Abstract
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to infect hundred thousands of people every day worldwide. Since it is a novel virus, research continues to update the possible therapeutic targets when new evidence regarding COVID-19 are gathered. This article presents an evidence-based hypothesis that activating the heme oxygenase-1 (HO-1) pathway is a potential target for COVID-19. Interferons (IFNs) have broad-spectrum antiviral activity including against SARS-CoV-2. Induction of HO-1 and increase in the heme catabolism end-product confer antiviral activity. IFN activation results in inhibition of viral replication in various viral infections. COVID-19 induced inflammation as well as acute respiratory distress syndrome (ARDS), and coagulopathies are now known major causes of mortality. A protective role of HO-1 induction in inflammation, inflammation-induced coagulation, and ARDS has been reported. Based on an association of HO-1 promoter polymorphisms and disease severity, we propose an evaluation of the status of these polymorphisms in COVID-19 patients who become severely ill. If an association is established, it might be helpful in identifying patients at high risk. Hence, we hypothesize that HO-1 pathway activation could be a therapeutic strategy against COVID-19 and associated complications.
Keywords: SARS-CoV-2, COVID-19, Heme Oxygenase-1, Antiviral activity, Type-1 IFNs, Inflammation, HO-1 promoter polymorphism, Coagulopathy
Graphical abstract
1. Introduction
The novel coronavirus SARS-CoV-2-related pneumonia called COVID-19 has brought enormous personnel and economic loss in recent times [1]. According to the World Health Organization, approximately 27 million people have been infected and more than 0.89 million have died since writing of this manuscript (September 10, 2020). At present, despite tireless efforts, no definitive therapies or vaccines are available. Hence, there is an urgent need to explore new therapeutic options. In this review, based on published data, we hypothesize that targeting the heme oxygenase pathway (HO) could be beneficial in COVID-19 and related complications.
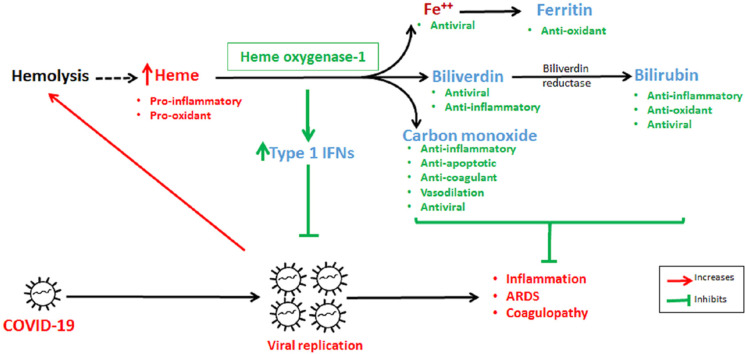
HO, an evolutionary conserved ubiquitous enzyme, has recently gained a lot of attention in different disease conditions due to its pleiotropic effects [2]. HO exists as two main isoforms, HO-1 and HO-2. Although, a splice-variant of HO-2 identified as HO-3 (third isoform) also exists, but is elusive and poorly understood [3,4]. HO-1, the well characterized inducible isoform also known as heat shock protein-32, is encoded by a gene called HMOX1, which is transcriptionally upregulated up to 100-fold in the presence of stimuli like infections, radiation, toxins and injuries such as ischemia/reperfusion injury, acute lung injury, etc [2,5]. HO-1 oxidatively catabolizes heme to ferrous iron, carbon monoxide (CO), and biliverdin (BV). BV is then converted to bilirubin (BR) in an energy consuming reaction by biliverdin reductase. This process makes BR more electrophilic than BV, thereby comparatively increasing affinity of BR towards Keap1-Nrf2, which in turn facilitates Nrf2-dependant antioxidant gene induction [6]. Under oxidative stress condition, BR again is converted to BV, which further regenerates back to BR and this cycle goes on and affords significant protection to endothelial cells [7]. The end-products, CO and BV/BR are known for their antioxidant, anti-inflammatory, anti-apoptotic effects, etc [8,9]. In the last decade, HO-1 and heme metabolism end-products like ferrous iron, CO, BV, and BR have been explored extensively and successfully for antiviral effects against wide range of viruses like HIV, HCV, HBV, influenza, dengue, Ebola, respiratory syncytial virus, enterovirus 71, pseudorabies, human herpes simplex virus, porcine reproductive and respiratory syndrome virus, etc [[10], [11], [12], [13], [14], [15]].
Currently, there is no direct pre-clinical or clinical evidence to confirm the therapeutic role of HO-1 modulation in COVID-19. We propose the beneficial role of HO-1 induction or administration of heme degradation end-products in combating SARS-CoV-2 infection. Our hypothesis is based on an established role of interferons (IFNs) in inhibiting replication of various viruses including coronaviruses, along with the well documented role of HO-1 in inducing type-1 IFN expression. We also discuss the possible association between HO-1 promoter polymorphisms and severity in individuals with COVID-19. Additionally, to support our hypotheses with the available evidences, we reviewed the beneficial role of HO-1 modulation in various inflammatory conditions and coagulation disorders similar to what is observed in COVID-19 infection with a focus on the lungs which is the prime organ affected.
2. Role of HO-1 as an antiviral agent
After a viral infection, type 1 IFNs are among the first cytokines produced by the host cells to inhibit viral replication. However, like other viruses, SARS-CoV-2 suppresses type 1 IFN induction as well as translation and also suppresses IFN stimulated genes in order to survive and replicate [[16], [17], [18], [19]]. IFNs act by inhibiting viral replication [20] and produce immunomodulatory effects by increasing natural killer cell cytotoxicity and proliferation, and expression of major histocompatibility complex-1 [21]. Furthermore, IFNs have been reported to possess broad-spectrum antiviral activity including against SARS-CoV [22,23].
Recently, there has been considerable in vitro evidence showing a potential role of IFNs in inhibiting SARS-CoV-2. SARS-CoV-2 is more sensitive to IFNs (EC50, IFN-α:1.35 IU/ml, IFN-β: 0.76 IU/ml) than SARS-CoV (EC50, IFN-α: 4950 IU/ml, IFN-β: 95 IU/ml) [24,25]. Clementi and co-workers [26] showed inhibition of SARS-CoV-2 replication by IFN-β-1a when administered after virus infection. Another study reported a dose-dependent inhibition of SARS-CoV-2 by IFNs in simian Vero E6 and human Calu-3 epithelial cell lines [27]. Clinically, in mild to moderate COVID-19 patients, IFN in combination with lopinavir/ritonavir was associated with a shortened duration of viral shedding and hospital stay when compared with either drugs alone [28]. A trial to assess IFN nasal drops to prevent COVID-19 in medical staff is ongoing (NCT04320238).
Earlier reports suggest age as an important risk factor, as old macaques with SARS-CoV infections showed severe lung pathology, higher pro-inflammatory cytokine expression, but low IFN expression compared with young macaques [29]. Similarly, monocytes from humans >65 years showed intact pro-inflammatory cytokine responses but defective IFN production after influenza A virus infection [30]. This age-dependent data further supports an important role of IFNs as a possible reason for increased severity of COVID 19 in aged populations.
Hence, based on the potential role of IFN in inhibiting SARS-CoV-2, we hypothesize early use of HO-1 activators as a potential strategy to combat COVID-19 by inducing IFNs production. Given below are a few examples of HO-1 activators and its downstream heme catabolism end-products, CO and BV showing antiviral activity through activation/restoration of IFNs.
-
•
Celastrol, a HO-1 inducer, augmented type 1 IFN production and decreased viral replication by inhibiting NS3/4a protease in hepatitis-C infection [31,32].
-
•
CoPP, a HO-1 inducer, reduced hRSV replication and titres by 3 log units via enhanced expression of IFN in human alveolar epithelial cells (A549 cells) both in vivo and in vitro [33].
-
•
Andrographolide showed anti-hepatitis-C virus activity by upregulating HO-1-dependent IFN in human hepatoma cells [34].
-
•
A phytocompound, lucidone, suppressed hepatitis-C virus replication by inducing HO-1 and biliverdin-dependent antiviral IFNs [35].
-
•
Sulforaphane and curcumin are associated with suppression of hepatitis-C viral replication by upregulating HO-1 activation-dependent IFN release [36,37].
-
•
A flavonoid, 6-demethoxy-4′-O-methylcapillarisin, from Artemisia rupestris L. inhibited influenza virus replication by augmenting HO-1-dependent IFNs production [13].
Apart from type 1 IFN-dependent viral replication inhibition, HO-1 pathway end-products, iron (Fe2+), BV, BR, and CO also produce direct antiviral effects via inhibition of RNA-dependent RNA polymerase and viral proteases. Fe2+ inhibits viral replication in HCV-transfected hepatoma cell lines via high affinity binding with Mg2+ binding sites present on a HCV RNA polymerase, thereby inhibiting the enzymatic activity and viral replication [38]. In another experiment, treatment of HCV-infected hepatoma cell lines with an iron donor at the time of infection, but not when pre-treated, significantly decreased the expression of viral proteins (core and non-structural protein 3) and viral RNA [39]. HCV infection also decreases cellular Fe2+ levels to bypass iron-dependent inhibition of viral replication [40]. In in vitro studies, BR inhibited the viral replication of human herpesvirus-6, herpes simplex virus type-1, and the enterovirus [15,41]. BV inhibits viral replication in HCV-infected hepatoma cell line by inhibiting NS3/4A protease [42]. Since, these viral proteases show high homology with coronavirus proteases [43], there is a strong possibility that BV or HO-1 inducers may inhibit coronavirus proteases as well. CO-releasing molecule-2, an exogenous CO donor, inhibits viral replication of enterovirus 71 and bovine viral diarrhea virus [44]. HO-1 activation has also been reported to produce antiviral effects in other viral infections like hepatitis B [45], HIV [46], dengue [47], Ebola [48], and Zika [49].
Additionally, various other pharmacological agents have also been reported to induce HO-1 expression, including rapamycin [50], resveratrol [51], proton pump inhibitors [52,53], statins [54], niacin [55] and aspirin [56], etc. Thus, based on the evidences discussed above, there is a strong possibility that HO-1 inducers or HO-1 pathway by-products could play decisive roles in controlling SARS-CoV-2 infections via either restoration of IFN production or inhibition of viral proteases and RNA polymerases.
3. Effect of HO-1 activation on inflammation and coagulation
3.1. Mechanism of inflammation-induced coagulation after viral infection
In general, infection initiates inflammation by releasing various pathogen-associated molecular patterns, triggering resident macrophages or immune cells to release various cytokines like IL-6, TNF-α, etc. These events cause disturbance in epithelial-endothelial barrier integrity by recruiting circulating neutrophils and macrophages to the site of injury [57,58]. Pro-inflammatory cytokines expose and upregulate the expression of tissue factor (TF) present on alveolar epithelial cells, macrophages, endothelial cells and fibroblasts present in blood vessels adventitia and platelets [59]. Exposed TF initiates the coagulation cascade by activating and forming complexes with factors VII, Xa, converting pro-thrombin to thrombin and further fibrinogen to fibrin; ultimately leading to the formation of clots [59,60]. Thrombin, in turn, activates the inflammatory cascade by binding to thrombin receptors (protease activated receptor, PAR 1/3/4) on mast cells causing mast cell degranulation, on endothelial cells/monocytes/macrophages causing release of various chemokines, cytokines, adhesion molecules and on platelets causing further formation of thrombin [61]. Moreover, TF/factor VIIa and TF/factor VIIa/Xa complex activate PAR 2 receptors and cause upregulation of adhesion molecules, further affecting endothelial cell integrity by increasing inflammatory cell infiltration [61,62].
To counteract coagulation, a physiological anti-coagulation cascade is activated releasing fibrinolysis activators and increasing the levels of fibrin degradation products (FDPs) [63]. FDPs themselves induce the release of inflammatory cytokines from peripheral monocytes and thus increase the expression of adhesion molecules, again inducing the vicious cycle of inflammation and coagulation [61,64]. In later stages of sepsis, pro-inflammatory cytokines induce a long-lasting release of plasminogen-activation inhibitors (PAI) from endothelium thereby decreasing fibrin degradation and causing multi-organ damage [61]. Thus, the balance between coagulants and anticoagulants get disturbed with increasing severity of inflammation.
All these events lead to narrowing of the blood vessels due to platelet aggregation and adhesion as well as activation of white blood cells on endothelial surfaces. Free radicals are released, causing lipid peroxidation of the circulating RBC membranes, decreasing their deformability, ultimately leading to aggregation, entrapment, and rupture of RBCs causing hemolysis [65,66]. Hemolysis leads to release of free hemoglobin (Hb), a potential source of free radicals and known to further aggravate inflammation [67]. Increased free Hb is scavenged either by soluble plasma proteins like haptoglobin (Hp), hemopexin (Hpx), albumin or, microglobulin via cell surface receptors like CD163/LDL receptor-related protein 1 for Hb/heme-Hpx complexes present on the surface of circulating monocytes or macrophages. Lysosomal proteases degrade Hb to liberate a potent pro-inflammatory and pro-oxidant molecule known as heme. Heme is finally degraded by HO-1 to CO, BV, and ferrous ions [68,69]. Two-fold increase in the free Hb levels were found in the broncho-alveolar lavage of ARDS patients compared to plasma indicating erythrocytes egress and hemolysis inside alveoli due to a compromised epithelial endothelial barrier [70]. Therefore, the inflammatory and prothrombotic state in ARDS, along with decrease in Hb scavengers lead to increase in cell free Hb. This aggravates the secondary endothelial damage, oxidative stress, and inflammation and the cycle goes on [66,71].
Hence, inflammation-induced activation of the coagulation cascade along with a reduction in activity of fibrinolytic system can trigger changes in coagulation parameters ranging from mild disturbances to overt microvascular thrombosis depending upon the severity of inflammation. Examples of inflammation-induced coagulation disturbances include sepsis-induced disseminated intravascular coagulation (DIC), ruptured atherosclerotic plaque, ARDS, etc [59,61].
3.2. COVID-19 induced inflammation and coagulopathy
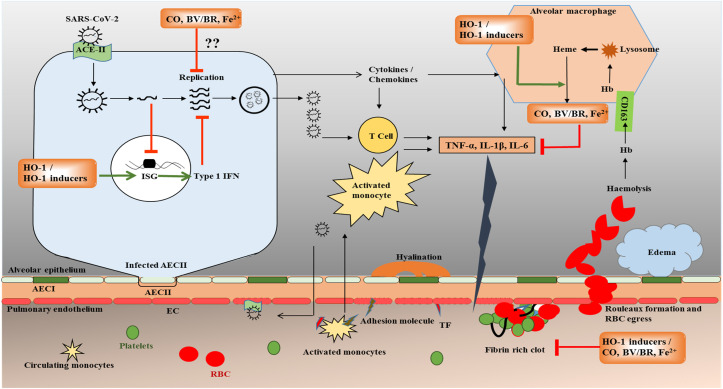
Briefly, SARS-CoV-2 enters the body through angiotensin-converting enzyme 2 (ACE-2) receptors on granular pneumocytes known as type-II alveolar epithelial cells (AEC-II) [72,73]. AEC-II, an immunologically active cell, secretes a variety of cytokines and chemokines in response to infections, thereby activating resident alveolar macrophages and T cells [72] (Fig. 1 ). Infection-induced inflammatory responses activate both innate and adaptive immunity, triggering apoptosis of lymphocytes causing lymphocytopenia, a consistent finding present in hospitalized COVID-19 patients. In later stages, with increase in replication rates, the virus infects endothelial cells of the pulmonary vasculature through ACE-2 receptors, thereby aggravating the inflammatory response with apoptosis of the endothelial cells [74,75]. In fact, the damaged endothelial cells, by exposing tissue factors and adhesion molecules, activate platelets, causing platelet degranulation followed by platelet aggregation ultimately leading to the formation of microthrombi [76]. Break in epithelial-endothelial integrity causes an increase in capillary permeability and subsequent infiltration with inflammatory cells (monocytes and neutrophils) and RBCs into alveolar space leading to pulmonary edema, alveolar hemorrhages, and hyalinization of respiratory epithelium, thus inclining the disease towards ARDS [72,77]. Further evidence has shown that SARS-CoV-2 infection can cause ARDS and subsequent hemolysis in critically ill patients with raised cell-free hemoglobin [78]. However, a controversial preliminary report suggests that COVID-19 attacks RBCs at later stages, denatures Hb and inhibits heme metabolism [79]. Similar to SARS-CoV, COVID-19 patients with ARDS also have altered pro-coagulants (increase in D-dimers and fibrinogen) and anticoagulants (increase in plasminogen activator inhibitor, which prevents fibrin degradation) levels [80,81]. This imbalance between pro and anticoagulant levels leads to the formation of thrombi and may cause complications like deep vein thrombosis, stroke, myocardial infarction, DIC, and multi-organ failure.
Fig. 1.
Hypothetical mechanism of HO-1 induction and heme degradation end-products such as CO, BV, and BR in COVID-19. SARS-CoV-2 virus infects nasal, bronchial epithelial cells and alveolar epithelial cell type II (AECII) via angiotensin converting enzyme-2 (ACE-2) receptors. In AECII, SARS-CoV-2 replicates and interferes with interferon induction and signaling, thereby stimulating infected cells to release inflammatory signaling molecules like cytokines and chemokines. These molecules in turn stimulate resident alveolar macrophages, and T-cells to release inflammatory mediators like TNF-α, IL-1β and IL-6. With further disease progression, these inflammatory mediators activate endothelial cells (EC) in pulmonary capillaries thereby inducing the expression of adhesion molecules. Adhesion molecules, in turn cause activation and recruitment of activated monocytes to alveoli where they release various inflammatory mediators, thus altering the balance between pro-inflammatory and anti-inflammatory cytokines. All these events ultimately affect epithelial-endothelial integrity, thereby further increasing the egress of monocytes and red blood cells. Pro-inflammatory cytokines also induce expression of tissue factor (TF) on ECs which when comes in contact with platelets; activate coagulation cascade forming a fibrin rich clot. In later stages, virus affects EC directly causing apoptosis, loss of barrier integrity thereby activating further inflammation and coagulation. Increased heme released after hemolysis as observed in the COVID-19 patients with ARDS, further increases pro-inflammatory cytokines. We hypothesize that HO-1 induction may provide protection in the initial stage of COVID-19 by targeting viral replication by upregulating the transcription of type 1 IFNs and in later stages by targeting inflammation and coagulation. ISG – Interferon-stimulated genes. Green arrows indicate activation and red arrows indicate inhibition. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Laboratory findings of COVID-19 infected hospitalized patients have significant increase in the levels of inflammatory markers mainly IL-6 (>50% patients), ferritin (>70% patients), total bilirubin (>18% patients), erythrocyte sedimentation rate (>85% patients), anemia (>11% patients), and lymphocytopenia (>35% patients) [77,82,83]. Approximately, 17–40% of patients presenting with ARDS during or at the time of hospitalization have significantly increased pro-thrombin time and D-dimer levels [82,84]. Among critically ill patients and non-survivors, ARDS was present in approximately 50–90% of patients and raised D-dimer levels and fibrin degradation produts in 80–100% of patients [77,83,84]. In a study conducted by Tang and co-workers [85], 71.6% of the non-survivors and only 0.6% of survivors met the criteria of DIC, indicating DIC to be an important cause of death. Additional evidence suggests that approximately 42% of hospitalized ARDS patients developed thrombotic complications with pulmonary embolism being the most common [86]. Except for thromboembolic complications, there is no significant difference in the ARDS disease pattern among COVID-19 and non-COVID-19 patients [86]. Looking at the currently available data, ARDS and thromboembolic complications are the major causes of severity and mortality in COVID-19 patients.
3.3. HO-1 as an anti-inflammatory agent
HO-1 and its downstream end-products confer cell survival by providing protection against oxidative stress, inflammation, and acts as a chaperone to remove degraded proteins as demonstrated by many in vitro and in vivo models of inflammation and acute lung injury. In response to oxidative stress and inflammation, HO-1 is upregulated in many cells including endothelial cells, basophils, monocytes, macrophages, neutrophils, and vascular smooth muscle cells [87]. This section focuses mainly on the anti-inflammatory role of HO-1 in different lung disease models (Fig. 1).
In a carrageenan-induced lung inflammation model, at 48 h, the mononuclear cells of inflammatory lesion expressed 8-fold higher HO-1 expression when compared to peripheral mononuclear cells. This increase correlated with a reduction in inflammation. Pre-treatment with HO-1 inhibitor aggravated inflammation, whereas, with HO-1 inducer, a decrease in inflammation was reported [88]. Furthermore, HO-1 transfection or pre-treatment with HO-1 inducers reduced LPS-induced pro-inflammatory cytokine production and high-mobility group box 1 (HMGB1) release from macrophages through CO generation. Also, HO-1 induction or CO supplementation decreased mortality in in vivo model of septic shock induced by LPS or cecal ligation and puncture along with decrease in plasma levels of HMGB1 as well as serum levels of TNF-α and IL-1β [89].
CO showed anti-inflammatory and anti-apoptotic effects in in vivo and in vitro models of ischemia/reperfusion injury in lungs by modulating P38-MAPK pathway [90]. The anti-inflammatory effects of CO were also evident by a decrease in LPS-induced reactive oxygen species-dependent toll-like receptor 4 (TLR4) recruitment to the lipid raft, thereby initiating immune cell-mediated inflammatory signaling [91]. In an in vivo model of acute lung injury induced by LPS, BV pre-treatment decreased alveolitis, pulmonary edema, and reduced inflammatory cells in bronchoalveolar lavage. BV also decreased the levels of NF-KB expression, a transcription factor, responsible for LPS-induced cytokine production and inflammation [92]. Recent evidence revealed that moderate increase in plasma levels of BR via HO-1 pathway provide protection against inflammation and endothelial dysfunction [6]. BR showed antioxidant and anti-inflammatory effects by scavenging nitric oxide and reactive oxygen species [93,94].
ARDS in COVID-19 patients often require external ventilator support which itself can cause lung injury [95]. Edema, endothelial and epithelial damage followed by a release of cytokines and chemokines, activation, recruitment and extravasation of leukocytes are the characteristics features of ventilator-induced lung injury (VILI) [96]. There has been extensive evidence to support a protective role of HO-1 activation in VILI by decreased pro-inflammatory cytokine expression including TNF-α, IL-8, decreased neutrophil, etc., and increased expression of anti-inflammatory cytokines like IL-10 [97,98].
Although, HO-1 expression has beneficial effects, its overexpression may be deleterious. Excess release of CO may blunt the anti-inflammatory response by either activating prostaglandin-endoperoxide synthase enzyme thereby increasing the production of pro-inflammatory cytokines [99] or inhibiting stress induced inflammatory response by suppressing hypothalamus pituitary adrenal axis and release of vasopressin [100]. Similarly, high serum BR levels may cause central nervous system toxicity like bilirubin encephalopathy [101]. Studies have also shown hormetic effect of HO-1 inducer, curcumin. Prolonged supplementation with curcumin (1–4 g/day for 6 months) was responsible for an increase in cholesterol levels which was opposite to effects observed with short-term treatment [102,103]. Therefore, hormetic response should be considered while targeting induction of HO-1 system.
3.4. HO-1 as an anti-thrombotic agent
Increasing evidences support the anti-thrombotic role of HO-1 induction during arterial or venous injury. Two independent groups have shown inhibition of thrombus formation in a ferric chloride-induced model of microvascular or platelet thrombosis in mice by induction of HO-1 [104,105]. Fujita and co-workers [106] showed that CO protected against the development of fibrin clots in the microvasculature by inhibiting the expression of gene encoding PAI-1, thereby derepressing fibrinolysis, when administered in HO-1-deficient mice model of ischemic lung injury. Furthermore, HO-1 gene induction or CO administration exhibited anti-inflammatory and anti-thrombotic effects in a model of arterial thrombosis produced in apoE-deficient hypercholesterolemic mice [107]. Moreover, Hmox1−/− mice had increased endothelial cell injury with high levels of TF and von Willebrand factor causing formation of platelet-rich micro-thrombi and apoptosis. Exogenous administration of CO and BV rescued thrombotic events and pro-thrombotic state indicating their role in formation of arterial thrombosis [108]. Another study reported that HO-1 activation significantly decreased exaggerated inflammatory responses as depicted by significant reduction in expression of inflammatory markers like E-selectin, P-selectin, IL-6, and MCP-1 along with decrease in clot size and inferior vena cava wall thickness in comparison to HO-1-deficient mice [109].
It is also noteworthy that the HO-1 inducer, hemin, increased the expression of IL-10, levels of anticoagulant-activated protein C, prothrombin time, and activated partial thromboplastin time in a model of sepsis via HO-1 activation. Histopathological analysis revealed decreased number of thrombi along with decrease in inflammatory changes in liver and lungs [110]. Further, HO-1 gene transfection or induction by hemin or administration of HO-1 end-products, CO and BV, reported decreased clot size in a venous thrombosis model of mice. Hemin-induced HO-1 upregulation also decreased clot formation and levels of PAI along with a decrease in the levels of heme suggesting anti-thrombotic effects either directly by HO-1 or through CO and BV [111]. Treatment of HO-1-deficient mice with CO-releasing agent in an allogenic aortic transplant model markedly improved survival rates along with significant reductions in platelet aggregation and arterial thrombosis [112]. Gabre and co-workers [113] also demonstrated thrombus resolution, and reduction in IL-6 production after treatment with activated protein C via increasing HO-1 expression in a mouse model of venous thrombosis.
Taken together, studies suggest that HO-1 induction or use of heme degradation end-products like CO, BV, and BR produce anti-thrombotic effects by decreasing endothelial injury, expression of adhesion molecules, and inflammatory responses along with decrease in the levels of pro-coagulant factors like tissue factor, von Willebrand factor, and PAI, thus supporting their potential therapeutic role in COVID-19. Since, inflammation induced-coagulopathy plays a key role in COVID-19-associated mortality; its attenuation by HO-1 induction may be a promising strategy to limit the devastating consequences of COVID-19 (Fig. 1).
4. HO-1 genetic polymorphism and disease severity
To support our hypothesis that HO-1 is a potential target in COVID-19, we present the association of HO-1 promoter polymorphisms and disease severity in various conditions like acute lung injury, thromboembolism and diabetes. Two studies have shown that HO-1 deficiency in two individuals was associated with severe hemolysis, raised inflammatory markers, nephritis, endothelial dysfunction, coagulation abnormalities, and death of both patients because of intracranial hemorrhage [114,115]. Similar complications were seen in Hmox1−/− deficient mice [116]. Tsur et al. [117] reported significant decrease in number of viable fetus in heterozygous HO-1+/− dams in comparison to wild type HO-1+/+. Treatment with pravastatin significantly improved heterozygous HO-1+/− fetal survival indicating the protective role of statin in HO-1 deficiency. The diverse and vital role of HO-1 in regulating physiological functions may be explained by various promoter polymorphisms. Kimpara and co-workers [118] observed the highly polymorphic nature of GT dinucleotide repeats in the HO-1 promoter region in the Japanese population. Later, many other reports showed that (GT)n repeat length can effect HO-1 transcription. On the basis of (GT)n repeat length, alleles can be classified into 3 categories: short (S, GT repeats < 25), middle (M, GT repeats 25–29), and long (L, GT repeats ≥ 30) [119,120]. Apart from (GT)n repeat polymorphism, two single nucleotide polymorphisms (SNPs) present in HO-1 gene promoter region are G (-1135)A and T (-413)A. The T (-413)A SNP, also known as rs2071746 polymorphism is reported to alter HO-1 expression [121]. The T (-413)A SNP with AA or AT genotype, in comparison to TT genotype was associated with high basal HO-1 expression [122]. Evidence suggested that patients with A allele carrier were associated with reduced type 2 diabetes induced albuminuria [123], ischemic heart disease, and atherosclerotic stroke [122,124]. In contrast, there was no association of these alleles with decrease in the risk of developing coronary artery disease [125], rheumatoid arthritis [126], lung function decline [127] and HIV induced encephalitis [46]. Furthermore, AA or AT genotype were associated with increased incidence of hypertension [128]. Hence, the role of T (-413)A SNP in altering disease severity requires further research.
Studies have shown an association between disease severity and (GT)n repeat polymorphism. The presence of the S allele was associated with increased HO-1 expression and decreased disease severity, whereas, the presence of the L allele was associated with decreased HO-1 expression and increased disease severity. Susceptibility to chronic pulmonary emphysema was significantly decreased in smokers carrying the S allele and thus having high HO-1 expression [119]. Similarly, in chronic obstructive pulmonary disease, decline in lung function was high in patients carrying the L allele [129]. Moreover, heavy smokers carrying the L allele showed a steep decline in lung function over a period of 8 years compared with those carrying an M/S allele [130]. A study on the (GT)n repeat lengths and end outcome after angioplasty showed a significant association between the presence of the L allele and restenosis [131]. Another study reported lower rates of percutaneous coronary intervention, coronary bypass surgeries, and cases of myocardial infarction in patients suffering from peripheral artery disease carrying either the heterozygous or homozygous S allele in comparison to non-carriers [132]. Furthermore, a strong association between the presence of L allele and progressive atherosclerosis with increase in the levels of oxidized lipoprotein and decreased antioxidant defense mechanism was observed in high risk patients [133]. Mustafa and co-workers [134] reported a two-fold increase in the risk of venous thromboembolism recurrence in patients carrying the heterozygous/homozygous L allele in comparison with the other two alleles.
With regard to the viral infections, recent data showed a lower risk of HIV-induced neuroencephalitis and neurocognitive impairment in patients with S allele [46,135]. Seu et al. [136] reported high viral loads and soluble CD14 in HIV-infected patients on antiretroviral therapy having the L allele. Also, decreased hepatitis-C viral replication was associated with increased HO-1 expression in humanized mice carrying the S allele [137]. Ethnic differences in the GT repeat lengths and disease severity has also been reported with high risk of recurrent and provoked venous thromboembolism in black patients carrying the L allele GT ≥ 35. Similarly, positive correlations between a decline in lung functions, risk of coronary artery diseases, and atherosclerosis with the L allele were more prominent in Japanese and European populations [129,130,133,138]. Meta-analysis in various diseases revealed positive associations between the presence of L allele and susceptibility of developing type 2 diabetes, coronary artery disease, restenosis after percutaneous coronary intervention, and squamous cell carcinoma [[139], [140], [141]].
Considering the above findings, presence of these polymorphisms may also be associated with COVID-19-affected patients becoming severely ill and being at high risk of acute lung injury as well as thromboembolism.
5. Conclusion
Abundant literature emphasizes the important role of IFNs in counteracting various viral infections including SARS-CoV-2. Similarly, significant data are available demonstrating antiviral effects of various HO-1 inducers acting through stimulation of IFNs. In this article, we presented evidence showing a beneficial role of HO-1 induction in inflammation-induced coagulation as seen in COVID-19 patients. Considering the association between promoter polymorphism and disease severity, we propose the need to identify the GT repeat lengths in severe COVID-19 patients. Based on the evidence presented above, we believe that there is a need to test the potential role of inducing HO-1, as a therapeutic approach not only as an antiviral strategy, but also as a treatment for COVID-19 associated complications like inflammation and coagulopathy.
References
- 1.Torales J., O'Higgins M., Castaldelli-Maia J.M., Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int. J. Soc. Psychiatr. 2020;66:317–320. doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 2.Ryter S.W., Choi A.M.K. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. 2016;167:7–34. doi: 10.1016/j.trsl.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi S., Omata Y., Sakamoto H., Higashimoto Y., Hara T., Sagara Y., Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–250. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Mccoubrey W.K., Huang T.J., Maines M.D. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 5.Morse D., Choi A.M.K. Heme oxygenase-1: from bench to bedside. Am. J. Respir. Crit. Care Med. 2005;172:660–670. doi: 10.1164/rccm.200404-465SO. [DOI] [PubMed] [Google Scholar]
- 6.Nitti M., Furfaro A.L., Mann G.E. Heme oxygenase dependent bilirubin generation in vascular cells: a role in preventing endothelial dysfunction in local tissue microenvironment? Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen T., Hortmann M., Oelze M., Opitz B., Steven S., Schell R., Knorr M., Karbach S., Schuhmacher S., Wenzel P., Münzel T., Daiber A. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell. Cardiol. 2010;49:186–195. doi: 10.1016/j.yjmcc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Brouard S., Otterbein L.E., Anrather J., Tobiasch E., Bach F.H., Choi A.M.K., Soares M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000;192:1015–1025. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 10.Espinoza J.A., Leon M.A., Cespedes P.F., Gomez R.S., Canedo-Marroquín G., Riquelme S.A., Salazar-Echegarai F.J., Blancou P., Simon T., Anegon I., Lay M.K., Gonzalez P.A., Riedel C.A., Bueno S.M., Kalergis A.M. Heme oxygenase-1 modulates human respiratory syncytial virus replication and lung pathogenesis during infection. J. Immunol. 2017;199:212–223. doi: 10.4049/jimmunol.1601414. [DOI] [PubMed] [Google Scholar]
- 11.Espinoza J.A., González P.A., Kalergis A.M. Modulation of antiviral immunity by heme oxygenase-1. Am. J. Pathol. 2017;187:487–493. doi: 10.1016/j.ajpath.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhang A., Duan H., Li N., Zhao L., Pu F., Huang B., Wu C., Nan Y., Du T., Mu Y., Zhao Q., Sun Y., Zhang G., Hiscox J.A., Zhou E.M., Xiao S. Heme oxygenase-1 metabolite biliverdin, not iron, inhibits porcine reproductive and respiratory syndrome virus replication. Free Radic. Biol. Med. 2017;102:149–161. doi: 10.1016/j.freeradbiomed.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Zhong M., Wang H., Ma L., Yan H., Wu S., Gu Z., Li Y. DMO-CAP inhibits influenza virus replication by activating heme oxygenase-1-mediated IFN response. Virol. J. 2019;16:21. doi: 10.1186/s12985-019-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang A., Wan B., Jiang D., Wu Y., Ji P., Du Y., Zhang G. The Cytoprotective Enzyme Heme oxygenase-1 suppresses pseudorabies virus replication in vitro. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santangelo R., Mancuso C., Marchetti S., Di Stasio E., Pani G., Fadda G. Bilirubin: an endogenous molecule with antiviral activity in vitro. Front. Pharmacol. 2012;3:1–8. doi: 10.3389/fphar.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thoms M., Buschauer R., Ameismeier M., Koepke L. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 2020;369:1249–1255. doi: 10.1126/science.abc8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konno Y., Kimura I., Uriu K., Fukushi M., Irie T., Koyanagi Y., Nakagawa S., Sato K. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is further increased by a naturally occurring elongation variant. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sa Ribero M., Jouvenet N., Dreux M., Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenoever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Andrea M., Ravera R., Gioia D., Gariglio M., Landolfo S. The interferon system: an overview. Eur. J. Paediatr. Neurol. 2002;6:A41–A46. doi: 10.1053/ejpn.2002.0573. [DOI] [PubMed] [Google Scholar]
- 21.Biron C.A., Nguyen K.B., Pien G.C., Cousens L.P., Salazar-Mather T.P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 22.Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A.M., Rimmelzwaan G.F., Van Amerongen G., Van Riel D., De Jong T., Itamura S., Chan K.H., Tashiro M., Osterhaus A.D.M.E. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroher U., DiCaro A., Li Y., Strong J.E., Aoki F., Plummer F., Jones S.M., Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-α. J. Infect. Dis. 2004;189:1164–1167. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antivir. Res. 2020;179:104811. doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clementi N., Ferrarese R., Criscuolo E., Diotti R.A., Castelli M., Scagnolari C., Burioni R., Antonelli G., Clementi M., Mancini N. Interferon-β-1a inhibition of severe acute respiratory syndrome–coronavirus 2 in vitro when administered after virus infection. J. Infect. Dis. 2020;222:722–725. doi: 10.1093/infdis/jiaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felgenhauer U., Schoen A., Gad H.H., Hartmann R., Schaubmar A.R., Failing K., Drosten C., Weber F. Inhibition of SARS-CoV-2 by type I and type III interferons. J. Biol. Chem. 2020;2 doi: 10.1074/jbc.ac120.013788. jbc.AC120.013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung I.F.N., Lung K.C., Tso E.Y.K., Liu R., Chung T.W.H., Chu M.Y., Ng Y.Y., Lo J., Chan J., Tam A.R., Shum H.P., Chan V., Wu A.K.L., Sin K.M., Leung W.S., Law W.L., Lung D.C., Sin S., Yeung P., Yip C.C.Y., Zhang R.R., Fung A.Y.F., Yan E.Y.W., Leung K.H., Ip J.D., Chu A.W.H., Chan W.M., Ng A.C.K., Lee R., Fung K., Yeung A., Wu T.C., Chan J.W.M., Yan W.W., Chan W.M., Chan J.F.W., Lie A.K.W., Tsang O.T.Y., Cheng V.C.C., Que T.L., Lau C.S., Chan K.H., To K.K.W., Yuen K.Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smits S.L., De Lang A., Brand J.M.A.V.D., Leijten L.M., Van Ijcken W.F., Eijkemans M.J.C., Van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D.M.E., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molony R.D., Nguyen J.T., Kong Y., Montgomery R.R., Shaw A.C., Iwasaki A. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aan2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng C.K., Hsu S.P., Lin C.K., Wu Y.H., Lee J.C., Young K.C. Celastrol inhibits hepatitis C virus replication by upregulating heme oxygenase-1 via the JNK MAPK/Nrf2 pathway in human hepatoma cells. Antivir. Res. 2017;146:191–200. doi: 10.1016/j.antiviral.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Z., Meleah Mathahs M., Schmidt W.N. Vol. 208. 2013. pp. 1653–1663. (Restoration of Type I Interferon Expression by Heme and Related Tetrapyrroles through Inhibition of NS3/4A Protease). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espinoza J.A., León M.A., Céspedes P.F., Gómez R.S., Canedo-marroquín G., Sebastían A., Salazar-echegarai F.J., Blancou P., Simon T., Anegon I., Lay M.K., Pablo A., Riedel C.A., Bueno S.M., Kalergis M., Canedo-marroquı G., Blancou P., Simon T., Anegon I., Lay M.K., Riedel C.A., Bueno S.M., Kalergis A.M. Heme Oxygenase-1 modulates human respiratory syncytial virus replication and lung pathogenesis during infection. J. Immunol. 2017;199:212–223. doi: 10.4049/jimmunol.1601414. [DOI] [PubMed] [Google Scholar]
- 34.Lee J.C., Tseng C.K., Young K.C., Sun H.Y., Wang S.W., Chen W.C., Lin C.K., Wu Y.H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014;171:237–252. doi: 10.1111/bph.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W.C., Wang S.Y., Chiu C.C., Tseng C.K., Lin C.K., Wang H.C., Lee J.C. Lucidone suppresses hepatitis c virus replication by Nrf2-mediated heme oxygenase-1 induction. Antimicrob. Agents Chemother. 2013;57:1180–1191. doi: 10.1128/AAC.02053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J.S., Chen W.-C., Tseng C.-K., Lin C.-K., Hsu Y.-C., Chen Y.-H., Lee J.-C. Sulforaphane suppresses hepatitis C virus replication by up-regulating heme oxygenase-1 expression through PI3K/Nrf2 pathway. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen M.H., Lee M.Y., Chuang J.J., Li Y.Z., Ning S.T., Chen J.C., Liu Y.W. Curcumin inhibits HCV replication by induction of heme oxygenase-1 and suppression of AKT. Int. J. Mol. Med. 2012;30:1021–1028. doi: 10.3892/ijmm.2012.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fillebeen C., Maria Rivas-Estilla A., Bisaillon M., Ponka P., Muckenthaler M., Hentze M.W., Koromilas A.E., Pantopoulos K. Iron inactivates the RNA polymerase NS5B and suppresses subgenomic replication of hepatitis C virus. J. Biol. Chem. 2005;280:995–999. doi: 10.1074/jbc.M412687200. [DOI] [PubMed] [Google Scholar]
- 39.Fillebeen C., Pantopoulos K. Iron inhibits replication of infectious hepatitis C virus in permissive Huh7.5.1 cells. J. Hepatol. 2010;53:995–999. doi: 10.1016/j.jhep.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 40.Fillebeen C., Pantopoulos K. Hepatitis C virus infection causes iron deficiency in Huh7.5.1 cells. PLoS One. 2013;8:83307. doi: 10.1371/journal.pone.0083307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagami T., Taji S., Takahashi M., Yamanishi K. Antiviral activity of a bile pigment, biliverdin, against human herpesvirus 6 (HHV-6) in vitro. Microbiol. Immunol. 1992;36:381–390. doi: 10.1111/j.1348-0421.1992.tb02037.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Z., Wilson A.T., Luxon B.A., Brown K.E., Mathahs M.M., Bandyopadhyay S., McCaffrey A.P., Schmidt W.N. Biliverdin inhibits hepatitis C virus nonstructural 3/4A protease activity: mechanism for the antiviral effects of heme oxygenase? Hepatology. 2010;52:1897–1905. doi: 10.1002/hep.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baez-Santos Y.M., John S.E. St, Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antivir. Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Z., Pu F., Zhang X., Yan Y., Zhao L., Zhang A., Li N., Zhou E.M., Xiao S. Carbon monoxide and biliverdin suppress bovine viral diarrhoea virus replication. J. Gen. Virol. 2017;98:2982–2992. doi: 10.1099/jgv.0.000955. [DOI] [PubMed] [Google Scholar]
- 45.Protzer U., Seyfried S., Quasdorff M., Sass G., Svorcova M., Webb D., Bohne F., Hösel M., Schirmacher P., Tiegs G. Antiviral activity and hepatoprotection by heme oxygenase-1 in hepatitis B virus infection. Gastroenterology. 2007;133:1156–1165. doi: 10.1053/j.gastro.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Gill A.J., Garza R., Ambegaokar S.S., Gelman B.B., Kolson D.L. Heme oxygenase-1 promoter region (GT)n polymorphism associates with increased neuroimmune activation and risk for encephalitis in HIV infection. J. Neuroinflammation. 2018;15:1–15. doi: 10.1186/s12974-018-1102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tseng C.K., Lin C.K., Wu Y.H., Chen Y.H., Chen W.C., Young K.C., Lee J.C. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci. Rep. 2016;6 doi: 10.1038/srep32176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill-Batorski L., Halfmann P., Neumann G., Kawaoka Y. The cytoprotective enzyme heme oxygenase-1 suppresses ebola virus replication. J. Virol. 2013;87:13795–13802. doi: 10.1128/jvi.02422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Kalamouni C., Frumence E., Bos S., Turpin J., Nativel B., Harrabi W., Wilkinson D.A., Meilhac O., Gadea G., Despres P., Krejbich-Trotot P., Viranaïcken W. Subversion of the heme oxygenase-1 antiviral activity by zika virus. Viruses. 2019;11 doi: 10.3390/v11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visner G.A., Lu F., Zhou H., Liu J., Kazemfar K., Agarwal A. Rapamycin induces heme oxygenase-1 in human pulmonary vascular cells: implications in the antiproliferative response to rapamycin. Circulation. 2003;107:911–916. doi: 10.1161/01.CIR.0000048191.75585.60. [DOI] [PubMed] [Google Scholar]
- 51.Chen C.Y., Jang J.H., Li M.H., Surh Y.J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 52.Becker J.C., Grosser N., Waltke C., Schulz S., Erdmann K., Domschke W., Schröder H., Pohle T. Beyond gastric acid reduction: proton pump inhibitors induce heme oxygenase-1 in gastric and endothelial cells. Biochem. Biophys. Res. Commun. 2006;345:1014–1021. doi: 10.1016/j.bbrc.2006.04.170. [DOI] [PubMed] [Google Scholar]
- 53.Ghebremariam Y.T., Cooke J.P., Gerhart W., Griego C., Brower J.B., Doyle-Eisele M., Moeller B.C., Zhou Q., Ho L., de Andrade J., Raghu G., Peterson L., Rivera A., Rosen G.D. Pleiotropic effect of the proton pump inhibitor esomeprazole leading to suppression of lung inflammation and fibrosis. J. Transl. Med. 2015;13:1–20. doi: 10.1186/s12967-015-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali F., Zakkar M., Karu K., Lidington E.A., Hamdulay S.S., Boyle J.J., Zloh M., Bauer A., Haskard D.O., Evans P.C., Mason J.C. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J. Biol. Chem. 2009;284:18882–18892. doi: 10.1074/jbc.M109.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu B.J., Chen K., Barter P.J., Rye K.A. Niacin inhibits vascular inflammation via the induction of heme oxygenase-1. Circulation. 2012;125:150–158. doi: 10.1161/circulationaha.111.053108. [DOI] [PubMed] [Google Scholar]
- 56.Jian Z., Tang L., Yi X., Liu B., Zhang Q., Zhu G., Wang G., Gao T., Li C. Aspirin induces Nrf2-mediated transcriptional activation of haem oxygenase-1 in protection of human melanocytes from H2O2-induced oxidative stress. J. Cell Mol. Med. 2016;20:1307–1318. doi: 10.1111/jcmm.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han S., Mallampalli R.K. The acute respiratory distress syndrome: from mechanism to translation. J. Immunol. 2015;194:855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. Jama. 2020;2019:1–13. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 59.van der Poll T. Tissue factor as an initiator of coagulation and inflammation in the lung. Crit. Care. 2008;12 doi: 10.1186/cc7026. S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monroe D.M., Key N.S. The tissue factor-factor VIIa complex: procoagulant activity, regulation, and multitasking. J. Thromb. Haemostasis. 2007;5:1097–1105. doi: 10.1111/j.1538-7836.2007.02435.x. [DOI] [PubMed] [Google Scholar]
- 61.Petaja J. Inflammation and coagulation. An overview. Thromb. Res. 2011;127:34–37. doi: 10.1016/S0049-3848(10)70153-5. [DOI] [PubMed] [Google Scholar]
- 62.H. Zhang, X. Zeng, S. He, Evaluation on potential contributions of protease activated receptors related mediators in allergic inflammation, Mediat. Inflamm.. 2014 (2014) 1-20. doi:10.1155/2014/829068. [DOI] [PMC free article] [PubMed]
- 63.Rosenberg R.D., Rosenberg J.S. Natural anticoagulant mechanisms. J. Clin. Invest. 1984;74:1–6. doi: 10.1172/JCI111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robson S.C., Shephard E.G., Kirsch R.E. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1β, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br. J. Haematol. 1994;86:322–326. doi: 10.1111/j.1365-2141.1994.tb04733.x. [DOI] [PubMed] [Google Scholar]
- 65.Piagnerelli M., Zouaoui Boudjeltia K., Vanhaeverbeek M., Vincent J.L. Red blood cell rheology in sepsis. Intensive Care Med. 2003;29:1052–1061. doi: 10.1007/s00134-003-1783-2. [DOI] [PubMed] [Google Scholar]
- 66.Janz D.R., Ware L.B. The role of red blood cells and cell-free hemoglobin in the pathogenesis of ARDS. J. Intensive Care. 2015;3:1–7. doi: 10.1186/s40560-015-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belcher J.D., Beckman J.D., Balla G., Balla J., Vercellotti G. Heme degradation and vascular injury. Antioxidants Redox Signal. 2010;12:233–248. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaer D.J., Schaer C.A., Buehler P.W., Boykins R.A., Schoedon G., Alayash A.I., Schaffner A. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107:373–380. doi: 10.1182/blood-2005-03-1014. [DOI] [PubMed] [Google Scholar]
- 69.Nielsen M.J., Møller H.J., Moestrup S.K. Hemoglobin and heme scavenger receptors. Antioxidants Redox Signal. 2010;12:261–273. doi: 10.1089/ars.2009.2792. [DOI] [PubMed] [Google Scholar]
- 70.Gaggar A., Patel R.P. There is blood in the water: hemolysis, hemoglobin, and heme in acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;311:L714–L718. doi: 10.1152/ajplung.00312.2016. [DOI] [PubMed] [Google Scholar]
- 71.Fredenburgh L.E., Perrella M.A., Mitsialis S.A. The role of heme oxygenase-1 in pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2007;36:158–165. doi: 10.1165/rcmb.2006-0331TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L., Banovich N.E., Barbry P., Brazma A., Collin J., Desai T.J., Duong T.E., Eickelberg O., Falk C., Farzan M., Glass I., Gupta R.K., Haniffa M., Horvath P., Hubner N., Hung D., Kaminski N., Krasnow M., Kropski J.A., Kuhnemund M., Lako M., Lee H., Leroy S., Linnarson S., Lundeberg J., Meyer K.B., Miao Z., Misharin A.V., Nawijn M.C., Nikolic M.Z., Noseda M., Ordovas-Montanes J., Oudit G.Y., Pe’er D., Powell J., Quake S., Rajagopal J., Tata P.R., Rawlins E.L., Regev A., Reyfman P.A., Rozenblatt-Rosen O., Saeb-Parsy K., Samakovlis C., Schiller H.B., Schultze J.L., Seibold M.A., Seidman C.E., Seidman J.G., Shalek A.K., Shepherd D., Spence J., Spira A., Sun X., Teichmann S.A., Theis F.J., Tsankov A.M., Vallier L., van den Berge M., Whitsett J., Xavier R., Xu Y., Zaragosi L.E., Zerti D., Zhang H., Zhang K., Rojas M., Figueiredo F. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.’ Alonzo D.D., De Fenza M., Pavone V. COVID-19 and pneumonia: a role for the uPA/uPAR system. Drug Discov. Today. 2020;25:1528–1534. doi: 10.1016/j.drudis.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lancman G., Marcellino B.K., Thibaud S., Troy K. Coombs-negative hemolytic anemia and elevated plasma hemoglobin levels in COVID-19. Ann. Hematol. 2020:1–3. doi: 10.1007/s00277-020-04202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu W., Li H. COVID-19 disease: ORF8 and surface glycoprotein inhibit heme metabolism by binding to porphyrin. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11938173.V3. [DOI] [Google Scholar]
- 80.Whyte C.S., Morrow G.B., Mitchell J.L., Chowdary P., Mutch N.J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID‐19. J. Thromb. Haemostasis. 2020;18:1548–1555. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gralinski L.E., Bankhead A., Jeng S., Menachery V.D., Proll S., Belisle S.E., Matzke M., Webb-Robertson B.J.M., Luna M.L., Shukla A.K., Ferris M.T., Bolles M., Chang J., Aicher L., Waters K.M., Smith R.D., Metz T.O., Law G.L., Katze M.G., McWeeney S., Baric R.S. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. mBio. 2013;4 doi: 10.1128/mBio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.M., Meziani F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alcaraz M., Fernandez P., Guillen M. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr. Pharmaceut. Des. 2005;9:2541–2551. doi: 10.2174/1381612033453749. [DOI] [PubMed] [Google Scholar]
- 88.Willis D., Moore A.R., Frederick R., Willoughby D.A. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat. Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 89.Tsoyi K., Tae Y.L., Young S.L., Hye J.K., Han G.S., Jae H.L., Ki C.C. Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo. Mol. Pharmacol. 2009;76:173–182. doi: 10.1124/mol.109.055137. [DOI] [PubMed] [Google Scholar]
- 90.Mishra S., Fujita T., Lama V.M., Nam D., Liao H., Okada M., Minamoto K., Yoshikawa Y., Harada H., Pinsky D.J. Carbon monoxide rescues ischemic lungs by interrupting MAPK-driven expression of early growth response 1 gene and its downstream target genes. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5191–5196. doi: 10.1073/pnas.0600241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakahira K., Hong P.K., Xue H.G., Nakao A., Wang X., Murase N., Drain P.F., Wang X., Sasidhar M., Nabel E.G., Takahashi T., Lukacs N.W., Ryter S.W., Morita K., Choi A.M.K. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J. Exp. Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarady-Andrews J.K., Liu F., Gallo D., Nakao A., Overhaus M., Öllinger R., Choi A.M., Otterbein L.E. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L1131–L1137. doi: 10.1152/ajplung.00458.2004. [DOI] [PubMed] [Google Scholar]
- 93.Mancuso C., Barone E., Guido P., Miceli F., Di Domenico F., Perluigi M., Santangelo R., Preziosi P. Inhibition of lipid peroxidation and protein oxidation by endogenous and exogenous antioxidants in rat brain microsomes in vitro. Neurosci. Lett. 2012;518:101–105. doi: 10.1016/j.neulet.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 94.Barone E., Trombino S., Cassano R., Sgambato A., De Paola B., Di Stasio E., Picci N., Preziosi P., Mancuso C. Characterization of the S-denitrosylating activity of bilirubin. J. Cell Mol. Med. 2009;13:2365–2375. doi: 10.1111/j.1582-4934.2008.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bein T., Grasso S., Moerer O., Quintel M., Guerin C., Deja M., Brondani A., Mehta S. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med. 2016;42:699–711. doi: 10.1007/s00134-016-4325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Belperio J.A., Keane M.P., Lynch J.P., Strieter R.M. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin. Respir. Crit. Care Med. 2006;27:350–364. doi: 10.1055/s-2006-948289. [DOI] [PubMed] [Google Scholar]
- 97.Constantin M., Choi A.J.S., Cloonan S.M., Ryter S.W. Therapeutic potential of heme oxygenase-1/carbon monoxide in lung disease. Int. J. Hypertens. 2012 doi: 10.1155/2012/859235. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang L., Zhao B., Chen Y., Ma L., Chen E.Z., Mao E.Q. Inflammation and edema in the lung and kidney of hemorrhagic shock rats are alleviated by biliary tract external drainage via the heme oxygenase-1 pathway. Inflammation. 2015;38:2242–2251. doi: 10.1007/s10753-015-0208-z. [DOI] [PubMed] [Google Scholar]
- 99.Mancuso C., Perluigi M., Cini C., De Marco C., Giuffrida Stella A.M., Calabrese V. Heme oxygenase and cyclooxygenase in the central nervous system: a functional interplay. J. Neurosci. Res. 2006;84:1385–1391. doi: 10.1002/jnr.21049. [DOI] [PubMed] [Google Scholar]
- 100.Mancuso C., Preziosi P., Grossman A.B., Navarra P. The role of carbon monoxide in the regulation of neuroendocrine function. Neuroimmunomodulation. 1997;4:225–229. doi: 10.1159/000097340. [DOI] [PubMed] [Google Scholar]
- 101.Mancuso C. Bilirubin and brain: a pharmacological approach. Neuropharmacology. 2017;118:113–123. doi: 10.1016/j.neuropharm.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 102.Scuto M.C., Mancuso C., Tomasello B., Ontario M.L., Cavallaro A., Frasca F., Maiolino L., Salinaro A.T., Calabrese E.J., Calabrese V. Curcumin, hormesis and the nervous system. Nutrients. 2019;11:1–17. doi: 10.3390/nu11102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baum L., Cheung S.K.K., Mok V.C.T., Lam L.C.W., Leung V.P.Y., Hui E., Ng C.C.Y., Chow M., Ho P.C., Lam S., Woo J., Chiu H.F.K., Goggins W., Zee B., Wong A., Mok H., Cheng W.K.F., Fong C., Lee J.S.W., Chan M.H., Szeto S.S.L., Lui V.W.C., Tsoh J., Kwok T.C.Y., Chan I.H.S., Lam C.W.K. Curcumin effects on blood lipid profile in a 6-month human study. Pharmacol. Res. 2007;56:509–514. doi: 10.1016/j.phrs.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 104.Peng L., Mundada L., Stomel J.M., Liu J.J., Sun J., Yet S.F., Fay W.P. Induction of heme oxygenase-1 expression inhibits platelet-dependent thrombosis. Antioxidants Redox Signal. 2004;6:729–735. doi: 10.1089/1523086041361677. [DOI] [PubMed] [Google Scholar]
- 105.Lindenblatt N., Bordel R., Schareck W., Menger M.D., Vollmar B. Vascular heme oxygenase-1 induction suppresses microvascular thrombus formation in vivo. Arterioscler. Thromb. Vasc. Biol. 2004;24:601–606. doi: 10.1161/01.ATV.0000118279.74056.8a. [DOI] [PubMed] [Google Scholar]
- 106.Fujita T., Toda K., Karimova A., Yan S.F., Naka Y., Yet S.F., Pinsky D.J. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat. Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y.H., Tsai H.L., Chiang M.T., Chau L.Y. Carbon monoxide-induced early thrombolysis contributes to heme oxygenase-1-mediated inhibition of neointimal growth after vascular injury in hypercholesterolemic mice. J. Biomed. Sci. 2006;13:721–730. doi: 10.1007/s11373-006-9093-7. [DOI] [PubMed] [Google Scholar]
- 108.True A.L., Olive M., Boehm M., San H., Westrick R.J., Raghavachari N., Xu X., Lynn E.G., Sack M.N., Munson P.J., Gladwin M.T., Nabel E.G. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ. Res. 2007;101:893–901. doi: 10.1161/circresaha.107.158998. [DOI] [PubMed] [Google Scholar]
- 109.Tracz M.J., Juncos J.P., Grande J.P., Croatt A.J., Ackerman A.W., Katusic Z.S., Nath K.A. Induction of heme oxygenase-1 is a beneficial response in a murine model of venous thrombosis. Am. J. Pathol. 2008;173:1882–1890. doi: 10.2353/ajpath.2008.080556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.P.S. Hassaan, R.A. Mehanna, A.E. Dief, The potential role of hemopexin and heme oxygenase-1 inducer in a model of sepsis, Phys. J. 2015 (2015) 1–10. doi:10.1155/2015/208485.
- 111.Nath K.A., Grande J.P., Belcher J.D., Garovic V.D., Croatt A.J., Hillestad M.L., Barry M.A., Nath M.C., Regan R.F., Vercellotti G.M. Antithrombotic effects of heme-degrading and heme-binding proteins. Am. J. Physiol. Heart Circ. Physiol. 2020;318:H671–H681. doi: 10.1152/ajpheart.00280.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen B., Guo L., Fan C., Bolisetty S., Joseph R., Wright M.M., Agarwal A., George J.F. Carbon monoxide rescues heme oxygenase-1-deficient mice from arterial thrombosis in allogeneic aortic transplantation. Am. J. Pathol. 2009;175:422–429. doi: 10.2353/ajpath.2009.081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gabre J., Chabasse C., Cao C., Mukhopadhyay S., Siefert S., Bi Y., Netzel-Arnett S., Sarkar R., Zhang L. Activated protein C accelerates venous thrombus resolution through heme oxygenase-1 induction. J. Thromb. Haemostasis. 2014;12:93–102. doi: 10.1111/jth.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yachie A., Niida Y., Wada T., Igarashi N., Kaneda H., Toma T., Ohta K., Kasahara Y., Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Radhakrishnan N., Pruthi P.K., Sawhney S., Piplani T. Human heme oxygenase-1 deficiency presenting. J. Pediatr. Hematol. Oncol. 2011;33:74–78. doi: 10.1097/MPH.0b013e3181fd2aae. [DOI] [PubMed] [Google Scholar]
- 116.Koizumi S. Human heme oxygenase-1 deficiency: a lesson on serendipity in the discovery of the novel disease. Pediatr. Int. 2007;49:125–132. doi: 10.1111/j.1442-200X.2007.02353.x. [DOI] [PubMed] [Google Scholar]
- 117.Tsur A., Kalish F., Burgess J., Nayak N.R., Zhao H., Casey K.M., Druzin M.L., Wong R.J., Stevenson D.K. Pravastatin improves fetal survival in mice with a partial deficiency of heme oxygenase-1. Placenta. 2019;75:1–8. doi: 10.1016/j.placenta.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 118.Kimpara T., Takeda A., Watanabe K., Itoyama Y., Ikawa S., Watanabe M., Arai H., Sasaki H., Higuchi S., Okita N., Takase S., Saito H., Takahashi K., Shibahara S. Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum. Genet. 1997;100:145–147. doi: 10.1007/s004390050480. [DOI] [PubMed] [Google Scholar]
- 119.Yamada N., Yamaya M., Okinaga S., Nakayama K., Sekizawa K., Shibahara S., Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am. J. Hum. Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hirai H., Kubo H., Yamaya M., Nakayama K., Numasaki M., Kobayashi S., Suzuki S., Shibahara S., Sasaki H. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood. 2003;102:1619–1621. doi: 10.1182/blood-2002-12-3733. [DOI] [PubMed] [Google Scholar]
- 121.Exner M., Minar E., Wagner O., Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic. Biol. Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 122.Cao L., Zhang Z., Cai B., Bai W., Zhang Y., Sen W., Xie X., Sun W., Cai Q., Li Z., Liu D., Xiong Y., Ma M., Liu X., Xu G. Association of heme oxygenase-1 gene rs2071746 polymorphism with vascular outcomes in patients with atherosclerotic stroke. J. Neurol. Sci. 2014;344:154–157. doi: 10.1016/j.jns.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 123.Lee E.Y., Lee Y.H., Kim S.H., Chung K.S., Kwon O., Kim B.S., Nam C.M., Park C.S., Lee B.W., Kang E.S., Cha B.S., Lee H.C. Association between heme oxygenase-1 promoter polymorphisms and the development of albuminuria in type 2 diabetes: a case-control study. Med. (United States). 2015;94:1–8. doi: 10.1097/MD.0000000000001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ono K., Goto Y., Takagi S., Baba S., Tago N., Nonogi H., Iwai N. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis. 2004;173:313–317. doi: 10.1016/j.atherosclerosis.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 125.Lublinghoff N., Winkler K., Winkelmann B.R., Seelhorst U., Wellnitz B., Boehm B.O., Marz W., Hoffmann M.M. Genetic variants of the promoter of the heme oxygenase-1 gene and their influence on cardiovascular disease ( the Ludwigshafen Risk and Cardiovascular Health Study ) BMC Med. Genet. 2009;10:1–9. doi: 10.1186/1471-2350-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rueda B., Oliver J., Robledo G., Lopez-Nevot M.A., Balsa A., Pascual-Salcedo D., Gonzalez-Gay M.A., Gonzalez-Escribano M.F., Martin J. HO-1 promoter polymorphism associated with rheumatoid arthritis. Arthritis Rheum. 2007;56:3953–3958. doi: 10.1002/art.23048. [DOI] [PubMed] [Google Scholar]
- 127.Tanaka G., Aminuddin F., Akhabir L., He J.Q., Shumansky K., Connett J.E., Anthonisen N.R., Abboud R.T., Paré P.D., Sandford A.J. Effect of heme oxygenase-1 polymorphisms on lung function and gene expression. BMC Med. Genet. 2011;12:1–8. doi: 10.1186/1471-2350-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ono K., Mannami T., Iwai N. Association of a promoter variant of the haeme oxygenase-1 gene with hypertension in women. J. Hypertens. 2003;21:1497–1503. doi: 10.1097/00004872-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 129.Nakayama K., Kikuchi A., Yasuda H., Ebihara S., Sasaki T., Ebihara T., Yamaya M. Heme oxygenase-1 gene promoter polymorphism and decline in lung function in Japanese men. Thorax. 2006;61:921. doi: 10.1136/thx.2006.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guenegou A., Leynaert B., Benessiano J., Pin I., Demoly P., Neukirch F., Boczkowski J., Aubier M. Association of lung function decline with the heme oxygenase-1 gene promoter microsatellite polymorphism in a general population sample. Results from the European Community Respiratory Health Survey (ECRHS), France. J. Med. Genet. 2006;43:1–6. doi: 10.1136/jmg.2005.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Exner M., Schillinger M., Minar E., Mlekusch W., Schlerka G., Haumer M., Mannhalter C., Wagner O. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J. Endovasc. Ther. 2001;8:433–440. doi: 10.1583/1545-1550(2001)008<0433:HOGPMP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 132.Dick P., Schillinger M., Minar E., Mlekusch W., Amighi J., Sabeti S., Schlager O., Raith M., Endler G., Mannhalter C., Wagner O., Exner M. Haem oxygenase-1 genotype and cardiovascular adverse events in patients with peripheral artery disease. Eur. J. Clin. Invest. 2005;35:731–737. doi: 10.1111/j.1365-2362.2005.01580.x. [DOI] [PubMed] [Google Scholar]
- 133.Pechlaner R., Willeit P., Summerer M., Santer P., Egger G., Kronenberg F., Demetz E., Weiss G., Tsimikas S., Witztum J.L., Willeit K., Iglseder B., Paulweber B., Kedenko L., Haun M., Meisinger C., Gieger C., Müller-Nurasyid M., Peters A., Willeit J., Kiechl S. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with progressive atherosclerosis and incident cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2015;35:229–236. doi: 10.1161/atvbaha.114.304729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mustafa S., Weltermann A., Fritsche R., Marsik C., Wagner O., Kyrle P.A., Eichinger S. Genetic variation in heme oxygenase 1 (HMOX1) and the risk of recurrent venous thromboembolism. J. Vasc. Surg. 2008;47:566–570. doi: 10.1016/j.jvs.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 135.Garza R., Gill A.J., Bastien B.L., Garcia-Mesa Y., Gruenewald A.L., Gelman B.B., Tsima B., Gross R., Letendre S.L., Kolson D.L. Heme oxygenase-1 promoter (GT) n polymorphism associates with HIV neurocognitive impairment. Neurol. Neuroimmunol. Neuroinflammation. 2020;7:710. doi: 10.1212/NXI.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seu L., Burt T.D., Witte J.S., Martin J.N., Deeks S.G., Mccune J.M. Variations in the heme oxygenase-1 microsatellite polymorphism are associated with plasma CD14 and viral load in HIV-infected African-Americans. Gene Immun. 2012;13:258–267. doi: 10.1038/gene.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kah J., Volz T., Lütgehetmann M., Groth A., Lohse A.W., Tiegs G., Sass G., Dandri M. Haem oxygenase-1 polymorphisms can affect HCV replication and treatment responses with different efficacy in humanized mice. Liver Int. 2017;37:1128–1137. doi: 10.1111/liv.13347. [DOI] [PubMed] [Google Scholar]
- 138.Kaneda H., Ohno M., Taguchi J., Togo M., Hashimoto H., Ogasawara K., Aizawa T., Ishizaka N., Nagai R. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors, Arterioscler. Thromb. Vasc. Biol. 2002;22:1680–1685. doi: 10.1161/01.ATV.0000033515.96747.6F. [DOI] [PubMed] [Google Scholar]
- 139.Bao W., Song F., Li X., Rong S., Yang W., Wang D., Xu J., Fu J., Zhao Y., Liu L. Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am. J. Epidemiol. 2010;172:631–636. doi: 10.1093/aje/kwq162. [DOI] [PubMed] [Google Scholar]
- 140.Zhang M.M., Zheng Y.Y., Gao Y., Zhang J.Z., Liu F., Yang Y.N., Li X.M., Ma Y.T., Xie X. Heme oxygenase-1 gene promoter polymorphisms are associated with coronary heart disease and restenosis after percutaneous coronary intervention: a meta-analysis. Oncotarget. 2016;7:83437–83450. doi: 10.18632/oncotarget.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qiao H., Sai X., Gai L., Huang G., Chen X., Tu X., Ding Z. Association between heme oxygenase 1 gene promoter polymorphisms and susceptibility to coronary artery disease: a HuGE review and meta-analysis. Am. J. Epidemiol. 2014;179:1039–1048. doi: 10.1093/aje/kwu024. [DOI] [PubMed] [Google Scholar]