Abstract
Objective
This study aimed to investigate the potential determining epidemiological and clinical risk factors affecting the survival of esophageal cancer (EC) patients across multiple hospitals in China.
Methods
This was a multicenter study comprising of newly diagnosed EC cases from Beijing, Hebei, Henan, Hubei, Zhejiang, and Guangdong Province of China. Their baseline characteristics and treatment methods data were collected from their medical records. The EpiData software was used for data quality control. The Kaplan‐Meier method was used to estimate their overall survival (OS), and the Cox's proportional hazard regression model was used to estimate hazard ratios (HR) and 95% confidence interval (CI).
Results
The 3‐ and 5‐year OS rates of the 5283 investigated EC patients were 49.98% and 39.07%, respectively. Their median survival was 36.00 months. The median survival time of females was longer than that of males (females vs. males: 45.00 vs. 33.00, P < 0.001). The 5‐year OS rate of patients who never‐smoked was higher than that of smokers (never‐smokers vs smokers: 40.73% vs. 37.84%, P = 0.001). There was no significant difference in the 5‐year OS rate between drinkers and never‐drinkers (drinkers vs never‐drinkers: 34.22% vs. 29.65%, P = 0.330). In multivariate analysis, pathological stage (stage II: HR = 1.80, 95% CI = 1.40‐2.31; stage III: HR = 2.62, 95% CI = 2.06‐3.34; stage IV: HR = 3.90, 95% CI = 2.98‐5.09), poor differentiation/undifferentiated (HR = 1.34, 95% CI = 1.11‐1.63), not married status (HR = 2.45, 95% CI = 1.49‐4.04), production and service personnel (HR = 1.36, 95% CI = 1.01‐1.83) and farming/fishing (HR = 1.40, 95% CI = 1.12‐1.76) were independent prognostic risk factors for poor EC survival. Tumors in the thoracic or abdominal part of the esophagus, female and family history of any cancer were independent factors predictive of a good EC OS.
Conclusion
Gender, marital status, occupation, family history of any cancer, tumor topographical site, differentiation status, and pathological stage were associated with the survival rate of EC. This study reveals important clinical characteristics of esophageal cancer patients in China and provides helpful information for their clinical management and surveillance.
Keywords: esophageal cancer, overall survival, epidemiology, lifestyle, clinical factors, risk factors, multicenter study
List of abbreviations
- AJCC
American Joint Committee on Cancer
- BMI
body mass index
- CI
confidence interval
- DFS
disease‐free survival
- DSS
disease‐specific survival
- EAC
esophageal adenocarcinoma
- EC
esophageal cancer
- ESCC
esophageal squamous cell carcinoma
- FHC
family history of any cancer
- FHEC
a family history of EC
- HIS
health information system
- HR
hazard ratio
- ICD‐O‐3
the International Classification of Diseases for Oncology, Third Edition
- NCCN
by the National Comprehensive Cancer Network
- OS
overall survival
- PSM
propensity score‐matched
1. INTRODUCTION
Esophageal cancer (EC) is one of the most common gastrointestinal malignancies worldwide. The latest global statistics showed that there were 572,000 new EC cases and 509,000 EC‐related deaths worldwide in 2018, ranking it as seventh and sixth in these respective categories [1]. According to the newly published 2018 China Cancer Report [2], the number of newly diagnosed EC cases in China was 258,000 and was ranked as the sixth most common incidence cancer type. EC was ranked fourth in terms of the number of related deaths, with 193,000 registered deaths. The incidence of EC varies from high‐ to low‐incidence areas. For instance, Cixian had a high incidence of EC (80.12/105), which had a higher incidence rate by 4.16‐fold than the national level (19.24/105) [3]. There are two main pathological types of EC: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). ESCC is the most common histological type in developing countries, such as China [4, 5]. The main treatments for EC are surgical resection, radiotherapy, and chemotherapy. Although great progress has been made in the treatment of EC, its prognosis is still poor, with a 5‐year survival rate below 25% [6].
Many studies related to EC survival in recent years mainly focused on treatment methods including surgery [6], radiotherapy [7], and chemotherapy [8]. For instance, Spence et al [9] found that low‐dose aspirin usage did not increase the survival of EC patients. In a retrospective study by Gabriel et al [10], a total of 1309 EC patients were stratified into two groups according to metastasized status and underwent either neoadjuvant chemoradiation plus surgery or surgery alone. The results suggested that the hazard ratio (HR) of clinically metastatic lymph node patients who underwent neoadjuvant chemoradiation with surgery was 0.52 (95% CI [confidence interval] = 0.42‐0.66). There are limited studies that investigated the association between lifestyle factors, such as smoking, drinking, marriage, occupation, body mass index (BMI), blood type, family history of any cancer (FHC) [11, 12, 13], and the survival of patients with EC. However, the results were inconsistent with other reports. Pan et al [14] performed a meta‐analysis, comprising of fourteen studies with a total of 4,823 cases, investigating the impact of BMI on EC survival. Their results showed that high BMI was an independent favorable factor (HR = 0.81; 95% CI = 0.73‐0.89) in EAC patients. In contrast, among patients with ESCC, high BMI was associated with a worse prognosis (HR, 2.26; 95% CI = 1.29‐3.24). In 2015, Wang et al [15] first studied the effect of ABO blood type on EC survival and found no relationship between blood type and EC survival. In contrast, Qin et al [16] analyzed 548 ESCC patients who underwent cytoreductive surgery and found that the 5‐year overall survival (OS) rates for A, B, O, and AB blood type patients were 41.20%, 49.70%, 44.00%, and 29.80%, respectively. The HR for type AB was 2.58, which was higher than those for the non‐AB blood types. However, most of these studies were single‐center, and/or with limited sample sizes. Additionally, the relationship between EC survival and lifestyle factors remains debatable.
Therefore, the aim of this study was to use a large number of multicenter cases to determine the lifestyle and clinical risk factors related to the survival of EC patients in China. We believe that our findings could act as a guidance to help concerned authorities to improve the management of EC patients.
2. METHODS AND MATERIALS
2.1. Data
Data were collected from 6 provincial hospitals, 8 municipal hospitals, and 4 county hospitals across 6 regions in China which were from the Hebei Province (the Fourth Hospital of Hebei Medical University, the First Hospital of Shijiazhuang, and Cixian Cancer Hospital), Beijing (the Beijing Cancer Hospital, Cancer Hospital Chinese Academy of Medical Sciences, and the First People's Hospital of Dongcheng), Henan Province (Henan Cancer Hospital, the People's Hospital of Jiyuan, the Third People's Hospital of Luoyang, and Dongfang Hospital of Luoyang), Zhejiang Province (Zhejiang Cancer Hospital, Haining Traditional Chinese Medicine Hospital, and Zhejiang Jinhua Guangfu Hospital), Hubei Province (Hubei Cancer Hospital, the First Hospital of Danjiangkou, and the First People's Hospital of Xiangyang), and Guangdong Province (the People's Hospital of Xiaolan, and the People's Hospital of Zhongshan) (Figure 1).
FIGURE 1.
Distribution of the participating hospitals comprising of provincial (blue color), and municipal and county (orange color) hospitals
Trained investigators extracted information including the baseline characteristics (gender, age at diagnosis, marital status, occupation, height and weight at admission, blood type, history of smoking, alcohol consumption, and FHC), tumor‐related information (pathological type, tumor topographical site, differentiation status, and pathological stage), treatment‐related information (surgery, radiotherapy, chemotherapy, and targeted therapy). These data were extracted from patients’ medical records using the health information system (HIS; Dayinjunhui company, Beijing, China). The tumor topographical site classification was coded as C15.0‐C15.9 according to the International Classification of Diseases for Oncology, Third Edition (ICD‐O‐3). C15.0, C15.1, C15.2 is defined as the cervical, thoracic, and abdominal esophagus respectively. C15.8 is defined as an overlapping lesion of the esophagus.
2.2. Inclusion and exclusion criteria
Patients who met the following inclusion criteria were included: (1) newly diagnosed as esophageal cancer between January 1, 2011, and December 31, 2013; (2) their diagnosis and treatment criteria were in accordance with the esophageal cancer guidelines issued by the National Comprehensive Cancer Network (NCCN) 2010; and (3) their initial diagnosis and treatments were performed in the above‐mentioned hospitals and had complete clinical records. The sampling of medical records in the provincial hospitals was performed as follows: the medical records of patients meeting the inclusion criteria were organized by month, and these records were randomly divided into two groups (odd numbers and even numbers) according to a random number table. There was no significant difference between the two groups in gender and age (P > 0.05). We included the even number group in the analysis. All the medical records from the municipal and county hospitals were included. Patients meeting the following criteria were excluded: (1) diagnosed with multiple primary or metastatic cancers; (2) non‐local residents; and (3) received treatments such as surgery, radiotherapy, and/or chemotherapy prior to admission to the above‐mentioned hospitals.
2.3. Exposure evaluation and ascertainment
Based on the occupation information in the patients’ medical records, occupations were divided into three categories: (1) enterprise personnel (e.g.: government officials, office clerks, technicians and so on), (2) production and service personnel (e.g.: factory workers, shop assistants and so on), and (3) fishing/farming (including those working in agriculture, forestry, animal husbandry, and fisheries).
BMI was calculated using the height and weight data retrieved at the time of the first diagnosis from the HIS medical record. According to the Asian standard, BMI was categorized into three groups, underweight (< 18.5 kg/m2), normal weight (18.5‐23 kg/m2), and overweight or obese ≥23 kg/m2).
According to the admission records, the patients were defined as smokers if they had smoked ≥100 cigarettes in one's lifetime. Drinkers were defined as individuals who drank any alcoholic beverage one or more times per week. Patients who had any cancer or EC among first‐degree or second‐degree relatives were defined as patients having an FHC or a family history of EC (FHEC). The first‐degree relatives included father, mother, or siblings; the second‐degree relatives were restricted to grandparents, uncles, and aunts. The pathological types and differentiation status in all patients were confirmed by pathological examination. EC patients were staged according to the 2007 American Joint Committee on Cancer (AJCC) TNM classification.
2.4. Follow up
Patients were followed‐up by passive and active methods until December 31, 2017, at every hospital. Active follow‐ups were performed every 3 months by telephone calls or extracting re‐admission information from the HIS system. Additionally, passive follow‐ups were carried out by the staff of the hospital. They linked the patient records with the data of the local population‐based cancer registries which could provide patients’ survival status. The survival time of patients who were alive and censored was calculated from the date of diagnosis of EC until the last date of follow‐up, and the survival time of patients who died was calculated from the date of diagnosis until the patients’ death.
2.5. Quality control
We standardized the format of the variables and used the EpiData software (version 3.1, EpiData Association, Denmark) to assess the quality of the data from all the eighteen participating hospitals. In a standardized format database, we selected 10% of the patients from each hospital with a random number table method. All the sampling medical records including the basic and clinical information were checked by two other staff members. The follow‐up rate of all hospitals was ensured above 85%.
2.6. Statistical analyses
All statistical analyses were performed using the SPSS software (version 21.0, SPSS Inc., Chicago, IL, USA) and the Stata software (version 12.0, StataCorp LLC, Texas, USA). The 3‐ and 5‐year OS rates and median survival time were analyzed using the Kaplan‐Meier method. Differences between the survival curves were determined using the log‐rank test. Cox's proportional hazard regression models were used to calculate the HR and 95%CI in the univariate and multivariate survival analyses. A multivariate Cox's proportional hazard regression model was performed using the associated risk factors with P values less than 0.10 in univariate analysis. Two‐sided P values < 0.05 were considered statistically significant.
3. RESULTS
3.1. Clinical characteristics of the investigated EC patients
A total of 5283 patients with EC were included in this study. As of December 31, 2017, there were 1333 (25.23%) patients who were still alive, 3158 (59.78%) deaths, and 2575 (48.74%) patients who had died due to EC. A total of 792 (14.99%) cases were lost to follow‐up. The follow‐up rate was 85.0%.
The age of the EC patients included in this study was between 16 and 90 years old, with a median age of 62 years old. A total of 3974 (75.22%) patients were male, and 1309 patients (24.78%) were female. Patients who were married accounted for 97.92% (5173/5283) of the entire cohort. In this study, 2615 (49.50%) were normal weight (18.5‐23.0 kg/m2). There were 1518 (28.73%), 1502 (28.43%), 1438 (27.22%), and 455 (8.61%) patients with A, B, O, and AB blood types, respectively. In total, 2742 (51.90%) patients were smokers, and 2189 (41.43%) were drinkers. Patients with an FHC accounted for 20.63% (1090/5283) of the entire cohort.
The investigated tumor characteristics included tumor topographical site, pathological type, differentiation status, and pathological stage. As shown in Table 1, the thoracic part was the most common topographical site which accounted for 59.11% (3123/5283) of the entire cohort. Squamous cell carcinoma was the main pathological type which accounted for 85.29% (4506/5283) of the entire cohort. Moderately differentiated was the most common histological type; 32.22% (1702/5283). Advanced stage including pathological stage III and IV accounted for 41.72% (2204/5283).
TABLE 1.
Clinical characteristics of the 5283 investigated esophageal cancer patients
| No. of patients | No. of deaths | |
|---|---|---|
| Characteristics | n (%) | n (%) |
| Total no. of cases | 5283 (100) | 3158 (100) |
| Gender | ||
| Male | 3974 (75.22) | 2431 (76.98) |
| Female | 1309 (24.78) | 727 (23.02) |
| Age (years) | ||
| ≤44 | 117 (2.21) | 61 (1.93) |
| 45‐54 | 860 (16.28) | 477 (15.10) |
| 55‐64 | 2215 (41.93) | 1268 (40.15) |
| 65‐74 | 1568 (29.68) | 977 (30.94) |
| ≥75 | 523 (9.90) | 375 (11.87) |
| Marital status | ||
| Married | 5173 (97.92) | 3082 (97.59) |
| Not married | 93 (1.76) | 66 (2.09) |
| Missing data | 17 (0.32) | 10 (0.32) |
| Occupation | ||
| Enterprise personnel | 502 (9.50) | 253 (8.01) |
| Production and service personnel | 565 (10.69) | 290 (9.18) |
| Farming/fishing | 3910 (74.01) | 2390 (75.68) |
| Missing data | 306 (5.79) | 225 (7.12) |
| BMI * (kg/m2) | ||
| <18.5 | 381 (7.21) | 241 (7.63) |
| 18.5‐23.0 | 2615 (49.50) | 1553 (49.18) |
| ≥23.0 | 1431 (27.09) | 878 (27.80) |
| Missing data | 856 (16.20) | 486 (15.39) |
| Blood type | ||
| A | 1518 (28.73) | 840 (26.60) |
| B | 1502 (28.43) | 943 (29.86) |
| O | 1438 (27.22) | 887 (28.09) |
| AB | 455 (8.61) | 270 (8.55) |
| Missing data | 370 (7.00) | 218 (6.90) |
| Smoking status | ||
| Never‐smoker | 2489 (47.11) | 1441 (45.63) |
| Smoker | 2742 (51.90) | 1677 (53.10) |
| Missing data | 52 (0.98) | 40 (1.27) |
| Drinking status | ||
| Never‐drinker | 2250 (42.59) | 1540 (48.77) |
| Drinker | 2189 (41.43) | 1423 (45.06) |
| Missing data | 844 (15.98) | 195 (6.17) |
| Family history of any cancer | ||
| Absent | 4115 (77.89) | 2522 (79.86) |
| Present | 1090 (20.63) | 575 (18.21) |
| Missing data | 78 (1.48) | 61 (1.93) |
| Tumor topographical site | ||
| Cervical part | 575 (10.88) | 352 (11.15) |
| Thoracic part | 3123 (59.11) | 1804 (57.12) |
| Abdominal part | 895 (16.94) | 545 (17.26) |
| Overlapping lesion of the esophagus | 452 (8.56) | 295 (9.34) |
| Missing data | 238 (4.51) | 162 (5.13) |
| Pathological type | ||
| Squamous cell carcinoma | 4506 (85.29) | 2646 (83.79) |
| Adenocarcinoma | 125 (2.37) | 79 (2.50) |
| Adenosquamous carcinoma | 67 (1.27) | 44 (1.39) |
| Other types | 161 (3.05) | 109 (3.45) |
| Missing data | 424 (8.03) | 280 (8.87) |
| Differentiation status | ||
| High differentiation | 805 (15.24) | 413 (13.08) |
| Moderate differentiation | 1702 (32.22) | 1006 (31.86) |
| Poor/Undifferentiated | 1414 (26.77) | 893 (28.28) |
| Missing data | 1362 (25.78) | 846 (26.79) |
| Pathological stage | ||
| I | 741 (14.03) | 273 (8.64) |
| II | 1413 (26.75) | 753 (23.84) |
| III | 1319 (24.97) | 852 (26.98) |
| IV | 885 (16.75) | 659 (20.87) |
| Missing data | 925 (17.51) | 621 (19.66) |
Body mass index
3.2. Survival and univariate analysis
The median follow‐up time for the 5283 EC cases was 33.70 (range: 1.00, 100.63) months. The 3‐ and 5‐year OS rate of all patients were 49.98% (95% CI = 48.60%‐51.34%) and 39.07% (95% CI = 37.68%‐40.46%), respectively. The median survival time for the investigated EC cases was 36.00 months (95% CI = 34.00‐39.00). The 5‐year OS of EC in provincial hospitals was 41.26%, which was higher than those in municipal and county hospitals (28.06%; Supplementary Table 1). The 5‐year OS rates of patients who underwent surgery only, radiotherapy only, and chemotherapy only were 52.13%, 25.91%, and 24.33%, respectively.
As shown in Table 2, the median survival time of females was longer than that of males (male vs female: 33.00 vs. 45.00 months; P < 0.001). Smokers and drinkers had comparatively lower median survival time than never smokers and non‐drinkers. The 5‐year OS rates of patients with A, B, O, and AB blood types were 44.01%, 36.28%, 36.59%, and 39.78%, respectively. However, the 3‐ and 5‐year OS rates of patients with FHC were 56.34% and 46.36%, respectively, which were higher than those of patients without FHC (3‐year OS: 48.55%; 5‐year OS: 37.39%). The 5‐year OS rates of patients with cancer in the thoracic esophagus were 41.26%, which were higher than those of patients with tumors in other topographical sites (cervical part, abdominal part and overlapping lesion of the esophagus). The 5‐year OS rates of ESCC and EAC were 40.09% and 37.46%, respectively. The 5‐year OS rate of pathological stage I was 61.90%. Supplementary Fig. S1‐3 shows the survival curves stratified by different factors.
TABLE 2.
Overall survival rate and median survival time of the 5283 esophageal cancer patients
| Overall survival rate (%, 95% CI) | ||||
|---|---|---|---|---|
| Characteristics | 3‐year | 5‐year | Median survival time (months‐ 95% CI) | P value * |
| All patients | 49.98 (48.60‐51.34) | 39.07 (37.68‐40.46) | 36.00 (34.00‐39.00) | |
| Age(year) | <0.001 | |||
| ≤44 | 54.31 (44.62‐63.02) | 46.32 (36.69‐55.39) | 44.00 (‐ ** ) | |
| 45‐54 | 50.92 (47.46‐54.26) | 43.65 (40.17‐47.07) | 37.00 (29.69‐44.32) | |
| 55‐64 | 52.28 (50.14‐54.36) | 41.30 (39.13‐43.47) | 40.00 (35.89‐44.11) | |
| 65‐74 | 49.77 (47.24‐52.24) | 36.73 (34.21‐39.25) | 35.00 (31.26‐38.74) | |
| ≥75 | 38.28 (34.03‐42.51) | 27.49 (23.48‐31.65) | 22.00 (18.35‐25.65) | |
| Gender | <0.001 | |||
| Male | 48.16 (46.57‐49.72) | 37.73 (36.14‐39.32) | 33.00 (30.74‐35.26) | |
| Female | 55.55 (52.76‐58.24) | 43.19 (40.34‐46.01) | 45.00 (39.78‐50.22) | |
| Marital status | 0.004 | |||
| Married | 50.09 (48.69‐51.46) | 39.26 (37.85‐40.66) | 36.00 (33.63‐38.38) | |
| Not married | 40.93 (30.71‐50.87) | 27.32 (17.23‐38.04) | 25.00 (14.24‐35.76) | |
| Occupation | <0.001 | |||
| Enterprise personnel | 58.94 (54.46‐63.13) | 48.75 (44.05‐53.27) | 54.00 (43.67‐64.33) | |
| Production and service personnel | 55.67 (51.39‐59.73) | 47.73 (43.30‐52.03) | 49.00 (38.58‐59.42) | |
| Farming/fishing | 48.86 (47.25‐50.44) | 37.86 (36.27‐39.46) | 34.00 (31.60‐36.40) | |
| BMI *** (kg/m2) | 0.014 | |||
| < 18.5 | 43.41 (38.29‐48.41) | 34.90 (29.87‐39.98) | 25.00 (19.68‐30.32) | |
| 18.5‐23.0 | 50.03 (48.07‐51.96) | 39.85 (37.88‐41.82) | 36.00 (32.71‐39.29) | |
| ≥23.0 | 48.50 (45.83‐51.12) | 37.16 (34.52‐39.79) | 34.00 (30.23‐37.77) | |
| Blood type | 0.028 | |||
| A | 52.45 (49.86‐54.97) | 44.01 (41.39‐46.60) | 40.00 (34.12‐45.89) | |
| B | 49.88 (47.29‐52.41) | 36.28 (33.70‐38.86) | 36.00 (32.56‐39.44) | |
| O | 47.88 (45.24‐50.47) | 36.59 (33.95‐39.23) | 32.00 (28.47‐35.53) | |
| AB | 49.25 (44.50‐53.82) | 39.78 (35.05‐44.46) | 35.00 (26.85‐43.15) | |
| Smoking status | 0.001 | |||
| Never‐smoker | 53.03 (51.01‐55.00) | 40.73 (38.68‐42.77) | 41.00 (37.46‐44.54) | |
| Smoker | 47.48 (45.57‐49.36) | 37.84 (35.93‐39.74) | 31.00 (28.25‐33.75) | |
| Drinking status | 0.330 | |||
| Never‐drinker | 43.94 (41.84‐46.02) | 29.65 (27.67‐31.65) | 29.00 (27.09‐30.91) | |
| Drinker | 43.69 (41.57‐45.78) | 34.22 (32.14‐36.31) | 27.00 (24.81‐29.19) | |
| Family history of any cancer | <0.001 | |||
| Absent | 48.55 (46.99‐50.09) | 37.39 (35.83‐38.95) | 33.00 (30.83‐35.17) | |
| Present | 56.34 (53.29‐59.26) | 46.36 (43.22‐49.44) | 51.00 (43.95‐58.05) | |
| Family history of esophageal cancer | <0.001 | |||
| Absent | 49.25 (47.78‐50.70) | 38.13 (36.66‐39.61) | 35.00 (32.00‐38.00) | |
| Present | 57.74 (53.54‐61.70) | 48.32 (43.97‐52.53) | 55.00 (46.00‐69.00) | |
| Tumor topographical site | <0.001 | |||
| Cervical part | 45.41 (41.21‐49.51) | 36.10 (31.90‐40.30) | 28.00 (22.74‐33.26) | |
| Thoracic part | 53.08 (51.29‐54.84) | 41.26 (39.43‐43.07) | 41.00 (37.73‐44.27) | |
| Abdominal part | 46.96 (43.60‐50.25) | 37.64 (34.27‐40.99) | 30.00 (25.32‐34.68) | |
| Overlapping lesion of esophagus | 43.11 (38.49‐47.64) | 35.39 (30.91‐39.90) | 24.00 (20.01‐27.99) | |
| Pathological stage | <0.001 | |||
| I | 72.08 (68.65‐75.21) | 61.90 (58.08‐65.49) | 84.00 (59.95‐108.05) | |
| II | 58.69 (56.04‐61.23) | 46.60 (43.81‐49.34) | 52.00 (46.28‐57.72) | |
| III | 43.26 (40.52‐45.97) | 32.80 (30.10‐35.52) | 27.00 (24.45‐29.55) | |
| IV | 37.40 (34.18‐40.63) | 26.17 (23.23‐29.20) | 23.00 (20.37‐25.63) | |
| Pathological type | 0.008 | |||
| Squamous cell carcinoma | 50.75 (49.26‐52.22) | 40.09 (38.58‐41.59) | 38.00 (35.00‐41.00) | |
| Adenocarcinoma | 46.12 (36.80‐54.93) | 37.46 (28.28‐46.61) | 30.00 (20.00‐54.00) | |
| Adenosquamous carcinoma | 46.22 (34.00‐57.58) | 32.73 (21.54‐44.37) | 30.00 (15.00‐42.00) | |
| Other types | 43.19 (35.21‐50.91) | 28.09 (20.85‐35.76) | 29.00 (22.33‐38.00) | |
| Differentiation status | <0.001 | |||
| High differentiation | 60.34 (56.00‐64.39) | 49.50 (45.01‐53.83) | 59.00 (50.33‐67.67) | |
| Moderate differentiation | 51.86 (48.83‐54.81) | 41.33 (38.25‐44.39) | 39.00 (33.17‐44.83) | |
| Poor/Undifferentiated | 47.41 (44.10‐50.64) | 35.98 (32.67‐39.30) | 29.00 (35.21‐42.79) | |
P value for the 5‐year OS of each subgroup
95% CI can not be estimated
Body mass index
The factors associated with EC survival on univariate analysis are shown in Table 3. To further investigate the reasons for the higher survival rate of patients with an FHC, we analyzed the distributions of differentiation status and pathological stage based on the presence or absence of FHC. Patients with FHC had a better survival rate than with patients without FHC (P < 0.001). Supplementary Table 2 shows that the proportion of patients with moderate and high differentiation status in the FHC group was 49.36%, which was higher than that of patients without FHC (46.88%). In addition, patients with FHC were more likely to have pathological stages I and II EC (44.22%) and the proportion of pathological stage I and II without FHC was 40.07% (P = 0.064). We also further analyzed the survival of patients with FHEC. The results were consistent with those for patients with FHC, in which the proportions of patients with and without FHEC with pathological stages I and II were 46.25% and 40.31%, respectively (P = 0.027; Supplementary Table 2)
TABLE 3.
Univariate and multivariate analyses demonstrating the association between clinical characteristics, lifestyle factors, and esophageal cancer survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Characteristics | HR (95%CI) | P value | HR (95%CI) | P value |
| Gender | <0.001 | <0.001 | ||
| Male | 1.00 | 1.00 | ||
| Female | 0.84 (0.77‐0.91) | 0.67 (0.56‐0.81) | ||
| Age(years) | <0.001 | |||
| ≤44 | 1.00 | |||
| 45‐54 | 1.15 (0.88‐1.50) | |||
| 55‐64 | 1.13 (0.87‐1.46) | |||
| 65‐74 | 1.26 (0.97‐1.63) | |||
| ≥ 75 | 1.75 (1.34‐2.30) | |||
| Marital status | 0.004 | <0.001 | ||
| Married | 1.00 | 1.00 | ||
| Not married | 1.43 (1.12‐1.82) | 2.45 (1.49‐4.04) | ||
| Occupation | <0.001 | 0.010 | ||
| Enterprise personnel | 1.00 | 1.00 | ||
| Production and service personnel | 1.07 (0.90‐1.26) | 1.36 (1.01‐1.83) | ||
| Farming/fishing | 1.27 (1.12‐1.45) | 1.40 (1.12‐1.76) | ||
| BMI ** (kg/m2) | 0.009 | |||
| < 18.5 | 1.00 | |||
| 18.5‐23.0 | 0.81 (0.71‐0.93) | |||
| ≥23.0 | 0.86 (0.75‐0.99) | |||
| Blood type | 0.038 | |||
| A | 1.00 | |||
| B | 1.13 (1.03‐1.24) | |||
| O | 1.13 (1.03‐1.24) | |||
| AB | 1.08 (0.94‐1.23) | |||
| Smoking status | 0.001 | |||
| Never‐smoker | 1.00 | |||
| Smoker | 1.13 (1.05‐1.21) | |||
| Drinking status | 0.335 | |||
| Never‐drinker | 1.00 | |||
| Drinker | 0.97 (0.90‐1.04) | |||
| Family history of any cancer | <0.001 | <0.001 | ||
| Absent | 1.00 | 1.00 | ||
| Present | 0.78 (0.71‐0.85) | 0.73 (0.61‐0.87) | ||
| Tumor topographical site | <0.001 | 0.013 | ||
| Cervical part | 1.00 | 1.00 | ||
| Thoracic part | 0.83 (0.74‐0.93) | 0.65 (0.50‐0.83) | ||
| Abdominal part | 0.98 (0.85‐1.11) | 0.72 (0.55‐0.95) | ||
| Overlapping lesion of esophagus | 1.06 (0.90‐1.23) | 0.69 (0.51‐0.94) | ||
| Pathological type | 0.002 | |||
| Squamous cell carcinoma | 1.00 | |||
| Other types * | 1.24 (1.08‐1.42) | |||
| Differentiation status | <0.001 | 0.010 | ||
| High differentiation | 1.00 | 1.00 | ||
| Moderate differentiation | 1.27 (1.10‐1.47) | 1.19 (0.99‐1.43) | ||
| Poor/Undifferentiated | 1.46 (1.26‐1.69) | 1.34 (1.11‐1.63) | ||
| Pathological stage | <0.001 | <0.001 | ||
| I | 1.00 | 1.00 | ||
| II | 1.60 (1.39‐1.83) | 1.80 (1.40‐2.31) | ||
| III | 2.40 (2.09‐2.75) | 2.62 (2.06‐3.34) | ||
| IV | 2.90 (2.52‐3.34) | 3.90 (2.98‐5.09) | ||
Other types included adenocarcinoma, adenosquamous carcinoma, and other types.
Body mass index
3.2.1. Stratified survival analysis by smoking status
We categorized the patients into never‐smokers and smokers (current and former smokers) according to their history of smoking. The 5‐year OS rate of patients who were never‐smokers was 40.73%, which was higher than that in the smoker group (37.84%; P = 0.001). The 5‐year OS rate of never‐smokers with FHC was 50.36% (95% CI = 45.61%‐54.90%), which was higher than that of smokers who had FHC (43.37%; 95% CI = 39.15%‐47.50%; P = 0.011); and the 5‐year OS rates of patients without FHC in the two groups were 38.50% and 36.40%, respectively (P = 0.015). In the never smoker group, the 5‐year OS rates of patients with pathological stage III and IV were 34.18% and 28.78%, respectively; while they were 32.18% and 24.30% in the smoker group, respectively. The 5‐year survival rates of patients with tumors in the cervical and thoracic parts were 38.73% and 42.36% in the never‐smoker group, respectively, and in the smoker group, they were 33.12% and 40.30%.
In the never‐smoker group, the median survival time of females was 46.00 (95% CI = 40.73‐51.27) months, which was higher than that of males (P = 0.001). Patients with normal and high BMI lived longer than patients with lower BMI (median survival time: normal BMI: 42.00 months; high BMI: 36.00 months; lower BMI: 28.00 months; P = 0.001). ESCC patients survived longer than patients with other pathological types (P = 0.003). However, among smokers, there were no significant differences in survival based on gender, BMI, or pathological type. In the group of smokers, the median survival time for married patients were better than those for unmarried patients (P = 0.008). There was no significant difference in the survival of EC patients by blood type when stratified by smoking status (Supplementary Table 3).
3.2.2. Stratified survival analysis by drinking
To further analyze the effect of drinking on the patients’ survival, the patients were divided into two groups based on their drinking status. The median survival times of patients in the never drinker and drinker groups were 29.00 and 27.00 months, respectively (P = 0.596).
The 5‐year OS rates of patients with low, normal, and high BMI in the never‐drinker group were 35.02%, 28.60%, and 36.08% (P = 0.011), while the 5‐year OS rates were 33.78%, 34.67%, and 37.67% (P = 0.009) in the drinkers' group, respectively. For patients in an advanced stage, the drinker group had a poorer prognosis than the never‐drinker group. The median survival times of patients with pathological stage IV were 25.00 months in the never‐drinker group, which corresponded to 15.00 months in the drinker group (P < 0.001). The median survival times of patients with poor differentiation/undifferentiated tumors in the never‐drinker and drinker groups were 26.00 and 21.00 months (P = 0.957), respectively. There was no significant association between blood type, smoking or tumor topographical site, and EC survival in the never‐drinker and drinker groups.
In the group of never drinkers, females had a better 5‐year OS rate (33.04%, 95% CI = 29.96‐36.15) than males (27.04%, 95% CI = 24.49‐29.65) (male vs. female: P = 0.004). A better 5‐year survival rate was also observed in married patients (P < 0.001) compared with their counterparts. In the drinker's group, the median survival time of patients with FHC was 35 months, which was longer than that of patients without FHC (P < 0.001; Supplementary Table S4).
3.3. Multivariate analysis of clinical characteristics, lifestyle factors and EC survival
Multivariate analyses indicated that advanced pathological stage (stage II: HR = 1.80, 95% CI = 1.40‐2.31; stage III: HR = 2.62, 95% CI = 2.06‐3.34; stage IV: HR = 3.90, 95% CI = 2.98‐5.09), poor differentiation/undifferentiated (HR = 1.34, 95% CI = 1.11‐1.63), not‐married status (HR = 2.45, 95% CI = 1.49‐4.04), production and service personnel (HR = 1.36, 95% CI = 1.01‐1.83), farming/fishing (HR = 1.40, 95% CI = 1.12‐1.76) were identified as independent prognostic factors for poor EC OS. Tumor topographical sites of EC was found to affect EC survival. The survival of EC patients whose tumor were located in the thoracic, abdominal, and overlapping part of the esophagus was better than those located in the cervical part (P = 0.013). Female (vs male, P < 0.001) and having FHC (vs absent FHC, P < 0.001) were independent protective factors affecting the prognosis of EC (Table 3 and Figure 2).
FIGURE 2.
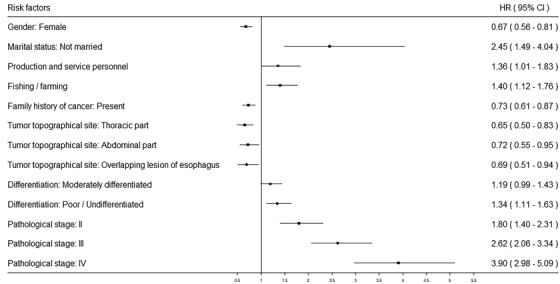
Forest plot of the significant risk factors related to the overall survival of esophageal cancer patients
4. DISCUSSION
This was a large multicenter study comprising of 5283 patients across several provinces in China. The latest population‐based study in China in 2009‐2011 showed that the age‐standardized 5‐year EC survival rate was 30.3%, and the 5‐year EC net survival of the population of the United States in 2010‐2014 was 20.0% [17, 18, 19]. Findings from the present study showed that the 5‐year OS rate was 39.07% in the investigated 18 hospitals and we found that gender, marital status, occupation, family history of any cancer, tumor topographical site, differentiation status, and pathological stage were associated with the survival rate of EC.
In our study, not married status was a negative prognostic factor for EC survival, with an HR of 2.45 (95% CI = 1.49‐4.04). This result was consistent with previous studies. Du et al [20] identified that marital status was related to the survival of EC patients in the U.S. In their study, single (HR = 1.14, 95%CI = 1.11‐1.17), divorced (HR = 1.16, 95% CI = 1.13‐1.19), and widowed (HR = 1.22, 95%CI = 1.19‐1.26) patients had a higher risk of death compared to married patients. Zhang et al [21] showed that unmarried patients had poorer OS than married patients (HR = 1.22). The reasons why the single status affected the survival of EC patients may be related to their mental state, income, spiritual support. Subsequently, unmarried patients may have less financial and psychological support during their treatment than married patients.
Previous studies were mainly focused on the relationship between EC survival and occupation with harmful chemical substances exposure [22, 23]. Clin et al [24] performed a 10‐year follow‐up of 14,515 male workers to determine the correlation between occupational asbestos exposure and EC mortality. Their results indicated that the HR for asbestos exposure was 1.40. Our study suggested that the survival of patients who worked as the production and service personnel and farming/fishing was worse than that of patients who were enterprise personnel. This may be due to their low level of awareness of cancer prevention and control. Patients in farming/fishing could be more likely diagnosed with advanced‐stage disease, leading to poor survival from EC.
Studies found that smoking was related to the survival of patients with various cancers, including prostate [25], lung [26], oral, and pharyngeal cancer [27]. Researchers also found that smoking was a poor prognosis factor of EC survival. In a prospective study including 411 EAC cases, patients in the highest smoking pack‐year group had a poor prognosis (HR = 2.29) [28]. In a recent study, Spreafico et al [29] explored the negative effect of smoking on EAC survival (HR = 1.22). As the number of smoking pack‐years increased, the risk of death increased. Similar results were also found in our study that the 5‐year OS rate of smokers (HR = 1.13) was 37.84%, which was lower than that of never smokers (never smoker vs smoker: P = 0.001).
Alcohol consumption has been proved to be related to many diseases, including cancer [30, 31, 32]. Some studies have investigated the effect of alcohol consumption on EC survival [33, 34]. For instance, compared with ESCC patients who were life‐long non‐drinkers, the HRs of those who consumed 7‐20 drinks/week or ≥21 drinks/week were 2.21 (95% CI = 1.27‐3.84) or 2.08 (95% CI = 1.18‐3.69) [12]. Huang et al [13] conducted a cohort study to evaluate the effect of alcohol consumption on EC. Their results indicated that the HR for the OS of drinkers was 1.22 (95% CI = 1.06‐1.41) and the HR for disease‐free survival (DFS) in drinkers was 1.16 (95% CI = 1.01‐1.34). In our study, the median survival time of drinkers was 27.00 months, which was shorter than that of never drinkers (29.00 months) (never drinker vs drinker: P = 0.596).
BMI is a reliable indicator of the nutritional and metabolic status of a population and a sensitive prognostic index for EC patients [35, 36]. However, the associations between BMI and the survival of patients with EC have been inconsistent. Our study suggested that the 5‐year OS rates of patients with high and normal BMI were higher than those of patients with low BMI (P = 0.014). Deng et al [36] conducted a propensity score‐matched (PSM) study to investigate whether a high BMI had any impact on the long‐term survival of 132 EC patients who were treated with curative esophagectomy. Cox multivariate analysis showed that there was no significant difference between patients with a high BMI and those with a normal BMI. The same results were also observed in other studies. Takemoto et al [37] and Hasegawa et al [38] both suggested that BMI might not be a risk factor for EC survival. In addition, there were studies suggesting that the effect of BMI on EC survival was related to smoking status [29, 39]. For example, Sun et al [39] indicated that patients with low BMI (< 18.5) had a 2.22 times higher risk of EC‐related death than patients with a high BMI (≥18.5) among never smokers. However, Yoon et al [40] conducted a study in EAC patients to investigate the impact of BMI by stratifying the patients according to smoking status. The HRs for disease‐specific survival (DSS), DFS, and OS were 2.11, 2.03, and 1.97, respectively, in the obese group when compared with the normal weight group among never smokers. In different smoking status, the prognostic impacts of obesity and low BMI on ESCC survival varied, but could not be eliminated.
There is little information available about the relationship between blood type and EC survival. In this present multicenter study, univariate analysis results indicated that the HRs for B and O blood types were both1.13. However, the ABO blood type was not significantly associated with survival in multivariate analysis. In recent years, the results of studies focused on the role of blood type in the survival of EC were inconsistent. Wang et al [15] demonstrated that ABO blood type was not related to survival among EC patients who underwent esophagectomy. Shiratori et al [41] demonstrated that the 5‐year OS rate of patients with non‐B blood type was 30.2%, which was lower than that of patients with type B blood (58.8%) among 181 Japanese ESCC patients. The 5‐year OS rates of patients with blood types A, B, O, and AB were 50.0%, 45.4%, 50.8%, and 60.7%, respectively, in the study by Sun et al [42].
There was an interesting result observed in patients with FHC in our study. In the smoking and drinking subgroups, univariate and multivariate analyses demonstrated that patients with FHC had better OS than patients without FHC. Previous studies have shown that patients with FHEC have a poor prognosis. For example, Gao et al [43] conducted a study consisting of 600 ESCC hospital‐based patients in Shanxi, China, and their results suggested that an increased risk of ESCC was related to FHC (OR = 1.72). In another hospital‐based cohort study by Yuequan et al [44], the 3‐year OS rate and 5‐year OS rate of patients with FHEC were 38.6% and 22.9%, respectively. FHEC was an independent risk factor in their multivariate analysis. Ghadimi et al [45] used a gamma frailty model to analyze the effect of FHC in 359 EC patients. Their results also indicated that FHC was a significant risk predictor for the survival of patients with EC. The 3‐ and 5‐year OS rates of patients with FHC were 56.34% and 46.36%, respectively, in our study, which were higher than those of patients without FHC and conflicted with the results of previous studies. The results of subgroup analyses showed that the distributions of differentiation status and TNM stage were different between patients with and without FHC or FHEC. The proportion of patients with poor differentiation/undifferentiated histological status with FHC was 23.39%, which was lower than that of patients without FHC. The proportion of patients with stage I and II and with FHC was 44.22%, which was higher than that of patients without FHC (40.07%). The same results were observed in the group of patients with FHEC. This might be because patients with FHC were defined as a high‐risk population and prior to be included in the cancer screening. Nevertheless, the specific effect of FHC on the survival of EC patients’ needs further investigation.
One limitation of this study is that different diagnostic assessments and treatment levels in the various hospitals might have influenced the results. In future studies, we will investigate more cases in municipal and county hospitals. A second limitation is that smoking status, drinking status, and other variables were self‐reported and recorded in the medical records. Hence, never‐smokers may have been misclassified, especially in participants who were ex‐smokers and had stopped smoking fewer than 15 years before. The proportion of the missing data of some variables, such as differentiation status was relatively high. However, it was within a reasonable range. The analysis of treatment methods was classified as surgery only, radiotherapy only, and chemotherapy only. We will analyze the detailed effects of treatment on EC survival in future studies. Further data are required to confirm these findings and to increase the precision of the effect for these exposures.
As conclusion, gender, marital status, occupation, family history of any cancer, tumor topographical site, differentiation status, and pathological stage were independent risk factors for EC survival in China. We expect that the findings could act as a guidance for enhancing the awareness of EC prevention and to change unhealthy lifestyles; and could assist authorities seeking to develop better surveillance strategies to increase the survival and quality of life of EC patients.
DECLARATIONS
AUTHORSHIP
WQC and YTH designed the research. YTH, TTG, DL, YYL, and BES were contributors in writing the manuscript. TTG, LBD, XBS, NW, KRW, and MZ collected the data. YTH, TTG, and DL performed data analyses. All authors have read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
COMPETING INTERESTS
The authors declare that they have no potential conflicts of interest.
FUNDING
This study was funded by the National Key Research and Development Program of China (2018YFC1313100) and a grant from the National Natural Scientific Foundation of China (81871922).
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We gratefully thank the following hospitals for their cooperation with regard to providing, sorting, and verifying the cancer data: the Fourth Hospital of Hebei Medical University, the First Hospital of Shijiazhuang, Cixian Cancer Hospital, the Beijing Cancer Hospital, Cancer Hospital of the Chinese Academy of Medical Sciences, the First People's Hospital of Dongcheng, Henan Cancer Hospital, the People's Hospital of Jiyuan, the Third People's Hospital of Luoyang, Dongfang Hospital of Luoyang, Zhejiang Cancer Hospital, Haining Traditional Chinese Medicine Hospital, Zhejiang Jinhua Guangfu Hospital, Hubei Cancer Hospital, the First Hospital of Danjiangkou, the First People's Hospital of Xiangyang, the People's Hospital of Xiaolan, and the People's Hospital of Zhongshan
He Y, Liang D, Du L, et al. Clinical characteristics and survival of 5283 esophageal cancer patients: a multicenter study from eighteen hospitals across six regions in China. Cancer Commun. 2020;40:531–544. 10.1002/cac2.12087
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Chen W SK, Zheng R, Zeng H, Zhang S, Xia C, Yang Z, Li H, Zou X, He J. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23(4):233‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry MA, Lerco MM, Ribeiro PW, Rodrigues MA. Epidemiological features of esophageal cancer. Squamous cell carcinoma versus adenocarcinoma. Acta Cirurgica Brasileira. 2014;29(6):389‐93. [DOI] [PubMed] [Google Scholar]
- 5. Oh TK, Kim K, Jheon SH, Do SH, Hwang JW, Jeon YT, et al. Long‐Term Oncologic Outcomes, Opioid Use, and Complications after Esophageal Cancer Surgery. J Clin Med. 2018;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orazbayev BA, Musulmanbekov K, Bukenov A. Analysis of Treatment Results of the Thoracic Part of Oesophageal Cancer. Open access Macedonian Journal Of Medical Sciences. 2019;7(1):82‐7. 10.3889/oamjms.2019.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones CM, Spencer K, Hitchen C, Pelly T, Wood B, Hatfield P, et al. Hypofractionated Radiotherapy in Oesophageal Cancer for Patients Unfit for Systemic Therapy: A Retrospective Single‐Centre Analysis. Clin Oncol. 2019. 10.1016/j.clon.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 8. Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta‐analysis. Lancet Oncol. 2011;12(7):681‐92. 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 9. Spence AD, Busby J, Johnston BT, Baron JA, Hughes CM, Coleman HG, et al. Low‐Dose Aspirin Use Does Not Increase Survival in 2 Independent Population‐Based Cohorts of Patients With Esophageal or Gastric Cancer. Gastroenterology. 2018;154(4):849‐60. [DOI] [PubMed] [Google Scholar]
- 10. Gabriel E, Attwood K, Du W, Tuttle R, Alnaji RM, Nurkin S, et al. Association Between Clinically Staged Node‐Negative Esophageal Adenocarcinoma and Overall Survival Benefit From Neoadjuvant Chemoradiation. JAMA Surg. 2016;151(3):234‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tramacere I, La Vecchia C, Negri E. Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta‐analysis. Epidemiology. 2011;22(3):344‐9. 10.1097/EDE.0b013e31821092cd. [DOI] [PubMed] [Google Scholar]
- 12. Thrift AP, Nagle CM, Fahey PP, Russell A, Smithers BM, Watson DI, et al. The influence of prediagnostic demographic and lifestyle factors on esophageal squamous cell carcinoma survival. Int J Cancer. 2012;131(5):E759‐68. 10.1002/ijc.27420. [DOI] [PubMed] [Google Scholar]
- 13. Huang Q, Luo K, Yang H, Wen J, Zhang S, Li J, et al. Impact of alcohol consumption on survival in patients with esophageal carcinoma: a large cohort with long‐term follow‐up. Cancer Sci. 2014;105(12):1638‐46. 10.1111/cas.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pan W, Sun Z, Xiang Y, Fang W. The correlation between high body mass index and survival in patients with esophageal cancer after curative esophagectomy: evidence from retrospective studies. Asia Pac J Clin Nutr. 2015;24(3):480‐8. [DOI] [PubMed] [Google Scholar]
- 15. Wang W, Liu L, Wang Z, Wei M, He Q, Ling T, et al. Impact of ABO blood group on the prognosis of patients undergoing surgery for esophageal cancer. BMC Surgery. 2015;15:106 10.1186/s12893-015-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin J, Wu SG, Sun JY, Lin HX, He ZY, Li Q. Effect of blood type on survival of Chinese patients with esophageal squamous cell carcinoma. OncoTargets and Therapy. 2015;8:947‐53. 10.2147/OTT.S81936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003‐15: a pooled analysis of 17 population‐based cancer registries. The Lancet Global health. 2018;6(5):e555‐e67. 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 18. Zarean E, Azizmohammad Looha M, Amini P, Mahmoudi M, Azimi T. Factors Affecting Long‐Survival of Patients with Esophageal Cancer Using Non‐Mixture Cure Fraction Model. Asian Pac J Cancer Prev. 2018;19(6):1677‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000‐14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet. 2018;391(10125):1023‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Du L, Kim JJ, Chen B, Zhu S, Dai N. Marital status is associated with superior survival in patients with esophageal cancer: a Surveillance, Epidemiology, and End Results study. Oncotarget. 2017;8(56):95965‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang QW, Lin XL, Zhang CH, Tang CY, Zhang XT, Teng LM, et al. The influence of marital status on the survival of patients with esophageal cancer: a population‐based, propensity‐matched study. Oncotarget. 2017;8(37):62261‐73. 10.18632/oncotarget.19446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manuwald U, Velasco Garrido M, Berger J, Manz A, Baur X. Mortality study of chemical workers exposed to dioxins: follow‐up 23 years after chemical plant closure. Occup Environ Med. 2012;69(9):636‐42. 10.1136/oemed-2012-100682. [DOI] [PubMed] [Google Scholar]
- 23. Lin S, Wang X, Yano E, Yu I, Lan Y, Courtice MN, et al. Exposure to chrysotile mining dust and digestive cancer mortality in a Chinese miner/miller cohort. Occup Environ Med. 2014;71(5):323‐8. 10.1136/oemed-2013-101360. [DOI] [PubMed] [Google Scholar]
- 24. Clin B, Thaon I, Boulanger M, Brochard P, Chamming's S, Gislard A, et al. Cancer of the esophagus and asbestos exposure. Am J Ind Med. 2017;60(11):968‐75. 10.1002/ajim.22769. [DOI] [PubMed] [Google Scholar]
- 25. Darcey E, Boyle T. Tobacco smoking and survival after a prostate cancer diagnosis: A systematic review and meta‐analysis. Cancer Treat Rev. 2018;70:30‐40. 10.1016/j.ctrv.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 26. Minnix JA, Karam‐Hage M, Blalock JA, Cinciripini PM. The importance of incorporating smoking cessation into lung cancer screening. Transl Lung Cancer Res. 2018;7(3):272‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo Y, Logan HL, Marks JG, Shenkman EA. The relationships among individual and regional smoking, socioeconomic status, and oral and pharyngeal cancer survival: a mediation analysis. Cancer Med. 2015;4(10):1612‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hardikar S, Onstad L, Blount PL, Odze RD, Reid BJ, Vaughan TL. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett's esophagus. PLoS One. 2013;8(1):e52192 10.1371/journal.pone.0052192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spreafico A, Coate L, Zhai R, Xu W, Chen ZF, Chen Z, et al. Early adulthood body mass index, cumulative smoking, and esophageal adenocarcinoma survival. Cancer Epidemiol. 2017;47:28‐34. 10.1016/j.canep.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 30. Radojicic J, Zaravinos A, Spandidos DA. HPV, KRAS mutations, alcohol consumption and tobacco smoking effects on esophageal squamous‐cell carcinoma carcinogenesis. Int J Biol Markers. 2012;27(1):1‐12. 10.5301/JBM.2011.8737. [DOI] [PubMed] [Google Scholar]
- 31. Kunzmann AT, Coleman HG, Huang WY, Berndt SI. The association of lifetime alcohol use with mortality and cancer risk in older adults: A cohort study. PLoS Med. 2018;15(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kowalski A, Striley CW, Varma DS, Egan KM, Yaghjyan L. Interactions between Alcohol Consumption and Adjuvant Hormone Therapy in Relation to Breast Cancer‐Free Survival. J Breast Cancer. 2018;21(2):158‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu N, Zhu Y, Kadel D, Pang L, Chen G, Chen Z. The prognostic influence of body mass index, resting energy expenditure and fasting blood glucose on postoperative patients with esophageal cancer. BMC Gastroenterology. 2016;16(1):142 10.1186/s12876-016-0549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peng Q, Chen H, Huo JR. Alcohol consumption and corresponding factors: A novel perspective on the risk factors of esophageal cancer. Oncol Lett. 2016;11(5):3231‐9. 10.3892/ol.2016.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun P, Zhang F, Chen C, An X, Li YH, Wang FH, et al. Comparison of the prognostic values of various nutritional parameters in patients with esophageal squamous cell carcinoma from Southern China. J Thorac Dis. 2013;5(4):484‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng HY, Alai G, Li G, Luo J, Zhuo ZG, Lin YD. High BMI has no impact on the survival of Chinese patients with lower thoracic esophageal adenocarcinoma treated with curative esophagectomy: a propensity score‐matched study. Dis Esophagus. 2019;32(1). [DOI] [PubMed] [Google Scholar]
- 37. Takemoto K, Shiozaki A, Fujiwara H, Konishi H, Morimura R, Murayama Y, et al. [High BMI does not influence short‐ and long‐term outcomes of patients with esophageal cancer treated with esophagectomy]. Gan To Kagaku Ryoho. 2014;41(12):1991‐3. [PubMed] [Google Scholar]
- 38. Hasegawa T, Kubo N, Ohira M, Sakurai K, Toyokawa T, Yamashita Y, et al. Impact of body mass index on surgical outcomes after esophagectomy for patients with esophageal squamous cell carcinoma. Journal of Gastrointestinal Surgery: Official Journal of the Society for Surgery of the Alimentary Tract. 2015;19(2):226‐33. 10.1007/s11605-014-2686-y. [DOI] [PubMed] [Google Scholar]
- 39. Sun P, Zhang F, Chen C, Ren C, Bi XW, Yang H, et al. Prognostic impact of body mass index stratified by smoking status in patients with esophageal squamous cell carcinoma. OncoTargets and therapy. 2016;9:6389‐97. 10.2147/OTT.S111843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoon HH, Lewis MA, Shi Q, Khan M, Cassivi SD, Diasio RB, et al. Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2011;29(34):4561‐7. 10.1200/JCO.2011.37.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shiratori F, Shimada H, Yajima S, Suzuki T, Oshima Y, Nanami T, et al. Relationship between ABO blood group and clinicopathological factors and their effect on the survival of Japanese patients with esophageal squamous cell carcinoma. Surg Today. 2017;47(8):959‐65. [DOI] [PubMed] [Google Scholar]
- 42. Sun P, Chen C, Zhang F, An X, Li XY, Li YH, et al. The ABO blood group predicts survival in esophageal squamous cell carcinoma in patients who ever smoked: a retrospective study from China. Tumour Biology : the Journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35(7):7201‐8. 10.1007/s13277-014-1960-7. [DOI] [PubMed] [Google Scholar]
- 43. Gao Y, Hu N, Han X, Giffen C, Ding T, Goldstein A, et al. Family history of cancer and risk for esophageal and gastric cancer in Shanxi, China. BMC Cancer. 2009;9:269 10.1186/1471-2407-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yuequan J, Shifeng C, Bing Z. Prognostic factors and family history for survival of esophageal squamous cell carcinoma patients after surgery. Ann Thorac Surg. 2010;90(3):908‐13. 10.1016/j.athoracsur.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 45. Ghadimi MR, Rasouli M, Mahmoodi M, Mohammad K. Prognostic factors for the survival of patients with esophageal cancer in Northern Iran. Journal of Research in Medical Sciences : the Official Journal of Isfahan University of Medical Sciences. 2011;16(10):1261‐72. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.


