Abstract
The diagnosis of melanoma can be challenging, especially in metastatic lesions, due to the ability of melanoma cells to morphologically mimic carcinoma, sarcoma and even lymphoma cells. Moreover, melanomas can exhibit negative immunostaining for the melanoma markers HMB-45 and MART-1/Melan-A, often used in the diagnosis of this tumor. KBA.62 is a recently described antibody that reacts with benign and malignant melanocytic proliferations. In this study, we report our experience with KBA.62 and S100 protein immunostaining in the diagnosis of metastatic melanoma on fine-needle aspiration and effusion samples. We reviewed 60 cytology samples from 58 patients with metastatic melanoma. Our results showed that KBA.62 stained 75% of the cases and S100 protein 87% of the cases. KBA.62 and S100 protein stained the majority of metastatic melanomas that were negative for HMB-45 and MART-1; KBA.62 stained 73% of the cases and S100 protein 73% of the cases. The majority (85%) of the cases negative for HMB-45 and MART-1 were positive for KBA.62 and/or S100 protein. Additionally, we also observed that KBA.62 staining was positive in the majority of epithelioid and spindle cell type melanoma cells. In conclusion, the performances of KBA.62 and S100 protein were similar and both markers are useful in the diagnosis of metastatic melanoma in cytology material, especially when the tumor cells lack expression of HMB-45 and MART-1.
Keywords: KBA.62, S100 protein, immunocytochemistry/immunohistochemistry, cytology, melanoma
INTRODUCTION
Fine-needle aspiration (FNA) is a safe, rapid, minimally invasive and cost-effective diagnostic tool for metastatic malignant melanoma (MMM). However, accurate diagnosis of MMM in FNA samples can sometimes be challenging, due in part to small sample size and the ability of melanoma cells to show varying morphologic appearances which can mimic carcinoma, sarcoma or lymphoma cells.1–3 Equally challenging is diagnosing MMM in effusion samples since melanoma cells can also mimic mesothelial cells. Immunohistochemistry plays an important role as an ancillary tool in the diagnosis of melanoma. However, melanomas, even in surgical excision specimens, may on occasion exhibit negative or focal staining for HMB-45, MART-1/Melan-A and S100 protein, markers that are considered gold standard in the diagnosis of melanoma.
KBA.62 was described in 1995 as a new monoclonal antibody against a melanoma associated antigen.4 It has been reported to be immunoreactive in benign and malignant melanocytic proliferations. Since then, there have been only a few published reports on this marker.5–8 A recent study demonstrated KBA.62 positivity in non-melanocytic neoplasms including leiomyosarcoma, gastrointestinal stromal tumor, malignant peripheral nerve sheath tumor, synovial sarcoma, and carcinomas mainly with squamous cell/stratified epithelial differentiation.8 We did not perform KBA.62 staining of non-melanoma malignancies and that is a limitation of our study; however, Aung et al found KBA.62 to be an useful complement marker for detecting melanomas that are negative for S100 protein, HMB-45 and Melan-A.8 In addition, Kaufman et al demonstrated a similar reactivity of KBA.62 to melanoma when compared to HMB-45 and S100 protein, and proposed using KBA.62 in conjunction with S100 protein and cytokeratin antibodies in the diagnosis of melanoma that are negative for three melanocyte-specific markers.5 However, to the best of our knowledge, there are no reported studies of KBA.62 expression in cytology samples of melanoma.
S100 protein is one of the most sensitive markers for melanoma, being expressed in 96–100% of primary and metastatic melanomas on tissue samples,5,7,9 and in 7–100% of fine-needle aspirates and effusion samples, including a series of 81 FNA cases of MMM.10–15 The sensitivity of the highly specific melanoma markers HMB-45 and MART-1 on cytology samples of MMM is reported to be approximately 78–100%14,16 and 88–100%,12,15,17 respectively. However, the specificity of S100 protein for melanomas is limited (75–87%).5,18 In this study, we demonstrate our experience with KBA.62 and S100 protein immunostaining in the diagnosis of MMM on cytology samples.
MATERIALS AND METHODS
We searched our files for the period between 1999 and 2010 for cytology cases where a diagnosis of MMM was made and immunostains for KBA.62, S100, HMB-45 and MART-1 were performed.
Fine-needle aspirations were performed either by a radiologist under image guidance or by a cytopathologist using a 23 or 25-gauge needle if the lesion was palpable. In addition, in some cases ex-vivo aspirations were obtained from surgically excised MMM lesions. Effusion samples were submitted to the cytology laboratory fresh without preservative.
Direct smears from the aspirated material were air-dried for Diff-Quik® staining or fixed in Carnoy’s fixative (95% ethanol and glacial acetic acid) for Papanicolaou staining. The smears were assessed for specimen adequacy by the cytopathologist. Cells obtained from needle rinses and effusion samples were centrifuged to prepare cytospin slides and/or fixed in formalin and embedded in paraffin for cell block preparations. Cell blocks were cut as 4μ sections and stained with hematoxylin and eosin (H&E) or left unstained for immunohistochemical staining. Cytospins were stained with Diff-Quik® or left unstained for immunocytochemical studies.
Immunostaining was performed on either acetone-fixed cytospins or formalin-fixed paraffin-embedded (FFPE) cell block sections using the Ventana automated immunostainer (Ventana Medical Systems, Tucson, AZ) using the following antibodies: HMB-45 (1:100, DAKO, Carpinteria, CA), KBA.62 (1:1, Immunotech/Beckman Coulter, Fullerton, CA), MART-1 (1:10, courtesy of Dr. S. Rosenberg, NCI Surgery Branch, Bethesda, MD), S100 protein (1:1, Zymed/Invitrogen, Carlsbad, CA), and cytokeratin AE1/AE3 (1:100 dilution, DAKO, Carpinteria, CA). Cytoplasmic staining for MART-1 and HMB-45, membranous staining for KBA.62, and both nuclear and cytoplasmic staining for S100 protein were considered positive staining. Staining was not quantitated, but scored as focal or diffuse. Immunostaining for HMB-45 and MART-1 was performed on either cytospins or cell block sections. Immunostaining for S100 and KBA.62 was performed on cell block sections. Appropriate positive and negative tissue controls, as well as negative sample controls were included in all cases.
The final diagnosis of MMM was made based on correlation of the cytologic features with the immunohistochemical findings and morphological comparison of pathology material from the patient’s primary tumor or metastatic tumors when available.
RESULTS
We retrospectively identified 60 cytologic samples of MMM from 58 patients. In 2 patients, 2 concurrent metastatic sites had been aspirated. Of the 60 samples, 25 were FNAs performed by a cytopathologist or radiologist, 31 were ex-vivo aspirates from surgical excisions, and 4 were effusion samples. The anatomic locations included skin and soft tissue (n=18), lymph node (n=7), liver (n=26), lung (n=3), pancreas (n=1), ovary (n=1), pleural fluid (n=3) and peritoneal fluid (n=1).
Of the 60 samples, 26 were positive for both HMB-45 and MART-1, 8 were positive for MART-1 and negative for HMB-45, and 26 were negative for both HMB45 and MART-1 (Table 1). Staining for KBA.62 showed moderate to strong membranous staining, either focally (22%) or diffusely (78%), in 45 of the 60 samples (75%) (Table 1) (Figure 1 D, E, and F). KBA.62 staining was positive in 20 out of 26 cases positive for both HMB-45 and MART-1 (77%) and in 6 out of 8 cases that were positive for either HMB-45 or MART-1 (75%). Interestingly, KBA.62 staining was positive in 19 of the 26 cases (73%) that were negative for both HMB-45 and MART-1 (Table 1).
Table 1:
Immunostaining for HMB-45, MART-1, KBA.62 and S100 protein in cytology specimens of metastatic melanomas
| KBA.62 (+) | KBA.62 (−) | KBA.62 (NC) | S100 (+) | S100 (−) | S100 (NC) | ||
|---|---|---|---|---|---|---|---|
| HMB-45 (+) and MART-1 (+) | n = 26 | 20 (77%) | 6 (23%) | 0 (0%) | 26 (100%) | 0 (0%) | 0 (0%) |
| HMB-45 (+) and MART-1 (−) | n = 0 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HMB-45 (−) and MART-1 (+) | n = 8 | 6 (75%) | 2 (25%) | 0 (0%) | 7 (88%) | 1 (12%) | 0 (0%) |
| HMB-45 (−) and MART-1 (−) | n = 26 | 19 (73%) | 5 (19%) | 2 (8%) | 19 (73%) | 4 (15%) | 3 (12%) |
| Total | n = 60 | 45 (75%) | 13 (22%) | 2 (3%) | 52 (87%) | 5 (8%) | 3 (5%) |
NC: noncontributory
Figure 1.
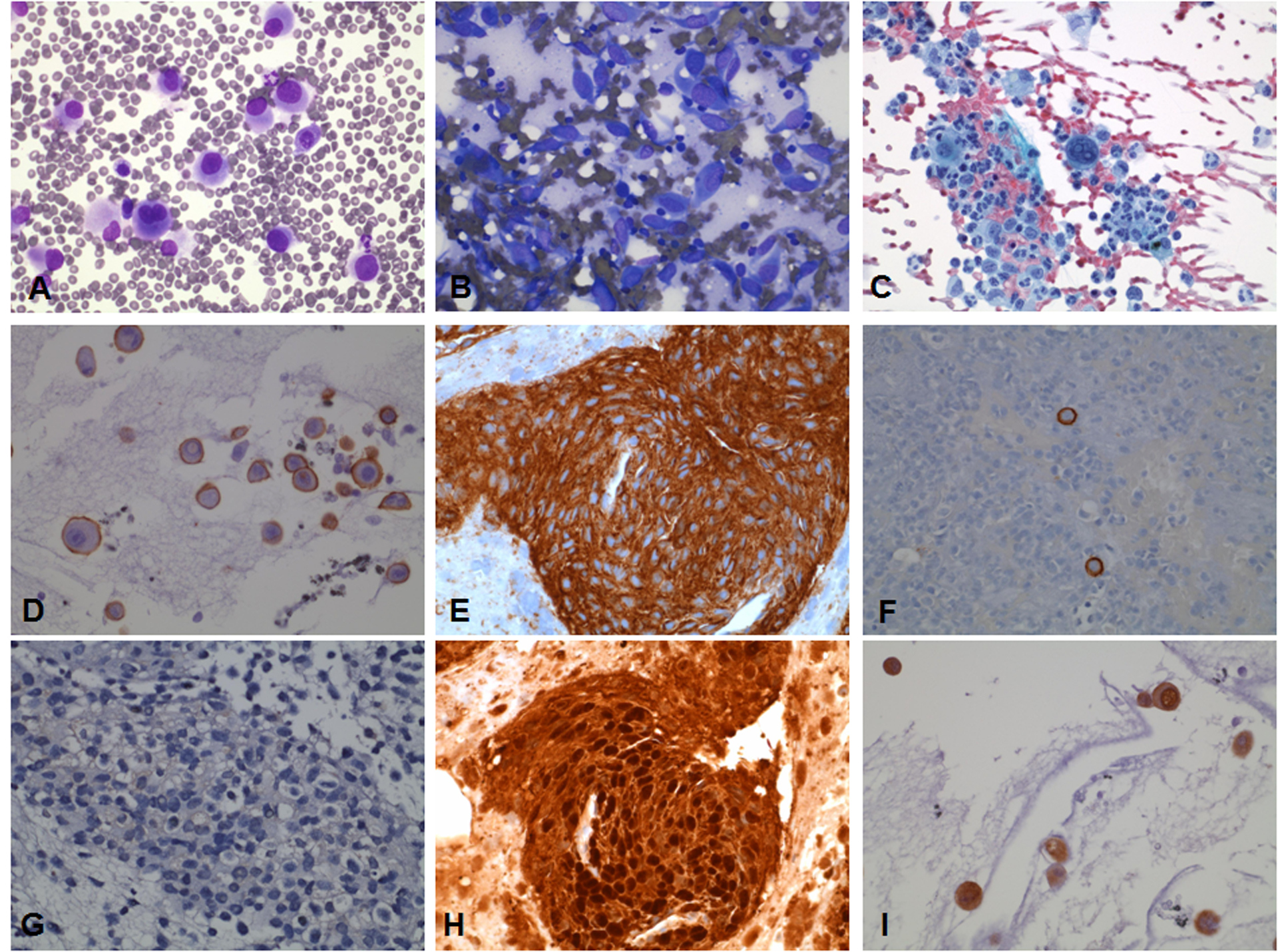
FNA of epithelioid (A, D, G) and spindle cell types of metastatic melanoma (B, E, H) and metastatic melanoma in a pleural effusion (C, F, I) stained with Diff-Quik (A, B), Papanicolaou (C), immunostained with KBA.62 (D, E, F) and S100 protein (G, H, I). KBA.62 immunostaining shows strong membranous staining in epithelioid and spindle cell type melanoma as well as in melanoma cells in a pleural effusion. S100 protein shows nuclear and cytoplasmic staining in a spindle cell type melanoma and a metastatic melanoma to pleural fluid. S100 protein is negative in this case of epithelioid cell type melanoma.
Staining for S100 protein showed nuclear and cytoplasmic staining, either focally or diffusely, in 52 of the 60 samples (87%) (Table 1) (Figure 1 H and I). S100 protein staining was positive in all 26 cases positive for both HMB-45 and MART-1 (100%) and in 7 out of 8 cases that were positive for either HMB-45 or MART-1 (88%). S100 protein staining was positive in 19 of the 26 cases (73%) that were negative for both HMB-45 and MART-1 (Table 1).
KBA.62 and S100 protein showed similar rates of immunoreactivity in the cases that were negative for both HMB-45 and MART-1 (19 out of 26 cases; 73%) (Table 1). 22 out of 26 cases (85%) negative for both HMB-45 and MART-1 were positive for either KBA.62 and/or S100 protein. Of the 8 cases that were either negative or noncontributory (scant number of atypical cells or not the expected staining pattern) for S100 protein (Table 2), 7 were also negative for both MART-1 and HMB-45. Three out of the 7 cases were strongly positive for KBA.62 (Table 2), negative for cytokeratin AE1/AE3 and morphologically similar to the patient’s prior material. In two cases, staining for all four markers, S100 protein, MART-1, HMB-45, and KBA.62 were negative. The remaining two cases were noncontributory for KBA.62 (Tables 1 and 2). These cases were morphologically similar to prior material from the same patient and stained negatively for cytokeratin AE1/AE3, therefore, they were interpreted as morphologically consistent with MMM.
Table 2:
KBA.62 immunostaining in metastatic melanomas stained for S100 protein
| KBA.62 (+) | KBA.62 (−) | KBA.62 (NC) | ||
|---|---|---|---|---|
| S100 (+) | n =52 | 42 | 10 | 0 |
| S100 (−) | n =5 | 1 (strong +) | 3 | 1 |
| S100 (NC) | n =3 | 2 (strong +) | 0 | 1 |
NC: noncontributory
We also looked into KBA.62 expression in relation to the morphologic type of melanoma cells. The majority of MMM demonstrated epithelioid cell morphology (39 out of 60 cases) (Table 3) (Figure 1A). Five cases were pure spindle cell type (Figure 1B) and 11 showed mixed morphology with both epithelioid and spindle cell type. KBA.62 was comparably positive in the majority of epithelioid (28 out of 39 cases, 72%), spindle cell (4 out of 5 cases, 80%) and mixed morphology (9 out of 11 cases, 82%) melanomas (Table 3; Figure 1). In effusion samples, it is more difficult to classify MMM based on cell morphology. Nonetheless, the majority of MMM in effusion samples (3 out of 4 cases, 75%) were positive for KBA.62 (Figure 1C, F, I).
Table 3:
KBA.62 immunostaining in metastatic melanomas based on cell morphology
| Cell Type | KBA.62 (+) | KBA.62 (−) | KBA.62 (NC) | |
|---|---|---|---|---|
| Epithelioid | n =39 | 28 (72%) | 10 (26%) | 1 (2%) |
| Spindled | n =5 | 4 (80%) | 1 (20%) | 0 (0%) |
| Mixed | n =11 | 9 (82%) | 1 (9%) | 1 (9%) |
| Unspecified (effusions) | n =4 | 3 (75%) | 1 (25%) | 0 (0%) |
| Degenerated cells | n =1 | 1 (100%) | 0 (0%) | 0 (0%) |
NC: noncontributory
DISCUSSION
In this study, we demonstrated a moderate to strong membranous staining for KBA.62 in cytology samples of MMM, consistent with prior reports on surgical pathology materials. Furthermore, positive immunostaining for KBA.62 was comparable to S100 protein, a very sensitive marker for melanoma, and was seen in the majority of the cases that were negative for the commonly used melanoma markers HMB-45 and MART-1.
Immunostaining for S100 protein in combination with pan-cytokeratin is widely used to evaluate poorly differentiated malignant tumors that could represent malignant melanoma. Cytokeratin positivity in malignant melanoma is exceedingly rare; in a previous study, we found only a single case out of 117 FNA samples of MMM that was positive for low molecular weight cytokeratin.19 Therefore, an immunoprofile of positivity for S100 protein and negative immunostaining for cytokeratin strongly supports a diagnosis of melanoma. Several studies have shown that S100 protein is a very sensitive marker for melanoma on cytology samples, including a large series of MMM FNAs in which S100 protein stained 100% of the cases.10–15 In our study, immunoreactivity for S100 protein was observed in 87% of the cases. S100 protein immunostain may be negative in up to 4% of metastatic melanomas in tissue samples and in up to 93% in cytology material.9,11,20 False negative or weak S100 protein immunostaining may be encountered in FNA material due to insufficient sampling, staining over destained Papanicolaou slides or use of alcohol-based fixative.1,11 In such cases, KBA.62 staining can be a helpful additional stain. We have found that 3 of our 8 cases that were negative or noncontributory for S100 protein showed a strong and diffuse staining for KBA.62.
Kaufman et al compared KBA.62, Melan-A and HMB-45 immunoreactivity in metastatic melanomas and showed that all 3 markers had identical staining results with a sensitivity of 0.85 for HMB-45, 0.86 for Melan-A and 0.86 for KBA.62.5 In our hands KBA.62 stained 75% of MMM in cytologic samples. Expression of HMB-45 and MART-1/Melan-A can be lost in spindle/desmoplastic cell type melanomas.21,22 On the other hand, HMB-45 and MART-1 can be expressed in tumors other than melanoma, such as clear cell sarcoma of soft parts, angiomyolipoma and lymphangioleiomyomatosis.23–25 MART-1-positive cells may also be present in lymph nodes from patients without melanoma.26 In our study, KBA.62 stained the majority of metastatic melanomas that were negative for HMB-45 and MART-1 and the majority of cases that were positive for MART-1 and/or HMB-45, suggesting that KBA.62 is a useful additional marker for the diagnosis of melanoma. Interestingly, KBA.62 also stained the majority of epithelioid and spindle cell type of MMM cells.
In conclusion, KBA.62 and S100 protein are useful contributory markers for the diagnosis MMM in cytology samples, especially in cases where the tumor cells lack expression of other commonly used melanoma markers. KBA.62 expression is comparable to the S100 protein expression since both were positive in the majority of cytologic specimens of MMM that were non-immunoreactive for HMB-45 and MART-1 and immunoreactive for HMB-45 and/or MART-1. KBA.62 stains the majority of epithelioid and spindled cell types of melanoma cells. In cytology samples, as previously proposed for surgical specimens, the KBA.62, S100 protein and cytokeratin immunostaining panel would help to confirm the diagnosis of melanoma in cases that are negative for HMB-45 and MART-1/Melan-A.
Acknowledgments:
We thank Dr. Steven A. Rosenberg for supplying part of the cytologic samples. This study was supported in part by the Intramural Research Program of the NIH, National Cancer Institute.
REFERENCES
- 1.Piao Y, Guo M, Gong Y. Diagnostic challenges of metastatic spindle cell melanoma on fine-needle aspiration specimens. Cancer 2008;114:94–101. [DOI] [PubMed] [Google Scholar]
- 2.Beaty MW, Fetsch P, Wilder AM, Marincola F, Abati A. Effusion cytology of malignant melanoma. A morphologic and immunocytochemical analysis including application of the MART-1 antibody. Cancer 1997;81:57–63. [DOI] [PubMed] [Google Scholar]
- 3.Murali R, Doubrovsky A, Watson GF, McKenzie PR, Lee CS, McLeod DJ, Uren RF, Stretch JR, Saw RP, Thompson JF, Scolyer RA. Diagnosis of metastatic melanoma by fine-needle biopsy: analysis of 2,204 cases. Am J Clin Pathol 2007;127:385–97. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Knafo E, al Saati T, Aziza J, Ralfkiaer E, Selves J, Gorguet B, Delsol G. Production and characterisation of an antimelanoma monoclonal antibody KBA.62 using a new melanoma cell line reactive on paraffin wax embedded sections. J Clin Pathol 1995;48:826–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann O, Koch S, Burghardt J, Audring H, Dietel M. Tyrosinase, melan-A, and KBA62 as markers for the immunohistochemical identification of metastatic amelanotic melanomas on paraffin sections. Mod Pathol 1998;11:740–6. [PubMed] [Google Scholar]
- 6.Kocan P, Jurkovic I, Boor A, Dudrikova K, Krajcar R, Benicky M, Kromydaki A. Immunohistochemical study of melanocytic differentiation antigens in cutaneous malignant melanoma. A comparison of six commercial antibodies and one non-commercial antibody in nodular melanoma, superficially spreading melanoma and lentigo maligna melanoma. Cesk Patol 2004;40:50–6. [PubMed] [Google Scholar]
- 7.Pages C, Rochaix P, al Saati T, Valmary-Degano S, Boulinguez S, Launay F, Carle P, Lauwers F, Payoux P, Le Guellec S, Brousset P, Lamant L. KBA.62: a useful marker for primary and metastatic melanomas. Hum Pathol 2008;39:1136–42. [DOI] [PubMed] [Google Scholar]
- 8.Aung PP, Sarlomo-Rikala M, Lasota J, Lai J, Wand Z, Miettinen M. KBA62 and PNL2: 2 new melanoma marlers – immunohistochemical analysis of 1563 tumors including metastatic, desmoplastic, and mucosal melanomas and their mimics. Am J Surg Pathol 2012;36:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aisner DL, Maker A, Rosenberg SA, Berman DM. Loss of S100 antigenicity in metastatic melanoma. Hum Pathol 2005;36:1016–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasiell K, Tani E, Skoog L. Fine needle aspiration cytology and immunocytochemistry of metastatic melanoma. Cytopathology 1991;2:137–47. [DOI] [PubMed] [Google Scholar]
- 11.Simmons TJ, Martin SE. Fine-needle aspiration biopsy of malignant melanoma: a cytologic and immunocytochemical analysis. Diagn Cytopathol 1991;7:380–6. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda K, Tate G, Iezumi K, Suzuki T, Kitamura T, Mitsuya T. Effusion cytomorphology and immunocytochemistry of malignant melanoma: five cases of melanotic melanoma and one case of amelanotic melanoma. Diagn Cytopathol 2009;37:516–21. [DOI] [PubMed] [Google Scholar]
- 13.Lai R, Redburn J, Nguyen GK. Cytodiagnosis of metastatic amelanotic melanomas by fine-needle aspiration biopsy. Adjunctival value of immunocytochemistry and electron microscopy. Cancer 1998;84:92–7. [PubMed] [Google Scholar]
- 14.Gupta RK, Kenwright DN, Fauck R, Lallu S, Naran S. The usefulness of a panel of immunostains in the diagnosis and differentiation of metastatic malignancies in pericardial effusions. Cytopathology 2000;11:312–21. [DOI] [PubMed] [Google Scholar]
- 15.Hookim K, Roh MH, Willman J, Placido J, Weigelin HC, Fields KL, Pang J, Betz BL, Knoepp SM. Application of immunocytochemistry and BRAF mutational analysis to direct smears of metastatic melanoma. Cancer Cytopathol 2012;120:52–61. [DOI] [PubMed] [Google Scholar]
- 16.Longatto Filho A, de Carvalho LV, Santos GC C, Oyafuso MS, Lombardo V, Bortolan J, Neves JI. Cytologic diagnosis of melanoma in serous effusions. A morphologic and immunocytochemical study. Acta Cytol 1995;39:481–4. [PubMed] [Google Scholar]
- 17.Fetsch PA, Marincola FM, Filie A, Hijazi YM, Kleiner DE, Abati A. Melanoma-associated antigen recognized by T cells (MART-1). The advent of a preferred immunocytochemical antibody for the diagnosis of metastatic malignant melanoma with fine-needle aspiration. Cancer 1999;87:37–42. [PubMed] [Google Scholar]
- 18.Ordonez NG, Ji XL, Hickey RC. Comparison of HMB-45 monoclonal antibody and S-100 protein in the immunohistochemical diagnosis of melanoma. Am J Clin Pathol 1988;90:385–90. [DOI] [PubMed] [Google Scholar]
- 19.Fetsch PA, Marincola FM, Abati A. Cytokeratin positivity in fine-needle aspirates of metastatic malignant melanoma: fact or fiction? Diagn Cytopathol 1999;20:393–6. [DOI] [PubMed] [Google Scholar]
- 20.Argenyi ZB, Cain C, Bromley C, Nguyen AV, Abraham AA, Kerschmann R, LeBoit PE. S-100 protein-negative malignant melanoma: fact or fiction? A light-microscopic and immunohistochemical study. Am J Dermatopathol 1994;16:233–40. [PubMed] [Google Scholar]
- 21.Skelton HG, Maceira J, Smith KJ, McCarthy WF, Lupton GP, Graham JH. HMB45 negative spindle cell malignant melanoma. Am J Dermatopathol 1997;19:580–4. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Chu AY, Pasha TL, Elder DE, Zhang PJ. Immunoprofile of MITF, tyrosinase, melan-A, and MAGE-1 in HMB45-negative melanomas. Am J Surg Pathol 2002;26:82–7. [DOI] [PubMed] [Google Scholar]
- 23.Hisaoka M, Ishida T, Kuo TT, Matsuyama A, Imamura T, Nishida K, Kuroda H, Inayama Y, Oshiro H, Kobayashi H, Nakajima T, Fukuda T, Ae K, Hashimoto H. Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol 2008;32:452–60. [DOI] [PubMed] [Google Scholar]
- 24.Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch 2008;452:119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fetsch PA, Fetsch JF, Marincola FM, Travis W, Batts KP, Abati A. Comparison of melanoma antigen recognized by T cells (MART-1) to HMB-45: additional evidence to support a common lineage for angiomyolipoma, lymphangiomyomatosis, and clear cell sugar tumor. Mod Pathol 1998;11:699–703. [PubMed] [Google Scholar]
- 26.Yan S, Brennick JB. False-positive rate of the immunoperoxidase stains for MART1/MelanA in lymph nodes. Am J Surg Pathol 2004;28:596–600. [DOI] [PubMed] [Google Scholar]


