Abstract
Over 16 million cases worldwide, severe acute respiratory syndrome coronavirus 2 has profoundly affected healthcare as we know it. Given reports of gastrointestinal involvement and viral shedding in the stool, it is unsurprising there are concerns that endoscopic equipment may be a potential vector of viral transmission. Here, we provide an overview of existing practices for endoscope reprocessing, recent developments in the field, and challenges in the COVID-19 environment. Current multi-society guidelines do not advise any change to endoscope disinfection protocols but emphasize strict adherence to recommended practices. However, endoscopy reprocessing staff may benefit from supplemental personal protective equipment measures, especially in high risk situations. Because thorough endoscope reprocessing is highly operator dependent, adequate training of personnel is critical for proper manual cleaning and disinfection of endoscopes that have potential to harbor virus. Bacterial contamination of duodenoscopes has caused outbreaks of infection from multidrug-resistant organisms, highlighting vulnerable areas. The emphasis of current studies is on optimization of disinfection and drying, minimization of simethicone use, and on quality control of endoscope reprocessing with sampling and microbiological culturing. Recent advances include novel approaches to endoscope sterilization, infection barrier methods, and design of partially or fully disposable duodenoscopes. Overall, the available data indicate that, when correctly executed, current reprocessing practices are sufficient in preventing SARS-COV-2 transmission.
Keywords: COVID pandemic, Endoscopic disinfection, Disposable endoscope, Duodenoscope-related infection, PPE
COVID-19 Pandemic Impact on Endoscopy Units
The coronavirus disease 2019 (COVID-19) pandemic imposed a severe burden on endoscopy units all over the world. A March 2020 multicenter survey from northern Italy revealed near universal exposure to COVID-19 positive patients in endoscopy units: 97.6% experienced a reduction in endoscopic activities and 50% performed endoscopic procedures on COVID-19 positive patients.1 In addition to droplet and airborne transmission, contamination with fomites and subsequent self-inoculation of the nose, eyes, and mouth is considered a contributor to high rates of viral transmission.2 Person to person transmission of COVID-19 or severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) in the healthcare setting has been described: a 1% rate of suspected and 0.12% of confirmed COVID-19 infection after endoscopic procedures was reported in a recent survey.3 , 4
Human coronaviruses can remain infectious on plastic surfaces for up to 9 days at room temperature. SARS-COV-2 was detected on polyvinyl chloride, silicone, rubber, and Teflon surfaces for up to 5 days at room temperature, with a similar initial inoculum titer across varying media types.5 Available data on surface stability of SARS-COV-2 are consistent with prior studies on human coronaviruses.6 While specific data regarding the transmissibility of coronavirus from inanimate objects to hands via touch are lacking at this time, prior modeling of influenza A and parainfluenza noted transmissibility rates of 31.6% and 1.5% of viral load, respectively, underscoring the potential of fomite transmission.7 , 8 Furthermore, real-world environment and surface testing demonstrated virus-positive samples from various areas within a COVID-19 patient room, highlighting the potential for exposure that staff may encounter.9
Gastrointestinal (GI) complains are frequent among COVID-19 patients, with 7.4% rate of diarrhea and 4.6% of nausea and vomiting.10 Several studies demonstrated that viral RNA could be detected in small and large intestinal biopsies of human coronavirus patients and in stool of COVID-19 patients with or without GI symptoms.10, 11, 12, 13, 14 Clinical implications of these findings on viral transmissibility through the GI tract are not fully understood; despite presence of viral RNA in the stool, viable virus was not isolated in a recent detailed virological assessment of hospitalized COVID-19 patients.15
Given the evidence of GI tract involvement and the resiliency of the SARS-COV-2 virus on inanimate surfaces, it is unsurprising that concerns have been raised about endoscopic equipment being a potential vector of viral transmission. Here, we discuss best practice guidelines on endoscope reprocessing, recent developments in the field, and current challenges in the COVID-19 pandemic environment.
Current State of Endoscope Reprocessing
Current multisociety best practice guidelines (endorsed jointly by AGA, ASGE, ACG, SGNA, ASCRS, and SAGES) advise no changes to established endoscope reprocessing procedures during the COVID-19 pandemic.16 Based on available evidence, the standard protocol including bedside precleaning followed by leak testing, manual cleaning and high-level disinfection (HLD) is sufficient in eradicating SARS-COV-2.16, 17, 18 Common chemical liquid disinfectants utilized in HLD of flexible endoscopes, including varying combinations and concentrations of glutaraldehyde, ortho-phthalaldehyde, peracetic acid, and hydrogen peroxide are virucidal for SARS-COV-2.19 , 20 The US Environmental Protection Agency recently issued an updated list of chemical disinfectant products for various pathogens including SARS-COV-2.20
Historically, the risk of viral transmission related to endoscopy has been rare to nonexistent.21 , 22 Previous reports of hepatitis C transmission were due to nonadherence to aseptic techniques in drug administration, lapses in reprocessing protocols, and failure to adequately disinfect reusable biopsy forceps.21 , 22 Multicenter prospective studies of endoscopic procedures on hepatitis C carriers or hepatitis B seropositive patients did not demonstrate post-procedural transmission following appropriate disinfections protocols.23 , 24 To date, there have been no reported cases of endoscope-associated SARS-COV-2 transmission.
Based on the Spaulding classification schema for disinfection of medical equipment, flexible endoscopes are “semi-critical” devices and should at minimum undergo HLD.21 HLD is achieved by complete immersion of the endoscope and associated removable parts in an approved chemical disinfectant at a specified temperature and duration. HLD is defined as a 6-log reduction of Mycobacteria and ensures that devices are essentially free of all microorganisms, apart from low-level bacterial spores that do not increase transmission risk.25 Certain devices in endoscopic procedures that are in contact with sterile tissues or anatomical spaces, such as sphincterotomes and biopsy forceps are classified as critical devices and must be sterile.22
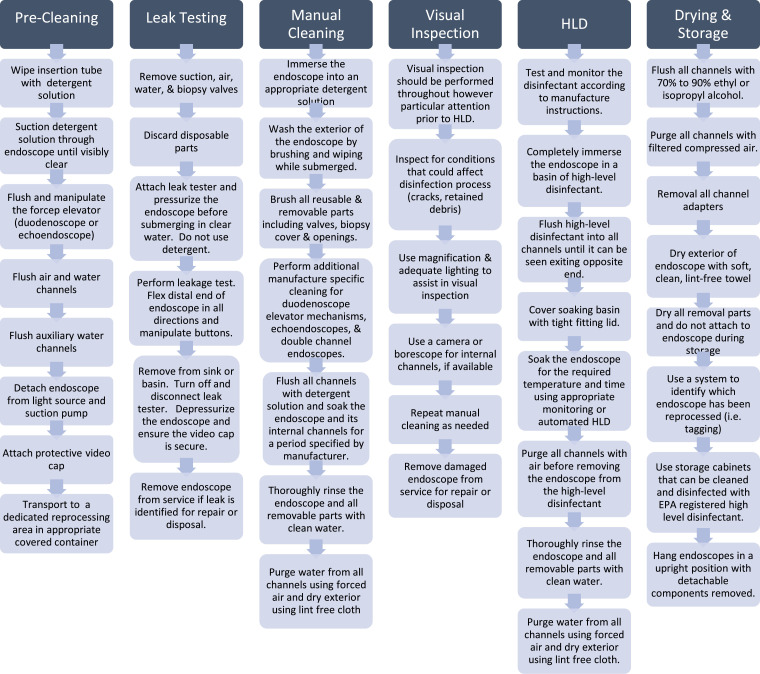
Endoscope reprocessing is a multistep sequential process, with HLD as its cornerstone. The standard protocol for endoscope reprocessing is detailed in Figure It is impossible to overemphasize manual cleaning as the most critical step in disruption of biofilm and removal of microbial burden. Manual cleaning is performed using a detergent solution and meticulous cleaning with appropriate brushes of endoscope interior, exterior, and working channel. FDA and manufacturer-specified instructions should be followed for disinfection of elevator mechanisms and double channel endoscopes.22 , 25
Figure 1.
Endoscope reprocessing summary
Because thorough endoscope reprocessing is highly operator dependent, extensive training of staff is critical for effective infection control. Along with general infection control measures within the endoscopy unit, validation of processes, and competency should be assessed per institutional policy at least annually. The recent ASGE Infection Control Summit which included representatives of main stakeholders in the field (researchers, regulatory agencies and manufacturers), emphasized the importance of developing evidence-based curricula for reprocessing staff training and supported the FDA proposal of standardizing duodenoscope durability testing.67 In the current COVI-19 pandemic, recommendations include limiting the number of reprocessing staff to those most experienced and to consider additional training sessions to reinforce existing protocols.16
Adequate personal protection of staff members is critical for endoscope disinfection, which requires handling and manipulation of flexible endoscopes that are heavily contaminated with bodily fluids including stool, mucosal secretions and blood—all of which have demonstrated potential to harbor virus. A recent prospective single-center study highlighted the significance of unrecognized face splashes, reporting that nearly half of postprocedure face shields were positive for bacterial colony forming units, thus highlighting the importance of appropriate personal protective equipment (PPE) for all personnel.26 Current multisociety best practice guidance advises that during the COVID-19 pandemic, reprocessing staff should continue to don appropriate PPE including gloves, gown, and face shields; a N95 respirator is recommended if available (Table 1 ).16 Use of a N95 respirator, especially in high-risk situations, is supported by the data from recent viral inoculum studies on SARS-COV-2 aerosolization, which estimated a half-life of aerosolized SARS-COV-2 of 1.1-1.2 hours.6
Table 1.
PPE for reprocessing staff.
|
PPE for reprocessing staff | |
| CLOTHING | Medical scrubs |
| HAIR | Scrub cap or bouffant cap |
| EYES | Goggles or face shield |
| FACE | Water resistant mask or large drape face shield |
| RESPIRATORY | N95 respiratory OR FFP2 equivalent* |
| TORSO | Water resistant gown |
| HANDS | Gloves (long sleeve if available) |
| FEET | Shoe covers (bootleg covers if available) |
if available.
The joint ANS/AAMI ST91 comprehensive guide to endoscope reprocessing recommends that reprocessing personnel should change into scrubs upon arrival to work and should have ready access to clean scrubs.18 All personal accessories such as jewelry and watches should be removed. The appropriate hand hygiene, and donning and doffing of PPE should be performed in a separate dedicated area as outlined in the accompanying video27, 28, 29 (Video).
Social distancing should be practiced within the reprocessing areas as well as frequent cleaning of all surfaces using chemical agents known to inactivate SARS-COV-2. As more data become available regarding seroconversion and viral immunity, deploying seroconverted staff in high-risk scenarios may be preferable, including in endoscope reprocessing areas.30
Challenges in Endoscope Reprocessing
FDA supplemental measures to reduce infection transmission
In August 2015, in response to multiple multidrug resistant organism (MDRO) infections linked to duodenoscope use, the Food and Drug Administration (FDA) strongly recommended that all endoscopy units adopt a series of supplemental measures to reduce infection transmission.31 FDA recommendations included adoption of one or more of the following measures: culture surveillance of endoscopes, Ethylene oxide (EtO) sterilization, use of a liquid chemical sterilant processing system, and/or repeat HLD. FDA recommendations led to rapid changes in endoscope reprocessing practices in the United States. Based on a recent survey of 249 endoscopy units,32 most centers had implemented at least one of the supplemental measures; the most common was repeat HLD (63%) followed by culture surveillance (53%).
Repeat HLD was proposed by the FDA as a logical next step in the effort to mitigate infection transmission risk. In practice, it is unclear if this recommendation achieves the desired goal. The FDA did not provide clear guidance on what is double HLD: whether 2 cycles of manual cleaning each combined with HLD versus a single cycle of manual cleaning followed by 2 rounds of HLD constituted “double HLD”; with most units adopting the easier latter approach. It could be argued that the second manual cleaning is a crucial step and provides the most benefit due to mechanical biofilm disruption.
Two recent randomized prospective trials comparing single versus double HLD, both using single cycle of manual cleaning, failed to show difference in postreprocessing endoscope culture positivity rate. In a prospective trial at four endoscopy units, Bartles et al randomized 45 duodenoscopes and linear echoendoscopes to single or double HLD.33 In total, 5850 specimens were obtained from 2925 encounters. Eight specimens from 5 scopes were positive for high-risk organisms; 3 of the scopes came from the double HLD group. All 8 positive cultures had been obtained from the elevator mechanism samples. The investigators found no significant difference between single and double HLD, even when stratified for culture positive growth, facility, sample type, or time from cleaning to culture (taking into account weekends and holidays).
Snyder et al performed a single-center randomized prospective trail comparing single HLD, double HLD, and single HLD with EtO sterilization.34 The primary and secondary outcome were MDRO culture-positivity after reprocessing, and reprocessing failure rate with cultures positive for aerobic bacteria, respectively. The trial was terminated early due to an insufficient number of events to assess primary outcome; there was no difference in the secondary outcome. Both trials illustrate significant challenges in conducting randomized studies to evaluate the effectiveness of current reprocessing protocols due to the rarity of endoscope reprocessing failure events. Of note, to our knowledge, there is no published data comparing the effectiveness of single versus double manual cleaning; however, the latter approach is significantly more labor and time intensive.
In August 2019, the FDA instructed endoscopy units “to initiate quality control program that includes sampling, microbiological culturing and other monitoring methods”.35 Data on the effectiveness of current endoscope culturing protocols is limited. Higa et al reported the results of the "culture and quarantine" program for duodenoscopes using an extensive bacteria culturing surveillance protocol from 2014 to 2017.36 A 0.7% culture-positive rate for high-concern organisms was reported from a total of 4307 samples. Their surveillance program resulted in the identification and withdrawal of 2 high-frequency positive culture duodenoscopes from circulation. However, quarantine alone did not appear to reduce the overall rate of infection.36 Instead, the authors attributed the decline in positive culture rate to the optimization of cleaning practices.
There is limited clinical evidence regarding liquid chemical sterilant and EtO use as an adjunct to current HLD practices. The use of liquid chemical sterilants is restricted by the heat sensitivity of endoscopes, though liquid chemical sterilization is believed to cause less endoscope wear and tear than EtO sterilization. Some agents used in HLD can also be utilized as sterilizing agents, however, the sterilization protocol and the required duration of treatment are prohibitive.37 A recent single-center randomized controlled trial comparing dual HLD (two cycles of manual cleaning with HLD) versus liquid chemical sterilization (Steris 1E system) demonstrated no difference in postreprocessing culture positivity rates.68 EtO sterilization successfully prevented recurrence of positive MDRO culturing at the site of one of the original outbreaks.38 A review of cases worldwide reported that 6 of 23 Carbapenem-Resistant Enterobacteriaceae or MDRO-related outbreaks were treated by adding EtO sterilization; this measure successfully terminated 3 of those 6 outbreaks.39 However, there are multiple barriers to widespread use of EtO including cost, potential toxicity to reprocessing staff, and possible damage to endoscopes.
Attention has also turned to endoscope channel wear and tear as a potential nidus of infection. Borescope inspection, a process used for endoscope repair, was proposed to evaluate endoscope channels for occult damage that can harbor bacteria. A borescope is a cylindrical tool with a light source that can be advanced forward and retrograde in the working channel for detailed inspection. Several studies have attempted to better characterize the correlation between borescope findings and endoscope postreprocessing bacterial growth. These studies presented quantitative data obtained from cultures and ATP bioluminescence, an assay that measures ATP, a surrogate marker for organic matter and a potential substrate for microbial growth.40, 41, 42, 43, 44 Ofstead et al showed that a significant amount of organic residue remains in the working channel; approximately half of examined endoscopes had residual liquid; common skin and GI flora bacterial species were frequently found. Damage to the working channel, persistent debris, and residual fluid were identified on borescope examination in this study, which were not apparent on visual inspection.40
Effects of drying protocols on biofilm formation
Another approach to reducing infection risk is optimization of drying protocols. Biofilm formation is a key factor in persistent bacterial contamination of endoscopes, and residual moisture facilitates bacterial growth. Routine wear and tear has also been implicated: damaged surfaces in the endoscope could provide a potential space for biofilm development. While endoscope reprocessing guidelines emphasize the importance of thoroughly drying the endoscope, there is limited data on the optimal dryness threshold and best methods to achieve it.45 , 46
A recent study45 compared the efficacy of standard drying cabinets commonly used in the United States for endoscope storage versus automated drying cabinets. Automated drying yielded dry inner channels after 1 hour versus 24 hours of storage in a standard cabinet. After 48 hours of drying in automated cabinet, the Pseudomonas aeruginosa inoculated endoscopes had 7 log and 9 log fewer recovered organisms from colonoscopes and duodenoscopes respectively; in contrast, standard drying cabinets allowed bacterial growth.45
To identify the optimum time for endoscope drying, Barakat et al46 compared a manual air-drying protocol versus 5-minute and 10-minute automated drying. Extending the automated drying time to 10 minutes yielded the best results: zero droplets were identified on borescope examination at 48 and 72 hours post-drying. Automated drying was also associated with lesser ATP bioluminescence values in the endoscope working channel. Of significant concern were the study findings of “pools of liquid” on borescope examination after standard reprocessing with an alcohol flush and a 1-minute air purge. Interestingly, vertical versus coiled endoscope storage did not affect residual channel fluid content in the automated drying group. In the manual drying group, vertical storage yielded fewer water droplets.46
Use of simethicone
Simethicone is a commonly used additive in endoscopy to remove bubbles from the mucosal surface. It is often mixed in water irrigation bottle or administered via a syringe during the procedure and significantly improves visualization.47 However, all three major endoscope manufacturers recommend against the use of simethicone in endoscopy as it can provide a potential source for bacterial growth. Simethicone is water and alcohol insoluble, making its removal challenging. If necessary, administration with a syringe at the lowest concentration possible is recommended. Several studies described residual simethicone in endoscopes after following manufacturer recommended reprocessing protocols.48, 49, 50 The impact of simethicone use on water droplet retention and residual biomatter was investigated in recent study49 using borescope inspection of the working channel and ATP bioluminescence measurement. The mean volume of simethicone solution used per procedure was higher when added to the irrigation water bottle versus injection via a syringe. Use of medium/high concentrations of simethicone resulted in retention of more fluid droplets and greater ATP bioluminescence values in the working channel. In this study the endoscopes that underwent a second reprocessing cycle retained no fluid droplets and had ATP bioluminescence values comparable to water use alone. This finding supports the manufacturer (Olympus America, Inc.) recommendations of 2 HLD cycles for endoscopes exposed to simethicone.49
Novel Approaches to Endoscope Reprocessing
The FDA required postmarket surveillance studies from all 3 major endoscope manufacturers to assess the “real-world” failure rate of endoscope reprocessing and to determine if reprocessing personnel can adhere to manufacturers’ reprocessing instructions. The 2019 findings demonstrated unacceptably high postreprocessing contamination rates ranging from 4% to 6% and the failure to perform critical steps of manual cleaning in almost one-third of participants.51 The inherent limitations of current endoscope reprocessing practices, highlighted by postmarketing studies, led to significant advances in both disinfection protocols and infection barrier methods, and triggered the development of novel partially and fully disposable endoscopes.
Endoscope sterilization
Low temperature plasma-activated gas (PAG) may represent a promising new method for sterilization. PAG was shown to have antimicrobial effects on a wide range of organisms including spores, fungi, and drug-resistant bacteria due to the production of ultraviolet light and free radicals. High frequency voltage applied at atmospheric pressure to gases such as hydrogen, nitrogen, or inert gases generates reactive oxygen and nitrogen species; these interact with the surface of interest without impacting its bulk properties. While this method has not been used in endoscopy to date, it has been commercially available since the 1990s and utilized for sterilization of total joint replacement components.52
A recent proof-of-concept bench study53 showed that application of argon PAG for 9 minutes resulted in dispersal of 48-hour biofilms without regrowth. Importantly, when the current was turned off, ozone concentration fell to less than detectable levels in 30 seconds. Finally, no evidence of structural damage was seen on electron microscopy after PAG exposure.53
Argon plasma is readily available and has already been FDA-approved in GI procedures for coagulation purposes. The plasma generating apparatus is housed in a compact (10 × 15 × 8 inch) box and reasonably cost-effective (<$2000). Another potential benefit is shortening of processing time by obviating the need for rinsing and drying after HLD. While argon PAG is a promising new method for endoscope disinfection, a commercial device tailored to GI use will need to be developed and studies performed with commonly used endoscopes to establish comparability to current HLD and sterilization techniques.52 , 53
Barrier method
An “endoscopic contamination prevention sheath” presents an alternative barrier approach to decreasing endoscope contamination, focusing particularly on the elevator mechanism. Scopeseal (GI Scientific, LLC) is a single-use novel infection control device designed to protect the tip of the duodenoscope while preserving functionality.54 Approved in October 2019 by the FDA for use with the Olympus TJF-Q180V duodenoscope as well commonly used accessories up to 10.7 Fr in diameter, it consists of 3 main components: an optical lens covering the camera, a port sealing the washing and insufflation nozzle, and the proprietary Working Channel Extension. The Working Channel Extension is a flexible, thin-walled catheter extending from the inside of a biopsy channel and traversing just beyond the elevator. The device bypasses the vulnerable space around the elevator completely, thus avoiding contamination during ERCP and post-procedure flushing of the biopsy channel.
Scopeseal's utility was recently demonstrated in a bench study;55 the integrity of its barrier function was tested with bacterial inoculum to outside of the duodenoscope and to the elevator mechanism, evaluating both outside-in and inside-out contamination protection. The ERCP accessories were also evaluated in a fluorescent dye-immersion test for any inadvertent exposure. No bacterial or inadvertent dye exposure were detected confirming compete seal with two-way protection from microbial contamination.55
Thus far, there have been no reports of the use of Scopeseal in real-life clinical scenarios. It may be a viable option in the category of disposable duodenoscope accessories, but studies will need to evaluate its comparable or improved cost-effectiveness vis-à-vis disposable tip duodenoscopes.
Partially and fully disposable duodenoscopes
In 2019, the FDA issued several safety communications recommending that endoscopy units transition from fixed-end duodenoscopes to those with partially or completely disposable components.35 , 56 As of August 2020, the FDA has cleared 6 such duodenoscope models. All 3 of the major endoscope manufacturers have produced models with variations on a disposable distal tip (Table 2 ).
Table 2.
Characteristics of disposable duodenoscopes.
|
EvisExera III TJF-Q190V (Olympus) |
ED34-i10T (Pentax) |
ED34-i10T2 (Pentax) |
ED-580XT (Fujifilm) |
EXALT Model D (Boston Scientific) |
aScopeDuodeno (Ambu) |
|
| Disposable component | Endcap | Endcap | Endcap | Endcap | Entire endoscope | Entire endoscope |
| Field of view (degrees) | 100 | 100 | 100 | 100 | 108 | 130 |
| Depth of view (mm) | 5-60 | 4-60 | 4-60 | 4-60 | 5-60 | Not available |
| Working length (mm) | 1240 | 1250 | 1250 | 1250 | 1240 | 1240 |
| Instrument channel (mm) | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 |
| Insertion tube diameter (mm) | 11.3 | 11.6 | 11.6 | 11.3 | 11.3 | 11.3 |
| Distal end diameter (mm) | 13.5 | 13 | 13 | 13.1 | 15.1 | 13.7 |
| Distal end with endcap (mm) | 13.5 | 13.8 | 13.4 | 14.9 | 15.1 | 13.7 |
The ED34-i10T model (Pentax Medical, USA) was the first disposable endcap duodenoscope cleared for use by the FDA.57 Since then, it has been largely replaced by the ED34-i10T2 which consists of a disposable endcap with an elevator.58 This current model is the subject of ICECAP, an ongoing Canadian randomized controlled trial investigating the persistent bacterial contamination rate and therapeutic efficacy of ED34-i10T2. Eligible patients will be allocated to ERCP with the ED34-i10T2 model or its previous iteration, ED 34-i10T. Results of the study are pending at this time.59
The ED-580XT model (Fujifilm Healthcare USA) with a disposable distal endcap60 was recently studied61 to verify if a detachable endcap indeed decreases bacterial contamination post-HLD by evaluating ATP bioluminescence and cultures at 72 hours. No endoscopes in the disposable endcap group were found positive for bacterial culture while one device in the fixed endcap group was positive for coagulase-negative Staphylococcus. The authors also validated a threshold value for ATP bioluminescence, determining that<40 RLU corresponded to the absence of positive cultures in both endoscope groups.61
Finally, EvisExera III TJF-Q190V (Olympus America, Inc.) is a recently developed model with a disposable endcap which must be removed by tearing across a predetermined seam, preventing reuse.62
The EXALT Model D (Boston Scientific) duodenoscope is of particular interest to the GI community as the first fully disposable duodenoscope. It was approved in December 2019 by the FDA.63 A recent bench study64 compared the utility of the EXALT Model D to 3 reusable duodenoscopes (Olympus Q180V, Pentax ED-3470TK, Fujifilm ED-530XT) in an anatomic model. Four tasks were designed to simulate clinical use: guidewire locking with elevator, placement and removal of a plastic stent, placement and removal of a metal stent, and basket sweeping. Endpoints of interest included the following: the ability to complete all 4 tasks, time for completion of each task, ratings on navigation/pushability by 6 experienced endoscopists, tip control, and image quality.64 All tasks were completed in both the reusable and disposable endoscope groups. There were no significant differences in the time-to-completion of the tasks. However, the performing endoscopists did rate the EXALT Model D lower on navigation/pushability. Overall, the disposable model had comparable functionality, although maneuverability may have been impacted.64 Data on clinical applications of single-use duodenoscope is limited, a recent multi-center case study reported on 58 ERCP procedures of various complexity; nearly all of the procedures could be performed entirely with single-use duodenoscope.69 A randomized trial of single-use versus reusable duodenoscopes in low complexity ERCP procedures demonstrated comparable technical performance of single-use duodenoscope with significantly lower number of cannulation attempts but with worse scores on maneuverability, image stability, and air-water button functionality.70 As the first fully disposable duodenoscope, its projected costs are also an important factor in potential replacement of currently used instruments. A recent cost-benefit analysis suggested that replacing one reusable duodenoscope in a center performing approximately 200 ERCPs/year would incur a 10x increase in cost.65
The most recent addition to the field is AScopeDuodeno (Ambu Inc.), which was approved by the FDA for marketing in July 2020.66 Similar to the EXALT Model D, this is a combination of a disposable duodenoscope and reusable processor. Currently, there are no available reports regarding its performance in clinical practice, and postmarketing studies are underway.
Future Directions
The coronavirus pandemic has brought new and rapidly evolving challenges throughout the medical field. Evidence of nosocomial transmission in healthcare settings has enhanced attention to both occupational safety and appropriate disinfectant practices, including in the endoscopic unit. In the COVID-19 pandemic, it is even more important for endoscope reprocessing personnel to have adequate protective equipment. Historical data concerning pathogen transmission in endoscopic procedures emphasize the importance of strict adherence to regimented protocols in maintaining the integrity of endoscope reprocessing. Furthermore, recent public health experiences have highlighted the challenges and shortcomings of existing practices and spurred innovative approaches to obviate them. A number of unanswered questions remain, first and foremost about “real world” effectiveness of novel methods of endoscope reprocessing and of endoscopes with disposable components. Where the balance lies between the risks, albeit quite low, of endoscope associated postprocedural infection and the cost (and environmental impact) of the disposable devices, remains to be determined. Overall, prior experience and available data suggest that, when correctly executed, current reprocessing practices are sufficient in preventing SARS-COV-2 transmission.
Acknowledgments
Special thanks to Jimmy Leal, central services technician, for his demonstration of appropriate donning of PPE.
Footnotes
Conflicts of interest Dr Reicher is a consultant for Boston Scientific. Dr Chua and Dr Halim have no conflicts of interest to disclose.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tige.2020.10.001.
Appendix. Supplementary materials
Video: PPE donning for endoscope reprocessing.
References
- 1.Repici A, Pace F, Gabbiadini R, et al. Endoscopy units and the coronavirus disease 2019 outbreak: a multicenter experience from Italy [published online ahead of print, 2020 Apr 10] Gastroenterology. 2020;159(1):363–366. doi: 10.1053/j.gastro.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinzerling A, Stuckey MJ, Scheuer T, et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient - Solano county, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):472–476. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Repici A, Aragona G, Cengia G, et al. Low risk of covid-19 transmission in GI endoscopy [published online ahead of print, 2020 Apr 22] Gut. 2020;69(11):1925–1927. doi: 10.1136/gutjnl-2020-321341. [DOI] [PubMed] [Google Scholar]
- 5.Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Doremalen N, Bushmaker DH, Morris MG, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Eng J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bean B, Moore BM, Sterner B, et al. Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982;146(1):47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 8.Ansari SA, Springthorpe VS, Sattar SA, et al. Potential role of hands in the spread of respiratory viral infections: studies with human parainfluenza virus 3 and rhinovirus 14. J Clin Microbiol. 1991;29(10):2115–2119. doi: 10.1128/jcm.29.10.2115-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong SWH, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parasa S, Desai M, Thoguluva Chandrasekar V, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA. Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore [published online ahead of print, 2020 Mar 3] JAMA. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung WK, To KF, Chan PK, et al. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125(4):1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J. 2020;133(9):1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 16.ASGE, SGNA, ACG, AGA, and ASCRS. Management of endoscopes, endoscope reprocessing and storage areas during the COVID-19 pandemic. Available at: https://fascrs.org/getattachment/5ef92409-cade-4928-96cf-91ca53ebad93/GI-Society-management-of-endoscope-fleet.pdf?lang=en-US
- 17.World Health Organization. World Health Organization; Geneva: 2020. Rational use of personal protective equipment for coronavirus disease (COVID-19): interim guidance, 27 February 2020. Available at: https://extranet.who.int/iris/restricted/handle/10665/331215
- 18.Instrumentation AftAoM . 2015. Flexible and semi-rigid endoscope processing in health care facilities (ANSI/AAMI ST91: 2015) Arlington, VA. [Google Scholar]
- 19.Lichtenstein D, Alfa MJ. Cleaning and disinfecting gastrointestinal endoscopy equipment. Clinical Gastrointestinal Endoscopy. 2019 32-50.e5. [Google Scholar]
- 20.List N: Disinfectants for use against SARS-COV-2, Environmental Protection Agency. Available at: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19.
- 21.ASGE Quality Assurance in Endoscopy Committee. Calderwood AH, Day LW, et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018;87(5):1167–1179. doi: 10.1016/j.gie.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Reprocessing Guideline Task Force. BT Petersen, Cohen J, et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017;85(2):282–294. doi: 10.1016/j.gie.2016.10.002. e1. [DOI] [PubMed] [Google Scholar]
- 23.Mikhail NN, Lewis DL, Omar N, et al. Prospective study of cross-infection from upper-GI endoscopy in a hepatitis C-prevalent population. Gastrointest Endosc. 2007;65(4):584–588. doi: 10.1016/j.gie.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Villa E, Pasquinelli C, Rigo G, et al. Gastrointestinal endoscopy and HBV infection: no evidence for a causal relationship. A prospective controlled study. Gastrointest Endosc. 1984;30(1):15–17. doi: 10.1016/s0016-5107(84)72286-3. [DOI] [PubMed] [Google Scholar]
- 25.Herrin A, Loyola M, Bocian S, et al. Standards of infection prevention in reprocessing flexible gastrointestinal endoscopes. Gastroenterol Nurs. 2016;39(5):404–418. doi: 10.1097/SGA.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 26.Johnston ER, Habib-Bein N, Dueker JM, et al. Risk of bacterial exposure to the endoscopist's face during endoscopy. Gastrointest Endosc. 2019;89(4):818–824. doi: 10.1016/j.gie.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Sequence for donning personal protective equipment (PPE). Available at: https://www.cdc.gov/hai/pdfs/ppe/ppe-sequence.pdf
- 28.Centers for Disease Control and Prevention. Using personal protective equipment (PPE). Available at:https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html
- 29.Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. Updated July 15 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html
- 30.Gupta S, Shahidi N, Gilroy N, et al. A proposal for the return to routine endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020:1–8. doi: 10.1016/j.gie.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration. Available at: http://wayback.archive-it.org/7993/20170722150658/https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm454766.htm
- 32.Thaker AM, Muthusamy VR, Sedarat A, et al. Duodenoscope reprocessing practice patterns in US endoscopy centers: a survey study. Gastrointest Endosc. 2018;88(2):316–322. doi: 10.1016/j.gie.2018.04.2340. [DOI] [PubMed] [Google Scholar]
- 33.Bartles RL, Leggett JE, Hove S, et al. A randomized trial of single versus double high-level disinfection of duodenoscopes and linear echoendoscopes using standard automated reprocessing. Gastrointest Endosc. 2018;88(2):306–313. doi: 10.1016/j.gie.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Snyder GM, Wright SB, Smithey A, et al. Randomized comparison of 3 high-level disinfection and sterilization procedures for duodenoscopes. Gastroenterology. 2017;153(4):1018–1025. doi: 10.1053/j.gastro.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration. FDA recommend healthcare facilities and manufacturers begin transitioning to duodenoscopes with disposable components to reduce risk of patient infection. August 29, 2019. Available at:https://www.fda.gov/medical-devices/safety-communications/fda-recommending-transition-duodenoscopes-innovative-designs-enhance-safety-fda-safety-communication
- 36.Higa JT, Choe J, Tombs D, et al. Optimizing duodenoscope reprocessing: rigorous assessment of a culture and quarantine protocol. Gastrointest Endosc. 2018;88(2):223–229. doi: 10.1016/j.gie.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 37.McDonnell G, Ehrman M, Kiess S. Effectiveness of the SYSTEM 1E liquid chemical sterilant processing system for reprocessing duodenoscopes. Am J Infect Control. 2016;44(6):685–688. doi: 10.1016/j.ajic.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Naryzhny I, Silas D, Chia K. Impact of ethylene oxide gas sterilization of duodenoscopes after a carbapenem-resistant Enterobacteriaceae outbreak. Gastrointest Endosc. 2016;84(2):259–262. doi: 10.1016/j.gie.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 39.Muscarella LF. Use of ethylene-oxide gas sterilisation to terminate multidrug-resistant bacterial outbreaks linked to duodenoscopes. BMJ Open Gastroenterol. 2019;6(1) doi: 10.1136/bmjgast-2019-000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ofstead CL, Heymann OL, Quick MR, et al. Residual moisture and waterborne pathogens inside flexible endoscopes: evidence from a multisite study of endoscope drying effectiveness. Am J Infect Control. 2018;46:689–696. doi: 10.1016/j.ajic.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Ofstead CL, Wetzler HP, Heymann OL, et al. Longitudinal assessment of reprocessing effectiveness for colonoscopes and gastroscopes: results of visual inspections, biochemical markers, and microbial cultures. Am J Infect Control Control. 2017;45(2):e26–e33. doi: 10.1016/j.ajic.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 42.Ofstead CL, Wetzler HP, Eiland J, et al. Assessing residual contamination and damage inside flexible endoscopes over time. Am J Infect Control. 2016;44(12):1675–1677. doi: 10.1016/j.ajic.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 43.Thaker AM, Kim S, Sedarat A, et al. Inspection of endoscope instrument channels after reprocessing using a prototype borescope. Gastrointest Endosc. 2018;88(4):612–619. doi: 10.1016/j.gie.2018.04.2366. [DOI] [PubMed] [Google Scholar]
- 44.Barakat MT, Girotra M, Huang RJ, et al. Scoping the scope: endoscopic evaluation of endoscope working channels with a new high-resolution inspection endoscope (with video) Gastrointest Endosc. 2018;88:601–611. doi: 10.1016/j.gie.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perumpail RB, Marya NB, McGinty BL, et al. Endoscope reprocessing: comparison of drying effectiveness and microbial levels with an automated drying and storage cabinet with forced filtered air and a standard storage cabinet. Am J Infect Control. 2019;47(9):1083–1089. doi: 10.1016/j.ajic.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Barakat MT, Huang RJ, Banerjee S. Comparison of automated and manual drying in the elimination of residual endoscope working channel fluid after reprocessing (with video) Gastrointest Endosc. 2019;89(1):124–132. doi: 10.1016/j.gie.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang W, Yeh MK, Hsu H, et al. Efficacy of simethicone and N‐acetylcysteine as premedication in improving visibility during upper endoscopy. J Gastroenterol & Hepatol. 2014;29(4):769–774. doi: 10.1111/jgh.12487. [DOI] [PubMed] [Google Scholar]
- 48.Alfa M.J., Singh H. Impact of wet storage and other factors on biofilm formation and contamination of patient-ready endoscopes: a narrative review. Gastrointest Endosc. 2020;91(2):236–247. doi: 10.1016/j.gie.2019.08.043. [DOI] [PubMed] [Google Scholar]
- 49.Barakat MT, Huang RJ, Banerjee S. Simethicone is retained in endoscopes despite reprocessing: impact of its use on working channel fluid retention and adenosine triphosphate bioluminescence values (with video) Gastrointest Endosc. 2019;89(1):115–123. doi: 10.1016/j.gie.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ofstead CL, Wetzler HP, Johnson EA, et al. Simethicone residue remains inside gastrointestinal endoscopes despite reprocessing. Am J Infect Control. 2016;44(11):1237–1240. doi: 10.1016/j.ajic.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 51.US Food and Drug Administration. 522 Postmarket Surveillance Study PS150003 / PSS001. 9/1/2017. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm?t_id=354&c_id=3692
- 52.Kurtz SM, Zagorski M. Elsevier; 2016. Packaging and sterilization of UHMWPE. UHMWPE Biomaterials Handbook (Third Edition) pp. 21–32. [Google Scholar]
- 53.Bhatt S, Mehta P, Chen C. et al. Efficacy of low-temperature plasma-activated gas disinfection against biofilm on contaminated GI endoscope channels. Gastrointest Endosc. 2019;89(1):105–114. doi: 10.1016/j.gie.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 54.GI Scientific, LLC. US FDA clears Scopeseal, the only single-use disposable device that seals the duodenoscope's elevator area and significantly reduces scope contamination during ERCP procedures. Available at: https://www.giscientific.com/scopeseal
- 55.Pasricha PJ, Miller S, Carter F, et al. A novel and effective disposable device that provides 2-way protection to the duodenoscope from microbial contamination. Gastrointest Endosc. 2020;92(1):199–208. doi: 10.1016/j.gie.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 56.US Food and Drug Administration. The FDA continues to remind facilities of the importance of following duodenoscope reprocessing instructions: FDA Safety Communication. 2019: Available at:https://www.fda.gov/medical-devices/safety-communications/fda-continues-remind-facilities-importance-following-duodenoscope-reprocessing-instructions-fda
- 57.Pentax Medical, USA. ED34-i10T Duodenoscope. Available at:https://www.pentaxmedical.com/pentax/download/fstore/uploadFiles/Pdfs/Downloads/AMER_GI_PRO-S_CMK-665-Rev%20-A_ED34-i10T-SS_01.2017.pdf
- 58.Pentax Medical, USA. ED34-i10T2 Duodenoscope. Available at:https://www.pentaxmedical.com/pentax/download/fstore/uploadFiles/Pdfs/Brochures/EMEA_GI_BR_DEC%20Brochure_10_17.pdf
- 59.Forbes N, Elmunzer BJ, Allain T, et al. Infection control in ERCP using a duodenoscope with a disposable cap (ICECAP): rationale for and design of a randomized controlled trial. BMC Gastroenterol. 2020;20(1):1–9. doi: 10.1186/s12876-020-01200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujifilm. Fujifilm ED-580XT duodenoscope. Available at: https://www.fujifilmhealthcare.com/endoscopy/fujifilm-580-series-interventional-endoscopes/fujifilm-ed-580xt-duodenoscope
- 61.Ridtitid W, Pakvisal P, Chatsuwan T, et al. A newly designed duodenoscope with detachable distal cap significantly reduces organic residue contamination after reprocessing. Endoscopy. 2020 doi: 10.1055/a-1145-3562. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 62.Olympus America, Inc. TJF-Q190V duodenoscope. Available at:https://www.olympus-europa.com/medical/rmt/media/Content/Content-MSD/Documents/Brochures/E0428371_TJF-Q190V_A4_Flyer_EN_ABC02-2_FINAL_42669.pdf
- 63.Boston Scientific. EXALT Model D: single use duodenoscope. Available at: https://www.bostonscientific.com/content/gwc/en-US/products/single-use-scopes/exalt–model-d.html
- 64.Ross AS, Bruno MJ, Kozarek RA, et al. Novel single-use duodenoscope compared with 3 models of reusable duodenoscopes for ERCP: a randomized bench-model comparison. Gastrointest Endosc. 2020;91(2):396–403. doi: 10.1016/j.gie.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 65.Bang JY, Sutton B, Hawes R, et al. Concept of disposable duodenoscope: at what cost? Gut. 2019;68(11):1915–1917. doi: 10.1136/gutjnl-2019-318227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ambu Ascope Duodeno. Sterile single-use problem solved. Available at: https://www.ambuusa.com/endoscopy/gastroenterology/duodenoscopes/product/ambu-ascope-duodeno
- 67.Day L, W, Kwok K, Visrodia K, Petersen B., T American Society of Gastrointestinal Endoscopy Infection Control Summit: updates, challenges and the future of infection control in GI endoscopy. Gastrointestinal Endoscopy. 2020 doi: 10.1016/j.gie.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 68.Gromski M, A, Sieber M, S, Sherman S, et al. Double high-level disinfection versus liquid chemical sterilization for reprocessing of duodenoscopes used for ERCP: a prospective, randomized study. Gastrointestinal Endoscopy. 2020 doi: 10.1016/j.gie.2020.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muthusamy V, R, Bruno M, J, Kozarek R, A, et al. Clinical evaluation of a single-use duodenoscope for Endoscopic Retrograde Cholangiopancreatography. Clinical Gastroenterology and Hepatology. 2020;18:2108–2117. doi: 10.1016/j.cgh.2019.10.052. [DOI] [PubMed] [Google Scholar]
- 70.Bang J, Y, Hawes R, Varadarajulu S. Equivalent performance of single-use and reusable duodenoscopes in a randomized trial. Gut. 2020;0:1–7. doi: 10.1136/gutjnl-2020-321836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video: PPE donning for endoscope reprocessing.