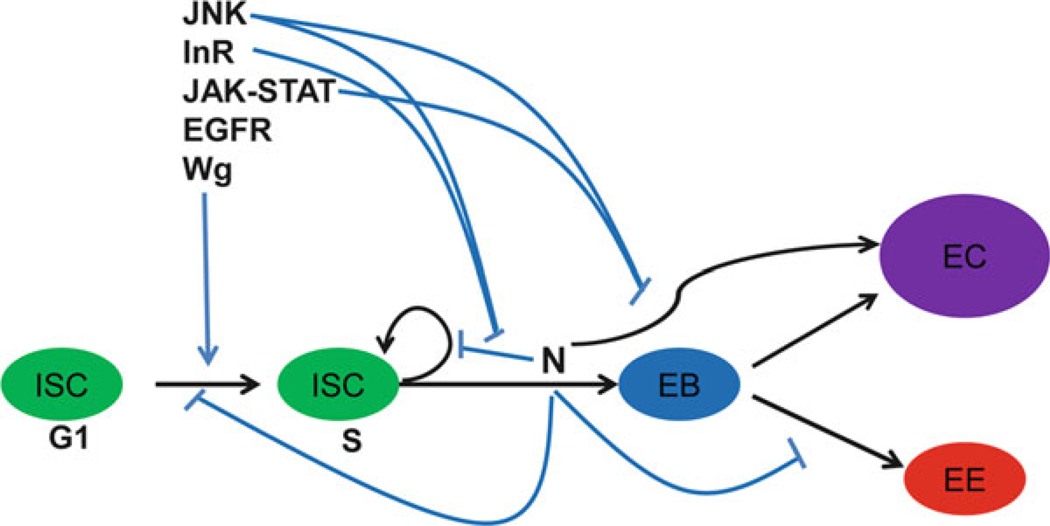
Besides the core N signal transduction pathway, several other signaling pathways (as discussed later) also regulate ISC proliferation, self-renewal, and differentiation [7]. These signaling pathways can either independently perform their functions on ISCs or function through their interplay with the N signaling pathway. The following sections briefly describe the potential interactions of the N signaling pathway and other signaling pathways (Fig. 5.4).
Fig. 5.4. External signals regulate ISC proliferation and differentiation.
In response to tissue stress and damage JNK, InR, JAK-STAT, EGFR and Wg signaling pathways modulate ISC proliferative response. JNK, InR and JAK-STAT signaling pathways also interact with N signaling to regulate ISC self-renewal and differentiation
5.4.1. JNK Signaling Regulates ISC Proliferation and Differentiation
Aged flies and flies under oxidative stress show an activated Jun N-terminal kinase (JNK) signaling pathway activity in ISCs and/or EBs [31], resulting in ISC proliferation and accumulation of misdifferentiated ISC daughter cells. Some of these daughter cells co-express the ISC marker Dl and the EB marker Su(H)GBE-lacZ and some esg expressing/Su(H)GBE-lacZ expressing cells are polyploid EC-like cells but do not express EC markers. The first type of cells are similar to the partially committed cells described above [22], the second type of cells are possibly the partially differentiated EBs. One possibility is that the activated JNK signaling partially suppresses the N signal transduction pathway (Fig. 5.4). The decrease in N activity may cause incomplete commitment of ISC to EB and result in cells that co-express the ISC marker Dl and the EB marker Su(H)GBE-lacZ. In addition, suppressing N activity can also block EB to EC differentiation and result in cells that still express EB markers, but are polyploid. Moreover, activated JNK signaling enhances the phenotypes observed due to low N activity; but, these phenotypes can be suppressed by reducing the dosage of Dl activity by half [31]. However, this model does not explain why reducing the dosage of Dl activity by half suppresses the phenotypes associated with the activated JNK signaling. One possibility is that the mis-differentiation phenotypes are dosage sensitive to the level of N signaling. A combination of reduction of Dl activity by half, together with activated JNK signaling might shift N activity level close to strong loss-of-function mutations of the N pathway. Strong N loss of function (LoF) mutations are known to cause ISC and EE tumors instead of mis-differentiation phenotypes. With this interpretation in mind, it is interesting to note that reducing N signaling by exposure to moderate levels (0.5 mM) of the γ-secretase inhibitor DAPT prevents JNK-induced mis-differentiation but occasionally causes ISC and EE tumors [31].
5.4.2. Insulin Receptor (InR) Regulates ISC Proliferation and Asymmetric Division
Throughout life, adult organs continually remodel to variable nutrient and environmental conditions. In the adult Drosophila midgut, it has recently been reported that the insulin signaling controls organ resizing through regulating ISCs [32]. The Drosophila insulin/insulin growth factor (IGF)-like peptide 3 (dILP3) is up-regulated when food is abundant in the nearby visceral muscle activating the insulin receptor (InR) signal transduction pathway in ISCs to drive midgut growth. The ISCs activated by the InR signaling direct a growth program through two altered modes of behavior: accelerated division rates as well as a switch to symmetric division from asymmetric division. Together, these altered modes result in expansion of ISCs and a net increase in total intestinal cells. The reverse process happens upon withdrawal of food. It is unclear how the InR signaling directly affects ISC proliferation and asymmetric division. One way to achieve these outcomes can be through partial suppression of the N signal transduction pathway (Fig. 5.4). However, activation of the InR signaling by nutrient does not result in mis-differentiation of ISCs as manifested in flies that have elevated JNK signaling. One can envisage two different scenarios. One, the InR signaling may only interface with the N signaling in regulating ISC proliferation and self-renewal but not in ISC commitment and EB differentiation. Alternatively, the InR signaling may control ISC proliferation and asymmetric division through regulating cell adhesion. Dl-N interaction requires cell adhesion because both Dl and N are transmembrane proteins. EBs attach to ISCs in order to let Dl expressed in ISCs to activate the N signal transduction pathway in EBs. E-cadherin (E-cad) has been reported to be required for stable attachment between EBs and ISCs [33, 34]. Choi et al. have demonstrated that nutrient deprivation and reduced insulin signaling suppress ISC proliferation due to prolonged contact between ISCs and newly formed daughter (EBs) [34]. They further showed that the disruption of cell adhesion through down-regulating the cell adhesion molecule E-cadherin (E-cad) can lead to increased ISC proliferation and potential increase in symmetric division [33, 34].
5.4.3. JAK-STAT Signaling Regulates ISC Proliferation and Differentiation
The JAK-STAT signal-transduction pathway also plays a key role in ISC regulation [29, 35–39]. The ligand of the Drosophila JAK-STAT signal-transduction pathway is provided by one of three leptin-like (IL-6 family) cytokines called the Unpaireds (Upd, Upd2, and Upd3) [40, 41]. JAK-STAT signal-transduction pathway is activated in both ISCs and EBs: in ISCs, JAK-STAT signaling regulates ISC proliferation; in EBs, the signaling regulates EB differentiation. JAK-STAT signaling can regulate ISC proliferation and EB differentiation through either independent mechanism or by suppressing N signal transduction pathway (Fig. 5.4).
Cells in the intestine are constantly exposed to numerous insults, from tissue damage to bacterial infection, resulting in a constant turn-over. It was recently shown that these events initially affect differentiated ECs, causing either EC ablation or activated JNK-mediated stress signaling in the ECs [7, 36–38, 42, 43]. The affected ECs signal the stem and progenitor cells to induce compensatory ISC division and differentiation, which is believed to be triggered by the up-regulation of ligands of the JAK-STAT signal transduction pathway. The JAK-STAT ligands, unpaired (Upd, Upd2 and Upd3), are known to be induced in damaged ECs, triggering the activation of the JAK-STAT pathway in ISCs and EBs, resulting in ISC and EB proliferation to replenish the damaged epithelium. Depleting Upd in ECs or blocking the JAK-STAT signaling pathway in ISCs and EB can completely suppress the mitotic response caused by EC damage and render the flies more susceptible to infection, indicating that JAK-STAT signaling is necessary for intestinal regeneration. Interestingly, over-activating JAK-STAT signaling in ISCs and EBs can mimic the proliferation caused by EC damage.
5.4.4. EGFR Signaling Regulates ISC Proliferation
It has recently been reported that the visceral muscle expressed Vein (Vn), one of the EGFR ligands, activates the EGFR signaling pathway in ISCs to regulate ISC proliferation [31, 35, 44]. vn knockdown, specifically in the visceral muscle, causes decreased ISC proliferation [31, 44]. Further, several components of the EGFR signaling pathway, including the three ligands of EGFR [Vn, spitz (Spi), and keren (Kn)], are dramatically induced in regenerating gut epithelium induced by cell death, JNK-mediated stress signaling, and pathogenic bacterial infection-mediated gut regeneration [36, 45]. The activated EGFR regulates ISC proliferation through the RAS/RAF/MAPK pathway for epithelial regeneration. The JAK-STAT and EGFR pathways may cross-talk in regulating ISC proliferation. It has been established that ISC proliferation induced by ectopic Upd can be completely inhibited by down-regulating the EGFR signaling in ISCs; and, likewise, ISC proliferation can be suppressed by blocking JAK-STAT signaling by over-expressing the EGFR ligand Vn [45, 46].
5.4.5. Wingless (WG) Signaling Regulates ISC Proliferation
Lin et al. have reported that the circular muscle cells express Wingless (WG) that can cross the basement membrane and activate the WG signal-transduction pathway in ISCs, thereby regulating ISC self-renewal [20]. They demonstrated that the disruption of WG signaling in flies by a mutation of frizzled (fz), fz2, disheveled (dsh), or armadillo (arm) can result in slower division of ISCs but with faster turn over than wild-type ISCs. Conversely, by the over-activation of WG signaling (by overexpressing wg, expressing constitutively activated arm, or generating null ISC clones of shaggy (sgg)) they observed an increase ISC proliferation that did not disturb ISC differentiation. Using epistatic analysis, Lin et al. further demonstrated that N acts downstream of WG pathway, suggesting that a hierarchy of WG/N signaling pathways controls the balance between the self-renewal and differentiation of ISCs [20]. However, several pieces of evidence dispute this model. First, the loss of Drosophila Adenomatous polyposis coli (Apc) or Axin or the expression of constitutively activated arm (armS10) all activate the WG signal-transduction pathway in the posterior midgut [19]. ISCs that lack Apc or Axin or that express armS10 exhibit disturbed proliferation, but their N-mediated ISC self-renewal is normal [19]. Second, wg is mainly expressed in epithelial cells at the junctions of foregut/midgut and midgut/hindgut [47, 48], and also in a small band of visceral muscle cells of the midgut. It is likely that WG from the small band of visceral muscle cells unlikely regulates the widely distributed ISCs in the midgut.
Taken together, WG signaling may only play a mild role (in comparison with other signals described above) in regulating ISC proliferation and further studies are needed to identify the source of the ligand WG.
5.5. Drosophila and Mammalian ISCs: A Comparison
The Drosophila midgut is a functional equivalent of the mammalian small intestine. The anatomy and cell renewal in the Drosophila midgut is similar to the mammalian small intestine: the intestinal epithelium in both systems is a tube composed of epithelial cells with absorptive and secretory functions; N signaling controls absorptive versus secretory fate decisions in the intestinal epithelium; cell renewal in both systems starts from SCs in the basal cell layer, and the differentiated cells then move toward the lumen. However, it is clear that the SCs in the two systems are regulated in different ways. In the mammalian small intestine, the slowly cycling SCs first generate the rapidly cycling Transit amplifying (TA) daughter cells, which then differentiate into the four terminally differentiated cell types; in the Drosophila midgut, the SCs are the only proliferating cells, and rapidly cycling TA cells do not exist.
Recent advances in mammalian SC research suggests that both quiescent (out of cell cycle and in a low metabolic state) and active (in cell cycle and not able to retain DNA labels) SC subpopulations coexist in several tissues [49, 50]. The two kinds of SCs are usually in separate yet adjoining locations and coordinately function not only to preserve stem cell’s long-term proliferation potential, but also to provide an emergency supply of progeny for sudden injuries or growth spurts. The mouse small intestine also has two types of SCs, the SCs at the +4 position are slow-cycling and label-retaining [51, 52], whereas the crypt base columnar (CBC) at the crypt base are fast-cycling and non-label-retaining [53, 54]. Both +4 position and CBC SCs give rise to all intestinal epithelial lineages. It has recently been demonstrated that the +4 position SCs could give rise to the CBC SCs and the CBC SCs could also convert into +4 position SCs, suggesting a bidirectional lineage relationship between quiescent and active SCs in intestine [55, 56]. However, a second type of SCs has not yet been identified in the Drosophila intestine. It is not known whether two types of SCs also coordinately maintain tissue homeostasis in the adult Drosophila digestive system.
Although WG signaling has a role in the Drosophila midgut, the function of the WNT signaling in mouse small intestine is quite different from that in the Drosophila midgut. WNT signaling regulates SC self-renewal and blocks SC differentiation. Overactivation of the WNT signaling results in the formation of SC tumor; the WG signaling regulates only the SC proliferation and not the SC self-renewal and daughter cell fate determination. The outcome of perturbing WG signaling in the Drosophila midgut is much less severe than that observed following perturbations of the WNT signal in the mammalian small intestine.
The Hedgehog (HH)-BMP relay signaling from the crypt or intervillus pocket delivers a long-range signal to both inhibit the formation of crypts and promote the formation of villi in mouse small intestine. No such function for the HH-BMP signaling has been reported to date in the Drosophila midgut.
N signaling seems to have opposite functions in stem cells in the mammalian and Drosophila intestinal epithelium: blocking N activity in mice causes the depletion of the progenitor cell compartment by promoting differentiation; in the Drosophila midgut, it induces overproliferation of SCs due to impaired differentiation. Elevating N signaling leads to expansion of the progenitor cells in the mammalian crypt but induces the SC differentiation in the Drosophila midgut.
In the mammalian small intestine, the localization and sorting of SCs, TA cells, and terminally differentiated cells is regulated by the Eph/ephrin signaling. However, signaling pathway(s) that regulate cell distribution in the Drosophila midgut have not been identified to date. It will be interesting to find whether the Drosophila counterpart of the Eph/ephrin signaling or a different signaling controls the sorting process in the Drosophila intestine.
In the mammalian intestine SC system, several SC markers (such as Lgr5, Prominin 1, and Bmi) are currently available, the techniques used to trace SC lineage have been developed, and the SC identities have been well characterized. In the Drosophila gut, the SC lineages have been established and several powerful tools are available to perform genetic manipulations in the SCs. The pace of advances in the study of intestinal SCs will be accelerated in the next few years through combining the mouse genetic manipulation and in vitro culture with the powerful Drosophila genetic screens.
5.6. Other Adult Stem Cells
In addition to ISCs identified in the posterior midgut, SCs are also identified in other locations of the adult Drosophila digestive system (Fig. 5.5).
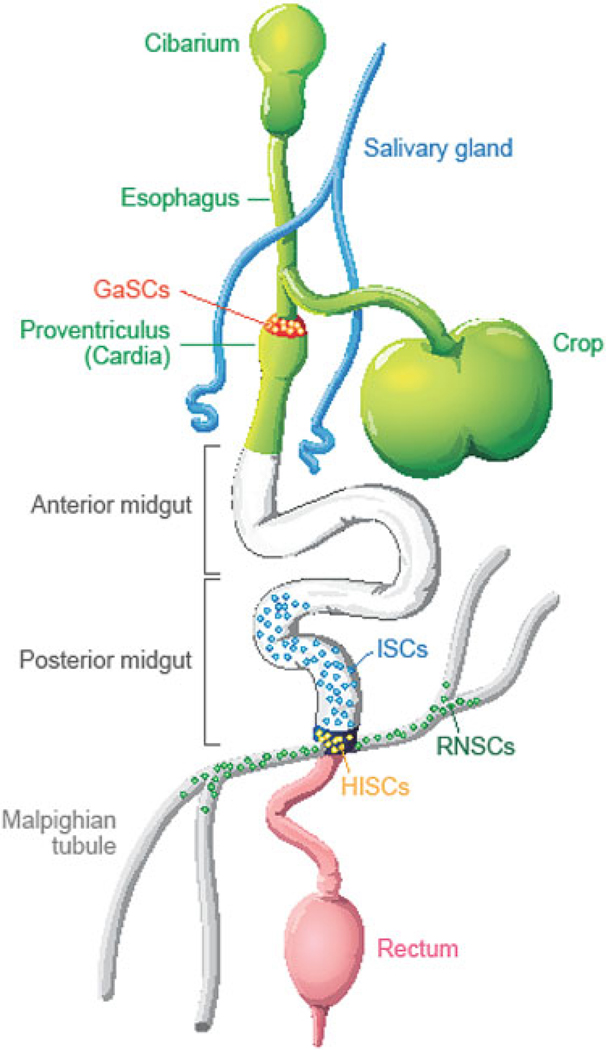
Fig. 5.5. Stem cells in adult Drosophila digestive system.
Schematic diagram of Drosophila digestive system including cardia, anterior midgut, posterior midgut, malpighian tubules, hindgut and rectum. Five types of region and organ-specific multipotent adult stem cells have been identified in the Drosophila digestive system: intestinal stem cells (ISCs) in the posterior midgut; hindgut intestinal stem cells (HISCs) at the midgut/hindgut junction; renal and nephric stem cells (RNSCs) in the Malpighian Tubules; type I gastric stem cells (GaSCs) at foregut/midgut junction; and type II gastric stem cells (GSSCs) at the middle of the midgut
5.6.1. The Multipotent Renal and Nephric Stem Cells (RNSCs) in Malpighian Tubules
The Drosophila Malpighian tubules (MTs) are connected to the midgut/hindgut junction and act as the excretory and osmoregulatory organ system (fly kidney). MTs consist of three domains: the ureter, lower tubule, and upper tubule. Multipotent renal and nephric stem cells (RNSCs) were identified in the ureter and lower tubules using a positively marked mosaic lineage-labeling technique and cellular markers [57]. There are ~100 RNSCs in one pair of anterior MTs that express the esg-lacZ and Stat-GFP (a reporter of the JAK-STAT signaling) markers. RNSCs undergo asymmetric division to give rise to a RNSC and a renal blast (RB). The RB can either differentiate into a mature renalcyte (RC) in the ureter/lower tubule or move toward the distal upper tubule to differentiate into principal and stellate cells [57, 58]. The autocrine JAK-STAT signaling regulates RNSC proliferation and self-renewal. Over-activation of JAK-STAT signaling by overexpressing Upd leads to a dramatic increase in RNSCs. On the contrary, the loss of JAK-STAT signaling function results in premature RNSC differentiation [58]. Further, mutations causing the loss of tumor suppressor salvador (sav) or scrrible (scr) or activation of the oncogene Ras can transform normal RNSCs into cancer SC-like cells [30]. In wild-type MTs, each SC generates one self-renewing and one differentiating daughter cell. However, in flies with loss-of-function sav or scrib or gain-of-function Ras mutations, both daughter cells grow and behave like SCs, leading to the formation of tumors in MTs (Fig. 5.6). Ras functions down- stream of Sav, Scrib, as well as the JAK-STAT signal transduction pathway in regulating SC transformation. The Ras-transformed SCs exhibited many of the hallmarks of cancer, such as increased proliferation, reduced cell death, failure to differentiate, and enhanced migration, through the up-regulation of Cyclin E, dMyc, DIAP, MMP1, and several other genes. Several signal transduction pathways (including MEK/MAPK, RhoA, PKA, and TOR) cooperatively mediate the function of Ras in the SC transformation.
Fig. 5.6. Ras-induced stem cell tumor.
(a) GFP labeled RNSC lineage Malpighian tubules. (b) Expression of Rasv12 in RNSC leads to stem cell tumors in Malpighian tubules
5.6.2. The Quiescent Hindgut Intestine Stem Cells (HISCs)
The Drosophila hindgut is functionally similar to the mammalian large intestine/colon and comprises three structures: the pylorus, ileum, and rectum [6, 59]. Based on lineage tracing and BrdU-labeling experiments, the hindgut intestine stem cells (HISCs) are identified in an anterior narrow segment, named the hindgut proliferation zone (HPZ) [11]. HISCs express the Stat-GFP and Wg markers. Within the HPZ, the anteriorly expressed WG functions as a niche signal to maintain HISCs in a slow-cycling, self-renewing mode. The slowly proliferating HISCs then gives rise to fast-proliferating progeny similar to TA cells in the mammalian crypt. These fast proliferating cells migrate posteriorly and enter into the posterior of the HPZ where the HH signal drives them out of the cell cycle to the onset of differentiation. However, other studies based on clonal marking and BrdU incorporation have shown no active SCs and little cell turnover in adult hindgut tissue and the adult hindgut is not generated by SCs at the anterior of the pylorus during larval/pupal development [59]. Fox and Spradling further found that the HISCs are quiescent and only proliferate to replenish lost cells in response to severe hindgut epithelium damage.
5.6.3. The Active Adult Gastric Stem Cells (GaSCs) at the Foregut/Midgut Junction
The Drosophila cardia (proventriculus) is located at the foregut/midgut junction and functions as a gastric valve. The cardia, anterior midgut, and crop together function as a stomach in Drosophila. Using clonal analysis and molecular marker labeling, multipotent gastric stem cells (GaSCs) were identified at the foregut/midgut junction in the cardia (proventriculus) [47]. GaSCs express the Stat-GFP and Wg markers and are actively dividing (double in ~ every 2 days). GaSCs can generate differentiated daughter cells that migrate either upward to anterior midgut or downward to esophagus and crop. GaSCs have some similar features with HISCs. GaSCs also first give rise to fast-proliferating TA-like cells which then differentiate into terminally differentiated cells. WG signaling regulates GaSC self-renewal, HH signaling regulates GaSC differentiation, and JAK- STAT signaling regulates GaSCs proliferation.
5.6.4. The Quiescent Adult Gastric Stem Cells (GSSCs) at Middle of the Midgut
The adult Drosophila copper cells are located in the middle of midgut and function as acid-secreting cells similar to mammalian gastric parietal cells. Multipotent SCs have been recently identified based on cell lineage tracing and genetic analysis [60]. The SCs can produce the acid-secreting copper cells, interstitial cells, and EE cells. Since the copper cells perform part of the stomach functions by secreting acid, the multipotent SCs were also named gastric stem cells (GSSCs). The GSSCs express escargot (esg) marker and are largely quiescent but can be induced to regenerate the gastric epithelium in response to environmental challenge. WG signaling may regulate GSSC maintenance.
In summary, the Drosophila digestive system is maintained by region and organ-specific multipotent SCs. These SCs share certain molecular markers and signaling pathways and yet each has unique properties. STAT-GFP is a marker of ISCs, RNSCs, GaSCs, and HISCs but not GSSCs; ESG is a marker of ISCs, RNSCs, and GSSCs but not HISCs and GaSCs; WG is a marker of HISCs and GaSCs but not ISCs, RNSCs, and GSSCs. JAK-STAT pathway regulates SC proliferation and works in combination with other signals to control SC fates in the four types of digestive SCs (ISCs, RNSCs, HISCs, and GaSCs). The other signals can be different in different SC systems. For example, JAK-STAT signaling mainly collaborates with the N signaling in ISCs [7, 39, 42], with the Ras-Raf signaling in RNSCs [30], with WG and HH signaling in GaSCs [47], and HISCs [48, 59]. Further, each type of SCs has different degree of quiescence. ISCs divide once every 24 h [3, 4], GaSCs divide once every 48 h [47], RNSCs divide once in about 1 week [30, 57], and the quiescent HISCs and GSSCs only divide during stress-induced tissue repair [48, 59, 60]. The uniqueness and diversity of SCs in the Drosophila digestive system provides an ideal genetic model system to study SC biology and future studies using the system will pave the way for significant implications of SCS in regenerative medicine to alleviate human health.
5.7. Perspective: Future Direction
The past 6 years have witnessed the discovery of five types of SCs in adult Drosophila digestive system and the signal transduction pathways that regulate the behavior of these SCs, uncovering crucial roles of SCs in tissue regeneration and animal aging.
Nevertheless, many open questions remain to be answered. For example, the differentiation of AMPs is in accord with metamorphosis progression that is regulated by morphogenetic hormones like 20-hydroxy ecdysone and juvenile hormone. We still do not know how the hormone signals are connected to AMP differentiation. In adult Drosophila posterior midgut, asymmetric N signaling from ISC to EB regulates ISC proliferation and asymmetric division. Dl is only expressed in ISCs and N signaling is only activated in EBs. We still do not know what regulates Dl ISC-specific expression. N is expressed in both ISCs and EBs, it is still unclear why the signaling is only activated in EBs. Besides the core N signaling, other signals including InR, JNK, JAK-STAT, EGFR, and WG signals also regulate ISC proliferation, asymmetric division, and differentiation. These signals and N signal must interplay to decide the final outcomes on ISCs. But we still do not know how exactly these signals interact and cross-talk with N signal transduction pathway. For example, InR signaling regulates ISC proliferation and asymmetric division. InR can achieve the outcomes either through directly blocking N signaling at some points in the N signal transduction pathway or through regulating duration of the E-cad-mediated ISC-EB connection. Further studies are necessary to solve this puzzle.
In comparison with ISCs, other four types of SCs have been studied barely. The five types of SCs have unique properties, are at different locations, have varied degrees of quiescence, and are regulated by various signal transduction pathways. Further studies to compare these SCs are necessary and important to fully understand SCs. Particularly, a constitutively activated form of Ras (RasV12) can only transform the fly kidney RNSCs but not other SCs to cancer SC-like cells. The unique backgrounds or combinations of signal transduction pathways may determine the outcomes of individual SCs. Further studies of this phenomenon may help us to understand why some oncogenes or tumor suppressors only affect tumor formation in certain organs. Furthermore, both quiescent and active SC subpopulations coexist in several tissues in mammals. The two kinds of SCs are usually in separate yet adjoining locations and coordinately function not only to preserve long-term proliferation potential of SCs, but also to provide an emergency supply of progeny for sudden injuries or growth spurts. However, the quiescent/active SC pair has not yet been identified in the Drosophila digestive system. It is interesting to find out whether such an arrangement exist or not in the Drosophila. Nonetheless, we anticipate that the SC research using the adult fly digestive system in next few years will play an important role in our understanding SC biology in general and SC applications in regenerative medicine and cancer treatment.
References
- 1.Weissman IL (2000) Stem cells: units of development, units of regeneration, and units in evolution. Cell 100(1):157–168 [DOI] [PubMed] [Google Scholar]
- 2.Hakim RS, Baldwin K, Smagghe G (2010) Regulation of midgut growth, development, and metamorphosis. Annu Rev Entomol 55:593–608 [DOI] [PubMed] [Google Scholar]
- 3.Saric A, Kalafatic M, Rusak G, Kovacevic G et al. (2007) Postembryonic development of Drosophila melanogaster Meigen, 1830 under the influence of quercetin. Entomol News 118(3):235–240 [Google Scholar]
- 4.Slama L, Farkas R (2005) Heartbeat patterns during the postembryonic development of Drosophila melanogaster. J Insect Physiol 51(5):489–503 [DOI] [PubMed] [Google Scholar]
- 5.Yamashita Y (2009) Asymmetric stem cell division and pathology: insights from Drosophila stem cell systems. J Pathol 217(2):181–185 [DOI] [PubMed] [Google Scholar]
- 6.Xie T (2009) Stem cell in the adult Drosophila hindgut: just a sleeping beauty. Cell Stem Cell 5(3):227–228 [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Edgar BA (2009) EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136(3):483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathur D, Bost A, Driver I, Ohlstein B (2010) A transient niche regulates the specification of Drosophila intestinal stem cells. Science 327(5962):210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micchelli CA, Sudmeier L, Perrimon N, Tang S et al. (2011) Identification of adult midgut precursors in Drosophila. Gene Expr Patterns 11(1–2):12–21 [DOI] [PubMed] [Google Scholar]
- 10.Micchelli CA (2012) The origin of intestinal stem cells in Drosophila. Dev Dyn 241(1):85–91 [DOI] [PubMed] [Google Scholar]
- 11.Takashima S, Adams KL, Ortiz PA, Ying CT et al. (2011) Development of the Drosophila enteroendocrine lineage and its specification by the Notch signaling pathway. Dev Biol 353(2):161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takashima S, Younossi-Hartenstein A, Ortiz PA, Hartenstein V (2011) A novel tissue in an established model system: the Drosophila pupal midgut. Dev Genes Evol 221(2):69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takashima S, Younossi-Hartenstein A, Ortiz PA, Hartenstein V (2011) A novel tissue in an established model system: the Drosophila pupal midgut. Dev Genes Evol 221(2):69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carel JC, Leger J (2008) Clinical practice. Precocious puberty. N Engl J Med 358(22):2366–2377 [DOI] [PubMed] [Google Scholar]
- 15.Issigonis M, Matunis E (2010) Previews niche today, gone tomorrow—progenitors create short-lived niche for stem cell specification. Cell Stem Cell 6(3):191–193 [DOI] [PubMed] [Google Scholar]
- 16.King-Jones K, Thummel CS (2005) Nuclear receptors—a perspective from Drosophila. Nat Rev Genet 6(4):311–323 [DOI] [PubMed] [Google Scholar]
- 17.Minakuchi C, Zhou X, Riddiford LM (2008) Kruppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech Dev 125(1–2):91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thummel CS (1996) Flies on steroids—Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet 12(8):306–310 [DOI] [PubMed] [Google Scholar]
- 19.Lee WC, Beebe K, Sudmeier L, Micchelli CA (2009) Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development 136(13):2255–2264 [DOI] [PubMed] [Google Scholar]
- 20.Lin G, Xu N, Xi R (2008) Paracrine wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455(7216):1119–1123 [DOI] [PubMed] [Google Scholar]
- 21.Ohlstein B, Spradling A (2007) Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315(5814):988–992 [DOI] [PubMed] [Google Scholar]
- 22.Perdigoto CN, Schweisguth F, Bardin AJ (2011). Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development 138(21):4585–4595 [DOI] [PubMed] [Google Scholar]
- 23.Bardin AJ, Perdigoto CN, Southall TD, Brand AH et al. (2010) Transcriptional control of stem cell maintenance in the Drosophila intestine. Development 137(5):705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knoblich JA (2008) Mechanisms of asymmetric stem cell division. Cell 132(4):583–597 [DOI] [PubMed] [Google Scholar]
- 25.Hutterer A, Knoblich JA (2005) Numb and alpha-adaptin regulate sanpodo endocytosis to specify cell fate in Drosophila external sensory organs. EMBO Rep 6(9):836–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Borgne R, Schweisguth F (2003) Unequal segregation of neuralized biases Notch activation during asymmetric cell division. Dev Cell 5(1):139–148 [DOI] [PubMed] [Google Scholar]
- 27.Neumuller RA, Knoblich JA (2009) Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev 23(23):2675–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajan A, Tien AC, Haueter CM, Schulze KL et al. (2009) The Arp2/3 complex and WASp are required for apical trafficking of delta into microvilli during cell fate specification of sensory organ precursors. Nat Cell Biol 11(7):815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beebe K, Lee WC, Micchelli CA (2010) JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol 338(1):28–37 [DOI] [PubMed] [Google Scholar]
- 30.Zeng X, Singh SR, Hou D, Hou SX (2010) Tumor suppressors Sav/Scrib and oncogene Ras regulate stem-cell transformation in adult Drosophila malpighian tubules. J Cell Physiol 224(3):766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biteau B, Jasper H (2011) EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development 138(6):1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien LE, Soliman SS, Li X, Bilder D (2011) Altered modes of stem cell division drive adaptive intestinal growth. Cell 147(3):603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda K, Takemura M, Umemori M, Adachi-Yamada T (2008) E-cadherin prolongs the moment for interaction between intestinal stem cell and its progenitor cell to ensure Notch signaling in adult Drosophila midgut. Genes Cells 13(12):1219–1227 [DOI] [PubMed] [Google Scholar]
- 34.Choi NH, Lucchetta E, Ohlstein B (2011) Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc Natl Acad Sci USA 108(46):18702–18707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin G, Xu N, Xi R (2010) Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of Drosophila intestinal stem cells. J Mol Cell Biol 2(1):37–49 [DOI] [PubMed] [Google Scholar]
- 36.Buchon N, Broderick NA, Kuraishi T, Lemaitre B (2010) Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol 8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchon N, Broderick NA, Poidevin M, Pradervand S et al. (2009) Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5(2):200–211 [DOI] [PubMed] [Google Scholar]
- 38.Cronin SJ, Nehme NT, Limmer S, Liegeois S et al. (2009) Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325(5938):340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu W, Singh SR, Hou SX (2010) JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem 109(5):992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arbouzova NI, Zeidler MP (2006) JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 133(14):2605–2616 [DOI] [PubMed] [Google Scholar]
- 41.Harrison DA, McCoon PE, Binari R, Gilman M et al. (1998) Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev 12(20):3252–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ et al. (2011) EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8(1):84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amcheslavsky A, Jiang J, Ip YT (2009) Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4(1):49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu N, Wang SQ, Tan D, Gao Y et al. (2011) EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol 354(1):31–43 [DOI] [PubMed] [Google Scholar]
- 45.Iordanou E, Chandran RR, Blackstone N, Jiang L (2011) RNAi interference by dsRNA injection into Drosophila embryos. J Vis Exp (50):2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Losick VP, Morris LX, Fox DT, Spradling A (2011) Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell 21(1):159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh SR, Zeng X, Zheng Z, Hou SX (2011) The adult Drosophila gastric and stomach organs are maintained by a multipotent stem cell pool at the foregut/midgut junction in the cardia (proventriculus). Cell Cycle 10(7):1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR et al. (2008) The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454(7204):651–655 [DOI] [PubMed] [Google Scholar]
- 49.Blanpain C, Fuchs E (2009) Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10(3):207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L, Clevers H (2010) Coexistence of quiescent and active adult stem cells in mammals. Science 327(5965):542–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Potten CS (1974) The epidermal proliferative unit: the possible role of the central basal cell. Cell Tissue Kinet 7(1):77–88 [DOI] [PubMed] [Google Scholar]
- 52.Sangiorgi E, Shuhua Z, Capecchi MR (2008) In vivo evaluation of PhiC31 recombinase activity using a self-excision cassette. Nucleic Acids Res 36(20): e134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng H, Leblond CP (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141(4):537–561 [DOI] [PubMed] [Google Scholar]
- 54.Barker N, van Es JH, Kuipers J, Kujala P et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449(7165): 1003–1007 [DOI] [PubMed] [Google Scholar]
- 55.Takeda N, Jain R, LeBoeuf MR, Wang Q et al. (2011) Interconversion between intestinal stem cell populations in distinct niches. Science 334(6061): 1420–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang J, Tian L, Peng C, Abdou M et al. (2011) DPP- mediated TGFbeta signaling regulates juvenile hormone biosynthesis by activating the expression of juvenile hormone acid methyltransferase. Development 138(11):2283–2291 [DOI] [PubMed] [Google Scholar]
- 57.Singh SR, Liu W, Hou SX (2007) The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell 1(2):191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh SR, Hou SX (2008) Lessons learned about adult kidney stem cells from the malpighian tubules of Drosophila. J Am Soc Nephrol 19(4):660–666 [DOI] [PubMed] [Google Scholar]
- 59.Fox DT, Spradling AC (2009) The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell 5(3):290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strand M, Micchelli CA (2011) Quiescent gastric stem cells maintain the adult Drosophila stomach. Proc Natl Acad Sci USA 108(43):17696–17701 [DOI] [PMC free article] [PubMed] [Google Scholar]