Objective:
Almost half of patients with heart failure (HF) have cognitive impairment. While exercise relates to better cognitive health, a hallmark of HF is exercise intolerance. The study objective was to explore whether light-to-moderate exercise improves cognitive function in patients with HF. Methods: This was an exploratory parallel design study of 69 patients with symptomatic HF (mean age=65, SD = 10), recruited from VA and University of California, San Diego Healthcare Systems. Participants were randomized to Tai Chi (TC) (n = 24), resistance band (RB) exercise (n = 22) or treatment as usual (TAU) (n = 23). The primary outcome was change in Montreal Cognitive Assessment (MoCA) scores. We further explored if changes in Beck Depression Inventory – IA (BDI-IA) scores or inflammation biomarkers, CRP, TNFα and IL-6 related to altered cognitive function. Results: There was a fixed effect of group for MoCA scores changes (F = 8.07, p = .001). TC and RB groups had greater MoCA score increases versus TAU, but no differences were found between TC and RB. Depression symptom changes predicted altered MoCA scores (ΔR2 = .15, Δ = −.413, p = .001). However, group did not interact with depression symptom levels for MoCA alterations (p = .392). Changes in CRP levels predicted MoCA scores (ΔR2 = .078, Δ = −.283, p = .01), but group did not interact with CRP levels for MoCA alterations (p = .689). Conclusions: Light-to-moderate exercises, TC and RB may improve cognitive function. However, the mechanisms remain unclear. ClinicalTrials.gov: NCT01625819
Heart failure (HF) affects approximately 6.5 million adults in the United States (1). Fifty percent of patients with HF and 90% of all HF deaths occur in adults over 70 years of age (2). With age, diminishing cardio-protective and repair systems associated with adverse remodeling are related to progression of disease processes that markedly increase risk for development of HF (3). Simultaneously, cognitive impairment (CI) is increasingly recognized as a common co-morbidity of heart failure (HF), affecting 25 to 70% of patients, varying with patient age, disease severity and method of cognitive assessment (4–6). In HF, mild cognitive impairment (MCI) is the most common form of cognitive dysfunction and increases risk of dementia with an annual conversion rate of 10–15% (7). MCI is related to reduced patient decision-making capacity, and adequate self-care (8), and diminished disease self-management (9). In turn, HF patients with cognitive impairment have a five-fold increase in mortality (10).
The Institute of Medicine (IOM) lists physical activity as its primary recommendation for maintaining cognitive health in older persons (11). Because dementia is associated with considerable neuronal damage for which there is a lack of pharmacological treatments, developing behavioral interventions that prevent, slow-down, or reverse cognitive deterioration are imperative (12). Few older adult patients with HF attend traditional cardiac rehabilitation. Barriers include high copays, low exercise capacity and pain13, HF symptom severity, frailty (13) and co-morbidities (14). However, lighter intensity exercise may be more acceptable for older adults with HF. Physical exercise of at least light to moderate intensity (e.g. aerobic exercise, resistance training, multicomponent training, and Tai Chi) of a duration of 45–60 min per week was revealed in a meta-analysis from 36 studies of adults over 50 years of age, to produce significant cognitive improvements regardless of the cognitive status of participants (15). A recent study of 2354 men and women with a mean age of 53 years, found that physical activity of light-intensity was related to greater brain volume and less brain aging (16). A meta-analysis revealed global cognitive function improvements in cognitively impaired adults (ranging from MCI to dementia) practicing Tai Chi compared with both non-intervention controls (Hedge’s g=0.35; p=0.004) and active interventions (Hedge’s g=0.30; p=0.002) (17). However, there have been few intervention studies examining exercise training on cognitive function in patients with HF. Moreover, there is limited research examining mechanisms by which exercise may improve cognitive function in patients with HF.
Research indicates that greater depressive symptom severity is associated with worse cognitive performance (18, 19). This is relevant to HF, since clinically elevated depression symptom levels are present in up to 30% of patients with HF and are associated with increased clinical events, health care use, and mortality (20–22). Recently, our team reported from our parent study that practicing Tai Chi and resistance exercises led to reduced depression symptoms in patients with HF (23). Moreover, Alosco et al., found an indirect pathway between depression symptoms and cognitive function through cardiovascular fitness in patients with HF (24). While these findings from a large cohort study were provocative and compelling, the study was observational. However, it is yet unknown whether exercise related reductions in depression symptoms can improve cognitive function in patients with HF.
Studies suggest that low-grade inflammation may contribute to various age-related diseases (25); biomarkers of inflammation are also elevated in patients with HF (26). Moreover, relationships are evident between peripheral inflammation markers and cognitive function. For example, our previous cross-sectional study revealed that patients with HF and MCI had greater circulating peripheral vascular inflammation marker levels compared to patients without MCI (27). Another study found a negative relationship between peripheral inflammation biomarkers, C-reactive protein (CRP) and interleukin- 6 (IL-6), and cognitive function in patients with HF (28). A meta-analysis revealed that older adults with high circulating IL-6 were 1.42 times more likely to have cognitive decline (29). However, not all studies found relationships: in the Longitudinal Aging Study Amsterdam of 1,284 participants with HF showed no relationship between declining cognitive function and peripheral IL-6 levels (30). In turn, research suggests exercise can be protective of inflammation increases as people age (31). For example, older adults who exercise have lower levels of circulating IL-6 (32). A meta-analysis revealed exercise training led to reduced CRP (33). Less is known about patients with HF, whether exercise can reduce inflammatory processes and consequently improves cognitive function.
The present study was a sub-study from our parent study: Exploring Tai Chi as a Behavioral Intervention for Heart Failure. The primary aims of the parent study were to examine effects of light-to-moderate intensity exercise on cardiac function (percent left ventricular ejection fraction, %LVEF; End Systolic Volume, ESV; End Diastolic Volume, EDV). Secondary aims were to assess physical (6-minute walk test), and psychological function (depression symptoms). Results from primary and secondary aims are reported elsewhere (23). Considering prior research in individuals without HF depicting improved cognitive function with mild-to-moderate exercise and because of the high risk of cognitive impairment in patients with HF, our primary objective was to preliminarily explore effects of mild-to-moderate exercise on cognitive function in patients with HF. To examine potential mechanisms, we explored whether depression symptoms and inflammation biomarkers relate to cognitive function changes, as a response to the mild-to-moderate exercises. We previously reported depression symptoms as a dependent outcome measure in response to participation in the parent RCT (23). However, cognitive function and its relationship with depression symptoms and inflammation biomarkers were not previously reported.
METHODS
Trial Design:
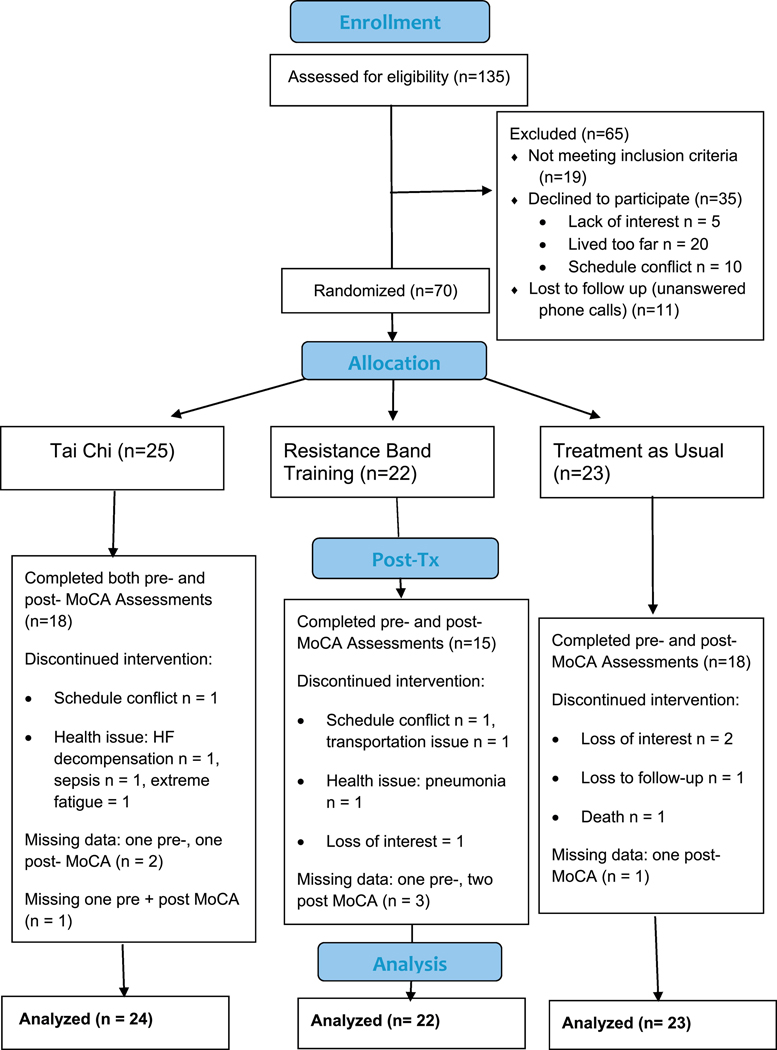
The parent study is registered at ClinicalTrials.gov: NCT01625819. Study details were reported previously (23). Briefly, this was a randomized-controlled trial (RCT) using a parallel design. Participants were randomized to one of three conditions: Tai Chi (TC), resistance band training (RB) or treatment as usual (TAU). This sub-study examined 69 patients with HF who received either pre- or post- Montreal Cognitive Assessment in addition to the assessments of the parent study (see CONSORT diagram, Figure 1), including depression symptoms, inflammation biomarkers and demographic information. The protocol for the parent study and cognitive testing sub-study received approval by VASDHS and UCSD Institutional Review Boards.
Figure 1.
CONSORT diagram
Participants:
Participants were recruited, and the study was performed at the Psychiatry Department at the University of California, San Diego (UCSD) and the VA San Diego Healthcare System (VASDHS). Inclusion criteria for the parent study consisted of a diagnosis with American Heart Association/American College of Cardiology classification, Stage C symptomatic HF (both preserved (HFpEF) and reduced (HFrEF) ejection fraction subtypes) of at least 3 months, clinically stable: not having been hospitalized for a 3-month period, on stable doses of neurohormonal blocking agents and diuretics for at least 3 months, no cardiac surgeries for at least 6 months. Exclusion criteria included the presence of any psychiatric diagnosis (other than major depression) (see Table 1 for participant characteristics).
Table 1.
Baseline Subject Characteristics
| N | Total 69 | TC 24 | RB 22 | TAU 23 | P value |
|---|---|---|---|---|---|
| Age | 65 (10) | 63 (9) | 65 (9) | 67(7) | .52 |
| Sex (% male) | 89 | 92 | 86 | 87 | .81 |
| Race (% white) | 68 | 68 | 82 | 54 | .29 |
| %LVEF | 46 (14) | 44 (13) | 46 (14) | 46 (12) | .85 |
| Smokers (n) | 9 | 4 | 3 | 2 | .68 |
| HFpEF (n) | 29 | 10 | 10 | 9 | .79 |
| BMI (kg/m2) | 32(8) | 32(8) | 33(8) | 31(6) | .64 |
| Marital (married) | 30 | 32 | 23 | 29 | .57 |
| MoCA score | 24(4) | 24(3) | 22(4) | 24(4) | .14 |
| BDI | 9 (7) | 10 (6) | 12 (8) | 8 (6) | .17 |
| CRP (mg/L) | 2.5 (3.5) | 7(4.8) | 1.6 (1.1) | 2.0 (3.0) | .33 |
| IL-6 (pg/ml) | 1.5 (.84) | 1.5 (.85) | 1.9 (.93) | 1.3(.75) | .34 |
| TNFα (pg/ml) | 3.2 (1.2) | 3.3 (1.4) | 3.8 (1.1) | 2.7 (.54) | .08 |
Data are reported as mean ± SD or ‘n’. Analysis of variance or Kruskal-Wallis test were used to determine significant baseline group differences. TC = Tai Chi; RB = resistance band; TAU = treatment as usual; BDI = Beck Depression Index; BMI = body mass index; HFpEF = heart failure with preserved ejection fraction; LVEF = left ventricular ejection fraction; MoCA=Montreal Cognitive Assessment; CRP = C-reactive protein; IL-6 = interleukin-6; TNFa = tumor necrosis factor- alpha
Interventions:
Interventions were previously described (23). Briefly, participants were randomly assigned to three conditions. The two active groups consisted of Tai Chi Chuan-Short Form (Yang-style, first third) (TC) and RB training that was based on the U.S. Department of Veterans Affairs “Move” program Move (39). TC and RB participants attended classes twice per week for 60 minutes per session for 16- weeks and were also asked to practice at home for 10–20 minutes per day on non-class days. The treatment as usual (TAU) group did not take part in an intervention. As described previously (23), RB replaced an education control condition in response to NIH study section reviewer recommendations. Exercises for both groups were each led by an experienced instructor, performed in groups of 6 – 8 participants held at the UCSD Medical Center. RB exercise was chosen as a comparison to TC due to the psychosocial and light-to-moderate physical exertion level similarities. Both the TC and the RB require minimal equipment, can be performed in community centers, clinics, and practiced at home. For the present study, the instructors had more than 10 years of experience instructing chronically ill and older adults. Both interventions had standardized manuals that were followed by instructors, with written materials provided to both groups to support home practice. All participants received standard of care throughout the study including regular visits to their primary care physicians, cardiologist, and other health specialists. In both the TC and RB intervention groups, participants were asked to exercise at an intensity using the Borg ratings of perceived exertion (RPE) of 11–13 (“fairly light” to “somewhat hard”). The Borg RPE scale ranges from 6 to 20 with 6 described as “no exertion” and a score of 20 is “maximum exertion.” (40) The parent study (23) found a 16% dropped out, with no group differences in drop-out rates (p >.5). The TC group attended a median of 87% of classes and practiced a median of 74 min/wk and the RB group attended 81% of classes and practiced 60.8 min/wk. There were no differences between the two active intervention groups for class attendance (p > .5) or practice time outside of class (p > .5). There were no adverse events (harms) associated with the interventions.
Outcomes:
Outcomes were assessed at baseline and after 16- weeks (post- interventions). Montreal Cognitive Assessment (MoCA) was added to the parent study as an exploratory measure, prior to the parent study’s commencement to gather preliminary information on cognitive function in response to mild-to-moderate exercise. One of three versions of the MoCA (34) were randomly assigned to each participant and counterbalanced for follow-up visits. The MoCA is a one-page test, that takes approximately 10 minutes to complete. It assesses cognitive domains including, short-term memory recall, executive function, verbal abstraction, visuospatial abilities, working memory, concentration, attention, language, and orientation. One point was added for subjects with 12 years or fewer of formal education, for a possible maximum of 30 points. Lower scores indicate lower cognitive function. Among HF, MoCA exhibits a sensitivity of 64% and specificity of 66% to detect cognitive impairment, compared with a full neuropsychological battery; furthermore, discriminant function analyses indicate patient differentiation of impairment on multiple domains (35). A review of the literature suggests that a stringent score of less than 24 yielded the best diagnostic accuracy for determining MCI versus healthy cognitive aging (36).
The 21-item Beck Depression Inventory −1A (BDI-IA):
shows high reliability and ability to discriminate between depressed and non-depressed participants with broad application in research and clinical practice (37). The BDI has often been used to measure depression in patients with cardiovascular disease (CVD).
Biomarkers:
Blood was drawn from participants between 0800 h and 0900 h. We assessed inflammation biomarkers known to be associated with cognitive impairment and depression including IL-6, C-reactive protein (CRP), and TNFa. (28, 29, 38) Whole blood was drawn into a 10 mL vacutainer tube preserved with EDTA after a ten-minute rest period, while participants were in an upright sitting position. Whole blood samples were placed on ice immediately, centrifugation occurred within 30 minutes, and plasma was stored at −80 ° C. Commercial high sensitivity ELISA (Meso Scale Discovery, Rockville, MD) were used to determine circulating levels of biomarkers and performed in duplicate. The assay sensitivities of these markers range from 0.1 to 10.1 pg/mL.
Sample Size, Randomization and Blinding:
Since the present investigation was exploratory, a power analysis was not performed prior to collecting cognitive data. However, a post-hoc power analysis indicated a power of 0.80 to detect a medium effect size (η2 = .06) (42) with an alpha of 0.05 to determine main effects of group on MoCA scores over time. Following the baseline visit, the study coordinator generated the randomization sequence without stratification using a computer-generated algorithm and randomized participants to one of three groups: TC, RB or TAU. The sequence was concealed from the principal investigator, recruitment personnel, patients and research assessors until interventions were assigned. Assessors, recruiters and principal investigator remained blinded to group allocation.
Statistical Methods:
Analyses were performed using SPSS version 25 (IBM Corp, Armonk, NY). Skewness and data distribution were determined by the Kolmogorov-Smirnov test and were transformed logarithmically for variables that were not normally distributed (i.e. pro-inflammatory markers, IL-6, TNFα and CRP). Baseline differences between groups were examined using analysis of variance (ANOVA) for continuously measured variables and Kruskal-Wallis test for non-continuous variables. Primary outcomes were analyzed with an intent-to-treat mixed-effects model to examine the efficacy of TC compared with RB exercises and TAU over 16 weeks of treatment (41). The analyses for group differences over time in cognitive function consisted of a model that included group, time, and group × time interaction as fixed effects with a heterogeneous first order autoregressive covariance structure, while adjusting for %LVEF and age. Exploratory linear regression analyses were performed with mean substitution for missing data to examine associations among BDI and biomarker changes, and MoCA scores over time, while adjusting for %LVEF, age and body mass index (BMI). Also, group x BDI and biomarker interactions, on changes in MoCA scores were examined.
RESULTS
Participant Flow, Recruitment and Baseline Demographics:
A CONSORT diagram of participant flow for the present sub-study is presented in Figure 1. Participants from the parent and sub-study were recruited beginning 3/17/2011 and active patient participation ended 04/30/2015 at the conclusion of grant funding. Registration for ClinicalTrials.gov was completed 4/18/12 and is still open for analyses. We did not perform analyses from the study data prior to the clinical trials registration. Of the 70 participants in the parent study, MoCA measures pre- or post- 16-weeks were collected from 69 participants by a trained research assistant blinded to group assignment (TC: N = 24, RB: N = 22, TAU: N = 24). The baseline median age of the participants was 65 (range = 44 – 89), VA patients were 70% of the sample, 90% of participants were male, MoCA scores averaged 24 (SD = 4) and BDI scores averaged 10 (SD = 7) suggesting mildly elevated depression symptoms (43). A total of 41% of participants were below cutoff for MCI (≤ 23) (36). Primary analyses revealed that the groups did not differ regarding age, race, gender, education, smoking status, body mass index, depression symptoms, cognitive function scores, and inflammation biomarkers (see Table 1 for baseline participant characteristics). Also, those who dropped out of the study or had missing MoCA’s did not differ from those who had complete data (p’s > .10).
Primary Outcomes
Group differences in cognitive function (see Table 2):
Table 2.
Pre- and post- 16 weeks
| TC (n = 18) | RB (n = 15) | TAU (n = 18) | ||
|---|---|---|---|---|
| MoCA* | pre | 24.3 (3) | 22.9 (4) | 24.7 (2) |
| post | 25.2 (3) | 24.9 (4) | 23.6 (3) | |
| BDI* | pre | 9.6(6) | 11.9 (7) | 8.0 (6) |
| post | 6.1 (5) | 8.6 (6) | 7.0 (6) | |
| CRP mg/L | pre | 3.7 (4.8) | 1.6 (1.1) | 2.0 (3.0) |
| post | 4.5 (4.4) | 1.4 (1.5) | 2.2 (2.4) | |
| IL-6 pg/ml | pre | 1.5 (.8) | 1.9 (.9) | 1.3 (.7) |
| post | 2.0 (1.4) | 1.8 (1.6) | 1.3 (.7) | |
| TNFa pg/ml | pre | 3.3 (1.2) | 3.8 (1.1) | 7 (.5) |
| post | 3.0 (1.2) | 3.8 (1.8) | 2.7 (1.3) | |
Repeated measures analysis of variance (ANOVA)
(p< .05)
Data are reported as mean ± SD. TC = Tai Chi; RB = resistance band; TAU = treatment as usual; BDI = Beck Depression Index; MoCA=Montreal Cognitive Assessment; CRP = C-reactive protein; IL-6 = interleukin-6; TNFα = tumor necrosis factor- alpha
This was an intent-to-treat study of 69 participants from the parent study. A mixed-effects model revealed significant fixed effect group differences over time for MoCA scores (group x time interaction, F = 8.11, p = .001), while adjusting for %LVEF and age. Compared with the TAU group, which decreased there were significant estimated fixed effect mean increases in MoCA scores over time for the TC group [95% CI, 0.55 to 4.1, p = .011] and the RB group [95% CI, 1.7 to 5.4, p < .001]. There were no differences over time between TC and RB groups [95% CI, −3.3 to 7.5, p = .21].
Exploratory analyses:
Relationships among biomarkers, depression and cognitive scores:
Analyses were performed from 69 participants using mean imputation for missing data (see CONSORT diagram). Depression symptoms in both TC and RB groups were reduced over the 16-week interventions compared with TAU (p’s < .05) (as reported in the parent study (23)). While baseline depression scores were not significantly related to baseline MoCA scores (p > .1), changes in BDI-IA negatively predicted changes in MoCA scores (ΔR2 = .15, Δ = −.413, p < .001), while adjusting for %LVEF and age. However, there was not a significant interaction between group x BDI-IA on MoCA scores (ΔR2 = .007, Δ = .244, p = .392). Similarly, changes in CRP levels predicted alterations in MoCA scores (ΔR2 = .078, Δ = −.283, p = .011), while adjusting for %LVEF, BMI and age, but not an interaction between group x CRP on MoCA scores (ΔR2 = .002, Δ = .104, p = .689). (see Table 3). There was a trend for changes in IL-6 to predict alterations in MoCA scores (ΔR2 = .043, B = −.209, p = .062) and TNFα did not predict alterations in cognitive function over 16-weeks (p = .97). Neither IL-6 or TNFα-interacted with group for alterations in MoCA scores (p > .20). Similar findings were revealed by excluding cases listwise, whereby changes in depression symptoms and CRP levels (p’s < .05), and a trend for IL-6 predicted reductions in MoCA scores (p’s < .07) and similar lack of interactions with group for MoCA scores (p’s > .30).
Table 3.
Linear Regressions: Dependent variable = post treatment- MoCA.
| ΔR2 | Beta | P | |
|---|---|---|---|
| Age** | .106 | −.326 | .006 |
| %LVEF | .002 | −.044 | .706 |
| MoCA (pre- tx)** | .095 | .327 | .006 |
| ΔBDI** | .150 | −.413 | .001 |
| Group | .010 | −.102 | .329 |
| Group X ΔBDI | .007 | .244 | .392 |
| Age** | .106 | −.326 | .006 |
| %LVEF | .002 | −.044 | .706 |
| MoCA (pre- tx)** | .095 | .327 | .006 |
| ΔCRP* | .078 | −.283 | .010 |
| Group | .029 | −.173 | .107 |
| Group X ΔCRP | .002 | .104 | .689 |
| Age** | .106 | −.326 | .006 |
| %LVEF | .002 | −.044 | .706 |
| MoCA (pre- tx)** | .095 | .327 | .006 |
| ΔIL-6 | .042 | −.207 | .061 |
| Group | .039 | −.204 | .066 |
| Group X ΔIL-6 | .016 | −.317 | .236 |
| Age** | .106 | −.326 | .006 |
| %LVEF | .002 | −.044 | .706 |
| MoCA (pre- tx)** | .095 | .327 | .006 |
| ΔTNFα | .001 | .006 | .957 |
| Group | .028 | −.173 | .130 |
| Group X ΔTNFα | .005 | −.207 | .520 |
BDI = Beck Depression Index; MoCA=Montreal Cognitive Assessment; CRP = C-reactive protein; IL-6 = interleukin-6; TNFα = tumor necrosis factor- alpha;
p < .05,
p<.01
DISCUSSION
As hypothesized, practicing TC and RB exercises for 16- weeks related to improved cognitive function scores compared with the TAU (see Table 2). Our findings correspond with studies in non-HF groups, including studies of older adults showing light-to-moderate exercise increased cognitive function (15, 17). Importantly, CI in older patients with HF can result in a vicious circle resulting in worsening HF prognosis, since older patients are more likely to be non-compliant due to difficulties adhering to complex medical regimens (3). Future studies are needed to examine whether light-to-moderate intensity exercises in patients with HF may improve the ability to comply with medically necessary self-care routines.
Studies suggest depression symptoms are related to worsening cognition in older adults, such as a study of over 8000 men and women, age 65 and older from the US Health and Retirement Study (44). In a study of over 300 patients with HF (mean age = 69) there was a correspondence between depression symptoms and cognitive function (18). In another study of older adults with HF, a model was tested showing a relationship between depression symptoms and cognitive function, which was mediated through cardiovascular fitness (24). However, there are a lack of exercise intervention-studies in patients with HF that have prospectively examined changes in depression symptoms on cognitive function. In the present study of older adults with HF, our baseline data did not find a relationship between depression symptoms and cognitive function; whereas, reductions in depression symptoms over 16-weeks were related to cognitive improvements. However, while both light-to-moderate exercise groups had improved depression symptoms and cognitive scores, we did not find relationships among group, depression symptom changes and cognitive function. This may suggest that depression and cognitive outcomes were independent responses to mind-to-moderate exercise. Although, the lack of relationship may be due to the small sample size and should be examined more fully in a larger-scaled randomized controlled trial.
Prior reports observed relationships between elevated inflammation biomarker levels and cognitive function (27, 28). Our present study found that higher baseline levels of TNFα were related to lower cognitive function and increases in CRP levels over 16-weeks were related to lower cognitive function. However, we did not find a relationship among group, inflammation biomarker change and cognitive function. Also, we did not find group differences in inflammation biomarker levels over time. Our findings may be similar to other exercise studies including the HF-ACTION study which also found no reductions in inflammation markers including CRP, IL-6, or TNFα in response to exercise practice (45, 46). Notably, patients with HF have elevated inflammation levels (47), which may preclude shifting these levels to a clinically meaningful degree with exercise.
As previously stated, the primary aims of the parent study are published elsewhere (23). The main limitation of this exploratory sub-study is a modest sample size resulting in limited statistical power and results should be interpreted with caution. Because of a deficiency of eligible patients with symptomatic HF from two clinic sites, our small modular R01 enrolled 70 participants, from the projected 135 participants. The present sub-study should be repeated with a larger multi-site investigation. Having a small sample size barred the inclusion of important covariates that may affect cognitive function, depression and inflammation. Moreover, we could not address differences between HF with preserved ejection fraction (HFpEF) and reduced ejection fraction (HFrEF) effectively. However, both groups are known to experience cognitive impairment (49) and therefore, the findings are likely relevant to both groups. Other limitations include a lack of measurement of exercise intensity, although participants in both groups were asked to perform activities at a Borg scale level of light to moderate difficulty, by increasing the size of body movements for the TC group and increasing the resistance for the RB group. Moreover, TC and resistance exercises are determined by other investigators to be light-to-moderate intensity in older adults (15, 17). Another major limitation was the small number of women in the study, since most patients were recruited from the VA hospital. This limits the generalizability of the trial findings.
Summary and conclusion: Participation in both TC and RB exercises were associated with greater improvements in cognitive function scores compared with TAU. As we previously reported, depression symptoms also declined more in the TC and RB groups compared with TAU (23); while, the sub-study saw no differences between groups in inflammation factors over the 16-week period. It was also observed that improved cognitive function scores over time for the whole cohort were related to reduced depression symptoms and CRP levels. However, group participation did not appear to influence these relationships. This suggests that while depression symptoms and inflammation factors may be related to cognitive function in patients with HF, they may not be mechanistic factors for improvements in cognitive function in response to mild-to-moderate exercise. These results should be considered exploratory and a larger, fully powered study is needed to evaluate the efficacy of TC and resistance training and for preventing cognitive decline in aging and the mechanisms that may be involved.
Highlights.
Around half of patients with heart failure have cognitive impairment.
The present study found light-to-moderate exercise interventions were related to increased cognitive function scores compared with treatment-as-usual, in patients with heart failure.
Depression symptoms and inflammation biomarker, C-reactive protein levels were related to cognitive function among the whole cohort.
Acknowledgments
This research was supported by R01HL096784
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11:e004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, Subcommittee AHASCaSS. Heart disease and stroke statistics−−2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8:143–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, Ding Y, Kim J, Sloan R, Jaynes H, Shaw RM. Cognitive deficits in chronic heart failure. Nurs Res. 2010;59:127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon JA, Moffitt P, Perez-Moreno AC, Walters MR, Broomfield NM, McMurray JJV, Quinn TJ. Cognitive Impairment and Heart Failure: Systematic Review and Meta-Analysis. J Card Fail. 2017;23:464–75. [DOI] [PubMed] [Google Scholar]
- 6.Alagiakrishnan K, Mah D, Dyck JR, Senthilselvan A, Ezekowitz J. Comparison of two commonly used clinical cognitive screening tests to diagnose mild cognitive impairment in heart failure with the golden standard European Consortium Criteria. Int J Cardiol. 2017;228:558–62. [DOI] [PubMed] [Google Scholar]
- 7.Espinosa A, Alegret M, Valero S, Vinyes-Junque G, Hernandez I, Mauleon A, Rosende-Roca M, Ruiz A, Lopez O, Tarraga L, Boada M. A longitudinal follow-up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. J Alzheimers Dis. 2013;34:769–80. [DOI] [PubMed] [Google Scholar]
- 8.Davis KK, Himmelfarb CR, Szanton SL, Hayat MJ, Allen JK. Predictors of heart failure self-care in patients who screened positive for mild cognitive impairment. J Cardiovasc Nurs. 2015;30:152–60. [DOI] [PubMed] [Google Scholar]
- 9.Dardiotis E, Giamouzis G, Mastrogiannis D, Vogiatzi C, Skoularigis J, Triposkiadis F, Hadjigeorgiou GM. Cognitive impairment in heart failure. Cardiol Res Pract. 2012;2012:595821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feola M, Garnero S, Vallauri P, Salvatico L, Vado A, Leto L, Testa M. Relationship between Cognitive Function, Depression/Anxiety and Functional Parameters in Patients Admitted for Congestive Heart Failure. Open Cardiovasc Med J. 2013;7:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Io Medicine. Cognitive Aging: Progress in Understanding and Opportunities for Action. Blazer DG, Yaffe K, Liverman CT, editors. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 12.Gary RA, Brunn K. Aerobic exercise as an adjunct therapy for improving cognitive function in heart failure. Cardiol Res Pract. 2014;2014:157508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denfeld QE, Winters-Stone K, Mudd JO, Hiatt SO, Chien CV, Lee CS. Frequency of and Significance of Physical Frailty in Patients With Heart Failure. Am J Cardiol. 2017;119:1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conraads VM, Deaton C, Piotrowicz E, Santaularia N, Tierney S, Piepoli MF, Pieske B, Schmid JP, Dickstein K, Ponikowski PP, Jaarsma T. Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2012;14:451–8. [DOI] [PubMed] [Google Scholar]
- 15.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52:154–60. [DOI] [PubMed] [Google Scholar]
- 16.Spartano NL, Davis-Plourde KL, Himali JJ, Andersson C, Pase MP, Maillard P, DeCarli C, Murabito JM, Beiser AS, Vasan RS, Seshadri S. Association of Accelerometer-Measured Light-Intensity Physical Activity With Brain Volume: The Framingham Heart Study. JAMA Netw Open. 2019;2:e192745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wayne PM, Walsh JN, Taylor-Piliae RE, Wells RE, Papp KV, Donovan NJ, Yeh GY. Effect of tai chi on cognitive performance in older adults: systematic review and meta-analysis. J Am Geriatr Soc. 2014;62:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins MA, Dolansky MA, Schaefer JT, Fulcher MJ, Gunstad J, Redle JD, Josephson R, Hughes JW. Cognitive Function in Heart Failure Is Associated With Nonsomatic Symptoms of Depression But Not Somatic Symptoms. J Cardiovasc Nurs. 2015;30:E9–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, Garcia S, Josephson R, van Dulmen M, Hughes J, Rosneck J, Gunstad J. The interactive effects of cerebral perfusion and depression on cognitive function in older adults with heart failure. Psychosom Med. 2013;75:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherwood A, Blumenthal JA, Trivedi R, Johnson KS, O’Connor CM, Adams KF Jr., Dupree CS, Waugh RA, Bensimhon DR, Gaulden L, Christenson RH, Koch GG, Hinderliter AL. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med. 2007;167:367–73. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, Califf RM, Krishnan RR, O’Connor CM. Relationship between depressive symptoms and longterm mortality in patients with heart failure. Am Heart J. 2007;154:102–8. [DOI] [PubMed] [Google Scholar]
- 22.Friedmann E, Thomas SA, Liu F, Morton PG, Chapa D, Gottlieb SS. Relationship of depression, anxiety, and social isolation to chronic heart failure outpatient mortality. Am Heart J. 2006;152:940 e1–8. [DOI] [PubMed] [Google Scholar]
- 23.Redwine L, Wilson K, Pung M, Chinh K, Rutledge T, Mills P, Smith B. A randomized study examining the effects of mild-to-moderate group exercises on cardiovascular, physical, and psychological well-being in patients with heart failure. Journal of Cardiopulmonary Rehabilitation and Prevention. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alosco ML, Spitznagel MB, van Dulmen M, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Hughes J, Rosneck J, Gunstad J. Depressive symptomatology, exercise adherence, and fitness are associated with reduced cognitive performance in heart failure. J Aging Health. [Research Support, N.I.H., Extramural]. 2013;25:459–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Benedetto S, Muller L, Wenger E, Duzel S, Pawelec G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev. 2017;75:114–28. [DOI] [PubMed] [Google Scholar]
- 26.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redwine LS, Pung MA, Wilson K, Chinh K, Duffy AR. Differential Peripheral Inflammatory Factors Associated with Cognitive Function in Patients with Heart Failure. Neuroimmunomodulation. 2018;25:146–52. [DOI] [PubMed] [Google Scholar]
- 28.Athilingam P, Moynihan J, Chen L, D’Aoust R, Groer M, Kip K. Elevated levels of interleukin 6 and C-reactive protein associated with cognitive impairment in heart failure. Congest Heart Fail. 2013;19:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradburn S, Sarginson J, Murgatroyd CA. Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-demented Adults: A Meta-Analysis of Prospective Studies. Front Aging Neurosci. 2017;9:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–7. [DOI] [PubMed] [Google Scholar]
- 31.Santos-Parker JR, LaRocca TJ, Seals DR. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ. 2014;38:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrer MD, Capo X, Martorell M, Busquets-Cortes C, Bouzas C, Carreres S, Mateos D, Sureda A, Tur JA, Pons A. Regular Practice of Moderate Physical Activity by Older Adults Ameliorates Their Anti-Inflammatory Status. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med. 2017;51:670–6. [DOI] [PubMed] [Google Scholar]
- 34.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins MA, Gathright EC, Gunstad J, Dolansky MA, Redle JD, Josephson R, Moore SM, Hughes JW. The MoCA and MMSE as screeners for cognitive impairment in a heart failure population: a study with comprehensive neuropsychological testing. Heart Lung. 2014;43:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018;33:379–88. [DOI] [PubMed] [Google Scholar]
- 37.Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Rev Bras Psiquiatr. 2013;35:416–31. [DOI] [PubMed] [Google Scholar]
- 38.Trollor JN, Smith E, Baune BT, Kochan NA, Campbell L, Samaras K, Crawford J, Brodaty H, Sachdev P. Systemic inflammation is associated with MCI and its subtypes: the Sydney Memory and Aging Study. Dement Geriatr Cogn Disord. 2010;30:569–78. [DOI] [PubMed] [Google Scholar]
- 39.Affairs USDoV. Move weight management program. Available from: https://www.move.va.gov/docs/NewHandouts/PhysicalActivity/P20_ResistanceTubesAndBands.pdf. [Google Scholar]
- 40.Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16:55–8. [DOI] [PubMed] [Google Scholar]
- 41.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–7. [DOI] [PubMed] [Google Scholar]
- 42.Richardson JTE. Eta squared and partial eta squared as measures of effect size in educational research. Educational Research Review. 2011;6:135–47. [Google Scholar]
- 43.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. [DOI] [PubMed] [Google Scholar]
- 44.Donovan NJ, Wu Q, Rentz DM, Sperling RA, Marshall GA, Glymour MM. Loneliness, depression and cognitive function in older U.S. adults. Int J Geriatr Psychiatry. 2017;32:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prescott E, Hjardem-Hansen R, Dela F, Teisner AS, Nielsen H. Exercise training in older patients with systolic heart failure: adherence, exercise capacity, inflammation and glycemic control. Scand Cardiovasc J. 2009;43:249–55. [DOI] [PubMed] [Google Scholar]
- 46.Ahmad T, Fiuzat M, Mark DB, Neely B, Neely M, Kraus WE, Kitzman DW, Whellan DJ, Donahue M, Zannad F, Pina IL, Adams K, O’Connor CM, Felker GM. The effects of exercise on cardiovascular biomarkers in patients with chronic heart failure. Am Heart J. 2014;167:193–202.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dick SA, Epelman S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ Res. 2016;119:159–76. [DOI] [PubMed] [Google Scholar]
- 48.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45:626–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albabtain M, Brenner MJ, Nicklas JM, Hummel SL, McCormick MP, Pawlowski JL, Remington TL, Gure TR, Dorsch MP, Bleske BE. Hyponatremia, Cognitive Function, and Mobility in an Outpatient Heart Failure Population. Med Sci Monit. 2016;22:4978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]