Abstract
Objective
Sarilumab is a human monoclonal antibody that blocks IL-6 from binding to membrane-bound and soluble IL-6 receptor-α. We assessed the long-term safety of sarilumab in patients from eight clinical trials and their open-label extensions.
Methods
Data were pooled from patients with rheumatoid arthritis who received at least one dose of sarilumab in combination with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs; combination group) or as monotherapy (monotherapy group). Treatment-emergent adverse events (AEs) and AEs and laboratory values of special interest were assessed.
Results
2887 patients received sarilumab in combination with csDMARDs and 471 patients received sarilumab monotherapy, with mean exposure of 2.8 years and 1.7 years, maximum exposure 7.3 and 3.5 years, and cumulative AE observation period of 8188 and 812 patient-years, respectively. Incidence rates per 100 patient-years in the combination and monotherapy groups, respectively, were 9.4 and 6.7 for serious AEs, 3.7 and 1.0 for serious infections, 0.6 and 0.5 for herpes zoster (no cases were disseminated), 0.1 and 0 for gastrointestinal perforations, 0.5 and 0.2 for major adverse cardiovascular events, and 0.7 and 0.6 for malignancy. Absolute neutrophil counts <1000 cells/mm3 were recorded in 13% and 15% of patients, respectively. Neutropenia was not associated with increased risk of infection or serious infection. Analysis by 6-month interval showed no signal for increased rate of any AE over time.
Conclusion
The long-term safety profile of sarilumab, either in combination with csDMARDs or as monotherapy, remained stable and consistent with the anticipated profile of a molecule that inhibits IL6 signalling.
Keywords: sarilumab, IL-6 inhibition, integrated safety analysis, long-term safety, biologic DMARD
Rheumatology key messages
This analysis represents the most comprehensive long-term safety report of sarilumab in RA to date.
Sarilumab’s long-term safety profile was consistent with phase III studies, with no new safety concerns.
Neutropenia was not associated with increased risk of infection or serious infection.
Introduction
IL-6 is a pleiotropic cytokine that plays a role in metabolic, homeostatic and regenerative processes [1]. IL-6 levels increase in response to infection or injury, promoting and coordinating pro-inflammatory activities. In autoimmune conditions such as RA, persistently elevated IL-6 levels can contribute to chronic inflammation and disease progression [2].
Sarilumab is a human monoclonal antibody that binds membrane-bound and soluble IL-6 receptor (IL-6R)-α to inhibit IL-6 cis- and trans-signalling [3]. Sarilumab is approved for the treatment of adults with moderately to severely active RA [3]. The efficacy and tolerability of sarilumab administered subcutaneously as monotherapy and in combination with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) have been demonstrated in active-comparator- and placebo-controlled phase III trials in adults with RA [4–6]. Long-term data on sarilumab as monotherapy and in combination with csDMARDs are being collected in patients with RA originally enrolled in eight trials, including those who continued into extension trials [4–11].
The aim of this post hoc analysis, the first integrated safety report of sarilumab in patients with RA, including up to 7.3 years of sarilumab exposure in combination with csDMARDs and up to 3.5 years as monotherapy, was to provide precise adverse event (AE) incidence rates (IRs) and to investigate changes in IRs over time for AEs of special interest (AESIs).
Methods
Data were pooled from patients with RA who received ⩾1 dose of sarilumab in combination with csDMARDs, or as monotherapy. Details of the contributing trials (MOBILITY, NCT01061736; TARGET, NCT01709578; ASCERTAIN, NCT01768572; MONARCH, NCT02332590; ACT11575, NCT01217814; ONE, NCT02121210; COMPARE, NCT01764997; and EASY, NCT02057250) [4–11] and open-label extensions (including EXTEND) [12] are provided in Supplementary Fig. S1, available at Rheumatology online. All trials were conducted in accordance with Good Clinical Practice and the principles laid down in the Declaration of Helsinki. All study protocols and patient information materials were approved by appropriate ethical review boards, and all patients provided written informed consent. Key exclusion criteria shared across the trials were prior treatment with an anti-IL-6R antagonist; history of malignancy; and history of inflammatory bowel disease, severe diverticulitis, or previous gastrointestinal perforation. At randomization, sarilumab dosage was 150 mg or 200 mg in monotherapy trials and predominantly 150 mg or 200 mg in csDMARD combination trials. In EXTEND, sarilumab starting dosage was 200 mg and dose reduction to 150 mg was permitted for protocol-specified laboratory abnormalities or at investigator discretion. Protocol-specified sarilumab dose modifications for neutropenia, thrombocytopenia and increased alanine aminotransferase (ALT) were consistent with recommendations in the sarilumab prescribing information (Supplementary Table S1, available at Rheumatology online) [3, 13]. Exposure was calculated as last dose date minus first dose date plus 14 days, regardless of unplanned intermittent discontinuations. The AE observation period included 60 days after the last dose of sarilumab.
AEs, including serious AEs (SAEs: including AEs that required inpatient hospitalization or prolongation of existing hospitalization) and prespecified AESIs, were collected at every visit. Samples for laboratory analysis, including haematology and clinical chemistry, were collected during screening, and pre-dose on treatment day 1, then at least every 2 weeks until week 12, at least every 12 weeks up to week 96, and at least every 24 weeks thereafter. AEs and AESIs were categorized according to Standardized Medical Dictionary for Regulatory Activities (MedDRA) Queries (narrow definitions) and High-Level Terms, except for infection (MedDRA primary system organ class), opportunistic infection (case-report form checkbox), and overdose (administering ⩾2 doses in <11 calendar days [once every 2 weeks (q2w) schedule] or <6 days [weekly (qw) schedule]; case-report form checkbox; reported as an AE per protocol). Serious infections were defined as infections requiring hospitalization and/or intravenous antibiotics. Major adverse cardiovascular events (MACE) were reviewed by an independent cardiovascular adjudication committee, and suspected gastrointestinal perforations were confirmed by medical review. Thromboembolic events were not prespecified as an AESI in the study protocols but are reported here post hoc based on the MedDRA high-level group term ‘Embolism and thrombosis’. IR by 6-month interval was analysed for serious AEs, serious infections, AEs leading to discontinuation, malignancies, MACE, injection-site reactions, absolute neutrophil count (ANC) <1000 cells/mm3, ALT >3× upper limit of normal (ULN), and platelet count <100 giga/L. The exact method was used to calculate 95% confidence intervals (95% CI) for proportions. For ANC, ALT and platelet count, the largest abnormality during follow-up is reported. Incidences of infection and serious infection were calculated by maximum neutropenia grade recorded at any time during the study. In addition, for infections that occurred within 12 weeks after an ANC assessment, incidences of infection and serious infection were calculated by the last ANC assessment before onset of the infection.
Results
Patient population and exposure
At the data cutoff of 15 January 2018, a total of 2887 patients had received at least one dose of sarilumab in combination with csDMARDs (predominantly methotrexate) and 471 patients had received at least one dose of sarilumab monotherapy (Supplementary Table S2, available at Rheumatology online). Most patients received sarilumab 200 or 150 mg q2w subcutaneously, except 151 patients from MOBILITY Part A (in combination with methotrexate) who received 100 mg qw, 150 mg qw, or 100 mg q2w subcutaneously. Both pooled study populations included patients with intolerance or inadequate response to csDMARDs, and the combination group also included those with inadequate response or intolerance to tumour necrosis factor antagonists and those with inadequate response to adalimumab plus methotrexate. Patients in the combination group had longer disease duration and greater prior exposure to biologic disease-modifying antirheumatic drugs (DMARDs) than those in the monotherapy group (Table 1).
Table 1.
Demographics and baseline characteristics
| Characteristic | Combination n = 2887 | Monotherapy n = 471 |
|---|---|---|
| Age, mean (s.d.), years | 51.8 (12.2) | 52.0 (12.6) |
| Female, n (%) | 2346 (81.3) | 389 (82.6) |
| Weight, mean (s.d.), kg | 75.6 (18.9) | 73.1 (17.5) |
| BMI ≥30 kg/m2, n (%) | 974 (33.8) | 127 (27.0) |
| Duration of RA, mean (s.d.), years | 9.4 (8.4) | 8.3 (8.4) |
| Prior biologic DMARD use, n (%) | 1118 (38.7) | 40 (8.5) |
| Baseline medications, n (%) | ||
| MTX without other csDMARD | 2654 (91.9) | 0 |
| MTX with or without other csDMARD | 2730 (94.6) | 0 |
| ≥2 other csDMARDs | 94 (3.3) | 0 |
| Oral corticosteroids | 1727 (59.8) | 253 (53.7) |
| NSAIDs | 2019 (69.9) | 323 (68.6) |
| Mean (s.d.) dose of csDMARDs at baseline | ||
| MTX, mg/week | 16.2 (5.1) | N/A |
| Leflunomide, mg/day | 19.3 (2.6) | N/A |
| Sulfasalazine, g/day | 1.65 (0.66) | N/A |
| Hydroxychloroquine, mg/day | 474 (258) | N/A |
csDMARD: conventional synthetic DMARD; N/A: not applicable.
Mean exposure to sarilumab in the combination group was 2.8 years, cumulative exposure was 7985.5 patient-years, maximum exposure was 7.3 years, and 773 patients (27%) were treated for ⩾240 weeks (∼5 years). In the monotherapy group, mean exposure was 1.7 years, cumulative exposure was 798.7 patient-years, maximum exposure was 3.5 years, and 384 patients (82%) were treated for ⩾60 weeks. Cumulative duration of observation for AEs was 8187.7 patient-years in the combination group and 812.4 patient-years in the monotherapy group.
Adverse events
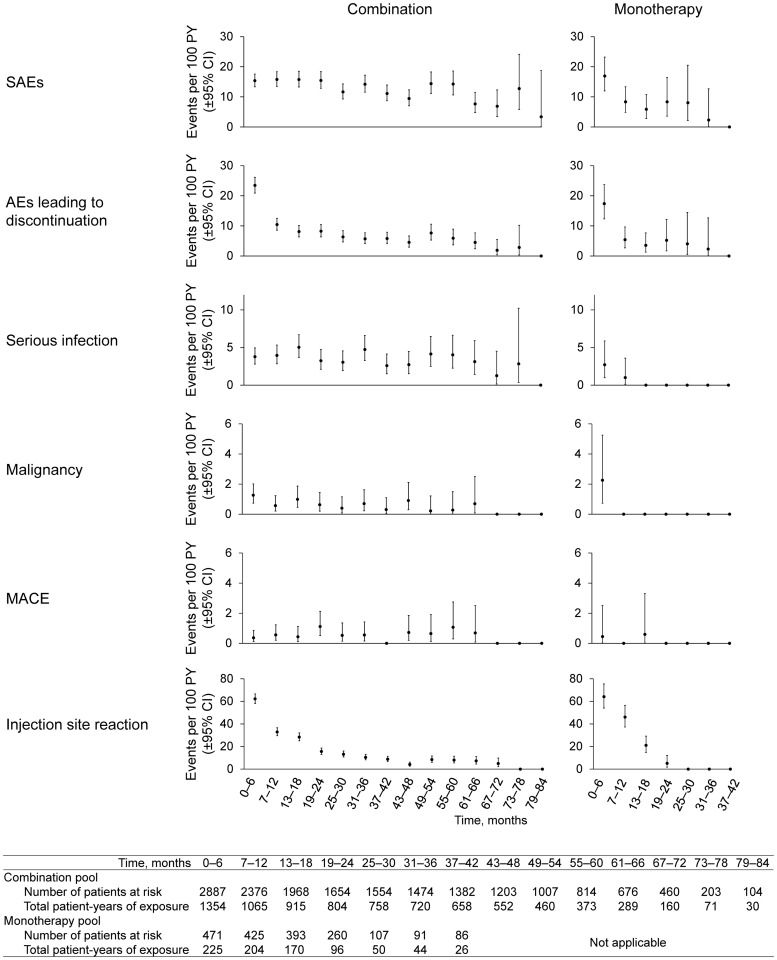
The overall incidence and exposure-adjusted IR of AEs were similar between combination and monotherapy (Table 2). The incidence and exposure-adjusted rate of SAEs and AEs leading to discontinuation were numerically lower with monotherapy compared with combination therapy. The most common AEs were neutropenia, injection-site erythema and upper respiratory tract infection with combination therapy, and neutropenia, injection-site erythema and nasopharyngitis with monotherapy. The most common SAEs were pneumonia, osteoarthritis and RA with combination therapy, and osteoarthritis, atrial fibrillation, neutropenia and RA with monotherapy. The most common AEs leading to discontinuation were neutropenia, ALT increased and herpes zoster (all non-disseminated) with combination therapy, and neutropenia, injection-site erythema and RA with monotherapy. There was no signal for an increased rate over time for any of the AEs analysed by 6-month interval (Fig. 1).
Table 2.
Investigator-reported all-cause AEs
| Combination (n = 2887) | Monotherapy (n = 471) | |||
|---|---|---|---|---|
| Cumulative total AE observation period, PY | 8187.7 | 812.4 | ||
| n (%) | IR/100 PY (nE) | n (%) | IR/100 PY (nE) | |
| Summarya | ||||
| Any AE | 2489 (86.2) | 144.2 (2489) | 386 (82.0) | 151.8 (386) |
| SAE | 685 (23.7) | 9.4 (685) | 52 (11.0) | 6.7 (52) |
| AE leading to discontinuation | 705 (24.4) | 8.7 (705) | 53 (11.3) | 6.6 (53) |
| AE leading to death | 31 (1.1) | 0.4 (31) | 5 (1.1) | 0.6 (5) |
| AEs with IR ≥5.0 per 100 PY in either groupb | ||||
| Neutropenia | 536 (18.6) | 13.8 (1132) | 85 (18.0) | 27.7 (225) |
| Injection-site erythema | 216 (7.5) | 13.3 (1091) | 38 (8.1) | 25.6 (208) |
| URTI | 386 (13.4) | 7.7 (634) | 37 (7.9) | 5.9 (48) |
| Accidental overdosec | 381 (13.2) | 6.7 (552) | 41 (8.7) | 6.6 (54) |
| Urinary tract infection | 309 (10.7) | 5.9 (481) | 33 (7.0) | 5.9 (48) |
| Nasopharyngitis | 294 (10.2) | 5.2 (426) | 55 (11.7) | 9.8 (80) |
| ALT increasedd | 309 (10.7) | 5.0 (412) | 26 (5.5) | 3.8 (31) |
| Bronchitis | 250 (8.7) | 4.3 (349) | 46 (9.8) | 7.1 (58) |
| SAEs with IR ≥0.3 per 100 PY in either groupb | ||||
| Pneumonia | 44 (1.5) | 0.6 (47) | 1 (0.2) | 0.1 (1) |
| Osteoarthritis | 36 (1.2) | 0.5 (43) | 4 (0.8) | 0.5 (4) |
| Rheumatoid arthritis | 34 (1.2) | 0.4 (35) | 2 (0.4) | 0.2 (2) |
| Cellulitis | 23 (0.8) | 0.3 (25) | 0 | 0 |
| Neutropenia | 22 (0.8) | 0.3 (23) | 2 (0.4) | 0.2 (2) |
| Atrial fibrillation | 9 (0.3) | 0.1 (10) | 3 (0.6) | 0.5 (4) |
| AEs leading to discontinuation with IR ≥0.3 per 100 PY in either groupb | ||||
| Neutropenia | 90 (3.1) | 1.1 (90) | 10 (2.1) | 1.5 (12) |
| ALT increased | 67 (2.3) | 0.8 (67) | 3 (0.6) | 0.4 (3) |
| Herpes zostere | 38 (1.3) | 0.5 (38) | 3 (0.6) | 0.4 (3) |
| Rheumatoid arthritis | 25 (0.9) | 0.3 (25) | 4 (0.8) | 0.5 (4) |
| Pneumonia | 24 (0.8) | 0.3 (24) | 1 (0.2) | 0.1 (1) |
| Injection-site erythema | 13 (0.5) | 0.2 (13) | 6 (1.3) | 0.7 (6) |
IR over time at risk of first event.
IR over cumulative total AE observation period.
Overdose was defined as administering ≥2 doses in <11 calendar days (once every 2 weeks schedule) or <6 days (weekly schedule).
Individual events were reported and laboratory abnormalities were not necessarily persistent.
All cases of herpes zoster were non-disseminated.
AE: adverse event; ALT: alanine aminotransferase; IR: incidence rate; nE: number of events; PY: patient-years; SAE: serious adverse event; URTI: upper respiratory tract infection.
Fig. 1.
Incidence rates of selected AEs by 6-month interval
AE: adverse event; csDMARD: conventional synthetic DMARD; MACE: major adverse cardiovascular event; PY: patient-years.
Laboratory abnormalities
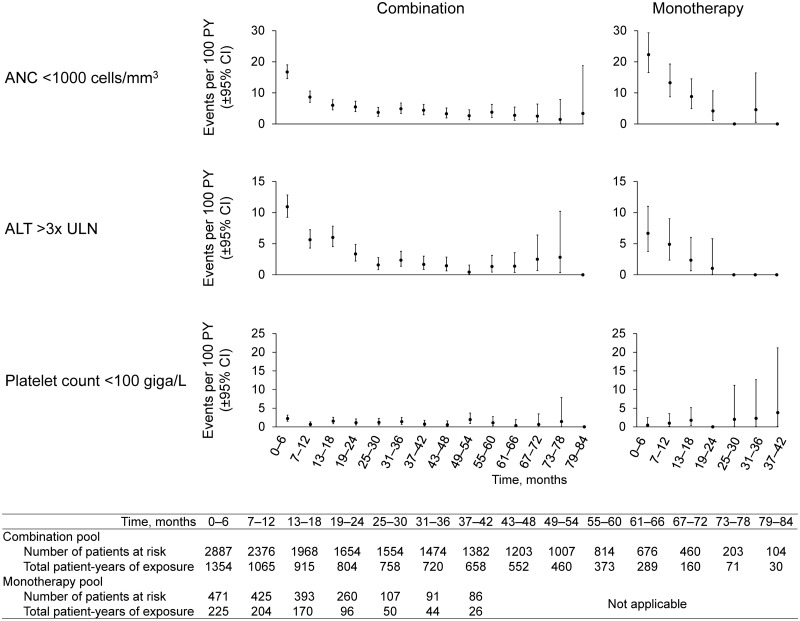
Leucopenia was reported as an AE in 21% and 20% of patients treated with combination therapy and monotherapy, respectively (IR 18.1 and 30.0 per 100 patient-years, respectively; Table 3). ANC values <1000 cells/mm3, the level at which dose interruption/reduction is recommended, were recorded in 13% and 15% of patients treated with combination therapy and monotherapy, respectively (Supplementary Table S3, available at Rheumatology online). Analysis by 6-month interval showed that incidence of ANC <1000 cells/mm3 was greatest during the first 6 months of treatment and declined thereafter (Fig. 2). ANC normalized on treatment in 257 (70%) of the 365 patients with ANC <1000 cells/mm3 in the combination group, and in 57 (81%) of the 70 patients with ANC <1000 cells/mm3 in the monotherapy group (Supplementary Table S2, available at Rheumatology online).
Table 3.
Investigator-reported all-cause AEs of special interest
| Combination (n = 2887) | Monotherapy (n = 471) | |||
|---|---|---|---|---|
| Cumulative total AE observation period, PY | 8187.7 | 812.4 | ||
| n (%) | IR/100 PY (nE) | n (%) | IR/100 PY (nE) | |
| Infections | 1582 (54.8) | 54.4 (4451) | 225 (47.8) | 54.9 (446) |
| Serious infections | 232 (8.0) | 3.7 (301) | 7 (1.5) | 1.0 (8) |
| Opportunistic infections | 72 (2.5) | 0.9 (76) | 6 (1.3) | 0.7 (6) |
| Tuberculosisa | 4 (0.1) | <0.1 (4) | 1 (0.2) | 0.1 (1) |
| Herpes zosterb | 51 (1.8) | 0.6 (53) | 4 (0.8) | 0.5 (4) |
| Leucopeniac | 618 (21.4) | 18.1 (1482) | 92 (19.5) | 30.0 (244) |
| Thrombocytopeniac | 101 (3.5) | 1.8 (147) | 5 (1.1) | 1.0 (8) |
| Hepatic disorders | 448 (15.5) | 8.9 (726) | 39 (8.3) | 7.1 (58) |
| Confirmed GI perforations | 9 (0.3) | 0.1 (9) | 0 | 0 |
| Upper | 3 (0.1) | <0.1 (3) | 0 | 0 |
| Lower | 6 (0.2) | 0.1 (6) | 0 | 0 |
| GI ulcerations | 33 (1.1) | 0.5 (38) | 1 (0.2) | 0.1 (1) |
| MACE | 41 (1.4) | 0.5 (45) | 2 (0.4) | 0.2 (2) |
| Elevation in lipidsc | 334 (11.6) | 6.1 (498) | 17 (3.6) | 2.2 (18) |
| Hypersensitivity | 308 (10.7) | 5.4 (444) | 37 (7.9) | 5.9 (48) |
| Anaphylaxis | 0 | 0 | 0 | 0 |
| Injection-site reactions | 333 (11.5) | 23.6 (1934) | 48 (10.2) | 34.3 (279) |
| Malignancy | 52 (1.8) | 0.7 (56) | 4 (0.8) | 0.6 (5) |
| Malignancy excluding NMSC | 38 (1.3) | 0.5 (38) | 3 (0.6) | 0.5 (4) |
| Lupus-like syndrome | 5 (0.2) | 0.1 (5) | 0 | 0 |
| Demyelinating disorders | 0 | 0 | 1 (0.2) | 0.1 (1) |
| Thromboembolic eventsd | 46 (1.6) | 0.8 (67) | 3 (0.6) | 0.4 (3) |
All cases of tuberculosis were reported as opportunistic infections.
Herpes zoster was reported as an opportunistic infection per protocol requirement; no cases of herpes zoster were disseminated.
Individual events were reported and laboratory abnormalities were not necessarily persistent.
Thromboembolic events were not prespecified as AEs of special interest and were summarized post hoc using a database search with the Medical Dictionary for Regulatory Activities System Organ Class ‘Vascular disorders’ and High-Level Group Term ‘Embolism and thrombosis’.
AE: adverse event; GI: gastrointestinal; IR: incidence rate; MACE: major adverse cardiovascular events (comprising cardiovascular death, myocardial infarction, stroke and hospitalization for either unstable angina and/or transient ischaemic attack); NMSC: non-melanoma skin cancer; PY: patient-years.
Fig. 2.
Incidence rates of selected laboratory abnormalities by 6-month interval
ALT: alanine aminotransferase; ANC: absolute neutrophil count; csDMARD: conventional synthetic DMARD; PY: patient-years; ULN: upper limit of normal.
ALT increase was reported as an AE in 11% and 6% of patients treated with combination therapy and monotherapy, respectively (IR 5.0 and 3.8 per 100 patient-years, respectively; Table 2). ALT elevations were observed in 65% and 48% of patients with combination therapy and monotherapy, respectively (Supplementary Table S4, available at Rheumatology online). ALT elevations >3× ULN, the level at which dose interruption is recommended, were observed in 10% and 6% of patients with combination therapy and monotherapy, respectively. Analysis by 6-month interval showed that incidence of ALT >3× ULN was greatest during the first 6 months of treatment and declined thereafter (Fig. 2). ALT normalized on treatment in 162 (55%) of the 296 patients with ALT >3× ULN in the combination group and in 17 (65%) of the 26 patients with ALT >3× ULN in the monotherapy group (Supplementary Table S4, available at Rheumatology online). Bilirubin elevations >1.5× ULN were observed in 135 patients (4.7%) and 25 patients (5.3%) with combination therapy and monotherapy, respectively, of whom 43 (1.5%) and 6 (1.3%) had elevations >2× ULN. There were no cases of Hy’s law attributable to sarilumab treatment.
Thrombocytopenia was reported at a rate of 1.8 and 1.0 per 100 patient-years with combination therapy and monotherapy, respectively (Table 3). Platelet counts <100 giga/L, the level at which dose interruption is recommended, were observed in 2.8% and 1.3% of patients with combination and monotherapy, respectively (Supplementary Table S5, available at Rheumatology online). Analysis by 6-month interval showed no increased incidence of platelet count <100 giga/L over time (Fig. 2). Platelet counts normalized on treatment in 47 (59%) of the 80 patients with platelet count <100 giga/L in the combination group and in four (67%) of the six patients with platelet count <100 giga/L in the monotherapy group (Supplementary Table S5, available at Rheumatology online).
Infections
Overall infection rates were 54.4 and 54.9, and serious infection rates were 3.7 and 1.0 per 100 patient-years for combination therapy and monotherapy, respectively (Table 3). The most common serious infections with combination therapy were pneumonia (n = 44; 1.5%), cellulitis (n = 23; 0.8%), and erysipelas (n = 9; 0.3%). Incidence of serious infections was low with monotherapy (n = 7; 1.5%), and no type of serious infection occurred in more than one patient. The rates of opportunistic infections, including herpes zoster and tuberculosis, were 0.9 and 0.7 per 100 patient-years with combination therapy and monotherapy, respectively. All cases of herpes zoster were non-disseminated.
With both combination therapy and monotherapy, incidences of infection and of serious infection were similar between patients with and without a recorded event of neutropenia at any time during the study (Table 4). Moreover, incidence of infection and serious infection did not increase with increasing severity of neutropenia. Of the total 4451 and 446 infections observed with combination therapy and monotherapy, respectively, 3943 (89%) and 434 (97%) occurred within 12 weeks after an ANC assessment. ANC values were normal at the last ANC assessment before infection for the majority of infections occurring within 12 weeks after an ANC assessment (3452/3943 [88%] and 370/434 [85%] of infections with combination therapy and monotherapy, respectively; Supplementary Table S6, available at Rheumatology online). Similar results were observed for serious infection: ANC values were normal at the last ANC assessment before serious infection for 244/261 (93%) and 8/8 (100%) serious infections occurring within 12 weeks of an ANC assessment with combination therapy and monotherapy, respectively.
Table 4.
Incidence of infection by lowest ANC during the study
| Combination (n = 2879)a | Monotherapy (n = 470)a | |||||
|---|---|---|---|---|---|---|
| Lowest ANC (neutropenia grade) | Lowest ANC n (%) | Infection n (%) | Serious infection n (%) | Lowest ANC n (%) | Infection n (%) | Serious infection n (%) |
| ≥LLN | 1382 (48.0) | 720 (25.0) | 106 (3.7) | 188 (40.0) | 93 (19.8) | 5 (1.1) |
| <LLN | 1497 (52.0) | 862 (29.9) | 126 (4.4) | 282 (60.0) | 132 (28.1) | 2 (0.4) |
| ≥1500 cells/mm3 – LLN (1) | 564 (19.6) | 332 (11.5) | 54 (1.9) | 112 (23.8) | 49 (10.4) | 0 (0) |
| ≥1000–<1500 cells/mm3 (2) | 568 (19.7) | 329 (11.4) | 48 (1.7) | 101 (21.5) | 52 (11.1) | 2 (0.4) |
| ≥500–<1000 cells/mm3 (3) | 318 (11.0) | 186 (6.5) | 22 (0.8) | 64 (13.6) | 29 (6.2) | 0 (0) |
| <500 cells/mm3 (4) | 47 (1.6) | 15 (0.5) | 2 (<0.1) | 5 (1.1) | 2 (0.4) | 0 (0) |
Number of patients with ≥1 post-baseline ANC assessment.
ANC: absolute neutrophil count; LLN: lower limit of normal.
Adverse events of special interest
Injection-site reactions were reported in 12% and 10% of patients with combination therapy and monotherapy, respectively (Table 3). There was a marked decline in incidence of injection-site reaction over time (Fig. 1). AEs of hypersensitivity occurred in 11% and 8% of patients with combination therapy and monotherapy, respectively. The most common hypersensitivity events (⩾1.0% incidence) were injection-site rash (n = 45; 1.6%), rash (n = 40; 1.4%) and urticaria (n = 29; 1.0%) with combination therapy, and rash (n = 5; 1.1%) with monotherapy. There were no events of anaphylaxis.
The exposure-adjusted incidence of malignancy was 0.7 and 0.6 per 100 patient-years (Table 3). The most common malignancy types with combination were basal cell carcinoma (n = 9; 0.3%), squamous cell carcinoma of skin (n = 4; 0.1%), breast cancer (n = 3; 0.1%) and malignant melanoma (n = 3; 0.1%). No more than one patient (0.2%) had the same malignancy type with monotherapy. The age- and sex-adjusted standardized incidence ratio for all malignancy types vs the general population in the US National Cancer Institute Surveillance and Epidemiology and End Results database, 2015, was 1.10 (95% CI 0.85, 1.43) with combination therapy and 0.94 (0.39, 2.25) with monotherapy. Compared with a reference population of patients with RA (Clinformatics Data Mart, 2000–2014; OptumInsight, Eden Prairie, MN, USA), the SIRs for all malignancy types were 0.55 (95% CI 0.42, 0.71) and 0.47 (0.20, 1.14) with combination therapy and monotherapy, respectively, and for malignancies excluding non-melanoma skin cancer, the SIRs were 0.38 (0.28, 0.52) and 0.39 (0.151, 1.03), respectively.
Nine patients had gastrointestinal perforations (three upper and six lower gastrointestinal tract) with combination therapy, giving an overall IR of 0.1 per 100 patient-years. The mean age of these nine patients at enrolment was 60 years (range 47–77). Six of the nine patients had been treated with concomitant corticosteroids, seven had been treated with nonsteroidal anti-inflammatory drugs (NSAIDs), four experienced AEs of diverticulitis during the study and none had a history of diverticulitis prior to baseline. There were no gastrointestinal perforations with monotherapy. Patients with a history of diverticulitis at baseline (n = 24 with combination [0.8%] and n = 6 with monotherapy [1.3%]) had no gastrointestinal-related AEs during sarilumab treatment. The majority of these patients were also receiving concomitant NSAIDs and/or corticosteroids (19/24 and 5/6, respectively).
Elevation in lipids was reported as an AE with an incidence of 6.1 and 2.2 per 100 patient-years with combination therapy and monotherapy, respectively (Table 3). Increases were observed in total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C; Supplementary Table S7, available at Rheumatology online). Mean HDL/LDL ratio remained generally stable throughout follow-up (Supplementary Fig. S2, available at Rheumatology online). At baseline, 319 (11%) and 56 patients (12%) in the combination and monotherapy groups, respectively, were receiving lipid-modifying agents (predominantly statins), of whom 236 and 46 had no change in prescription of lipid-modifying agent during the study, 68 and 9 changed medication, and 15 and 1 stopped their medication. A total of 307 (11%) and 21 patients (4%) in the combination and monotherapy groups, respectively, initiated lipid-modifying therapy after initiating study drug. There were 47 MACE in 43 patients overall (Supplementary Table S8, available at Rheumatology online).
Other medically relevant events
Thromboembolic events (reported by the investigators and evaluated post hoc; not a prespecified AESI) occurred at a rate of 0.8 and 0.4 per 100 patient-years with combination therapy and monotherapy, respectively, including 0.2 and 0.2 per 100 patient-years for pulmonary embolism and 0.2 and 0.1 per 100 patient-years for deep vein thrombosis.
Discussion
This integrated analysis of ∼9000 patient-years of cumulative patient exposure to sarilumab represents the most comprehensive investigation of sarilumab long-term safety to date. Results were consistent with the safety findings of sarilumab phase III trials, and consistent with the anticipated safety profile of IL-6 signalling inhibition [4–6, 14]. No signal was observed for an increased IR of any of the AEs or laboratory assessments analysed over time by 6-month interval. For several AEs, including serious infections, thrombocytopenia, ALT elevation, MACE and lipid elevations, the incidence was markedly lower with monotherapy than with combination therapy. Moreover, incidences of SAEs and AEs leading to discontinuation were lower with monotherapy than with combination therapy. These disparities likely reflect differences in the patient populations recruited into the combination and monotherapy trials, and in the case of serious infections and thrombocytopenia, possibly also the additional burden of taking more than one immunomodulator.
The IRs for SAEs and serious infections observed with sarilumab were no greater than those observed with other biologic and targeted synthetic DMARDs in long-term studies [15–19]. Focussing on DMARDs that target IL-6 signalling, the IRs for AEs and SAEs with sarilumab monotherapy (151.8 and 6.7 per 100 patient-years, respectively) and sarilumab combination therapy (144.2 and 9.4, respectively) may compare favourably with those observed for tocilizumab (224.5 and 13.6, respectively; all-exposed population: all doses, combination and monotherapy, exposure >36 months) [18]. Similarly, the IRs for serious infection with sarilumab monotherapy and combination therapy, 1.0 and 3.7 per 100 patient-years, respectively, may compare favourably with the IR of 4.5 observed with tocilizumab (all-exposed population) [18].
Consistent with previous analyses and with this class of therapy [4–6, 14], neutropenia was common with sarilumab treatment, as evidenced by investigator-reported AEs of leucopenia as well as protocol-mandated study measures of ANC. However, in this dataset, patients with neutropenia at any time during the study were no more likely to develop infections or serious infections than patients without neutropenia. Furthermore, the last ANC recorded before onset of infection or serious infection was normal in most cases. The absence of an association between neutropenia and infection in the sarilumab clinical trial populations is supported by the analyses of infection rate by maximum grade of neutropenia during the study (lowest ANC), which found no increase in infection rate with increasing maximum grade of neutropenia. Moreover, although the IR for leucopenia was numerically greater with sarilumab monotherapy than with combination therapy, the IR for serious infection was lower with monotherapy than with combination therapy. Evidence from pharmacodynamic studies suggests that the disconnect between neutropenia and infection with sarilumab treatment might be a consequence of neutrophil margination, whereby blockade of the effects of IL-6 results in migration of neutrophils from the circulation into extravascular pools without impairing their function [20, 21]. In vitro and in vivo studies on the effect of inhibition of IL-6 signalling on neutrophils found no effect on apoptosis, priming of respiratory burst, expression of adhesion molecules or chemotaxis [22].
Effective treatment of RA is associated with an increase in TC, LDL-C and HDL-C levels, without a change in TC: HDL-C ratio and without concomitant increase in the risk of MACE [23]. Monitoring lipid levels is recommended 1–2 months after initiating sarilumab and every 6 months thereafter [3]. Owing to a disease–drug interaction involving anti-IL-6R agents and simvastatin [24], the LDL-lowering effect of simvastatin is reduced by 5–6% in patients with RA taking concomitant sarilumab [25]. In the present analysis, sarilumab treatment was associated with increases in TC, LDL-C and HDL-C, whereas HDL/LDL ratio remained generally stable. The changes were not associated with an elevated risk of MACE in this study population. Exposure-adjusted incidences of MACE with sarilumab combination and monotherapy (0.5 and 0.2 per 100 patient-years, respectively) were no greater than the incidence in the general RA population (1.4 per 100 patient-years without exposure to DMARDs, 1.1 with exposure to DMARDs, and 1.2 overall) [26]. The absence of an excess in MACE despite the increase in lipid levels might be a manifestation of the ‘lipid paradox’, which describes the weaker association between LDL-C level and cardiovascular risk among patients with RA compared with the general population [27]. Moreover, inhibition of IL-6 signalling might exert effects on cardiovascular risk outside any effects on lipid levels; advances in the understanding of the role of inflammation in atherosclerosis have led to the suggestion that targeting the actions of IL-6 might prove beneficial in reducing the inflammatory response implicated in development of coronary artery disease [28, 29].
Gastrointestinal perforation is a rare but serious condition, and patients with RA may be at higher risk than the general population [30, 31]. The incidence of gastrointestinal perforations observed with sarilumab (IRs of 0.1 per 100 patient-years and 0 with combination therapy and monotherapy, respectively) was lower than reported with tocilizumab (0.3 per 100 patient-years) [32]. It is notable that the majority of patients who experienced gastrointestinal perforations with sarilumab were taking concomitant NSAIDs and/or corticosteroids, which are a known risk factor for gastrointestinal perforations in patients with RA [30]. Indeed, the IR of 0.1 per 100 patient-years for the sarilumab combination group irrespective of NSAID/corticosteroid use is similar to the rate reported for biologic DMARDs without glucocorticoids in an administrative database analysis of 143 000 patients with RA (0.10 per 100 patient-years without glucocorticoids and 0.19 per 100 patient-years with glucocorticoids) [30]. However, the protocol exclusion of patients with a history of severe diverticulitis, another recognized risk factor for gastrointestinal perforation [30], may have mitigated against the risk of gastrointestinal perforation in this population. Sarilumab prescribing information lists diverticulitis under warnings and precautions and recommends the prompt evaluation of acute abdominal signs or symptoms [3].
Patients with RA are at approximately two-fold greater risk of venous thromboembolism compared with the general population, and assessment of thromboembolic events has become an important factor in the assessment of drug safety in RA [33, 34]. The IRs for thromboembolic events with sarilumab combination therapy and monotherapy (0.8 and 0.4 per 100 patient-years, respectively) were within the range of IRs reported in population-level analyses of patients with RA treated with DMARDs (0.4–0.8 per 100 patient-years) [33].
The IR for malignancy with sarilumab was similar to the general population, lower than a reference population of patients with RA, and remained stable throughout the observation period, suggesting no excess of malignancies with sarilumab.
Elevation in liver enzymes is a recognized effect of IL-6 signalling inhibition, and the profile of ALT increases seen with sarilumab was similar to that reported with tocilizumab [35]. The approximately doubled incidence of AEs of ALT elevation with sarilumab in combination with csDMARDs (predominantly methotrexate) compared with sarilumab monotherapy might reflect the known hepatoxic effects of methotrexate [36].
One limitation of this analysis is that cumulative patient-years of exposure to sarilumab in combination with csDMARDs was ∼10 times greater than exposure to sarilumab monotherapy; consequently, the level of evidence is lower for monotherapy than for combination therapy. Moreover, where the incidence of an adverse event is low, it is not possible to appropriately determine a differential rate between combination and monotherapy because too few events occurred with monotherapy to allow a meaningful comparison. Another limitation, common to all prospective long-term analyses, is that attrition of patients who develop AEs, SAEs or serious infections tends to enrich the long-term population with patients who are best able to tolerate treatment.
In conclusion, no new safety concerns emerged in this integrated analysis of up to 7 years’ sarilumab treatment representing almost 9000 years cumulative exposure. The long-term safety profile of sarilumab, either in combination with csDMARDs or as monotherapy remained stable and consistent with the anticipated profile of an IL-6 signalling inhibitor. Safety follow-up is ongoing in the sarilumab clinical development programme for both combination treatment and monotherapy.
Supplementary Material
Acknowledgements
The authors thank the patients and their families as well as the investigators and other study staff involved in the studies. Medical writing support was provided by Matt Lewis PhD (Adelphi Communications Ltd.), and funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Funding: This study was supported by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Disclosure statement: R.F. has received research support and/or consulting fees from AbbVie, ACEA, Amgen, AstraZeneca, BMS, Celgene, Celltrion, EMD Serono, Eli Lilly, GSK, Merck, Novartis, Pfizer, Roche, Samsung, Sandoz, Sanofi-Genzyme, Taiho and UCB. M.G. has received research grants or consulting fees from R-Pharm, Roche/Genentech, and Sanofi Genzyme. Y.L. and S.W. are employees of Sanofi Genzyme and may hold stock and/or stock options in the company. G.St.J. is an employee of Regeneron and may hold stock and/or stock options in the company. D.vdH. has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi, Eli-Lilly, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB. J.J.G.-R. has received research support and/or consulting fees from Biogen, Gilead, Eli Lilly, Merck Sharp & Dohme, Pfizer and Roche. J.A.M.-C. has received consulting fees and/or participated in Speakers’ Bureau for Pfizer, Merck Sharp and Dohme, Sanofi–Aventis, Novartis, Bristol-Myers Squibb, Roche, Boehringer Ingelheim, Schering–Plough, Abbott, UCB, Eli Lilly and Gilead. M.S. has received consulting fees from R-Pharm. A.K. has received consulting fees and/or participated in Speakers’ Bureau for AbbVie, Pfizer, Genentech, UCB, Sanofi/Regeneron, Celgene, Horizon and Merck. G.B. has received research support and/or consulting fees from AbbVie, Lilly, Merck Sharpe & Dohme, Pfizer, Roche, Sanofi and UCB.
References
- 1. Schett G. Physiological effects of modulating the interleukin-6 axis. Rheumatology 2018;57:ii43–50. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka T, Kishimoto T.. Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int J Biol Sci 2012;8:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kevzara Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf (1 March 2019, date last accessed).
- 4. Genovese MC, Fleischmann R, Kivitz AJ. et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a Phase III study. Arthritis Rheumatol 2015;67:1424–37. [DOI] [PubMed] [Google Scholar]
- 5. Fleischmann R, van Adelsberg J, Lin Y. et al. Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol 2017;69:277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burmester GR, Lin Y, Patel R. et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis 2017;76:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kivitz A, Baret-Cormel L, van Hoogstraten H. et al. Usability and patient preference phase 3 study of the sarilumab pen in patients with active moderate-to-severe rheumatoid arthritis. Rheumatol Ther 2018;5:231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emery P, Rondon J, Parrino J. et al. Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis. Rheumatology 2019;58:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COMPARE Clinical Trial Record. www.clinicaltrials.gov/ct2/show/NCT01764997 (1 March 2019, date last accessed).
- 10.ACT11575 Clinical Trial Record. www.clinicaltrials.gov/ct2/show/NCT01217814 (1 March 2019, date last accessed).
- 11.Wells AF, Parrino J, Mangan EK et al./collab>. Immunogenicity of sarilumab monotherapy in patients with rheumatoid arthritis who were inadequate responders or intolerant to disease-modifying antirheumatic drugs. Rheumatol Ther 2019; Advance Access published 14 May 2019, doi: 10.1007/s40744-019-0157-3. [DOI] [PMC free article] [PubMed]
- 12.EXTEND Clinical Trial Record. www.clinicaltrials.gov/ct2/show/NCT01146652 (1 March 2019, date last accessed).
- 13.Kevzara Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/kevzara-epar-product-information_en.pdf (1 March 2019, date last accessed).
- 14. Kim GW, Lee NR, Pi RH. et al. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res 2015;38:575–84. [DOI] [PubMed] [Google Scholar]
- 15. Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP.. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013;72:517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen SB, Tanaka Y, Mariette X. et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleischmann R, Wollenhaupt J, Takiya L. et al. Safety and maintenance of response for tofacitinib monotherapy and combination therapy in rheumatoid arthritis: an analysis of pooled data from open-label long-term extension studies. RMD Open 2017;3:e000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Genovese MC, Rubbert-Roth A, Smolen JS. et al. Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: a cumulative analysis of up to 4.6 years of exposure. J Rheumatol 2013;40:768–80. [DOI] [PubMed] [Google Scholar]
- 19. Klareskog L, Gaubitz M, Rodriguez-Valverde V. et al. Assessment of long-term safety and efficacy of etanercept in a 5-year extension study in patients with rheumatoid arthritis. Clin Exp Rheumatol 2011;29:238–47. [PubMed] [Google Scholar]
- 20. Kovalenko P, Paccaly A, Boyapati A. et al. Pharmacodynamic (PD) model of neutrophil margination to describe transient effect of single-dose sarilumab on absolute neutrophil count (ANC) in patients with rheumatoid arthritis (RA). J Pharmacokinet Pharmacodyn 2017; 44(Suppl 1):S11–S143. Abstract T-033. [Google Scholar]
- 21. Lok LSC, Farahi N, Juss JK. et al. Effects of tocilizumab on neutrophil function and kinetics. Eur J Clin Invest 2017;47:736–45. [DOI] [PubMed] [Google Scholar]
- 22. Wright HL, Cross AL, Edwards SW, Moots RJ.. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology 2014;53:1321–31. [DOI] [PubMed] [Google Scholar]
- 23. Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M.. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology 2014;53:2143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S.. Disease-drug-drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther 2011;89:735–40. [DOI] [PubMed] [Google Scholar]
- 25. Lee EB, Daskalakis N, Xu C. et al. Disease-drug interaction of sarilumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacokinet 2017;56:607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ogdie A, Yu Y, Haynes K. et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis 2015;74:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liao KP, Liu J, Lu B, Solomon DH, Kim SC.. Association between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol 2015;67:2004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hartman J, Frishman WH.. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev 2014;22:147–51. [DOI] [PubMed] [Google Scholar]
- 29. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016;118:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Curtis JR, Lanas A, John A, Johnson DA, Schulman KL.. Factors associated with gastrointestinal perforation in a cohort of patients with rheumatoid arthritis. Arthritis Care Res 2012;64:1819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gout T, Ostor AJ, Nisar MK.. Lower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: a systematic literature review. Clin Rheumatol 2011;30:1471–4. [DOI] [PubMed] [Google Scholar]
- 32. Van Vollenhoven R, Keystone E, Furie R. et al. Gastrointestinal safety in patients with rheumatoid arthritis treated with tocilizumab: data from Roche clinical trials. Arthritis Rheum 2009;60(Suppl S10):S602. Abstract 1613. [Google Scholar]
- 33. Scott IC, Hider SL, Scott DL.. Thromboembolism with Janus kinase (JAK) inhibitors for rheumatoid arthritis: how real is the risk? Drug Saf 2018;41:645–53. [DOI] [PubMed] [Google Scholar]
- 34. Ungprasert P, Srivali N, Spanuchart I, Thongprayoon C, Knight EL.. Risk of venous thromboembolism in patients with rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol 2014;33:297–304. [DOI] [PubMed] [Google Scholar]
- 35. Genovese MC, Kremer JM, van Vollenhoven RF. et al. Transaminase levels and hepatic events during tocilizumab treatment: pooled analysis of long-term clinical trial safety data in rheumatoid arthritis. Arthritis Rheumatol 2017;69:1751–61. [DOI] [PubMed] [Google Scholar]
- 36. West SG. Methotrexate hepatotoxicity. Rheum Dis Clin North Am 1997;23:883–915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.