Abstract
Stem cells have an enormous capacity of self-renewal, as well as the ability to differentiate into specialized cell types. Proper control of these two properties of stem cells is crucial for animal development, growth control, and reproduction. Germline stem cells (GSCs) are a self-renewing population of germ cells, which generate haploid gametes (sperms or oocyte) that transmit genetic information from generation to generation. In Drosophila testis and ovary, GSCs are anchored around the niche cells. The cap cells cluster in females and hub cells in males act as a niche to control GSC behavior. With highly sophisticated genetic techniques in Drosophila, tremendous progress has been made in understanding the interactions between stem cells and niches at cellular and molecular levels. Here, we provide details of genetic, immunofluorescence labeling, and in situ hybridization techniques in identification and characterization of stem cells in Drosophila male and female germline niches.
Keywords: Drosophila, Testis, Ovary, Germline stem cells, Niches
1. Introduction
In recent years, the stem cell field has opened a new venue in regenerative and reproductive medicine. Stem cells have an enormous ability to self-renew as well as produce diverse types of differentiated cells [1–4]. Stem cells provide an opportunity to dissect the cellular and molecular mechanisms controlling embryonic development, cellular differentiation, and organ maintenance and also have great potential in developing novel cell-based therapies. In order for stem cells to function properly, a tight balance between proliferation and differentiation should be maintained because over-proliferation of stem cells results in tumor formation [5], while under-proliferation results in loss of stem cell population, which results in an inability to form the specific tissue or organ [1, 6]. A large body of research suggests that stem cells are regulated by specific microenvironments, known as niches, which is a subset of neighboring stromal cells and extracellular substrates. The stromal cells usually secrete growth factors to regulate stem cell function [1].
Germline stem cells (GSCs) serve as a reservoir for the continuous production of gametes in all organisms. The existence of stem cells in the germline was proposed over a century ago [7]. Recent studies in C. elegans, Drosophila, and mouse have provided detailed molecular mechanisms, which regulate GSC division and maintenance. GSCs are known to exist in the testes and ovaries of all animal species [1, 6, 8–10]. Although the niche hypothesis was first postulated for hematopoietic stem cells, the GSC niches in Drosophila are the best studied because of well-defined structures and availability of molecular markers [2, 6, 9]. Using Drosophila as a model organism, tremendous progress has been made in understanding molecular mechanisms underlying interactions between stem cells and niches. GSCs are present in the gonads of Drosophila females and males. The proper maintenance and correct differentiation of GSCs are essential for fertility and fecundity.
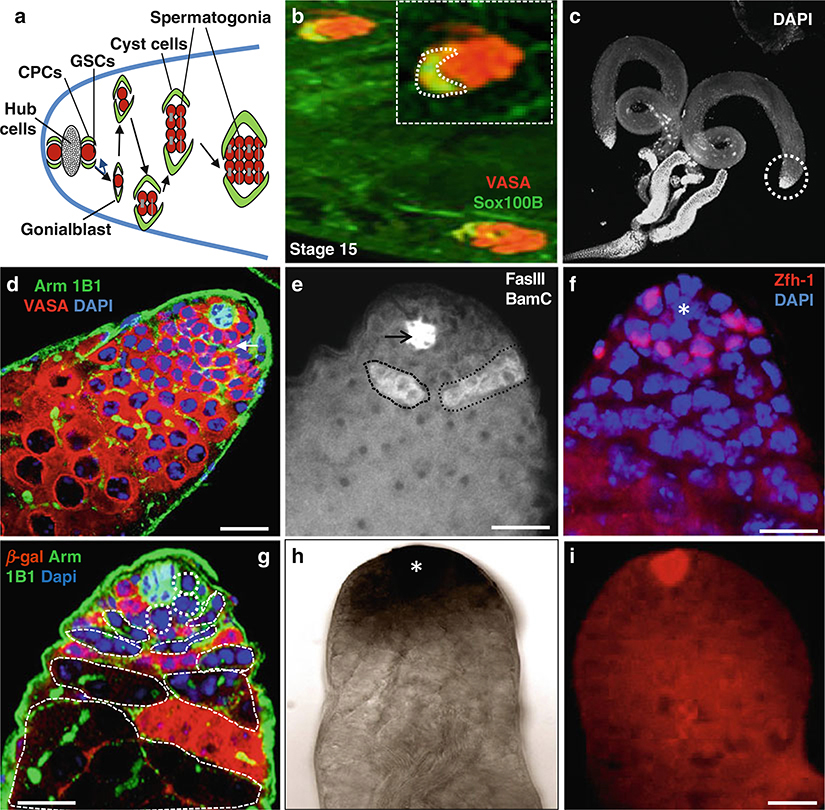
The Drosophila testis provides an excellent in vivo system to study stem cells’ niche interactions at the cellular and molecular levels [3, 11–33]. In Drosophila males, the stem cell niche and the germline and somatic stem cells (also known as cyst progenitor cells (CPCs)) are located at the closed anterior apex of each testis. Each testis has 5–9 GSCs that are encysted by two CPCs. Both GSCs and CPCs are physically attached to a group of 12 nondividing somatic cells called the hub [6, 11–15] (Fig. 1), a niche structure, which supports the self-renewal of GSCs and CPCs [6]. Each GSC divides in an asymmetric way with the mitotic spindle orientated perpendicular to the hub [12–14]. One of the daughter cells remains in contact with the hub, inherits the mother centriole, and retains GSC identity, while the other daughter cell, called a gonialblast (GB), inherits the daughter centriole and initiates differentiation (Fig. 1) [12–14]. Similarly, CPCs self-renew and produce daughters, which differentiate into somatic cyst cells (SCC) [18, 19]. CPCs also produce hub cells [20]. GSCs and gonialblasts contain a spectrosome. The gonialblast will undergo four rounds of mitotic division with incomplete cytokinesis to form 16 interconnected spermatogonia, which contain a branched fusome. SCC will grow without further division; but they become elongated, and form a thin layer around the spermatogonial cyst [21–27]. However, the germ cells form spermatocytes that will increase in size and ultimately undergo meiosis and differentiate into sperm [27]. The adhesion between niche cells and stem cells controls self-renewal in the Drosophila testis germline [6, 11–33]. In addition, there are several signaling pathways known to control the behavior of GSCs/CPCs and their niches in Drosophila testes [ 11–42 ].
Fig. 1.
Immunostaining and in situ hybridization of Drosophila testes. (a) Schematic diagram highlights the tip of the testes, which usually contain five to nine GSCs; only two GSCs are shown in this diagram, surrounded by about twice as many cyst progenitor cells (CPCs). Both GSCs and CPCs anchor around the hub cells, which act as niche cells. The testis proliferation center consists of hub, GSCs, CPCs, gonialblasts, and 2- to 16-cell spermatogonia. (b) Wild-type stage 15 embryonic testes stained with anti-Vasa (red ) to mark the germline and anti-Sox100B (green) representing the male- specific somatic gonadal precursor (msSGP) cells. (c) Wild-type testes stained with DAPI. (d) Wild-type testis stained with anti-Arm (green) to mark the hub cells, 1B1 to mark the spectrosomes and fusome (green), and anti-Vasa (red) marks all germ cells including GSCs. (e) Wild-type testis stained with anti-BamC to mark the spematogonial cells (white color positive cells inside black dotted lines) and anti-FasII to mark hub cells (black arrow). (f) Wild-type testis stain with anti-Zfh-1 (red) to mark cyst stem cells. (g) Wild type 6 days after heat shock GSC clones highlighted by dotted lines. Testis stained with anti-β-galactosidase (red), anti-Arm and anti1b1 (green). (h) Wild-type testis with Gef26 mRNA expression. (j) Wild-type testis with upd mRNA expression (red) using Fast red FISH. Dapi marks DNA (blue) in (d, f, g). Scale bars: 20 μm (b); 10 μm (d-G, I)
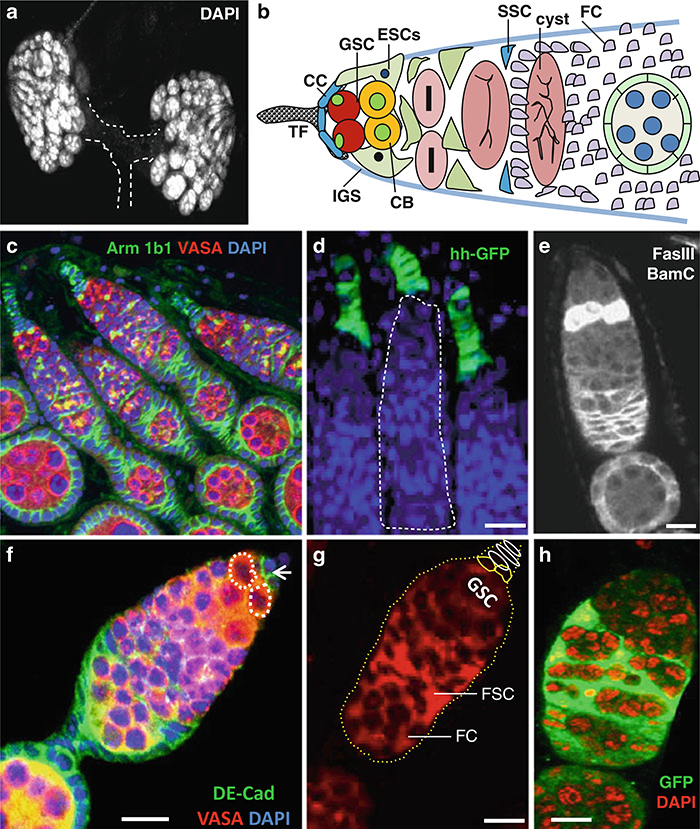
The adult Drosophila contains two ovaries and each ovary is composed of approximately 16–20 tubes called ovarioles, each with a specialized structure called germarium. The anterior tip of each germarium contains three types of stem cells: GSCs, escort stem cells (ESCs), and follicle stem cells (FSCs). The female GSC niche contains 5–7 nondividing somatic cap cells, which physically anchor 2–3 GSCs in each germarium [43–45]. There are 8–10 terminal fi lament (TF) cells anterior to cap cells connecting the germarium with the inner germarium sheath (IGS) cells (Fig. 2).
Fig. 2.
Immunostaining and in situ hybridization of Drosophila ovary. (a) Wild-type ovary stained with DAPI. (b) Schematic diagram highlights the tip of the ovary, which contains 2–3 GSCs and types of cells, depicted in the figure. (c) Wild-type ovary stained with anti-Arm (green) to mark the cap cells, anti-1B1 (green) to mark the fusome and spectrosomes (green), as well as niche cells and anti-Vasa (red) mark all germ cells including GSCs. (d) hh-Gal4 UAS-GFP line stained with anti-GFP (green) marks the terminal fi lament cells and cap cells. (e) Wild-type ovary stained with anti-Decad (green) and anti-Vasa (red). (f) Wild-type ovary stained with anti-BamC to and anti-FasIII. (g) ) Wild-type ovary with Gef26 mRNA expression. (h) Wild type 6 days after heat shock somatic stem cells clones (GFP-green), Dapi marks DNA (red). Dapi marks DNA (blue) in (c, d, f). Scale bars: 50 μm (c); 20 μm (d); 10 μm (e-h)
Through asymmetric division, GSC produces self-renewing GSC, and a differentiating daughter cell called cystoblast (CB), which moves away from niche and forms an interconnected 16-cell cyst by incomplete cytokinesis. The cystoblasts move away from the niche wrapped by differentiated escort cells produced from ESCs. The cyst cells are encysted by the escort cells until they reach the 16-cell stage. Only 1 out of 16 germ cells can become an oocyte and the remaining cells will then become nurse cells to support the growth of the oocyte. Studies have shown that cap cells and ESCs interact together to form the GSC niche [43]. Furthermore, it has been shown that DE cadherin is required for anchoring GSCs in their niche [44]. Several signaling pathways are responsible for niche stem cells’ interaction and maintenance in the Drosophila ovary [29–45]. There are 4–6 ESCs and their progeny are called escort cells [42]. Each germarium contains 40–50 IGS cells [26] and about 18 escort cells [42]. In addition, there are 2–3 FSCs located in the middle of each germarium across from each other. FSCs divide and produce a population of mitotically active follicle progenitor cells, which proliferate in the egg chambers of stage 1 to stage 6, and produce several types of differentiated cells that cover egg chambers including a follicle cell monolayer [43]. IGS cells and cap cells act as an FSC niche and FSC behavior is regulated by several signaling pathways [26, 27, 44]. In this chapter, we provide the protocols (immunostaining, generation of germline and somatic clones, and in situ hybridization, see Figs. 1 and 2) to identify and characterize germline and somatic stem cells in the Drosophila testis and ovary.
2. Materials
Prepare and store all reagents at room temperature (unless otherwise indicated).
2.1. Culturing Drosophila
Control and transgenic Drosophila lines.
Drosophila food: 175 g Brewer’s yeast, 525 g corn meal, 103 g sugar, 75 g agar, 17.5 g baker’s yeast, and methyl paraben solution (add 26.6 g methyl paraben in 15.4 propionic acid and 105 ml ethanol—dissolve by heating and stir). To prepare the fly food add 7.91 l of double-distilled water to the above gradients and mix well, autoclave for 45 min, take out, and mix thoroughly. When cooked food temperature is 85 °C add the methyl paraben solution and mix well.
Autoclave for food preparation.
Fly food dispenser.
Fly plastic vials.
Plastic bottles.
Foam or cotton plugs.
Morgue containing 70 % alcohol for discarding the dead flies.
Fly trap to make sure that flies escaped during crossing have been trapped.
Fly culture incubators (18, 25, and 29 °C).
2.2. Isolation of Testis and Ovary
3–5-day-old male and female flies of control (Oregon-R) and transgenic lines.
Dissecting solution ( Drosophila Ringer’s solution): 130 mM NaCl, 4.7 mM KCl, 1.9 mM CaCl2, and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 6.9. Dissolve 7.5 g NaCl, 0.35 g KCl, 0.21 g CaCl2, and 2.38 g HEPES in approximately 1 l distilled water and stir to dissolve. Adjust to pH 7.2 with 1 N HCl and make the final volume of 1 l with distilled water. Store the dissecting solution in a glass bottle at 4 °C or it can be stored at room temperature for a short time.
Drosophila anesthesia CO2 station.
Drosophila CO2 fly pads.
Paint brush.
Dissecting tweezers.
Glass microslides.
Plastic dropper.
Kimwipes.
Dissecting microscope.
Ice.
70 % (v/v) ethanol.
Pipet (20, 200, 1,000 μl).
Pipet tips (200, 1,000 μl).
2.3. Generation of Germline and Somatic Clones
Stocks for generating FLP-mediated recombination germline clones: hsFLP; FRT82B arm-lacZ; FRT40A arm-lacZ. These stocks are available from the Bloomington Stock Center (http://www.flybase.org).
Stocks for generating CPC clones using MARCM system: c587-Gal4.UAS-2XEYFP/FM7; FRT40A-tub-Gal80/Cyo; FRT40A-w+/Cyo; +/TM3, Sb, hs-Flp. For details, see ref. 33.
37 °C water bath tank for heat-shock regime.
25 °C incubator to maintain fly crosses.
Antibodies: Mouse or rabbit anti-GFP and mouse or rabbit anti-β-galactosidase.
2.4. Immunostaining and Microscopy of Testis and Ovary
Phosphate-buffered saline (PBS): 130 mM NaCl, 7 mM Na2HPO4, 3 mM NaH2PO4, and adjust to pH 7.4 with HCl. Store at room temperature. For longer stability of the solution, store at 4 °C.
Triton X-100 or Tween-20 (Sigma).
PBX solution: 0.1 % Triton X-100.
Fixation solution: 4 % (w/v) paraformaldehyde in 1× PBX.
Gloves.
Parafilm.
Eppendorf tubes.
15-ml conical tubes.
1.5-ml microcentrifuge tubes.
Centrifuge.
Blocking solution: 2 % normal goat serum (Vector laboratories) in 1× PBX. Store at 4 °C.
Bovine serum albumin (BSA; Sigma).
Minivortex (VWR Scientific Products).
Tube shaker.
Aluminum foil.
Microcentrifuge tube rack (Fisher Scientific).
Primary antibodies: Rabbit anti-Vasa (1:2,000), Mouse anti1b1 (1: 20), Rat anti-Decad (DSHB, 1:20), Mouse anti-FasIII (DSHB, 1:100), Mouse anti-Arm (DSHB, 1:200), Mouse anti-BamC (DSHB, 1:10), Guinea pig anti-Zfh-1 (1:4000), Rat anti-Tj (1:400), Rabbit anti-Sox 100B (1:1,000), Rabbit or Mouse anti-β-galactosidase (Invitrogen, 1:500), and Rabbit or Mouse anti-GFP (Invitrogen, 1:500) for GFP-fusion protein lines. The above antibodies can be stored at 4 °C for short term. For long- term storage, use −20 °C with 50 % glycerol or −80 °C. For details about the antibodies used in GSCs, see refs. [33–61].
Secondary antibodies: Goat anti-Mouse, Goat anti-Guinea pig, Goat anti-Rat, and Goat anti-Rabbit, conjugated to Alexa Fluor 488 or Alexa Fluor 594 or Texas Red (Invitrogen). Store in the dark at 4 °C. Use 1:200–500 dilutions in 1× PBX.
DAPI (4,6-Diamidino-2-phenyldole dihydrochloride) (Invitrogen) to stain DNA. Store in the dark at 4 °C.
50 % glycerol in 1× PBS.
4, –20, and −80 °C freezers.
Permanent marker.
Microscope cover glass.
Microscope slides.
Light, fluorescent, and confocal microscope.
Microslide plastic folder.
Computer and software for image processing.
2.5. Collection, Preparation, Fixation, and Staining of Embryos
Drosophila adult flies.
Grape juice.
Culture plate.
Washing buffer (10×): 70 g NaCl and 3 ml Triton X-100, dissolve in 1 l of water.
Nylon mesh.
50 % Clorox bleach.
Triton X-100.
Paintbrush.
Eppendorf tubes.
Glass scintillation vial.
5× PEM: 0.5 M Pipes, pH 6.9, 10 mM MgSo4, 5 mM EGTA, pH 7.0.
Fixation solution: 1.375 ml dH2O, 0.5 ml 5× PEM, 0.625 ml 16 % formaldehyde, 2.5 ml heptane.
Plate form shaker.
Pasteur pipet.
Heptane.
Methanol.
2 % Normal goat serum.
1× PBX.
Primary antibodies. See Subheading 2.4.
Secondary antibodies. See Subheading 2.4.
50 % glycerol.
2.6. In Situ Hybridization
Dissecting solution (Drosophila Ringer’s solution): See Subheading 2.2.
4 % paraformaldehyde (Sigma).
HEPES buffer: 0.1 M HEPES pH 6.9, 2 mM MsSo4, 1 mM ethylene glycol tetraacetic acid (EGTA).
PBT: 1× PBS, 0.1 % Tween-20.
Proteinase K (Sigma).
Glycine.
Water bath.
Moist chamber.
Ice.
96-well plate.
RNAse-free water.
Hybridization buffer: 50 % deionized formamide, 5× SSC, 100 μg/ml sonicated salmon sperm DNA, 50 μg/ml heparin, 0.1 % Tween-20.
N-methylthiotetrazole (NMTT): 100 mM NaCl, 50 mM mgCl2, 100 mM Tris pH 9.5, 0.1 % Tween-20.
Alkaline phosphatase conjugated anti-DIG antibody (Boehringer Mannheim).
NBT (Nitro-Blue Tetrazolium) (Boehringer Mannheim).
X-phosphate (Boehringer Mannheim).
Fast Red tablets (Santa Cruse). To use the tablet for staining, dissolve one Fast Red tablet in 2 ml 0.1 M Tris–HCl, pH 8.2. Filter using 0.2 μm filter. Shake for 1–3 min. Use the prepared solution within 30 min after preparation. Tablet should be stored at −20 °C (see Note 1).
Glycerol.
DAPI solution.
3. Methods
3.1. Generation of Germline Clones
Clones of mutant GSCs were generated by Flp-mediated mitotic recombination, as described previously [43].
To generate the stocks for GSC clonal analysis, produce the flies carrying an armadillo-lacZ transgene in trans to the mutant-bearing chromosome using standard crosses [ 30 ].
Take the 3–5-day-old adult males or females carrying an arm-lacZ transgene in trans to the mutant-bearing chromosome and heat-s hock them for 1 h at 37 °C for 3 consecutive days, separated by 8–12 h of interval in each heat shock (see Note 2).
After the heat shock, transfer the males to fresh food every day at room temperature.
Remove the testis and ovary after 2 days, 4 days, 6 days, and 2 weeks after the last heat-shock treatment for antibody staining.
3.2. Generation of Somatic Clones
CPC clones can be generated using the MARCM system [61].
Cross the c587-Gal4.UAS-2XEYFP/FM7; FRT40A-tub-Gal80/Cyo virgin females with males of FRT40A-w+/Cyo; +/TM3, Sb, hs-Flp.
Heat-shock 3–5-day-old males and females carrying a tub-Gal80 transgene in trans to the mutant-bearing chromosome for 1 h at 37 °C for 2 days separated by 8–12 h of interval on each heat shock.
Transfer the males and females at 25 °C to fresh food vial every day.
Remove the testis and ovary 2, 4, 6 days and 2 weeks after the final heat-shock regime for antibody staining.
3.3. Isolation of Testis and Ovary
Collect adult males and females after 3–5 days of emergence under CO2 station.
Place a slide under dissecting microscope and put drops of dissecting solution on the slide.
Take the males and females using tweezers. Put one tweezers at the thorax region and with other tweezers take out the terminalia and isolate the ovaries and testes.
Transfer the dissected testis and ovary into separate tubes containing dissecting solution in ice.
For ovary, it is better to dissociate ovaries into ovarioles and then fragment each ovariole into pieces with fi ne tungsten needles before putting in dissecting solution or fixation solution.
3.4. Immunofluorescence Staining of Testis and Ovary
Fix the tissues in 4 % formaldehyde for 30 min.
Remove the fixative solution and wash the testes and ovaries three times for 2 min each in 1× PBX (see Note 3).
Block the tissues in 2 % normal goat serum for overnight at 4 °C or 30 min at room temperature.
Prepare the primary antibodies in specific concentration in 1× PBX. Incubate the tissues with primary antibodies overnight at 4 °C.
Then wash the tissues at room temperature for 15 min in 1× PBX three times.
Dilute the secondary antibodies in desired concentration and incubate the tissue for 2 h at room temperature (see Note 4).
Remove the secondary antibodies and wash the tissue for 15 min in 1× PBX three times.
After the final wash, counterstain the tissues in DAPI for 5 min.
Rinse the tissues with 1× PBS, put 50 % glycerol as a mounting medium in the tubes, and put the tubes in 4 °C.
Next day, using the dissecting microscope, put the tissue on a glass slide, arrange the testis and ovary in the desired direction, and cover with glass slides.
Image the tissues using confocal microscopy.
The number of GSCs and somatic stem cells can be determined using serial confocal reconstructions of the entire testis and ovary tip. Details of the specific markers expressed in each cell type in the testis and ovary are presented using the above protocol in Figs. 1 and 2.
Using the above protocols, many diverse types of antibodies can be tested to study male and female GSCs. For references and details of the antibodies, see refs. [9–60].
3.5. Collection, Preparation, Fixation, and Staining of Embryos
Collect embryos on grape juice plates overnight.
Rinse embryos in washing buffer into a nylon mesh.
Dechorionate by putting embryos in 50 % Clorox for 5 min at room temperature.
Rinse embryos twice with washing buffer.
Transfer the embryos to fixation solution containing heptanes in a glass scintillation vial, put the vials on shaker, and shake at a moderate speed for 25 min.
Discard the aqueous (bottom) layer of solution with the help of a Pasteur pipet.
Transfer the embryos in 10 ml of heptane.
Add 10 ml of methanol to the embryos and shake vigorously for 30–60 s to help in devitellinization of embryos.
Transfer the devitellinized embryos to a fresh tube and rinse with methanol.
Embryos can be stored at −20 °C for extended period for future staining.
To stain the embryos, take a desired number of embryos in Eppendorf tubes.
Rehydrate the embryos into the following order: 9:1 methanol:1× PBX; then put 7:3 methanol:1× PBX, then put 5:5 methanol:1× PBX; then put 3:7 methanol:1× PBX, then put 1:9 methanol:1× PBX, and then wash with PBX two times.
Remove PBX and incubate the embryos in 2 % normal goat serum.
The primary and secondary antibody dilution, incubation, washing, and microscopy can be done in a similar way as mentioned in Subheading 3.4.
3.6. In Situ Hybridization (See Note 5)
Probes can be prepared using SP6/T7DIG RNA labeling kit (Roche) following the manufacturer’s instructions.
Dissect the 3–5-day-old males and females as mentioned in Subheading 3.3.
Fix the tissues for 30 min in 4 % paraformaldehyde in HEPES buffer at room temperature.
Wash the tissues three times for 5 min each in 1× PBX.
Incubate the tissues in 50 μg/ml proteinase K for 5 min (see Note 6). Stop the reaction with 2 mg/ml glycine for 2 min and wash the tissues two times for 5 min each with 1× PPX.
Fix and wash the tissues again if using step 5. If not, wash the tissues for 10 min in 1:1 ratio of 1× PBX:hybridization buffer.
Pre-hybridize the tissues for about 1 h at 65 °C in a water bath (for RNA probes) in hybridization buffer. It is advised to preheat the hybridization buffer.
Denature the probe by heating at 70 °C for 10 min in a water bath, and then rapidly cooling in ice.
Then heat the hybridization buffer at 65 °C in a water bath, mix the probe (in a ratio 1:50 probe:hybridization buffer), and put on tissue.
Hybridize overnight at 65 °C in a water bath. Make sure to have moist chamber where you can place the tube. No need to shake.
Next day, after hybridization, wash the tissues six times for 30 min each in hybridization buffer in a water bath at 65 °C.
Then wash for 15 min in 4:1 ratio hybridization buffer:1× PBX at room temperature.
Then wash for 15 min in 3:2 ratio hybridization buffer:1× PBX at room temperature.
Then wash for 15 min in 2:3 ratio hybridization buffer:1× PBX at room temperature.
Then wash for 15 min in 1:4 ratio hybridization buffer:1× PBX at room temperature.
Then wash two times for 15 min each in 1× PBX at room temperature.
For nonfluorescent staining with NBT and X-phosphate, incubate the tissue overnight in a 1:2,000 dilution of alkaline phosphatase- conjugated anti-DIG antibody in 1× PBX at 4 °C; then three times, 15 min each, with 1× PBX; and after that, three times for 15 min each with NMTT. Prepare the color reaction by adding 4.5 μl NBT and 3.5 μl X-phosphate in 1 ml NMTT. Incubate the tissue for 10–30 min depending on the color reaction; once you see the color changing in the tissue by looking at the dissecting microscope, it is better to stop the reaction by putting 1× PBX. Put in 50 % glycerol for 1 h and tissue can be put in 90 % glycerol overnight. It is better to mount in 90 % glycerol. Image can be taken using the microscope with bright-fi eld and DIC capabilities.
For fluorescent in situ hybridization (FISH), we used Fast Red staining procedure by following the manufacturer’s instructions. Follow the above method from steps 1 to 17, and then incubate tissues in 2 % blocking solution. Add primary antibody anti-DIG, incubate the tissues at 37 °C for 1 h, wash, and in the place of secondary antibody use Fast Red solution. Incubate the tissues with 1 mg/ml of Fast Red solution and monitor the reaction to prevent overstaining. Stop the reaction by washing the tissues in water or 1× PBS. Put the 50 % glycerol for mounting. Images can be taken using a confocal microscope (some of the images using the above procedures are provided in Figs. 1 and 2). With the above procedure, two-color and three-color FISH can also be performed.
Acknowledgments
M.K.S. is supported by the Knight’s Templar Eye Foundation and start-up support from the University of Dayton, OH. We thank Robin Permut for editing the manuscript.
Footnotes
The Fast Red working solution should be filtered to remove non-dissolved substrate particles.
8–12 h of interval in each heat shock was used to allow flies to recover from heat shock because shorter interval can kill the flies.
Proper care should be taken while staining the ovary because during washing ovarioles can be washed out.
Wrap the tubes with foil during secondary antibody staining to avoid exposure of light and fading of the signal.
All the materials should be RNase free; always use gloves and use DEPC-treated water.
Since Drosophila tissues are soft, it is not necessary to use proteinase K.
References
- 1.Morrison SJ, Spradling AC (2008) Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132:598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh SR, Chen X, Hou SX (2005) JAK/STAT signaling regulates tissue outgrowth and male germline stem cell fate in Drosophila. Cell Res 15:1–5 [DOI] [PubMed] [Google Scholar]
- 3.Singh SR, Zhen W, Zheng Z, Wang H, Oh SW, Liu W, Zbar B, Schmidt LS, Hou SX (2006) The Drosophila homolog of the human tumor suppressor gene BHD interacts with the JAK-STAT and Dpp signaling pathways in regulating male germline stem cell maintenance. Oncogene 25:5933–5941 [DOI] [PubMed] [Google Scholar]
- 4.Singh SR, Liu W, Hou SX (2007) The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell 1:191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111 [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Singh SR, Zheng Z, Oh SW, Chen X, Edwards K, Hou SX (2006) Rap-GEF signaling controls stem cell anchoring to their niche through regulating DE-cadherin-mediated cell adhesion in the Drosophila testis. Dev Cell 10:117–126 [DOI] [PubMed] [Google Scholar]
- 7.Wilson E (1896) The cell in development and inheritance. Macmillan, New York [Google Scholar]
- 8.Nishimiya-Fujisawa C, Sugiyama T (1993) Genetic analysis of developmental mechanisms in hydra. XX. Cloning of interstitial stem cells restricted to the sperm differentiation pathway in Hydra magnipapillata. Dev Biol 157:1–9 [DOI] [PubMed] [Google Scholar]
- 9.Lin H, Spradling AC (1993) Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Dev Biol 159:140–152 [DOI] [PubMed] [Google Scholar]
- 10.Hou SX, Singh SR (2008) Germline stem cells. Methods Mol Biol, 450, Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 11.Gilboa L, Lehmann R (2004) How different is Venus from Mars? The genetics of germ-line stem cells in Drosophila females and males. Development 131:4895–4905 [DOI] [PubMed] [Google Scholar]
- 12.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT (2007) Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science 315:518–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng J, Türkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM (2008) Centrosome misorientation reduces stem cell division during ageing. Nature 456:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng XR, Brawley CM, Matunis EL (2009) Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell 5:191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gönczy P, DiNardo S (1996) The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development 122:2437–2447 [DOI] [PubMed] [Google Scholar]
- 16.Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M (1979) The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res 69:180–190 [DOI] [PubMed] [Google Scholar]
- 17.Fuller MT, Spradling AC (2007) Male and female Drosophila germline stem cells: two versions of immortality. Science 316:402–404 [DOI] [PubMed] [Google Scholar]
- 18.Yamashita YM, Jones DL, Fuller MT (2003) Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301:1547–1550 [DOI] [PubMed] [Google Scholar]
- 19.Yamashita YM, Fuller MT (2008) Asymmetric centrosome behavior and the mechanisms of stem cell division. J Cell Biol 180:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voog J, D’Alterio C, Jones DL (2008) Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature 454:1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT (2001) Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294:2542–2545 [DOI] [PubMed] [Google Scholar]
- 22.Tulina N, Matunis E (2001) Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294: 2546–2549 [DOI] [PubMed] [Google Scholar]
- 23.Brawley C, Matunis E (2004) Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304: 1331–1334 [DOI] [PubMed] [Google Scholar]
- 24.Schulz C, Kiger AA, Tazuke SI, Yamashita YM, Pantalena-Filho LC, Jones DL, Wood CG, Fuller MT (2004) A misexpression screen reveals effects of bag-of-marbles and TGF beta class signaling on the Drosophila male germline stem cell lineage. Genetics 167:707–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar A, Parikh N, Hearn SA, Fuller MT, Tazuke SI, Schulz C (2007) Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr Biol 17: 1253–1258 [DOI] [PubMed] [Google Scholar]
- 26.Kiger AA, White-Cooper H, Fuller MT (2000) Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature 407:750–754 [DOI] [PubMed] [Google Scholar]
- 27.Tran J, Brenner TJ, DiNardo S (2000) Somatic control over the germline stem cell lineage during Drosophil a spermatogenesis. Nature 407:754–757 [DOI] [PubMed] [Google Scholar]
- 28.Shivdasani AA, Ingham PW (2003) Regulation of stem cell maintenance and transit amplifying cell proliferation by TGF-beta signaling in Drosophila spermatogenesis. Curr Biol 13:2065–2072 [DOI] [PubMed] [Google Scholar]
- 29.Bunt SM, Hime GR (2004) Ectopic activation of Dpp signalling in the male Drosophila germline inhibits germ cell differentiation. Genesis 39:84–93 [DOI] [PubMed] [Google Scholar]
- 30.Kawase E, Wong MD, Ding BC, Xie T (2004) Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development 131:1365–1375 [DOI] [PubMed] [Google Scholar]
- 31.Schulz C, Wood CG, Jones DL, Tazuke SI, Fuller MT (2002) Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 129:4523–4534 [DOI] [PubMed] [Google Scholar]
- 32.Leatherman JL, Dinardo S (2008) Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell 3:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh SR, Zheng Z, Wang H, Oh SW, Chen X, Hou SX (2010) Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling. J Cell Physiol 223:500–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toledano H, D’Alterio C, Czech B, Levine E, Jones DL (2012) The let-7-Imp axis regulates ageing of the Drosophila testis stem-cell niche. Nature 485:605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan S, Mahowald AP, Fuller MT (2012) The receptor regulates adhesion between Drosophila male germline stem cells and the niche. Development 139:1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monk AC, Siddall NA, Fraser B, McLaughlin EA, Hime GR (2011) Differential roles of HOW in male and female Drosophila germline differentiation. PLoS One 6(12):e28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inaba M, Yuan H, Yamashita YM (2011) String (Cdc25) regulates stem cell maintenance, proliferation and aging in Drosophila testis. Development 138:5079–5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casper AL, Baxter K, Van Doren M (2011) No child left behind encodes a novel chromatin factor required for germline stem cell maintenance in males but not females. Development 138:3357–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Q, Wang Y, Vargas E, DiNardo S (2011) maguis required for germline stem cell self- renewal through BMP signaling in the Drosophila testis. Dev Biol 357:202–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinardo S, Okegbe T, Wingert L, Freilich S, Terry N (2011) Lines and bowl affect the specification of cyst stem cells and niche cells in the Drosophila testis. Development 138:1687–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leatherman JL, Dinardo S (2010) Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol 12:806–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cherry CM, Matunis EL (2010) Epigenetic regulation of stem cell maintenance in the Drosophila testis via the nucleosome-remodeling factor NURF. Cell Stem Cell 6: 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie T, Spradling AC (2000) A niche maintaining germ line stem cells in the Drosophila ovary. Science 290:328–330 [DOI] [PubMed] [Google Scholar]
- 44.Song X, Xie T (2002) DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci USA 99:14813–14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song X, Call GB, Kirilly D, Xie T (2007) Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development 134:1071–1080 [DOI] [PubMed] [Google Scholar]
- 46.Xie T, Spradling AC (1998) Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94:251–260 [DOI] [PubMed] [Google Scholar]
- 47.Liu N, Han H, Lasko P (2009) Vasa promotes Drosophila germline stem cell differentiation by activating mei-P26 translation by directly interacting with a (U)-rich motif in its 3′ UTR. Genes Dev 23:2742–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen R, Weng C, Yu J, Xie T (2009) eIF4A controls germline stem cell self-renewal by directly inhibiting BAM function in the Drosophila ovary. Proc Natl Acad Sci USA 106:11623–11628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Li Z, Cai Y (2008) The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J Cell Biol 180:721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decotto E, Spradling AC (2005) The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell 9:501–510 [DOI] [PubMed] [Google Scholar]
- 51.Nystul T, Spradling A (2007) An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell 1:277–285 [DOI] [PubMed] [Google Scholar]
- 52.Lu W, Casanueva MO, Mahowald AP, Kato M, Lauterbach D, Ferguson EL (2012) Niche-associated activation of rac promotes the asymmetric division of Drosophila female germline stem cells. PLoS Biol 10(7):e1001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rojas-Ríos P, Guerrero I, González-Reyes A (2012) Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol 10(4):e1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Maines JZ, Tastan OY, McKearin DM, Buszczak M (2012) Mei-P26 regulates the maintenance of ovarian germline stem cells by promoting BMP signaling. Development 139: 1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing Y, Kurtz I, Thuparani M, Legard J, Ruohola- Baker H (2012) Loss-of-function screen reveals novel regulators required for Drosophila germline stem cell self-renewal. (G3) Bethesda 2:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Pan L, Wang S, Zhou J, McDowell W, Park J, Haug J, Staehling K, Tang H, Xie T (2011) Histone H3K9 trimethylase eggless controls germline stem cell maintenance and differentiation. PLoS Genet 7(12):e1002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.König A, Yatsenko AS, Weiss M, Shcherbata HR (2011) Ecdysteroids affect Drosophila ovarian stem cell niche formation and early germline differentiation. EMBO J 30:1549–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris RE, Pargett M, Sutcliffe C, Umulis D, Ashe HL (2011) Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev Cell 20:72–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia L, Jia S, Huang S, Wang H, Zhu Y, Mu Y, Kan L, Zheng W, Wu D, Li X, Sun Q, Meng A, Chen D (2010) The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient BMP response. Cell 143:978–990 [DOI] [PubMed] [Google Scholar]
- 60.Singh SR, Hou SX (2008) Immunohistological techniques for studying the Drosophila male germline stem cell. Methods Mol Biol 450:45–59 [DOI] [PubMed] [Google Scholar]
- 61.Lee T, Luo L (2001) Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci 24:251–254 [DOI] [PubMed] [Google Scholar]