Abstract
Objective
The aim of this study was to determine the effect of bariatric surgery on the incidence of RA in participants of the Swedish Obese Subjects (SOS) study.
Methods
The SOS is a longitudinal study aiming to assess the effect of bariatric surgery on mortality and obesity-related diseases. This report includes 2002 subjects with obesity who underwent bariatric surgery and 2034 matched controls; none of them had RA at baseline. Cases of incident RA were identified through the Swedish National Patient Register by searching for International Classification of Diseases codes. Both intention-to-treat analyses and per-protocol analyses are reported. In the per-protocol analysis, participants from the control group who underwent bariatric surgery later on during follow-up were censored at the time of surgery.
Results
During follow-up, 92 study participants developed RA. The median follow-up was 21 years (range 0–29). Bariatric surgery was neither associated with the incidence of RA in the intention-to-treat analysis [hazard ratio (HR) 0.92 (95% CI 0.59, 1.46), P = 0.74], nor in the per-protocol analysis [HR 0.86 (95% CI 0.54, 1.38), P = 0.53]. Weight change at the 2 year follow-up, expressed as the change in BMI compared with baseline, did not associate with the development of RA. Higher serum CRP levels and smoking associated with the future development of RA independent of other factors.
Conclusions
We did not detect any association between bariatric surgery and the incidence of RA in subjects affected by obesity followed up for up to 29 years.
ClinicalTrials.gov
Keywords: rheumatoid arthritis, surgery, adipose tissue, clinical trials and methods, epidemiology
Rheumatology key messages
Obesity has been reported as a risk factor for the development of RA.
Bariatric surgery was not associated with a lower risk of incident RA in subjects with obesity.
Both higher baseline CRP and smoking were associated with a greater risk for RA development.
Introduction
RA is a chronic inflammatory autoimmune disease affecting primarily the joints. The disease is characterized by the breakdown of self-tolerance mechanisms in genetically susceptible individuals and by the activation of both innate and adaptive immunity, leading to autoantibody formation (RF and ACPA), synovial inflammation and cartilage and bone damage. The pathogenesis of RA is complex and far from fully understood [1]. Several factors have been identified that represent a risk for the development of RA, including female sex, genetic predisposition and smoking [2]. Large meta-analyses have also shown that obesity is among the risk factors for the development of RA, especially among women [3–6]. Moreover, in subjects with RA, obesity is associated with higher disease activity and lower chance to achieve sustained remission [7–10]. Subjects with obesity and RA also have a worse response to therapy, including treatment with biologic DMARDs [11–13].
Bariatric surgery–induced weight loss in subjects with RA has been associated with lower disease activity, a decrease in inflammatory markers and a decreased use of DMARDs [14]. However, it is not known if bariatric surgery is able to prevent the development of RA in individuals with obesity.
The Swedish Obese Subjects (SOS) study is a longitudinal controlled study on the effect of bariatric surgery on mortality and on the incidence of obesity-related diseases [15, 16]. We have recently shown that, in individuals with obesity, bariatric surgery associates with a lower risk of developing gout and psoriasis, two common inflammatory diseases [17, 18]. The aim of this study was to determine whether bariatric surgery associated with a lower incidence of RA in SOS study participants.
Methods
SOS study design
The SOS is a longitudinal non-randomized study including 4047 subjects with obesity recruited at 25 surgical departments and 480 primary health care centres in Sweden between 1 September 1987 and 31 January 2001, as previously described [15]. Briefly, after a recruitment campaign in the media and at surgical departments and primary health care centres, 5335 subjects were found eligible at the matching examination. Of these, 2010 individuals chose to undergo bariatric surgery and constituted the surgery group. A matched control group of 2037 subjects was created, based on data from the matching examination. Matching was not performed at an individual level, but by using the method of sequential treatment assignment on the basis of 18 matching variables, as previously reported (sex, age, weight, height, hip circumference, waist circumference, systolic blood pressure, triglycerides, total cholesterol, postmenopausal status, daily smoking, diabetes, four psychosocial variables associated with death risk and two personality traits related to treatment preferences) [16, 20]. Ethical reasons did not allow randomization since six of seven Swedish ethics review boards considered the relatively high death rate following bariatric surgery unacceptable for randomization when the study was approved in 1987 [15, 19]. The surgery patient and the conventionally treated matched patient started the study on the same day (i.e. the day of the surgery). As previously reported, the matching process unexpectedly created a surgery group having a slightly higher mean body weight, a younger age and more severe risk factors than the control group [15].
Inclusion and exclusion criteria were identical for both groups and all the participants from the control group were, in principle, eligible for surgery. Inclusion criteria were age 37–60 years and BMI ⩾34 for men and ⩾38 for women. Exclusion criteria were previous surgery for gastric or duodenal ulcer, earlier bariatric surgery, gastric ulcer during the previous 6 months, ongoing or active malignancy during the past 5 years, myocardial infarction during the past 6 months, bulimic eating pattern, drug or alcohol abuse, psychiatric or cooperative problems contraindicating bariatric surgery or other rare conditions [21].
Subjects from the surgery group underwent bariatric surgery at baseline (19% gastric banding, 68% vertical banded gastroplasty and 13% gastric bypass). The control group received conventional non-surgical obesity treatment at their centres of registration (ranging from intensive lifestyle modifications to no treatment of any kind) and no attempt was made to standardize the non-surgical treatment [22]. All the subjects gave their written or oral informed consent to participate in the study. Seven Swedish local ethics review boards approved the study protocol. The study is registered at ClinicalTrials.gov with identifier NCT01479452.
Clinical and biochemical assessments
Centralized laboratory examinations were performed at matching, at baseline and at the 2-, 10-, 15- and 20-year follow-ups at the Central Laboratory of Sahlgrenska University Hospital. ESR was measured at the participants’ health care centres at the time of health examination visits. CRP levels at baseline were measured with an ultrasensitive immunoturbidimetric method (Sentinel, Milan, Italy) using the Architect c8200 analyser (Abbott Laboratories, Abbott Park, IL) in Helsinki, Finland between October 2010 and April 2011. A sufficient number of serum samples for the measurement of CRP were available for 3693 participants at baseline, when excluding subjects with prevalent RA at baseline.
Health examinations were performed at matching, at baseline and after 6 months and 1, 2, 3, 4, 6, 8, 10, 15 and 20 years. BMI was calculated as weight in kilograms divided by the square of the height in metres. Changes in BMI at the 2 year follow-up were calculated as [(value at follow-up – baseline value)/baseline value] × 100. The variable called ‘obesity duration’ was created based on patients’ self-reported weight at different ages and age at baseline. The first reported BMI (calculated from self-reported weight) ⩾30 after 20 years of age was considered as obesity debut. Obesity duration was defined as the time period from obesity debut to baseline age.
Outcome
The endpoint of this report was the diagnosis of RA, which is not a predefined endpoint of the SOS study. The primary endpoint of the SOS study was mortality. Secondary endpoints included type 2 diabetes and cardiovascular disease [21]. To identify SOS study participants with a diagnosis of RA, the Swedish National Patient Register was searched for the following International Classification of Diseases (ICD) codes for RA: 712.38 and 712.39 (ICD-8), 714.0–2 (ICD-9) and M05, M06.0, M06.8 and M06.9 (ICD-10). The National Patient Register includes medical records from both inpatient and outpatient visits all over Sweden. Inpatient care visits have been documented since 1964, but the register reached national coverage only in 1987. The outpatient register does not include data about primary care, only visits to hospital-based medical specialists, and became nationwide in 2001. Information about serostatus was obtained by a review of the ICD codes; subjects who were seronegative at the diagnosis of RA but later developed seropositivity according to the ICD codes were classified as seropositive.
Based on the National Patient Register, all study participants who had a diagnosis of RA before inclusion in the study were considered as having ‘a prevalent RA’ and were therefore excluded from the current study. The subjects who developed RA after baseline were considered as having incident RA. Participants were followed up until diagnosis of RA, death, migration or end of follow-up. The cut-off date for the current analyses was 31 December 2016. At the end of follow-up, 924 study participants died or were censored due to emigration. Information on death or migration was obtained from the Cause of Death Register and the Register of the Total Population [23].
Statistical analysis
Baseline characteristics of the study population are shown as mean (s.d.) or number (%). Baseline continuous variables were compared by general linear model test, whereas categorical variables were compared by χ2 tests.
Kaplan–Meier method estimates of cumulative incidence rates have been used to assess time to incident RA after inclusion. Comparisons between groups were performed by log-rank test. Hazard ratios (HRs) and corresponding 95% CIs for the risk of RA were calculated with Cox proportional hazard models.
The intention-to-treat principle was used in all analyses unless otherwise specified. In the per-protocol analysis, participants from the control group who underwent bariatric surgery during follow-up were censored at the time of surgery. Among those subjects, study participants who developed RA were censored only if the diagnosis was made after the day of the surgery. Two-sided P-values <0.05 were considered statistically significant. Statistical analyses were performed with the Statistical Package for Social Science (version 24.0; IBM, Armonk, NY, USA).
Results
Baseline characteristics
We identified a total of 11 individuals with prevalent RA at baseline, i.e. having a reported diagnosis of RA before baseline. Therefore, after exclusion of the 11 subjects with prevalent RA, this report included 4036 subjects: 2002 from the surgery group and 2034 from the control group. Compared with participants from the control group, subjects in the surgery group had higher BMI, CRP and ESR and were more likely to be smokers, as previously reported [15]. They also were slightly younger compared with participants from the control group (Table 1). As previously reported, bariatric surgery resulted in a sustained reduction in BMI [−23% (95% CI −24, −23) at the 2 year follow-up; −17% (95% CI −17, −16) at the 10 year follow-up], whereas the change was minimal in the control group [+0.1% (95% CI −0.3, 0.5) at the 2 year follow-up; +1.7% (95% CI −1.0, 2.4) at the 10 year follow-up] [15].
Table 1.
Baseline characteristics of study participants stratified by treatment group
| Characteristic | Control group (n = 2034) | Surgery group (n = 2002) | P-value |
|---|---|---|---|
| Age, years, mean (s.d.) | 49 (6) | 47 (6) | <0.001 |
| Sex, male, n (%) | 590 (29) | 589 (29) | 0.77 |
| BMI, kg/m2, mean (s.d.) | 40 (5) | 42 (5) | <0.001 |
| Obesity duration, years, mean (s.d.) | 16 (8) | 16 (8) | 0.68 |
| ESR, mm/h, mean (s.d.) | 15 (11) | 16 (11) | 0.001 |
| CRPa, mg/L, mean (s.d.) | 6.8 (7.4) | 8.1 (8.3) | <0.001 |
| Current or former smoker, n (%) | 1192 (59) | 1374 (69) | <0.001 |
Measurements of CRP were available for 3693 study participants at baseline.
Incidence of RA during follow-up
Ninety-two participants in the SOS study developed RA during a follow-up for up to 29 years [median follow-up 21 years (range 0–29)]. Baseline characteristics of study participants stratified by incident RA are shown in Table 2. The percentage of men was lower in the group of SOS participants who developed RA compared with those who did not; moreover, participants who developed RA had higher serum levels of CRP at baseline. Of the 92 study participants who developed RA, 51 were seropositive; serostatus was unknown for 17 subjects.
Table 2.
Baseline characteristics of study participants stratified by RA diagnosis during follow-up
| Characteristic | Incident RA (n = 92) | No incident RA (n = 3944) | P-value |
|---|---|---|---|
| Bariatric surgery, n (%) | 47 (51) | 1955 (50) | 0.77 |
| Age, years, mean (s.d.) | 48 (6) | 48 (6) | 0.55 |
| Sex, male, n (%) | 18 (20) | 1161 (29) | 0.04 |
| BMI, kg/m2, mean (s.d.) | 42 (5) | 41 (5) | 0.16 |
| Obesity duration, years, mean (s.d.) | 17 (8) | 16 (8) | 0.68 |
| ESR, mm/h, mean (s.d.) | 18 (11) | 15 (10) | 0.06 |
| CRPa, mg/L, mean (s.d.) | 10.1 (12.5) | 7.4 (7.7) | 0.002 |
| Current or former smoker, n (%) | 66 (72) | 2500 (63) | 0.10 |
| Seropositive, n (%)b | 51 (68) | – |
Measurements of CRP were available for 3693 study participants at baseline.
Data were obtained by a review of the ICD codes from the National Patient Register. Subjects who were seronegative at the diagnosis of RA but later developed seropositivity according to the ICD codes are classified here as seropositive. Seventeen subjects are not included due to unknown status.
Bariatric surgery and the incidence of RA
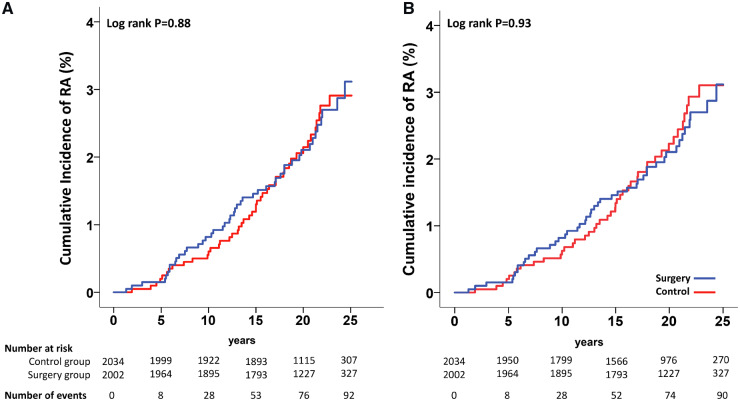
Among the 92 subjects who developed RA during follow-up, 47 belonged to the surgery group (2.3%) and 45 to the control group (2.2%). Bariatric surgery was not associated with the incidence of RA during follow-up, as shown in Fig. 1A (log-rank P = 0.88). Similarly, we could not detect any association between bariatric surgery and RA when only seropositive RA (no = 51) was considered as the outcome in the analysis (log-rank P = 0.52). Although previous studies have shown that the association between higher BMI and development of RA is stronger in women [6], we could not detect any interaction between bariatric surgery and sex on the incidence of RA (P = 0.92).
Fig. 1.
Cumulative incidence of RA in the SOS study
(A) Intention-to-treat analysis. (B) Per-protocol analysis.
We also performed a per-protocol analysis. A total of 285 participants from the control group who underwent bariatric surgery after baseline were censored at the time of the surgery, including two subjects who developed RA after the day of the surgery. Three subjects from the control group developed RA before undergoing bariatric surgery and therefore where not censored. The per-protocol analysis confirmed no association between bariatric surgery and RA (log-rank P = 0.93; Fig. 1B).
Multivariable analysis for the incidence of RA
In a multivariable analysis including risk factors for RA, bariatric surgery was not associated with the incidence of the disease [intention to treat: HR 0.92 (95% CI 0.59, 1.46), P = 0.74; per protocol: HR 0.86 (95% CI 0.54, 1.38), P = 0.53; Table 3]. Baseline CRP levels were significantly associated with a higher risk of developing RA independently of bariatric surgery and other factors [intention to treat: HR 1.28 (95% CI 1.06, 1.55), P = 0.01; per protocol: HR 1.29 (95% CI 1.07, 1.56), P = 0.007; Table 3]. Smoking was also associated with an increased risk for RA, although the association reached significance only in the per-protocol analysis [intention to treat: HR 1.63 (95% CI 0.99, 2.67), P = 0.05; per protocol: HR 1.69 (95% CI 1.02, 2.81), P = 0.04; Table 3]. Neither sex nor baseline ESR, BMI and obesity duration were risk factors for RA in this cohort of subjects with obesity.
Table 3.
Multivariable analysis for bariatric surgery and the incidence of RA
| Variables | Intention to treat | Per protocol | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Surgery vs control group | 0.92 (0.59, 1.46) | 0.74 | 0.86 (0.54, 1.38) | 0.53 |
| Men vs women | 0.64 (0.37, 1.13) | 0.13 | 0.66 (0.37, 1.16) | 0.15 |
| Age, per 10 years | 1.00 (0.68, 1.48) | 0.99 | 0.97 (0.65, 1.45) | 0.89 |
| BMI, per 10 kg/m2 | 1.03 (0.62, 1.71) | 0.90 | 1.04 (0.63, 1.74) | 0.87 |
| Obesity duration, per 10 years | 1.13 (0.85, 1.50) | 0.40 | 1.16 (0.87, 1.55) | 0.31 |
| ESR, per 10 mm/h | 1.04 (0.85, 1.27) | 0.68 | 1.04 (0.85, 1.27) | 0.69 |
| CRP, per 10 mg/L | 1.28 (1.06, 1.55) | 0.01 | 1.29 (1.07, 1.56) | 0.007 |
| Current or former smoker, yes vs no | 1.63 (0.99, 2.67) | 0.05 | 1.69 (1.02, 2.81) | 0.04 |
The adjusted HRs were calculated using a Cox proportional hazards model based on baseline data.
BMI changes and the incidence of RA
To determine whether changes in BMI rather than bariatric surgery were associated with the development of RA, we performed a multivariable Cox regression analysis including BMI change at the 2 year follow-up, sex, age, ESR, CRP and smoking. Changes in BMI at the 2 year follow-up were not associated with the development of RA in the intention-to-treat analysis or the per-protocol analysis [HR 0.96 (95% CI 0.82, 1.12), P = 0.57 and HR 0.99 (95% CI 0.83, 1.17), P = 0.87, respectively].
Discussion
Obesity is one of the described risk factors for the development of RA and it negatively affects disease activity and treatment outcomes [3, 4, 7–9]. Although it has been shown that bariatric surgery–induced weight loss results in decreased disease activity and improved response to treatment, the effect of bariatric surgery on the incidence of RA is not known [14]. In this study we could not detect any association between bariatric surgery and the incidence of RA in subjects with obesity followed up for up to 29 years. Baseline CRP levels and smoking, but not ESR, were associated with a greater risk of developing RA during follow-up.
In the same cohort of >4000 subjects with obesity, we have recently shown that bariatric surgery prevented the development of gouty arthritis and psoriasis, but it did not affect the incidence of PsA [17, 18]. RA and PsA are both inflammatory joint disorders that are very different at a clinical, immunological and pathogenic level, although they share some features and common risk factors, such as obesity [24]. In our previous article, the incidence of PsA was similar in the control and bariatric surgery groups, although there was a non-significant trend for a lower incidence in the surgery group. Our current results show no trends for a lower incidence of RA in the bariatric surgery group either in the intention-to-treat analysis or the per-protocol analysis.
Previous studies have shown that obesity augments RA disease activity and decreases the likelihood to achieve a sustained remission despite treatment [7–9]. Although the negative effect of excess weight on RA disease activity and response to treatment is widely known, the impact of obesity per se as a risk factor for the development of RA is still debated. Some studies show no increased risk for RA in subjects with obesity [25–27]. Conversely, several studies, including large meta-analyses, have shown that a higher BMI increases the risk of developing RA, although often the increased risk due to obesity was rather modest and possibly only limited to women [3–6, 28–30]. In our analyses, we could not detect any significant association between bariatric surgery as well as change in BMI 2 years after surgery and the incidence of RA in subjects with obesity. Moreover, no interaction was observed between bariatric surgery and sex. However, it is important to point out that our results do not necessarily rule out that obesity is a risk factor for RA. It is possible to speculate that long-term obesity, associated with a mild inflammatory state [31], has triggered the development of asymptomatic preclinical RA and that such changes could not be counterbalanced by the subsequent weight loss. On the other hand, our results show that obesity duration did not affect the incidence of RA. Another possible explanation is that the role of obesity in the pathogenesis of RA is rather marginal, as also suggested by the HRs for the risk of RA that in most studies, including meta-analyses, were <1.5 [4, 5, 32]. It is also important to note that many study participants from the surgery group still remain affected by obesity even after profound weight loss following bariatric surgery (about −23% of the original BMI), and this allows for speculations about the possibility that an even greater degree of weight loss might be needed to provide protection from the development of RA.
Serum from patients with preclinical RA shows signs of activation of the immune system, as suggested by the presence of ACPAs, chemokines and cytokines up to 10 years before the onset of the disease [33, 34]. CRP is also known to be elevated years before the diagnosis of RA [34–36]. Our results show that elevated CRP levels at baseline preceded RA in subjects with obesity followed-up for up to 29 years. This association was independent of bariatric surgery as well as other risk factors for RA, including smoking.
RA was not among the primary nor secondary aims of the SOS trial, hence the study was not designed for this outcome. As expected, the number of subjects who developed RA during follow-up was limited, suggesting that our study might be underpowered to detect a difference in the incidence of RA between the surgery and the control group. Moreover, compared with the surgery group, the control group had a less severe risk profile for RA at baseline, including lower BMI, lower serum CRP and lower prevalence of smokers, and to a certain extent this might have smoothed the effect of bariatric surgery on the development of RA. Another limitation of the study is that the diagnosis of RA was retrieved only through the Swedish National Patient Register by screening ICD codes and therefore we may have missed some patients diagnosed with RA. However, it is important to point out that the National Patient Register is a record of all the diagnoses made in public hospitals all over Sweden and private practices are still uncommon in the country [37]. Moreover, in Sweden, the diagnosis of RA can be only made by a rheumatologist, thus increasing the chances that the record of RA diagnoses that we have is accurate. We also have to acknowledge that the information about serostatus is missing for 17 subjects. This limits the possibility of a more detailed analysis.
In this study we did not observe any association between bariatric surgery and the incidence of RA in subjects with obesity during a long follow-up. Further longitudinal studies in larger cohorts are needed to confirm this finding.
Acknowledgments
We thank the staff members at 480 primary health care centres and 25 surgical departments in Sweden who participated in the study. Christina Torefalk is acknowledged for valuable administrative support. This study was presented at the 2018 American College of Rheumatology Annual Meeting (abstract number 75401).
Funding: This work was supported by the Knut and Alice Wallenberg Foundation and the Wallenberg Centre for Molecular and Translational Medicine at the University of Gothenburg, Gothenburg, Sweden, the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (grant R01DK105948 to LC), the Swedish Research Council (grant K2013-54X-11285-19), the Sahlgrenska University Hospital Regional Agreement on Medical Education and Research grants, the Ministry of Culture and Science of the State of North Rhine-Westphalia and the German Federal Ministry of Health. This study was also partially supported by a grant from the German Federal Ministry of Education and Research to the German Center for Diabetes Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders.
Disclosure statement: AR reports that until 2018, part of the salary for her full professor position at the Sahlgrenska Academy at the University of Gothenburg was covered by a grant from AstraZeneca IMed RIA (Respiratory, Inflammation, Autoimmunity) in compensation for advice regarding basic research in inflammation at the company. LC reports receiving lecture fees from AstraZeneca and Johnson & Johnson. The other authors have declared no conflicts of interest.
References
- 1. McInnes IB, Schett G.. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Aletaha D, Barton A. et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018;4:18001. [DOI] [PubMed] [Google Scholar]
- 3. Feng J, Chen Q, Yu F. et al. Body mass index and risk of rheumatoid arthritis: a meta-analysis of observational studies. Medicine (Baltimore) 2016;95:e2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qin B, Yang M, Fu H. et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther 2015;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ljung L, Rantapaa-Dahlqvist S.. Abdominal obesity, gender and the risk of rheumatoid arthritis – a nested case–control study. Arthritis Res Ther 2016;18:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou Y, Sun M.. A meta-analysis of the relationship between body mass index and risk of rheumatoid arthritis. EXCLI J 2018;17:1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vidal C, Barnetche T, Morel J, Combe B, Daïen C.. Association of body mass index categories with disease activity and radiographic joint damage in rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol 2015;42:2261–9. [DOI] [PubMed] [Google Scholar]
- 8. Schulman E, Bartlett SJ, Schieir O. et al. Overweight and obesity reduce the likelihood of achieving sustained remission in early rheumatoid arthritis: results from the Canadian Early Arthritis Cohort study. Arthritis Care Res (Hoboken) 2018;70:1185–91. [DOI] [PubMed] [Google Scholar]
- 9. Sandberg ME, Bengtsson C, Kallberg H. et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann Rheum Dis 2014;73:2029–33. [DOI] [PubMed] [Google Scholar]
- 10. Ellerby N, Mattey DL, Packham J, Dawes P, Hider SL.. Obesity and comorbidity are independently associated with a failure to achieve remission in patients with established rheumatoid arthritis. Ann Rheum Dis 2014;73:e74. [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez-Gay MA, Gonzalez-Juanatey C.. Rheumatoid arthritis: obesity impairs efficacy of anti-TNF therapy in patients with RA. Nat Rev Rheumatol 2012;8:641–2. [DOI] [PubMed] [Google Scholar]
- 12. Gremese E, Carletto A, Padovan M. et al. Obesity and reduction of the response rate to anti-tumor necrosis factor α in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res (Hoboken) 2013;65:94–100. [DOI] [PubMed] [Google Scholar]
- 13. Ottaviani S, Gardette A, Tubach F. et al. Body mass index and response to infliximab in rheumatoid arthritis. Clin Exp Rheumatol 2015;33:478–83. [PubMed] [Google Scholar]
- 14. Sparks JA, Halperin F, Karlson JC, Karlson EW, Bermas BL.. Impact of bariatric surgery on patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2015;67:1619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sjöström L, Narbro K, Sjöström CD. et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52. [DOI] [PubMed] [Google Scholar]
- 16. Carlsson LM, Peltonen M, Ahlin S. et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704. [DOI] [PubMed] [Google Scholar]
- 17. Maglio C, Peltonen M, Neovius M. et al. Effects of bariatric surgery on gout incidence in the Swedish Obese Subjects study: a non-randomised, prospective, controlled intervention trial. Ann Rheum Dis 2017;76:688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maglio C, Peltonen M, Rudin A, Carlsson L.. Bariatric surgery and the incidence of psoriasis and psoriatic arthritis in the Swedish Obese Subjects study. Obesity (Silver Spring) 2017;25:2068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brolin RE. Results of obesity surgery. Gastroenterol Clin North Am 1987;16:317–38. [PubMed] [Google Scholar]
- 20. Pocock SJ, Simon R.. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15. [PubMed] [Google Scholar]
- 21. Sjöström L, Larsson B, Backman L. et al. Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord 1992;16:465–79. [PubMed] [Google Scholar]
- 22. Zentenius E, Andersson-Assarsson JC, Carlsson LMS, Svensson PA, Larsson I.. Self-reported weight-loss methods and weight change: ten-year analysis in the Swedish Obese Subjects study control group. Obesity (Silver Spring) 2018;26:1137–43. [DOI] [PubMed] [Google Scholar]
- 23. Ludvigsson JF, Almqvist C, Bonamy AE. et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125–36. [DOI] [PubMed] [Google Scholar]
- 24. Veale DJ, Fearon U.. What makes psoriatic and rheumatoid arthritis so different? RMD Open 2015;1:e000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hernandez Avila M, Liang MH, Willett WC. et al. Reproductive factors, smoking, and the risk for rheumatoid arthritis. Epidemiology 1990;1:285–91. [DOI] [PubMed] [Google Scholar]
- 26. Turesson C, Bergstrom U, Pikwer M, Nilsson JA, Jacobsson LT.. A high body mass index is associated with reduced risk of rheumatoid arthritis in men, but not in women. Rheumatology (Oxford) 2016;55:307–14. [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez LA, Tolosa LB, Ruigomez A, Johansson S, Wallander MA.. Rheumatoid arthritis in UK primary care: incidence and prior morbidity. Scand J Rheumatol 2009;38:173–7. [DOI] [PubMed] [Google Scholar]
- 28. Linauskas A, Overvad K, Symmons D. et al. Body fat percentage, waist circumference and obesity as risk factors for rheumatoid arthritis: a Danish cohort study. Arthritis Care Res (Hoboken) 2019;71:777–86. [DOI] [PubMed] [Google Scholar]
- 29. Wesley A, Bengtsson C, Elkan AC. et al. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: results from a population-based case-control study. Arthritis Care Res (Hoboken) 2013;65:107–12. [DOI] [PubMed] [Google Scholar]
- 30. Lahiri M, Luben RN, Morgan C. et al. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register–the EPIC-2-NOAR Study). Ann Rheum Dis 2014;73:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Debnath M, Agrawal S, Agrawal A, Dubey GP.. Metaflammatory responses during obesity: pathomechanism and treatment. Obes Res Clin Pract 2016;10:103–13. [DOI] [PubMed] [Google Scholar]
- 32. George MD, Baker JF.. The obesity epidemic and consequences for rheumatoid arthritis care. Curr Rheumatol Rep 2016;18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johansson L, Pratesi F, Brink M. et al. Antibodies directed against endogenous and exogenous citrullinated antigens pre-date the onset of rheumatoid arthritis. Arthritis Res Ther 2016;18:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deane KD, O’Donnell CI, Hueber W. et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum 2010;62:3161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nielen MM, van Schaardenburg D, Reesink HW. et al. Increased levels of C-reactive protein in serum from blood donors before the onset of rheumatoid arthritis. Arthritis Rheum 2004;50:2423–7. [DOI] [PubMed] [Google Scholar]
- 36. Masi AT, Rehman AA, Elmore KB, Aldag JC.. Serum acute phase protein and inflammatory cytokine network correlations: comparison of a pre-rheumatoid arthritis and non-rheumatoid arthritis community cohort. J Innate Immun 2013;5:100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lapidus J. Private health insurance in Sweden: fast-track lanes and the alleged attempts to stop them. Health Policy 2017;121:442–9. [DOI] [PubMed] [Google Scholar]