Abstract
Purpose of Review:
Limited data exist on the prevalence, determinants, and outcomes of hepatitis delta virus (HDV) infection among HIV/hepatitis B virus (HBV)-coinfected persons. This review provides current evidence on the epidemiology, natural history, and treatment of HDV infection in patients with HIV/HBV coinfection and highlights future research needs.
Recent Findings:
Cross-sectional studies in Europe, Africa, South America, and Asia show that the prevalence of HDV among HIV/HBV-coinfected patients ranges from 1.2%–25%. No studies have evaluated the prevalence of HDV infection among HIV/HBV-coinfected patients in the United States. HDV infection increases the risk of hepatic decompensation and hepatocellular carcinoma among HIV/HBV-coinfected patients. HDV treatment remains limited to pegylated interferon-alpha, which results in sustained virologic response in <25%.
Summary:
Data on the epidemiology, natural history, and treatment of HDV among HIV/HBV-coinfected persons remain limited. More research is needed to address these knowledge gaps in order to better manage HDV coinfection in HIV/HBV-coinfected patients.
Keywords: Hepatitis delta infection, hepatitis D virus, HDV, HIV/HBV coinfection, hepatitis B infection
Introduction
Coinfection with hepatitis delta virus (HDV) is estimated to occur in approximately 5–10.6% of patients with chronic hepatitis B virus (HBV), affecting up to 72 million people worldwide [1–4]. The prevalence of HDV infection is thought to be rising in many parts of the world due to increased injection drug use, sexual transmission, and immigration of persons from areas of high endemicity [3, 5–8]. HDV coinfection is associated with more severe hepatitis and higher rates of hepatic decompensation, hepatocellular carcinoma, and death compared to infection with chronic HBV alone [2, 9]. Despite being associated with worse outcomes, testing for HDV infection among chronic HBV-infected patients is often neglected in clinical practice [10]. Moreover, treatment options remain limited to pegylated interferon-alpha (PEG-IFN-α) therapy, which results in sustained virologic response in <25% [11–14].
Few data exist on the prevalence, determinants, and outcomes of HDV infection among HIV/HBV-coinfected persons. The prevalence of HDV coinfection among HIV/HBV-coinfected patients has been estimated to range from 1.2% to 25% [9, 15, 16]. HDV coinfection has been associated with an increased risk of liver complications, particularly hepatic decompensation and hepatocellular carcinoma, and death among HIV/HBV-coinfected patients [2, 6, 9, 17–23]. Notably, no studies have examined the effectiveness of PEG-IFN-α or whether it reduces the risk of liver complications and mortality among HIV/HBV-coinfected patients.
In this article, we first provide a general overview of the virology, pathogenesis, natural history, and epidemiology of HDV infection. Next, we review current data on the epidemiology and clinical course of HDV infection among HIV/HBV-coinfected patients. We then discuss the efficacy of available treatment options and novel therapies being investigated for HDV infection. We conclude by highlighting the existing research needs for the field.
Virology and Pathogenesis of HDV
HDV is a small (35–37 nm in diameter) RNA virus that was discovered in 1977 [5, 24]. The HDV virion contains a circular negative single-stranded RNA genome and both small and large hepatitis delta antigen (HDAg) proteins [25]. The virion is defective, as it requires the hepatitis B surface antigen (HBsAg) for infection [25]. The HBsAg serves as an envelope protein, allowing HDV to enter hepatocytes [26]. Both HBV and HDV bind to heparan sulfate proteoglycans to adsorb onto hepatocytes [27] and utilize the sodium taurocholate co-transporting polypeptide receptor to enter the cells [26, 28]. Unlike conventional RNA viruses, HDV employs host RNA polymerases for viral replication [25, 29]. The small HDAg is important for the initiation of viral replication [25]. The large HDAg is essential for the assembly of new virion particles and undergoes several post-translational modifications, including prenylation (i.e., addition of hydrophobic molecules), which is being evaluated as a potential therapeutic target [25, 30, 31].
Eight HDV genotypes have been identified [5, 24]. HDV genotype 1 is the most common worldwide and predominates in North America, Europe, and the Middle East. Knowledge regarding the clinical course based on HDV genotype remains limited, though HDV genotype 3 infection has been associated with fulminant hepatitis and severe liver disease in cohort studies of HBV-infected persons in South America [32–34].
The pathogenesis of HDV is poorly understood, especially in the setting of HIV/HBV coinfection. Observational studies suggest that HDV infection induces liver injury primarily by generating a host-mediated immune response that results in hepatic inflammation, similar to chronic HBV and hepatitis C virus (HCV) infections [35, 36]. An immune-mediated process is suggested by the prominent necroinflammatory pattern observed on liver histology among patients with either chronic HDV coinfection or superinfection [36, 37]. In addition, one study found that HDV infection was associated with the generation of HDAg-specific CD4+ T-lymphocyte responses in the peripheral blood of patients with HDV infection [38]. In this study, the CD4+ T-lymphocytes generated high levels of inflammatory cytokines, particularly interferon-gamma [38]. Whether or not the HDAg-specific T-lymphocyte response and cytokine production plays a role in defense against HDV infection, immunopathogenesis, or both remains unknown. In the setting of HIV coinfection, HIV-induced reductions in CD4+ T-lymphocyte count have been shown to increase HDV viremia, suggesting that cell-mediated immunity may contribute to the control of HDV infection in HIV [39]. Whether or not HDV has cytopathic activity remains controversial. One study supports HDV cytotoxicity during acute HDV infection [40], whereas other studies do not support this [35, 41]. A study performed among transgenic mice expressing HDAg did not result in hepatocyte injury [35]. Similarly, a cohort study of liver transplant recipients who expressed HDAg after liver transplantation demonstrated that HDAg persistence did not manifest hepatocyte injury [41].
The interactions between hepatitis B, C, and D remain poorly understood. A well-described phenomenon with viral hepatitis coinfection is reciprocal interference, where one hepatitis virus suppresses the replication of other hepatitis viruses [42]. Several studies have found HDV to dominate HBV and HCV, suppressing replication of both viruses and resulting in lower levels of HBV and HCV viremia, regardless of HIV status [43–47].
Diagnosis and Clinical Course of HDV Infection
Diagnostic tests for HDV infection can assess the presence of immunoglobulin M (IgM) or immunoglobulin G (IgG) antibody to HDAg (anti-HDV) in serum or plasma, HDV RNA in serum or plasma, and HDAg in serum or within tissue specimens by direct immunohistochemical staining. Initial diagnosis of HDV infection typically involves testing for total anti-HDV antibodies (IgM and IgG), which usually are present after 4 weeks of acute infection, by using an enzyme immunoassay or radioimmunoassay. Anti-HDV IgM appears in serum 2–4 weeks after the time of initial infection, can persist with chronic infection, and may correlate with disease activity [48–52]. Anti-HDV IgG develops several weeks after anti-HDV IgM and persists with chronic HDV infection [52, 53].
In those who are HDV antibody-positive, HDV RNA should be used to confirm active HDV infection. The detection of HDV RNA by reverse transcriptase PCR amplification can be qualitative or quantitative [54]. Although assays for the detection of anti-HDV have been standardized and are now commercially available, assays for HDV RNA have not been standardized and have varying sensitivity [54, 55]. Levels of HDV viremia may be transient and fluctuate over time [56]. HDV RNA levels have not been shown to correlate with the severity of liver disease [57]. HDV RNA testing is also used to assess virologic response to antiviral treatment.
Current guidelines on HDV testing vary by region. The American Association for the Study of Liver Diseases recommends HDV antibody testing only in select patients, specifically HIV-infected individuals, persons who inject drugs, men who have sex with men, those at risk for sexually transmitted infections, immigrants from areas of high HDV endemicity, and patients identified with low HBV DNA levels but elevated liver aminotransferases [12]. Despite these recommendations, HDV screening is low in the United States (US). One study evaluating HDV testing in the Veterans Health Administration found that only 8.5% of HBsAg-positive patients ever underwent anti-HDV antibody screening [10]. In contrast, the European Association for the Study of Liver Diseases and the Asian-Pacific Association for the Study of the Liver recommend testing for HDV infection in all HBsAg-positive patients [13, 14]. Even with these recommendations, HDV screening remains suboptimal and occurs only in up to 46% of HBsAg-positive individuals [58].
The clinical course of HDV infection varies and typically depends on the mode of infection, defined as coinfection or superinfection. Simultaneous HBV/HDV coinfection is usually self-limited, though may cause an acute or fulminant hepatitis that is associated with higher mortality than that of acute HBV infection alone [3, 25]. HBV/HDV coinfection results in HDV viral clearance in more than 90% of cases as a result of resolution of HBV infection [3, 17, 25]. In contrast, HDV superinfection of HBV carriers is frequently associated with acute hepatitis and more likely results in persistent HDV replication [3, 25], with up to 90% of cases progressing to chronic HDV infection [17, 46, 59].
Regardless of the mode of infection, once chronic HDV infection occurs, it is associated with more severe hepatitis and accelerated progression of liver fibrosis to cirrhosis. Chronic HDV infection is also associated with a higher risk of hepatic decompensation, hepatocellular carcinoma, and all-cause mortality among chronic HBV-infected persons [2, 6, 9, 17–23, 60, 61]. In one cohort study of 299 chronic HDV/HBV-coinfected patients in Italy (13 of whom had HIV coinfection), the annual incidence of hepatocellular carcinoma was 2.8% per year over a mean follow-up of 19 years [18]. In a cohort study of 200 chronic HBV-infected patients with compensated cirrhosis, HDV infection was associated with a 3-fold increased risk of hepatocellular carcinoma over a median of 6.6 years [21]. The incidence of hepatocellular carcinoma was not compared by HIV status in either study.
Epidemiology of HDV Infection
HDV infection is a significant source of healthcare and economic burden. Globally, it is estimated that 5–10.6% of individuals with chronic HBV are coinfected with HDV, representing up to 72 million people worldwide [2–4, 62]. The prevalence of HDV/HBV coinfection varies geographically. HDV coinfection is highly endemic in the Mediterranean basin, Vietnam, Pakistan, Iran, Mongolia, Romania, Central Africa, West Africa, and the Amazon Basin, with estimates of prevalence exceeding 20% in these regions [63]. The prevalence of HDV coinfection has been reported to be as high as 42% in the Brazilian Amazon [64], and 75% among HIV-infected injection drug users in Taiwan [65]. In the US, HDV/HBV coinfection is associated with higher healthcare utilization and costs than HBV monoinfection [66].
As a result of HBV vaccination, the prevalence of HDV infection has declined since the 1990s in certain parts of Europe, particularly Italy [67, 68], Spain [69], and Turkey [70], where the prevalence has stabilized around 8–11% [8, 67, 71]. However, a resurgence of HDV infection has been observed in some countries due to increases in injection drug use, unprotected sex, and immigration of persons from highly endemic regions [3, 6–8].
In the US, studies have found the prevalence of HDV infection to range widely. A cross-sectional study in the mid-1980s reported the prevalence of HDV infection to be 3.8% in HBsAg-positive blood donors [72]. Some recent cross-sectional studies in the US have found the prevalence of HDV to be similarly low among HBsAg-positive individuals, with estimates of 2.2% in the US Midwest [73], 3.4% in the US Veterans Health Administration [10], and 8% in northern California [74]. However, one cross-sectional analysis from the 2011–2016 National Health and Nutrition Examination Survey found that 42% of HBsAg-positive individuals were HDV antibody-positive [75]. Among HBsAg-positive injection drug users, the frequency of HDV infection is high, with studies reporting the prevalence to range from 42% to 67% [76–78].
Epidemiology of HDV in HIV/HBV Coinfection
Coinfection with HBV occurs in 6–14% of HIV-infected individuals in North America and Europe [79, 80] and 10–20% in Asia and Africa [81–83], resulting in 3–6 million persons with HIV/HBV coinfection worldwide [84]. Despite the prevalence of HIV/HBV coinfection and clinical impact of HDV infection, few studies have evaluated the epidemiology of HDV infection among HIV/HBV-coinfected patients. The prevalence in these studies varies by geographic location.
A cross-sectional study of 422 HIV/HBV-coinfected patients with stored serum samples in the EuroSIDA cohort found the prevalence of HDV infection to be 14.5%, of whom 87% had HDV viremia. In this study, HDV-infected patients were more likely to have used injection drugs or have HCV coinfection. A cross-sectional analysis within the Swiss HIV Cohort Study determined that the HDV prevalence was 15.4% among a sample of 771 HIV/HBV-coinfected patients, of whom 63% had HDV viremia. Injection drug use and HCV coinfection were also risk factors for HDV infection in this study. Additionally, the prevalence of HDV infection was 20% in a cross-sectional analysis of 85 HIV/HBV-coinfected patients from Spain [85], 22.2% in a sample of 162 HIV/HBV-coinfected patients from Taiwan [86], and 25% among 72 HIV/HBV-coinfected patients from Guinea-Bissau in Africa [16]. Three cross-sectional studies of HIV/HBV-coinfected injection drug users in Taiwan reported the prevalence of HDV infection to range from 75% to 84% [65, 87, 88]. One possible explanation for the high prevalence of HDV infection among HIV/HBV-coinfected persons is the shared route of transmission through injection drug use. Table 1 summarizes the existing studies that have examined the prevalence and risk factors of HDV infection in HIV/HBV coinfection. Notably, no studies have determined the prevalence of HDV infection among HIV/HBV-coinfected patients in the US, limiting our understanding of the epidemiology of HDV coinfection in US settings.
Table 1.
Studies examining the prevalence and risk factors of hepatitis delta virus infection among HIV/hepatitis B virus-coinfected persons.
| Reference (Year) | Setting | No. Anti-HDV Antibody-Positive Among HBsAg-Positive | Anti-HDV Antibody Prevalence | Significant Risk Factors for HDV Infection in HTVTHBV Coinfection |
|---|---|---|---|---|
| Beguelin et al. (2017) [9] | Swiss HIV Cohort Study | 119/771 | 15.4% |
|
| Coffie et al. (2017) [123] | IeDEA West Africa Cohort | 10/67 | 14.9% | Not Reported |
| Hsieh et al. (2016) [87] | Taiwan (injection drug users) | 48/57 | 84.2% | Not Reported |
| Katwesigye et al. (2016) [124] | Uganda | 6/198 | 3.2% | Not Reported |
| Lin et al. (2015) [65] | Taiwan (injection drug users) | 197/263 | 74.9% | Not Reported |
| Lee et al. (2015) [125] | Taiwan | 7/64 | 10.9% | Not Reported |
| Honge et al. (2014) [16] | Guinea-Bissau | 18/72 | 25% | Not Reported |
| Hung et al. (2014) [126] | Taiwan | 38/375 | 10.1% | Not Reported |
| Fernandez et al. (2014) [85] | Spain | 17/85 | 20% |
|
| Thio et al. (2013) [127] | Multi-national study | 15/113 | 13.3% | Not Reported |
| Soriano et al. (2011) [15] | Eurosida | 61/422 | 14.5% |
|
| Chang et al. (2011) [88] | Taiwan (injection drug users) | 135/179 | 75.4% |
|
| Mendes-Correa et al. (2011) [128] | Brazil | 1/86 | 1.2% | Not Reported |
| Sheng et al. (2007) [86] | Taiwan | 36/162 | 22.2% |
|
Abbreviations: HBV=hepatitis B virus; HBsAg=hepatitis B surface antigen; HDV=hepatitis delta virus; MSM=men who have sex with men.
Significant alcohol use defined as >60 g/day of alcohol intake.
Clinical Course of HDV Infection in HIV/HBV Coinfection
There is limited information about the natural history of HDV infection in HIV/HBV-coinfected patients. Existing studies suggest that HIV/HBV/HDV triple infection is associated with worse outcomes compared to HIV/HBV coinfection [9, 15, 44, 85, 86]. In the 2011 EuroSIDA study, HDV antibody-positivity was associated with a more than 4-fold increased risk of liver-related death and more than 2-fold increased risk of all-cause mortality over a median follow-up of 7.5 years [15]. Similarly, in the 2017 Swiss HIV Cohort Study, HDV coinfection increased the risk of hepatocellular carcinoma 9-fold and the risk of liver-related death 8-fold over a median follow-up of 8.7 years [9]. Moreover, a 2014 Spanish study found that HIV/HBV/HDV infection increased the risk of hepatic decompensation or death 7-fold over a median 81 months of follow-up [85]. Finally, a 2007 cohort study from Taiwan found that HDV infection was associated with an increased incidence of cirrhosis, hepatic decompensation, and death over a median of 54.7 months, though this study only included 29 HIV/HBV/HDV-infected patients, all of whom were non-cirrhotic at start of follow-up [86]. Taken together, these data suggest that HDV coinfection increases the risk of liver complications, such as hepatic decompensation or hepatocellular carcinoma, and mortality among HIV/HBV-coinfected patients.
Factors associated with liver complications remain unknown among HIV/HBV/HDV-infected patients. HIV-related immune dysfunction has been shown to enhance HBV replication and accelerate HBV-related liver disease [89]. Thus, the level of HIV-related immune function may be an important determinant of HDV-induced liver disease. The independent effects of HDV, HIV, and HBV viremia, as well as other traditional liver disease risk factors (e.g., body mass index, alcohol consumption, tobacco use, diabetes mellitus, HCV coinfection) have not been evaluated among triply infected persons and are in need of study in this population.
Treatment of HDV Infection
All patients with HIV/HBV coinfection should initiate an antiretroviral therapy (ART) regimen that contains tenofovir alafenamide (TAF) or tenofovir disoproxil fumarate (TDF), emtricitabine or lamivudine, and a third antiretroviral drug, regardless of the CD4+ cell count [90]. Tenofovir is preferred as the anti-HBV backbone because virologic efficacy is high [91–93], and the risk of HBV resistance has been shown to be low among both HBV-monoinfected [94, 95] and HIV/HBV-coinfected patients [96–98]. If tenofovir cannot be safely used, the nucleoside analogue entecavir is an alternative, but it should only be used when added to an ART regimen that has resulted in HIV suppression [99]. Entecavir’s activity can be limited in patients with prior lamivudine treatment or resistance [100], which is common in HIV/HBV-coinfected patients [101, 102]. The main goal of HBV-active ART is sustained suppression of HBV DNA to an undetectable level and clearance of HBsAg to reduce the likelihood of liver-related complications and HBV person-to-person transmission [103–107]. Given the high likelihood of HBV reactivation upon discontinuation of HBV-active nucleos(t)ide analogues [108], HBV-active ART is typically continued indefinitely in HIV/HBV-coinfected patients to achieve persistent HBV control.
Nucleos(t)ide analogues have been shown to be generally ineffective in achieving HDV suppression. Lamivudine, adefovir, and entecavir do not affect HDV RNA levels when used alone or in combination with interferon-alpha (IFN-α) [11, 109, 110]. Whether or not tenofovir contributes to reducing HDV RNA remains unclear, with studies reporting mixed results. In a Spanish study of 19 HIV/HBV-coinfected patients treated with TDF for a median of 58 months, 53% of patients had undetectable HDV RNA [111]. In contrast, a French cohort study did not find significant declines in HDV RNA level among either 4 HIV/HBV-coinfected patients treated with TDF-based ART plus IFN-α or 13 coinfected individuals treated with TDF-based ART alone [112]. Similarly, a recent study of 21 HIV/HBV-coinfected patients in the Swiss HIV Cohort Study who received TDF-containing ART demonstrated no significant reductions in HDV RNA levels after a median of 5 years of therapy [113].
There is no approved therapy for the treatment of chronic HDV infection, but a 12–18-month course of PEG-IFN-α is often used [12–14]. However, the rate of sustained virologic response (SVR), defined as absence of HDV viremia 6 or more months after completion of therapy, has been low with this treatment [107]. The efficacy of IFN-α therapy was evaluated in a trial of 42 patients with chronic HDV infection who were randomly assigned to receive either 9 million or 3 million units of standard IFN-α−2a (three times a week for 48 weeks) compared to no treatment [114]. In approximately half of the patients who received either dosage of IFN-α−2a, serum alanine aminotransferase levels normalized, serum HDV RNA becomes undetectable, and histologic improvement occurred. However, HDV infection relapse was common after treatment was discontinued, and only 17% achieved SVR at 6 months after completion of therapy [114]. In a prospective cohort study evaluating the effectiveness of a 48-week course of PEG-IFN-α administered to 104 chronic HDV-infected patients, SVR at 24 weeks post-treatment was observed in only 24 (23.1%) patients [115]. Failure to achieve SVR to PEG-IFN-α is more likely among patients with a <3 log10 decrease in HDV RNA over the initial 6 months of antiviral treatment [116]. Notably, patients with HIV infection were excluded from these studies.
New treatment options for HDV infection are currently being investigated in clinical trials and include myrcludex-B, lonafarnib, REP 2139-Ca, and pegylated interferon-lambda. Myrcludex-B (also known as bulevirtide) is a synthetic lipopeptide that inhibits the liver-specific sodium taurocholate co-transporting polypeptide receptor and can block entry of both HBV and HDV [117, 118]. In a phase 2 clinical trial, myrcludex-B was shown to reduce HDV RNA levels significantly at week 24 of treatment when used alone or in combination with PEG-IFN-α [119]. However, only 1/7 (14.3%) of patients achieved SVR [119]. Lonafarnib is a farnesyltransferase inhibitor that prevents prenylation of the large HDAg, which ultimately disrupts HDV virion assembly and release. Ritonavir acts as a booster, allowing the use of a lower dose of lonafarnib. Lonafarnib has been shown to reduce HDV RNA levels rapidly in a dose-dependent manner and has been more effective when used in combination with PEG-IFN-α [30, 31]. Phase 3 trials of myrcludex-B and lonafarnib are ongoing. REP 2139-Ca is a synthetic nucleic acid polymer that inhibits the attachment of HBV and HDV to heparan sulfate proteoglycans. In one clinical trial in which REP 2139-Ca was followed by PEG-IFN-α, significant reductions in HDV RNA, reductions in quantitative HBsAg levels, and the development of anti-HBs-positivity were reported [120, 121]. Finally, pegylated interferon-lambda has been shown to reduce HDV RNA levels in 50% of treated patients [122] and is currently being studied in combination with lonafarnib. Figure 1 illustrates current and potential future targets for HDV treatment.
Figure 1.
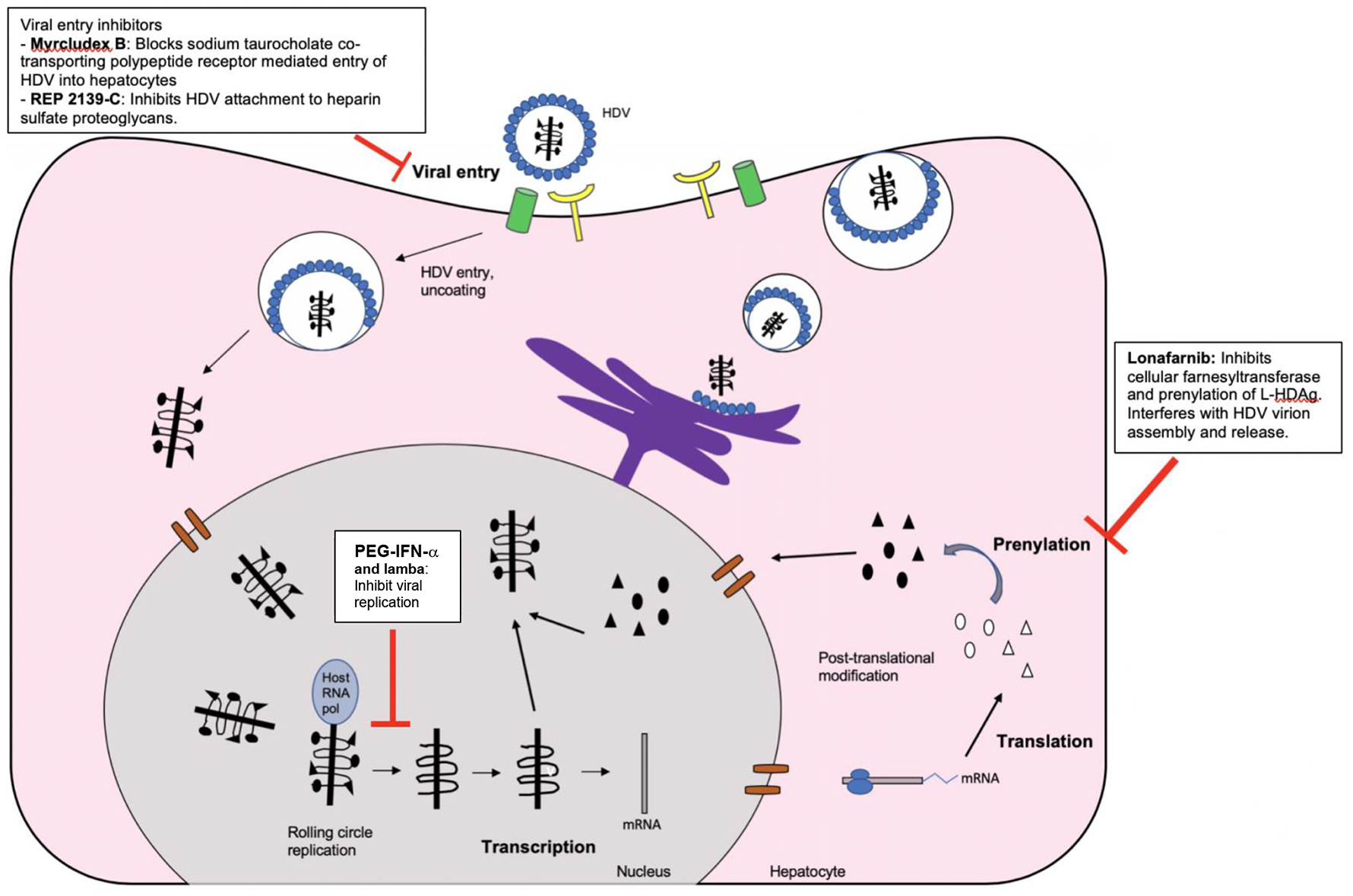
Current and potential future targets for therapeutic interventions against chronic hepatitis delta virus infection.
Abbreviations: HDAg=hepatitis delta antigen; HDV=hepatitis delta virus; Host RNA pol=host RNA polymerase; HBsAg=hepatitis B surface antigen; L-HDAg=large hepatitis delta antigen; mRNA=messenger RNA; PEG-IFN-α=pegylated interferon alpha; S-HDAg=small hepatitis delta antigen

Research Needs
Many questions remain unanswered regarding HIV/HBV/HDV coinfection, and dedicated research in this area is needed. More basic research is needed to understand the pathogenesis of HDV infection among HIV/HBV-coinfected patients and to elucidate the complex interactions between HIV, HBV, and HDV. Understanding these viral interactions in the context of HIV infection may help identify targets for antiviral therapy. In addition, the epidemiology of HDV infection among HIV/HBV-coinfected patients has not been thoroughly studied, especially in the US. More data on the prevalence and risk factors for HDV infection among HIV/HBV-coinfected persons are needed to identify high-risk subgroups that warrant HDV testing. Moreover, the effects of viral factors (e.g., HDV genotype and HDV, HIV, and HBV viremia), host factors (e.g., HIV-related immune function, antiretroviral therapy use), and traditional determinants of liver disease (e.g., obesity, alcohol consumption, tobacco use, diabetes mellitus, HCV coinfection) on outcomes such as hepatic decompensation and hepatocellular carcinoma remain incompletely understood among triply infected persons. Such studies are needed to identify the factors that might be modified to attenuate liver disease progression in this group. Finally, research is needed in novel therapies of HDV infection among HIV/HBV/HDV-infected patients to reduce the risk of adverse liver outcomes and prolong survival in these individuals.
Conclusion
HDV coinfection occurs in up to 25% of persons with HIV/HBV coinfection. HDV coinfection has been associated with an increased risk of hepatic decompensation, hepatocellular carcinoma, and death among HIV/HBV-coinfected patients. Despite the clinical impact of HDV coinfection, treatment options remain limited to PEG-IFN-α-based therapy, which results in SVR in fewer than 25% of treated patients, but the effectiveness of this treatment has not been evaluated in HIV/HBV/HDV-infected patients. More studies are needed to understand the interactions between HIV, HBV and HDV infections; determine the epidemiology of HDV infection among HIV/HBV-coinfected patients; and evaluate novel treatment strategies in triply infected persons to increase rates of SVR and reduce the risk of liver complications and mortality. These knowledge gaps must be overcome in order to appropriately understand and manage HDV coinfection in HIV/HBV-coinfected patients.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Human and Animal rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
REFERENCES
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Wedemeyer HM, Michael. Epidemiology, pathogenesis and managementof hepatitis D: update and challenges ahead. Nature Review Gastroenterology and hepatology 2010; 7: 31–40. [DOI] [PubMed] [Google Scholar]
- 2.Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet 2011; 378(9785): 73–85. [DOI] [PubMed] [Google Scholar]
- 3.Rizzetto M Hepatitis D: Thirty years after. Journal of Hepatology 2009; 50(5): 1043–50. [DOI] [PubMed] [Google Scholar]
- 4.Chen HY, Shen DT, Ji DZ, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut 2018; 68: 512–21. [DOI] [PubMed] [Google Scholar]; •• This systematic review and meta-analysis found that the prevalence of HDV among HBsAg-positive individuals may be higher than was previously reported.
- 5.Rizzetto M Hepatitis D virus: introduction and epidemiology. Cold Spring Harb Perspect Med 2015; 5(7): a021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wedemeyer H, Heidrich B, Manns MP. Hepatitis D virus infection--not a vanishing disease in Europe! Hepatology 2007; 45(5): 1331–2; author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 7.Erhardt A, Knuth R, Sagir A, Kirschberg O, Heintges T, Häussinger D. Socioepidemiological data on hepatitis delta in a German university clinic--increase in patients from Eastern Europe and the former Soviet Union. Z Gastroenterol 2003; 41(6): 523–6. [DOI] [PubMed] [Google Scholar]
- 8.Cross TJ, Rizzi P, Horner M, et al. The increasing prevalence of hepatitis delta virus (HDV) infection in South London. J Med Virol 2008; 80(2): 277–82. [DOI] [PubMed] [Google Scholar]
- 9.Béguelin C, Moradpour D, Sahli R, et al. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J Hepatol 2017; 66(2): 297–303. [DOI] [PubMed] [Google Scholar]; •• This cross-sectional study found the prevalence of HDV infection to be 15.45% in the Swiss HIV Cohort Study.
- 10.Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J Hepatol 2015; 63(3): 586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wedemeyer H, Yurdaydìn C, Dalekos GN, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011; 364(4): 322–31. [DOI] [PubMed] [Google Scholar]
- 12.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Clin Liver Dis (Hoboken) 2018; 12(1): 33–4. [DOI] [PMC free article] [PubMed] [Google Scholar]; • These are the most updated American Association for the Study of Liver Disease guidelines on hepatitis B virus management.
- 13.EASL 2017 Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67(2): 370–98. [DOI] [PubMed] [Google Scholar]
- 14.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016; 10(1): 1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soriano V, Grint D, d’Arminio Monforte A, et al. Hepatitis delta in HIV-infected individuals in Europe. AIDS 2011; 25(16): 1987–92. [DOI] [PubMed] [Google Scholar]
- 16.Hønge BL, Jespersen S, Medina C, et al. Hepatitis B and delta virus are prevalent but often subclinical co-infections among HIV infected patients in Guinea-Bissau, West Africa: a cross-sectional study. PLoS One 2014; 9(6): e99971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buti M, Homs M, Rodriguez-Frias F, et al. Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. J Viral Hepat 2011; 18(6): 434–42. [DOI] [PubMed] [Google Scholar]
- 18.Romeo R, Del Ninno E, Rumi M, et al. A 28-year study of the course of hepatitis delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 2009; 136(5): 1629–38. [DOI] [PubMed] [Google Scholar]
- 19.Yurdaydın C, Idilman R, Bozkaya H, Bozdayi AM. Natural history and treatment of chronic delta hepatitis. J Viral Hepat 2010; 17(11): 749–56. [DOI] [PubMed] [Google Scholar]
- 20.Gheorghe L, Iacob S, Simionov I, et al. Natural history of compensated viral B and D cirrhosis. Rom J Gastroenterol 2005; 14(4): 329–35. [PubMed] [Google Scholar]
- 21.Fattovich G, Giustina G, Christensen E, et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut 2000; 46(3): 420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji J, Sundquist K, Sundquist J. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst 2012; 104(10): 790–2. [DOI] [PubMed] [Google Scholar]
- 23.Fattovich G, Boscaro S, Noventa F, et al. Influence of hepatitis delta virus infection on progression to cirrhosis in chronic hepatitis type B. J Infect Dis 1987; 155(5): 931–5. [DOI] [PubMed] [Google Scholar]
- 24.Rizzetto M, Canese MG, Aricò S, et al. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977; 18(12): 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grabowski J, Wedemeyer H. Hepatitis delta: immunopathogenesis and clinical challenges. Dig Dis 2010; 28(1): 133–8. [DOI] [PubMed] [Google Scholar]
- 26.He W, Ren B, Mao F, et al. Hepatitis D virus infection of mice expressing human sodium taurocholate co-transporting polypeptide. PLoS Pathog 2015; 11(4): e1004840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verrier ER, Colpitts CC, Bach C, et al. A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatology 2016; 63(1): 35–48. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Wands J. Hepatitis B and D viral receptors. Hepatology 2016; 63(1): 11–3. [DOI] [PubMed] [Google Scholar]
- 29.Chang J, Nie X, Chang HE, Han Z, Taylor J. Transcription of hepatitis delta virus RNA by RNA polymerase II. J Virol 2008; 82(3): 1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh C, Canini L, Dahari H, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis 2015; 15(10): 1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wedemeyer HPK, Deterding K, et al. A phase 2 dose-escalation study of lonafarnib plus ritonavir in patients with chronic hepatitis D: final results from the Lonafarnib with ritonavir in HDV-4 (LOWR HDV-4) study. Journal of Hepatology 2017; 66(1): S24. [Google Scholar]
- 32.Su CW, Huang YH, Huo TI, et al. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology 2006; 130(6): 1625–35. [DOI] [PubMed] [Google Scholar]
- 33.Casey JL, Niro GA, Engle RE, et al. Hepatitis B virus (HBV)/hepatitis D virus (HDV) coinfection in outbreaks of acute hepatitis in the Peruvian Amazon basin: the roles of HDV genotype III and HBV genotype F. J Infect Dis 1996; 174(5): 920–6. [DOI] [PubMed] [Google Scholar]
- 34.Gomes-Gouvêa MS, Soares MC, Bensabath G, et al. Hepatitis B virus and hepatitis delta virus genotypes in outbreaks of fulminant hepatitis (Labrea black fever) in the western Brazilian Amazon region. J Gen Virol 2009; 90(Pt 11): 2638–43. [DOI] [PubMed] [Google Scholar]
- 35.Guilhot S, Huang SN, Xia YP, La Monica N, Lai MM, Chisari FV. Expression of the hepatitis delta virus large and small antigens in transgenic mice. J Virol 1994; 68(2): 1052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Govindarajan S, De Cock KM, Redeker AG. Natural course of delta superinfection in chronic hepatitis B virus-infected patients: histopathologic study with multiple liver biopsies. Hepatology 1986; 6(4): 640–4. [DOI] [PubMed] [Google Scholar]
- 37.Liao B, Zhang F, Lin S, et al. Epidemiological, clinical and histological characteristics of HBV/HDV co-infection: a retrospective cross-sectional study in Guangdong, China . PLoS One 2014; 9(12): e115888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nisini R, Paroli M, Accapezzato D, et al. Human CD4+ T-cell response to hepatitis delta virus: identification of multiple epitopes and characterization of T-helper cytokine profiles. J Virol 1997; 71(3): 2241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roingeard P, Dubois F, Marcellin P, et al. Persistent delta antigenaemia in chronic delta hepatitis and its relation with human immunodeficiency virus infection. J Med Virol 1992; 38(3): 191–4. [DOI] [PubMed] [Google Scholar]
- 40.Bichko V, Netter HJ, Wu TT, Taylor J. Pathogenesis associated with replication of hepatitis delta virus. Infect Agents Dis 1994; 3(2–3): 94–7. [PubMed] [Google Scholar]
- 41.Samuel D, Zignego AL, Reynes M, et al. Long-term clinical and virological outcome after liver transplantation for cirrhosis caused by chronic delta hepatitis. Hepatology 1995; 21(2): 333–9. [PubMed] [Google Scholar]
- 42.Raimondo G, Brunetto MR, Pontisso P, et al. Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis B virus/hepatitis C virus-coinfected patients. Hepatology 2006; 43(1): 100–7. [DOI] [PubMed] [Google Scholar]
- 43.Arribas JR, González-García JJ, Lorenzo A, et al. Single (B or C), dual (BC or BD) and triple (BCD) viral hepatitis in HIV-infected patients in Madrid, Spain. AIDS 2005; 19(13): 1361–5. [DOI] [PubMed] [Google Scholar]
- 44.Mathurin P, Thibault V, Kadidja K, et al. Replication status and histological features of patients with triple (B, C, D) and dual (B, C) hepatic infections. J Viral Hepat 2000; 7(1): 15–22. [DOI] [PubMed] [Google Scholar]
- 45.Jardi R, Rodriguez F, Buti M, et al. Role of hepatitis B, C, and D viruses in dual and triple infection: influence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral replicative interference. Hepatology 2001; 34(2): 404–10. [DOI] [PubMed] [Google Scholar]
- 46.Smedile A, Dentico P, Zanetti A, et al. Infection with the delta agent in chronic HBsAg carriers. Gastroenterology 1981; 81(6): 992–7. [PubMed] [Google Scholar]
- 47.Pontisso P, Ruvoletto MG, Fattovich G, et al. Clinical and virological profiles in patients with multiple hepatitis virus infections. Gastroenterology 1993; 105(5): 1529–33. [DOI] [PubMed] [Google Scholar]
- 48.Wranke A, Heidrich B, Ernst S, et al. Anti-HDV IgM as a marker of disease activity in hepatitis delta. PLoS One 2014; 9(7): e101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farci P, Gerin JL, Aragona M, et al. Diagnostic and prognostic significance of the IgM antibody to the hepatitis delta virus. JAMA 1986; 255(11): 1443–6. [PubMed] [Google Scholar]
- 50.Borghesio E, Rosina F, Smedile A, et al. Serum immunoglobulin M antibody to hepatitis D as a surrogate marker of hepatitis D in interferon-treated patients and in patients who underwent liver transplantation. Hepatology 1998; 27(3): 873–6. [DOI] [PubMed] [Google Scholar]
- 51.Shattock AG, Morris MC. Evaluation of commercial enzyme immunoassays for detection of hepatitis delta antigen and anti-hepatitis delta virus (HDV) and immunoglobulin M anti-HDV antibodies. J Clin Microbiol 1991; 29(9): 1873–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aragona M, Macagno S, Caredda F, et al. Serological response to the hepatitis delta virus in hepatitis D. Lancet 1987; 1(8531): 478–80. [DOI] [PubMed] [Google Scholar]
- 53.Noureddin M, Gish R. Hepatitis delta: epidemiology, diagnosis and management 36 years after discovery. Curr Gastroenterol Rep 2014; 16(1): 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Gal F, Brichler S, Sahli R, Chevret S, Gordien E. First international external quality assessment for hepatitis delta virus RNA quantification in plasma. Hepatology 2016; 64(5): 1483–94. [DOI] [PubMed] [Google Scholar]
- 55.Olivero A, Smedile A. Hepatitis delta virus diagnosis. Semin Liver Dis 2012; 32(3): 220–7. [DOI] [PubMed] [Google Scholar]
- 56.Schaper M, Rodriguez-Frias F, Jardi R, et al. Quantitative longitudinal evaluations of hepatitis delta virus RNA and hepatitis B virus DNA shows a dynamic, complex replicative profile in chronic hepatitis B and D. J Hepatol 2010; 52(5): 658–64. [DOI] [PubMed] [Google Scholar]
- 57.Zachou K, Yurdaydin C, Drebber U, et al. Quantitative HBsAg and HDV-RNA levels in chronic delta hepatitis. Liver Int 2010; 30(3): 430–7. [DOI] [PubMed] [Google Scholar]
- 58.Manesis EK, Vourli G, Dalekos G, et al. Prevalence and clinical course of hepatitis delta infection in Greece: a 13-year prospective study. J Hepatol 2013; 59(5): 949–56. [DOI] [PubMed] [Google Scholar]
- 59.Negro F Hepatitis D virus coinfection and superinfection. Cold Spring Harb Perspect Med 2014; 4(11): a021550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rizzetto M, Alavian SM. Hepatitis delta: the rediscovery. Clin Liver Dis 2013; 17(3): 475–87. [DOI] [PubMed] [Google Scholar]
- 61.Amougou MA, Noah DN, Moundipa PF, Pineau P, Njouom R. A prominent role of Hepatitis D Virus in liver cancers documented in Central Africa. BMC Infect Dis 2016; 16(1): 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hadziyannis SJ. Review: hepatitis delta. J Gastroenterol Hepatol 1997; 12(4): 289–98. [DOI] [PubMed] [Google Scholar]
- 63.Rizzetto M, Ponzetto A, Forzani I. Hepatitis delta virus as a global health problem. Vaccine 1990; 8 Suppl: S10–4; discussion S21–3. [DOI] [PubMed] [Google Scholar]
- 64.Braga WS, Castilho MaC, Borges FG, et al. Hepatitis D virus infection in the Western Brazilian Amazon - far from a vanishing disease. Rev Soc Bras Med Trop 2012; 45(6): 691–5. [DOI] [PubMed] [Google Scholar]
- 65.Lin HH, Lee SS, Yu ML, et al. Changing hepatitis D virus epidemiology in a hepatitis B virus endemic area with a national vaccination program. Hepatology 2015; 61(6): 1870–9. [DOI] [PubMed] [Google Scholar]
- 66.Elsaid MI, Li Y, John T, Narayanan N, Catalano C, Rustgi VK. Economic and healthcare burdens of hepatitis delta: a study of commercially insured adults in the United States. Hepatology 2019. Available from: 10.1002/hep.31055. [DOI] [PubMed] [Google Scholar]; •• This was a case control study that utilized claims data to determine the economic burden of HDV infection, and found that HDV infection was associated with increased healthcare costs.
- 67.Gaeta GB, Precone DF, Cozzi-Lepri A, Cicconi P, D’Arminio Monforte A. Multiple viral infections. J Hepatol 2006; 44(1 Suppl): S108–13. [DOI] [PubMed] [Google Scholar]
- 68.Sagnelli E, Stroffolini T, Ascione A, et al. Decrease in HDV endemicity in Italy. J Hepatol 1997; 26(1): 20–4. [DOI] [PubMed] [Google Scholar]
- 69.Navascués CA, Rodríguez M, Sotorrío NG, et al. Epidemiology of hepatitis D virus infection: changes in the last 14 years. Am J Gastroenterol 1995; 90(11): 1981–4. [PubMed] [Google Scholar]
- 70.Değertekin H, Yalçin K, Yakut M. The prevalence of hepatitis delta virus infection in acute and chronic liver diseases in Turkey: an analysis of clinical studies. Turk J Gastroenterol 2006; 17(1): 25–34. [PubMed] [Google Scholar]
- 71.Gaeta GB, Stroffolini T, Smedile A, Niro G, Mele A. Hepatitis delta in Europe: vanishing or refreshing? Hepatology 2007; 46(4): 1312–3. [DOI] [PubMed] [Google Scholar]
- 72.Nath N, Mushahwar IK, Fang CT, Berberian H, Dodd RY. Antibodies to delta antigen in asymptomatic hepatitis B surface antigen-reactive blood donors in the United States and their association with other markers of hepatitis B virus. Am J Epidemiol 1985; 122(2): 218–25. [DOI] [PubMed] [Google Scholar]
- 73.Safaie P, Razeghi S, Rouster SD, Privitera I, Sherman KE. Hepatitis D diagnostics: utilization and testing in the United States. Virus Res 2018; 250: 114–7. [DOI] [PubMed] [Google Scholar]
- 74.Gish RG, Yi DH, Kane S, et al. Coinfection with hepatitis B and D: epidemiology, prevalence and disease in patients in Northern California. J Gastroenterol Hepatol 2013; 28(9): 1521–5. [DOI] [PubMed] [Google Scholar]
- 75.Patel EU, Thio CL, Boon D, Thomas DL, Tobian AAR. Prevalence of hepatitis B and hepatitis D virus infections in the United States, 2011–2016. Clin Infect Dis 2019; 69(4): 709–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This cross-sectional study found that 42% of HBsAg-positive individuals are HDV antibody positive.
- 76.Kucirka LM, Farzadegan H, Feld JJ, et al. Prevalence, correlates, and viral dynamics of hepatitis delta among injection drug users. J Infect Dis 2010; 202(6): 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ponzetto A, Seeff LB, Buskell-Bales Z, et al. Hepatitis B markers in United States drug addicts with special emphasis on the delta hepatitis virus. Hepatology 1984; 4(6): 1111–5. [DOI] [PubMed] [Google Scholar]
- 78.Novick DM, Farci P, Croxson TS, et al. Hepatitis D virus and human immunodeficiency virus antibodies in parenteral drug abusers who are hepatitis B surface antigen positive. J Infect Dis 1988; 158(4): 795–803. [DOI] [PubMed] [Google Scholar]
- 79.Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis 2003; 188(4): 571–7. [DOI] [PubMed] [Google Scholar]
- 80.Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005; 19(6): 593–601. [DOI] [PubMed] [Google Scholar]
- 81.Lee HC, Ko NY, Lee NY, Chang CM, Ko WC. Seroprevalence of viral hepatitis and sexually transmitted disease among adults with recently diagnosed HIV infection in Southern Taiwan, 2000–2005: upsurge in hepatitis C virus infections among injection drug users. J Formos Med Assoc 2008; 107(5): 404–11. [DOI] [PubMed] [Google Scholar]
- 82.Diop-Ndiaye H, Touré-Kane C, Etard JF, et al. Hepatitis B, C seroprevalence and delta viruses in HIV-1 Senegalese patients at HAART initiation (retrospective study). J Med Virol 2008; 80(8): 1332–6. [DOI] [PubMed] [Google Scholar]
- 83.Nyirenda M, Beadsworth MB, Stephany P, et al. Prevalence of infection with hepatitis B and C virus and coinfection with HIV in medical inpatients in Malawi. J Infect 2008; 57(1): 72–7. [DOI] [PubMed] [Google Scholar]
- 84.Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection--a global challenge. N Engl J Med 2012; 366(19): 1749–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernández-Montero JV, Vispo E, Barreiro P, et al. Hepatitis delta is a major determinant of liver decompensation events and death in HIV-infected patients. Clin Infect Dis 2014; 58(11): 1549–53. [DOI] [PubMed] [Google Scholar]
- 86.Sheng WH, Hung CC, Kao JH, et al. Impact of hepatitis D virus infection on the long-term outcomes of patients with hepatitis B virus and HIV coinfection in the era of highly active antiretroviral therapy: a matched cohort study. Clin Infect Dis 2007; 44(7): 988–95. [DOI] [PubMed] [Google Scholar]
- 87.Hsieh MH, Wang SC, Hsieh MY, et al. Hepatitis D virus infections among injecting drug users with and without human immunodeficiency virus infection in Taiwan. Kaohsiung J Med Sci 2016; 32(10): 526–30. [DOI] [PubMed] [Google Scholar]
- 88.Chang SY, Yang CL, Ko WS, et al. Molecular epidemiology of hepatitis D virus infection among injecting drug users with and without human immunodeficiency virus infection in Taiwan. J Clin Microbiol 2011; 49(3): 1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thio CL, Seaberg EC, Skolasky R, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360(9349): 1921–6. [DOI] [PubMed] [Google Scholar]
- 90.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA 2018; 320(4): 379–96. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• These are the most recent guidelines for the treatment of HIV infection.
- 91.Delaney WE, Ray AS, Yang H, et al. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother 2006; 50(7): 2471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 2011; 140(1): 132–43. [DOI] [PubMed] [Google Scholar]
- 93.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013; 381(9865): 468–75. [DOI] [PubMed] [Google Scholar]
- 94.Snow-Lampart A, Chappell B, Curtis M, et al. No resistance to tenofovir disoproxil fumarate detected after up to 144 weeks of therapy in patients monoinfected with chronic hepatitis B virus. Hepatology 2011; 53(3): 763–73. [DOI] [PubMed] [Google Scholar]
- 95.Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology 2013; 58(2): 505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sheldon J, Camino N, Rodes B, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther 2005; 10(6): 727–34. [PubMed] [Google Scholar]
- 97.De Vries-Sluijs TE, Reijnders JG, Hansen BE, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology 2010; 139(6): 1934–41. [DOI] [PubMed] [Google Scholar]
- 98.Lada O, Gervais A, Branger M, et al. Long-term outcome of primary non-responders to tenofovir therapy in HIV/HBV-co-infected patients: impact of HBV genotype G. Liver Int 2012; 32(1): 93–101. [DOI] [PubMed] [Google Scholar]
- 99.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV Infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA 2014; 312(4): 410–25. [DOI] [PubMed] [Google Scholar]
- 100.McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med 2007; 356(25): 2614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006; 20(6): 863–70. [DOI] [PubMed] [Google Scholar]
- 102.Kim HN, Rodriguez CV, Van Rompaey S, et al. Factors associated with delayed hepatitis B viral suppression on tenofovir among patients coinfected with HBV-HIV in the CNICS cohort. J Acquir Immune Defic Syndr 2014; 66(1): 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45(2): 507–39. [DOI] [PubMed] [Google Scholar]
- 104.Lai CL, Yuen MF. The natural history and treatment of chronic hepatitis B: a critical evaluation of standard treatment criteria and end points. Ann Intern Med 2007; 147(1): 58–61. [DOI] [PubMed] [Google Scholar]
- 105.Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008; 2(3): 263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS 2008; 22(12): 1399–410. [DOI] [PubMed] [Google Scholar]
- 107.Cornberg M, Lok AS, Terrault NA, Zoulim F, Faculty E-AHTEC. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. J Hepatol 2019. Available from: 10.1002/hep.31030. [DOI] [PubMed] [Google Scholar]; •• Recommendations for treatment endpoints for HBV and HDV therapies in clinical trials.
- 108.Dore GJ, Soriano V, Rockstroh J, et al. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS 2010; 24(6): 857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niro GA, Ciancio A, Tillman HL, et al. Lamivudine therapy in chronic delta hepatitis: a multicentre randomized-controlled pilot study. Aliment Pharmacol Ther 2005; 22(3): 227–32. [DOI] [PubMed] [Google Scholar]
- 110.Lau DT, Doo E, Park Y, et al. Lamivudine for chronic delta hepatitis. Hepatology 1999; 30(2): 546–9. [DOI] [PubMed] [Google Scholar]
- 111.Soriano V, Vispo E, Sierra-Enguita R, et al. Efficacy of prolonged tenofovir therapy on hepatitis delta in HIV-infected patients. AIDS 2014; 28(16): 2389–94. [DOI] [PubMed] [Google Scholar]
- 112.Boyd A, Miailhes P, Brichler S, et al. Effect of tenofovir with and without interferon on hepatitis D virus replication in HIV-hepatitis B virus-hepatitis D virus-infected patients. AIDS Res Hum Retroviruses 2013; 29(12): 1535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Beguelin CFN, Moradpour D, et al. Impact of tenofovir on hepatitis delta virus replication in the Swiss Human Immunodeficiency Virus cohort study. Clin Infect Dis 2017; 64(9): 1275–8. [DOI] [PubMed] [Google Scholar]; • This cohort study found that tenofovir-containing antiretrovieral therapy did not result in significant reductions in HDV RNA among HIV/HBV-coinfected patients in the Swiss HIV Cohort Study.
- 114.Abbas Z, Khan MA, Salih M, Jafri W. Interferon alpha for chronic hepatitis D. Cochrane Database Syst Rev 2011; (12): CD006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abbas Z, Memon MS, Mithani H, Jafri W, Hamid S. Treatment of chronic hepatitis D patients with pegylated interferon: a real-world experience. Antivir Ther 2014; 19(5): 463–8. [DOI] [PubMed] [Google Scholar]
- 116.Erhardt A, Gerlich W, Starke C, et al. Treatment of chronic hepatitis delta with pegylated interferon-alpha2b. Liver Int 2006; 26(7): 805–10. [DOI] [PubMed] [Google Scholar]
- 117.Gripon P, Cannie I, Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J Virol 2005; 79(3): 1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Slijepcevic D, Kaufman C, Wichers CG, et al. Impaired uptake of conjugated bile acids and hepatitis b virus pres1-binding in na(+) -taurocholate cotransporting polypeptide knockout mice. Hepatology 2015; 62(1): 207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bogomolov P, Alexandrov A, Voronkova N, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol 2016; 65(3): 490–8. [DOI] [PubMed] [Google Scholar]
- 120.Al-Mahtab M, Bazinet M, Vaillant A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment-naive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS One 2016; 11(6): e0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bazinet M, Pântea V, Cebotarescu V, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2017; 2(12): 877–89. [DOI] [PubMed] [Google Scholar]; •• This Phase 2 study that found that the combination of REP 2139 and pegylated interferon alfa-2a resulted in reductions in both HDV RNA and HBsAg.
- 122.Etzion OHS, Lurie Y, Gane E, Bader N, Yardeni D, Nevo-Shor A, et al. End of study results from LIMT HDV study: 36% durable virologic response at 24 weeks post-treatment with pegylated interferon lambda monotherapy in patients with chronic hepatitis delta infection. Journal of Hepatology 2019: E32. [Google Scholar]; •• Phase 2 study that found that pegylated interferon lambda resulted in virologic response in 36% at 24 weeks after therapy and with improved tolerability as compared to PEG-IFN-α.
- 123.Coffie PA, Tchounga BK, Bado G, et al. Prevalence of hepatitis B and delta according to HIV-type: a multi-country cross-sectional survey in West Africa. BMC Infect Dis 2017; 17(1): 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Katwesigye E, Seremba E, Semitala F, Ocama P. Low sero-prevalence of hepatitis delta antibodies in HIV/hepatitis B co-infected patients attending an urban HIV clinic in Uganda. Afr Health Sci 2016; 16(4): 1089–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee CY, Tsai HC, Lee SS, et al. Higher rate of hepatitis events in patients with human immunodeficiency virus, hepatitis B, and hepatitis D genotype II infection: a cohort study in a medical center in southern Taiwan. J Microbiol Immunol Infect 2015; 48(1): 20–7. [DOI] [PubMed] [Google Scholar]
- 126.Hung CC, Wu SM, Lin PH, et al. Increasing incidence of recent hepatitis D virus infection in HIV-infected patients in an area hyperendemic for hepatitis B virus infection. Clin Infect Dis 2014; 58(11): 1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thio CL, Smeaton L, Saulynas M, et al. Characterization of HIV-HBV coinfection in a multinational HIV-infected cohort. AIDS 2013; 27(2): 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mendes-Correa MC, Gomes-Gouvêa MS, Alvarado-Mora MV, et al. Hepatitis delta in HIV/HBV co-infected patients in Brazil: is it important? Int J Infect Dis 2011; 15(12): e828–32. [DOI] [PubMed] [Google Scholar]


