Abstract
Context
Crosstalk through receptor ligand interactions at the maternal-fetal interface is impacted by fetal sex. This affects placentation in the first trimester and differences in outcomes. Sexually dimorphic signaling at early stages of placentation are not defined.
Objective
Investigate the impact of fetal sex on maternal-fetal crosstalk.
Design
Receptors/ligands at the maternal-fetal surface were identified from sexually dimorphic genes between fetal sexes in the first trimester placenta and defined in each cell type using single-cell RNA-Sequencing (scRNA-Seq).
Setting
Academic institution.
Samples
Late first trimester (~10-13 weeks) placenta (fetal) and decidua (maternal) from uncomplicated ongoing pregnancies.
Main outcome measures
Transcriptomic profiling at tissue and single-cell level; immunohistochemistry of select proteins.
Results
We identified 91 sexually dimorphic receptor-ligand pairs across the maternal-fetal interface. We examined fetal sex differences in 5 major cell types (trophoblasts, stromal cells, Hofbauer cells, antigen-presenting cells, and endothelial cells). Ligands from the CC family chemokine ligand (CCL) family were most highly representative in females, with their receptors present on the maternal surface. Sexually dimorphic trophoblast transcripts, Mucin-15 (MUC15) and notum, palmitoleoyl-protein carboxylesterase (NOTUM) were also most highly expressed in syncytiotrophoblasts and extra-villous trophoblasts respectively. Gene Ontology (GO) analysis using sexually dimorphic genes in individual cell types identified cytokine mediated signaling pathways to be most representative in female trophoblasts. Upstream analysis demonstrated TGFB1 and estradiol to affect all cell types, but dihydrotestosterone, produced by the male fetus, was an upstream regulator most significant for the trophoblast population.
Conclusions
Maternal-fetal crosstalk exhibits sexual dimorphism during placentation early in gestation.
Keywords: first trimester placenta, sex differences, human pregnancy, single-cell RNA sequencing, placenta cell types, receptor-ligand
Sex differences in pregnancy outcomes have been well-documented for decades (1-11). Worldwide epidemiologic data demonstrate sexual dimorphism in maternal and fetal outcomes, all of which are intricately related to the function of the uteroplacental unit. Fetal growth is determined partly by fetal sex; male fetuses are larger throughout gestation and heavier at birth (2, 6, 12). Increased rates of macrosomia have been reported, and gestational diabetes and maternal obesity independently increase the risk of macrosomia in male fetuses only—females appear to be less susceptible to maternal factors promoting increased growth (3). The immune system also appears to be altered, leading to differences in outcomes as a result of fetal sex (13, 14). Prematurity, a major cause of neonatal morbidity and mortality in the United States, is more common in male fetuses, including periviable, preterm, and late preterm deliveries (4-7, 11), which is attributed to immune dysfunction, including an imbalance between innate and adaptive immune cells at the maternal-fetal interface (15). Fetal sex also impacts maternal health. Some epidemiologic studies have demonstrated an increased risk of preeclampsia with male fetuses (1, 6, 10), although this has not been found consistently (1, 7-9).
Despite the large body of literature documenting sex-dependent outcomes, the pathophysiologic mechanism for observed sex differences is poorly understood and likely occurs early in gestation during placentation. Crosstalk, specifically through receptor-ligand interactions at the maternal-fetal interface, may play a significant role in these sex differences. We identified sex differences in signaling at the maternal-fetal interface, including members of the transforming growth factor-β (TGF-β) superfamily, specifically TGFβ-1 and bone morphogenic proteins (BMPs). Of differentially expressed genes, ligands among individual cell types from the CC chemokine ligand (CCL) family were most highly representative in females, whereas interleukin 1 receptor antagonist (IL1RN), matrix metallopeptidase 9 (MMP9), and insulin-like growth factor 1 (IGF1) were highly expressed in males, with their corresponding receptors present on the maternal surface. Furthermore, upstream regulators of sexually dimorphic signaling demonstrated that TGFB1 and estradiol affect bulk placenta tissue and individual cell types but dihydrotestosterone, which is produced by the male fetus, was an upstream regulator that was most significant for the trophoblast population.
Materials and Methods
Patient recruitment
Subjects with spontaneously conceived singleton pregnancies were recruited into the Cedars Sinai Medical Center (CSMC) Prenatal Biorepository at time of chorionic villus sampling. With informed consent following the Institutional Review Board–approved protocol (Pro00008600), chorionic villi (placental tissues) and matching decidua (if available) at 10 to 13 weeks of gestational age were obtained that would otherwise be discarded. All pregnancies were healthy and had a normal karyotype.
Demographics and pregnancy outcomes
All pregnancies were genetically normal and resulted in healthy term live births, 5 females and 5 males. There is no statistically significant difference in birthweight between female and male infants (P = 0.47) (16).
Tissue storage and processing for bulk RNA-Seq
Tissue samples (5-15 mg) were stored with 250 μL RNAlater RNA Stabilization Reagent (QIAGEN, Hilden, Germany) at −80°C in the Cedars Sinai Medical Center Prenatal Repository until further processing. Tissue was processed as previously described (17). Briefly, tissue was thawed on ice with 600 μL of RLT Plus lysis buffer (QIAGEN) and 1% β-mercaptoethanol added to each sample. Tissue was homogenized by passing through increasingly thinner single-use needles (22G, 25G, 27G) attached to a 1 mL sterile, RNase-free syringe. The homogenates were loaded onto AllPrep spin columns and the remainder of the protocol was performed following manufacturer instructions for the AllPrep DNA/RNA Mini Kit (QIAGEN). The RNA elution was used for total RNA-Seq.
Bulk RNA-Seq of decidua and placenta tissue
RNA-Seq libraries were constructed using Illumina TruSeq Stranded Total RNA LT with Ribo-zero kits (Illumina, San Diego). Libraries were pooled at a 4 nM concentration and an average of 19.01 million 2 × 75 base pairs (bp) paired-end reads per sample were generated with Illumina NextSeq 500 using High Output 150-cycle flow cells. Reads were aligned to the human reference genome (build GRCh38 with Ensembl release version 82) using STAR (18). The DESeq2 Bioconductor package was used in the R statistical computing environment (http://www.R-project.org/) to normalize count data, estimate dispersion, and fit a negative binomial model for each gene (19-21). P values were adjusted for multiple comparisons using the Benjamini-Hochberg procedure to generate false discovery rate (FDR) values. To describe tissue expression, we utilized both the fragments per kilobase of transcript per million mapped reads (FPKM) value and a Z-score describing the position of a gene’s FPKM value compared to all other genes in a sample. The Z-score for a gene equals the number of standard deviations away from the mean FPKM of all genes (in a sample), such that a gene with Z-score of zero (0) has an FPKM equal to the mean FPKM of all genes (in that sample), and genes with a positive Z-score have an FPKM above the mean (in that sample). To determine which genes were expressed in each tissue type, we applied thresholds of mean FPKM >1 and mean Z-score >0 (n = 4 per tissue). Consequently, “expressed genes” describes genes which are consistently expressed in decidua or placental tissue. Our criteria for defining expressed genes in decidua is FPKM >1 and Z-score >0. This is similar to zFPKM method (22). Bulk placenta sex differences data comes from total RNA-Sequencing of chorionic villus sampling tissue (17 female and 22 male) available at National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) accession GSE109082, using the study-specific FPKM >1.281 expression threshold (12).
Identification of receptors and ligands in placenta
We used previously published dataset (NCBI GEO accession GSE109082) and filtered by FPKM >1.281 and P < 0.05 to select placenta-expressed sexually dimorphic genes. We mined the Human Protein Atlas for annotations on all protein-coding genes, data accessed on September 8, 2017 (23). We mined the CellPhoneDB database for receptors (protein_curated.csv) and ligands (non-receptors in interaction_curated.csv) (24), which we annotated with Ensembl Gene IDs using R package biomaRt (25, 26). To identify placenta-expressed genes, we cross-referenced Ensembl Gene IDs to bulk RNA-Seq datasets with the pandas Python package (27). The initial list of receptors was a merged list of 4 sources (i) All confirmed receptors identified by Human Protein Atlas, including nuclear receptors and G-protein coupled receptors; (ii) A manual filtering of genes with “receptor” in the gene description after removing false positives such as “non-receptor type” and manually verifying potential hits such as “receptor-like” using annotations from the UniProt protein knowledgebase and the NCBI Gene database (28, 29). Proteins that bound small molecules or extracellular proteins were added as receptors; (iii) protein-coding genes that had Gene Ontology (GO) terms “receptor” and “binding” and were also identified by the Human Protein Atlas as membrane proteins; and (iv) receptors identified by CellPhoneDB (24).
Similarly, the initial list of candidate ligands was compiled from a merged list of: (i) manually filtered genes with “ligand” in the gene description; (ii) genes identified as “predicted secreted” by Human Protein Atlas; (iii) genes identified as “plasma proteins” by Human Protein Atlas; and (iv) non-receptors in CellPhoneDB’s list of interacting pairs (interaction_curated.csv). Genes identified through both methods (eg, CRLF1) were sorted into the receptor category to resolve conflicts.
Placenta sexually dimorphic receptors and ligands (P < 0.05) were input into the Human Plasma Membrane Receptome (http://www.receptome.org/) and CellPhoneDB (https://www.cellphonedb.org/) to identify interacting receptors and ligands (output) (24, 30). To identify which output genes are expressed at the maternal-fetal interface, we cross-referenced the database output with our bulk RNA-Seq data. Since our goal is to identify cell-cell communication pathways relevant for maternal-fetal crosstalk, we kept receptor-receptor pairs and include these when discussing “receptor-ligand interactions” throughout the text, similar to previous publications (24).
Circos plots
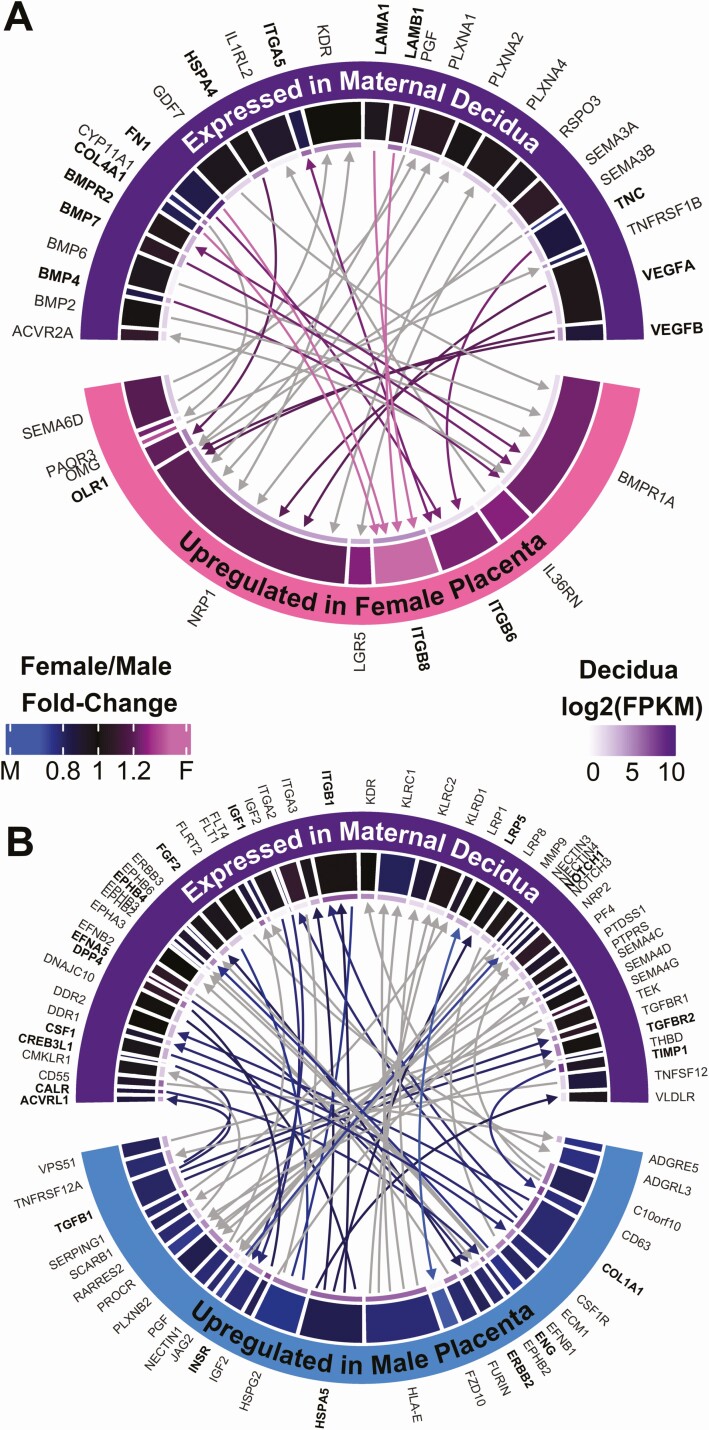
To visualize receptor-ligand interactions between placenta and decidua, circos plots were generated using chordDiagram function in the R package circlize 0.4.8 (RRID: SCR_002141) (21, 31). Gene inputs (“from” column) and gene outputs (“to” column) are interacting receptors and ligands discovered as described earlier. The fold-change heatmap colors were generated with function colorRamp2, using black as the middle color corresponding to 1:1 fold-change (31). The innermost thin grid represents the gene FPKM value in decidua, colors ranging from white (FPKM = 1) to the darker purples for higher expression. Links are comprised of lines and arrows, with arrowheads pointing to receptors (Fig. 1).
Figure 1.
Sexually dimorphic receptor-ligand interactions at the maternal-fetal interface. (A, B) Circos plots showing interactions between sexually dimorphic placenta genes and the maternal decidua. Arrowheads point to receptors. Overlap with upstream regulators is highlighted with bold text and color links. Other genes are not bold, with gray links. Grids represent fold-change sex differences (thick grid, blue-black-pink) and decidua FPKM (thin grid, white-purple, starting at FPKM = 1). (A) Female-upregulated placenta crosstalk to decidua. (B) Male-upregulated placenta crosstalk to decidua.
ScRNA-Seq: preparation of the first trimester placental cells
Fresh placental tissues of approximately 5 to 10 mg were placed in collection medium, transferred to the laboratory, and kept at 4 °C overnight before cell preparation. The collection medium was αMEM with sodium heparin (100 000 units/100 mL H2O, Sigma), gentamycin (0.5%, Invitrogen) and antibiotic-antimycotic (2%, Invitrogen). The tissue was washed in cold phosphate-buffered saline to remove maternal contamination and minced with a sterile scalpel, then mixed with 0.25% trypsin, 300 U/mL collagenase and 200 μg/mL DNAse I and incubated in a 37 °C, 5% CO2 incubator for 90 minutes with occasional agitation. After centrifugation at 1200 rpm for 10 minutes, cell pellets were carefully resuspended in Chang medium (Irvine Scientific). To avoid overpopulation of erythrocytes in the captured cell population, 1× Red Blood Cell (RBC) lysis buffer was applied to the cell suspension for 15 minutes at room temperature, after which the cells were carefully washed and resuspended in 100 μL Chang medium and strained using a 70-μm Flowmi cell strainer (Bel-Art) immediately before library construction.
ScRNA-Seq: library construction and sequencing
Single-cell RNA-Seq libraries were prepared per Single Cell 3′ v2 Reagent Kits User Guide (10X Genomics, Pleasanton, California). Single-cell suspensions were loaded on a Chromium Controller instrument (10X Genomics) to generate single-cell Gel Bead-In-EMulsions (GEMs). GEM-RT were performed in a Veriti 96-well thermal cycler (Thermo Fisher Scientific, Waltham, MA), following which, GEMs were harvested and the cDNAs were amplified and cleaned up with SPRIselect Reagent Kit. Indexed sequencing libraries were constructed using Chromium Single-Cell 3′ Library Kit for enzymatic fragmentation, end-repair, A-tailing, adapter ligation, ligation cleanup, sample index polymerase chain reaction (PCR), and PCR cleanup. The barcoded sequencing libraries were quantified using the KAPA Library Quantification Kit (KAPA Biosystems, Wilmington, MA). Sequencing libraries were loaded on a NovaSeq 6000 (Illumina, San Diego, CA) with a custom sequencing setting (26 bp for Read 1 and 91 bp for Read 2).
ScRNA-Seq: data processing and clustering analyses
Cell Ranger 2.0.0 (10X Genomics) was used to demultiplex reads and to convert raw base call files into fastq format. Reads alignment was performed by using STAR (version 2.5.1) (32) with hg38 transcriptome reference from Gencode 25 annotation, containing all protein-coding and long noncoding RNA genes. Raw expression counts for each gene in all samples were collapsed to unique molecular identifier (UMI) counts using Cell Ranger 2.0.0 (10X Genomics). Data filtration and normalization was performed using the workflow described in (33, 34) with scran 1.8.4 and scater 1.8.4 packages (35). Briefly, we filtered cells with log-library sizes that are more than 2 median absolute deviations (MADs) below median as well as cells with log-transformed expressed genes that are more than 2 MADs below median. Cells with mitochondrial proportions of 2 MADs higher than median were also removed. In addition, the low-abundance genes (average UMI counts <0.1) were excluded, yielding 7245 cells and 6806 genes in total for further analyses, with median genes of 1860 per cell and median UMI counts of 5654 per cell. The data matrix was then cell-specifically normalized by a deconvolution method using centered pool-based size factor (34) for similar cells with clustering. We applied a shared nearest neighbor graph-based algorithm to cluster the cells for global population. To obtain 2-dimensional visualization of the cell population, principal components analysis was first run on the normalized log-expression values. The top 10 principal components that explained the most variability were selected to perform t-distributed stochastic neighbor embedding (tSNE) for cells using default parameters. For tSNE, the perplexity parameter and the Ɵ parameter were set to 30 and 0.5, respectively, while the other parameters were left as default and total iterations was 1000. For subclustering analysis within the trophoblast population, an average UMI cutoff of 0.05 was applied to remove low-abundance genes, and normalization using the same method as above was performed. A total of 1465 cells and 8214 genes were used, and hierarchical clustering with a dynamic tree cut was performed to define subclusters of trophoblast cells. For analyses on both global and trophoblast populations, outliers in tSNE were excluded.
ScRNA-Seq: identification of differentially expressed genes
The edgeR 3.22.4 package (36) was applied to conduct differential expression analysis. Briefly, after initial filtration and normalization, the SingleCellExperiment object was converted to a DGEList object containing the UMI counts and calculated size factors. After dispersion estimation using a design matrix, differentially expressed genes (DEGs) were identified between any 2 cell types by fitting a negative binomial generalized log-linear model adjusted by samples; that is, using an additive model with “sample” as the blocking factor so that identified DEGs between cell types exist in every sample and therefore each sex, preventing bias from oversampling female placenta cells. The Benjamini-Hochberg method was applied for multiple test corrections to calculate false discovery rates (FDR). Pairwise comparison between cell types was performed, and the DEGs (Log2FC >1, FDR <0.01) that were specifically enriched for each cell type were depicted as a heatmap (genes that are expressed in at least 5% of cells from all conceptions were selected). For DEG analyses between sexes in each cell type, the DEGs (FDR <0.01) from both male and female conceptions were selected for enrichment analysis. A stricter cutoff of FDR <0.01 and |Log2FC| >1 was applied to highlight the most significant genes between sexes.
Enrichment analysis and upstream analysis
For bulk RNA-Seq analysis: Placenta-expressed (FPKM >1.281) receptors and ligands with sex differences at P < 0.05 were used for core analyses with Ingenuity Pathway Analysis software (RRID: SCR_008653). Inputs were Ensembl Gene IDs, P values, and log2 fold-change from DESeq2 (20).
For single-cell analysis: The Gene Ontology Consortium website (http://www.geneontology.org/) revealed Enriched Gene Ontology terms (biological processes) using the cluster-specific genes. Upregulated genes were used for enrichment analysis for fetal sexes (FDR <0.01) or comparing 2 subclusters of trophoblast cells (Log2FC >1, FDR <0.01). Bonferroni-corrected for P < 0.05 was used for multiple testing. For upstream regulator analyses using Ingenuity Pathway Analysis (RRID: SCR_008653), input was significantly and specifically expressed genes in each cell type following DEG analysis and pairwise comparison. Molecule types of upstream regulators included were: endogenous mammalian chemicals, cytokines, enzymes, G-protein coupled receptor, growth factor, ion channel, kinase, ligand-dependent nuclear receptor, mature miRNA, miRNA, peptidase, phosphatase, transcription regulator, translation regulator, transmembrane receptor, transporter.
Identification of receptor ligands at the single-cell level
DEGs (Log2FC >1, FDR <0.01) between fetal sexes identified in each cell type were cross-referenced with Human Plasma Membrane Receptome (http://www.receptome.org/) and Human Protein Atlas to select for known receptors/ligands among these DEGs, as well as to identify their interacting ligands/receptors. We then cross-referenced the partner genes (ligands/receptors) of these differentially expressed receptors/ligands at the single-cell level with our bulk RNA-Seq data from decidua and placenta. Only the pairs that have tissue-expressed partner genes (Z >0 and FPKM >1, either expressed in both placenta and decidua or only in decidua) were selected for presentation of the sexually dimorphic receptors/ligands at the maternal-fetal interface at the single-cell level.
Immunohistochemistry
Placental tissues (12-14 weeks) from patients undergoing termination were harvested and fixed in 10% formalin for 48 hours before paraffin embedding at 72 °C with EZ solution (Cat 950-100). Sections (4 μm) were dewaxed and incubated in antigen retrieval solution for 64 minutes. Tris solution CC1, PH 8 (Cat 950-124) at 98 °C was used for mucin-15 (MUC15) antigen retrieval and citrate solution CC2, PH 6 (Cat 950-123) at 91 °C was used for notum, palmitoleoyl-protein carboxylesterase (NOTUM) antigen retrieval. Following incubation with the inhibitor CM (Cat 760–4307) at room temperature for 12 minutes, sections were incubated with primary antibodies for 1 hour at room temperature (anti-MUC15 ab224468, abcam, 1:1000; anti-NOTUM, SAB3500082, Sigma, 1:8000; both were diluted with antibody dilution buffer Cat ADB250). For detection, Discovery Anti-Rabbit HQ RUO (Cat 760–4815) was used for 12 minutes at 37 °C for MUC15 and Discovery Anti-HQ HRP RUO (Cat 760–4820) was used for 12 minutes at room temperature. Chromogen DAB CM (Cat 760–4304) was applied for color development. All staining steps were carried out on a Ventana Discovery ULTRA instrument and all reagents were purchased from Roche except for the primary antibodies.
Results
Sexually dimorphic interactions at the maternal-fetal interface
Bulk RNA-Sequencing (RNA-Seq) for matched decidua (maternal) and placenta (chorionic villi) tissue was performed at weeks 11 to 13 of gestation, a critical time in placentation. Samples were separated by tissue type (decidua vs placenta) in Principal component 1, but of interest samples were also separated by fetal sex in Principal component 2 (37, 38).
To investigate sex differences in maternal-fetal crosstalk during placentation, we mined placental bulk RNA-Seq data from our previously published cohort (12) and identified 52 receptors and 90 ligands that were DEGs between sexes (P < 0.05, fold-change >1.2). These sexually dimorphic genes were cross-referenced with receptor-ligand databases and our decidua-placenta transcriptome to identify sexually dimorphic maternal-fetal crosstalk, yielding 91 receptor-ligand pairs. Then, in order to identify the most relevant signaling regulating sex differences in maternal-fetal crosstalk, we performed Ingenuity Pathway Analysis (IPA) upstream analysis on placenta receptors and ligands that were sexually dimorphic at P < 0.05.
In female fetuses, there were 32 receptor-ligand pairs between placenta and decidua (Fig. 1A). IPA upstream analysis verified 3 of the female-upregulated placenta receptors as significant upstream regulators of placenta sex differences: ITGB6, ITGB8, and OLR1 (P < 0.05, Fisher’s exact test). Their decidua-expressed ligands are mostly extracellular matrix components: ITGA5, FN1, TNC, COL4A1, LAMB1, LAMA1, as well as heat shock protein HSPA4. Additional significant upstream regulators from maternal decidua, including the ligands BMP4, BMP7, COL4A1, FN1, HSPA4, ITGA5, LAMA1, LAMB1, TNC, VEGFA, and VEGFB, as well as the receptor BMPR2, had crosstalk with female placenta through receptor-ligand interactions. The most significantly female-upregulated placental receptor, ITGB8 (P = 0.000104, FDR = 0.0372) was also a significant upstream regulator of sexually dimorphic placental receptors and ligands (P = 0.0349). The ITGB8 receptor interacts with decidua-expressed ligands COL4A1, FN1, LAMA1, and LAMB1, and all are also significant upstream regulators themselves, suggesting that these are critical sexually dimorphic interactions. Integrins play a significant role at the maternal-fetal interface and have been implicated in sexually dimorphic fetal growth and development in animal studies (39); future studies are needed to determine their role in sexual dimorphism in humans.
In male fetuses, there were 59 receptor-ligand interactions between decidua and placenta (Fig. 1B). Of these, IPA analysis verified several male-upregulated placental receptors (ENG, ERBB2, and INSR) and ligands (COL1A1, HSPA5, and TGFB1) expressed on the placental surface as also significantly upstream regulators (P < 0.05) of sexually dimorphic placental receptors and ligands overall. Decidua receptors that both (i) interact with male-upregulated placenta ligands and (ii) are upstream of placenta sexually dimorphic receptors and ligands include: ACVRL1, EPHB4, ITGB1, LRP5, NOTCH1, TGFBR2, with respective ligands in the male placenta that were DEGs among the sexes. In addition, the maternal decidua ligands CALR, CREB3L1, CSF1, DPP4, EFNA5, FGF2, IGF1, and TIMP1 were also significant upstream regulators that have male-upregulated receptors on the placenta. The most significantly male-upregulated placental receptor, FZD10 (1.48-fold higher in male vs female placenta, P = 0.000188, FDR = 0.0559), was not identified as a significant upstream regulator on IPA but its ligand, LRP5, which is expressed on the maternal decidua, was a significant upstream regulator (P = 0.0273, Fisher’s exact test).
Other critical upstream regulators of sexually dimorphic receptor/ligand pairs identified through IPA include the cytokines CSF1, IFNG, IL1B, IL6, IL10, SPP1, TNF, and WNT3A (Table 1). In addition, hormones were also identified as critical upstream regulators including beta-estradiol and its receptor, ESR1, as well as the androgen receptor. Other important endogenous chemicals identified include the sugar D-glucose and the hormone aldosterone which regulates cardiovascular and renal function.
Table 1.
Top 50 Upstream Regulators of Sexually Dimorphic Receptors and Ligands Expressed in Placenta
| Regulator | Molecule Type | P value | Molecule # |
|---|---|---|---|
| TNF | cytokine | 3.05E-14 | 62 |
| TGFB1* | growth factor | 3.57E-09 | 51 |
| IFNG | cytokine | 7.73E-09 | 43 |
| HIF1A | transcription regulator | 7.11E-07 | 19 |
| SP1 | transcription regulator | 1.12E-06 | 22 |
| TFR2 | transporter | 1.26E-06 | 4 |
| SPDEF | transcription regulator | 1.32E-06 | 8 |
| XBP1 | transcription regulator | 1.33E-06 | 13 |
| IL1B | cytokine | 2.49E-06 | 29 |
| HRAS | enzyme | 2.78E-06 | 23 |
| tretinoin | chemical—endogenous | 4.97E-06 | 40 |
| TP73 | transcription regulator | 5.41E-06 | 17 |
| APOE | transporter | 7.52E-06 | 15 |
| beta-estradiol | chemical—endogenous | 8.73E-06 | 45 |
| CSF1* | cytokine | 1.13E-05 | 12 |
| ERN1 | kinase | 1.99E-05 | 9 |
| KLF11 | transcription regulator | 2.25E-05 | 9 |
| WT1 | transcription regulator | 2.38E-05 | 12 |
| AR | ligand-dependent nuclear receptor | 2.47E-05 | 18 |
| FGF2* | growth factor | 2.92E-05 | 16 |
| SMAD4 | transcription regulator | 2.93E-05 | 12 |
| IL10 | cytokine | 3.14E-05 | 15 |
| D-glucose | chemical—endogenous | 3.17E-05 | 21 |
| ESR1 | ligand-dependent nuclear receptor | 3.94E-05 | 33 |
| TP53 | transcription regulator | 4.36E-05 | 42 |
| IL6 | cytokine | 5.33E-05 | 23 |
| SMARCA4 | transcription regulator | 5.61E-05 | 20 |
| IGF1* | growth factor | 5.90E-05 | 17 |
| palmitic acid | chemical—endogenous | 7.11E-05 | 11 |
| prostaglandin E2 | chemical—endogenous | 9.57E-05 | 13 |
| ERBB2 | kinase | 1.09E-04 | 22 |
| EGF | growth factor | 1.38E-04 | 17 |
| CEBPB | transcription regulator | 1.43E-04 | 15 |
| ITGB1* | transmembrane receptor | 2.23E-04 | 8 |
| DICER1 | enzyme | 2.36E-04 | 12 |
| SP3 | transcription regulator | 2.53E-04 | 10 |
| aldosterone | chemical—endogenous | 2.58E-04 | 8 |
| WNT3A | cytokine | 2.73E-04 | 11 |
| TCF4 | transcription regulator | 2.84E-04 | 14 |
| CNR1 | G-protein coupled receptor | 2.84E-04 | 8 |
| butyric acid | chemical—endogenous | 2.93E-04 | 15 |
| SPP1 | cytokine | 3.16E-04 | 9 |
| SFTPA1 | transporter | 3.39E-04 | 6 |
| CCN5 | growth factor | 3.58E-04 | 5 |
| TGFB2 | growth factor | 3.64E-04 | 7 |
| EDEM1 | enzyme | 4.10E-04 | 2 |
| HDGF | growth factor | 4.10E-04 | 2 |
| MMP8 | peptidase | 4.22E-04 | 3 |
| ELF3 | transcription regulator | 4.58E-04 | 4 |
| vitamin A | chemical—endogenous | 5.19E-04 | 4 |
P value is calculated using Fisher’s exact test.
*Bold indicates upstream regulator is also part of maternal-fetal crosstalk affected by fetal sex; see also Fig. 1.
Sexual dimorphism in individual cell types
Since the placenta consists of different cell types, we used single-cell RNA sequencing (scRNA-Seq) to evaluate sexual dimorphism at the single-cell level. Cells were collected without marker selection to maximize the cell populations captured. Five clusters (P1-P5) were separated from unsupervised clustering (Fig. 2A). We identified clusters by expression of conventional markers: trophoblast cells (KRT7, KRT8, CGA, EGFR), stromal fibroblast cells (COL3A1, PDGFRA, THY1), Hofbauer cells (CD14, CD163, CSF1R), antigen-presenting cells (APCs) (HLA-DRA, HLA-DPB1, CD52) and endothelial cells (PECAM1, CD93). Stromal fibroblast cells (P2) are the most abundant cell type (38.8%), followed by Hofbauer cells (P3, 29.4%), trophoblast cells (P1, 22.1%), APCs (P4, 8.5%), and endothelial cells (P5, 1.2%). DEG analysis and pairwise comparison yielded genes that are specific to each cluster corroborating the identification of each cluster (40, 41).
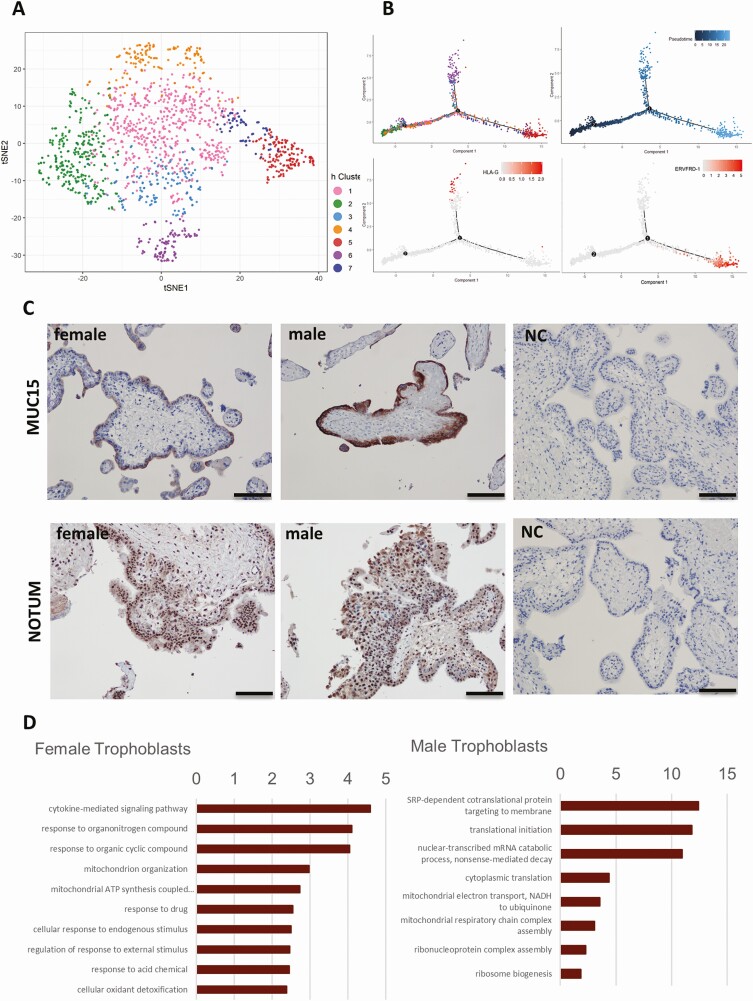
Figure 2.
Single-cell transcriptomic profiles and sexual dimorphism of the first trimester placenta. (A-i) Placental cell clusters visualized by t-Distributed Stochastic Neighbor Embedding (t-SNE) using scran and scater packages. Colors indicate cell types. (A-ii) The tSNE of male and female placenta cells with males colored in blue and females in pink. (B) The top sexually dimorphic genes (FDR <0.01, ILog2FCI >1) in each cluster are presented as a heatmap. Black bars on the left of the heatmap indicate genes on sex chromosomes. (C) Sexually dimorphic ligands in the first trimester placental cells interact with decidua-expressed receptors.
Of the 7245 cells that are selected for analysis, 4369 cells are from the female placentas and 2876 cells are from the male placentas (Fig. 2A). The top DEGs among sexes (ILog2FCI >1, FDR <0.01) for each cell type shown in Fig. 2B, and genes that are also specific markers to corresponding cell types are of particular interest, including MUC15, NOTUM, and MAGEA4 in trophoblasts; STC1 in stromal fibroblasts; FCGBP, CCL13, and RETN in Hofbauer cells; and IL1RN, MMP9, and GPR183 in APCs. The fold changes of top DEGs among sexes are shown in the Supplemental Material (37, 42).
Only trophoblasts cells have unique X chromosome genes upregulated in females compared with males, including MAGEA4 (melanoma associated antigen 4) and TMSB4X (thymosin beta 4) whereas XIST (X inactive specific transcript) is upregulated in all cell types except APCs, which are likely maternally derived. Essential for translational initiation and RNA modification, 3 genes on the Y chromosome (DDX3Y, EIF1AY, RPS4Y1) are upregulated in almost all cell types of male placentas compared with female placentas, except for DDX3Y and EIF1AY in APCs, and DDX3Y in endothelial cells. RPL36A (a ribosomal gene on the X chromosome) is only upregulated in endothelial cells of male placentas.
The majority of the DEGs between sexes are autosomal genes, with 17 significantly upregulated in females and 27 significantly upregulated in males (|Log2FC| >1, FDR <0.01). These genes are involved in cell-cell interaction, antigen presenting, small regulatory RNAs, and metabolic functions. Of the 17 genes upregulated in female placentas, some are uniquely upregulated in individual cell types of female placentas while others are shared between cell types. CCL3, CCL4, GTSF1, RNASE1, and CXCL8 are upregulated in female trophoblasts. GSTT2B and SNHG7 are upregulated in female stromal cells. In Hofbauer cells, RGS1, CCL13, and RETN are upregulated in females and F13A1 is upregulated in APCs. GADD45G is specifically upregulated in female endothelial cells. Genes that are upregulated in females in multiple cell types include HBG2 in stromal fibroblast cells and endothelial cells, HBB in stromal fibroblast cells and Hofbauer cells, GPR183 and CGA in Hofbauer cells and APCs, as well as MTRNR2L1 in APCs and endothelial cells. Similarly, of the 27 genes upregulated in males, HLA-C, MUC15, NOTUM, SNHG19, and SNHG25 are upregulated in male trophoblasts. BCYRN1, CTC-425F1.4, HLA-DRB1, and STC1 are upregulated in male stromal fibroblasts. APOC1, BDH1, and FCGBP are upregulated in male Hofbauer cells and BEST1, CH17-373J23.1, FBLIM1, IL1RN, MMP9, and NBEAL1 are upregulated in male APCs. Male endothelial cells have upregulation of HLA-A and IGF1 compared to females. Several genes are upregulated in males in more than one cell type which includes DONSON, expressed in Hofbauer cells, APCs, and endothelial cells, as well as MT1G, PRDM6, TMEM176A, and TMEM176B upregulated in male Hofbauer cells and APCs.
To understand what regulates the DEGs between fetal sexes at individual cell types, we performed upstream regulator analyses using IPA (the top 10 regulators are shown in Table 2). The ligands TGFβ-1 and β-estradiol regulate sexually dimorphic genes in trophoblasts, stromal fibroblast cells, and Hofbauer cells. In addition, the hormone dihydrotestosterone, which binds to the androgen receptor, impacts sexually dimorphic genes in the trophoblast population. Sex differences in Hofbauer cells and APCs, the 2 immune populations, are also regulated by cytokines such as TNF and the interleukin family.
Table 2.
Top 10 Upstream Regulators of Sexually Dimorphic Genes at Individual Cell Types in Placenta
| P1 (Trophoblast) | Molecule Type | P value | Molecule # |
|---|---|---|---|
| KRAS | enzyme | 1.31E-17 | 45 |
| MYC | transcription regulator | 1.38E-16 | 66 |
| TP53 | transcription regulator | 2.89E-16 | 88 |
| dihydrotestosterone | chemical—endogenous | 2.98E-16 | 45 |
| HRAS | enzyme | 7.34E-16 | 47 |
| TGFB1 | growth factor | 2.48E-15 | 85 |
| MYCN | transcription regulator | 4.93E-15 | 31 |
| EGF | growth factor | 9.08E-15 | 41 |
| beta-estradiol | chemical—endogenous | 1.13E-14 | 88 |
| STAT3 | transcription regulator | 2.46E-14 | 43 |
| P2 (Stromal) | Molecule Type | P value | Molecule # |
| TGFB1 | growth factor | 1.48E-50 | 302 |
| beta-estradiol | chemical—endogenous | 1.25E-47 | 312 |
| TP53 | transcription regulator | 2.49E-45 | 295 |
| MYCN | transcription regulator | 3.08E-45 | 101 |
| MYC | transcription regulator | 5.33E-45 | 215 |
| HRAS | enzyme | 1.37E-36 | 140 |
| KRAS | enzyme | 1.34E-35 | 124 |
| ERBB2 | kinase | 7.63E-33 | 151 |
| hydrogen peroxide | chemical—endogenous | 6.68E-27 | 108 |
| TNF | cytokine | 1.57E-26 | 246 |
| P3 (Hofbauer) | Molecule Type | P value | Molecule # |
| MYCN | transcription regulator | 2.41E-28 | 56 |
| beta-estradiol | chemical—endogenous | 6.24E-27 | 154 |
| TNF | cytokine | 6.26E-27 | 146 |
| TP53 | transcription regulator | 8.38E-27 | 148 |
| MYC | transcription regulator | 4.38E-25 | 107 |
| IFNG | cytokine | 1.09E-22 | 116 |
| IL1B | cytokine | 2.48E-22 | 90 |
| TGFB1 | growth factor | 6.51E-22 | 136 |
| tretinoin | chemical—endogenous | 8.46E-22 | 125 |
| KRAS | enzyme | 1.34E-17 | 59 |
| P4 (APCs) | Molecule Type | P value | Molecule # |
| MYCN | transcription regulator | 1.03E-15 | 26 |
| IL3 | cytokine | 2.56E-11 | 23 |
| IL5 | cytokine | 3.40E-11 | 21 |
| TNF | cytokine | 6.17E-11 | 55 |
| ALKBH1 | enzyme | 7.99E-11 | 6 |
| NSUN3 | enzyme | 7.99E-11 | 6 |
| STAT3 | transcription regulator | 4.22E-10 | 28 |
| IL1B | cytokine | 8.26E-10 | 35 |
| SIRT3 | enzyme | 1.86E-09 | 9 |
| KRAS | enzyme | 3.51E-09 | 25 |
| P5 (Endothelial) | Molecule Type | P value | Molecule # |
| MYCN | transcription regulator | 2.75E-06 | 5 |
| STAT5A | transcription regulator | 7.59E-05 | 4 |
| MYC | transcription regulator | 1.91E-04 | 6 |
| ZMPSTE24 | peptidase | 6.34E-04 | 2 |
| ZNF426 | transcription regulator | 8.32E-04 | 1 |
| RNF165 | enzyme | 8.32E-04 | 1 |
| CYTL1 | cytokine | 8.32E-04 | 1 |
| TPH2 | enzyme | 8.32E-04 | 1 |
| PTS | enzyme | 8.32E-04 | 1 |
| NOCT | transcription regulator | 8.32E-04 | 1 |
Input is the significantly and specifically expressed genes in each cell type using differentially expressed gene analysis and pairwise comparison.
Abbreviations: APCs, antigen-presenting cells.
*Includes other miRNAs with seed CCAGUGU. P value is calculated using Fisher’s exact test.
We also focused on DEGs among individual cell types that were known receptors or ligands, and their interacting partners that are decidua-expressed. Among the 44 differentially expressed genes identified, ligands among individual cell types from the CCL family were most highly representative in female placentas. Compared with male trophoblasts, CCL3 and CCL4 in female trophoblasts were significantly upregulated; the encoded ligands bind to the decidua-expressed receptors CCL1 and CCL5. Female Hofbauer cells express more abundant CCL13 that binds to the decidua-expressed CCR2. Antigen-presenting cells from male gestations express higher levels of IL1RN and MMP9 than that in female gestations; their products bind to decidual IL1R1 and LRP1, respectively. Insulin-like growth factor 1 (IGF1) was upregulated in male endothelial cells compared with female counterparts, the receptor IGF1R resides in decidua (Fig. 2D).
In addition, we compared bulk RNA-Seq to scRNA-Seq results to identify specific cell types with sexually dimorphic expression of receptors and ligands identified in bulk placenta. Of the 10 receptors and ligands upregulated in females and the 29 receptors and ligands upregulated in males, 7 genes from each group had low abundance (UMI <0.1) and were excluded. Several DEGs were identified among the individual cell types at FDR <0.1, with 1 upregulated in females and 12 upregulated in males in various cell types (43). IPA upstream analysis verified 5 DEGs as significant upstream regulators of placenta sex differences: ORL1, which was significantly upregulated in female Hofbauer cells (FDR = 0.06845) and 4 of the male-upregulated placenta receptors and ligands. In males, COL1A1 was significantly upregulated in stromal cells (FDR = 3.59E-43); ENG was significantly upregulated in trophoblasts (FDR = 0.0142), stromal cells (FDR = 5.90E-07), and Hofbauer cells (FDR = 3.76E-4); HSPA5 was significantly upregulated in stromal cells (FDR = 2.39E-7), Hofbauer cells (FDR = 0.0781), and APCs (FDR = 2.97E-3); and TGFB1 was significantly upregulated in stromal cells (FDR = 0.0223). In addition to ENG, IGF2, although not identified in IPA, was upregulated in males in multiple cell types including trophoblasts (FDR = 9.84E-9), stromal cells (FDR = 4.46E-9), Hofbauer cells (FDR = 8.11E-6), and endothelial cells (FDR = 0.064).
Sexually dimorphic trophoblast clusters
Since trophoblast cells are most proximal to the maternal interface, we performed subclustering analysis and identified 7 trophoblast subclusters, T1 to T7 (constituting 41.6%, 19.1%, 9.5%, 9.3%, 10%, 6.5%, and 4% of the trophoblast population, respectively). Markers of the progenitor cell type, cytotrophoblasts, are expressed throughout different subclusters: PARP1 is abundantly expressed in T1, T2, T3, and T4; ITGA6 is abundantly expressed in T4 and T6; TP63 expression is greatest in T4. The expression of a recently reported cytotrophoblast marker NRP2 (24) is highest in T3 and T4. Subclusters T5 and T6 are differentiated syncytiotrophoblasts (STBs) and extra-villous trophoblasts (EVTs), respectively, as characterized by conventional markers and differential gene expression analysis (Fig. 3A) (44). T5 specifically expresses the retroviral envelope genes ERVFRD-1 and ERVV-1 that are critical for cell fusion, hormone synthesis genes CYP19A1 (converts androgens to estrogens) and HSD11B2 (converts cortisol to cortisone), as well as solute carrier SLC family members such as SLC40A1 and SLC26A2. T6 specifically expressed genes important for cell adhesion, cell invasion, extracellular remodeling (TIMP3, SPON2, MFAP5), and immunomodulation (TGFB1, HLA-G).
Figure 3.
Sexual dimorphism in the first trimester trophoblasts. (A) tSNE of trophoblast subclusters using scran and scater packages. Colors indicate subclusters within the trophoblast population. (B) Pseudotime reconstruction of the developmental trajectory in the trophoblast population shows trophoblast subclusters (T1~T7), indicated by colors. The timepoints 1 and 2 represent hierarchical branching events. Pseudotemporal trajectory indicates developmental timeline. The distribution patterns of cells expressing EVT marker gene HLA-G and STB marker gene ERVFRD-1 in the pseudotemporal trajectory. (C) Representative images of MUC15 and NOTUM immunostaining in 3 female placentas and 4 male placentas, bar = 100 um. (D) The top 10 enriched GO terms are presented as −log10(P value) for either sex in trophoblasts. (Bonferroni-corrected for P < 0.05).
Consistent with the highly proliferative activity of the cytotrophoblasts in the first trimester placenta, a group of cell cycle regulators were specifically expressed in subclusters T2 (MKI67, CCNA2, CCNB1/2, CDK1), T4 (CDK6), and T6 (CDK7 and CCNE1). We reconstructed the developmental trajectory of cytotrophoblasts and observe a bifurcating pattern of trophoblast cell development (Fig. 3B). One branch ends with EVTs (T6) and the other ends with STBs (T5). T2 and T4 are the initial clusters and likely the stem cytotrophoblasts. T1, T3, and T7 occupy the intermediate stages, suggesting they are the transitional stages leading to the fully differentiated EVTs or STBs.
The sexually dimorphic transcripts from autosome genes in the trophoblast cluster were MUC15 and NOTUM, which were most highly expressed in STBs and EVTs, respectively, the 2 most terminally differentiated and functionally significant trophoblast populations. Immunohistochemistry confirms protein localization (Fig. 3C). Gene Ontology (GO) analysis identified distinctive enriched functions between male and female trophoblasts. (Fig. 3D). Male trophoblast cells are enriched in protein translation and certain mitochondrial and ribosomal functions, whereas female trophoblast cells are mostly enriched in the cytokine-mediated signaling pathway and responses to various compounds and stimuli.
Discussion
This is the first study looking at the impact of fetal sex on the maternal-fetal interface in the late first trimester. We identify cell-specific regulation in healthy ongoing pregnancies, ensuring that these interactions are indicative of normal placentation and uncomplicated human pregnancies. Differences among the sexes at the maternal-fetal interface include immunomodulation, hormonal regulation, and metabolism, consistent with sexually dimorphic clinical outcomes. We identified 91 receptor-ligand pairs across the maternal-fetal interface that are sexually dimorphic, among which there are 35 genes that are also significant upstream regulators of pathways critical in sexually dimorphic placentation. These 35 receptors and ligands may be key regulators of sex differences and maternal-fetal crosstalk in early pregnancy. To further dissect placental heterogeneity, we identified 5 major cell types (trophoblasts, stromal cells, Hofbauer cells, antigen-presenting cells, and endothelial cells) using scRNA-Seq and identified sex differences in individual cell types. Of differentially expressed genes, ligands among individual cell types from the CCL family were most highly representative in females, whereas IL1RN, MMP9, and IGF1 were highly expressed in males with their corresponding receptors present on the maternal surface. In trophoblast cells, which are most proximal to the maternal interface, subclustering analysis demonstrated 7 subclusters with the progenitor cytotrophoblasts differentiating into the syncytiotrophoblasts and extra-villous trophoblasts. Among the sexually dimorphic transcripts which are expressed on autosomes, MUC15 and NOTUM were also most highly expressed in syncytiotrophoblasts and extra-villous trophoblasts, respectively, the 2 most terminally differentiated and functionally significant trophoblast populations. GO analysis identified distinctive enriched functions between female and male trophoblasts. Furthermore, upstream regulators of sexually dimorphic genes demonstrated estradiol and TGFB1, whose expression is also upregulated in male placenta (P = 0.0462), to affect all cell types. However, dihydrotestosterone, which is produced by the male fetus, was an upstream regulator most significant for the trophoblast population.
Although the maternal-fetal interface is a highly immunologic state with presumed similarities among the fetal sexes (24), sex differences do exist with certain immune functions enhanced or altered in female first trimester placenta compared with placenta of male fetuses. We identified TNF as a significant global and cell type-specific upstream regulator of sexually dimorphic genes within the first trimester placenta, whereas IL1B, IL3, and IL5, were significant upstream regulators of the antigen-presenting cells as well as IL1B in Hofbauer cells. TNF-α, IL-1β, IL-6, IL-8, and IL-5 have all been found to be significantly increased in placentas of female fetuses whose mothers had mild asthma, with no changes observed in placentas of male fetuses (14), suggesting these are potential critical markers that affect maternal-fetal outcomes. We identified IL36RN to be upregulated in female first trimester placenta and it binds to decidua-expressed IL1RL2. IL1RL2 has been identified in the gene signature of metastasis promotion in tumor cells which may also be critical for differences in migration and invasion potential for proper implantation and subsequent placentation (45).
In females, CCL3 and CCL4 in trophoblasts and CCL13 in Hofbauer cells were upregulated. The cytokine-mediated signaling pathway was the most significant pathway identified in female trophoblasts suggesting sexually dimorphic immunologic response. CCL3, through decidua-expressed CCR1, is involved in recruitment of natural killer cells and monocyte migration from the maternal interface (46, 47). CCL13, with higher expression in female Hofbauer cells, binds to decidua-unique CCR2, and functions as a chemoattractant for various immune cells, suggesting that Hofbauer signaling for immune cell migration is sexually dimorphic, differentially impacting the maternal immune response. In addition, CCL13 is involved in the M2 phenotype of macrophages (48). Increased expression of CCL13 in females may increase the M2 phenotype, which mainly induces the Th2 response and has greater involvement in tissue repair than mounting a maternal response, consistent with the premise that male conceptions promote increased maternal innate and adaptive immunity compared with female conceptions (49).
TGFB1, expressed in decidua and placenta in bulk RNA-Seq analysis, is a key upstream regulator in all cell types, confirming that TGFβ-1 is a significant coordinator of placental development at the maternal-fetal interface (50). TGFB1 expression is sexually dimorphic, with upregulation in male first trimester placenta, specifically the stromal cells. TGFB1 suppresses the immune response (51) and inhibits trophoblast invasion (52), which may explain sex differences in the immunologic milieu at the maternal-fetal interface, and may be critical to decrease rejection of a male fetus which may be recognized as foreign, given that there is a higher rate of fetal loss during male gestation (53). TGFB1 is more highly expressed in the EVT subcluster compared with other subclusters in trophoblasts in our data, consistent with that of Vento-Tormo, et al (24). TGFB1 and its receptor increase during EVT differentiation (24). Furthermore, TGFβ-1 may coordinate invasion of EVTs with differentiation of STBs through its receptor TGFBR1, which is expressed in both EVT and STB subclusters in our study (data not shown) and by others (54).
Precise hormonal regulation is necessary for a healthy gestation, regulated through maternal-fetal crosstalk initiated by the mother, and later engaged in by the fetus, through the placenta. Estrogen and progesterone production by the STBs is fully established by 10 weeks, making the placenta the source of hormone production essential for pregnancy maintenance (55). β-Estradiol is a key upstream regulator in all placental cell types identified from placentas at 11 to 13 weeks gestation in our studies. Receptors for β-estradiol such as ESR1, are among the top upstream regulators in bulk tissues as well as trophoblast cells, suggesting at this gestational age, signaling to the maternal decidua is from hormones produced by the STBs, which via crosstalk regulate the placenta and trophoblasts. The critical role of precise hormonal regulation in placentation has been identified during aberrant hormonal states, including supraphysiologic hormone states, which lead to placental reprogramming resulting in elevated estradiol and progesterone production (56). Elevated hormone production affects transcriptional regulators of trophoblasts, such as GATA3, altering trophoblast migration and invasion, which can lead to placental dysfunction and adverse outcomes, including low birth weight and small for gestational age infants (56-60).
In addition to hormone production, trophoblast cells, which are the most proximal to the maternal interface, are also critical for invasion. Among the trophoblast population, autosomal MUC15 and NOTUM are upregulated in male trophoblasts. NOTUM is a polarity determinant that affects stem cell migration (61) and MUC15 is important for cell adhesion to the extracellular matrix (62). Collectively, these genes may play significant roles in sexually dimorphic trophoblast migration and invasion, providing insight into sexually dimorphic placental dysfunction and disease, such as preeclampsia (62).
Dihydrotestosterone, which is only expressed in male fetuses, is produced early in gestation from the developing testes (63). It regulates TGFB1 (64-66), which may be the key differentiating factor that affects trophoblast differentiation, invasion, and also immune modulation. In addition, testosterone affects trophoblast migration (67), which is the foundation for appropriate placentation that ultimately impacts fetal growth and development (68, 69).
Thus, in addition to its role in development of male external genitalia, fetal dihydrotestosterone production may also be a critical regulator of trophoblast migration and trophoblast development leading to sex differences in fetal growth. This may be in conjunction with other hormone regulators of growth that are upregulated in males, including the IGF signaling pathway (70) with INSR, upregulated in male placenta, binding IGF1 expressed in maternal decidua. Furthermore, upregulation of INSR in the male fetal placenta may explain the increased rates of macrosomia associated with male but not female fetuses in pregnancies with gestational diabetes (3).
Caucasians make up the largest demographic for chorionic villus sampling in our institution. As a result, all of the subjects in this study were Caucasian. It remains to be seen if sex differences at the maternal-fetal interface vary with race/ethnic background. Therefore, these results need to be interpreted with caution due to this limitation of a homogenous patient population. In addition, we did not see a difference in fetal weight, yet we and others have demonstrated that male fetuses are larger throughout gestation and heavier at birth (2, 6, 12). This may be due to the small sample size. We also recognize that for our bulk RNA-Seq, fold change differences were low, but our intention was to look at overall global differences highlighting those interactions that were also identified as upstream regulators. These limitations, as well as our limited validation, will require larger studies to corroborate with our findings and further studies will be necessary to better understand signaling at individual cell types in a sex-specific manner.
In summary, the maternal-fetal interface in ongoing human pregnancies is a hormonally and immunologically controlled environment for the establishment and development of the placenta. Hormonal regulation, initially from the maternal ovarian luteal cells followed by subsequent regulation from the fetal STBs, maintains the maternal surface to immunologically balance the invasion of the fetal trophoblasts that are critical for the establishment of the placenta, through maternal-fetal crosstalk. Although the TGF-β family is critical at the maternal-fetal interface, sexual dimorphism may be the result of fetal hormone production, specifically dihydrotestosterone, that affects TGFβ-1. This may impact immune and trophoblast function leading to sexually dimorphic outcomes in pregnancy, for both the fetus and the mother. Further studies are needed to evaluate sexual dimorphism at the maternal-fetal interface during placentation.
Acknowledgments
The authors would like to thank Rae Buttle, Erica Sauro, and Yayu Lin for patient recruitment and sample collection. We gratefully acknowledge the support from the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development R01 grants to MDP (R01HD074368, R01HD091773), the Ruth L. Kirschstein National Research Service Award to TLG (T32DK007770) and the National Institutes of Health U01 grant to MDP and HRT (U01 EB026421). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency was not involved in the design, analysis, or interpretation of the data reported.
Author Contributions: Conceptualization: M.D.P., B.R.S., H.R.T., S.A.K., C.R.F., S.S.R., E.T.W.; Data Curation: N.D., J.T., Y.W., C.Y., A.F.K., S.D.T., C.R.F.; Formal Analysis: N.D., T.L.G., T.S., A.F.K., S.D.T., C.R.F., M.D.P.; Funding Acquisition: M.D.P., J.W., S.S.R.; Investigation: T.S., T.L.G., N.T., B.L., J.T., Y.W., B.R.S., C.Y., A.F.K., S.D.T., C.R.F.; Methodology: T.S., T.L.G., N.D., R.D., E.L.C., J.T., Y.W., B.R.S., C.Y., A.F.K., S.D.T., C.R.F.; Project Administration: M.D.P., T.S., T.L.G.; Resources: B.L., J.W.; Software: N.D., J.T., Y.W., A.F.K., S.D.T. Supervision: M.D.P., S.S.R.; Validation and Visualization: N.D., T.L.G., Y.W., A.F.K., S.D.T., M.D.P.; Writing—Original Draft: T.S., T.L.G., N.D.; Writing—Review and Editing: T.S., T.L.G., M.D.P., B.R.S., H.R.T., S.A.K., C.R.F., S.S.R.
Glossary
Abbreviations
- APCs
antigen-presenting cells
- BMP
bone morphogenic protein
- CCL
CC family chemokine ligand
- DEG
differentially expressed gene
- DHT
dihydrotestosterone
- FC
fold-change
- FDR
false discovery rate
- EVT
extra-villous trophoblast
- FPKM
fragments per kilobase of exon per million reads
- GO
Gene Ontology
- IGF
insulin-like growth factor
- IL1RN
interleukin 1 receptor antagonist
- IPA
Ingenuity Pathway Analysis
- MADs
median absolute deviations
- MMP9
matrix metalloproteinase 9
- MUC15
mucin-15
- NCBI
National Center for Biotechnology Information
- NOTUM
notum, palmitoleoyl-protein carboxylesterase
- STB
syncytiotrophoblast
- TGF-β
transforming growth factor-β
- tSNE
t-distributed stochastic neighbor embedding
- UMI
unique molecular identifier
Additional Information
Disclosure Summary: The authors declare no conflicting or competing interests that is relevant to the subject matter or materials included in this work.
Data Availability
The datasets generated during and/or analyzed during the current study are publicly available at NCBI GEO with the following accessions: GSE131875 (superseries), GSE131696 (CVS scRNA-Seq), GSE131874 (matched CVS and decidua bulk RNA-Seq), and GSE109082 (CVS sex differences bulk RNA-Seq).
References
- 1. Basso O, Olsen J. Sex ratio and twinning in women with hyperemesis or pre-eclampsia. Epidemiology. 2001;12(6):747-749. [DOI] [PubMed] [Google Scholar]
- 2. Melamed N, Yogev Y, Glezerman M. Fetal gender and pregnancy outcome. J Matern Fetal Neonatal Med. 2010;23(4):338-344. [DOI] [PubMed] [Google Scholar]
- 3. Ricart W, López J, Mozas J, et al. ; Spanish Group for the study of the impact of Carpenter and Coustan GDM thresholds . Maternal glucose tolerance status influences the risk of macrosomia in male but not in female fetuses. J Epidemiol Community Health. 2009;63(1):64-68. [DOI] [PubMed] [Google Scholar]
- 4. Astolfi P, Zonta LA. Risks of preterm delivery and association with maternal age, birth order, and fetal gender. Hum Reprod. 1999;14(11):2891-2894. [DOI] [PubMed] [Google Scholar]
- 5. Zeitlin J, Saurel-Cubizolles MJ, De Mouzon J, et al. Fetal sex and preterm birth: are males at greater risk? Hum Reprod. 2002;17(10):2762-2768. [DOI] [PubMed] [Google Scholar]
- 6. Verburg PE, Tucker G, Scheil W, Erwich JJ, Dekker GA, Roberts CT. Sexual dimorphism in adverse pregnancy outcomes–a retrospective Australian Population Study 1981-2011. PLoS One. 2016;11(7):e0158807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elsmén E, Källén K, Marsál K, Hellström-Westas L. Fetal gender and gestational-age-related incidence of pre-eclampsia. Acta Obstet Gynecol Scand. 2006;85(11):1285-1291. [DOI] [PubMed] [Google Scholar]
- 8. Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M. Gender does matter in perinatal medicine. Fetal Diagn Ther. 2004;19(4):366-369. [DOI] [PubMed] [Google Scholar]
- 9. Shiozaki A, Matsuda Y, Satoh S, Saito S. Impact of fetal sex in pregnancy-induced hypertension and preeclampsia in Japan. J Reprod Immunol. 2011;89(2):133-139. [DOI] [PubMed] [Google Scholar]
- 10. Toivanen P, Hirvonen T. Sex ratio of newborns: preponderance of males in toxemia of pregnancy. Science. 1970;170(3954):187-188. [DOI] [PubMed] [Google Scholar]
- 11. Tyson JE, Parikh NA, Langer J, Green C, Higgins RD; National Institute of Child Health and Human Development Neonatal Research Network . Intensive care for extreme prematurity–moving beyond gestational age. N Engl J Med. 2008;358(16):1672-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonzalez TL, Sun T, Koeppel AF, et al. Sex differences in the late first trimester human placenta transcriptome. Biol Sex Differ. 2018;9(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mitchell AM, Palettas M, Christian LM. Fetal sex is associated with maternal stimulated cytokine production, but not serum cytokine levels, in human pregnancy. Brain Behav Immun. 2017;60:32-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scott NM, Hodyl NA, Murphy VE, et al. Placental cytokine expression covaries with maternal asthma severity and fetal sex. J Immunol. 2009;182(3):1411-1420. [DOI] [PubMed] [Google Scholar]
- 15. Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez-Lopez N. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol. 2016;13(4):462-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun TGonzalez TL, Deng N, et al. Table S1. Patient demographics and pregnancy outcomes. figshare. Posted February 27, 2020. 10.6084/m9.figshare.10314425.v1 [DOI] [Google Scholar]
- 17. Pisarska MD, Akhlaghpour M, Lee B, et al. Optimization of techniques for multiple platform testing in small, precious samples such as human chorionic villus sampling. Prenat Diagn. 2016;36(11):1061-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2012;29(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 22. Hart T, Komori HK, LaMere S, Podshivalova K, Salomon DR. Finding the active genes in deep RNA-seq gene expression studies. BMC Genomics. 2013;14:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28(12):1248-1250. [DOI] [PubMed] [Google Scholar]
- 24. Vento-Tormo R, Efremova M, Botting RA, et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. 2018;563(7731):347-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Durinck S, Moreau Y, Kasprzyk A, Davis S, De Moor B, Brazma A, Huber W. BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics 2005;21:3439-3440 [DOI] [PubMed] [Google Scholar]
- 26. Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc. 2009;4(8):1184-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKinney W. Data structures for statistical computing in Python. Proceedings of the 9th Python in Science Conference 2010:51-56 [Google Scholar]
- 28. Consortium TU. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158-D169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NCBI Resource Coordinators. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2017;45(D1):D12-D17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ben-Shlomo I, Yu Hsu S, Rauch R, Kowalski HW, Hsueh AJ. Signaling receptome: a genomic and evolutionary perspective of plasma membrane receptors involved in signal transduction. Sci STKE. 2003;2003(187):RE9. [DOI] [PubMed] [Google Scholar]
- 31. Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize Implements and enhances circular visualization in R. Bioinformatics. 2014;30(19):2811-2812. [DOI] [PubMed] [Google Scholar]
- 32. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lun AT, McCarthy DJ, Marioni JC. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with Bioconductor. F1000Res. 2016;5:2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lun AT, Bach K, Marioni JC. Pooling across cells to normalize single-cell RNA sequencing data with many zero counts. Genome Biol. 2016;17:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCarthy DJ, Campbell KR, Lun AT, Wills QF. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics. 2017;33(8):1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun TGonzalez TL, Deng N, et al. Supplemental information: sexually dimorphic crosstalk at the maternal-fetal interface. figshare. Posted June 19, 2020. 10.6084/m9.figshare.c.4741742.v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun TGonzalez TL, Deng N, et al. Figure S1. PCA of matched chorionic villi and decidua tissue from first trimester human pregnancies. figshare. Posted February 27, 2020. 10.6084/m9.figshare.10314254.v1 [DOI] [Google Scholar]
- 39. Stenhouse C, Hogg CO, Ashworth CJ. Association of foetal size and sex with porcine foeto-maternal interface integrin expression. Reproduction. 2019;157(4):317-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun TGonzalez TL, Deng N, et al. Table S2. Placental cell markers from single cell RNA sequencing. figshare. Posted February 27, 2020. 10.6084/m9.figshare.10314404.v1 [DOI] [Google Scholar]
- 41. Sun TGonzalez TL, Deng N, et al. Figure S2. Heatmap of differentially expressed genes from pairwise comparisons of placental cell clusters. figshare. Posted February 27, 2020. 10.6084/m9.figshare.10314380.v1 [DOI] [Google Scholar]
- 42. Sun T, Gonzalez TL, Deng N, et al. Table S3. Placental cell type-specific sexually dimorphic genes. figshare. Posted February 27, 2020. 10.6084/m9.figshare.10314407.v1 [DOI] [Google Scholar]
- 43. Sun TGonzalez TL, Deng N, et al. Table S4. Placenta receptors and ligands sexually dimorphic in both bulk and single cell RNA sequencing. figshare. Posted June 19, 2020. 10.6084/m9.figshare.12518456.v2 [DOI] [Google Scholar]
- 44. Sun TGonzalez TL, Deng N, et al. Table S5. Trophoblast subcluster markers in human first trimester. figshare. Posted June 19, 2020. 10.6084/m9.figshare.10314419.v2 [DOI] [Google Scholar]
- 45. Chen YC, Gonzalez ME, Burman B, et al. Mesenchymal stem/stromal cell engulfment reveals metastatic advantage in breast cancer. Cell Rep. 2019;27(13):3916-3926.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drake PM, Gunn MD, Charo IF, et al. Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1alpha. J Exp Med. 2001;193(10):1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175(12):8096-8104. [DOI] [PubMed] [Google Scholar]
- 48. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453-461. [DOI] [PubMed] [Google Scholar]
- 49. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626-638. [DOI] [PubMed] [Google Scholar]
- 50. Ni N, Li Q. TGFbeta superfamily signaling and uterine decidualization. Reprod Biol Endocrin. 2017;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yi Y, Cheng JC, Klausen C, Leung PCK. TGF-β1 inhibits human trophoblast cell invasion by upregulating cyclooxygenase-2. Placenta. 2018;68:44-51. [DOI] [PubMed] [Google Scholar]
- 53. Byrne J, Warburton D. Male excess among anatomically normal fetuses in spontaneous abortions. Am J Med Genet. 1987;26(3):605-611. [DOI] [PubMed] [Google Scholar]
- 54. Pavličev M, Wagner GP, Chavan AR, et al. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Res. 2017;27(3):349-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Strauss J, Barbieri R. Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Saunders, 7th ed. 2013. [Google Scholar]
- 56. Sun T, Lee B, Kinchen J, et al. Differences in first trimester maternal metabolomic profiles in pregnancies conceived from fertility treatments. J Clin Endocrinol Metab. 2018;104:1005-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee B, Kroener LL, Xu N, et al. Function and hormonal regulation of GATA3 in human first trimester Placentation. Biol Reprod. 2016;95(5):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jackson S, Hong C, Wang ET, Alexander C, Gregory KD, Pisarska MD. Pregnancy outcomes in very advanced maternal age pregnancies: the impact of assisted reproductive technology. Fertil Steril. 2015;103(1):76-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang ET, Ramos L, Vyas N, Bhasin G, Simmons CF, Pisarska MD. Maternal and neonatal outcomes associated with infertility. J Matern Fetal Neonatal Med. 2019;32(17):2820-2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shevell T, Malone FD, Vidaver J, et al. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106(5 Pt 1):1039-1045. [DOI] [PubMed] [Google Scholar]
- 61. Abnave P, Aboukhatwa E, Kosaka N, Thompson J, Hill MA, Aboobaker AA. Epithelial-mesenchymal transition transcription factors control pluripotent adult stem cell migration in vivo in planarians. Development. 2017;144(19):3440-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30(1):15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dawood MY, Saxena BB. Testosterone and dihydrotestosterone in maternal and cord blood and in amniotic fluid. Am J Obstet Gynecol. 1977;129(1):37-42. [DOI] [PubMed] [Google Scholar]
- 64. Qi W, Gao S, Wang Z. Transcriptional regulation of the TGF-beta1 promoter by androgen receptor. Biochem J. 2008;416(3):453-462. [DOI] [PubMed] [Google Scholar]
- 65. McCarthy TL, Centrella M. Androgen receptor activation integrates complex transcriptional effects in osteoblasts, involving the growth factors TGF-β and IGF-I, and transcription factor C/EBPδ. Gene. 2015;573(1):129-140. [DOI] [PubMed] [Google Scholar]
- 66. Chipuk JE, Cornelius SC, Pultz NJ, et al. The androgen receptor represses transforming growth factor-beta signaling through interaction with Smad3. J Biol Chem. 2002;277(2):1240-1248. [DOI] [PubMed] [Google Scholar]
- 67. Pan T, He G, Chen M, et al. Abnormal CYP11A1 gene expression induces excessive autophagy, contributing to the pathogenesis of preeclampsia. Oncotarget. 2017;8(52):89824-89836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Meakin AS, Saif Z, Tuck AR, Clifton VL. Human placental androgen receptor variants: potential regulators of male fetal growth. Placenta. 2019;80:18-26. [DOI] [PubMed] [Google Scholar]
- 69. Meakin AS, Clifton VL. Review: understanding the role of androgens and placental AR variants: insight into steroid-dependent fetal-placental growth and development. Placenta. 2019;84:63-68. [DOI] [PubMed] [Google Scholar]
- 70. Nawathe AR, Christian M, Kim SH, Johnson M, Savvidou MD, Terzidou V. Insulin-like growth factor axis in pregnancies affected by fetal growth disorders. Clin Epigenetics. 2016;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are publicly available at NCBI GEO with the following accessions: GSE131875 (superseries), GSE131696 (CVS scRNA-Seq), GSE131874 (matched CVS and decidua bulk RNA-Seq), and GSE109082 (CVS sex differences bulk RNA-Seq).