Abstract
Background
The novel coronavirus (COVID-19) pandemic has led surgical societies to recommend delaying diagnosis and treatment of suspected lung cancer for lesions less than 2 cm. Delaying diagnosis can lead to disease progression, but the impact of this delay on mortality is unknown. The COVID-19 infection rate at which immediate operative risk exceeds benefit is unknown. We sought to model immediate versus delayed surgical resection in a suspicious lung nodule less than 2 cm.
Methods
A decision analysis model was developed, and sensitivity analyses performed. The base case was a 65-year-old male smoker with chronic obstructive pulmonary disease presenting for surgical biopsy of a 1.5 to 2 cm lung nodule highly suspicious for cancer during the COVID-19 pandemic. We compared immediate surgical resection to delayed resection after 3 months. The likelihood of key outcomes was derived from the literature where available. The outcome was 5-year overall survival.
Results
Immediate surgical resection resulted in a similar but slightly higher 5-year overall survival when compared with delayed resection (0.77 versus 0.74) owing to the risk of disease progression. However, if the probability of acquired COVID-19 infection is greater than 13%, delayed resection is favorable (0.74 vs 0.73).
Conclusions
Immediate surgical biopsy of lung nodules suspicious for cancer in hospitals with low COVID-19 prevalence likely results in improved 5-year survival. However, as the risk of perioperative COVID-19 infection increases above 13%, a delayed approach has similar or improved survival. This balance should be frequently reexamined at each health care facility throughout the curve of the pandemic.
On March 11, 2020, the World Health Organization declared the novel coronavirus disease (COVID-19) to be a global pandemic.1 Physicians have refocused their efforts on combating COVID-19 as health systems struggle to adapt to the overwhelming burden of this disease. Difficult decisions must be made about which scheduled surgical cases merit the risk of COVID-19 exposure and which can be delayed.
The American College of Surgeons Commission on Cancer, The Society of Thoracic Surgeons, and the American Association of Thoracic Surgery established COVID-19 guidelines for the triage of surgical patients during the pandemic.2 , 3 The thoracic oncology guidelines recommend operating on known or presumed lung cancer in solid lung nodules greater than 2 cm during the early phase of the pandemic (semi-urgent or preparation phase), but delaying surgery for smaller nodules for 3 months.
Specific guidelines are necessary for lung cancer as delaying diagnosis may impact survival.4 Early detection is the only chance at true cure, but the majority of non-small cell lung cancers (NSCLC) are diagnosed at late stages (III-IV) when 5-year survival is low (less than 36%).5 Notably, the smaller lung nodules (less than 2 cm) recommended for delayed resection may have a greater than 50% risk of malignancy. If the diagnosis of suspicious lung nodules is not achieved through noninvasive methods, such as fluorodeoxyglucose-positron emission tomography scan, navigational bronchoscopy, and so forth, a surgical biopsy is recommended.6 The impact from delaying surgical biopsies during the COVID-19 pandemic on survival is unknown, and there are no published data or guidelines to aid surgeons in decision making with their patients. This issue will remain important as COVID-19 prevalence fluctuates across the country, or if similar infectious pandemics emerge in the future.
However, the risk of delaying diagnosis of lung cancer must be balanced with the risk of mortality from COVID-19. Early reports suggest that patients with significant comorbidities such as cancer or respiratory diseases are at higher risk for complications and death.7, 8, 9, 10 In addition, patients aged more than 60 years are at higher risk for complications and COVID-19-related mortality.10, 11, 12, 13 As the majority of patients with NSCLC fall into the above demographics, their high risk of complications must be considered.
The purpose of this study was to compare the effect on 5-year overall survival of delayed surgical biopsy of a lung nodule suspicious for cancer with possible perioperative COVID-19 infection within an early phase infection environment. We sought to model the optimal clinical decision of immediate surgical resection versus delayed surgical resection in a 1.5 to 2 cm lung nodule highly suspicious for lung cancer.
Patients and Methods
Decision Model Design
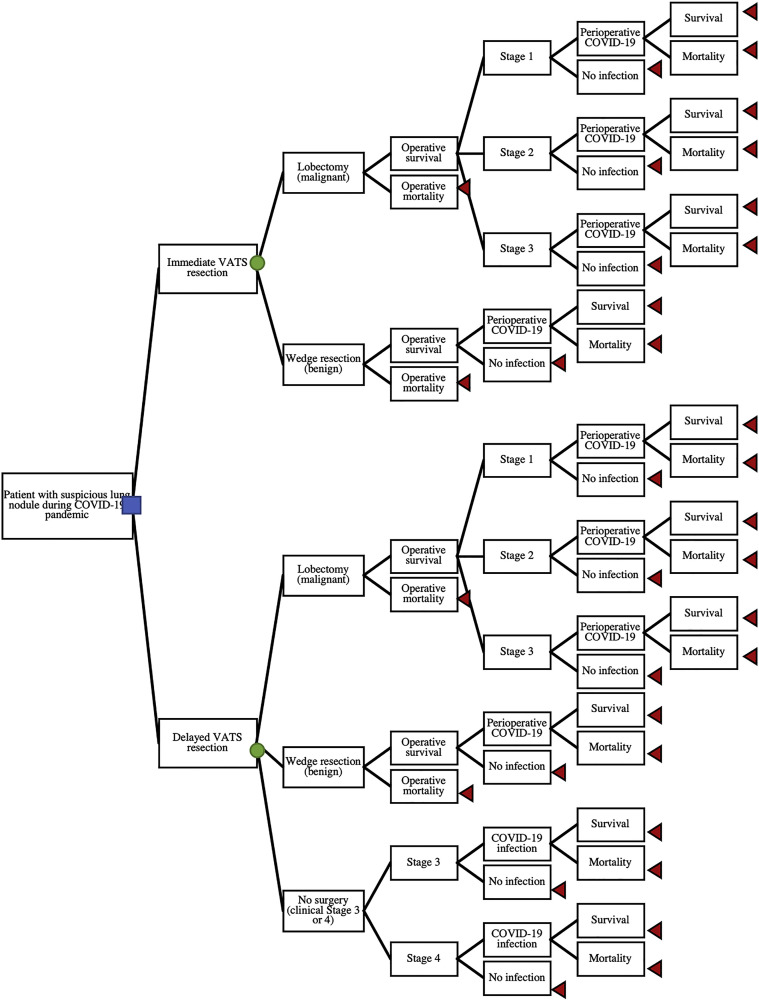
We developed a decision analysis model to evaluate two diagnostic strategies for a suspicious lung nodule requiring surgical biopsy because of a nondiagnostic image-guided or bronchoscopic-guided biopsy during the COVID-19 pandemic. The two diagnostic options evaluated were immediate minimally invasive resection or delayed minimally invasive resection after 3 months (Figure 1 ). The decision tree details the initial choice (the decision node) of immediate or delayed resection and follows branch points to the ultimate outcomes of death or 5-year overall survival (terminal nodes). If the surgical biopsy shows benign disease (first chance node), the patient follows the wedge resection branch with chance nodes for operative mortality, perioperative COVID-19 infection, and mortality due to COVID-19. If the surgical biopsy shows malignancy (first chance node), the patient follows the lobectomy branch. If the patient survives the surgery, there is a chance of stage 1, 2, or 3 disease at the time of diagnosis, and for any of these results there is a chance of perioperative COVID-19 infection and mortality. Finally, under the delayed resection choice there is a third option at the first chance node: no surgery owing to disease progression in the interim.
Figure 1.
Decision analysis tree for timing surgical biopsy for suspicious lung nodule during COVID-19 pandemic, displaying potential pathways base case patient could follow for either immediate or delayed resection of highly suspicious lung nodule. For either immediate or delayed resection, there is a chance of the nodule being malignant or benign; for a delayed scenario, there is also a chance of disease progression during the delay precluding operative intervention. All pathways have potential for COVID-19 infection and mortality. Blue square indicates decision node, whether to choose immediate or delayed resection. Green circles indicate chance nodes (probabilities detailed in Table 1). Red triangles indicate terminal nodes, death or 5-year overall survival. (VATS, video-assisted thoracoscopic surgery.)
We used TreeAge Pro version 2018 (TreeAge Software, Williamson, MA) to construct the decision tree model. Literature review and expert opinion (when published data were not available) were used to estimate model parameters and applicable ranges for sensitivity analysis.
Patients
Our base clinical case was a 65-year-old male smoker with mild chronic obstructive pulmonary disease presenting with a highly suspicious lung nodule requiring surgical biopsy for diagnosis. That assumes less-invasive means of obtaining a diagnosis have failed to make a definitive diagnosis. We chose a base case with a high probability of malignancy (65%) so that the argument to proceed with surgical biopsy would be compelling despite the COVID-19 pandemic. To reflect this, the characteristics of the nodule were chosen to be 1.5 to 2 cm, spiculated, and located in the upper lobe.14 The patient was presumed to have clinical stage IA NSCLC, and there were no indications for invasive preoperative mediastinal staging as imaging did not show concerning adenopathy. The patient was a candidate for thoracoscopic resection and presented to a hospital in the semi-urgent or preparatory phase of the COVID-19 pandemic.2 This scenario assumes COVID-19 in the community and hospital, but not to a degree that most hospital resources have been diverted to caring for COVID-19 patients. The patient had negative COVID-19 testing preoperatively.
Treatment Strategies
For both immediate and delayed resection of the lung nodule, the patient undergoes a minimally invasive wedge resection, either video-assisted thoracoscopic surgery (VATS) or robotic (both referred to as VATS moving forward for simplicity). If intraoperative pathology is benign, no further intervention is undertaken. If intraoperative pathology is positive for malignancy, this patient undergoes a completion lobectomy. For the model, we assume the surgeon is able to complete both operations minimally invasively.
The delayed resection follows a similar progression, but after a 3-month delay to allow for COVID-19 prevalence in the community (and therefore burden to the hospital) to decrease. Owing to the delay, there is the potential that the patient’s NSCLC has progressed to a more advanced stage. We assume the patient is reimaged with, at minimum, a computed tomography scan of the chest preoperatively. If the patient has not progressed significantly (clinical stage I-II), he would proceed with the same operative pathway as the immediate resection above. A patient with clinically advanced disease (stage III-IV) would not undergo surgical resection and would follow a different branch point in the decision analysis (see Figure 1, Table 1 ). He would still be accessing the health care system for diagnostic imaging and procedures and thus would still be at risk for COVID-19 (albeit a low risk due to the 3-month delay).
Table 1.
Model Variables
| Variables | Probability | Sensitivity Analysis Values | References |
|---|---|---|---|
| Lobectomy mortality | 0.02 | 0.01-0.05 | 15 |
| Wedge mortality | 0.02 | 0.01-0.05 | 16 |
| COVID-19 mortality | 0.29 | 0.15-0.52 | 7-10 |
| Immediate VATS resection | |||
| Lobectomy, malignancy | 0.65 | 0.3-1.0 | a |
| Stage 1 NSCLC | 0.75 | 17 | |
| Stage 2 NSCLC | 0.17 | 17 | |
| Stage 3 NSCLC | 0.08 | 17 | |
| COVID-19 infection, NSCLC | 0.021 | 0.007-0.1 | 7a |
| Wedge resection, benign nodule | 0.35 | a | |
| COVID-19 infection, benign nodule | 0.014 | 0.007-0.05 | a |
| Delayed VATS resection | |||
| Lobectomy, malignancy | 0.61 | 0.26-0.71 | a |
| Stage 1 NSCLC, postoperative | 0.72 | 0.5-0.75 | 17-19 |
| Stage 2 NSCLC, postoperative | 0.19 | 0.17-0.3 | 17-19 |
| Stage 3 NSCLC, postoperative | 0.09 | 0.08-0.2 | 17-19 |
| Wedge resection, benign nodule | 0.35 | a | |
| No surgery, clinical stage 3 or 4 | 0.04 | 0.0001-0.1 | |
| Stage 3 NSCLC, nonoperative | 0.5 | 16-19 | |
| Stage 4 NSCLC, nonoperative | 0.5 | 16-19 | |
| COVID-19 infection, NCSLC or benign nodule | 0.00001 | 0.01 | a |
NSCLC, non-small cell lung cancer; VATS, video-assisted thoracoscopic surgery.
Parameters set by research team for base case scenario.
Operative complications were assumed to be unchanged with a delay of the procedure and therefore omitted from the model. Operative mortality from either wedge or lobectomy resections were assumed to be equal for immediate or delayed procedures (Table 1).15 , 16
Model Variables
Non-small cell lung cancer
Event probabilities for the chance nodes were estimated using published reports derived from the literature (Table 1). The probability of malignancy was 65% for the base case. The distribution of NSCLC stages for immediate resection of a clinical stage IA NSCLC were derived from the literature.17 The distribution of NSCLC stages after delayed resection was calculated from an assumed shift in stage distribution due to the delay. That accounted for a reported doubling time in nodule size (stage I to II) and progression from localized to regional (stage III) or distant disease (stage IV) after a 3-month delay.18 , 19 Using previously published data by Edelsberg and colleagues,19 we assumed a 0.14 probability of doubling in size of nodule over the 3-month waiting period (shift from stage IA to later stage I or stage II). Similarly, from Gould and associates6 and the Surveillance, Epidemiology, and End Results program data on regional and distant spread, we assumed a 0.069 probability shift to stage III and 0.02 to stage IV during the delay. We assumed half of stage III disease would be identified on the interval computed tomography scan and half would be detected pathologically at the time of resection.
COVID-19
The COVID-19 variables were derived from the limited published reports available as of April 1, 2020. The risk of perioperative COVID-19 infection for our base case for immediate resection was set to reflect the research team’s local prevalence during the acute phase of the pandemic at 1.4% (Table 1). That was used as the probability of infection if the patient had benign disease. However, reports show hospitalized cancer patients are likely at an elevated risk of COVID-19 infection and mortality.7 Therefore, we elevated the probability of perioperative COVID-19 infection if the patient had a malignancy (2.1%). The term “perioperative COVID-19 infection” includes infections that are acquired in the hospital or community in the perioperative period.
For the delayed VATS resection, the probability of acquiring a perioperative COVID-19 infection was set to almost zero as the purpose of delaying surgery is to allow the prevalence of COVID-19 in the community to significantly decrease. This probability was used for benign and malignant nodules. The risk of COVID-19–related mortality after VATS resection was derived from reports of hospitalized or institutionalized patients with cancer or other comorbidities, all of whom had higher acuity disease.7, 8, 9, 10 The probability of mortality due to COVID-19 for the base case was set at 29%, higher than in the general population of 60- to 69-year-old patients owing to underlying comorbidities, which we believed was appropriate.
Five-Year Overall Survival
Five-year overall survival was chosen for the outcome to model the impact of cancer progression as well as potential long-term effects of perioperative COVID-19 infection. The 5-year overall survival of the base case with benign disease was determined from United States National Vital Statistics System.20 , 21 Similarly, 5-year overall survival for NSCLC by pathologic stage was established from the literature5 (Table 2 ). With limited available substage progression data for NSCLC (for example, stage IA vs IB), we used averaged 5-year overall survival data for the substages as an aggregate for stage I to IV disease.
Table 2.
Five-Year Overall Survival
| Variables | Values | Sensitivity Analysis Values | References |
|---|---|---|---|
| Without COVID-19 infection | |||
| Benign | 0.92 | 20, 21 | |
| Stage 1 NSCLC | 0.8 | 0.68-0.92 | 5 |
| Stage 2 NSCLC | 0.57 | 0.53-0.6 | 5 |
| Stage 3 NSCLC | 0.25 | 0.13-0.36 | 5 |
| Stage 4 NSCLC | 0.05 | 0.0001-0.1 | 5 |
| With perioperative COVID-19 infection | |||
| Benign | 0.77 | 0.65-0.91 | 20-26 |
| Stage 1 NSCLC | 0.67 | 0.57-0.77 | 20-26 |
| Stage 2 NSCLC | 0.48 | 0.40-0.50 | 20-26 |
| Stage 3 NSCLC | 0.21 | 0.11-0.29 | 20-26 |
| Stage 4 NSCLC | 0.04 | 0.0001-0.084 | 20-26 |
NSCLC, non-small cell lung cancer.
As COVID-19 is an emerging disease, there is no long-term follow-up of patients available for reference. To approximate the effect of COVID-19 on long-term survival after lung cancer resection, we utilized available data on the impact of significant complications after NSCLC resection on 5-year overall survival.22, 23, 24, 25, 26 The decrease in 5-year overall survival due to COVID-19 was set at 16% (range, 11% to 29%), and this was used to discount the survival for each NSCLC stage. For example, if the patient had stage I NSCLC and survived perioperative COVID-19, the likelihood of surviving 5 years would decrease from 80% to 67%.
Sensitivity Analyses
One-way sensitivity analyses were performed to account for uncertainty in key model parameters (Table 1, Table 2) and to approximate how differences in the base case could affect the model outcome. That is accomplished by altering one parameter at a time while holding all other variables constant at baseline values. To model the impact of different patient or nodule characteristics, the probability of malignancy of the nodule was varied. To account for uncertainty in stage progression and to approximate longer or shorter delays, the probability of each stage after the delay was varied. To account for variability in the grade or substage, the mortality of each stage of NSCLC was varied. To model the impact of different baseline patient characteristics, the probability of both COVID-19 infection and mortality was varied. Finally, the impact of different patient characteristics (including disease progression or interim infection) on operative mortality was modeled. Two-way sensitivity analysis was performed by simultaneously varying the probability of perioperative COVID-19 infection and COVID-19–related mortality to approximate the outcome of the model with different patient-level or community-level variables.
Results
For the base case scenario, choosing immediate VATS resection of the suspicious lung nodule resulted in improved 5-year overall survival when compared with delayed VATS resection after 3 months to allow for COVID-19 prevalence to decrease (0.77 and 0.74, respectively).
Sensitivity Analyses
Altering the probability of malignancy of the nodule, the probability of stage progression during the delay or the 5-year overall survival estimates attributed to each stage of NSCLC did not change the outcome of favoring immediate resection. If the model was altered to have no change in stage after the 3-month delay, then the choices of immediate or delayed resection were equivocal (0.77 each).
When the probability of perioperative COVID-19 infection was varied while holding all other parameters constant, choosing immediate resection was favored as long as the probability of infection was less than 10% for a cancer patient (6.7% for patient with benign disease). Delayed resection became the preferred choice when the probability of perioperative COVID-19 infection was greater than 13% for a cancer patient (8.7% for patient with benign disease). Altering the probability of COVID-19–related mortality did not affect the outcome.
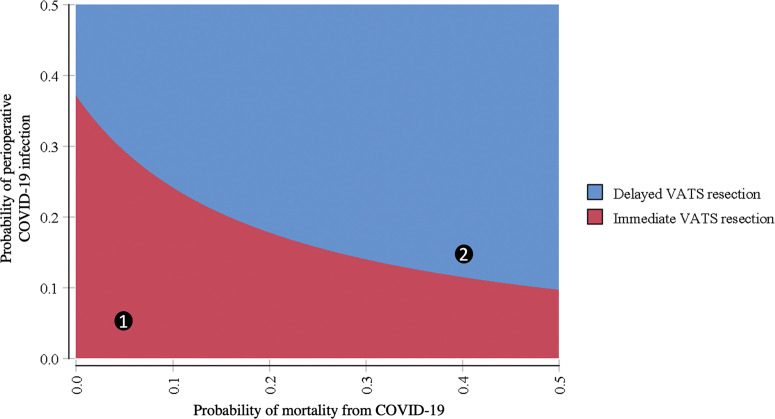
The two-way sensitivity analysis results are presented in Figure 2 . Delayed resection is increasingly preferred as the probability of either infection or mortality from COVID-19 are increased. For example, if the probabilities of infection and mortality were set to 5%, then immediate resection is favored (point 1 in Figure 2). If the probability of infection and mortality were set to 15% and 40%, respectively, then delayed resection is favored (point 2 in Figure 2).
Figure 2.
Two-way sensitivity analysis for probability of infection and mortality from COVID-19, displaying favored strategy, immediate video-assisted thoracoscopic surgery (VATS, red area) or delayed resection (blue area) across range of possible perioperative COVID-19 infection and mortality probabilities while holding all other model variables constant at baseline values. Point 1 favors immediate resection for probability of infection and mortality of 5%. Point 2 favors delayed resection for probability of infection and mortality of 15% and 40%, respectively.
Comment
Surgeons of all specialties are facing a new dilemma in triaging care of patients requiring nonemergent surgeries during the COVID-19 pandemic. They must balance the use of staff and resources with the potential harm to patients whose operations are delayed. As lung cancer survival can be significantly affected by delays in diagnosis and treatment,27 , 28 we created an informative simple model quantifying potential harm to a patient with a suspicious lung nodule if his care was delayed owing to the COVID-19 pandemic.
Our decision analysis model found that delaying resection for 3 months did affect 5-year overall survival, although not significantly (0.74 for delayed vs 0.77 for immediate). The model was strongly affected by the probability of COVID-19 infection; as the probability of infection exceeded 13%, delayed resection resulted in improved 5-year overall survival. Interestingly, realistic changes in stage progression or 5-year survival rates (to approximate more or less aggressive cancers) had little impact on the outcome. Similarly, when the nodule’s probability of malignancy was set to 100% (to mimic a biopsy-proven less than 2 cm NSCLC), immediate resection was still favored (0.69, vs 0.67 for delayed).
Although decision analysis model parameters are very narrow in scope by design, the sensitivity analyses allow for modeling a range of uncertainty. The two-way sensitivity analysis may serve as a helpful guide for clinicians to interpret the model for different patient-level and system-level factors at the time of resection. For example, older patients with a number of comorbidities are more likely to have severe infections and increased mortality from COVID-19.7, 8, 9, 10 , 13 Using Figure 2, if mortality exceeds 40% for these patients, delayed resection is likely preferred when infection prevalence exceeds 15%. Conversely, among younger patients with few comorbidities and a lower mortality rate of 5%, decision to delay resection may be only preferred when infection prevalence exceed 30%.
This model assumes that COVID-19 prevalence will decrease after 3 months. This assumption relies on the success of public health measures, such as social distancing, mask wearing, and widespread testing to combat viral spread. Otherwise, a 3-month delay would carry both risk of disease progression as well as risk of COVID-19 infection. For our base case we modeled a probability of infection of nearly zero as an ideal outcome of the pandemic. We modeled higher probabilities of infection after the delay, and as the probability of infection increased, proceeding immediately with surgery was more strongly favored. However, while ongoing COVID-19 after a 3-month delay did not have an impact on the overall outcome of the model, it could affect the resources available for nonemergent surgeries. To proceed with nonemergent surgeries during the pandemic, hospitals must be equipped to handle not only a surge in patients with COVID-19, but also postoperative patients with or without complications such as prolonged ventilation and intensive care unit stays.
Study Limitations
This study has several limitations. First, the paucity of literature on COVID-19 resulted in estimating several model parameters from similar but nonidentical clinical scenarios. We addressed this by analyzing a range of values for the COVID-19 parameters in our sensitivity analyses. Second, we did not model community-acquired COVID-19 infection separately from hospital-acquired infection. Whereas hospital-acquired infection seemed to drive the epidemic in China, we have not had the same experience thus far, but significant differences between these two infection rates could impact the outcome of the model. In addition, this model only applies to patients who require a surgical biopsy for diagnosis, and that may limit generalizability as many patients achieve diagnosis through bronchoscopy, image-guided biopsy, and so forth. Furthermore, our model assumed that negative preoperative testing for COVID-19 was accurate; we did not model complications and outcomes for a patient who had a false negative test. Finally, we did not account for surgical complications (including conversion to open thoracotomy) in the model. Although the probability should be nearly equivalent between the two strategies, if there was a predominance in one arm it could significantly impact the probability of perioperative COVID-19 infection or mortality with increased time in intensive care, multiple procedures, or need for post-acute care. Complications would also increase utilization of resources, which were not included in this model. Our model assumed adequate hospital and community resources were available to proceed with nonemergent surgeries.
Despite these limitations, we believe this simplified model provides a robust framework to inform the surgical decision and could be adapted for other, similar operative decision environments for the COVID-19 era. As the prevalence of COVID-19 fluctuates in communities, or other infectious pandemics arise, this model can be adapted to assist hospitals and surgeons to decide when to proceed with specific operations.
Conclusion
Proceeding with immediate VATS resection of a suspicious lung nodule during the COVID-19 pandemic resulted in a similar but slightly improved 5-year overall survival when compared with resection after a 3-month delay in our base case scenario. However, if the risk of perioperative COVID-19 was increased above 13%, delaying operations until prevalence decreases improved long-term survival. This balance should be frequently reexamined at each health care facility throughout the curve of the pandemic.
Acknowledgments
Dr Shipe received a research grant from the Agency for Health care Research (grant T32 HS026122). Dr Haddad received a research grant from National Institutes of Health (grant T32 CA106183-15). Dr Deppen and Dr. Grogan were supported by a research grant from Early Detection Research Network (NCI-5U24-CA0866368). Dr Grogan is supported by the Department of Veterans Affairs.
References
- 1.World Health Organization Coronavirus (COVID-19) events as they happen. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen Available at:
- 2.American College of Surgeons, Commission on Cancer COVID-19 guidelines for triage of thoracic patients. American College of Surgeons. https://www.facs.org/covid-19/clinical-guidance/elective-case/thoracic-cancer Available at:
- 3.Thoracic Surgery Outcomes Research Network COVID-19 guidance for triage of operations for thoracic malignancies: a consensus statement from Thoracic Surgery Outcomes Research Network. J Thorac Cardiovasc Surg. 2020;160:601–605. doi: 10.1016/j.jtcvs.2020.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R., Ward E., Brawley O., Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 5.Goldstraw P., Chansky K., Crowley J. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Gould M.K., Donington J., Lynch W.R. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl):e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;4720:2019–2021. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterpetti A.V. Lessons learned during the COVID-19 virus pandemic. J Am Coll Surg. 2020;230:1092–1093. doi: 10.1016/j.jamcollsurg.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 12.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;3099:1–9. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korean Society of Infectious Diseases, Korean Society of Pediatric Infectious Diseases, Korean Society of Epidemiology, Korean Society for Antimicrobial Therapy, Korean Society for Healthcare-associated Infection Control and Prevention, and Korea Centers for Disease Control and Prevention Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swensen S., Silverstein M., Ilstrup D., Schlek C., Edell E. The probability of malignancy in solitary pulmonary nodules. Arch Intern Med. 1997;157:849–855. [PubMed] [Google Scholar]
- 15.Kozower B.D., Sheng S., O’Brien S.M. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg. 2010;90:875–883. doi: 10.1016/j.athoracsur.2010.03.115. [DOI] [PubMed] [Google Scholar]
- 16.Starnes S.L., Reed M.F., Meyer C.A. Can lung cancer screening by computed tomography be effective in areas with endemic histoplasmosis? J Thorac Cardiovasc Surg. 2011;141:688–693. doi: 10.1016/j.jtcvs.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Grogan E.L., Deppen S.A., Ballman K.V. Accuracy of fluorodeoxyglucose-positron emission tomography within the clinical practice of the American College of Surgeons Oncology Group Z4031 trial to diagnose clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2014;97:1142–1148. doi: 10.1016/j.athoracsur.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould M.K., Sanders G.D., Barnett P.G. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med. 2003;138:724–735. doi: 10.7326/0003-4819-138-9-200305060-00009. [DOI] [PubMed] [Google Scholar]
- 19.Edelsberg J., Weycker D., Atwood M., Hamilton-Fairley G., Jett J.R. Cost-effectiveness of an autoantibody test (EarlyCDT-Lung) as an aid to early diagnosis of lung cancer in patients with incidentally detected pulmonary nodules. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochanek K.D., Murphy S.L., Xu J., Arias E. Deaths: final data for 2017. National Vital Statistics Reports. 2019. https://www.cdc.gov/nchs/products/index.htm Available at: [PubMed]
- 21.Xu J., Murphy S.L., Kochanek K.D., Arias E. Mortality in the United States, 2018. National Center for Health Statistics data brief. https://www.cdc.gov/nchs/data/databriefs/db355_tables-508.pdf#1 Available at:
- 22.Okada S., Shimada J., Kato D., Tsunezuka H., Teramukai S., Inoue M. Long-term prognostic impact of severe postoperative complications after lung cancer surgery. Ann Surg Oncol. 2019;26:230–237. doi: 10.1245/s10434-018-7061-x. [DOI] [PubMed] [Google Scholar]
- 23.Shinohara S., Kobayashi K., Kasahara C. Long-term impact of complications after lung resections in non-small cell lung cancer. J Thorac Dis. 2019;11:2024–2033. doi: 10.21037/jtd.2019.04.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rueth N.M., Parsons H.M., Habermann E.B. The long-term impact of surgical complications after resection of stage I nonsmall cell lung cancer: a population-based survival analysis. Ann Surg. 2011;254:368–374. doi: 10.1097/SLA.0b013e31822150fe. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., Li X., Li Y. The long-term impact of postoperative pulmonary complications after video-assisted thoracic surgery lobectomy for lung cancer. J Thorac Dis. 2017;9:5143–5152. doi: 10.21037/jtd.2017.10.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan H., Yin H., Wong S.L. Postoperative complications and long-term survival after complex cancer resection. Ann Surg Oncol. 2017;24:638–644. doi: 10.1245/s10434-016-5569-5. [DOI] [PubMed] [Google Scholar]
- 27.Samson P., Patel A., Garrett T. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2015;99:1906–1913. doi: 10.1016/j.athoracsur.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C.F., Chao Y.K. Saving time is saving lives: a delayed lobectomy predicts poorer overall survival in patients with clinical stage IA squamous cell carcinoma of the lung. J Thorac Dis. 2018;10(suppl 26):S3147–S3148. doi: 10.21037/jtd.2018.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]