Abstract
Objectives
Systemic juvenile idiopathic arthritis (sJIA) is a childhood arthritis with features of autoinflammation and high risk of macrophage activation syndrome (MAS). IL-18 has been shown to have key roles in sJIA and MAS. We aimed to examine IL-18 levels in sJIA in relation to disease activity and history of MAS and other disease biomarkers namely S100 proteins and CXCL9.
Methods
Total IL-18, CXCL9 and S100 proteins levels were determined in 40 sJIA patients, and IL-18 levels were compared between patients with regards to disease activity, history of MAS, and other biomarkers.
Results
Total IL-18 levels were significantly higher in patients with active sJIA (median 16 499 pg/ml; interquartile range (IQR) 4816–61 839), and remained persistently elevated even in the majority of patients with inactive disease (1164 pg/ml; IQR 587–3444). Patients with history of MAS had significantly higher IL-18 levels (13 380 pg/ml; IQR 4212–62 628) as compared with those without MAS history (956.5 pg/ml; IQR 276.3–4262.5). Total IL-18 performed well with area under the curve of 0.8145 and 0.84 in predicting disease activity and history of MAS, respectively. We observed moderate correlation between IL-18 and CXCL9 (R = 0.56), S100A8/A9 (R = 0.47) and S100A12 (R = 0.46). The correlation was stronger for ferritin (R = 0.74) and overall for those with active disease.
Conclusion
Total IL-18 levels were elevated in the majority of sJIA patients regardless of clinical features, but were higher in patients with active disease and history of MAS. Change in IL-18 may reflect increased disease activity or development of MAS.
Keywords: Interleukin-18 (IL-18), systemic juvenile idiopathic arthritis (sJIA), macrophage activation syndrome (MAS)
Rheumatology key messages
IL-18 is elevated in most patients with sJIA, with higher levels associated with active disease.
sJIA patients with a history of MAS had elevated IL-18 compared with those without MAS.
Change in IL-18 may reflect increased disease activity or development of MAS.
Introduction
Systemic JIA (sJIA) is a childhood arthritis distinguished from other subtypes of JIA by predominance of systemic inflammation and extra-articular features, including daily spiking fever, rash, lymphadenopathy, hepatosplenomegaly and serositis [1]. sJIA shares many features with classical autoinflammatory disorders, most notably response to IL-1 inhibition, and less resembles an autoimmune disease as other subtypes of JIA [2]. In particular, sJIA is associated with production of inflammatory cytokines including IL-1, IL-6 and IL-18 as well as S100 alarmin proteins [3, 4].
sJIA is also associated with a high risk of macrophage activation syndrome (MAS) [5]. MAS is a life-threatening syndrome of overwhelming inflammation, with fever, cytopenia, coagulopathy, liver and central nervous system dysfunction, and a systemic cytokine storm [5]. MAS has phenotypic overlap with primary haemophagocytic lymphohistocytosis, caused by genetic defects in the perforin cytolytic pathway. Indeed, many authors consider MAS as secondary haemophagocytic lymphohistocytosis in the setting of rheumatic disease [5]. Substantial evidence supports a role of IFNγ in primary hemophagocytic lymphohistocytosis pathogenesis [5]. Similarly, increasing evidence supports a central role for IFNγ in MAS [5], and the use of downstream chemokines such as CXCL9 as biomarkers [6].
IL-18 is an IL-1 family cytokine with both pro-inflammatory and immune-protective functions. Levels of IL-18 were noted to be elevated in patients with both sJIA and adult onset Still’s disease (AOSD), the adult counterpart of sJIA [4, 7, 8]. Excessive IL-18 may represent a potential pathogenic link between sJIA and MAS [4]. Notably, IL-18 elevation in MAS is very striking, with high circulating levels described in MAS broadly [9] and sJIA-induced MAS more specifically [10].
Knowing the possible role of IL-18 in sJIA and development of MAS, we examined serum IL-18 levels in sJIA patients in relation to disease activity, history of MAS, and other biomarkers namely S100 proteins and CXCL9. We hypothesized that in both sJIA and sJIA-associated MAS, IL-18 could serve to assess disease activity and development of MAS.
Methods
Patients and subjects
This study was approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center (CCHMC IRB), and written informed consent was obtained from all patients and/or their legal guardians. Consecutive patients presenting to clinic were enrolled. sJIA was diagnosed based on the International League of Associations for Rheumatology diagnostic criteria [1]. For several patients, samples were obtained and treatment initiated with disease duration <6 weeks, using the operational definition of sJIA [11]. Two patients were diagnosed after their 16th birthday and therefore would be strictly classified as AOSD. Patients were considered as having active sJIA if at the time of sample collection they had presence of any of the following: active arthritis; systemic features including rash, fever, adenopathy or hepatosplenomegaly; or elevated ESR or CRP based on local standards. Patients were considered to have clinically inactive disease (CID) based on the Wallace criteria [3]. Patients were considered to have MAS based on diagnosis of the treating physician, and also satisfying the 2016 MAS Classification Criteria [4].
Biomarker analysis
A single sample was obtained from each patient, serum aliquoted and stored at −80°C within 120 min of collection. Serum total IL-18 and CXCL9 levels were determined by the CCHMC Diagnostic Immunology Lab (DIL) using ELISA kits obtained from MBL (Woburn, MA, USA) and R&D Systems (Minneapolis, MN, USA), respectively. Serum S100A8/A9 and S100 A12 were determined using a specific ELISA kit obtained from ALPCO (Salem, NH, USA) and MBL, respectively.
Normal IL-18 ranges were calculated by the DIL using serum from normal adults and paediatric patients (no notable differences between these populations). A 2 s.d. range was determined based on 92 serum samples that span 7 weeks to 72 years old. The upper limit of normal was determined as 540 pg/ml.
Statistical analysis
Analysis was performed using Prism (GraphPad Software Inc., San Diego, CA, USA) and R (R Foundation for Statistical Computing, Vienna, Austria) software. Continuous variables were expressed as median and interquartile range (IQR). Group comparisons were analysed by the Mann–Whitney and Kruskal–Wallis tests of ANOVA. Correlations were expressed using Spearman’s rank correlation coefficient. A receiver operating characteristic (ROC) curve was used to quantify performance of IL-18 as a measure of disease activity and MAS.
Results
Cohort demographic and clinical features
The demographic and clinical features of the 40 sJIA patients at the time of sample collection is shown in Supplementary Table S1, available at Rheumatology online. Twenty-one patients had CID at time of IL-18 determination. Half of the study population had a previous history of MAS during the course of their illness. However, only two (5%) had overt MAS at time of sampling. Forty per cent of the patients had active arthritis (76% of active disease), but only 17.5% of patients (33% of active disease) had fever at the time of sample collection. Total serum IL-18 ranged from 164 to 240 986 pg/ml (upper limit normal 540 pg/ml), with median value of 4832.50 pg/ml (IQR 708.5–18556.5). Serum IL-18 levels in different subgroups are shown in Supplementary Table S2, available at Rheumatology online. Only seven patients had normal IL-18 levels regardless of disease activity.
Total IL-18 levels in relation to clinical features
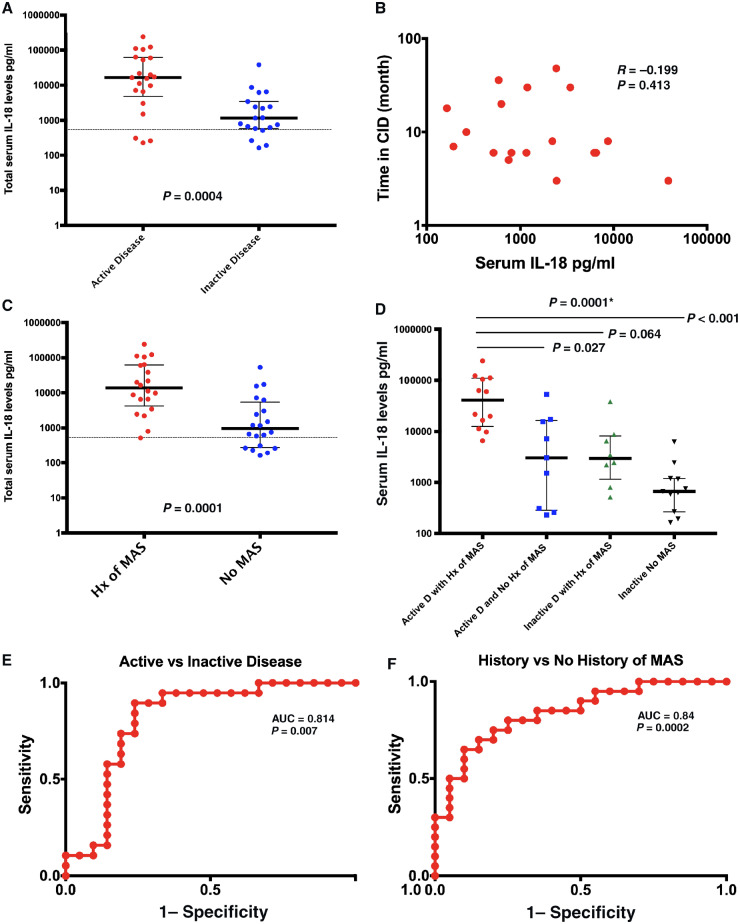
Median IL-18 levels were significantly higher in patients with active disease (16 499, IQR 4816–61 839) compared with those with CID (Fig. 1A, P = 0.0004). Despite this difference, IL-18 levels remained elevated in the majority (79%) of sJIA patients with CID (median 1164, IQR 587–3444; Fig. 1A). While there was a weak inverse correlation between IL-18 levels and time in CID, it was not statistically significant (Fig. 1B, P = 0.413).
Fig. 1.
IL-18 levels in relation to clinical features and ROC curves
(A) IL-18 levels in relation to disease activity. (B) Correlation between IL-18 levels and duration of CID. (C) IL-18 levels in relation to history of MAS. (D) ANOVA comparison of IL-18 levels in different groups stratified by disease activity and Hx of MAS. (E and F) ROC curve distinguishing (E) active vs inactive disease and (F) history of MAS vs no history of MAS. Horizontal line in (A) and (C) represents upper limit of normal for total serum IL-18 (540 pg/dl). *P-value for ANOVA group analysis. AUC: area under the curve; CID: clinically inactive disease; Hx MAS: history of macrophage activation syndrome; MAS: macrophage activation syndrome; ROC: receiver operating characteristic.
Patients with history of MAS had significantly elevated IL-18 compared with patients without MAS history (13 380, IQR 4212–62 628 vs 956.5, IQR 276.3–4626.5; Fig. 1C, P = 0.0001). While only two patients in the cohort had features of active MAS at sampling, those patients’ IL-18 was markedly elevated (91 522–240 986). Importantly, serum IL-18 levels remained above the normal range even in 70% of patients without history of MAS (Fig. 1C).
We stratified patients into four groups based on disease activity status and history of MAS: active disease with MAS history, active without MAS history, CID with MAS history and CID without MAS history. Comparing stratified groups, there were significant differences between groups with higher levels apparent in those with either active disease or history of MAS, and the highest levels in those with both active disease and history of MAS (Fig. 1D). Post hoc analysis showed significant differences in IL-18 only between patients with both active disease and MAS history compared with those with active disease but no history of MAS (P = 0.027), and those with both CID and no history of MAS (P < 0.0001).
Similar significant elevations in serum IL-18 were observed in patients with fever (P = 0.007) and arthritis (P = 0.017) (Supplementary Fig. S1A and B, available at Rheumatology online). Also, patients with elevated inflammatory markers (P = 0.0008) or active systemic features (P = 0.0225) had significantly higher IL-18 levels compared with their counterparts (Supplementary Fig. S1C and D, available at Rheumatology online).
IL-18 as biomarker of disease activity and MAS
ROC analysis was used to quantify the performance of serum IL-18 in predicting sJIA disease activity or history of MAS. IL-18 performed well with area under the curve of 0.8145 (P = 0.0007) and 0.84 (P = 0.0002) in predicting disease activity and history of MAS, respectively (Fig. 1E and F). This analysis indicated an optimal cut-off to detect active sJIA was 6368 pg/ml with a sensitivity of 76.2% and a specificity of 84.2%. The cut-off of 2438 pg/ml identified patients with a history of MAS with sensitivity of 85% and 70% specificity.
Correlation between IL-18 and other biomarkers
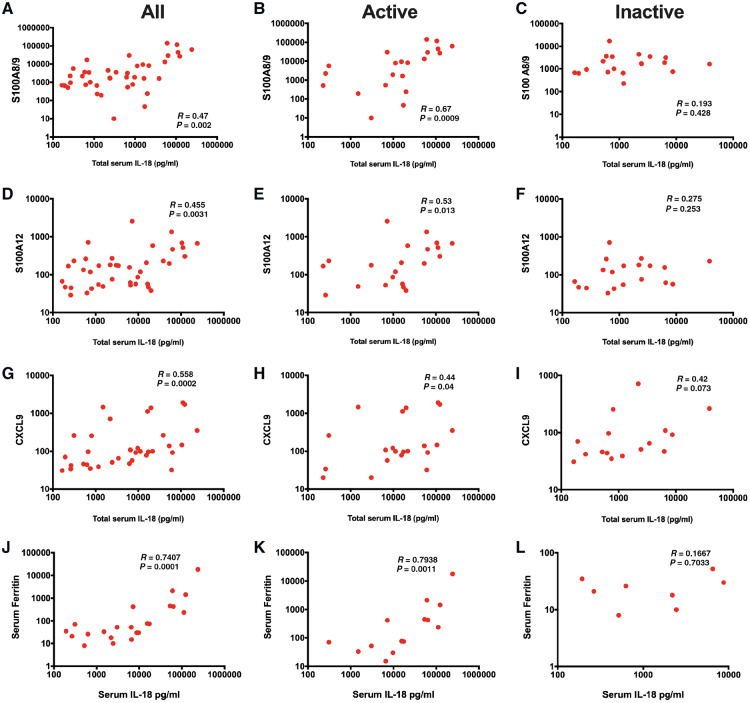
We examined the relationship between IL-18 and other emerging biomarkers for sJIA and MAS. S100A12 (calgranulin C) and S100A8/A9 (calprotectin) are proinflammatory ligands and alarmins, and proposed tools for diagnosing and measuring disease activity in sJIA [12, 13]. Our results demonstrated moderate correlation between total serum IL-18 and S100A8/A9 (R = 0.47, P = 0.002) and S100A12 proteins (R = 0.46, P = 0.003) (Fig. 2A–D). When stratifying by disease activity, IL-18 showed a sustained correlation with S100 proteins only among patients with active disease and not those with CID (Fig. 2B, C, E and F).
Fig. 2.
Correlation between total serum IL-18 and other biomarkers
(A) IL-18 and S100 A8/9 in all patients. (B) IL-18 and S100 A8/9 in patients with active disease. (C) IL-18 and S100 A8/9 in patients with inactive disease. (D) IL-18 and S100 A12 in all patients. (E) IL-18 and S100 A12 in patients with active disease. (F) IL-18 and S100 A12 in patients with inactive disease. (G) IL-18 and CXCL9 in all patients. (H) IL-18 and CXCL9 in patients with active disease. (I) IL-18 and CXCL9 in patients with inactive disease. (J) IL-18 and ferritin in all patients. (K) IL-18 and ferritin in patients with active disease. (L) IL-18 and ferritin in patients with inactive disease.
There is increasing evidence that IFNγ plays a major role in the pathogenesis of MAS complicating sJIA [6, 14]. We compared IL-18 levels with the IFNγ-induced chemokine CXCL9, which strongly correlates with laboratory parameters and severity of MAS [6]. Our results determined a moderate correlation between IL-18 and CXCL9 (R = 0.56, P = 0.0002; Fig. 2G). Similar to S100 proteins, when stratified by disease activity, IL-18 showed a sustained correlation with CXCL9 only among those with active disease at the time of sample collection (R = 0.44, P = 0.044; Fig. 2H and I). Stratification by history of MAS revealed no significant correlation between IL-18 and CXCL9 in those with or without history of MAS (Supplementary Fig. S1, available at Rheumatology online).
Finally, we examined the correlation between IL-18 and serum ferritin, a widely available biomarker for both sJIA and MAS. While serum ferritin was only available for a subset of patients (22; 55%), results demonstrated strong correlation with serum IL-18 (R = 0.74, P < 0.0001). When stratifying by disease activity, IL-18 showed a sustained strong correlation only among patients with active disease (Fig. 2J–L).
Discussion
Our results showed significant elevation of IL-18 not only in patients with active sJIA, but in the majority of patients even with CID. IL-18 was further elevated in patients with history of MAS, which could support increased susceptibility to MAS in patients with elevated IL-18.
Our findings complement an emerging body of literature in sJIA [7, 15] and AOSD [8], and support a key role of IL-18 both in the pathogenesis of sJIA, and as a biomarker of disease activity. Importantly, correlation with other validated biomarkers of disease activity such as ferritin, S100A12 and S100A8/9 proinflammatory ligands [13] was stronger in active disease and weaker (and non-significant) during CID. It is important to note that we observed persistent IL-18 elevation in most patients in the study including those with CID, and regardless of time in CID (Fig. 1B). We could not identify any unique feature with regard to the seven patients with normal IL-18 levels. We believe this indicates a slow recovery of IL-18 elevation after disease control, consistent with work showing persistent elevation despite good disease control for >6 months [16]. It is worth mentioning that how IL-18 changes over time and the impact of specific treatments is unknown, and could not be addressed in this study, but is a critical topic to explore in future studies. This might also point to other roles of IL-18 in patients with CID, or indicate ongoing inflammation despite effective treatment.
Whereas blocking IL-1 is highly efficacious in treating diseases prone to MAS such as sJIA, it does not prevent MAS [17], suggesting a critical role for other cytokines such as IL-18 in the development of MAS. This is particularly highlighted by gain of function mutation in NLRC4 (NLRC4-MAS), where both total and free IL-18 are chronically elevated [18]. In agreement with this, we found significantly higher IL-18 levels in children with history of MAS, regardless of underlying sJIA disease activity. In addition, while only two patients had active MAS at the time of sample collection, both had extremely high IL-18 (91 522–240 986). Together, this supports chronic elevation of IL-18 in patients prone to MAS, consistent with other studies [4, 19].
One key biological role of IL-18 is inducing IFNγ from NK cells and CTL, which plays a pivotal role in MAS complicating sJIA [6, 14]. However, levels of IL-18 only moderately correlated with CXCL9, a marker of MAS activity [6]. Stratifying results by disease activity, only patients with active disease showed significant correlation between IL-18 and CXCL9, indicating possible subclinical activation of IFNγ without overt MAS. While this question requires further study in a longitudinal cohort, it supports a model in which overproduction of IL-18 plays a major role in sJIA pathogenesis and as a tipping point for development of MAS [6].
With significant work suggesting the implications of imbalance between IL-18 and its high-affinity naturally secreted endogenous regulator IL-18 binding protein (IL-18BP) in MAS, correcting this IL-18/IL-18BP imbalance seems to be a rational strategy. A phase II trial of recombinant (r)IL-18BP in the treatment of AOSD found early signs of clinical and laboratory efficacy with response rates of 50% [20]. This response raises optimism for similar use of rIL-18BP in patients with IL-18 driven disease such as sJIA to prevent life threatening MAS.
Supplementary Material
Acknowledgements
S.Y., A.A.G. and G.S.S. conceived and designed the study. S.Y., N.F., R.B. and M.H. obtained patient samples and analysed biospecimens. S.Y., A.A.G., G.S.S., S.C., C.G. and C.G. analysed and interpreted the data. S.Y. wrote the first draft of the manuscript. All authors contributed to and approved the final version of the manuscript. We thank all of the patients and their families for their participation in this research, as well as their treating clinicians. We also thank the CCHMC Diagnostic Immunology Lab for performing IL-18 and CXCL9 levels. S.Y. was supported by the National Institute for Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health [T32 AR069512].
Funding: This work was supported by the National Institute for Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health [K08-AR072075] and the Systemic JIA Foundation.
Disclosure statement: S.Y., N.F., R.B., M.H. and G.S.S. declare that they have no competing interests. A.A.G. has received grant/research support from NovImmune, AB2Bio, and is a Consultant for Novartis, Juno; C.G. has received grants from Pfizer, Roche, AB2 Bio, Consultant and speaker’s fees from Roche, Pfizer, BMS, Merck, Sanofi, Regeneron, Eli Lilly, Novartis, AB2 Bio, and possesses shares in AB2 Bio; S.W.C. is a Consultant for AB2Bio; C.G.G.’s salary is supported by an unrestricted grant from AB2 Bio. The other authors have declared no conflicts of interest.
References
- 1. Petty RE, Southwood TR, Manners P. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 2. Mellins ED, Macaubas C, Grom AA.. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol 2011;7:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J.. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med 2005;201:1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimizu M, Nakagishi Y, Yachie A.. Distinct subsets of patients with systemic juvenile idiopathic arthritis based on their cytokine profiles. Cytokine 2013;61:345–8. [DOI] [PubMed] [Google Scholar]
- 5. Schulert GS, Grom AA.. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu Rev Med 2015;66:145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bracaglia C, de Graaf K, Pires Marafon D. et al. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis 2017;76:166–72. [DOI] [PubMed] [Google Scholar]
- 7. Jelusić M, Lukić IK, Tambić-Bukovac L. et al. Interleukin-18 as a mediator of systemic juvenile idiopathic arthritis. Clin Rheumatol 2007;26:1332–4. [DOI] [PubMed] [Google Scholar]
- 8. Girard C, Rech J, Brown M. et al. Elevated serum levels of free interleukin-18 in adult-onset Still's disease. Rheumatology (Oxford) 2016;55:2237–47. [DOI] [PubMed] [Google Scholar]
- 9. Mazodier K, Marin V, Novick D. et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood 2005;106:3483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev 2018;281:138–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeWitt EM, Kimura Y, Beukelman T. et al. Consensus treatment plans for new-onset systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2012;64:1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holzinger D, Frosch M, Kastrup A. et al. The Toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann Rheum Dis 2012;71:974–80. [DOI] [PubMed] [Google Scholar]
- 13. Shenoi S, Ou JN, Ni C. et al. Comparison of biomarkers for systemic juvenile idiopathic arthritis. Pediatr Res 2015;78:554–9. [DOI] [PubMed] [Google Scholar]
- 14. Put K, Avau A, Brisse E. et al. Cytokines in systemic juvenile idiopathic arthritis and haemophagocytic lymphohistiocytosis: tipping the balance between interleukin-18 and interferon-γ. Rheumatology (Oxford) 2015;54:1507–17. [DOI] [PubMed] [Google Scholar]
- 15. Shimizu M, Yokoyama T, Yamada K. et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology (Oxford) 2010;49:1645–53. [DOI] [PubMed] [Google Scholar]
- 16. Brachat AH, Grom AA, Wulffraat N. et al. Early changes in gene expression and inflammatory proteins in systemic juvenile idiopathic arthritis patients on canakinumab therapy. Arthritis Res Ther 2017;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schulert GS, Minoia F, Bohnsack J. et al. Effect of biologic therapy on clinical and laboratory features of macrophage activation syndrome associated with systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2018;70:409–19. [DOI] [PubMed] [Google Scholar]
- 18. Canna SW, de Jesus AA, Gouni S. et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet 2014;46:1140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weiss ES, Girard-Guyonvarc'h C, Holzinger D. et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood 2018;131:1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gabay C, Fautrel B, Rech J. et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still's disease. Ann Rheum Dis 2018;77:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.