Abstract
Objective
To describe clinical features associated with cancer outcomes of patients with immune checkpoint inhibitor (ICI)‐associated arthritis.
Methods
Observational study of patients with ICI‐arthritis enrolled in a single‐center registry. Arthritis phenotype and activity, medications, and cancer status were recorded at every visit. We used descriptive statistic, and Kaplan‐Meier curves using two‐sided log‐rank test and Cox regression analysis were used to identify factors associated with cancer progression‐free survival (PFS).
Results
Forty‐two patients with ICI‐arthritis were followed for a median (interquartile range [IQR]) of 7.4 (1.7, 14.7) months. Fifty‐seven percent were female, 33% had melanoma, and 69% received anti–programmed death ligand 1 monotherapy. Median time from ICI initiation to arthritis onset was 2.8 (0.8, 11.2) months. Sixty‐two percent had a rheumatoid arthritis (RA)‐like small‐joint presentation; 27% of all patients were rheumatoid factor and/or cyclic citrullinated peptide positive. Median (IQR) Clinical Disease Activity Index (CDAI) on presentation was 15 (8, 24); 62% required systemic glucocorticoids, 55% required disease‐modifying antirheumatic drugs (DMARDs), and 69% had ongoing arthritis at 6 months. Arthritis led to ICI discontinuation in five patients. In univariate analysis, baseline CDAI, DMARD use, earlier arthritis onset, and longer duration of follow‐up were associated with shorter PFS. In multivariable Cox regression analysis controlling for DMARD use and time to arthritis onset, CDAI was a significant predictor of cancer progression (hazard ratio 1.09, 95% confidence interval [CI] 1.00‐1.19, P = 0.05)
Conclusion
ICI‐arthritis most commonly presents with an RA‐like phenotype. High disease activity, as measured by CDAI, may portend cancer progression.
Significance & Innovations.
Immune checkpoint inhibitor (ICI)‐arthritis usually presents with a rheumatoid arthritis–like phenotype with symmetric small‐joint involvement of the wrists, hands, and feet, but other phenotypes include large‐joint involvement with enthesitis, arthralgia, and polymyalgia rheumatica.
Unlike other immune‐related adverse events, ICI‐arthritis usually persists, even after ICI discontinuation.
High ICI‐arthritis disease activity as measured by CDAI, rather than Common Terminology Criteria for Adverse Events grade, may be associated with cancer progression, although this needs to be confirmed in a larger cohort.
Studies are needed to define optimal ICI‐arthritis treatment strategies that do not worsen cancer survival.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) are being used to treat an ever‐widening array of cancers, prolonging survival in some patients even with advanced disease (1, 2, 3, 4, 5). ICI target inhibitory molecules, such as cytotoxic T lymphocyte‐associated protein 4 (CTLA‐4) and/or programmed cell death‐1 (PD‐1), or its ligand, PD‐L1, blocking pathways that normally serve to protect the body from excessive immune cell activation (6). As such, ICIs result in immune‐related adverse events (irAEs) in up to 90% of patients (7), including dermatologic, gastrointestinal, pulmonary, endocrine, and rheumatologic toxicities among others (8, 9). In one large prospective cancer cohort, the incidence of ICI‐associated inflammatory arthritis (ICI‐arthritis) was 3.8% (10). In this study, we describe the clinical characteristics of ICI‐arthritis and cancer outcomes in patients enrolled in a single‐center observational irAE registry.
PATIENTS AND METHODS
Study investigators (KC, AB) have a fast track referral service at Hospital for Surgery (HSS) that enables outpatients with irAE from Memorial Sloan Kettering Cancer Center andNew York Presbyterian Hospital/Cornell to be seen at HSS within 1 week. On May 1, 2018, a prospective registry was established, and all patients with irAE were invited to enroll, including patients already established in the investigators’ practices. The registry was approved by our institutional review board and all patients provided written consent. Seventeen patients had already established care at HSS prior to registry enrollment, one of whom was previously reported (11). The first patient visit prior to registry enrollment was August 1, 2016. At the time of their first HSS rheumatology visit and first registry visit, demographics, comorbidities, medications, past medical history, and detailed cancer history were obtained from the patient and from review of oncology records. Cancer was identified by primary site (eg, melanoma, non‐small‐cell lung cancer), and cancer stage was documented as locally advanced (stage III) or metastatic (stage IV). The specific ICI regimen was documented as well as the first date of its administration. At each visit, we documented cancer response (complete response, partial response, stable disease, or disease progression) based on the most recent imaging studies performed by the patient’s oncologist. Oncologists routinely perform CT and/or other imaging modalities every 3 months (or sooner if symptoms or signs warrant it) in patients on ICI in order to assess cancer status. Arthritis disease activity was measured using the Clinical Disease Activity Index (CDAI) (12), and functional status was measured using the Multidimensional Health Assessment Questionnaire (MD‐HAQ) (13). Common Terminology Criteria for Adverse Events (CTCAE) irAE grade (14) and maximum ever CTCAE irAE grade was documented at the baseline registry visit and updated at all subsequent visits. Rheumatoid factor (RF), anti–cyclic citrullinated peptide antibody (CCP), antinuclear antibody, erythrocyte sedimentation rate (ESR), and C‐reactive protein (CRP) were collected at the first rheumatology visit. We included registry patients in this study if they had inflammatory joint symptoms, and we grouped them according to their presenting phenotype: (a) inflammatory arthritis with any small‐joint involvement, (b) inflammatory arthritis with exclusively large‐joint involvement, (c) inflammatory arthralgia (joint pain without joint swelling, but with morning stiffness), or (d) a polymyalgia rheumatica (PMR)‐like syndrome. We excluded patients with mechanical joint pain (eg, osteoarthritis), nonarticular rheumatic syndromes (eg, sicca, myositis, eosinophilic fasciitis), or preexisting autoimmune disease. Time of arthritis onset was defined as the time from the date of the first ICI dose until the date of the first joint symptoms. Duration of follow‐up was measured from the date of the first rheumatology visit. Median steroid dose in the first 30 and 60 days was calculated from the date of steroid initiation for joint symptoms, even if steroids were started by the patient’s oncologist prior to the first rheumatology visit. Data collection for this study ended July 12, 2019. We received institutional funding from the HSS Rheumatology Council Research Grant Program.
Statistical analysis
Normality of continuous variables was assessed using the Shapiro‐Wilk normality test. All variables, except for age and time to progression, were found not to be normally distributed and reported as median and interquartile range (IQR). Categorical variables are summarized as frequencies and percentages. Comparison of continuous non‐normally distributed variables was conducted using nonparametric Mann‐Whitney U or Kruskal‐Wallis tests. Student t tests were used to compare the normally distributed age and time to progression. Analysis of discrete variables was performed using χ2 or Fisher exact tests. Logistic regression models were constructed to assess predictors of cancer progression. Backward stepwise modeling was performed, and variables were removed from the model if they had a P value greater than 0.1. Survival analyses were used to assess time to arthritis control (grade 0 on or off medications) and progression‐free survival (PFS), measured from ICI initiation until radiographic cancer progression. Kaplan‐Meier curves were used to visualize differences in time to arthritis control between patients who did and did not discontinue ICI treatment. Similarly, PFS between CDAI levels were compared using two‐sided log‐rank test. Because patients with locally advanced (stage III) cancer given ICI as adjuvant therapy are less likely to progress than patients given ICI for treatment of metastatic disease, we excluded them from the analysis of PFS. Cox regression models were used to identify factors associated with PFS. Because of the limited sample size available, variables in the model were limited to CDAI level, disease‐modifying antirheumatic drug (DMARD) usage, and time to arthritis onset. Statistical significance was defined as P values of 0.05 or below. All analyses were performed using SPSS version 23.0 (IBM Corp) and Stata version 14.0.
RESULTS
Of the 66 patients enrolled in the registry, we included the 42 with inflammatory arthritis, arthralgia, or PMR. Patient characteristics are shown in Table 1. Twenty‐four (57%) were female, 21 (50%) were current or former smokers, 14 (33%) had melanoma, and 29 (69%) received anti–PD‐(L)1 monotherapy. Median (IQR) time from ICI initiation to arthritis onset was 2.8 (0.8, 11.2) months, and median time to referral after arthritis onset was 3.2 (1.2, 7.2) months. Median ESR on presentation was 29 (16.5, 52.5) (normal <20 mm/h), and median CRP 1.1 (0.3, 3.1) (normal <0.8 mg/dL). Eleven (27%) patients tested positive for RF and/or anti‐CCP antibody. There was no difference in the percentage of ever smokers among patients who were CCP positive versus those who were CCP negative (78% vs 42%, P = 0.13).
Table 1.
Patient characteristics grouped by arthritis phenotype
| Variable |
Overall (N = 42) |
Small Joint (n = 26) |
Large Joint (n = 4) |
Arthralgia (n = 7) |
PMR (n = 5) | P value |
|---|---|---|---|---|---|---|
| Median [IQR] or N (%) | Median [IQR] or N (%) | Median [IQR] or N (%) | Median [IQR] or N (%) | Median [IQR] or N (%) | ||
| Demographics | ||||||
| Age, mean (SD) | 65.1 (11.9) | 66.5 (13.3) | 63.5 (9.2) | 57.3 (5.9) | 69.7 (9.4) [61.2‐78.0] | 0.25 |
| Female | 24 (57) | 15 (58) | 3 (75) | 3 (43) | 3 (60) | 0.771 |
| Race | ||||||
| White or Caucasian | 35 (83) | 22 (85) | 3 (75) | 5 (71) | 5 (100) | 0.610 |
| Black or African American | 3 (7) | 1 (4) | 1 (25) | 1 (14) | 0 (0) | … |
| Other | 4 (10) | 3 (12) | 0 (0) | 1 (14) | 0 (0) | … |
| Current/former smoker | 21 (50) | 14 (54) | 1 (25) | 4 (57) | 2 (40) | 0.683 |
| Cancer features | ||||||
| Type | ||||||
| Melanoma | 14 (33) | 10 (38) | 2 (50) | 1 (14) | 1 (20) | 0.531 |
| NSCLC | 7 (17) | 3 (12) | 1 (25) | 3 (43) | 0 (0) | … |
| Renal | 7 (17) | 4 (15) | 0 (0) | 2 (29) | 1 (20) | … |
| Urothelial | 2 (5) | 1 (4) | 0 (0) | 0 (0) | 1 (20) | … |
| Other | 12 (29) | 8 (31) | 1 (25) | 1 (14) | 2 (40) | … |
| Stage | ||||||
| Stage III | 5 (12) | 2 (8) | 0 (0) | 2 (26) | 1 (20) | … |
| Stage IV | 37 (88) | 24 (92) | 4 (100) | 5 (71) | 4 (80) | … |
| ICI regimen | ||||||
| PD‐(L)1 monotherapy | 29 (69) | 18 (69) | 1 (25) | 5 (71) | 5 (100) | 0.117 |
| Combination CTLA4+PD‐(L)1 | 13 (31) | 8 (31) | 3 (75) | 2 (29) | 0 (0) | … |
| Arthritis features | ||||||
| Onset after ICI initiation (mo) | 2.8 [0.8‐11.2] | 2.6 [0.6‐8.0] | 14.3 [6.5‐44.9] | 4.0 [1.0‐24.5] | 0.2 [0.0‐3.3] | 0.022 |
| Time to first rheumatology visit (mo) | 3.2 [1.2‐7.2] | 5.6 [1.8‐9.9] | 1.2 [0.5‐3.9] | 2.1 [1.1‐5.2] | 1.4 [0.5‐5.5] | 0.132 |
| Duration of rheumatology follow‐up (mo) | 7.4 [1.7‐14.7] | 9.0 [3.1‐24.3] | 9.6 [3.6‐26.6] | 1.0 [0.0‐4.8] | 7.6 [0.4‐10.4] | 0.016 |
| Persistent arthritis at last follow‐up | 34 (81) | 21 (81) | 3 (75) | 6 (86) | 4 (80) | 0.978 |
| Enthesitis or tenosynovitis | 10 (24) | 4 (15) | 2 (50) | 4 (57) | 0 (0) | 0.039 |
| CDAI at first office visit | 15.0 [8.0‐24.0] | 22.3 [11.0‐25.0] | 11.0 [5.6‐11.9] | 6.0 [4.5‐10.5] | 11.5 [7.0‐22.0] | 0.013 |
| <12 | 17 (44) | 7 (27) | 3 (75) | 5 (100) | 2 (50) | 0.011 |
| 12+ | 22 (56) | 19 (73) | 1 (25) | 0 (0) | 2 (50) | … |
| Maximum CDAI | 20.0 [10.0‐24.0] | 22.8 [15.0‐28.3] | 11.0 [5.6‐16.0] | 6.0 [4.5] | 15.5 [7.0‐30.8] | 0.004 |
| Maximum grade | ||||||
| 1.0 | 8 (20) | 4 (16) | 1 (25) | 3 (50) | 0 (0) | 0.184 |
| 2.0 | 20 (50) | 14 (56) | 3 (75) | 1 (17) | 2 (40) | … |
| 3.0 | 12 (30) | 7 (28) | 0 (0) | 2 (33) | 3 (60) | … |
| Laboratory results | ||||||
| ESR (mm/h) at first visit | 29.0 [16.5‐52.5] | 30.0 [18.0‐63.0] | 53.5 [10.3‐96.8] | 30.0 [11.0‐41.0] | 22.0 [13.5‐60.5] | 0.904 |
| CRP (mg/dL) at first visit | 1.1 [0.3‐3.1] | 1.5 [0.7‐3.1] | 1.4 [0.2‐9.0] | 1.0 [0.5‐1.1] | 1.1 [0.2‐12.3] | 0.935 |
| RF positive | 4 (10) | 4 (16) | 0 (0) | 0 (0) | 0 (0) | 0.417 |
| CCP positive | 9 (23) | 5 (20) | 1 (25) | 2 (29) | 1 (25) | 0.966 |
| RF and/or CCP positive | 11 (27) | 7 (28) | 1 (25) | 2 (29) | 1 (20) | 0.985 |
| Arthritis treatment | ||||||
| Median prednisone equivalent daily dose | ||||||
| In first 30 days after prednisone initiation or first rheumatology visit | 13.8 [6.9‐30.0] | 18.1 [10.0‐35.5] | 7.8 [0.0‐39.5] | 10.0 [0.0‐14.2] | 10.0 [2.5‐22.5] | 0.150 |
| In first 60 days after prednisone initiation or first rheumatology visit | 15.0 [7.3‐26.2] | 20.0 [10.0‐31.3] | 10.7 [1.5‐32.0] | 10.0 (0.0‐13.4) | 15.0 [3.0‐23.8] | 0.138 |
| DMARDs | ||||||
| No DMARDs | 19 (45) | 8 (31) | 3 (75) | 5 (71) | 3 (60) | 0.581 |
| cDMARDS only | 15 (36) | 11 (42) | 1 (25) | 2 (29) | 1 (20) | … |
| bDMARDS only | 2 (5) | 2 (8) | 0 (0) | 0 (0) | 0 (0) | … |
| cDMARDS plus bDMARDS | 6 (14) | 5 (19) | 0 (0) | 0 (0) | 1 (20) | … |
| ICI treatment discontinued | 20 (48) | 16 (62) | 1 (25) | 2 (29) | 1 (20) | 0.146 |
| Cancer outcome | ||||||
| Complete response | 11 (26) | 9 (35) | 0 (0) | 1 (14) | 1 (20) | 0.571 |
| Partial response | 5 (12) | 3 (12) | 0 (0) | 1 (14) | 1 (20) | … |
| Stable | 12 (29) | 5 (19) | 3 (75) | 3 (43) | 1 (20) | … |
| Progression | 14 (33) | 9 (35) | 1 (25) | 2 (29) | 2 (40) | … |
Significant P values in bold.
Abbreviations: bDMARD, biologic DMARD; CCP, cyclic citrullinated protein; CDAI, Clinical Disease Activity Index; cDMARD, conventional DMARD; CRP, C‐reactive protein; DMARD, disease‐modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; ICI, immune checkpoint inhibitor; NSCLC, non‐small‐cell lung cancer; PMR, polymyalgia rheumatica; RF, rheumatoid factor.
Median duration of follow‐up after the first rheumatology visit was 7.4 (1.7, 14.7) months. Sixty‐nine percent of patients had ongoing arthritis at 6 months and 58% at 12 months (Figure 1A). Twenty (48%) patients discontinued ICI therapy during follow‐up, 5 for joint pain and 15 for other reasons, primarily cancer progression. Arthritis duration was the same in patients who did versus did not discontinue ICI (Figure 1B).
Figure 1.
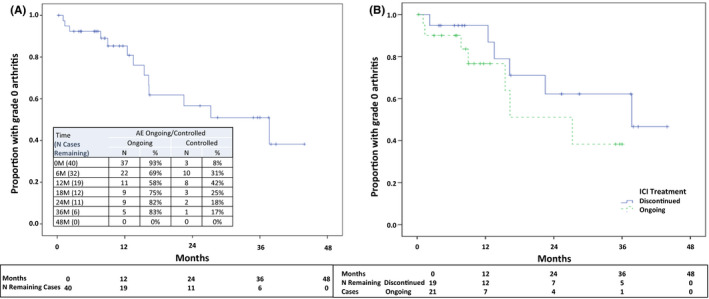
Kaplan‐Meier analysis: time to arthritis control, impact of immune checkpoint inhibitor (ICI) discontinuation.
Arthritis activity
Median [IQR] baseline CDAI was 15 [8, 24]. The median [IQR] patient global component score of the CDAI was 5 [3, 7] and the median physician global was 3 [2, 4]. Maximum CDAI was 20 [10, 24]. There was no difference in maximum CDAI between patients who received combination ICI versus monotherapy (P = 0.62). Only 8% of patients had a maximum CTCAE grade of 3. For patients with a maximum CTCAE arthritis grade of 1, 2, and 3, respectively, the corresponding maximum CDAI was 11 [6, 22], 18 [11, 24], and 24 [7.8, 34]. Baseline MD‐HAQ was 2.0 [1.0, 3.3].
Arthritis phenotypes
Twenty‐six (62%) patients had small‐joint involvement reminiscent of rheumatoid arthritis (RA), four (9%) had arthritis involving only large joints, seven (17%) had arthralgia without joint swelling, and five (12%) had a PMR‐like condition (Table 1). The median [IQR] swollen joint count was 4.5 [2, 8] in patients with the small‐joint phenotype and 1 [1, 1] in patients with the large‐joint phenotype. Patients with small‐joint involvement had a higher baseline CDAI than other phenotypes: 22.3 [11,25], versus 11 [5.6, 11.9] for patients with large‐joint arthritis, 6 [4.5, 10.5] for patients with arthralgia, 11.5 [7, 22] for patients with PMR (P = 0.013). Patients with large‐joint arthritis presented later than the other groups, 14.3 [6.5‐44.9] months after ICI initiation, and enthesitis was seen almost exclusively in patients with large‐joint involvement or arthralgia. Although there was no statistical difference between the phenotypes with regard to their ICI regimen, 18 of 26 (69%) patients with small‐joint arthritis received anti–PD‐(L)1 monotherapy, whereas 3 of 4 (75%) patients with large‐joint arthritis received anti–CTLA‐4/anti–PD‐1 combination therapy. Patients grouped by arthritis phenotypes were similar with regard to age, smoking status, type of malignancy, and RF/CCP seropositivity.
Arthritis treatment
Twenty‐six (62%) patients required systemic glucocorticoids (Table 1). The median (IQR) prednisone dose per day in the first 30 days of arthritis treatment was 13.8 (6.9, 30) mg. Twenty‐three (55%) required DMARDs: 15 (36%) a conventional DMARD (one or more), 2 (5%) a biologic DMARD alone, and 6 (14%) a combination of conventional and biologic DMARD. Hydroxychloroquine (HCQ) was the most commonly used DMARD (40% of patients). Other DMARDs used include methotrexate (21%), sulfasalazine (17%), tumor necrosis factor (TNF)‐inhibitors (14%), interleukin (IL)‐6R inhibitors (7%), and rituximab (5%). Figure 2 shows arthritis treatments that the patients received over time.
Figure 2.
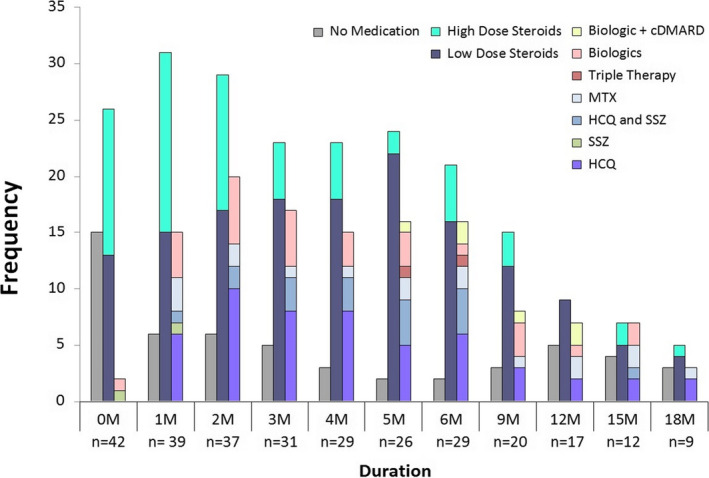
Immune checkpoint inhibitor (ICI)‐arthritis treatment over time. High‐dose steroid: prednisone >10 mg/d. Low‐dose steroid: prednisone ≤10 mg/d. Biologics used: infliximab, rituximab, tocilizumab. Abbreviations: cDMARD, conventional disease‐modifying antirheumatic drug; HCQ, hydroxychloroquine; MTX, methotrexate; SSZ, sulfasalazine.
Cancer outcomes
Thirty‐seven (88%) of the ICI‐arthritis patients had stage IV (metastatic) cancer. Of these, 13 (35%) had cancer progression during follow‐up (Table 2). Progressors had a longer duration of follow‐up than nonprogressors (8 [5.0, 25.8] vs 7.8 [1.3, 14.2] months, P = 0.039). Progressors were similar to nonprogressors with regard to age, sex, race, type of malignancy, smoking status, arthritis phenotypes, RF/CCP seropositivity, baseline ESR and CRP, and steroid dose. Progressors had a shorter time to arthritis onset (median [IQR] 2.0 [0.4, 13.8] vs 4.1 [1.0, 11.7] months, P = 0.05) and were more likely to have received a DMARD (77% vs 42%, P = 0.04). There was a trend toward more combination ICI therapy among progressors than nonprogressors (54% vs 25%, P = 0.079). Patients whose cancer progressed had a higher baseline CDAI than nonprogressors (23 [11.5, 31] vs 11.3 [6.8, 22.9], P = 0.007) and no patient with a baseline CDAI less than 10 progressed. In a logistic regression model, CDAI was found to be a statistically significant predictor of progression (odds ratio = 1.11, 95% CI [1.02, 1.20]). Figure 3 shows a Kaplan‐Meier analysis of PFS in patients with a baseline CDAI of 12 or greater versus less than 12. In multivariable Cox regression analysis, there was an association between baseline CDAI and cancer progression after controlling for time to arthritis onset and DMARD use (hazard ratio 1.09, 95% CI [1.00, 1.19], P = 0.050).
Table 2.
Predictors of cancer progression: univariate analysis
| Variable | Overall (N = 37) |
Nonprogressors (N = 24) |
Progressors (N = 13) | P value |
|---|---|---|---|---|
| Median [IQR] or N (%) | Median [IQR] or N (%) | Median [IQR] or N (%) | ||
| Demographics | ||||
| Age | 65.4 [58.5‐46.2] | 65.3 [58.6‐75.8] | 66.6 [57.0‐79.0] | 0.229 |
| Female | 21 (57) | 13 (54) | 8 (62) | 0.666 |
| Race | ||||
| White or Caucasian | 31 (84) | 22 (92) | 9 (69) | 0.210 |
| Black or African American | 3 (8) | 1 (4) | 2 (15) | … |
| Other | 3 (8) | 1 (4) | 2 (15) | … |
| Current/former smoker | 19 (51) | 13 (54) | 6 (46) | 0.642 |
| Cancer features | ||||
| Type | ||||
| Melanoma | 13 (35) | 8 (33) | 5 (38) | 0.790 |
| NSCLC | 5 (14) | 4 (17) | 1 (8) | … |
| Renal | 6 (16) | 3 (13) | 3 (23) | … |
| Urothelial | 1 (3) | 1 (4) | 0 (0) | … |
| Other | 12 (32) | 8 (33) | 4 (31) | … |
| ICI regimen | ||||
| PD‐(L)1 monotherapy | 24 (65) | 18 (75) | 6 (46) | 0.079 |
| Combination CTLA‐4+PD‐(L)1 | 13 (35) | 6 (25) | 7 (54) | … |
| Arthritis features | ||||
| Onset after ICI initiation (mo) | 3.0 [0.9‐11.5] | 4.1 [1.0‐11.7] | 2.0 [0.4‐13.8] | 0.050 |
| Time to first rheumatology visit (mo) | 3.1 [1.3‐6.9] | 2.5 [0.9‐6.9] | 5.0 [1.3‐7.3] | 0.214 |
| Duration of follow‐up (mo) | 8.0 [2.2‐18.6] | 7.8 [1.3‐14.2] | 8.0 [5.0‐25.8] | 0.039 |
| Persistent arthritis at last follow‐up | 30 (81) | 20 (83) | 10 (77) | 0.635 |
| CDAI at first office visit | 12.0 [7.3‐23.8] | 11.3 [6.8‐22.9] | 23.0 [11.5‐31.0] | 0.007 |
| <12 | 17 (47) | 14 (61) | 3 (23) | 0.041 |
| 12+ | 19 (53) | 9 (39) | 10 (77) | … |
| Maximum CDAI | 16.5 [10.0‐24.0] | 11.5 [7.0‐22.0] | 24.0 [16.3‐31.0] | 0.008 |
| Arthritis phenotypes | ||||
| Small joint | 23 (62) | 14 (58) | 9 (69) | 0.912 |
| Large joint | 4 (11) | 3 (13) | 1 (8) | … |
| Arthralgia | 6 (16) | 4 (17) | 2 (15) | … |
| PMR | 4 (11) | 3 (13) | 1 (8) | … |
| Maximum grade | ||||
| 1.0 | 7 (20) | 5 (23) | 2 (15) | 0.752 |
| 2.0 | 17 (49) | 11 (50) | 6 (46) | … |
| 3.0 | 11 (31) | 6 (27) | 5 (38) | … |
| Laboratory results | ||||
| ESR (mm/h) at first visit | 28.5 [16.3‐45.8] | 28.0 [9.0‐45.0] | 29.0 [20.5‐63.0] | 0.637 |
| CRP (mg/dL) at first visit | 1.0 [0.2‐2.9] | 1.0 [0.2‐1.9] | 1.1 [0.5‐4.6] | 0.954 |
| RF positivity | 4 (11) | 6 (8) | 2 (17) | 0.588 |
| CCP positivity | 9 (25) | 5 (21) | 4 (33) | 0.443 |
| RF and/or CCP positivity | 11 (31) | 6 (25) | 5 (42) | 0.446 |
| Arthritis treatment | ||||
| Median prednisone equivalent daily dose | ||||
| In first 30 days after prednisone initiation or first rheumatology visit | 14.2 [8.8‐30.0] | 13.8 [6.3‐20.6] | 15.6 [8.8‐35.0] | 0.108 |
| In first 60 days after prednisone initiation or first rheumatology visit | 15.0 [7.5‐25.2] | 14.2 [6.9‐20.0] | 18.8 [10.0‐29.2] | 0.157 |
| DMARDs | ||||
| No DMARDs | 17 (46) | 14 (58) | 3 (23) | 0.037 |
| cDMARDS only | 13 (35) | 6 (25) | 7 (54) | … |
| bDMARDS only | 2 (5) | 0 (0) | 2 (15) | … |
| cDMARDS plus bDMARDS | 5 (14) | 4 (17) | 1 (8) | … |
| ICI treatment discontinued | 17 (46) | 11 (46) | 6 (46) | 0.985 |
Significant P values in bold.
Abbreviations: bDMARD, biologic DMARD; CCP, cyclic citrullinated peptide; CDAI, Clinical Disease Activity Index; cDMARD, conventional DMARD; CRP, C‐reactive protein; CTLA‐4, cytotoxic T lymphocyte‐associated protein 4: DMARD, disease‐modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; ICI, immune checkpoint inhibitor; NSCLC, non‐small‐cell lung cancer; PD‐(L)1, programmed cell death ligand‐1; PMR, polymyalgia rheumatica; RF, rheumatoid factor.
Figure 3.
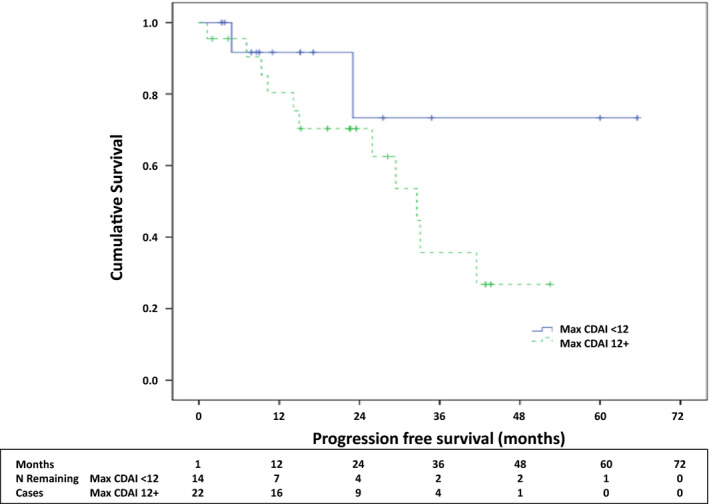
Kaplan‐Meier analysis of progression‐free survival stratified by baseline Clinical Disease Activity Index (CDAI).
DISCUSSION
In this large, real‐world observational cohort of patients with ICI‐arthritis recruited and followed in New York City, we demonstrate that most patients present with an RA‐like phenotype, regardless of type of ICI therapy, type of cancer, or serologic status. Most patients require systemic corticosteroids, and a substantial number require DMARDs. ICI‐arthritis persists, even in patients who stop their ICI. We also demonstrate that for every one‐point increase in baseline CDAI, the likelihood of cancer progression increased by 9%, independent of DMARD use and time to arthritis onset after ICI initiation.
Our finding that small‐joint arthritis, closely resembling that seen in RA, is the most frequently encountered phenotype is in keeping with two other published cohorts (15, 16). Of interest, the majority of patients with this phenotype had received PD‐(L)1 blockade. There is evidence that PD‐1 inhibition mimics the biology of RA. In a study by Guo et al, for example, a “nivolumab (anti–PD‐1) gene signature” was demonstrated in peripheral blood mononuclear cells from patients with active RA (17). PD‐1 expression is increased in the synovium of patients with RA at all stages of disease, and PD‐L1 expression is increased when disease is active (17, 18). The majority of patients with large‐joint arthritis in our study (half of whom also had enthesitis) had received combination ICI (anti–CTLA‐4 plus anti–PD‐1), raising the possibility that combination ICI triggers pathways seen in patients with spondyloarthritidesopathies. In keeping with this, a high level of IL‐17, a cytokine implicated in the pathogenesis of the spondyloarthritides (19), has been demonstrated in patients experiencing colitis due to anti–CTLA‐4 therapy (20).
The persistence of ICI‐arthritis demonstrated in our study has also been reported by others (12, 21). The majority of our patients had arthritis for 12 months or more, regardless of whether their ICI was discontinued. This may be explained by the pharmacokinetics of ICI binding to synovial tissue resident immune cells. For example, one case report demonstrated 100% PD‐1 receptor occupancy (ie, complete blockade) in a synovial biopsy taken from an ICI‐arthritis patient 200 days after their last dose of nivolumab (see figure 2 in Murray‐Brown et al (22)).
The majority of patients in our cohort (62%) required ongoing glucocorticoids to control their symptoms, and 55% required conventional and/or biologic DMARDs. The decision to start a DMARD is often driven by a need to taper glucocorticoid, as studies suggest that steroid doses greater than 7.5 to 10 mg/d negatively impact cancer outcomes (23, 24
). The choice of DMARD is always made in consultation with the patient and their oncologist and may be influenced by the type of cancer and whether the patient is enrolled in a clinical trial. In our cohort, HCQ was the most commonly used DMARD (40% of patients). This treatment approach is based largely on the perceived safety of HCQ relative to other commonly used DMARDs. Intriguingly, in addition to the anti‐inflammatory effect of HCQ, one potential added benefit is its inhibition of autophagy. HCQ has in fact been used in preclinical studies and early‐phase clinical trials to potentiate the response to chemotherapy in patients with advanced solid tumors (25, 26). Roberts et al studied HCQ systematically in ICI‐arthritis with good results (27). Unfortunately, no other ICI‐arthritis treatment has been systematically studied. Several retrospective studies of ICI‐induced enterocolitis have suggested that infliximab is not only effective but also safe (28, 29), but a retrospective study of multiple irAEs suggested that infliximab could negatively impact survival (30). Given our limited sample size, we were unable calculate the effect of individual DMARDs on PFS.
Our study suggested that there may be an association between high‐baseline ICI‐arthritis disease activity, as measured by CDAI, and cancer progression, and this association was not explained by immunosuppressive DMARD use. A number of studies have demonstrated an association between irAE and cancer survival (31, 32, 33), but few have analyzed whether irAE severity impacts cancer outcomes. Although one study demonstrated an association between low‐grade, but not high‐grade, irAEs on PFS (34), high‐grade irAEs are less common than low‐grade ones, and the study may not have been powered for the latter analysis. Weber et al demonstrated a stepwise improvement in overall survival in patients with higher numbers of (any grade) irAEs but failed to show a protective effect from high‐grade irAEs (35). This too may have been an issue of study power.
CDAI is a validated disease activity measure in RA that takes into account tender and swollen joint counts along with a patient and physician global scores (12). As such, we would expect the CDAI to parallel the small‐joint ICI‐arthritis phenotype, where typically more joints are affected—indeed our small‐joint arthritis patients had a higher CDAI than the other groups. However, although a higher CDAI predicted worse PFS, the small‐joint phenotype and maximum CTCAE grade did not. This suggests that the patient and physician global components of the CDAI capture important prognostic information that is not captured by joint counts or grade. For example, it is possible that large‐joint involvement is perceived to be more problematic by both physicians and patients. In fact, Cappelli et al demonstrated a significant lag in time to diagnosis among patients who presented with small‐joint involvement versus those who presented with large‐joint involvement (36). In general, the CTCAE grading system does a poor job of characterizing ICI‐arthritis severity (14). ICI‐arthritis rarely requires hospitalization and is therefore usually characterized as grades 1 to 2 (low grade), even if patients have significant impairment in their activities of daily living. In our cohort, patients with higher CDAI generally had a higher CTCAE grade, but there was considerable overlap. CTCAE grade is insensitive to the degree of synovitis and, in our study, grade was not a predictor of PFS. This suggests that in studies of ICI‐arthritis, traditional measures of arthritis activity, such as the CDAI, should be used in addition to grade.
Although this is one of the largest ICI‐arthritis cohorts published, our study does have some limitations. The number of patients is still relatively small, the cohort is heterogeneous with regard to cancer types and ICI therapies used, and referral bias may have led us to overestimate ICI‐arthritis severity. However, these real‐world data reflect the kinds of ICI‐arthritis that rheumatologists are likely to see in practice. Arthritis treatment in this study was based on clinician judgement rather than a protocol, which points to the need for treatment trials in ICI‐arthritis patients. Although we describe four arthritis phenotypes in our patients, this needs to be validated in studies from other centers given our sample size, particularly because the high rate of seropositivity in our cohort suggests the possibility of referral bias. Finally, because we could not include patients lost to follow‐up prior to the registry’s initiation, estimates of arthritis phenotype distribution, severity, and duration may have been biased. These weaknesses are balanced by several strengths, however. This is among the largest published series of ICI‐arthritis patients, and we provide a long duration of follow‐up. We offer extensive information about arthritis characteristics and arthritis treatments, and we demonstrate that ICI‐arthritis persists even when ICIs are discontinued. Finally, we provide evidence for a possible relationship between arthritis disease activity and cancer progression, although this will need to be validated in a larger cohort.
In summary, ICI‐arthritis is a long‐lasting condition that often requires immunosuppression, even after ICI discontinuation. Current treatments are based on expert opinion and depend on each center’s preferences and experience. Untangling the relationship between ICI‐arthritis disease severity (eg, CDAI), ICI‐arthritis treatment (eg, TNF inhibitors), and cancer survival will be critical to the development of safe treatment approaches.
AUTHOR CONTRIBUTIONS
Chan and Bass are primarily responsible for the content, drafting, and approval of the final version of the manuscript. Tirpack, Vitone, Benson, and Ghosh contributed to data visualization, drafting and final manuscript approval. Nguyen, Jannat‐Khah, and Bykerk made substantial contributions to drafting and final manuscript approval.
Study conception and design
Chan, Bykerk, Bass.
Acquisition of data
Chan, Tirpack, Vitone, Benson, Ghosh, Bass.
Analysis and interpretation of data
Chan, Nguyen, Jannat‐Khah, Bass.
This study was supported in part by institutional funding from the Hospital for Surgery Rheumatology Council Research grant program. In addition, Mr. Nguyen reports institutional grants outside the submitted work from the Clinical Translational Science Center (CTSC), National Center for Advancing Translational Sciences (NCATS) grant #UL1‐ RR024996. Dr. Bykerk served as consultant for BMS, Amgen, Gilead, UCB, and Pfizer, and received institutional grants >$10,000 from BMS and Amgen outside the submitted work; she is supported by NIH (National Institute of Allergy and Infectious Diseases/National Institute of Arthritis and Musculoskeletal and Skin Diseases) grant 1UH2AR067691‐01, gRANT11652401, and The Cedar Hill Foundation.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Larkin J, Chiarion‐Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolchok JD, Chiarion‐Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hellmann MD, Paz‐Ares L, Caro RB, Zurawski B, Kim SW, Costa EC, et al. Nivolumab plus ipilimumab in advanced non–small‐cell lung cancer. N Engl J Med September 2019;381:2020–31. [DOI] [PubMed] [Google Scholar]
- 6. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 8. Suarez‐Almazor ME, Kim ST, Abdel‐Wahab N, Diab A. Review: immune‐related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol 2017;69:687–99. [DOI] [PubMed] [Google Scholar]
- 9. Khoja L, Day D, Chen TW, Siu LL, Hansen AR. Tumour‐ and class‐specific patterns of immune‐related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017;28:2377–85. [DOI] [PubMed] [Google Scholar]
- 10. Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer—clinical aspects and relationship with tumour response: a single‐centre prospective cohort study. Ann Rheum Dis 2018;77:393–8. [DOI] [PubMed] [Google Scholar]
- 11. Smith MH, Bass AR. Arthritis after cancer immunotherapy: symptom duration and treatment response. Arthritis Care Res (Hoboken) 2019;71:362–6. [DOI] [PubMed] [Google Scholar]
- 12. Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42:244–57. [DOI] [PubMed] [Google Scholar]
- 13. Pincus T, Swearingen C, Wolfe F. Toward a multidimensional health assessment questionnaire (MDHAQ): Assessment of advanced activities of daily living and psychological status in the patient‐friendly health assessment questionnaire format. Arthritis Rheum 1999;42:2220–30. [DOI] [PubMed] [Google Scholar]
- 14. Division of Cancer Treatment and Diagnosis: National Institutes of Health . Common Terminology Criteria for Adverse Events (CTCAE), Cancer Therapy Evaluation Program. URL: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
- 15. Richter MD, Crowson C, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Rheumatic syndromes associated with immune checkpoint inhibitors: a single‐center cohort of sixty‐one patients. Arthritis Rheumatol 2019;71:468–75. [DOI] [PubMed] [Google Scholar]
- 16. Arnaud L, Lebrun‐Vignes B, Salem JE. Checkpoint inhibitor‐associated immune arthritis. Ann Rheum Dis 2019;78:e68. [DOI] [PubMed] [Google Scholar]
- 17. Guo Y, Walsh AM, Canavan M, Wechalekar MD, Cole S, Yin X, et al. Immune checkpoint inhibitor PD‐1 pathway is down‐regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS One 2018;13:e0192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuda K, Miyoshi H, Hiraoka K, Hamada T, Yoshida S, Ishibashi Y, et al. Clinicopathological value of programmed cell death 1 (PD‐1) and programmed cell death ligand 1 (PD‐L1) expression in synovium of patients with rheumatoid arthritis. Clin Exp Med 2018;18:487–94. [DOI] [PubMed] [Google Scholar]
- 19. Kirkham BW, Kavanaugh A, Reich K. Interleukin‐17A: a unique pathway in immune‐mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology 2014;141:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, et al. Baseline circulating IL‐17 predicts toxicity while TGF‐β1 and IL‐10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braaten TJ, Brahmer JR, Forde PM, Le D, Lipson EJ, Naidoo J, et al. Immune checkpoint inhibitor‐induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis 2020;79:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray‐Brown W, Weedon H, Wilsdon T, Proudman S, Walker J, Smith MD, et al. Nivolumab‐induced synovial tissue t cell infiltration, sustained pd1 occupancy and resolution of severe synovitis with infliximab therapy [abstract]. Arthritis Rheumatol 2018;70 Suppl 10 URL: https://acrabstracts.org/abstract/nivolumab‐induced‐synovial‐tissue‐t‐cell‐infiltration‐sustained‐pd1‐occupancy‐and‐resolution‐of‐severe‐synovitis‐with‐infliximab‐therapy/. [Google Scholar]
- 23. Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High‐dose glucocorticoids for the treatment of ipilimumab‐induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–14. [DOI] [PubMed] [Google Scholar]
- 24. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death‐1 and programmed death‐ligand 1 blockade in patients with non–small‐cell lung cancer. J Clin Oncol 2018;36:2872–78. [DOI] [PubMed] [Google Scholar]
- 25. Poklepovic A, Gewirtz DA. Outcome of early clinical trials of the combination of hydroxychloroquine with chemotherapy in cancer. Autophagy 2014;10:1478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mulcahy Levy JM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer 2017;17:528–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts J, Smylie M, Walker J, Basappa NS, Chu Q, Kolinsky M, et al. Hydroxychloroquine is a safe and effective steroid‐sparing agent for immune checkpoint inhibitor‐induced inflammatory arthritis. Clin Rhematol 2019;38:1513–9. [DOI] [PubMed] [Google Scholar]
- 28. Lesage C, Longvert C, Prey S, Maanaoui S, Dréno B, Machet L, et al. Incidence and clinical impact of anti‐TNFα treatment of severe immune checkpoint inhibitor‐induced colitis in advanced melanoma: the Mecolit Survey. J Immunother 2019;42:175–9. [DOI] [PubMed] [Google Scholar]
- 29. Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL Jr, Abdel‐Wahan N, et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune‐related enterocolitis. J Immunother Cancer 2018;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verheijden RJ, May AM, Blank CU, Aarts MJ, van den Berkmortel FW, van den Eertwegh AJ, et al. Association of anti‐TNF with decreased survival in steroid refractory ipilimumab and anti‐PD1‐treated patients in the Dutch Melanoma Treatment Registry. Clin Cancer Res 2020;26:2268–74. [DOI] [PubMed] [Google Scholar]
- 31. Freeman‐Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Toi Y, Sugawara S, Kawashima Y, Aiba T, Kawana S, Saito R, et al. Association of immune‐related adverse events with clinical benefit in patients with advanced non‐small‐cell lung cancer treated with nivolumab. Oncologist 2018;23:1358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Abu‐Sbeih H, Mao E, Ali N, Ali FS, Qiao W, et al. Immune‐checkpoint inhibitor‐induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer 2018;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Judd J, Zibelman M, Handorf E, O'Neill J, Ramamurthy C, Bentota S, et al. Immune‐related adverse events as a biomarker in non‐melanoma patients treated with programmed cell death 1 inhibitors. Oncologist 2017;22:1232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–92. [DOI] [PubMed] [Google Scholar]
- 36. Cappelli LC, Brahmer JR, Forde PM, Le DT, Lipson EJ, Naidoo J, et al. Clinical presentation of immune checkpoint inhibitor‐induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum 2018;48:553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


