Abstract
The novel coronavirus disease 2019 (COVID-19) pandemic is seriously challenging the healthcare system globally. Endothelial damage and increased coagulation activity have been reported in some patients with COVID-19, resulting in a variety of thrombotic events. We report the cases of four patients with various severities of COVID-19 who had presented with acute arterial thrombosis. Although these are rare events, they carry high morbidity and mortality and require prompt diagnosis and treatment. These cases highlight the major life- and limb-threatening clinical sequelae of COVID-19 that frontline medical providers must be aware can occur even in the absence of previous cardiovascular disease.
Keywords: Acute arterial thrombosis, Acute limb ischemia, Aortic occlusion and hypercoagulability, COVID-19
The coronavirus disease 2019 (COVID-19) has overwhelmed the healthcare system. Early reported studies on the clinical course have been crucial for developing treatment paradigms.1, 2, 3 The identification of a prothrombotic state has been reported and associated with increased mortality rates. Venous thrombotic events have been reported in critically ill patients with COVID-19, predominantly those who were already critically ill.4,5 Arterial events in the form of ischemic strokes and myocardial infarction have also been reported.1,5,6 We report four cases of acute arterial large-vessel thrombosis, in the absence of severe pulmonary symptoms, generalized sepsis, or severe peripheral vascular disease. The institutional review board approved the present study, and the patients provided consent for the report of their case details and images.
Case report
Patient 1: aortoiliac and femoral–popliteal thrombosis
A 39-year-old Hispanic male nonsmoker with no significant medical history had presented to the emergency room with acute lower extremity weakness of 6 hours' duration. The patient reported 1 week of dry cough and fevers. He tested positive for COVID-19. On examination, he had profound weakness in the left leg, mild weakness in the right leg, and absent pulses. A computed tomography (CT) angiogram revealed acute aortoiliac and left popliteal artery thrombosis (Fig 1). The findings from a hypercoagulable workup were unremarkable, and an echocardiogram did not reveal any source of potential embolism. Inflammatory markers were elevated during the acute phase of the infection (Table).
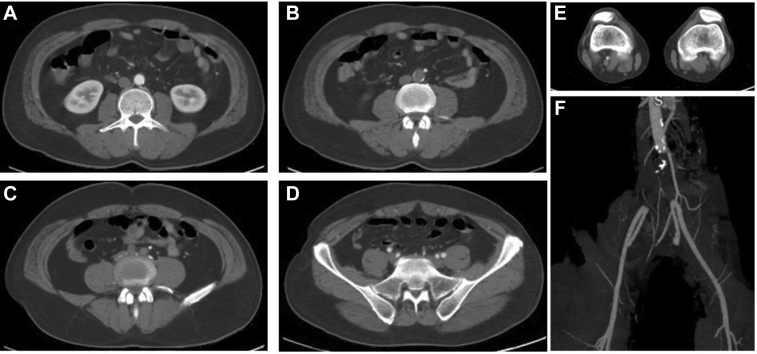
Fig 1.
Selected computed tomography (CT) angiograms of the aortoiliac occlusion of patient 1 showing patent infrarenal aorta (A), occlusive thrombus in the distal aorta (B) and iliac arteries (C), patent external and internal iliac arteries (D), and nonopacification of the left popliteal artery (E). F, Maximum intensity projection reconstruction showing full aortoiliac occlusion.
Table.
Demographic, clinical, and laboratory findings
| Pt. No. | Age, years; Sex | D-dimer, μg/mL | CRP, μg/mL | Ferritin, ng/mL | ICU stay required | Intubation duration, days | Arterial presentation after start of respiratory symptoms, days | Aortic thrombus | Mesenteric or renal thrombus | Infrainguinal thrombus | CVA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39; Male | 6.9 | 12.8 | 833 | No | <1 | 7 | Yes | No | Yes | No |
| 2 | 68; Male | >20 | >270 | 1270 | No | 0 | 5 | Yes | Yes | No | No |
| 3 | 56; Male | >20 | >150 | 1300 | No | 0 | 8 | No | Yes | Yes | Yes |
| 4 | 65; Male | >20 | >270 | 2280 | Postop | 12 | 7 | No | No | Yes | No |
CRP, C-reactive protein; CVA, cerebrovascular accident; ICU, intensive care unit; Postop, postoperatively; Pt. No., patient number.
The patient was taken emergently for surgical thromboembolectomy. A significant clot burden was removed (Fig 2). Palpable pulses were restored to the right foot, and Doppler signals were obtained in the left foot. Four compartment fasciotomies were performed on the left leg. The patient was extubated postoperatively. Therapeutic heparin was started postoperatively, and he was discharged with a prescription for apixaban 5 mg twice daily.
Fig 2.
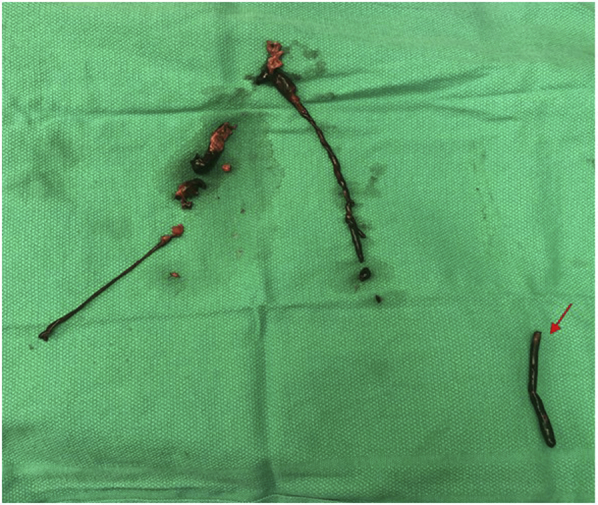
Aortoiliac thrombus and left popliteal thrombus (arrow). The appearance is classic of a fresh acute thrombus.
Patient 2: partial occlusion of the infrarenal aorta
A 60-year-old white male former smoker with a medical history of chronic emphysema, hypertension, and hyperlipidemia had been hospitalized after 5 days of respiratory symptoms and 1 day of left-sided abdominal discomfort. He tested positive for COVID-19. A CT scan with intravenous contrast was obtained to assess the abdominal pain. The CT scan showed semiocclusive thrombus in the infrarenal portion of the abdominal aorta, focal aortic thrombus at the level of the left renal artery with nonperfusion of the left kidney, an occluded inferior right renal artery with segmental infarction of the right lower renal pole (Fig 3). A transthoracic echocardiogram did not reveal any source of potential embolism. The lower extremity resting ankle brachial index was 0.89 and 0.98 for the right and left legs, respectively. The lower extremity pulse examination revealed palpable femoral pulses and Doppler pedal signals. The findings from the hypercoagulable workup were unremarkable. Inflammatory markers were elevated during the acute phase of the infection (Table).
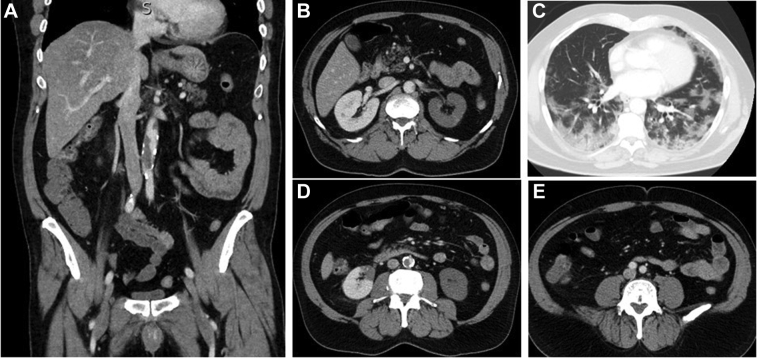
Fig 3.
Selected computed tomography angiograms of the partial aortic occlusion (A and D) with patient common iliac arteries (E) and bilateral renal infarcts (B and D). C, Chest computed tomography (CT) scan showing patchy infiltrates consistent with coronavirus disease 2019 pneumonia.
Despite the large infrarenal aortic thrombus, the iliac arteries were widely patent, and the patient remained asymptomatic. The patient was treated nonoperatively with intravenous heparinization. The patient was discharged home with a prescription for oral apixaban 5 mg twice daily. The patient was asymptomatic at 1 week after discharge.
Patient 3: femoral popliteal thrombosis
A 56-year-old black male nonsmoker with a history of hypertension and diabetes had presented to the emergency department with an ischemic leg. The patient had presented at the emergency department for generalized weakness 5 days earlier. He tested positive for COVID-19. Focal weakness to the left leg was noted, and the distal pulses were absent. A CT angiogram revealed acute occlusion of the femoral and popliteal arteries. A nonocclusive superior mesenteric artery thrombus was noted (Fig 4). Gastrointestinal symptoms were absent, and the superior mesenteric artery lesion was treated conservatively.
Fig 4.
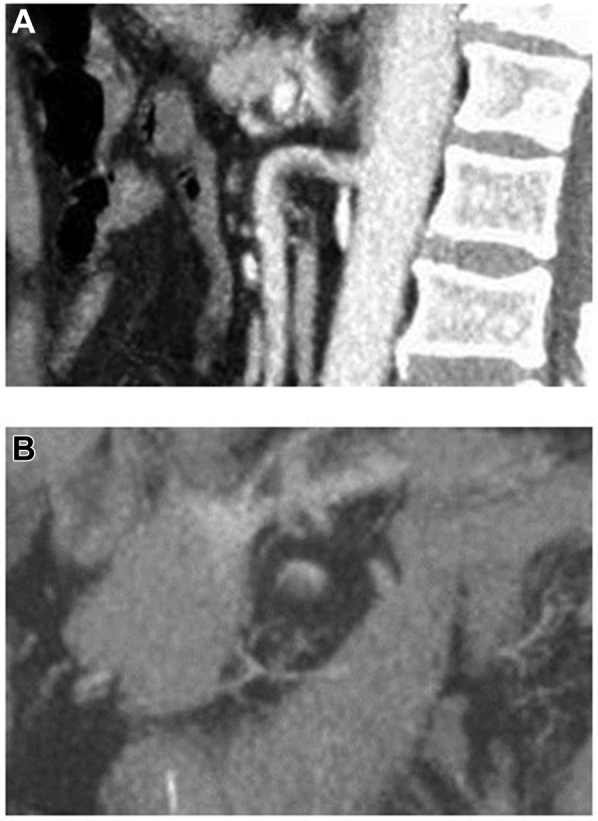
Superior mesenteric artery (SMA) with nonocclusive acute thrombus formation. A, Sagittal view of the nonocclusive thrombus seen primarily on the lesser curve of the SMA. B, Axial view of the nonocclusive thrombus in the SMA.
The patient was taken emergently to the operating room for surgical thrombectomy. An acute thrombus was removed from the superficial femoral artery through the tibial vessels. Minimal chronic arterial occlusive disease was found on direct evaluation of the vessels and intraoperative arteriogram. Poor distal flow in the lower calf and foot was present at completion. The patient underwent interval below-the-knee amputation. A change in mental status led to the performance of a head CT scan, which identified a 1.5-cm subacute ischemic infarction. Postoperatively, therapeutic anticoagulation therapy was continued, and the patient was discharged home. The hypercoagulable workup findings were unremarkable, and an echocardiogram did not reveal any source of potential embolism. Inflammatory markers were elevated during the acute phase of the infection (Table).
Patient 4: femoral popliteal thrombosis
A 65-year-old white male nonsmoker with diabetes had presented to hospital with fever, shortness of breath, and hypoxia. The patient tested positive for COVID-19. Three days after his admission, new-onset acute left lower extremity pain was reported. On examination, the left foot was cold and a loss of pulses had occurred below the femoral artery. An arterial duplex ultrasound scan revealed left popliteal artery occlusion.
The patient was taken for emergent thrombectomy, and an acute thrombus was removed from the popliteal and tibial arteries. The vessels were soft, without evidence of arterial occlusive disease. Distal perfusion was restored intraoperatively, and Doppler signals were insonated. The patient was extubated after 10 days in the intensive care unit and was subsequently discharged with therapeutic anticoagulation therapy. The findings from a hypercoagulable workup were unremarkable, and an echocardiogram did not reveal any source of potential embolism. Inflammatory markers were elevated during the acute phase of the infection (Table).
Discussion
Infection with SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2; COVID-19) has been shown to have a wide range of clinical presentations from asymptomatic in a large percentage of patients to devastating pulmonary failure, sepsis, and death.1,7,8 Hypercoagulability has been recognized as a significant cause of the morbidity in this disease, resulting in pulmonary parenchymal thrombosis,9 venous thrombosis and emboli,4,5 and stroke.10 Multiple causative factors have been implicated, including a cytokine storm associated with SARS-CoV-2,11 endotheliitis,12 and hypoxia.13
The cases we have presented have demonstrated the occurrence of limb- and organ-threatening large vessel arterial thrombotic events with a lack of an association with the severity of the pulmonary infection. Only 1 patient required prolonged intubation after surgery, and all recovered from their respiratory illness.
The occurrence of macrovascular thrombosis in patients infected with COVID-19 has not been well reported.1, 2, 3,7,8,14,15 Two series have identified venous thrombosis and thromboembolism in significant numbers. Klok et al5 found an incidence of 31% and Cui et al4 an incidence of 25% in intensive care unit patients. Large vessel arterial thrombosis with cerebrovascular thrombosis and stroke has been reported.10 As with our patients, these thromboses occurred early, without the presence of severe pulmonary disease. We found that major vessel arterial thrombosis occurred at a rate of ≤1% in patients hospitalized with COVID-19. Only 4 of 773 patients hospitalized in our institutions had been identified at the time of this report.
The choice of intervention in our cases was guided by the need to limit interventions that would expose these patients to stressful procedures, the desire to limit exposure of medical personnel, and the need to conserve resources. Severe ischemia in 3 patients necessitated an urgent open approach; however, in 2 patients, conservative management was effective.
Prophylactic anticoagulation therapy is currently recommended for in-hospital treatment of COVID-19.16, 17, 18 Our findings support consideration for prophylactic anticoagulation or antiplatelet therapy for outpatients with early COVID-19 symptoms,18 especially when the D-dimer levels are elevated (Table). The therapeutic anticoagulation duration should be guided by normalization of the inflammatory markers, clearance of infection, and the follow-up imaging findings. An evaluation to identify preexisting hypercoagulable states, sources of emboli, and other provocative factors (occult malignancy) should be pursued in all cases for optimal patient care and to assist in further research on the thrombotic effects of COVID-19.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Bhatraju P.K., Ghassemieh B.J., Nichols M. COVID-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Richardson S., Hirsch J., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5,700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magro C., Mulvey J., Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxley T., Mocco J., Majidi S. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong C., Lam C., Wu A. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varga Z., Flammer A.J., Steiger P. Endothelial infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans C. Hypoxia and HIF activation as a possible link between sepsis and thrombosis. Thrombosis J. 2019;17:16. doi: 10.1186/s12959-019-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Lu X., Chen H. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikdeli B., Madhavan M., Jiminez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kollias A., Kyriakoulis K., Dimakakos E. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]