Abstract
The administration of high-level spinal anesthesia for cesarean section may lead to significant hemodynamic changes. Bioreactance-based non-invasive cardiac output monitoring (NICOM™) provides an accurate monitoring system for parturients under spinal anesthesia. The present study hypothesized that baseline hemodynamic parameters obtained via the NICOM™ system could serve as predictive indicators for post-spinal anesthesia hypotension. Therefore, 80 full-term parturients with singleton pregnancies who underwent scheduled cesarean section were enrolled and allocated to either a supine position group or a 15˚ left tilt group. All parturients received standard pre-hydration with 750 ml of 0.9% saline. Baseline cardiac output index (CI), total peripheral resistance index (TPRI) and stroke volume (SV) were recorded using the NICOM™ system. Subsequently, spinal anesthesia with 2.4 ml of 0.5% hyperbaric bupivacaine, 10 µg of fentanyl and 0.2 mg of morphine was administered. Receiver operating characteristic (ROC) curves and multivariate logistic regression were used to analyze the data. A total of 40 parturients (51.9%) developed hypotension. The areas under the ROC curves were 0.666, 0.594 and 0.622 for the CI, TPRI and SV, respectively. The optimal cut-off value of the CI in predicting hypotension was 3.68 l/min/m2 (ROC, sensitivity=85.0%, specificity=48.6%). Furthermore, CI was considered as an independent factor for post-spinal anesthesia hypotension. In conclusion, the baseline CI obtained via the bioreactance-based NICOM™ system may serve as a predictor of post-spinal anesthesia hypotension in parturients regardless of patient position.
Keywords: spinal anesthesia, physiological monitoring, hypotension, cesarean section
Introduction
Post-spinal anesthesia hypotension during cesarean section may lead to severe maternal and fetal morbidity (1). Many techniques have been developed to prevent post-spinal anesthesia hypotension such as pre-hydration with crystalloids or colloids, preventive usage of ephedrine or phenylephrine and lower leg compression (2). However, a method that would allow the accurate prediction of patient risk of hypotension would be beneficial for the development of a management strategy for each individual patient.
Several methods for predicting the risk of post-spinal anesthesia hypotension have been investigated, including heart rate variability, perfusion index (PI), skin conductance, pleth variability index (PVi), sensory block level and bioimpedance-based hemodynamic monitoring. However, none of these methods has been widely adopted in clinical practice due to variability in the accuracy of results (3-8). The recently developed bioreactance-based non-invasive cardiac output monitoring (NICOM™) system (Cheetah Medical; Baxter International Inc.) provides an accurate monitoring method for parturients under spinal anesthesia (9). Unlike bioimpedance, bioreactance is accompanied by fewer adverse effects associated with body movement (9,10). However, frequent position alterations during induction of spinal anesthesia have been commonly observed. On the other hand, the bioreactance-based NICOM™ system has been indicated to minimize the inaccuracy due to alterations in the patient position during and after spinal anesthesia, such as the difference between the supine and left tilt (11). Volume status may affect post-spinal anesthesia hypotension (12). The present study hypothesized that baseline hemodynamic parameters, such as cardiac output index (CI), total peripheral resistance index (TPRI) and stroke volume (SV), obtained via the NICOM™ system, could be used to predict the risk of post-spinal anesthesia hypotension regardless of the patient's post-anesthetic position. Therefore, an open label, case controlled, observational study was designed to evaluate the association between the parameters measured with the NICOM™ system and the risk of post-spinal anesthesia hypotension.
Materials and methods
Ethics
The present prospective, observational study was designed as part of the trial of left tilt in preventing hypotension (Chinese Clinical Trial Registry no. ChiCTR-IOR-15007087). The present study was approved by the Institutional Review Board of the Changhua Christian Hospital (Changhua, Taiwan; protocol no. 150605) and registered in the Chinese Clinical Trial Register. Written informed consents were obtained from each participant before inclusion.
Study population
A total of 80 full-term, parturients with singleton pregnancies scheduled for elective cesarean delivery were included in the study. The exclusion criteria were as follows: Parturients with multiple pregnancies, current labor, a history of hypertension, pre-eclampsia, obesity [body mass index (BMI) >35 kg/m2], heart disease, diabetes, placenta previa and fetal distress.
Study protocol
All participants were allocated to either the supine group (supine position after spinal anesthesia) or the left tilt group (15˚ left lateral table tilt after spinal anesthesia). Group allocation was determined using a computerized random number table and the sealed envelope technique (13). All parturients received standard treatment in all aspects except the position of the body following anesthesia. Within 20 min of arrival at the operating room, all parturients were treated with 750 ml of 0.9% saline via a 20-gauge cannula. Patients were simultaneously subjected to pulse oximetry, electrocardiography and non-invasive blood pressure measurements using the NICOM™ system with a cuff on the left arm. Following automatic calibration of the NICOM™ system, three data points for blood pressure (mmHg), CI (l/min/m2), TPRI (dynes/s/cm5/m2) and SV (ml/beat) were averaged within 5 min of hydration with 2.5 min intervals as the baseline data.
A standard spinal anesthesia dose with 2.4 ml of 0.5% hyperbaric bupivacaine, 10 µg of fentanyl and 0.2 mg of morphine was injected to all parturients via a 25-gauge spinal needle at the L3-L4 interspace in the right lateral position. Patients in the supine group were placed in the supine position and those in the left tilt group in a 15 left lateral table tilt immediately after spinal anesthesia. Subsequently, blood pressure was recorded at 1, 3, 5, 7 and 10 min following spinal anesthesia. Post-spinal anesthesia hypotension was defined as a decrease in systolic blood pressure >20%, as previously described (14). For safety reasons, when hypotension occurred or when clinical signs of hypotension, such as nausea or vomiting were observed, parturients were treated with 10 mg ephedrine and its total dose was recorded. Ephedrine dosage >20 mg was considered as high dose usage. Following recording for 10 min, the sensory block level was determined using an alcohol pad and surgery was then performed.
Statistical analysis
Power analysis using data from our pilot study with 20 participants indicated that for a rate of 50% post-spinal anesthesia hypotension, 42 subjects were required to detect a difference between the low- and high-CI groups with a power of 0.8 and alpha of 0.05, which was comparable to that of previous study (14). Student's t-test, χ2 test or Fisher's exact test were performed when appropriate, to evaluate patient's characteristics, namely neonatal weight, Apgar score, level of anesthesia and pre-anesthetic CI, TPRI and SV. Receiver operating characteristic (ROC) curve analysis was performed to assess the diagnostic performance of the pre-anesthetic CI, TPRI and SV for hypotension and high dose ephedrine usage. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), cut-off value and area under the ROC curve (AUC) were analyzed. Furthermore, Youden's index was applied to determine the optimal cut-off value. The cumulative incidence rate of hypotension was evaluated via a Kaplan-Meier curve. Multivariate analyses were performed using Cox proportional-hazards and logistic regression models to evaluate the independent perioperative risk factors for hypotension and high dose ephedrine usage.
For all data, P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were carried out using the SPSS statistical software (version 22; SPSS, Inc.).
Results
Characteristics of participants
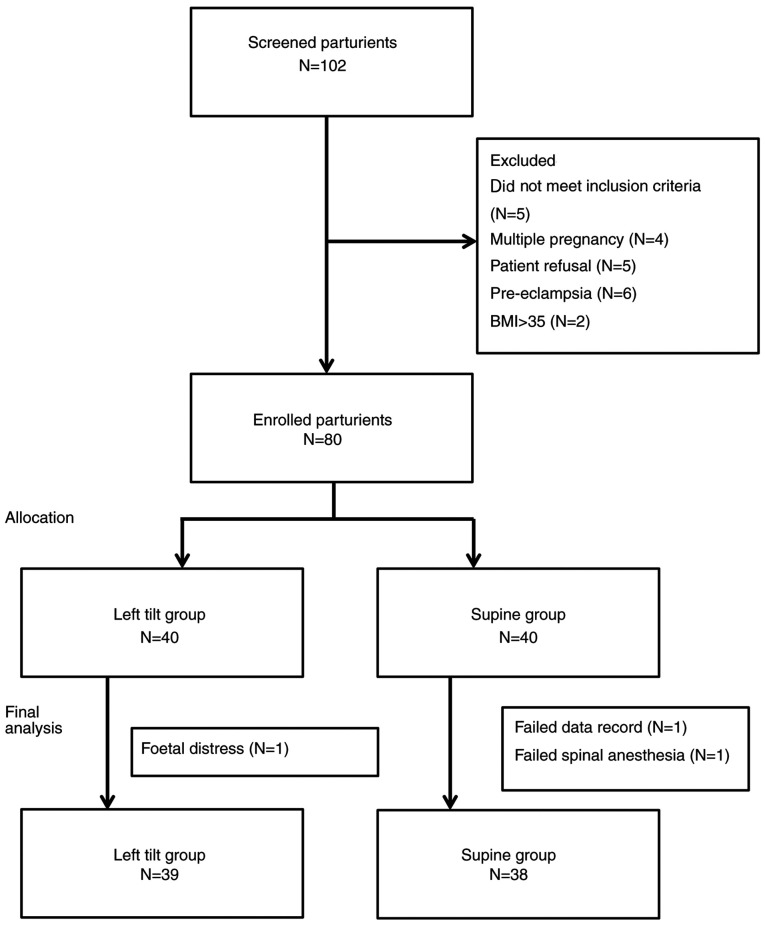
A total of 102 parturients were screened for eligibility between September 2015 and March 2016 and 80 of them met the inclusion criteria of the study (age range, 23-44 years; mean, 33.16 years). Among the 80 parturients, one in the left tilt group and two in the supine group were excluded from the analysis due to the development of fetal distress prior the scheduled operation (left tilt group), an inadequate sensory block level that required general anesthesia and missing data in the records (supine group; Fig. 1). The characteristics of the remaining 77 participants are presented in Tables I-III. Age, BMI, pre-anesthetic CI, TPRI, SV, sensory block level, incidence of hypotension, ephedrine dose, neonatal weight and Apgar score showed no statistically significant differences between the supine and left tilt groups (Table I).
Figure 1.
Study subject analysis flow diagram.
Table I.
Characteristics of the participants sorted by post-anesthesia position.
| Post-anesthesia position | |||
|---|---|---|---|
| Characteristics | Supine (N=38) | Left tilt (N=39) | P-value |
| Age (years) | 33.47±3.95 | 32.85±4.60 | 0.523 |
| Height (cm) | 159.48±6.45 | 159.67±5.37 | 0.895 |
| Weight (kg) | 67.26±9.01 | 69.18±10.47 | 0.393 |
| BMI | 26.43±3.10 | 27.13±3.96 | 0.390 |
| CI | 3.97±0.55 | 4.00±0.57 | 0.864 |
| TPRI | 1902.34±321.45 | 1917.23±357.10 | 0.848 |
| SV | 85.21±14.16 | 82.44±15.56 | 0.417 |
| SBP (baseline, mmHg) | 122.37±15.38 | 122.00±14.90 | 0.915 |
| SBP (0 min, mmHg) | 118.21±19.52 | 117.62±16.47 | 0.885 |
| SBP (2 min, mmHg) | 106.92±26.12 | 98.41±19.33 | 0.108 |
| SBP (5 min, mmHg) | 101.68±25.26 | 92.00±19.32 | 0.062 |
| SBP (7 min, mmHg) | 102.32±19.06 | 97.54±15.96 | 0.236 |
| SBP (10 min, mmHg) | 103.39±17.02 | 103.44±16.22 | 0.991 |
| Ephedrine dose (mg) | 12.11±11.19 | 13.59±12.03 | 0.577 |
| Apgar 1 min | 7.97±0.28 | 7.95±0.51 | 0.792 |
| Apgar 5 min | 8.97±0.16 | 9.00±0.23 | 0.564 |
| Neonatal weight (g) | 3040.26±425.60 | 3098.79±402.03 | 0.537 |
| Sensory block T3 (n, %) | 3, 7.9 | 3, 7.7 | 0.435 |
| Sensory block T4 (n, %) | 31, 81.6 | 35, 89.7 | |
| Sensory block T5 (n, %) | 4, 10.5 | 1, 2.6 | |
Data are presented as the mean ± SD. The P-value was calculated using Student's t-test or Fisher's exact test, as appropriate. BMI, body mass index; CI, cardiac output index; TPRI, total peripheral resistance index; SV, stroke volume; SBP, systolic blood pressure.
Table II.
Characteristics of the participants sorted by experience of hypotension.
| Hypotension | |||
|---|---|---|---|
| Characteristics | No (N=37) | Yes (N=40) | P-value |
| Age (years) | 32.84±4.35 | 33.45±4.24 | 0.534 |
| Height (cm) | 159.80±6.35 | 159.38±5.51 | 0.756 |
| Weight (kg) | 65.78±8.45 | 70.50±10.43 | 0.033a |
| BMI | 25.73±2.74 | 27.76±3.96 | 0.011a |
| CI | 3.82±0.53 | 4.14±0.54 | 0.012a |
| TPRI | 1962.97±367.69 | 1860.78±304.06 | 0.187 |
| SV | 80.53±13.79 | 86.85±15.31 | 0.062 |
| SBP (baseline, mmHg) | 119.92±12.81 | 124.28±16.73 | 0.206 |
| SBP (0 min, mmHg) | 115.46±14.29 | 120.18±20.65 | 0.251 |
| SBP (2 min, mmHg) | 108.38±14.55 | 97.28±28.12 | 0.032a |
| SBP (5 min, mmHg) | 102.03±16.80 | 91.93±26.54 | 0.052 |
| SBP (7 min, mmHg) | 102.92±15.07 | 97.10±19.44 | 0.149 |
| SBP (10 min, mmHg) | 100.89±13.66 | 105.75±18.64 | 0.194 |
| Ephedrine dose (mg) | 8.92±9.66 | 16.50±12.10 | 0.003a |
| Apgar 1 min | 7.97±0.16 | 7.95±0.55 | 0.809 |
| Apgar 5 min | 9.00±0.00 | 8.98±0.28 | 0.570 |
| Neonatal weight (g) | 3068.92±344.13 | 3070.83±470.85 | 0.984 |
| Sensory block T3 (n, %) | 1, 2.7 | 5, 12.5 | 0.134 |
| Sensory block T4 (n, %) | 32, 86.5 | 34, 85.0 | |
| Sensory block T5 (n, %) | 4, 10.8 | 1, 2.5 | |
| Supine (n, %) | 18, 48.6 | 20, 50.0 | 0.906 |
| Left tilt (n, %) | 19, 51.4 | 20, 50.0 | |
Data are presented as the mean ± SD. The P-value was calculated using Student's t-test, χ2 test or Fisher's exact test, as appropriate.
aP<0.05. BMI, body mass index; CI, cardiac output index; TPRI, total peripheral resistance index; SV, stroke volume; SBP, systolic blood pressure.
Table III.
Characteristics of the participants sorted by level of ephedrine received.
| High dose ephedrine | |||
|---|---|---|---|
| No (N=43) | Yes (N=34) | P-value | |
| TPRI | 2009.95±377.05 | 1783.32±228.77 | 0.002a |
| Sensory block T3 (n, %) | 0, 0.0 | 6, 17.6 | 0.006a |
| Sensory block T4 (n, %) | 39, 90.7 | 27, 79.4 | |
| Sensory block T5 (n, %) | 4, 9.3 | 1, 2.9 | |
Data are presented as the mean ± SD. The P-value was calculated using Student's t-test or Fisher's exact test, as appropriate.
aP<0.05. TPRI, total peripheral resistance index.
Among the 77 participants, 40 (51.9%) developed hypotension after spinal anesthesia. Within the hypotension group, BMI, pre-anesthetic CI and ephedrine dosage values were significantly higher compared with those in the non-hypotension group (P=0.011, P=0.012 and P=0.003, respectively; Table II). Furthermore, 32 parturients (44.2%) met the criteria for high dose ephedrine usage. Therefore, the high dose ephedrine group exhibited a lower mean TPRI and higher mean sensory block level compared with the low dose ephedrine group (P=0.002 and P=0.006, respectively; Table III).
ROC curve analysis
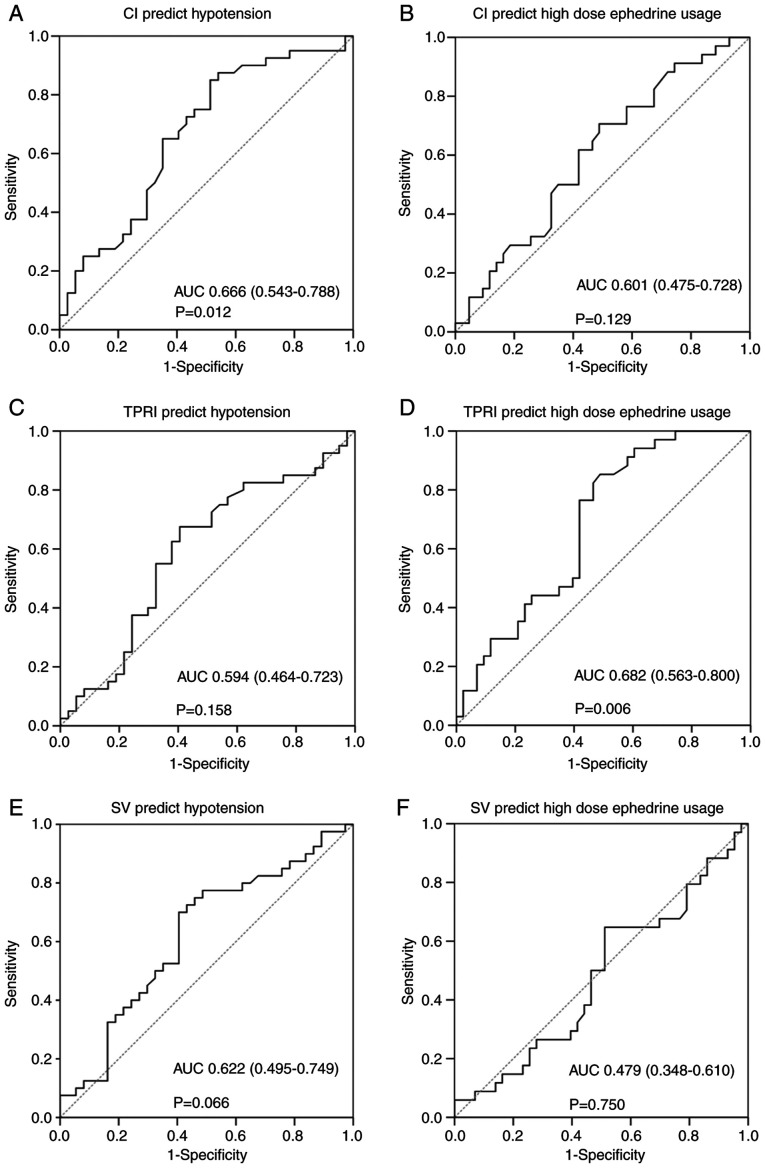
ROC curve analysis was performed to assess the diagnostic performance of pre-anesthetic CI, TPRI and SV in the detection of hypotension and high dose ephedrine usage. The results are presented in Table IV and Fig. 2. The AUCs of pre-anesthetic CI, TPRI and SV for hypotension prediction were 0.666, 0.594 and 0.622, respectively, with pre-anesthetic CI reaching statistical significance (P=0.012). In addition, the AUCs of pre-anesthetic CI, TPRI and SV for high dose ephedrine prediction were 0.601, 0.682 and 0.479, respectively, with pre-anesthetic TPRI also reaching statistical significance (P=0.006). The Youden's index was applied to determine the optimal cut-off values. Therefore, cut-off values of CI ≥3.68 (sensitivity=85%; specificity=48.6%; PPV=64.2%; NPV=75.0%) and TPRI ≥1,989 (sensitivity=85.3%; specificity=51.2%; PPV=58.0%; NPV=81.5%) were set for predicting hypotension and high dose ephedrine usage, respectively.
Table IV.
Receiver operating characteristic curve for CI, TPRI and SV as predictive indicators of post-spinal anesthesia hypotension and high dose ephedrine usage.
| A, Hypotension | ||||||
|---|---|---|---|---|---|---|
| AUC | 95% CI | Cut-off point | Sensitivity | Specificity | P-value | |
| CI | 0.666 | 0.543-0.788 | ≥3.68 | 0.850 | 0.486 | 0.012a |
| TPRI | 0.594 | 0.464-0.723 | ≥1926 | 0.675 | 0.595 | 0.158 |
| SV | 0.622 | 0.495-0.749 | ≥80.35 | 0.700 | 0.595 | 0.066 |
| B, High dose ephedrine | ||||||
| CI | 0.601 | 0.475-0.728 | ≥3.88 | 0.706 | 0.512 | 0.129 |
| TPRI | 0.682 | 0.563-0.800 | ≥1989 | 0.853 | 0.512 | 0.006a |
| SV | 0.479 | 0.348-0.610 | ≥79.4 | 0.647 | 0.488 | 0.750 |
aP<0.05. AUC, area under curve; 95% CI, confidence interval; CI, cardiac output index; TPRI, total peripheral resistance index; SV, stroke volume.
Figure 2.
Receiver operating characteristic curve for (A) baseline CI to predict post-spinal anesthesia hypotension, with the optimal cutoff point 3.68 using Youden's index. (B) Baseline CI to predict high dose ephedrine usage, with the optimal cutoff point 3.88. (C) Baseline TPRI to predict post-spinal anesthesia hypotension, with the optimal cutoff point 1926. (D) Baseline TPRI to predict high dose ephedrine usage, with the optimal cutoff point 1989. (E) Baseline SV to predict post-spinal anesthesia hypotension, with the optimal cutoff point 80.35. (F) Baseline SV to predict high dose ephedrine usage, with the optimal cutoff point 79.4. AUC, area under the curve; 95% confidence interval presented in parentheses; CI, cardiac output index; TPRI, total peripheral resistance index; SV, stroke volume.
Α Kaplan-Meier plot at the optimal cut-off point is shown in Fig. 3. The log-rank test was performed to evaluate the differences in hypotension occurrence time between the two groups. Mean hypotension occurrence time was 5.8 min with a 95% confidence interval (CI) of 4.8-6.8 min in parturients with CI ≥3.68 and 8.5 min (95% CI, 7.1-9.9 min) in parturients with CI <3.68. Pre-anesthetic CI was associated with hypotension (P=0.003, log-rank test), with an estimated hazard ratio of 1.862 (95% CI, 1.096-3.163; P=0.022) in the univariate analysis.
Figure 3.
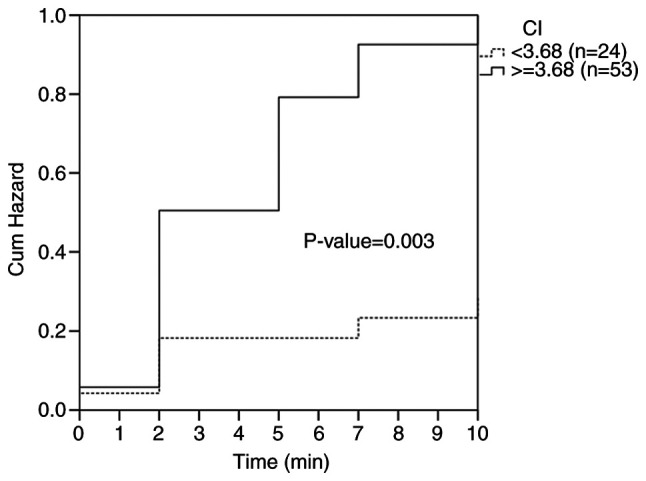
Development of hypotension during observation period according to Kaplan-Meier curve. Dotted line, CI <3.68; solid line, CI ≥3.68. CI, cardiac output index.
Multivariate logistic regression analysis
Using multivariate analysis, after adjusting for other confounding factors, BMI and CI were assessed for their association with risk of post-anesthetic hypotension. The hazard ratios were 1.120 and 2.122 for BMI and CI, respectively (P=0.040 and P=0.014). Furthermore, TPRI was independently associated with high dose ephedrine usage (odds ratio, 0.997; P=0.013). However, neither hypotension nor high dose ephedrine usage were associated with post-anesthetic position (Table V).
Table V.
Multivariate logistic regression for post-spinal anesthesia hypotension and high dose ephedrine usage.
| A, Regression analysis for hypotension | |||
|---|---|---|---|
| Factor | Hazard ratio | 95% CI | P-value |
| Supine position | 1.000 | ||
| Left tilt position | 0.891 | 0.467-1.700 | 0.725 |
| Age | 1.018 | 0.934-1.109 | 0.689 |
| BMI | 1.120 | 1.005-1.247 | 0.040a |
| Sensory block T3 | 1.000 | ||
| Sensory block T4 | 0.619 | 0.220-1.744 | 0.364 |
| Sensory block T5 | 0.082 | 0.008-0.855 | 0.037a |
| CI | 2.122 | 1.161-3.876 | 0.014a |
| Baseline SBP | 1.008 | 0.987-1.030 | 0.468 |
| B, Regression analysis for high dose ephedrine usage | |||
| Odds ratio | 95% CI | P-value | |
| Supine position | 1.000 | ||
| Left tilt position | 1.626 | 0.568-4.651 | 0.365 |
| Age | 0.987 | 0.862-1.130 | 0.849 |
| TPRI | 0.997 | 0.995-0.999 | 0.013a |
| Baseline SBP | 0.968 | 0.568-4.651 | 0.365 |
Cox proportional-hazards regression analysis for post-spinal hypotension and logistic regression analysis for high dose ephedrine usage.
aP<0.05. BMI, body mass index; CI, cardiac output index; SBP, systolic blood pressure; TPRI, total peripheral resistance index; 95% CI, confidence interval.
Discussion
Many attempts have been made to predict post-neuraxial anesthesia hypotension using PI, PVI or other hemodynamic parameters (5,15-17). PI represents a measure of peripheral perfusion, which is affected by the peripheral vascular tone. It has been reported that PI >3.5 is considered a fair predictor of post-spinal anesthesia hypotension (5,15). However, another study failed to replicate this result (16). This may be due to methodological differences between the two studies and factors that affect PI, such as anxiety and patient movement (16).
PVI is considered to be associated with intravascular volume and it has been proposed as a predictor of post-neuraxial anesthesia hypotension in some studies (3,17). However, one study contradicted this conclusion (16). The reliability of PVI in spontaneously breathing patients may be the greatest point of concern for this parameter. It has been demonstrated that heart rate variability is an effective indicator used to assess central nervous system autonomic function (4,18-20); however, due to the complexity of the analysis process the method is not widely applied in daily clinical practice (4).
The use of hemodynamic parameters obtained using thoracic electrical bioimpedance techniques to predict post-neuraxial anesthesia has been also reported (8). However, the effects of variations in body size and physical factors on electrical conduction have limited the clinical use of bioimpedance (21). Nevertheless, the accuracy and predictive value of the bioreactance-based NICOM™ device has been demonstrated during elective cesarean delivery under spinal anesthesia (9).
In the present study, the post-anesthetic hypotension rate was 51.9% and was consistent with that in previous reports (3,4,7). CI and BMI were considered independent predictors that could affect the risk of post-spinal anesthesia hypotension. Furthermore, a left tilt of 15 did not contribute to the risk for hypotension. Although former guidelines have suggested an immediate left tilt after spinal anesthesia (22), recent studies have reported no improvement in maternal and neonatal outcomes following a 15 left tilt (23). The results of the present study also supported this conclusion. Similar to previous studies, this study showed that a higher BMI was associated with a higher rate of post-spinal anesthesia hypotension under the same dosage (24,25). This may result from aortocaval compression and decreased cerebrospinal fluid volume (26,27).
The results of the present study also revealed that pre-anesthetic CI could be used to predict the risk of post-spinal anesthesia hypotension with an AUC of the ROC of 0.666 (95% CI, 0.543-0.788; P=0.012). Unlike a previous report (8), the results showed that TPRI had no predictive value on post-anesthetic hypotension. This difference may be associated with several factors, with the first being the differences in the patient group. For example, unlike the previous study that enrolled patients with both spinal and epidural anesthesia (8), in the present study only parturients who underwent spinal anesthesia were enrolled. However, whether the results could be extended to epidural anesthesia or combined spinal-epidural anesthesia remains unknown. The second factor is that the precision for spontaneous patient measurement between bioimpedance and bioreactance could contribute to the study results. It has been suggested that bioreactance is not affected by the precision of electrode placement or body movement during respiration (10). Thirdly, the time of baseline data acquisition and the types of fluid comprise could be another important issue. For example, the previous study obtained baseline data after hydration with 1,000 ml lactated Ringer's solution (8); however, in the present study only 750 ml 0.9% saline were used for pre-hydration. Pre-hydration affects the hemodynamic status (28). Therefore, the time of baseline data acquisition, before or after hydration, could affect the results. For the same reason, pre-hydration fluid with colloids or crystalloids and the volume of pre-hydration could also have an impact on the findings of the present study.
Consistent with a previous meta-analysis, a dose of 20 mg of ephedrine was considered as a high dose (29). Notably, rather than CI, TPRI was more closely associated with high dose ephedrine usage. It has been reported that ephedrine exhibits direct and indirect effects on the sympathetic system, and its responses are considered to be associated with the sympathetic tone (30-32). However, whether the baseline TPRI is an indicator of the sympathetic tone or not is another concern. The precise cause for the association between TPRI and high ephedrine requirement remains unknown and requires further research.
The present study has several limitations. Firstly, in this study, instead of a continuous blood pressure monitor, a non-invasive blood pressure monitoring device was used. Arterial cannulation is required for continuous blood pressure monitoring, which is considered unnecessary in clinical practice. Therefore, the participants were protected from additional risks. Secondly, the analysis was conducted for only 10 min following spinal anesthesia and not throughout the whole procedure. Massive blood loss and fluid shifting may occur during cesarean section (33) and may complicate the interpretation of the study results. Furthermore, there were no statistically significant differences between the hypotension and non-hypotension groups 5 min following spinal anesthesia due to timely treatment. Thirdly, the definition of hypotension could affect the cut-off point for the results. Hypotension was defined as a 20% decrease from the baseline systolic blood pressure, as previously reported (14). Fourthly, hemodynamic status is dynamic. In the current study, the baseline data were obtained within 15 min of hydration with 750 ml of 0.9% saline. Therefore, the current findings may be only applied to individuals subjected to the same protocol.
The present study demonstrated that baseline CI obtained via the bioreactance-based NICOM™ system could serve as a predictive indicator for post-spinal anesthesia hypotension in parturients regardless of the position of the patient. In addition, baseline TPRI could be used as a potential predictive indicator of poor ephedrine response. Therefore, the present anesthesia strategy was designed, including the prophylactic use of ephedrine or adjustment of pre-hydration volume according to the baseline hemodynamic data. This non-invasive method may provide guidance for individualized treatment or prophylaxis for parturients. However, further studies are required before the clinical application of the aforementioned findings.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
PHY and SET designed the study and collected the data. YJC analyzed the data. PHY and YJC prepared the initial manuscript, which was critically revised by SET. All authors read and approved the final manuscript for publication.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of Changhua Christian Hospital, protocol number 150605. Written informed consent were obtained from each parturient before inclusion.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Corke BC, Datta S, Ostheimer GW, Weiss JB, Alper MH. Spinal anaesthesia for caesarean section. The influence of hypotension on neonatal outcome. Anaesthesia. 1982;37:658–62. doi: 10.1111/j.1365-2044.1982.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 2.Chooi C, Cox JJ, Lumb RS, Middleton P, Chemali M, Emmett RS, Simmons SW, Cyna AM. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev. 2017;8(CD002251) doi: 10.1002/14651858.CD002251.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuwata S, Suehiro K, Juri T, Tsujimoto S, Mukai A, Tanaka K, Yamada T, Mori T, Nishikawa K. Pleth variability index can predict spinal anaesthesia-induced hypotension in patients undergoing caesarean delivery. Acta Anaesthesiol Scand. 2018;62:75–84. doi: 10.1111/aas.13012. [DOI] [PubMed] [Google Scholar]
- 4.Bishop DG, Cairns C, Grobbelaar M, Rodseth RN. Heart rate variability as a predictor of hypotension following spinal for elective caesarean section: A prospective observational study. Anaesthesia. 2017;72:603–608. doi: 10.1111/anae.13813. [DOI] [PubMed] [Google Scholar]
- 5.Toyama S, Kakumoto M, Morioka M, Matsuoka K, Omatsu H, Tagaito Y, Numai T, Shimoyama M. Perfusion index derived from a pulse oximeter can predict the incidence of hypotension during spinal anaesthesia for Caesarean delivery. Br J Anaesth. 2013;111:235–241. doi: 10.1093/bja/aet058. [DOI] [PubMed] [Google Scholar]
- 6.Ledowski T, Paech MJ, Browning R, Preuss J, Schug SA. An observational study of skin conductance monitoring as a means of predicting hypotension from spinal anaesthesia for caesarean delivery. Int J Obstet Anesth. 2010;19:282–286. doi: 10.1016/j.ijoa.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, He L, Ni JX. Level of sensory block after spinal anesthesia as a predictor of hypotension in parturient. Medicine (Baltimore) 2017;96(e7184) doi: 10.1097/MD.0000000000007184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouzounian JG, Masaki DI, Abboud TK, Greenspoon JS. Systemic vascular resistance index determined by thoracic electrical bioimpedance predicts the risk for maternal hypotension during regional anesthesia for cesarean delivery. Am J Obstet Gynecol. 1996;174:1019–1025. doi: 10.1016/s0002-9378(96)70343-5. [DOI] [PubMed] [Google Scholar]
- 9.Doherty A, Ohashi Y, Downey K, Carvalho JCA. Monitoramento não invasivo baseado na biorreatância revela instabilidade hemodinâmica significativa durante cesárea eletiva sob raquianestesia. Rev Bras Anestesiol. 2011;61:326–332. doi: 10.1016/S0034-7094(11)70038-1. (In Portugese) [DOI] [PubMed] [Google Scholar]
- 10.Keren H, Burkhoff D, Squara P. Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. Am J Physiol Heart Circ Physiol. 2007;293:H583–H589. doi: 10.1152/ajpheart.00195.2007. [DOI] [PubMed] [Google Scholar]
- 11.Jakovljevic DG, Trenell MI, MacGowan GA. Bioimpedance and bioreactance methods for monitoring cardiac output. Best Pract Res Clin Anaesthesiol. 2014;28:381–394. doi: 10.1016/j.bpa.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Bonica JJ, Kennedy WF, Akamatsu TJ, Gerbershagen HU. Circulatory effects of peridural block: 3. Effects of acute blood loss. Anesthesiology. 1972;36:219–227. doi: 10.1097/00000542-197203000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Shin W. How to do random allocation (randomization) Clin Orthop Surg. 2014;6:103–109. doi: 10.4055/cios.2014.6.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klöhr S, Roth R, Hofmann T, Rossaint R, Heesen M. Definitions of hypotension after spinal anaesthesia for caesarean section: Literature search and application to parturients. Acta Anaesthesiol Scand. 2010;54:909–921. doi: 10.1111/j.1399-6576.2010.02239.x. [DOI] [PubMed] [Google Scholar]
- 15.Duggappa DR, Lokesh MPS, Dixit A, Paul R, Raghavendra Rao RS, Prabha P. Perfusion index as a predictor of hypotension following spinal anaesthesia in lower segment caesarean section. Indian J Anaesth. 2017;61:649–654. doi: 10.4103/ija.IJA_429_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokose M, Mihara T, Sugawara Y, Goto T. The predictive ability of non-invasive haemodynamic parameters for hypotension during caesarean section: A prospective observational study. Anaesthesia. 2015;70:555–562. doi: 10.1111/anae.12992. [DOI] [PubMed] [Google Scholar]
- 17.Sun S, Huang SQ. Role of pleth variability index for predicting hypotension after spinal anesthesia for cesarean section. Int J Obstet Anesth. 2014;23:324–329. doi: 10.1016/j.ijoa.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Chamchad D, Arkoosh VA, Horrow JC, Buxbaum JL, Izrailtyan I, Nakhamchik L, Hoyer D, Kresh JY. Using heart rate variability to stratify risk of obstetric patients undergoing spinal anesthesia. Anesth Analg. 2004;99:1818–1821. doi: 10.1213/01.ANE.0000140953.40059.E6. [DOI] [PubMed] [Google Scholar]
- 19.Hanss R, Bein B, Ledowski T, Lehmkuhl M, Ohnesorge H, Scherkl W, Steinfath M, Scholz J, Tonner PH. Heart rate variability predicts severe hypotension after spinal anesthesia for elective cesarean delivery. Anesthesiology. 2005;102:1086–1093. doi: 10.1097/00000542-200506000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Prashanth A, Chakravarthy M, George A, Mayur R, Hosur R, Pargaonkar S. Sympatho-vagal balance, as quantified by ANSindex, predicts post spinal hypotension and vasopressor requirement in parturients undergoing lower segmental cesarean section: A single blinded prospective observational study. J Clin Monit Comput. 2017;31:805–811. doi: 10.1007/s10877-016-9906-9. [DOI] [PubMed] [Google Scholar]
- 21.Engoren M, Barbee D. Comparison of cardiac output determined by bioimpedance, thermodilution, and the fick method. Am J Crit Care. 2005;14:40–45. [PubMed] [Google Scholar]
- 22. NICE, NIfHaCE: Clinical guidelines and updates: Caesarean section. Available at: https://www.nice.org.uk/guidance/cg132/chapter/1-guidance. Accessed Feb 10, 2020. [Google Scholar]
- 23.Lee AJ, Landau R, Mattingly JL, Meenan MM, Corradini B, Wang S, Goodman SR, Smiley RM. Left lateral table tilt for elective cesarean delivery under spinal anesthesia has no effect on neonatal acid-base status: A randomized controlled trial. Anesthesiology. 2017;127:241–249. doi: 10.1097/ALN.0000000000001737. [DOI] [PubMed] [Google Scholar]
- 24.Nani FS, Torres ML. Correlation between the body mass index (BMI) of pregnant women and the development of hypotension after spinal anesthesia for cesarean section. Rev Bras Anestesiol. 2011;61:21–30. doi: 10.1016/S0034-7094(11)70003-4. [DOI] [PubMed] [Google Scholar]
- 25.Wang HZ, Chen HW, Fan YT, Jing YL, Song XR, She YJ. Relationship between body mass index and spread of spinal anesthsia in pregnant women: A randomized controlled trial. Med Sci Monit. 2018;24:6144–6150. doi: 10.12659/MSM.909476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaiser R. Anesthetic considerations in the obese parturient. Clin Obstet Gynecol. 2016;59:193–203. doi: 10.1097/GRF.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 27.Roofthooft E, Van de Velde M. Low-dose spinal anaesthesia for Caesarean section to prevent spinal-induced hypotension. Curr Opin Anaesthesiol. 2008;21:259–262. doi: 10.1097/ACO.0b013e3282ff5e41. [DOI] [PubMed] [Google Scholar]
- 28.Guinot PG, Bernard E, Defrancq F, Petiot S, Majoub Y, Dupont H, Lorne E. Mini-fluid challenge predicts fluid responsiveness during spontaneous breathing under spinal anaesthesia: An observational study. Eur J Anaesthesiol. 2015;32:645–649. doi: 10.1097/EJA.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 29.Lee A, Ngan Kee WD, Gin T. A dose-response meta-analysis of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for elective cesarean delivery. Anesth Analg. 2004;98:483–490. doi: 10.1213/01.ane.0000096183.49619.fc. [DOI] [PubMed] [Google Scholar]
- 30.Zaimis E. Vasopressor drugs and catecholamines. Anesthesiology. 1968;29:732–762. doi: 10.1097/00000542-196807000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Alsufyani HA, Docherty JR. Direct and indirect effects of ephedrine on heart rate and blood pressure in vehicle-treated and sympathectomised male rats. Eur J Pharmacol. 2018;825:34–38. doi: 10.1016/j.ejphar.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 32.Xia J, Yuan J, Lu X, Yin N. Prone position results in enhanced pressor response to ephedrine compared with supine position during general anesthesia. J Clin Anesth. 2016;31:94–100. doi: 10.1016/j.jclinane.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Duthie SJ, Ghosh A, Ng A, Ho PC. Intra-operative blood loss during elective lower segment caesarean section. Br J Obstet Gynaecol. 1992;99:364–367. doi: 10.1111/j.1471-0528.1992.tb13749.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.