Abstract
Diarrhea-predominant irritable bowel syndrome (IBS-D) is a common chronic functional gastrointestinal disorder. MicroRNAs (miRNAs) have been identified to be involved in different physiological and pathological processes. In this study, the role of miRNA-29a in the potential mechanism underlying the function of the intestinal mucosal barrier in IBS-D was analyzed. Human intestinal mucosal epithelia from patients with IBS-D (diagnosed as meeting the Rome IV criteria) and healthy volunteers were collected. An IBS-D mouse model was established via induction with trinitro-benzene-sulfonic acid (TNBS), and the mice were injected with miRNA-29a inhibitor. Using transmission electron microscopy (TEM), the epithelial ultrastructure of the human intestinal mucosa was examined. Using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis, the expression level of miRNA-29a was assessed. ELISA was used to analyze the activity of D-lactate (D-LA) and diamine oxidase (DAO). Through immunohistochemistry, RT-qPCR and western blotting, the expression of tight junction protein ZO-1 (ZO-1) and claudin-1 (CLDN1) was examined. In the human intestinal mucosal epithelia from patients with IBS-D, miRNA-29a was upregulated, ZO-1 and CLDN1 were downregulated, and the junctional complex (JC) was faint and discontinuous. In the IBS-D mouse model, treatment with miRNA-29a inhibitor downregulated D-LA and DAO activity, and increased the expression of ZO-1 and CLDN1 in the intestinal mucosal epithelium. In conclusion, the present study revealed that miRNA-29a is involved in the pathogenesis of IBS-D, probably by downregulating ZO-1 and CLDN1 expression, suggesting that miRNA-29a is likely to be an important regulator of intestinal barrier function and could be a possible therapeutic target for IBS-D.
Keywords: diarrhea-predominant irritable bowel syndrome, intestinal barrier, microRNA-29a, tight junction protein ZO-1, claudin 1
Introduction
IBS-D is a common chronic functional gastrointestinal disorder characterized by chronically recurring abdominal pain, diarrhea, discomfort that is relieved by defecation, or altered bowel habits, which correlate with impaired intestinal mucosal barrier function (1). Previous studies have demonstrated that the prevalence of IBS-D is 5-10% in the general population, and that it may result from immune activation, intestinal barrier dysregulation and low-grade inflammation (2-4). IBS-D severely affects quality of life; however, the pathophysiology of IBS-D is poorly understood.
Accumulating evidence indicates that intestinal barrier function is impaired in IBS-D (5). Tight junctions (TJs) regulate the paracellular permeability of the intestinal barrier. The cytosolic protein tight junction protein ZO-1 (ZO-1) is a vital protein in TJs; the transmembrane proteins, including claudin-1 (CLDN1), and the cytosolic protein bind to the actin cytoskeleton to control paracellular permeability (6). Recent evidence suggests that the impaired function of the intestinal mucosal barrier may be implicated in the pathological and pathogenic processes of IBS-D, indicated by an increase in intestinal permeability (7).
MicroRNAs (miRNAs) are small non-coding single-stranded RNA molecules, which serve a vital role in cell proliferation, apoptosis and differentiation (8). miRNA binding sites are usually located in the 3' untranslated region (UTR) of their target mRNAs and regulate translation (9). Numerous studies have demonstrated that miRNAs have been extensively involved in abundant physiological and pathological processes, which serve important roles in inflammation and the immune response (10,11). Recently, the correlation between miRNAs and IBS-D have showed that the change of miRNAs have affected IBS-D, including the function of intestinal mucosal barrier (12). miRNA-29a is an important member of the miRNA-29 family. A recent study showed that miRNA-29a regulates intestinal membrane permeability via the glutamine synthetase gene (GLUL) in IBS-D (13). Proteins regulated by miRNA-29a remain to be fully elucidated. Decreased ZO-1 and CLDN1 expression are observed in the colonic tract of patients with IBS-D, which contributes to weakening of the intestinal barrier (14). However, whether miRNA-29a is involved in the intestinal barrier pathophysiology of IBS-D by regulating ZO-1 and CLDN1 expression remains unclear.
In the present study, to evaluate the role of miRNA-29a in intestinal mucosal barrier function, specimens were collected from patients with IBS-D and IBS-D mouse models were established, to compare the changes in miRNA-29a, ZO-1 and CLDN1 in IBS-D.
Materials and methods
Patients
The study included 21 patients with IBS-D admitted to the Department of Gastroenterology at the First Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangzhou, China) between April 2017 and January 2018. There were 13 males and 8 females aged 21-56 years old (mean age, 35 years old; median age, 29 years old). All patients met the Rome IV criteria for IBS-D (15). There were 7 male and 9 female healthy volunteers (mean age, 30 years old; median age 28, years old) recruited as a control group. The human specimens collected were sigmoid colon mucosa. All patients underwent screening colonoscopies and provided written informed consent before the study. The experiment was approved by the Medical Research Ethics committee of The First Affiliated Hospital of Guangzhou University of Chinese Medicine (approval no. AF/JD-02/02), and was conducted in accordance with the principles expressed in the Declaration of Helsinki. The specimens collected from humans were stored at -80˚C.
Ultrastructural observation by TEM
The colonoscopic biopsies from three patients with IBS-D and three healthy volunteers were cut into 1 mm3 strips, and immediately fixed in 2.5% glutaraldehyde for 4 h at 4˚C. The strips were post-fixed in 2% osmium tetroxide in 0.1 mol/l PBS (pH 7.4) for 2 h. Sample strips were dehydrated in graded ethanol and subsequently embedded in Epon 812. Samples were cut into ultrathin sections (75 nm) and observed in a JEM-1400 electron microscope (Hitachi).
Animals
This study was approved by The Animal Experimental Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (approval no. TCMF1-2017009). The experimental procedure was performed according to the Guide for the Use and Care of Laboratory Animals. A total of 40 specific pathogen-free male C57BL/6J mice (weighing 20-25 g) were purchased from the Guangzhou Experimental Animals Center (Guangzhou, China; Certificate no. SCXK [Yue] 2013-0092). The mice were housed at a constant temperature of 20-22˚C in sawdust-lined plastic cages and maintained on a 12:12-h light-dark cycle. The standard chow diet and water were provided ad libitum.
Immunohistochemistry for ZO-1 and CLDN1 expression
The immunohistochemistry study was performed as described previously (16). The mouse tissue sections (4 µm thick) were immersed in PBS and placed in polycarbonate staining jars (Kartell) filled with 10 mM sodium citrate buffer solution (pH 6.0). Following exposure to microwaves for 15 min for antigen retreival, the tissues were incubated in a solution of 10% bovine serum albumin at room temperature for 10 min. Sections were incubated with rabbit polyclonal ZO-1 antibody (cat. no. 21773-1-AP; 187 µg/150 µl; 1:300; Proteintech Group, Inc.) and rabbit polyclonal antibody CLDN1 (cat. no. 4933, 1:200, Cell Signaling Technology, Inc.), respectively, incubated at 4˚C overnight. Following washing in PBS, polyperoxidase rabbit IgG secondary antibody (cat. no. SP-9001; 1:1,000; OriGene Technologies, Inc.) was incubated for 2 h at room temperature, and the sections were rinsed and cover-slipped. Subsequently, the sections were examined with a microscope (Nikon 80). All areas were analyzed under the same sensitivity captured with x200 magnification using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.). The positive staining area was selected in the colon mucosa and the optical density was automatically calculated. The average optical density values (IOD/area) in the colonic mucosa were calculated from 8 random fields per section. In total, 10 samples in each group were analyzed.
Establishment of the IBS-D mouse model
An experimental mouse model of IBS-D was established as per previous study (17,18). Then, 40 mice were randomly assigned into four groups: Control, model, miRNA-29a negative control and miRNA-29a inhibitor groups. A total of 10 mice were used in each group. Following a period of fasting, not including water, for 24 h, the mice were anesthetized with 5% diethyl ether (19,20). The use of ether was approved by the ethics committee. Due to the animal individual differences, the tolerance of anesthesia varies. We observed body reaction of mice during the process, including breath, heart rate, muscular tension and corneal reflex. These parameters were monitored to ensure the animals were fully anesthetized following the administration of ether (21). Under anesthesia, the mouse model of IBS-D was established by administration of TNBS into the proximal colon. Following treatment with TNBS (1 mg/mouse in 50% ethanol), the mice were subsequently maintained in a headstand for 2 min. The mice in the control group were administrated an equal volume of 50% ethanol instead of TNBS. Before the collection of tissue, the mice were euthanized by cervical dislocation under anesthesia, induced by the inhalation of 5% isoflurane. Cardio-respiratory arrest, absence of reflex was monitored to ensure the euthanasia.
ELISA of D-LA and DAO
Mouse blood was extracted from the abdominal aorta, and separated at 1,000 x g for 20 min at 4˚C. The serum D-LA and DAO were determined according to the protocols of the D-LA (cat. no. AE91431mo; AMEKO) and DAO ELISA kits (cat. no. AE90824mo; AMEKO). Samples were analyzed in duplicate in accordance with the product specification. A total of 10 samples in each group were analyzed. The experiment was repeated three times.
Intraperitoneal injection with miRNA-29a inhibitor
The experimental protocol follows previous study and some improvements were made (22, 23), 10 μg of miRNA-29a inhibitor (cat. no: miR200029-1-5, RiboBio, Guangzhou, China) or negative control (cat. no: miR2N00003-1-5, RiboBio, Guangzhou, China) in 100 μl sterile saline was administered by intraperitoneal injection of 10 mice in each group for one time. All mice were sacrificed 24 h after the administration of miRNA-29a inhibitor. The aseptic operation was needed. The sequence of miRNA-29a inhibitor: 3'-AUCGUGGUAGACUUUAGCCAAU-5'.
Reverse transcription-quantitative PCR (RT-qPCR)
The total miRNA from the tissues was extracted using a miRcute miRNA isolation kit (cat. no. DP501; Tiangen Biotech Co., Ltd.) and used as a template for reverse transcription with a cDNA miRcute Plus miRNA First-strand cDNA Synthesis kit (cat. no. KR211; Tiangen Biotech Co., Ltd.). Subsequently, PCR amplification was conducted with the miRcute miRNA RT-qPCR Detection kit (cat. no. FP411-01; Tiangen Biotech Co., Ltd.), with U6 as an internal reference.
The total mRNA from the tissues was extracted with TRIzol® (cat. no. 15596026; Invitrogen; Thermo Fisher Scientific, Inc.) and used as a template for reverse transcription into cDNA (cat. no. RR820A; Takara Biotechnology Co., Ltd.). Subsequently, PCR amplification was conducted in accordance with the protocol of the RT-qPCR kit, with β-actin as an internal reference. RT-PCR and quantitative analysis was conducted using the Bio-Rad real-time PCR detection system (Bio-Rad Laboratories, Inc.). The reaction conditions for miRNA-29a were as follows: 94˚C for 2 min (one cycle), then 94˚C for 20 sec, and 60˚C for 34 sec (35 cycles). The reaction conditions for ZO-1 and CLDN1 were as follows: 95˚C for 30 sec (one cycle), 95˚C for 5 sec and then 60˚C for 30 sec (40 cycles). The relative expression levels of miRNA-29a and ZO-1 and CLDN1 mRNA were calculated using the 2-ΔΔCq method (24). All the primer sequences for miRNA-29a, ZO-1, CLDN1, U6 and β-actin were synthesized by Sangon Biotech Co., Ltd. The primer sequences were 5'-TAGCACCATCTG AAATCGGTTA-3' for miRNA-29a; 5'-AGAGTGAACCAC GAGACGCTG-3' and 5'-TCTACTGTCCGTGCTATACATT GA-3' for ZO-1; 5'-CTGCCCCAGTGGAGGATT-3' and 5'-CA GCCCAGCCAGTGAAGA-3' for CLDN1. The mean miRNA and mRNA expression levels of the three RT-qPCR experiments were calculated for each case.
Western blotting
Protein levels of ZO-1 and CLDN1 were quantified by western blotting. Intestinal mucosa were lysed with radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Beijing China) at the ratio of 80 mg tissue/ml. The total protein concentration was measured using a bicinchoninic acid concentration determination reagent kit (Beyotime Institute of Biotechnology), according to the protocol. Equal amounts of protein (50 µg/well) were separated by SDS-PAGE and transferred to PVDF membranes (EMD Millipore). The membranes were blocked at room temperature and incubated overnight at 4˚C using the following primary antibodies: Rabbit polyclonal ZO-1 antibody (0.028 µg/150 µl; 1:2,000; cat. no. 21773-1-AP; Proteintech Group, Inc.) and rabbit polyclonal CLDN1 antibody (1:1,000; cat. no. 4933; Cell Signaling Technology, Inc.) and rabbit polyclonal β-actin antibody (0.013 µg/150 µl; 1:5,000; cat. no. 20536-1-AP; Proteintech Group, Inc.). All the antibodies were diluted with 5% bovine serum albumin in 1X TBST. The membranes were subsequently washed, followed by incubation with horseradish peroxidase-conjugated (HRP) goat anti-rabbit secondary antibodies (1:2,000; cat. no. SA00001-2; Proteintech Group, Inc.) for 1 h at room temperature. The bands were observed using a chemiluminescent HRP kit (cat. no. WBKLS0500; EMD Millipore) on a ChemiDoc™ imaging system (Bio-Rad Laboratories, Inc.) in accordance with the protocol.
Statistical analysis
Experimental data were analyzed with SPSS 17.0 software (SPSS, Inc.). The results are stated as the mean ± standard deviation. The differences between two groups were analyzed by Student's t-test. Multiple groups were analyzed using ANOVA (parametric) followed by Tukey's multiple comparison post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparisons of miRNA-29a and ZO-1 and CLDN1 mRNA in the colon mucosa of patients with IBS-D
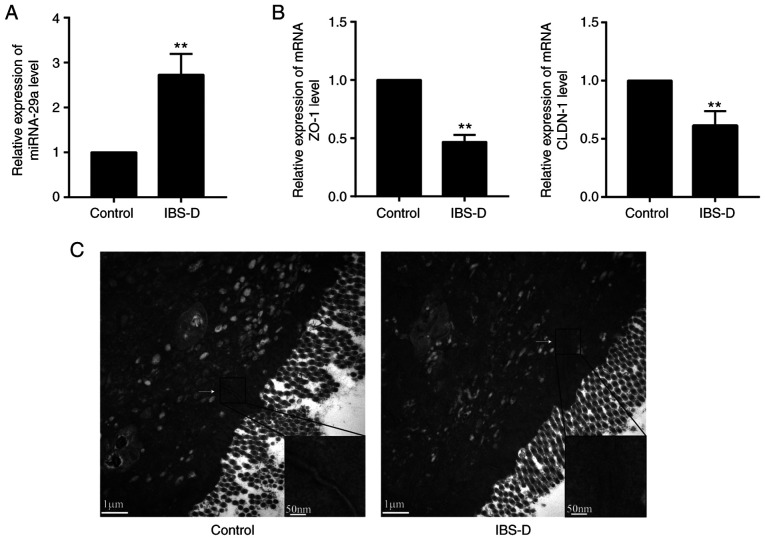
To investigate whether the colon mucosa of patients with IBS-D have different molecular expression patterns than healthy tissues, the present study analyzed the expression of miRNA-29a, ZO-1 and CLDN1 in the colon mucosa from patients with IBS-D by RT-qPCR. It was observed that the relative content of miRNA-29a in the IBS-D group was significantly upregulated compared with the control group (P<0.01; Fig. 1A). In addition, the ZO-1 and CLDN1 mRNA expression levels were significantly downregulated compared with the IBS-D group (P<0.01; Fig. 1B). These results suggested that miRNA-29a, ZO-1 and CLDN1 may serve important roles in the pathogenesis of IBS-D.
Figure 1.
Alterations in miRNA-29a, ZO-1, CLDN1 and the JC in the colon mucosa of patients with IBS-D. Relative expression of (A) miRNA-29a, (B) ZO-1 and CLDN1 was detected by reverse transcription-quantitative PCR. **P<0.01 vs. control. (C) Staining of the JC between colonic epithelium observed by transmission electron microscopy. n=3. Magnification, x20,000. IBS-D, diarrhea-predominant irritable bowel syndrome; miRNA, microRNA; ZO-1, tight junction protein ZO-1; CLDN1, claudin-1; JC, junctional complex.
Alterations in the JC in the colon mucosa of patients with IBS-D
To further assess the morphological alterations in the colon mucosa of patients with IBS-D, TEM was used to visualize the staining of the JC among colonic enterocytes. It was identified that the staining was strong and continuous in the control group, while the signals were faint and discontinuous in the IBS-D group (Fig. 1C). These results suggested that alterations in the JC may be implicated in the pathogenesis of IBS-D.
Comparisons of miRNA-29a, D-LA and DAO in IBS-D mice
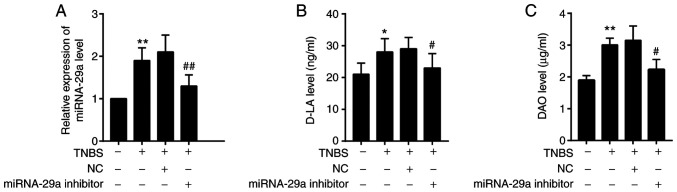
To analyze the content of miRNA-29a, RT-qPCR was performed (Fig. 2A). To further analyze the content of D-LA and DAO, ELISA was used (Fig. 2B and C). It was observed that miRNA-29a, D-LA and DAO were significantly upregulated in the IBS-D mouse model group (with TNBS treatment) compared with the control group (P<0.01, P<0.05 and P<0.01, respectively). The miRNA-29a, D-LA and DAO levels in IBS-D mice were significantly decreased by the miRNA-29a inhibitor, but remained higher compared with the control group (P<0.01, P<0.05 and P<0.05, respectively).
Figure 2.
Alterations in miRNA-29a and D-LA, DAO in the colon mucosa of IBS-D mice. (A) Level of miRNA-29a was measured by reverse transcription-quantitative PCR. Levels of (B) D-LA and (C) DAO were measured by ELISA. n=10. *P<0.05, **P<0.01 vs. control; #P<0.05, ##P<0.01 vs. IBS-D model. NC, negative control; miRNA, microRNA; TNBS, trinitro-benzene-sulfonic acid; D-LA, D-lactate; DAO, diamine oxidase.
miRNA-29a downregulation alleviates the impairment of the intestinal mucosal barrier and upregulates the presence of ZO-1 and CLDN1 in IBS-D mice
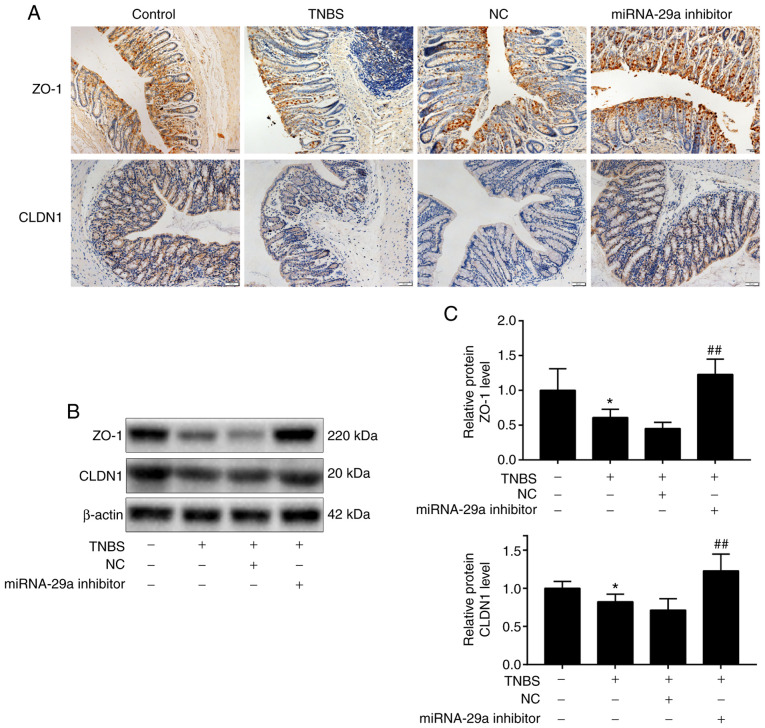
To further investigate the presence of miRNA-29a, ZO-1 and CLDN1 following administration of miRNA-29a inhibitor, immunohistochemistry, RT-qPCR and western blot analysis were used. The immunohistochemistry results (Fig. 3A) demonstrated that the presence of ZO-1 staining was distributed on the edges of intestinal epithelial cells in brown, while CLDN1 staining was distributed widely within the intestinal epithelial cells in brown. In the IBS-D mouse group, ZO-1 and CLDN1 staining was unevenly distributed or faded, and the presence of ZO-1 and CLDN1 was markedly reduced. However, following administration of miRNA-29a inhibitor, the downregulation of ZO-1 and CLDN1 presence heightened significantly. As presented in Table I, the average immunohistochemistry optical density values of ZO-1 and CLDN1 decreased significantly (P<0.01 and P<0.05, respectively) in the IBS-D group, compared with the control group. Nevertheless, the values of ZO-1 and CLDN1 in the miRNA-29a inhibitor group were significantly increased compared with the IBS-D group (P<0.05 and P<0.01, respectively). ZO-1 and CLDN1 were further evaluated by western blotting. As presented in Fig. 3B and C, compared with the control group, ZO-1 and CLDN1 protein levels were downregulated significantly (P<0.01 and P<0.05, respectively) in the IBS-D model group. Furthermore, the effect of miRNA-29a on the expression of ZO-1 and CLDN1 in IBS-D mice was evaluated by the administration of miRNA-29a inhibitor. Compared with the IBS-D model group, ZO-1 and CLDN1 protein levels were upregulated significantly (P<0.01) in the miRNA-29a inhibitor group. These results suggested that miRNA-29a reversed the TNBS-induced decrease in ZO-1 and CLDN1 protein levels.
Figure 3.
Alterations of ZO-1 and CLDN1 in the colon mucosa of mice. (A) Expression of ZO-1 and CLDN1 was detected by immunohistochemical staining. Magnification, x200. (B) Western blot analysis and (C) subsequent densitometry. n=10. *P<0.05 vs. control; ##P<0.01 vs. IBS-D mice model. NC, negative control; miRNA, microRNA; TNBS, trinitro-benzene-sulfonic acid; ZO-1, tight junction protein ZO-1; CLDN1, claudin-1.
Table I.
Comparison of ZO-1 and CLDN1 immunohistochemistry average optical density values.
| Group | ZO-1 | CLDN1 |
|---|---|---|
| Control | 183,692±66,541.2 | 138,695.2±36,541.6 |
| TNBS | 95,120.5±26,458.3b | 68,745.9±12,548.3a |
| NC | 105,750.9±43,257.6 | 59,457.2±11,563.1 |
| miRNA-29a inhibitor | 144,815.4±25,493.8c | 104,560.2±26,981.9d |
Values are presented as the mean ± standard deviation.
aP<0.05
bP<0.01 vs. control;
cP<0.05
dP<0.01 vs. IBS-D model. NC, negative control; miRNA, microRNA; TNBS, trinitro-benzene-sulfonic acid; ZO-1, tight junction protein ZO-1; CLDN1, claudin-1.
Discussion
The present study demonstrated that miRNA-29a expression was upregulated in intestinal mucosal of IBS-D patients and mice. Following administration of miRNA-29a inhibitor, the impairment of the intestinal mucosal barrier was alleviated. miRNA-29a inhibitor also decreased the expression of ZO-1 and CLDN1. This may be implicated in the role of the intestinal mucosal barrier in IBS-D.
miRNAs have been widely reported for their role in various human disorders (8,25,26). Recently, increasing evidence has demonstrated that miRNAs serve an important role in the pathogenesis of IBS-D (27-29). However, knowledge of the function of miRNAs in the progression of IBS-D remains limited. miRNA-29 is highly expressed in glioma tumorigenesis (30), pancreatic ductal adenocarcinoma (31) and chronic liver damage (32). It is noteworthy that miRNA-29 has been reported to be involved in the regulation of intestinal permeability and epithelial barrier function (33,34). Consistent with early research (35), the present results demonstrated that miRNA-29a was significantly increased in patients with IBS-D compared with the control, indicating that miRNA-29a serves a role in IBS-D.
CLDN1 is a key TJ protein that maintains and regulates intestinal permeability (36). ZO-1 has a vital role in maintaining the appropriate structure and function of TJs. ZO-1 and CLDN1 are the key proteins that form TJs, and their role in barrier permeability has been thoroughly examined and demonstrated to be crucial for the regulation of TJs (37,38). Studies have indicated that the administration of ZO-1 siRNA may affect and promote intestinal mucosal permeability (39). Moreover, although evident histomorphological changes in the colon mucosa were not observed in patients with IBS-D (40), the change in ultrastructure was observed by TEM in the present study. The present results demonstrated that the JC was discontinuous in IBS-D, and that ZO-1 and CLDN1 expression was significantly downregulated. Therefore, it may be speculated that the ultrastructural change is associated with the degradation of ZO-1 and CLDN1.
D-LA is a metabolite of bacterial fermentation, the level of which reflects the function of the intestinal mucosal epithelial barrier. The level of DAO in serum can be used as a marker to evaluate the integrity of the intestinal mucosa. To further determine the role of miRNA-29a, intraperitoneal injection with miRNA-29a inhibitor was performed, indicating that serum D-LA and DAO were decreased (P<0.05) in the model group and suggesting that intestinal mucosal barrier function may be improved by reducing the expression of miRNA-29a. Furthermore, increased ZO-1 and CLDN1 expression was also detected in IBS-D mice following administration of miRNA-29a inhibitor, suggesting that miRNA-29a serves an important role in the pathogenesis of IBS-D through regulation of ZO-1 and CLDN1 expression; this indicated that the regulation of TJs by ZO-1 and CLDN1 is important in maintaining the integrity of the epithelial barrier in response to miRNA-29a inhibitor.
There are several limitations to this study. First, colonoscopic biopsies from IBS-D patients were sent for RNA extraction and TEM. However, only the discontinuous distribution of JC was observed by TEM, and the alterations of various junctional proteins in IBS-D require further study and confirmation. Secondly, since at least two investigators evaluated and agreed to the clinical symptoms, the study was single blinded. Thirdly, a previous study showed that CLDN-1 is the direct target of miRNA-29a (34). We were unable to conclusively determine whether ZO-1 is a direct target of miRNA-29a. Nevertheless, we found that ZO-1 was significantly upregulated after administering the intraperitoneal injection of miRNA-29a inhibitor. Whether miRNA-29a regulates intestinal barrier function in IBS-D by upregulating an important pathway including ZO-1 requires verification in the future study.
In conclusion, the results of the present study further suggested that miRNA-29a may be involved in the pathogenesis of IBS-D by regulating intestinal mucosal barrier function. Thus, the results provide evidence that miRNA-29a is a possible molecular target for the treatment of IBS-D. Furthermore, the present results indicated that the expression of ZO-1 and CLDN1 in the colonic mucosa of IBS-D mice was significantly increased following intraperitoneal injection with miRNA-29a inhibitor.
Acknowledgements
The authors would like to thank Dr Xiaofei Li of the Karolinska Institute for manuscript correction.
Funding
The present study was supported by The General Program of National Nature Science Foundation of China (grant nos. 81673842 and 81703955) and The Special Clinical Program of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (grant no. 2019ⅡT08).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HT and YC designed the study and approved the final version of the manuscript. HZ and XX conducted the experiments. HZ wrote the manuscript. HZ, YS and YW contributed to the acquisition, analysis and interpretation of the case information, and obtained intestinal patient samples. HZ and XX established the IBS-D mouse model. DL and FX analyzed the patient data. YH and GH contributed to the study conception and drafting the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The patient study was approved by The Medical Research Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (approval no. AF/JD-02/02). All patients signed the informed consent. The animal study was approved by The Animal Experimental Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (approval no. TCMF1-2017009)
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: Involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 2.Martínez C, Lobo B, Pigrau M, Ramos L, González-Castro AM, Alonso C, Guilarte M, Guilá M, de Torres I, Azpiroz F, et al. Diarrhoea-predominant irritable bowel syndrome: An organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. doi: 10.1136/gutjnl-2012-302093. [DOI] [PubMed] [Google Scholar]
- 3.Matricon J, Meleine M, Gelot A, Piche T, Dapoigny M, Muller E, Ardid D. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009–1031. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 4.Martínez C, González-Castro A, Vicario M, Santos J. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome. Gut Liver. 2012;6:305–315. doi: 10.5009/gnl.2012.6.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–G785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 6.Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165–2173. doi: 10.1038/ajg.2011.257. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkman DJ, Popp JW. Mucosal Barrier Defects in Irritable Bowel Syndrome. Who Left the Door Open? Am J Gastroenterol. 2006;101:864–865. doi: 10.1111/j.1572-0241.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- 8.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 9.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 10.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 11.Xu XJ, Zhang YL, Liu L, Pan L, Yao SK. Increased expression of nerve growth factor correlates with visceral hypersensitivity and impaired gut barrier function in diarrhoea-predominant irritable bowel syndrome: A preliminary explorative study. Aliment Pharmacol Ther. 2017;45:100–114. doi: 10.1111/apt.13848. [DOI] [PubMed] [Google Scholar]
- 12.Martínez C, Rodiño-Janeiro BK, Lobo B, Stanifer ML, Klaus B, Granzow M, González-Castro AM, Salvo-Romero E, Alonso-Cotoner C, Pigrau M, et al. miR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut. 2017;66:1537–1538. doi: 10.1136/gutjnl-2016-311477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775–784. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcz-Villega E, McClean S, O'Sullivan M. Reduced E-cadherin expression is associated with abdominal pain and symptom duration in a study of alternating and diarrhea predominant IBS. Neurogastroenterol Motil. 2014;26:316–325. doi: 10.1111/nmo.12262. [DOI] [PubMed] [Google Scholar]
- 15.Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016;150:1262–1279. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Liu W, Peng QX, Peng JL, Yu LZ, Hu JL. Protective effect of huoxiang zhengqi oral liquid on intestinal mucosal mechanical barrier of rats with postinfectious irritable bowel syndrome induced by acetic acid. Evid Based Complement Alternat Med. 2014;2014(218383) doi: 10.1155/2014/218383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du C, Peng L, Kou G, Wang P, Lu L, Li Y. Assessment of Serum sTREM-1 as a Marker of Subclinical Inflammation in Diarrhea-Predominant Patients with Irritable Bowel Syndrome. Dig Dis Sci. 2018;63:1182–1191. doi: 10.1007/s10620-018-5002-y. [DOI] [PubMed] [Google Scholar]
- 18.Chao G, Wang Y, Ye F, Zhang S. Regulation of Colonic Mucosal MicroRNA Expression via Multiple Targets in Visceral Hypersensitivity Rats by Tongxieyaofang. Yonsei Med J. 2018;59:945–950. doi: 10.3349/ymj.2018.59.8.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raju D, Ilango K, Chitra V, Ashish K. Evaluation of Anti-ulcer activity of methanolic extract of Terminalia chebula fruits in experimental rats. J Pharm Sci Res. 2009;1:101–107. [Google Scholar]
- 20.van Herck H, Baumans V, Brandt CJWM, Hesp APM, Sturkenboom JH, van Lith HA, van Tintelen G, Beynen AC. Orbital sinus blood sampling in rats as performed by different animal technicians: The influence of technique and expertise. Lab Anim. 1998;32:377–386. doi: 10.1258/002367798780599794. [DOI] [PubMed] [Google Scholar]
- 21.van Herck H, Baumans V, de Boer SF, van der Gugten J, van Woerkom AB, Beynen AC. Endocrine stress response in rats subjected to singular orbital puncture while under diethyl-ether anaesthesia. Lab Anim. 1991;25:325–329. doi: 10.1258/002367791780809931. [DOI] [PubMed] [Google Scholar]
- 22.Liao XJ, Mao WM, Wang Q, Yang GG, Wu WJ, Shao SX. MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem Biophys Res Commun. 2016;469:288–293. doi: 10.1016/j.bbrc.2015.11.102. [DOI] [PubMed] [Google Scholar]
- 23.Deng Y, Zhou X, Xiang X, Ou Y, He J. Effect of miRNA-19a on gastrointestinal motility in rats with functional dyspepsia. Exp Ther Med. 2018;15:4875–4879. doi: 10.3892/etm.2018.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Viswambharan V, Thanseem I, Vasu MM, Poovathinal SA, Anitha A. miRNAs as biomarkers of neurodegenerative disorders. Biomarkers Med. 2017;11:151–167. doi: 10.2217/bmm-2016-0242. [DOI] [PubMed] [Google Scholar]
- 26.Issler O, Chen A. Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci. 2015;16:201–212. doi: 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- 27.Hou Q, Huang Y, Zhu S, Li P, Chen X, Hou Z, Liu F. miR-144 Increases Intestinal Permeability in IBS-D Rats by Targeting OCLN and ZO1. Cell Physiol Biochem. 2017;44:2256–2268. doi: 10.1159/000486059. [DOI] [PubMed] [Google Scholar]
- 28.Wohlfarth C, Schmitteckert S, Härtle JD, Houghton LA, Dweep H, Fortea M, Assadi G, Braun A, Mederer T, et al. miR-16 and miR-103 impact 5-HT4 receptor signalling and correlate with symptom profile in irritable bowel syndrome. Sci Rep. 2017;7(14680) doi: 10.1038/s41598-017-13982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–2977. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Duan N, Duan S. MiR-29a Inhibits Glioma Tumorigenesis through a Negative Feedback Loop of TRAF4/Akt Signaling. BioMed Res Int. 2018;2018(2461363) doi: 10.1155/2018/2461363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang C, Shi S, Meng Q, Liang D, Hua J, Qin Y, Zhang B, Xu J, Ni Q, Yu X. MiR-29a, targeting caveolin 2 expression, is responsible for limitation of pancreatic cancer metastasis in patients with normal level of serum CA125. Int J Cancer. 2018;143:2919–2931. doi: 10.1002/ijc.31654. [DOI] [PubMed] [Google Scholar]
- 32.Huang YH, Yang YL, Wang FS. The Role of miR-29a in the Regulation, Function, and Signaling of Liver Fibrosis. Int J Mol Sci. 2018;19(1889) doi: 10.3390/ijms19071889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q, Costinean S, Croce CM, Brasier AR, Merwat S, Larson SA, Basra S, Verne GN. MicroRNA 29 targets nuclear factor-κB-repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology. 2015;148:158–169.e8. doi: 10.1053/j.gastro.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue H, Zhang G, Geurts AM, Usa K, Jensen DM, Liu Y, Widlansky ME, Liang M. Tissue-specific effects of targeted mutation of Mir29b1 in rats. EBioMedicine. 2018;35:260–269. doi: 10.1016/j.ebiom.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin X, Zhou X, Liu D, Yun L, Zhang L, Chen X, Chai Q, Li L. MicroRNA-29 regulates high-glucose-induced apoptosis in human retinal pigment epithelial cells through PTEN. In Vitro Cell Dev Biol Anim. 2016;52:419–426. doi: 10.1007/s11626-015-9990-z. [DOI] [PubMed] [Google Scholar]
- 36.Pope JL, Bhat AA, Sharma A, Ahmad R, Krishnan M, Washington MK, Beauchamp RD, Singh AB, Dhawan P. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut. 2014;63:622–634. doi: 10.1136/gutjnl-2012-304241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, Lyonnet S, De Prost Y, Munnich A, Hadchouel M, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: A tight junction disease. Gastroenterology. 2004;127:1386–1390. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Masahiko I, Mikio F, Kazumasa M, Koji K. Mitinori Saitou and Tsukita AS: Direct Binding of Three Tight Junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH Termini of Claudins. J Cell Biol. 2000;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang N, Han Q, Wang G, Ma WP, Wang J, Wu WX, Guo Y, Liu L, Jiang XY, Xie XL, et al. Resveratrol Protects Oxidative Stress-Induced Intestinal Epithelial Barrier Dysfunction by Upregulating Heme Oxygenase-1 Expression. Dig Dis Sci. 2016;61:2522–2534. doi: 10.1007/s10620-016-4184-4. [DOI] [PubMed] [Google Scholar]
- 40.Liu DR, Xu XJ, Yao SK. Increased intestinal mucosal leptin levels in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2018;24:46–57. doi: 10.3748/wjg.v24.i1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.