Abstract
Objective
To examine the role of Tie2 signalling in macrophage activation within the context of the inflammatory synovial microenvironment present in patients with RA and PsA.
Methods
Clinical responses and macrophage function were examined in wild-type and Tie2-overexpressing (Tie2-TG) mice in the K/BxN serum transfer model of arthritis. Macrophages derived from peripheral blood monocytes from healthy donors, RA and PsA patients, and RA and PsA synovial tissue explants were stimulated with TNF (10 ng/ml), angiopoietin (Ang)-1 or Ang-2 (200 ng/ml), or incubated with an anti-Ang2 neutralizing antibody. mRNA and protein expression of inflammatory mediators was analysed by quantitative PCR, ELISA and Luminex.
Results
Tie2-TG mice displayed more clinically severe arthritis than wild-type mice, accompanied by enhanced joint expression of IL6, IL12B, NOS2, CCL2 and CXCL10, and activation of bone marrow-derived macrophages in response to Ang-2 stimulation. Ang-1 and Ang-2 significantly enhanced TNF-induced expression of pro-inflammatory cytokines and chemokines in macrophages from healthy donors differentiated with RA and PsA SF and peripheral blood-derived macrophages from RA and PsA patients. Both Ang-1 and Ang-2 induced the production of IL-6, IL-12p40, IL-8 and CCL-3 in synovial tissue explants of RA and PsA patients, and Ang-2 neutralization suppressed the production of IL-6 and IL-8 in the synovial tissue of RA patients.
Conclusion
Tie2 signalling enhances TNF-dependent activation of macrophages within the context of ongoing synovial inflammation in RA and PsA, and neutralization of Tie2 ligands might be a promising therapeutic target in the treatment of these diseases.
Keywords: rheumatoid arthritis, psoriatic arthritis, macrophages, Tie2, angiopoietins, inflammation, serum transfer-induced arthritis, synovial tissue explants
Rheumatology key messages
Tie2 overexpression enhances the severity of K/BxN serum transfer-induced arthritis.
Tie2 induces the expression of inflammatory mediators by macrophages from RA and PsA patients.
Ang-2 neutralization reduced the production of IL-6 and IL-8 by synovial tissue of RA patients.
Introduction
RA and PsA are clinically distinct diseases with different aetiologies, but both are characterized by synovial hyperplasia, elevated synovial expression of proinflammatory cytokines including TNF, IL-1β and IL-6, increased angiogenesis and eventual joint destruction [1, 2]. Tie2 is a tyrosine kinase receptor essential for vascular development and blood vessel remodelling through interaction with its ligands angiopoietin-1 (Ang-1) and Ang-2 [3]. Tie2 and its ligands are expressed in RA and PsA synovial tissue at higher levels than observed in healthy controls and OA patients [4–6]. In RA and PsA synovial tissue, Tie2 is expressed by fibroblast like synoviocytes (FLS), endothelial cells (ECs) and macrophages [5, 7]. Ang-1 and Ang-2 are produced by synovial ECs, macrophages and FLS, and their expression is induced by inflammatory mediators such as TNF, TGF-β and toll-like receptors agonists [4–6, 8–10]. Importantly, expression of Tie2, Ang-1 and Ang-2 is elevated in the early stages of RA and PsA, and Tie2 signalling may contribute to the onset and progression of RA [6, 11]. Moreover, inhibition of Tie2 signalling using gene therapy strategies, soluble receptors or neutralizing antibodies against Ang-2 or Tie2 reduces the incidence and severity of murine experimental arthritis [7, 12–15].
We have previously observed that phosphorylated Tie2, indicative of Tie2 activation, is predominantly observed in RA and PsA synovial macrophages [7]. Macrophages are one of the most predominant cell types present in the synovium of RA and PsA patients and contribute to the pathology of these diseases through the release of inflammatory cytokines, MMPs, chemokines, reactive oxygen and nitrogen intermediates [16–18]. Importantly, clinical disease activity in RA and PsA correlates strongly with macrophage numbers in the synovial tissue and with the production of macrophage-derived cytokines like TNF, IL-1β and IL-6. Reciprocally, decreased numbers of synovial macrophages and expression of macrophages products correlate strongly with the clinical efficacy of treatments in RA and PsA [19–22]. Myeloid Tie2 signalling has been most extensively studied in tumour-associated Tie2-expressing monocytes, which have immunosuppressive properties and play an essential role in the vascularization and growth of solid tumours [23–26]. However, we found in vitro that Tie2 is expressed by both M1 (pro-inflammatory) and M2 (immunoregulatory) macrophages differentiated from healthy donors. While stimulation of these cells with Ang-1 or Ang-2 had little effect on macrophage gene expression, Tie2 stimulation of both M1 and M2 macrophages enhanced TNF-induced expression of cytokines and chemokines [27].
In vitro polarization of cells is a useful model for studying potential immunomodulatory properties of macrophages under different disease conditions, and synovial macrophages from inflammatory arthritis patients display both M1 and M2 characteristics [28]. However, the complex cytokine and cell–cell interactions present in the inflamed joint confer a unique pro-inflammatory transcriptome to synovial macrophages [29, 30] that can alter macrophage responses to subsequent cytokine stimulation through signalling crosstalk, positive and negative feedback mechanisms, and epigenetic regulation [31–33]. As a result, a cytokine signalling pathway that by itself has robust effects on macrophage gene expression and activation may make inconsequential or unpredictable contributions to synovial pathology within a complex inflammatory environment. Notably, although expression of Tie2 and its ligands is altered in both RA and PsA synovial tissue, differences in neo-angiogenic patterning is one of the most readily distinguishing features between these diseases, which are otherwise very similar in cellular composition and gene expression [6, 34, 35]. Given these considerations, we here examined how Tie2 stimulation might regulate macrophage activation in the context of the synovial environment, using murine and ex vivo human models of inflammatory arthritis.
Methods
Methodology describing the histological analysis, isolation of murine bone marrow, human monocytes, flow cytometry, RT-PCR and quantitative (q)PCR arrays, immunoblotting, measurement of cytokine production and the culture, differentiation and stimulation of macrophages and synovial biopsy explants has been previously published and is provided in detail in the Supplementary Material, section Methods, available at Rheumatology online [27, 36, 37].
Generation and maintenance of Tie2-overexpressing mice
Transgenic mice inducibly overexpressing Tie2 in all cellular compartments normally expressing endogenous Tie2 (Tie2-TG) were described previously [38]. pTek-tTA and pTetOS-Tek mice (kindly provided by D. Dumont, Sunnybrook Research Institute, Toronto, ON, Canada), maintained as CD1 outbred lines, were backcrossed onto a wild-type (WT) C57BL/6J background for at least 10 generations prior to their use in experiments. Tie2-TG and WT control littermates were obtained by breeding the pTek-tTA and the pTetOS-Tek mouse lines and identification by genotyping. Mice were housed under conventional conditions at the animal facility of the Academic Medical Center (Amsterdam, The Netherlands) and were fed ad libitum. The drinking water of mice was supplemented with doxycycline (200 µg/ml, Sigma-Aldrich, Zwijndrecht, The Netherlands) until 2 weeks before initiation of experiments, at which time doxycycline was withdrawn to allow overexpression of Tie2. Animal experiments were approved by the Academic Medical Center animal ethics committee.
Serum-transfer arthritis and clinical scoring
K/BxN serum was collected from 4- to 8-week-old arthritic K/BxN mice (provided by C. Benoist and D. Mathis, Harvard Medical School, Boston, MA, USA), pooled and stored at –80°C until use. Arthritis was induced by transfer of 100 μl of K/BxN serum into 12- to 14-week-old mice (five WT and five Tie2-TG) by intraperitoneal injection on day 0 and day 2. Mice were sacrificed on day 14 after serum transfer. Arthritis severity was assessed in each of the four limbs, every 2 days by two blinded observers, using a semi-quantitative clinical score (0: no swelling; 1: slight swelling and erythema of the ankle, wrist or digits; 2: moderate swelling and erythema; 3: severe swelling and erythema; and 4: maximal inflammation with joint rigidity; maximum possible score 16 per mouse).
Patients
Synovial biopsies were obtained by needle arthroscopy as previously described [39] from clinically active inflamed joints of RA and PsA patients fulfilling the ACR/EULAR criteria for RA and the classification criteria for PsA, respectively [40–42]. Clinical characteristics of patients providing synovial tissue, peripheral blood, serum and SF for cellular material are detailed in supplementary Tables S1–S3, available at Rheumatology online. All patients provided written informed consent prior to their inclusion in this study, and this study was approved by the Medical Ethics Committee of the Academic Medical Center, University of Amsterdam (MEC 07/253) and the University Medical Center Utrecht (MEC 13-696 and 13-697).
Statistical analyses
Statistical analysis was performed using Windows GraphPad Prism 5 (GraphPad Software, Inc.). Potential differences between experimental groups were analysed by non-parametric, Kruskal–Wallis test and Friedman test, or parametric analysis of variance test as appropriate. P-values <0.05 were considered statistically significant.
Results
Increased arthritis severity in mice overexpressing Tie2
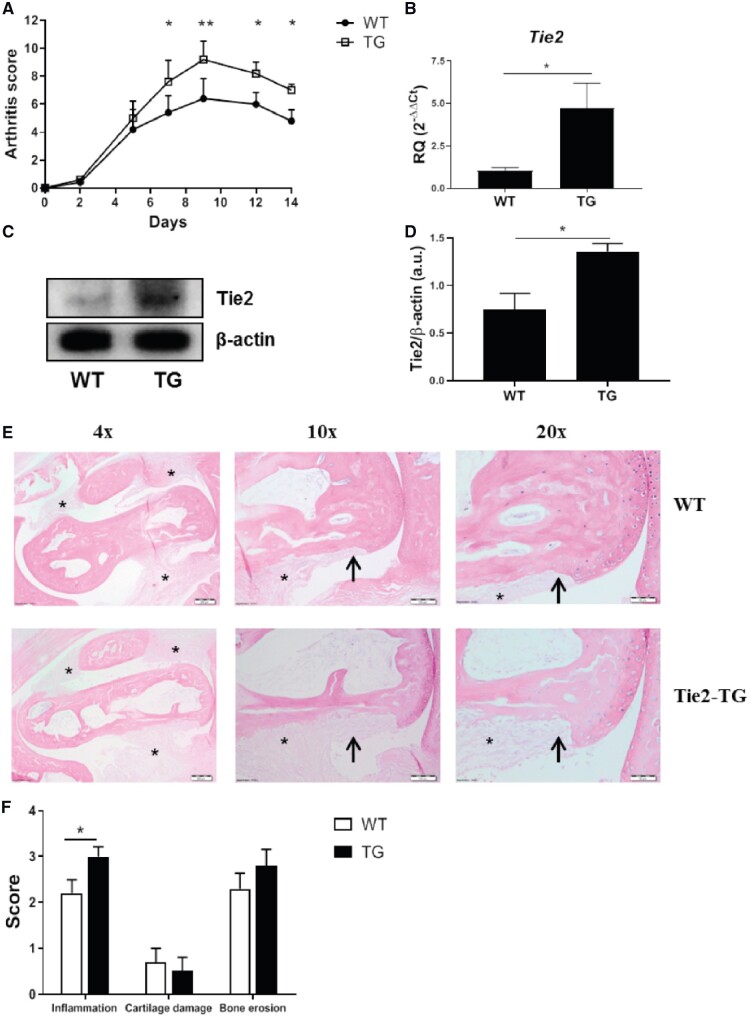
Interfering with Tie2 signalling by administration of soluble recombinant Tie2 or neutralizing anti-Ang-2 antibodies is protective in murine models of arthritis [7, 13], but it is unclear whether the overexpression of Tie2 observed in the synovium of RA patients is sufficient to enhance inflammatory pathways in vivo [4–6]. Therefore, we determined the consequences of altered Tie2 expression on disease activity in the K/BxN serum transfer murine arthritis model, using mice in which removal of deoxycycline from the drinking water allows overexpression of transgenic Tie2 in all cell types expressing endogenous Tie2 [38]. The administration of K/BxN serum led to arthritis in all of the WT and Tie2-TG mice (data not shown). The clinical severity of disease was significantly higher in Tie2-TG mice (Fig. 1A). Importantly, we observed an overexpression of Tie2 in the total joint tissue of Tie2-TG arthritic mice, as well as in isolated bone marrow-derived macrophages (BMDM, Fig. 1B–D and supplementary Fig. S1A, available at Rheumatology online). Histological analysis of the tibiotalar and forefoot joints revealed a significant increase in synovial inflammation in Tie2-TG mice compared with WT mice, and a trend towards increased bone erosion. Tie2 overexpression did not influence cartilage damage in this arthritis model (Fig. 1E and F). To determine the molecular mechanisms associated with the more severe clinical pathology observed in Tie2-TG mice, we analysed joint mRNA expression of inflammatory mediators, either identified previously as Tie2-inducible gene products in human macrophages or well-documented contributors to pathology in murine arthritis [27]. In WT mice, the induction of arthritis up-regulated expression of Il12b, Tnf and Ccl3, compared with non-arthritic WT mice. In Tie2-TG arthritic mice, expression of Il6, Il12, Nos, Il1, C-C motif ligand 2 Ccl2, C-X-C motif chemokine Cxcl10, Tnf, Ccl3, Mmp3 and Tnfrsf11a (the gene that encodes Receptor activator of nuclear factor κβ) was enhanced compared with non-arthritic WT and Tie2-TG mice. Comparing expression between arthritic WT and Tie2-TG mice, we observed that expression of Il6, Il12B, Nos, Ccl2 and Cxcl10 was significantly higher in Tie2-TG mice (Fig. 2A). We also analysed the expression of the Tie2 ligands. The expression of Ang1 and Ang2 was similar between the non-arthritic WT and Tie2-TG mice. Arthritis did not modulate the expression of Ang1 and induced a slight up-regulation of Ang2 in both WT and Tie2-TG mice arthritic mice, but differences were not significant (supplementary Fig. S1B, available at Rheumatology online).
Fig. 1.
Increased arthritis severity in mice overexpressing Tie2
(A) Daily global arthritic scores of WT and Tie2-TG mice. *P < 0.05 and **P < 0.01 compared with WT mice. (B, C) mRNA expression (B) and representative immunoblot (C) of Tie2 in the joints of WT and Tie2-TG mice. qPCR data are shown as relative quantity respect to WT control mice, as described in the Methods. Bars represent the means and s.e.m. of five mice. *P < 0.05. (D) Densitometric analysis of Tie2 protein expression. Data are shown as relative expression with respect to β-actin expression. Bars represent the mean and s.e.m. of five mice. *P < 0.05. (E) Representative images of joint pathology in indicated mice visualized by haematoxylin and eosin staining. Representative areas of synovial cellular infiltration (asterisk) and bone erosion (black arrows) are noted. (F) Inflammation, cartilage damage and bone erosion scores of mice in each group. Data are mean ± s.e.m. for each group (n = 10 ankle joints per group). *P < 0.05. qPCR: quantitative PCR; Tie2-TG: transgenic mice inducibly overexpressing Tie2; WT: wild-type.
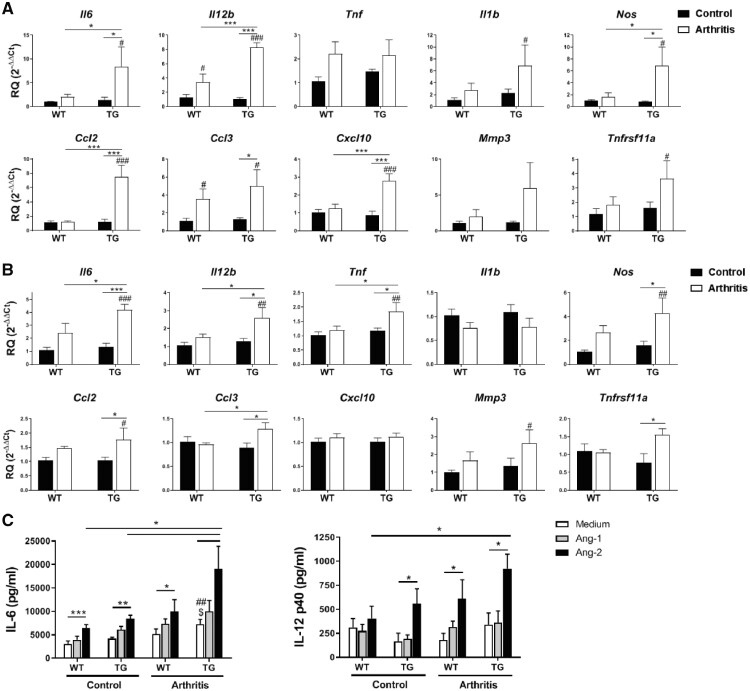
Fig. 2.
Enhanced expression of inflammatory mediators in arthritic mice overexpressing Tie2
(A, B) mRNA expression of inflammatory mediators in the forepaws (A) and BMDM (B) of control and arthritic WT or Tie2-TG mice. Data are shown as relative quantity with respect to WT control mice, as described in the Methods. Bars represent the means and s.e.m. of five mice. (C) IL-6 and IL-12p40 expression in M1-differentiated BMDM from control and arthritic WT and Tie2-TG mice after 24 h incubation in medium, Ang-1 or Ang-2 (both 200 ng/ml). Bars represent the means and s.e.m. of five mice. *P < 0.05, **P < 0.01 and ***P < 0.001; ##P < 0.01 and ###P < 0.001 compared with WT control mice; $P < 0.05 compared with TG control mice. Ang-1 and -2: angiopoietin-1 and -2; BMDM: bone marrow-derived macrophages; Tie2-TG: transgenic mice inducibly overexpressing Tie2; WT: wild-type.
To determine whether Tie2 overexpression in murine macrophages could contribute to the enhanced pathology observed in arthritic Tie2-TG mice, we analysed the mRNA expression of these inflammatory mediators by unpolarized BMDM from untreated and arthritic WT and Tie2-TG mice. The expression of Il6, Il12b, Tnf and Ccl3, genes displaying enhanced expression in Tie2-TG joints, was significantly higher in BMDM from arthritic Tie2-TG compared with arthritic WT mice (Fig. 2B). We also examined the capacity of Ang-1 and Ang-2 to stimulate M1-differentiated BMDM. Basal production of IL-6, but not IL-12p40, was significantly higher in macrophages from Tie2-TG arthritic mice, compared with BMDM from healthy WT and Tie2-TG mice (Fig. 2C). Stimulating BMDM with Ang-1 had no significant effect on IL-6 or IL-12p40 secretion. However, Ang-2 induced a significant increase in IL-6 secretion in each of the four groups of macrophages analysed, and IL-6 production was higher in arthritic Tie2-TG macrophages than the other macrophage groups. Ang-2 also induced a significant increase of IL-12p40 secretion in WT arthritic, and in Tie2-TG control and arthritic mice. The production of IL-12p40 induced by Ang-2 in the arthritic Tie2-TG macrophages was higher compared with the other macrophage groups, although significant differences were reached only in comparison with healthy WT mice (Fig. 2C).
Therefore, overexpression of Tie2 leads to a more severe clinical phenotype in this arthritis model, marked by enhanced synovial expression of inflammatory mediators produced by macrophages in response to Tie2 signalling.
The synovial microenvironment of RA and PsA supports functional expression of macrophage Tie2
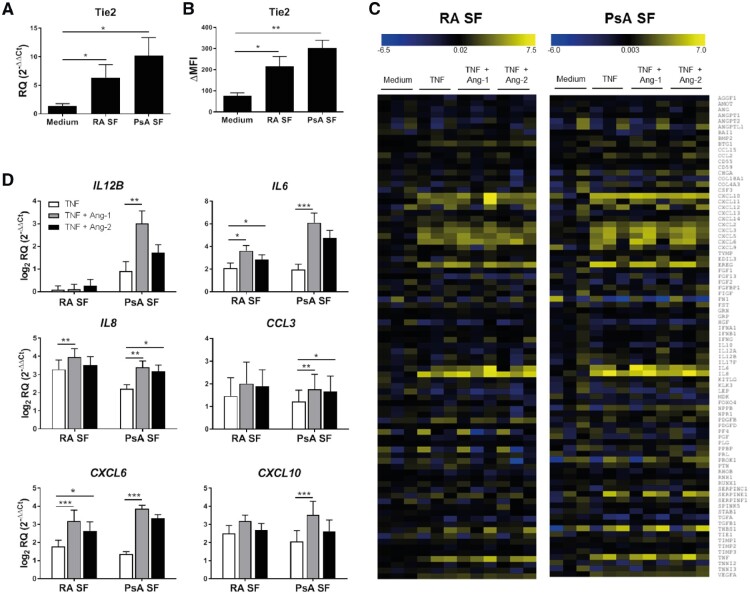
As Tie2 is activated in RA synovial macrophages [7], we determined how secreted products present in the synovial microenvironment might influence macrophage Tie2 expression and function. In one line of analysis, monocytes from peripheral blood (PB) of healthy donors (HD) were differentiated into macrophages with SF from RA (MΦRA SF) and PsA (MΦPsA SF) patients. Both RA and PsA SF induced significantly higher Tie2 mRNA (Fig. 3A) and protein (Fig. 3B) expression compared with macrophages differentiated with medium alone. However, serum of RA or PsA patients did not induce the expression of Tie2, suggesting that this effect is dependent upon the synovial microenvironment (supplementary Fig. S2, available at Rheumatology online).
Fig. 3.
Tie2 is expressed by macrophages differentiated with SF of RA or PsA patients and enhances the TNF-induced mRNA expression of cytokines and chemokines
(A, B) mRNA (A) and protein (B) Tie2 expression in macrophages differentiated with medium, or SF of RA or PsA patients for 7 days. qPCR data are shown as relative quantity, as described in the Methods. Surface expression was calculated as ΔMFI = (Median fluorescence intensity)positive staining – (Median fluorescence intensity)isotype staining. Values are the mean ± s.e.m. of seven independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001. (C) mRNA expression profiles of angiogenesis related genes in macrophages differentiated with SF of RA or PsA patients after 4 h incubation in medium alone or TNF (10 ng/ml) in the absence or presence of Ang-1 or Ang-2 (200 ng/ml for both, n = 3). Data are presented as a heat map where the lowest mRNA expression is showed in dark blue and the highest in yellow. (D) Validation of mRNA expression levels of selected genes analysed in (C). Data are shown as log2 relative quantity respect to unstimulated cells, as described in the Methods. Bars represent the means and s.e.m. of six independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001. Ang-1 and -2: angiopoietin-1 and -2; qPCR: quantitative PCR.
In MΦRA SF, Tie2 stimulation with Ang-1 or Ang-2 alone did not induce any significant change in mRNA expression of 84 angiogenesis-related genes (data not shown), negative results comparable to those previously observed examining M1 and M2 macrophages obtained from HD, where Tie2 signalling only influenced macrophage gene expression in combination with TNF stimulation [27]. We therefore stimulated MΦRA SF and MΦPsA SF with TNF in the absence or presence of Ang-1 or Ang-2. TNF stimulation up-regulated 13 genes at least 2-fold in MΦRA SF. Of these genes, Ang-1 and Ang-2 enhanced the TNF-induced expression of six and five genes, respectively (Fig. 3C and supplementary Table S6, available at Rheumatology online). In MΦPsA SF, TNF up-regulated 15 genes, of which Ang-1 and Ang-2 further enhanced the expression of 5 and 4 genes, respectively. To validate the array data, we analysed expression of IL12B, IL6, CXCL6, CXCL8, CXCL10 and CCL3 by qPCR. In MΦRA SF, Ang-1 significantly enhanced TNF-induced expression of IL6, IL8 and CXCL6 (Fig. 3D), while Ang-2 similarly enhanced expression of IL6 and CXCL6. In MΦPsA SF Ang-1 significantly enhanced TNF-induced expression of IL12B, IL6, IL8, CCL3, CXCL6 and CXCL10, while Ang-2 enhanced TNF-induced expression of IL8 and CCL3 (Fig. 3D). These results suggest that unidentified secreted factors present in the synovial microenvironment of RA and PsA patients can induce the expression of functional Tie2, which in turn can participate in the pro-inflammatory activation of macrophages.
Tie2 signalling promotes the pro-inflammatory activation of PB-derived RA and PsA macrophages
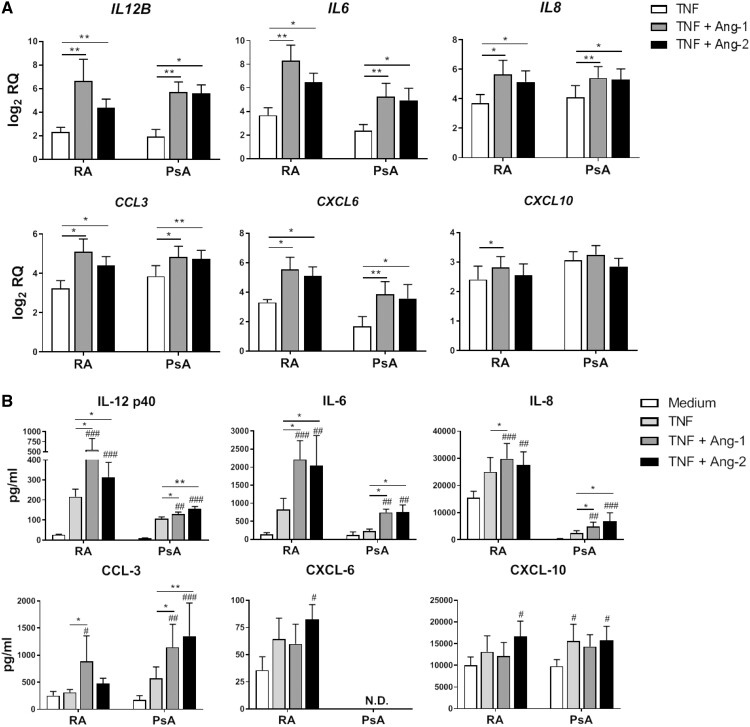
To determine whether systemic inflammation could also impact upon macrophage Tie2 expression and function, we obtained PB monocytes from HD and RA and PsA patients, and differentiated them into macrophages using either IFN-γ (MΦIFN-γ) or IL-10 (MΦIL-10), as macrophages present in RA and PsA synovial tissue are phenotypically similar to macrophages differentiated with these cytokines [27, 28]. Surface expression of Tie2 was similar in MΦIFN-γ and MΦIL-10 derived from HD and patients, and no differences were observed in Tie2 expression between macrophages derived from PB and SF of RA patients (supplementary Fig. S3A and B, available at Rheumatology online). In MΦIFN-γ, both Ang-1 and Ang-2 significantly enhanced TNF-induced expression of IL12B, IL6, IL8, CXCL6 and CCL3 by RA and PsA macrophages (Fig. 4A), and Ang-1 also increased CXCL10 expression in RA patient MΦIFN-γ. This pro-inflammatory effect of Tie2 stimulation was not unique to MΦIFN-γ, as Tie2 stimulation also enhanced expression of inflammatory mediators in MΦIL-10 from RA and PsA patients (supplementary Fig. S4, available at Rheumatology online). We also analysed the protein expression of these inflammatory mediators in tissue culture supernatants of stimulated MΦIFN-γ (Fig. 4B). In MΦIFN-γ differentiated from RA and PsA patients, Ang-1 and Ang-2 significantly enhanced TNF-induced secretion of IL-12p40, IL-6, IL-8 and CCL-3, but not CXCL-6 or CXCL-10. To determine whether other inflammatory cytokines involved in RA and PsA pathogenesis also cooperate with Tie2 signalling in the activation of macrophages, we stimulated MΦIFN-γ from RA and PsA patients with IL-1β and IL-6 in combination with Ang-1 or Ang-2. Ang-1, and to a lesser extent Ang-2, enhanced the IL-1β-induced expression of IL12B, IL6 and IL8. IL-6 slightly up-regulated the mRNA expression of these inflammatory mediators, but despite this modest effect, Ang-1 enhanced the expression of IL6 and IL8. Ang-2 also enhanced the IL-6-induced expression of IL6 and IL8, but the effect was less prominent (supplementary Fig. S5A and B, available at Rheumatology online). These results suggest a generalized pro-inflammatory role for Tie2 signalling within the context of other cytokines present in the synovium of RA and PsA patients. Finally, to understand how exposure of monocytes to the synovial microenvironment might influence Tie2 signalling, we differentiated RA patient SF monocytes with IFN-γ. Ang-1 and Ang-2 significantly enhanced TNF-induced expression of IL12B, IL6, IL8, CXCL6 and CCL3, but not CXCL10 (supplementary Fig. S6A, available at Rheumatology online). Enhanced Tie2-dependent production of IL-6 and IL-8 was also confirmed at the protein level (supplementary Fig. S6B, available at Rheumatology online). These results confirm that the pro-inflammatory effect of Tie2 signalling in macrophages is preserved in cells exposed to systemic alterations in cytokine levels and the synovial microenvironment present in patients with inflammatory arthritis.
Fig. 4.
Ang-1 and Ang-2 enhance the TNF-induced mRNA and protein expression of inflammatory mediators in RA and PsA macrophages
(A) mRNA expression of chemokines and cytokines by monocytes from PB of RA and PsA patients differentiated with IFN-γ after 4 h incubation in medium alone or TNF (10 ng/ml) in the absence or presence of Ang-1 or Ang-2 (200 ng/ml for both). Data are shown as log2 relative quantity with respect to unstimulated cells, as described in the Methods. Bars represent the means and s.e.m. of five independent experiments. *P < 0.05 and **P < 0.01. (B) Analysis of protein production by monocytes from PB of RA and PsA patients differentiated with IFN-γ after 24 h incubation in medium alone or TNF (10 ng/ml) in the absence or presence of Ang-1 or Ang-2 (200 ng/ml for both). Bars represent the means and s.e.m. of five independent experiments. *P < 0.05 and **P < 0.01; #P < 0.05, ##P < 0.01 and ###P < 0.001, compared with unstimulated cells. Ang-1 and -2: angiopoietin-1 and -2; PB: peripheral blood.
Tie2 signalling is sufficient and required for perpetuation of inflammation in RA synovial tissue
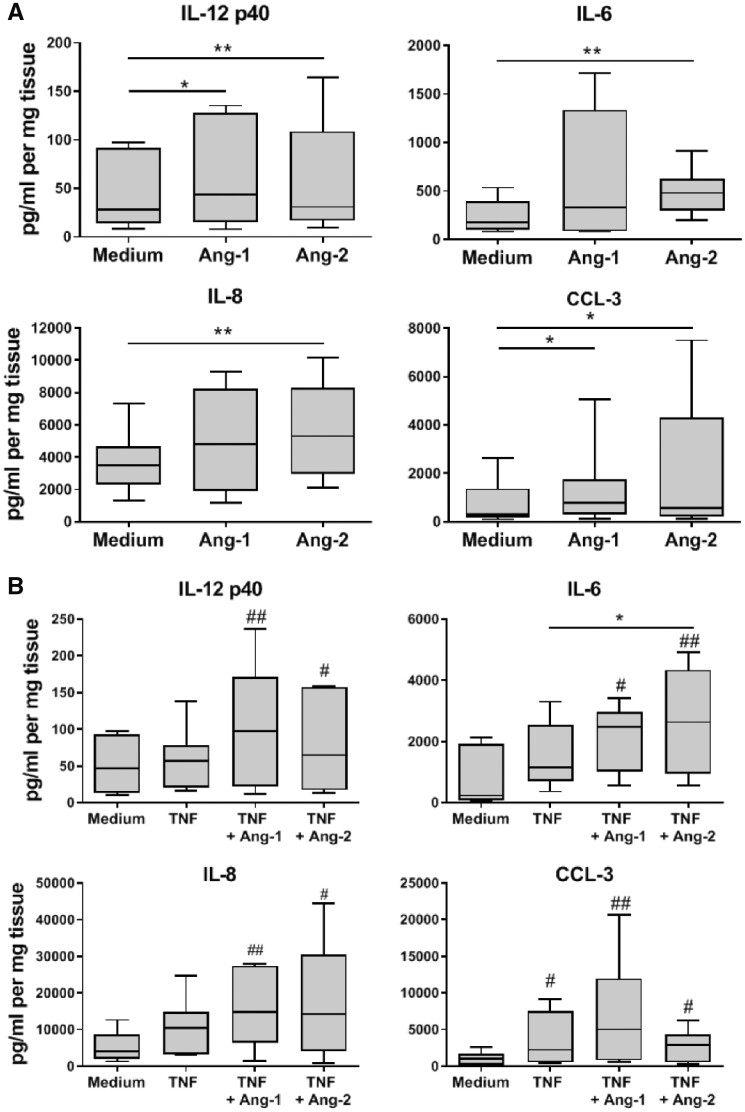
Lastly, we examined whether the pro-inflammatory effects of Tie2 signalling in isolated macrophages under conditions relevant to RA were preserved in the intact synovium of RA patients. Synovial tissue explants from RA patients were incubated in medium alone, or in the presence of Ang-1 or Ang-2, and secreted analytes were measured. We observed that Ang-2 alone significantly induced the secretion of IL-12p40, IL-6, IL-8 and CCL-3 by RA synovial explants (Fig. 5A). In contrast, Ang-1 only significantly induced the secretion of IL-12p40 and CCL-3. TNF induced the secretion of CCL-3, but had a small or negligible effect on the production IL-6, IL-12p40 and IL-8. In contrast, the combination of TNF with Ang-1 or Ang-2 significantly induced the production of IL-6, IL-12p40, IL-8 and CCL-3, compared with the unstimulated explants (Fig. 5B). Interestingly, the pro-inflammatory effect of Ang-1 and Ang-2 was not restricted to RA, as Ang-2 significantly induced the secretion of IL-6 in the synovial tissue explants of PsA patients (supplementary Fig. S7, available at Rheumatology online).
Fig. 5.
Tie2 signalling induces cytokine and chemokine production in RA synovial tissue
(A) IL-6, IL-12p40, IL-8 and CCL-3 production in supernatants of RA synovial tissue after 24 h incubation in medium, Ang-1 or Ang-2 (both 200 ng/ml). (B) IL-6, IL-12p40, IL-8 and CCL-3 production in supernatants of RA synovial tissue after 24 h incubation in medium alone or TNF (10 ng/ml) alone or in combination with Ang-1 or Ang-2 (both 200 ng/ml). Data are presented as box plots, where the boxes represent the 25th–75th percentiles, the lines within the box mark the median value and lines outside the boxes denote the 10th and 90th percentiles (n = 4–7). *P < 0.05 and **P < 0.01; #P < 0.05 and ##P < 0.01, compared with medium. Ang-1 and -2: angiopoietin-1 and -2.
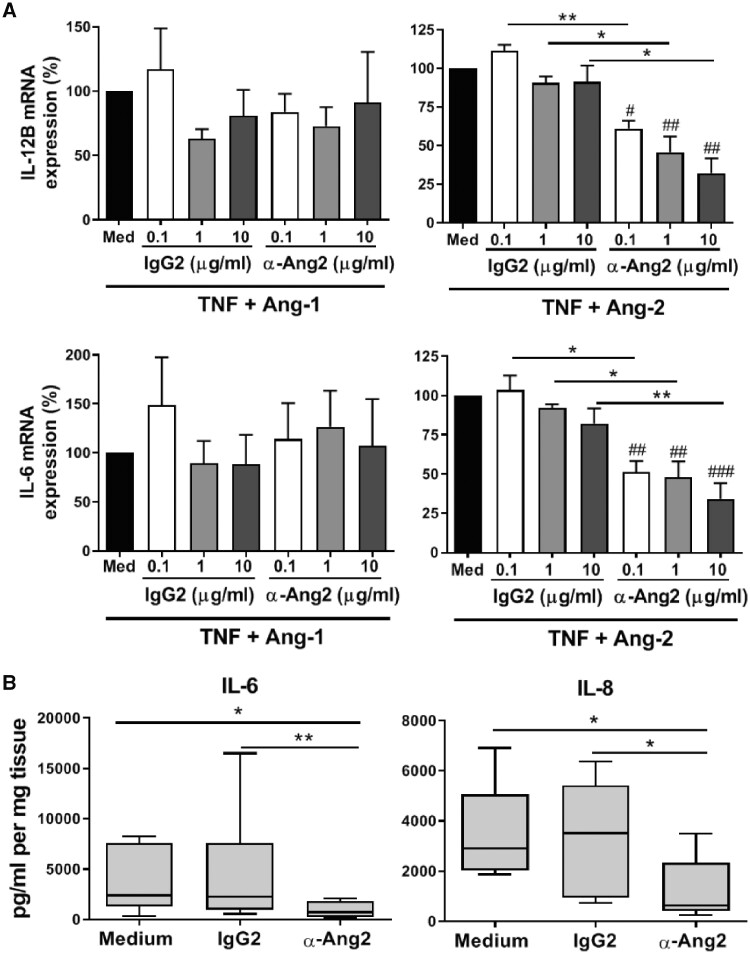
As Ang-2 appeared to regulate RA synovial tissue gene expression more robustly than Ang-1, we analysed the potential effects of blocking Ang-2 signalling in RA synovial explants using a neutralizing anti-Ang-2 antibody, which dose-dependently reduced IL6 and IL12B expression in HD MΦIFN-γ macrophages stimulated with TNF and Ang-2, but not those stimulated with TNF and Ang-1 (Fig. 6A). In the RA synovial tissue explant model, Ang-2 neutralization significantly reduced IL-6 and IL-8 production compared with explants that were left untreated or exposed to isotype control antibody (Fig. 6B), providing direct evidence that Tie2 signalling induces macrophage expression of inflammatory mediators in the synovium of arthritis patients, and that specific neutralization of Ang-2 can reduce ongoing synovial expression of pro-inflammatory mediators relevant to disease in RA and other forms of arthritis.
Fig. 6.
Ang-2 neutralization reduces cytokine production in RA synovial tissue
(A) mRNA expression of IL6 and IL12B in RA synovial tissue explants stimulated for 24 h with TNF (10 ng/ml) plus Ang-1 (left panels) or Ang-2 (right panels, both 200 ng/ml) in the absence (med) or presence of control IgG2 or anti-Ang-2 antibodies. Data are presented as percentage of expression relative to TNF in combination with Ang-1 or Ang-2. Bars represent the means and s.e.m. of seven independent experiments. (B) IL-6 and IL-8 production in supernatants of RA synovial tissue after 24 h incubation in medium alone, anti-Ang2 or IgG2 antibody (0.1 µg/ml). Data are presented as box plots, where the boxes represent the 25th–75th percentiles, the lines within the box mark the median value and lines outside the boxes denote the 10th and 90th percentiles (n = 6). *P < 0.05 and **P < 0.01; #P < 0.05, ##P < 0.01 and ###P < 0.001, compared with medium. Ang-1 and -2: angiopoietin-1 and -2.
Discussion
Expression of the Tie2 receptor tyrosine kinase and its ligands Ang-1 and Ang-2 is elevated in the synovium of RA and PsA patients compared with healthy and disease controls [7, 12, 13]. Intriguingly, expression and activation of Tie2 is elevated in the synovial tissue of early arthritis patients, associated with the eventual development of RA and disease progression [6, 11]. The potential importance of this to pathology in inflammatory arthritis is underscored by studies showing that inhibition of Tie2 signalling is protective in animal models of arthritis [7, 13–15]. Potential cellular targets of Tie2 signalling in the inflamed joint include ECs, FLS and macrophages. For example, toll-like receptor 2 and VEGF-mediated EC survival, migration and angiogenic tube formation is dependent upon Tie2 signalling [10, 43]. Tie2 stimulation of RA FLS promotes the chemotactic and tissue invasiveness of these cells, and TNF-dependent activation of macrophage chemokine and cytokine production is enhanced by Ang-1 and Ang-2 [7, 27, 44, 45]. An emerging unifying feature, from published observations and data presented here, is that Tie2 signalling cooperates with TNF and other agonists to promote cellular activation in inflammatory arthritis [7, 27, 46].
That Tie2 signalling exerts its effects predominantly within the context of cellular exposure to other cytokines, such as TNF and VEGF, is important for understanding and potentially targeting Tie2 contributions to pathology in inflammatory arthritis. First, macrophage responses to a given agonist are greatly influenced by the polarization conditions they are exposed to as they enter tissue. While synovial macrophages clearly make pro-inflammatory contributions in RA and PsA, their expression of both M1 (pro-inflammatory) and M2 (immunoregulatory) surface markers is consistent with exposure to IFN-γ and IL-10, respectively [47]. Global gene expression analysis of RA SF and synovial tissue macrophages confirms that the complex synovial microenvironment provides unique differentiation signals [30]. Second, responses of differentiated macrophage to a given cytokine are directly influenced by concurrent exposure to other cytokines and environmental influences. For example, IFN-γ, immune complexes and hypoxic conditions present in the inflamed synovium render macrophages refractory to immunoregulatory signalling by IL-10 [48, 49]. In contrast, TNF can tolerize macrophages to toll-like receptor 4 signalling while sensitizing them to type I IFN signalling [33].
Thus, it might not be possible to predict the contributions of macrophage Tie2 signalling to inflammatory arthritis using highly defined classical macrophage polarization models. To address this, we undertook three distinct experimental approaches, utilizing in vivo, in vitro and ex vivo models of RA. In vivo, Tie2 overexpression increased the clinical severity of murine serum-induced arthritis, consistent with previous data suggesting that elevated expression of angiopoietins and Tie2 in RA and PsA synovial tissue contributes to pathology [6, 11]. Enhanced disease activity in Tie2-TG mice was associated with the elevated expression of IL6, IL12B, CCL-2 and CXCL10, inflammatory mediators that we had previously identified as inducible by Tie2 signalling in human macrophages [27]. BMDM from Tie2-TG arthritic mice also produced higher amounts of IL-6 than WT BMDM. These findings suggest that macrophage Tie2 signalling can contribute directly to inflammation in experimental arthritis. We did not address direct effects of enhanced Tie2 signalling in EC or FLS in these in vivo studies, questions that warrant further investigation.
In vitro, we examined the potential influences of systemic and synovial environmental cues on myeloid Tie2 expression and function in RA and PsA. PB monocytes from RA and PsA patients retained the capacity to upregulate expression of Tie2 following differentiation in either IFN-γ or IL-10. Tie2 expressed by these macrophages was functional, as Ang-1 and Ang-2 stimulation enhanced the production of cytokines and chemokines induced by different inflammatory mediators (TNF, IL-1β and IL-6), including IL-6, IL-8, IL-12p40 and CCL-3. We observed little if any difference in Tie2 expression between RA and PsA PB-derived macrophages, and only a slight trend towards increased sensitivity to Ang-2 in PsA, indicating that systemic disease influences did not overtly impact on myeloid Tie2 signalling. However, differentiation of HD PB monocytes in RA and PsA SF significantly up-regulated macrophage Tie2 mRNA and protein expression. Ang-1 and Ang-2 stimulation of these cells enhanced TNF-dependent expression of a subset cytokines and chemokines similar to what we previously observed in polarized HD macrophages [27]. Finally, we confirmed that macrophages derived from RA SF also retained the capacity to produce more TNF-dependent IL-6 and IL-8 in the presence of Ang-1 or Ang-2. Together, these results provide evidence that the synovial cytokine environment promotes myeloid Tie2 expression, and that Tie2 can cooperate with inflammatory mediators involved in the pathology of RA and PsA to promote macrophage cytokine and chemokine production in both RA and PsA. In the various models used in these studies, some quantitative but inconsistent differences were observed in Tie2-dependent gene expression between RA and PsA. No clear qualitative differences that might explain distinct neo-angiogenic patterning and myofibroblast gene signatures were observed [6, 34], possibly a limitation of the power of this study, and global gene expression analysis in these macrophages may yet reveal disease-specific differences in macrophage Tie2 signalling.
Lastly, we sought to determine whether Tie2 signalling was sufficient and necessary to sustain inflammatory gene expression in intact synovial tissue from patients with inflammatory arthritis ex vivo. Ang-2 alone induced RA synovial explant production of IL-6, IL-8, IL-12p40 and CCL-3, while Ang-1 enhanced only the production of IL-12p40 and CCL-3. In PsA synovial tissue, both Ang-1 and Ang-2 promoted IL-6 production. These results were in contrast to those obtained from isolated macrophages, where Tie2 signalling by itself did not affect gene expression, but likely due to the presence of inflammatory mediators such as TNF in patient synovial tissue [50]. Combinatorial exposure of RA synovial membrane to TNF, which by itself had little effect on cytokine and chemokine production, with Ang-1 or Ang-2 significantly enhanced production of IL-6, IL-12p40, IL-8 and CCL-3. Reciprocally, RA synovial tissue production of IL-6 and IL-8 was suppressed in the presence of a human neutralizing anti-Ang-2 antibody.
This study provides evidence that myeloid Tie2 signalling is preserved in RA and PsA synovial tissue, and engagement of Ang-2/Tie2 signalling is sufficient and necessary to promote synovial inflammation in these diseases. In particular, the capacity of Tie2 and inflammatory signalling pathways to cooperatively regulate inflammatory gene expression in ECs, FLS and macrophages suggests clinical benefit in simultaneously targeting both pathways, an idea already substantiated by pre-clinical studies in animal models of arthritis [14, 15].
Supplementary Material
Acknowledgements
S.G. was supported by the Anxeles Alvariño postdoctoral programme (I + D + I Xunta de Galicia) and the European Social Fund (ESF). T.C. was supported by a Grant from the Portuguese national funding agency for science, research and technology: Fundação para a Ciência e a Tecnologia (SFRH/BD/93526/2013). D.L.B. is supported by a Vidi grant from the Dutch Scientific Organization.
Funding: This research was supported in part by the Dutch Arthritis Association Project Grants (NR 04-1-301 and NR 09-1-405) to K.A.R.
Disclosure statement: M.S. is formerly an employee at MedImmune for the duration of this collaboration. J.C. is an employee of MedImmune LLC. The other authors have declared no conflicts of interest.
References
- 1. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 2. McInnes IB, Schett G.. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017;389:2328–37. [DOI] [PubMed] [Google Scholar]
- 3. Huang H, Bhat A, Woodnutt G, Lappe R.. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer 2010;10:575–85. [DOI] [PubMed] [Google Scholar]
- 4. Jackson JR, Scott BB, Zaratin PF. et al. Constitutive expression of angiopoietin-1 and -2 and modulation of their expression by inflammatory cytokines in rheumatoid arthritis synovial fibroblasts. J Rheumatol 2002;29:230–9. [PubMed] [Google Scholar]
- 5. Shahrara S, Volin MV, Connors MA, Haines GK, Koch AE.. Differential expression of the angiogenic Tie receptor family in arthritic and normal synovial tissue. Arthritis Res 2002;4:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fearon U, Griosios K, Fraser A. et al. Angiopoietins, growth factors, and vascular morphology in early arthritis. J Rheumatol 2003;30:260–8. [PubMed] [Google Scholar]
- 7. Krausz S, Garcia S, Ambarus CA. et al. Angiopoietin-2 promotes inflammatory activation of human macrophages and is essential for murine experimental arthritis. Ann Rheum Dis 2012;71:1402–10. [DOI] [PubMed] [Google Scholar]
- 8. Gravallese EM, Pettit AR, Lee R. et al. Angiopoietin-1 is expressed in the synovium of patients with rheumatoid arthritis and is induced by tumour necrosis factor α. Ann Rheum Dis 2003;62:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott BB, Zaratin PF, Gilmartin AG. et al. TNF-alpha modulates angiopoietin-1 expression in rheumatoid synovial fibroblasts via the NF-kappa B signalling pathway. Biochem Biophys Res Commun 2005;328:409–14. [DOI] [PubMed] [Google Scholar]
- 10. Saber T, Veale DJ, Balogh E. et al. Toll-like receptor 2 induced angiogenesis and invasion is mediated through the tie2 signalling pathway in rheumatoid arthritis. PLoS One 2011;6:e23540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van De Sande MGH, De Launay D, De Hair MJH. et al. Local synovial engagement of angiogenic TIE-2 is associated with the development of persistent erosive rheumatoid arthritis in patients with early arthritis. Arthritis Rheum 2013;65:3073–83. [DOI] [PubMed] [Google Scholar]
- 12. DeBusk LM, Chen Y, Nishishita T. et al. Tie2 receptor tyrosine kinase, a major mediator of tumor necrosis factor α-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum 2003;48:2461–71. [DOI] [PubMed] [Google Scholar]
- 13. Chen Y, Donnelly E, Kobayashi H, DeBusk LM, Lin PC.. Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis Rheum 2005;52:1585–94. [DOI] [PubMed] [Google Scholar]
- 14. Hah YS, Koh YJ, Lim HS. et al. Double-antiangiogenic protein DAAP targeting vascular endothelial growth factor A and angiopoietins attenuates collagen-induced arthritis. Arthritis Res Ther 2013;15:R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanakaraj P, Puffer BA, Yao XT. et al. Simultaneous targeting of TNF and Ang2 with a novel bispecific antibody enhances efficacy in an in vivo model of arthritis. MAbs 2012;4:600–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamilton JA, Tak PP.. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum 2009;60:1210–21. [DOI] [PubMed] [Google Scholar]
- 17. Ambarus C, Yeremenko N, Tak PP, Baeten D.. Pathogenesis of spondyloarthritis: autoimmune or autoinflammatory? Curr Opin Rheumatol 2012;24:351–8. [DOI] [PubMed] [Google Scholar]
- 18. Li J, Hsu HC, Mountz JD.. Managing macrophages in rheumatoid arthritis by reform or removal. Curr Rheumatol Rep 2012;14:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haringman JJ, Gerlag DM, Zwinderman A. H. et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005;64:834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baeten D, Kruithof E, De Rycke L. et al. Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res Ther 2005;7:R359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor PC, Michael Peters A, Paleolog E. et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor α blockade in patients with rheumatoid arthritis. Arthritis Rheum 2000;43:38–47. [DOI] [PubMed] [Google Scholar]
- 22. Kane D, Gogarty M, O’Leary J. et al. Reduction of synovial sublining layer inflammation and proinflammatory cytokine expression in psoriatic arthritis treated with methotrexate. Arthritis Rheum 2004;50:3286–95. [DOI] [PubMed] [Google Scholar]
- 23. Pucci F, Venneri MA, Biziato D. et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood 2009;114:901–14. [DOI] [PubMed] [Google Scholar]
- 24. Venneri MA, De Palma M, Ponzoni M. et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 2007;109:5276–85. [DOI] [PubMed] [Google Scholar]
- 25. Murdoch C, Tazzyman S, Webster S, Lewis CE.. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol 2007;178:7405–11. [DOI] [PubMed] [Google Scholar]
- 26. De Palma M, Venneri MA, Galli R. et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005;8:211–26. [DOI] [PubMed] [Google Scholar]
- 27. García S, Krausz S, Ambarus CA. et al. Tie2 signaling cooperates with TNF to promote the pro-inflammatory activation of human macrophages independently of macrophage functional phenotype. PLoS One 2014;9:e82088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ambarus CA, Noordenbos T, de Hair MJ, Tak PP, Baeten DL.. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res Ther 2012;14:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mandelin AM, Homan PJ, Shaffer AM. et al. Transcriptional profiling of synovial macrophages using minimally invasive ultrasound-guided synovial biopsies in rheumatoid arthritis. Arthritis Rheumatol 2018;13:0–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palacios BS, Estrada-Capetillo L, Izquierdo E. et al. Macrophages from the synovium of active rheumatoid arthritis exhibit an activin a-dependent pro-inflammatory profile. J Pathol 2015;235:515–26. [DOI] [PubMed] [Google Scholar]
- 31. Kalliolias GD, Ivashkiv LB.. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 2016;12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gordon RA, Grigoriev G, Lee A, Kalliolias GD, Ivashkiv LB.. The interferon signature and STAT1 expression in rheumatoid arthritis synovial fluid macrophages are induced by tumor necrosis factor α and counter-regulated by the synovial fluid microenvironment. Arthritis Rheum 2012;64:3119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park SH, Kang K, Giannopoulou E. et al. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat Immunol 2017;18:1104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yeremenko N, Noordenbos T, Cantaert T. et al. Disease-specific and inflammation-independent stromal alterations in spondylarthritis synovitis. Arthritis Rheum 2013;65:174–85. [DOI] [PubMed] [Google Scholar]
- 35. Celis R, Cuervo A, Ramírez J, Cañete JD.. Psoriatic synovitis: singularity and potential clinical implications. Front Med 2019;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown JL, Cao ZA, Pinzon-Ortiz M. et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol Cancer Ther 2010;9:145–56. [DOI] [PubMed] [Google Scholar]
- 37. Davis BK. Isolation, culture, and functional evaluation of bone marrow-derived macrophages. In: Allen IC, ed. Mouse models of innate immunity: methods and protocols. Totowa, NJ: Humana Press, 2013: 27–35. [DOI] [PubMed] [Google Scholar]
- 38. Voskas D, Jones N, Van Slyke P. et al. A cyclosporine-sensitive psoriasis-like disease produced in Tie2 transgenic mice. Am J Pathol 2005;166:843–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smeets T. Analysis of the cell infiltrate and expression of proinflammatory cytokines and matrix metalloproteinases in arthroscopic synovial biopsies: comparison with synovial samples from patients with end stage, destructive rheumatoid arthritis. Ann Rheum Dis 2003;62:635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taylor W, Gladman D, Helliwell P. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 41. Aletaha D, Neogi T, Silman AJ. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 42. Aletaha D, Neogi T, Silman AJ. et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 43. Malik NM, Jin P, Raatz Y. et al. Regulation of the angiopoietin-tie ligand-receptor system with a novel splice variant of Tie1 reduces the severity of murine arthritis. Rheumatology (Oxford) 2010;49:1828–39. [DOI] [PubMed] [Google Scholar]
- 44. Hashiramoto A, Sakai C, Yoshida K. et al. Angiopoietin 1 directly induces destruction of the rheumatoid joint by cooperative, but independent, signaling via ERK/MAPK and phosphatidylinositol 3-kinase/Akt. Arthritis Rheum 2007;56:2170–9. [DOI] [PubMed] [Google Scholar]
- 45. Takahara K, Iioka T, Furukawa K. et al. Autocrine/paracrine role of the angiopoietin-1 and -2/Tie2 system in cell proliferation and chemotaxis of cultured fibroblastic synoviocytes in rheumatoid arthritis. Hum Pathol 2004;35:150–8. [DOI] [PubMed] [Google Scholar]
- 46. Fiedler U, Reiss Y, Scharpfenecker M. et al. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat Med 2006;12:235–9. [DOI] [PubMed] [Google Scholar]
- 47. Ambarus CA, Krausz S, van Eijk M. et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods 2012;375:196–206. [DOI] [PubMed] [Google Scholar]
- 48. Huynh L, Kusnadi A, Park SH. et al. Opposing regulation of the late phase TNF response by mTORC1-IL-10 signaling and hypoxia in human macrophages. Sci Rep 2016;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ji J-D, Tassiulas I, Park-Min K-H. et al. Inhibition of interleukin 10 signaling after Fc receptor ligation and during rheumatoid arthritis. J Exp Med 2003;197:1573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McInnes IB, Buckley CD, Isaacs JD.. Cytokines in rheumatoid arthritis—shaping the immunological landscape. Nat Rev Rheumatol 2016;12:63–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.