Abstract
Introduction
Alzheimer's disease (AD) is neuropathologically marked by amyloid beta (Aβ) plaques and neurofibrillary tangles. Little is known about isotopic compositions of human AD brains. Here we study this in comparison with control subjects for copper and zinc.
Methods
We use mass‐spectrometry methods, developed to study extraterrestrial materials, to compare the copper and zinc isotopic composition of 10 AD and 10 control brains.
Results
Copper and zinc natural isotopic compositions of AD brains are statistically different compared to controls, and correlate with Braak stages.
Discussion
The distribution of natural copper and zinc isotopes in AD is not affected by the diet, but is a consequence of Aβ plaques and tau fibril accumulation. This is well predicted by the changes of the chemical bonding environment caused by the development of Aβ lesions and accumulation of tau proteins. Future work will involve testing whether these changes affect brain functions and are propagated to body fluids.
Keywords: Alzheimer's disease, amyloid beta, copper, isotopes
1. INTRODUCTION
Metals are concentrated in the amyloid beta (Aβ) plaques and tau filaments of the neocortex in patients with Alzheimer's disease (AD), 1 , 2 , 3 and changes in metal homeostasis associated with AD have been tested as a diagnostic tool. Furthermore, it has also been shown that zinc and copper can induce the precipitation of Aβ and possibly tau, with zinc being especially critical with respect to aggregation. 3 , 4 , 5 However, studies focusing on elemental abundance in the serum of AD patients are inconsistent, 6 as they may be affected by factors unrelated to AD, such as intestinal absorption. 7
Technical advances in geo‐cosmochemistry now allow for ultra‐precise measurements of metal natural isotopic compositions, for example, to study the origin of the Moon. 8 More recently these techniques have shown that the copper and zinc isotopic composition of blood is distinct from that in the brain, with a magnitude comparable to that observed in cosmic/planetary processes. 9 , 10 , 11 These natural isotopic variations are controlled by the difference of speciation of the elements between bodily reservoirs, and notably are not affected by diet. 9 Equilibrium isotopic fractionation is due to the difference of energy between systems and, in general, heavier isotopes become more concentrated where the element forms the strongest bonds. Because accumulation of Aβ plaques and tau proteins induces changes of bonding environments in the AD brain, a change in brain isotopic composition should be induced, and by mass balance this change should be mirrored in body fluids (an approach similar to what has already been successfully in cancer research 12 ). For example, zinc in Aβ plaques binds to three histidine residues in the N‐terminal hydrophilic region Aβ16 of the Aβ peptide, 13 , 14 whereas in normal brain it is mostly bound to cysteine within metallothionein. 15 Zinc bound to histidine is isotopically heavier than cysteine by ≈0.6‰ at 298K because it forms tighter bonds, 9 and therefore we would expect the AD brains to be heavier than controls due to a net change in the bonding environment. This difference of bound energy is enhanced for elements that would be present in different redox states between different phases. This is not the case for zinc (or Zn, always +2), but it is for copper (or Cu, +1 or +2), and therefore copper should present larger isotopic variations.
In summary, the formation of Aβ plaques induces a change in the bonding environments of metals in AD brains and this change should manifest as disturbances in metal isotope compositions, where the general direction (positive or negative) and magnitude of these changes are theoretically constrained. By mass balance, the inverse of the change of isotopic composition in the brain should be transferred to other organs and body fluids. Although the full scope of these theoretical suggestions requires further scrutiny, recent zinc isotopic work on Aβ aggregation experiments agree with theoretical estimates, lending evidence to the validity of this framework. 16
The isotopic effects of Aβ plaques accumulation have been tested on APPswe/PSEN1dE9 and control mice for copper and zinc isotopes. 17 , 18 The brains of APPswe/PSEN1dE9 mice that developed Aβ plaques were enriched in the light isotope of copper, 18 and the heavy isotopes of zinc. 17 These isotopic differences are consistent with the changes of copper and zinc speciation associated with the formation of Aβ plaques. 17 , 18 Furthermore, the copper isotopic composition of the brains and serum were correlated, implying copper transport between these two reservoirs, in particular a transfer of Cu(I) from the brain to the serum, illustrating the potential to be used as detection tools for the formation of Aβ fibrils in the brain. However, it has been shown that serum isotopic compositions for human and mouse models can be different, and the human serum values reported herein are heavier than what is reported for mice by ≈0.5‰ [eg,12, 19, 20, 21]. It is therefore fundamental to validate these discoveries on human samples.
Here we test whether isotopic variations of human brains are associated with the development of the AD on 20 well‐characterized human brain (frontal cortex) samples: 10 with clear AD diagnostics (Braak stages V‐VI) and 10 controls (Braak stages 0‐II).
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources (eg, ISI web of Science, PubMed) and meeting abstracts and presentations. All relevant publications are cited.
Interpretation: Our findings of a difference in zinc and copper natural isotopic composition between Alzheimer's disease (AD) and control brains validate the hypothesis that the change of metal speciation due to the accumulation of amyloid beta (Aβ) and tau proteins affect the metal homeostasis of the brain.
Future Directions: This article presents the first observation of natural isotopic fractionation induced by the AD in brains. Future work will be related to providing a theoretical and experimental framework for the fractionation induced by tau accumulation, and investigate whether these effects are propagated to body fluids and whether they affect brain function.
2. METHODS
2.1. Brains
Frozen prefrontal brain tissues from patients with AD Braak stages V‐VI (see Table S1) were obtained from the Center of Cognitive Neurology, Lariboisière Hospital with approval by the Ethical Committee of Paris Diderot University Hospitals (CEERB Bichat University Hospital, Paris, France). All AD patients had a history of progressive dementia, satisfying National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders criteria for probable AD, 22 and satisfying neuropathological criteria for AD, 23 whereas all controls satisfied no neuropathological AD criteria.
2.2. Chemical purification and mass‐spectrometry
Copper and zinc isotopic measurements were performed at the Institut de Physique du Globe de Paris, France, following the protocol described before. 11 , 17 , 18
It is not feasible to obtain precise absolute isotopic ratios, and therefore the convention is to report isotopic compositions relative to a standard 8 :
| (1) |
| (2) |
The sample‐to‐sample comparisons (eg, AD vs control) are relativistic and discussed in terms of being heavier (or lighter) than one another.
3. RESULTS
The isotopic data are reported in Table S1. The copper isotopic composition of 19 samples (9 AD) and the zinc isotopic composition of 18 samples (9 AD) were possible to measure. The copper isotopic composition of human brains (average δ65Cu = +0.60 ± 0.13, for controls) falls within what was measured for mouse brain (+0.56 ± 0.28 for controls, 18 ). Human brain is therefore enriched in the heavy isotope of copper compared to serum (δ65Cu = 0.10 ± 0.22, 12 ), as observed in mice. 18 The human brain is enriched in the light isotopes of zinc (δ66 Zn = −0.6 ± 0.20 for the controls) as for Gottingen minipigs (−0.80 ± 0.05) and mice (δ66 Zn = −0.20 ± 0.27, 17 ).
δ65Cu is not statistically dependent (r2 = 0.04 and P = .1) on age for both controls (22 to 97 years) and AD (66 to 95 years) (Figure S1a). The same is true for δ66 Zn (Figure S1b), albeit the controls show a weak (but statistically insignificant, r2 = 0.37, P = .08) positive correlation with the age.
Neither the copper nor the zinc isotopic composition is statistically dependent on sex for both controls and AD (Figure S2), and in the following both male and female are treated together.
AD brains are isotopically lighter for copper (δ65Cu = 0.35 ± 0.13) than control patients (δ65Cu = 0.60 ± 0.14) (Figure 1A), which is statistically significant (P = .0002). The zinc isotopic composition of AD and control brains are also statistically distinct (Figure 1B), but this difference is weaker than in the case of copper (P = .024), the difference between control and AD brains.
FIGURE 1.
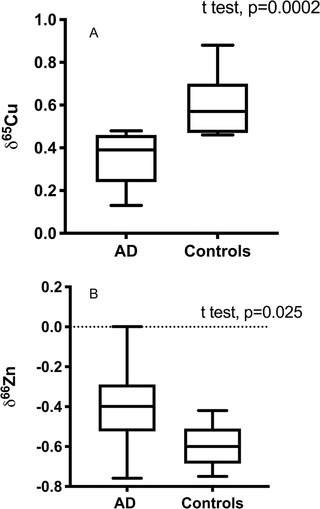
Effect of Alzheimer's disease (AD) on copper (A) and zinc (B) isotopic composition of the brains. Unpaired t tests show a significant effect of AD for copper isotopes (P = .0002) and to a lesser extent on zinc (P = .025). AD brains are more enriched in the lighter isotopes of copper than controls
In summary, the brains of AD patients have different copper and zinc stable isotopic composition compared to controls (see Figure 1), with a difference of −0.18 for δ65Cu and 0.20 for δ66 Zn. There is a statistical interaction between δ65Cu and δ66 Zn (two‐way analysis of the variance, P = .0005), and this is also seen in the δ65Cu‐δ66 Zn correlation (Figure S3), suggesting that zinc and copper isotope variations have similar origins.
4. DISCUSSION
Our results reveal that the copper and zinc isotopic composition of AD brains is different from controls in a statistically meaningful way.
The copper and zinc isotopic variations are in the same direction as observed previously in 12month‐old APPswe/PSEN1dE9 mice, with δ65Cu decreasing and δ66 Zn increasing in the brain of AD mice compared to controls, 17 , 18 but this effect is stronger in humans (Figure 1).
Both δ65Cu and δ66 Zn vary with Braak stage in a statistically meaningful correlation, reflecting an input from tangles (Figure 2). This shows that the changes in the isotopic composition correlate with the evolution of AD, and is a strong indication that an extra cause of the isotopic changes of the brains could be associated with the accumulations of neurofibrillary tangle in neurons. This necessitates the deconvolution of potential mechanistic drivers for the observed isotopic compositions, in particular, questioning the role of tangles and Aβ peptide aggregation. Furthermore, this result questions the impact of such accumulations on brain functions, which by default asks if their associated isotopic changes are of medical or diagnostic utility.
FIGURE 2.
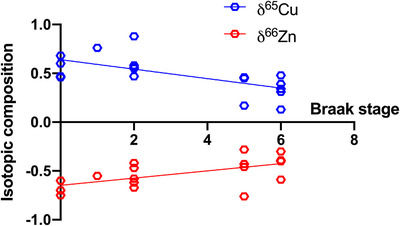
Isotopic composition of copper and zinc as a function of the Braak stage. Both δ65Cu and δ66 Zn vary systematically with the Braak stage, with P values of 0.0028 and 0.0142, respectively
The AD‐associated isotopic change in the brain must be related to the disruption of copper and zinc homeostasis. The heavy isotope enrichment of zinc in AD is consistent with the change in speciation in AD brains due to the development of Aβ plaques. Grossly assuming that these isotopic differences between amino acids represent the isotopic difference between Aβ and normal brain (zinc bound to histidine is isotopically heavier than cysteine by ≈0.6‰ at 298K 9 ), the AD brain should be enriched by ≈50% in Zn, in line with typical estimates (eg, factor 2 in Zn 2 ). The accumulation of isotopically heavy Aβ is therefore a well‐reasoned explanation for the zinc composition of AD brain.
Copper has multiple redox states (Cu+ and Cu2+), and isotopic fractionation is enhanced by redox change, likely explaining the larger and statistically more significant isotopic shift observed for copper relative to zinc. Copper is primarily bound as Cu2+ to cysteine in normal brain, whereas in Aβ plaques it can be Cu+. 24 , 25 Reduced phases, such as Cu+ here, are isotopically lighter than oxidized phases (Cu2+). Therefore, even in the absence of theoretical calculations, the enrichment of Aβ in Cu+ may qualitatively explain the low δ65Cu of AD brains.
An additional effect is the binding of copper and zinc to tau tangles. 26 Cu+ can bind strongly to histidine residues of the tau proteins, 26 which would be enriched in the light isotope compared to normal brains (Cu2+ bound to cysteine) and would enhance the effect seen with Aβ aggregation. On the other hand, Zn2+ can bind to histidine in tau proteins, 27 which would therefore be isotopically heavier than normal brain. The effect of tau proteins is further supported by the correlation between copper and zinc isotopic compositions and the Braak stages (Figure 2), a metric that is strongly based on neurofibrillary tangles. For copper especially, the effect of isotopically light tau proteins may explain the larger copper isotopic difference in humans than in mice models that do not develop tau accumulation.
In summary, copper and zinc natural isotopic compositions of brain tissue from AD patients are modified compared to controls and correlates with the Braak stages. The isotopic excursions observed within the brain are similar in magnitude to what is observed in Moon samples. Given that much knowledge has been added to the cosmochemistry cannon by exploring isotopic variation, 8 this underscores great promise for the use of isotopes in medical research. These changes are not dependent on the sex, diet, or the age of the patients. These isotopic fractionations are well predicted by the changes of speciation of copper and zinc due to the development of Aβ lesions and the accumulation of tau proteins. The next step is to test whether the changes in isotopic composition affect brain functions and whether these changes are propagated to body fluids.
CONFLICT OF INTEREST
There are not conflicts of interest.
AUTHOR CONTRIBUTIONS
Frédéric Moynier, Marie Le Borgne, Jacques Hugon, Brandon Mahan, and Claire Paquet conceived the study. Jacques Hugon and Claire Paquet performed the sample collection. Frédéric Moynier, Brandon Mahan, and Esther Lahoud performed the isotopic measurements. Frédéric Moynier wrote the manuscript. All authors contributed to the interpretations and helped in writing the manuscript.
ETHICAL STATEMENT
The work follows the approval by the Ethical Committee of Paris Diderot University Hospitals (CEERB Bichat University Hospital, Paris, France).
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank the CNRS via a grant of the mission pour les initiatives transverses et interdisciplinaires (MITI) Defi ISOTOP (grant MAMI) and the Université Sorbonne Paris Cité for a Chaire d'Excellence. Parts of this work were supported by IPGP multidisciplinary program PARI, and by Region île‐de‐France SESAME Grant no. 12015908. We thank two contructive reviewers who greatly improve the quality of the manuscript and the editor for efficient handling of the manuscript.
Moynier F, Borgne ML, Lahoud E, et al. Copper and zinc isotopic excursions in the human brain affected by Alzheimer's Disease. Alzheimer's Dement. 2020;12:e12112. 10.1002/dad2.12112
[Online correction added on March 02, 2022: appearance of the author's name is incorrect in the original published version. The fifth author's name was updated to read as “Francois Mouton‐Liger”]
DATA AVAILABILITY STATEMENT
All data are available in supplementary materials.
REFERENCES
- 1. Miller LM, Wang Q, Telivala TP, Smith RJ, Lanzirotti A, Miklossy J. Synchrotron‐based infrared and X‐ray imaging shows focalized accumulation of Cu and Zn co‐localized with beta‐amyloid deposits in Alzheimer's disease. J Struct Biol. 2006;155:30‐37. [DOI] [PubMed] [Google Scholar]
- 2. Religa D, Strozyk D, Cherny RA, et al. Elevated cortical zinc in Alzheimer disease. Neurology. 2006;67:69‐75. [DOI] [PubMed] [Google Scholar]
- 3. Bush AI. The metallobiology of Alzheimer's disease. Trends Neurosci. 2003;26:207‐214. [DOI] [PubMed] [Google Scholar]
- 4. Kitazawa M, Cheng D, Laferla F. Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J Neurochem. 2009;108:1550‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roman AY, Devred F, Byrne D, et al. Zinc induces temperature‐dependent reversible self‐assembly of tau. J Mol Biol. 2019;431:687‐695. [DOI] [PubMed] [Google Scholar]
- 6. Bagheri S, Squitti R, Haertle T, Siotto M, Saboury AA. Role of copper in the onset of Alzheimer's disease compared to other metals. Front Aging Neurosci. 2017;9:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vasto S, Mocchegiani E, Malavolta M, et al. Zinc and inflammatory/immune response in aging. Ann N Y Acad Sci. 2007;100:111‐122. [DOI] [PubMed] [Google Scholar]
- 8. Moynier F, Vance D, Fujii T, Savage P. The isotope geochemistry of copper and zinc. In: Teng F‐Z, Watkins J, Dauphas N, eds. Non‐Traditional Stable Isotopes: Mineralogical Society of America. 2017:543‐600. [Google Scholar]
- 9. Moynier F, Fujii T, Shaw A, Le Borgne M. Heterogeneous distribution of natural zinc isotopes in mice. Metallomics. 2013;5:693‐699. [DOI] [PubMed] [Google Scholar]
- 10. Balter V, Lamboux A, Zazzo A, et al. Contrasting Cu, Fe, and Zn isotopic patterns in organs and body fluids of mice and sheep, with emphasis on cellular fractionation. Metallomics. 2013;5:1470‐1482. [DOI] [PubMed] [Google Scholar]
- 11. Mahan B, Moynier F, Jorgensen AL, Habekost M, Siebert J. Examining the homeostatic distribution of metals and Zn isotopes in Go¨ttingen minipigs. Metallomics. 2018;10:1264‐1281. [DOI] [PubMed] [Google Scholar]
- 12. Balter V, Nogueira da Costa A, Bondanese VP, et al. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc Natl Acad Sci U S A. 2015;112:982‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bush A, Pettingell W, Multhaup G, et al. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265:1464‐1467. [DOI] [PubMed] [Google Scholar]
- 14. Pithadia AS, Lim MH. Metal‐associated amyloid‐beta species in Alzheimer's disease. Curr Opin Chem Biol. 2012;16:67‐73. [DOI] [PubMed] [Google Scholar]
- 15. Krezel A, Hao Q, Maret W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch Biochem Biophys. 2007;463:188‐200. [DOI] [PubMed] [Google Scholar]
- 16. Poitrasson F, Sabater L, Henry M, et al. Zinc isotope study of neurotoxic peptide aggregation. Goldschmidt Conference. Hawaii 2020. p. 2099.
- 17. Moynier F, Foriel J, Shaw A, Le Borgne M. Zinc isotopic behavior during Alzheimer's disease. Geochem Perspect Lett. 2017;3:142‐150. [Google Scholar]
- 18. Moynier F, Creech J, Dallas J, Le Borgne M. Serum and brain natural copper stable isotopes in a mouse model of Alzheimer's disease. Sci Rep. 2019;9:11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Telouk P, Puisieux A, Fujii T, et al. Copper isotope effect in serum of cancer patients. A pilot study. Metallomics. 2015;7:299‐308. [DOI] [PubMed] [Google Scholar]
- 20. Costas‐Rodriguez M, Anoshkina Y, Lauwens S, Van Vlierberghe H, Delanghe J, Vanhaecke F. Isotopic analysis of Cu in blood serum by multi‐collector ICP‐mass spectrometry: a new approach for the diagnosis and prognosis of liver cirrhosis?. Metallomics. 2015;7:491‐498. [DOI] [PubMed] [Google Scholar]
- 21. Lauwens S, Costas‐Rodriguez M, Van Vlierberghe H, Vanhaecke F. Cu isotopic signature in blood serum of liver transplant patients: a follow‐up study. Sci Rep. 2016;6:30683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duyckaerts C, Delatour B, Potier M‐C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5‐36. [DOI] [PubMed] [Google Scholar]
- 24. Atwood C, Moir R, Huang X, et al. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;22:12817‐12826. [DOI] [PubMed] [Google Scholar]
- 25. Cheignon C, Tomas M, Bonnefont‐Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018;14:450‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bacchella C, Gentili S, Bellotti D, et al. Binding and reactivity of copper to R1 and R3 fragments of tau Protein. Inorg Chem. 2019;59:274‐286. [DOI] [PubMed] [Google Scholar]
- 27. Huang Y, Wu Z, Cao Y, Lang M, Lu B, Zhou B. Zinc binding directly regulates tau toxicity independent of tau hyperphosphorylation. Cell Rep. 2014;8:831‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
All data are available in supplementary materials.


