Abstract
Objectives
The aim of this study was to devise reproducible biopsy criteria for distinguishing pulmonary large cell neuroendocrine carcinoma (LCNEC) from non-small cell lung carcinoma (NSCLC).
Methods
Tissue microarrays of LCNEC and NSCLC were generated from resection specimens and used as biopsy surrogates. They were stained for neuroendocrine markers, Ki-67, napsin-A, and p40, and independently analyzed by standardized morphologic criteria by four pathologists. Tumors were scored based on morphology, neuroendocrine marker expression, and Ki-67 proliferative index.
Results
The average total score for LCNEC was significantly higher than for NSCLC (5.65 vs 0.51, P < .0001). Utilizing a cutoff score of 4 or higher showed 100% sensitivity and 99% specificity for LCNEC diagnosis, with an excellent agreement among four pathologists (98%).
Conclusions
The proposed semiquantitative approach based on a combination of specific morphologic and immunophenotypic features may be a useful tool for biopsy diagnosis of LCNEC.
Keywords: Large cell neuroendocrine carcinoma of the lung, Biopsy diagnosis, Scoring criteria
Large cell neuroendocrine carcinoma (LCNEC) of the lung is considered a rare entity, representing approximately 3% of primary lung malignancies.1 Although originally considered a subtype of pulmonary large cell carcinoma,2 in the 2015 World Health Organization (WHO) classification3 LCNEC has been grouped with other neuroendocrine tumors, as its clinical and molecular features are more closely related to small cell lung carcinoma (SCLC). Current diagnostic criteria include: (1) non-small cell cytologic features, (2) high mitotic rate of more than 10 mitoses per 2 mm2, and (3) neuroendocrine differentiation by morphology and immunohistochemistry, with the former characterized by organoid nesting, trabecular growth pattern, peripheral palisading, and/or rosette-like structures.3 Although additional frequently encountered features of LCNEC include extensive necrosis, prominent nucleoli, and a Ki-67 labeling index (LI) greater than 40%,3 they are not currently required for the diagnosis of LCNEC. Molecular analysis suggests two major subtypes, one more closely related to small cell carcinoma and one more closely related to adenocarcinoma, but with distinct differences from each.4,5
Among neuroendocrine tumors, the distinction between SCLC and LCNEC is considered the most difficult.6 However, among an unselected population of lung carcinomas diagnosed on biopsy, the distinction is most commonly between non-small cell carcinomas vs LCNEC because LCNEC shares cytologic features such as large cell size and prominent nucleoli with non-small cell lung carcinoma (NSCLC) by definition. In this differential, neuroendocrine stains are not generally recommended because generic NSCLC can express these markers as well. More specific features of LCNEC such as palisading and organoid appearance are difficult to visualize on small biopsies. Immunohistochemistry for p40 largely resolves the problem of distinction with a nonkeratinizing basaloid squamous cell carcinoma. However, the problem of distinction from a solid poorly differentiated adenocarcinoma and large cell carcinoma remains.
Watanabe et al7 were able to diagnose LCNEC on biopsy specimens, as evidenced by concordance with six corresponding resected cases using a combined scoring system that included morphology, neuroendocrine stains, and Ki-67. However, that study did not provide guidelines as to the minimum number of criteria that must be met to make the LCNEC diagnosis. Furthermore, to our knowledge, this approach has not been validated in a larger independent cohort. We report here a more detailed evaluation of this approach using a larger cohort, taking advantage of a tissue microarray as a biopsy surrogate. In our hands, this approach is generally accurate and reproducible for diagnosis of LCNEC in small tissue samples.
Materials and Methods
Tissue Microarray Construction
A natural language search of the Yale pathology information system was performed for all lung resection cases from 1983 to 2014 containing “large cell” and “neuroendocrine” in the final diagnosis field. All cases were re-reviewed by two of the authors (R.J.H. and M.K.B.). Twenty-seven cases that were either diagnosed and signed out as LCNEC or were morphologically compatible with LCNEC on re-review were identified. For the LCNEC tissue microarrays (TMAs), of the 27 selected resection cases, six were negative for neuroendocrine markers. Two of these neuroendocrine marker-negative cases showed diffuse nuclear positivity for p40, consistent with squamous cell carcinoma. Another two neuroendocrine marker-negative cases were strongly and diffusely napsin-A positive, consistent with adenocarcinoma, while the remaining two were negative for napsin-A or p40, and were morphologically most consistent with large cell carcinoma, not otherwise specified (NOS). These six cases were reclassified in all further analyses. The existing NSCLC TMA included one LCNEC case, which was included in our analysis, resulting in a total of 22 confirmed LCNECs. This last case, however, could not be used in the analysis of intratumor heterogeneity (see below for details) due to inability to retrieve the corresponding tissue block for additional sampling. Thus only 21 cases on the LCNEC TMA were used for that part of the study.
Three replicate TMAs were constructed, each of which included the 27 initially selected putative LCNECs (21 confirmed as LCNEC and six reclassified as other types of NSCLC) and four additional definite non-LCNEC cases (to serve as internal controls and for normalization between the three blocks) comprising one adenocarcinoma, one squamous cell carcinoma, one small cell carcinoma, and one atypical carcinoid, for a total of 31 cases. H&E-stained sections from corresponding representative paraffin-embedded, formalin-fixed blocks containing tumor material were used to define diagnostic areas (ie, composed of viable tumor). To determine diagnostic reproducibility and to account for intratumor heterogeneity, three random representative 1-mm cores were obtained from different areas of the tumor for each case and then inserted in a grid pattern into three corresponding recipient paraffin blocks using a tissue arrayer (Beecher Instruments).
The final LCNEC cohort included eight males and 14 females, ranging in ages from 53 to 81 years old, with a median age at diagnosis of 64.5 years. For this study, the patients were deidentified making it exempt from institutional review board oversight. As a result, no additional clinical information was able to be collected at the time of the study, including treatment or survival data.
A second TMA of primary lung cancer resection specimens was analyzed for comparison. This TMA had been previously constructed from institutional paraffin archives, including 238 cases of NSCLC (adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, large cell carcinoma, and NSCLC, NOS), eight carcinoid tumors including one atypical carcinoid, one small cell carcinoma, and 33 tumors without histologic classification.
Immunohistochemistry
Sections (4 μm) were cut from each TMA block, mounted onto glass slides, and deparaffinized. Epitope retrieval was performed using Leica Bond ER 1 for 40 minutes on the stainer. Consecutive sections were incubated with napsin-A (Novus, clone TMU-A), p40 (Biocare, clone BC28), Ki-67 (Dako, clone MIB-1), chromogranin (Dako, clone A), synaptophysin (Leica, clone 27G12), and CD56 (Dako, clone NCAM) antibodies. Detection was carried out using Leica Bond Polymer Refine DAB detection kit or Ventana Ultraview DAB detection kit, depending on the antibody. Napsin-A and p40 immunostains were used for confirmation of tumor histotype as adenocarcinoma or squamous cell carcinoma, respectively. Any strength of neuroendocrine marker staining was considered positive, which was determined by consensus between two of the authors (R.J.H. and M.K.B.).
Ki-67 LI in the tumors was assessed independently by two pathologists, and the average was used for primary data analysis. Secondary analysis was performed to include neuroendocrine marker staining and Ki-67 LI interpretation from all four participating pathologists.
Morphologic Assessment and Scoring
Previously described morphologic criteria3 were independently assessed on H&E-stained sections of the TMA by four pathologists, including three experienced pathologists and one trainee (PGY2 resident) using the scoring scheme outlined in Table 1. Architectural features included peripheral palisading, organoid nesting, and rosette-like structures, each of which received a score of 1 if present, with a maximum score of 3 if all three features were identified. An additional one point was assigned for the presence of extensive necrosis. Three points were assigned for one or more neuroendocrine marker positivity, the presence of which was required for LCNEC diagnosis, and a Ki-67 LI 40% or greater earned an additional point.
Table 1.
Alternative Scoring Scheme for Pulmonary LCNEC With Nucleoli Excluded and Total Score Threshold Decreased to 4 or Higher
| Features | Score |
|---|---|
| Morphologic | |
| Palisading | 1 |
| Rosette-like structures | 1 |
| Organoid nesting | 1 |
| Necrosisa | 1 |
| Immunophenotypic | |
| Ki-67 ≥ 40% (high) | 1 |
| Neuroendocrine marker positivityb | 3 |
| Total scorec | 8 |
LCNEC, large cell neuroendocrine carcinoma.
aExtensive central or geographic necrosis only.
bOne positive neuroendocrine marker (chromogranin, synaptophysin, or CD56) is sufficient to assign a score of 3, and at least one is necessary for diagnosis of LCNEC.
cTotal score 4 or higher is required for diagnosis of LCNEC.
Nucleolar prominence was considered in the original scoring scheme, but it appeared to contribute little to differentiating LCNEC from NSCLC. This may not be surprising as this is a commonly shared feature with NSCLC in general. We therefore excluded nucleolar prominence from the final proposed scoring scheme.
Statistical Analysis
JMP version 14.0 software (SAS Institute) was used for statistical evaluation of the data. Receiver operating characteristic curve analysis was performed to determine the cutoff total score (≥4) for assigning tumors into the LCNEC diagnostic category (Table 1). Sensitivity and specificity of the total score for LCNEC diagnosis were manually calculated. Continuous average total scores (among four participating pathologists) for LCNEC and NSCLC were compared by one-way analysis of variance. The mean (among triplicate TMAs) of the LCNEC average total scores and standard error of the mean (SEM) was used to represent the range of all possible scores for the corresponding tumor and to define intratumor heterogeneity. Ki-67 LI was dichotomized into low and high, based on a 40% cutoff, and its sensitivity and specificity with regards to LCNEC diagnosis were manually calculated. The significance of the scored morphologic characteristics, and the associations of tumor histotype, neuroendocrine markers, and napsin-A immunoreactivities with Ki-67 LI were determined using Fisher exact test and χ2 analysis. Pairwise Cohen κ was used to assess agreement on morphology between participating pathologists, and the range for all pathologist pairs was reported along with the standard deviation.
Results
Immunophenotypic Characterization of the LCNEC Cohort
Neuroendocrine marker immunoreactivity was variable among the confirmed LCNECs (Supplemental Table 1; all supplemental materials can be found at American Journal of Clinical Pathology online). Eight cases (36%) were positive for all three neuroendocrine markers (synaptophysin, chromogranin, and CD56), 10 cases were positive for two markers (45%), and four cases were positive for one marker (18%). In our series, the most sensitive neuroendocrine marker was synaptophysin, similar to prior reports.8 This contrasts with small cell carcinoma, in which CD56 is the most commonly expressed marker (90%-100%).8,9
In our NSCLC TMA, only 2.7% of NSCLCs displayed variable staining with neuroendocrine markers (Supplemental Table 1). Tumors that were neuroendocrine marker positive included two large cell carcinomas, two squamous cell carcinomas, and one unspecified NSCLC. This is slightly lower than the typical reported range of 6% to 20%.10-12 Four of these five cases displayed only focal and weak immunoreactivity for neuroendocrine markers. The one case with diffuse moderate staining with chromogranin was also patchy and weakly positive for synaptophysin and was one of the 33 tumors on the TMA with missing histologic classification. Based on our morphologic assessment, however, this case is likely to have been a true LCNEC. Three of the four weakly focally staining cases were positive for only one marker. The remaining one case, which was positive for two markers (synaptophysin and CD56), was classified on the original resection material as large cell carcinoma with focal neuroendocrine differentiation. Morphologically, this case did not have the typical LCNEC features and its average total score by the proposed criteria was 3.25 (Supplemental Table 1), which is below the proposed diagnostic threshold for LCNEC.
There was no correlation between the number, type, or strength of neuroendocrine marker immunoreactivity and the total score (P = .5), as expected by design. When immunohistochemistry for neuroendocrine markers was scored proportionally to the number and strength of marker expression, true LCNEC cases were missed, resulting in decreased sensitivity.
Morphologic Characterization and Scoring Criteria for LCNEC Diagnosis on Biopsy Material
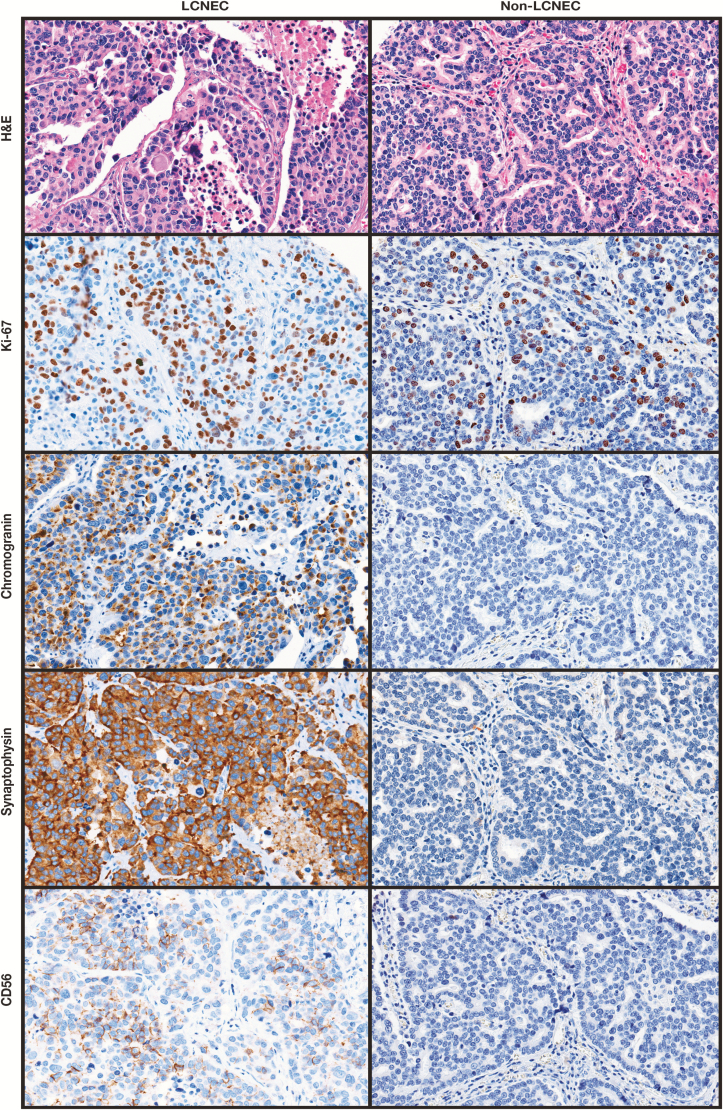
As described in “Materials and Methods,” scoring criteria were applied that included Ki-67 LI in addition to defined morphologic and immunophenotypic features (Table 1 and Image 1). Of the three scored morphologic criteria, architectural pattern and necrosis were significantly different between NSCLC and LCNEC Table 2. The presence of two or more architectural criteria (palisading, rosette-like structures, and organoid nesting) was the most specific morphologic feature for diagnosis of LCNEC (98%) but lacked sufficient sensitivity (63%). In contrast to the report by Watanabe et al,7 nucleolar prominence was neither sensitive nor specific for detecting LCNEC in our cohort (Table 2). It is unclear if the difference was due to utilized criteria for determining nucleolar prominence, as that was not explicitly defined in the previous study. Alternatively, because prominent nucleoli are a common feature of high-grade adenocarcinomas and squamous cell carcinomas of the lung as is also evident from our data (Table 2), it may not provide additional value to this differential diagnosis. Nucleolar prominence was therefore excluded from the proposed scoring scheme.
Image 1.
Morphologic and immune phenotypes of sample large cell neuroendocrine carcinoma (LCNEC) and non-LCNEC tumors (original magnification, ×20). Total score for LCNEC was 7 (architecture = 2, necrosis = 1, neuroendocrine marker positivity = 3, high Ki-67 labeling index [≥40%] = 1). Total score for non-LCNEC was 3 (architecture = 2, necrosis = 0, neuroendocrine marker positivity = 0, high Ki-67 labeling index [≥40%] = 1).
Table 2.
Morphologic Scores for LCNEC and NSCLC Cases
| Architecture | Necrosis | Prominent Nucleolia | ||||||
|---|---|---|---|---|---|---|---|---|
| Specimen | Score | Score | Score | |||||
| 0 | 1 | 2 | 3 | 0 | 1 | 0 | 1 | |
| NSCLC, No. (%) | 188 (82) | 38 (16) | 4 (2) | 0 | 170 (74) | 60 (26) | 102 (44) | 128 (56) |
| LCNEC, No. (%) | 3 (14) | 5 (23) | 8 (36) | 6 (27) | 6 (27) | 16 (73) | 14 (64) | 8 (36) |
| Probability | <.0001b | <.0001b | .09 | |||||
LCNEC, large cell neuroendocrine carcinoma; NSCLC, non-small cell lung carcinoma.
aNucleolar prominence was considered in primary analysis but ultimately was excluded from the proposed scoring scheme (Table 1) on the basis of its inability to differentiate between LCNEC and NSCLC.
bStatistically significant (P > .05).
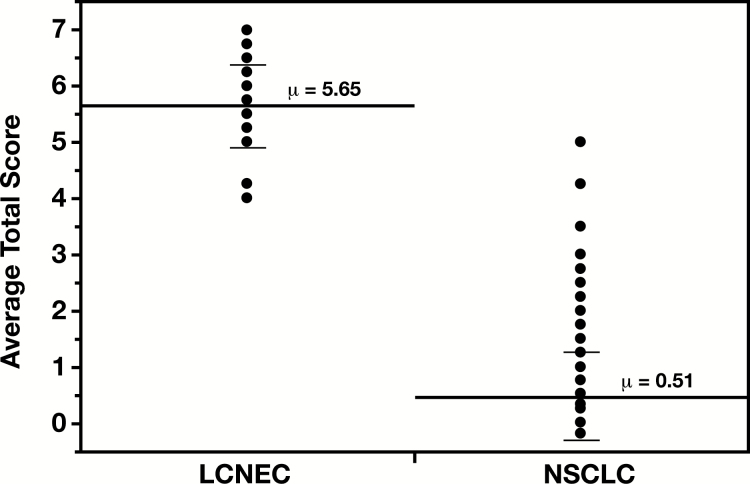
While the vast majority of NSCLCs had total scores less than 2, 100% of LCNECs had total scores of at least 4 Table 3. The average total scores ranged between 0 and 5 for NSCLCs, with a median of 0.25 and a mean of 0.51 (±0.81 SEM). For LCNECs, the range was 4 to 7, with a median of 6 and a mean of 5.63 (±0.73 SEM). The difference between the mean total scores for the two diagnostic categories was statistically significant (P < .0001) Figure 1. While napsin-A positivity among NSCLCs predicted a lower total score (data not shown), its significance on the morphologic score of LCNECs could not be assessed due to a low number of such cases (two of 22; average total scores of 4.25 and 5.25).
Table 3.
Distribution of Average Total Scores in NSCLC and LCNEC
| Average Total Score | NSCLC, No. (%) | LCNEC, No. (%) |
|---|---|---|
| 0-2 | 164 (93) | 0 (0) |
| 2< to <4 | 10 (6) | 0 (0) |
| ≥ 4 | 2 (1) | 22 (100) |
| Total | 176 | 22 |
LCNEC, large cell neuroendocrine carcinoma; NSCLC, non-small cell lung carcinoma.
Figure 1.
Average total scores in large cell neuroendocrine carcinoma (LCNEC) and non-small cell lung carcinoma (NSCLC). Each dot represents an individual case but cases with overlapping total scores (average from four pathologists) are displayed as a single dot. The mean total scores (long solid lines) with the corresponding values (μ) and the standard deviations (short solid lines) for each phenotypic category are displayed on the graph. The mean total score for LCNEC (5.65) was significantly higher than that for NSCLC (0.51, P < .0001).
Finally, and most notably, utilizing the total score cutoff of 4 or higher resulted in the diagnosis of LCNEC with a 90% to 99% specificity and 95% to 100% sensitivity among individual pathologists. When the average total scores from the four participating pathologists were used, LCNEC was correctly diagnosed in all 22 cases (100% sensitivity), and the specificity reached 99%, even when other neuroendocrine tumors (carcinoids and small cell carcinomas) in both TMAs were included in the analysis. The sensitivity and specificity held up in secondary analysis (100% and 98.9%, respectively), which included neuroendocrine marker and Ki-67 LI evaluation by all four participating pathologists rather than a consensus/average from two pathologists in our primary analysis. The specificity was 100% if other neuroendocrine tumors were excluded. This highlights that additional validation with modification of the scoring criteria components could be considered to specifically address the differential diagnosis of LCNEC vs SCLC vs atypical carcinoid.
Proliferative Activity (Ki-67 LI)
Compared to the NSCLC cohort, a significantly greater proportion of LCNEC cases displayed high proliferative activity, defined as Ki-67 LI greater than or equal to 40% (P < .0001) Table 4. Thus, elevated Ki-67 LI was a highly specific (93%), albeit not very sensitive (64%), feature of LCNEC, and thereby provided a significant value to accurate diagnosis of LCNEC.
Table 4.
Comparison of Ki-67 Labeling Index by Fisher Exact Test
| Ki-67 LI | ||
|---|---|---|
| Fisher Exact Test | Low (< 40%) | High (≥ 40%) |
| Histotype | ||
| LCNEC | 8 | 14 |
| NSCLC | 195 | 15 |
| Probability | P < .0001 | |
| LCNEC and NSCLC, napsin-A | ||
| Negative | 96 | 24 |
| Positive | 74 | 2 |
| Probability | P = .0004 | |
| LCNEC only, chromogranin | ||
| Negative | 1 | 9 |
| Positive | 8 | 4 |
| Probability | P = .01 | |
LCNEC, large cell neuroendocrine carcinoma; LI, labeling index; NSCLC, non-small cell lung carcinoma.
Additionally, among all examined cases (both NSCLC and LCNEC), those expressing napsin-A by immunohistochemistry, including both weakly napsin-A positive LNCECs, had significantly lower proliferative activity with 96% displaying Ki-67 LI less than 40% (P = .0004; Table 4). It was also noted that the majority of LCNEC lesions that were immunoreactive for chromogranin displayed low Ki-67 LI (90%; P = .01; Table 4). This lower proliferative activity is likely a reflection of a more mature/differentiated phenotype as is implied by the corresponding immunophenotype.
Intratumor Heterogeneity
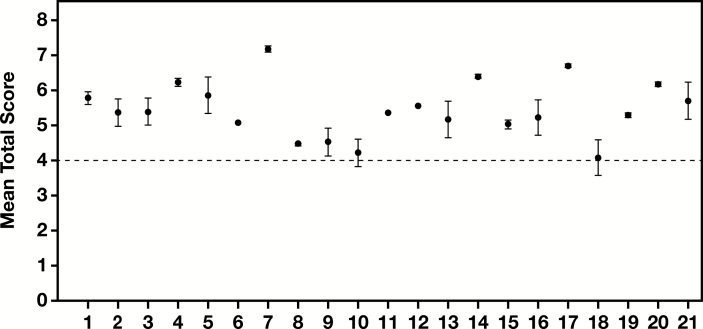
To examine reproducibility of the proposed scoring system and to assess intratumor heterogeneity, three different parts of the tumor represented on the three LCNEC TMAs were separately scored by all four pathologists. The LCNEC case from the archived NSCLC TMA utilized in all other parts of the study could not be used for this analysis due to the inability to access corresponding tissue blocks, leaving a total of 21 cases for this assessment. The SEM ranged from 0.02 to 0.53, with a median of 0.12, reflecting a good overall reproducibility and minimal intratumor heterogeneity. In addition, of the 21 LCNEC cases, 19 (90%) were consistently scored above the diagnostic threshold for LCNEC diagnosis (total score ≥4) Figure 2. This further highlights the reproducibility of the scoring system and the overall homogeneity of the tumor. The remaining two cases (10%) had mean total scores very close to the cutoff of 4, which made them fall outside the LCNEC diagnostic interpretation when SEMs were considered (score <4; Figure 2). This reflects reduced diagnostic reproducibility of the proposed scoring system for tumors that are on the proposed diagnostic cusp. Independent scoring of neuroendocrine marker immunohistochemistry and Ki-67 LI by four participating pathologists did not significantly alter these findings (data not shown).
Figure 2.
Intratumor heterogeneity and reproducibility of the proposed large cell neuroendocrine carcinoma (LCNEC) scoring system. Each dot represents an individual case and the bars represent standard errors of the mean (SEM). A horizontal dotted line is drawn at a mean score of 4, which is the diagnostic cutoff for LCNEC based on the proposed scoring criteria.
Agreement Among Pathologists
There was moderate agreement among pathologist on the major morphologic criteria Table 5, with greater variability for architecture (κ = 0.42-0.55), which is attributed to its multivariable/ordinal (comprised of three distinct scoring categories adding up to a final score of 0-3) rather than binary nature. Although there was some disagreement for each individual component of the score, the final classification into LCNEC vs non-LCNEC diagnosis demonstrated strong agreement among pathologists (κ = 0.93-0.98; Table 5). The κ values were not altered significantly when Ki-67 LI and neuroendocrine marker immunohistochemistry were scored independently by all four pathologists (Supplemental Table 2). The lower limit of the κ range (0.74) was attributed to one of the four pathologists scoring neuroendocrine markers slightly more liberally than the other three despite the predefined criteria for positivity.
Table 5.
Analysis of Agreement Among Participating Pathologists: Pairwise Cohen κ Ranges for Each Morphologic Category and Final Diagnosis
| Category | Cohen κ range | Standard Deviation (σ) |
|---|---|---|
| Architecturea | 0.42-0.55 | 0.04 |
| Necrosis | 0.61-0.74 | 0.04 |
| Final diagnosisb | 0.93-0.98 | 0.03 |
aOrdinal data, with a score range 0-3.
bFinal diagnosis was binarized into large cell neuroendocrine carcinoma and non-large cell neuroendocrine carcinoma based on a total cutoff score of 4 or higher.
Based on the total score cutoff of 4 or higher, there was complete agreement (100%) among the four pathologists on the final assignment of tumors to LCNEC vs non-LCNEC diagnostic category in 202 of 208 analyzed cases (97%), with an average agreement of 99% ± 5% for all examined tumors. When considered separately, the agreement was significantly better for non-LCNEC than for LCNEC cases Table 6. This latter finding is primarily due to two factors: a small cohort size and a small number of evaluating pathologists. While the former is a function of low LCNEC incidence and a single institution experience, the latter assigns substantial weight to each assigned score. Specifically, in eight of nine LCNEC cases with diagnostic disagreement (89%), there was only one divergent opinion (one of four pathologists assigning a score below the threshold), and only one case which was assigned to the LCNEC diagnostic category by half of the pathologists and to the non-LCNEC category by the other half. These divergent opinions did not correlate with the pathologists’ experience. Excluding neuroendocrine tumors other than LCNEC from the analysis resulted in further improvement in interobserver agreement, which was not affected significantly in the secondary analysis with independent neuroendocrine immunohistochemistry and Ki-67 LI scoring by all four participating pathologists (Supplemental Table 2).
Table 6.
Analysis of Agreement Among Participating Pathologists: Diagnostic Agreement Based on the Total Score Cutoff of 4 or Highera
| Percent Cases With 100% Agreement | % Agreement ± SD | |||
|---|---|---|---|---|
| Diagnostic Category | Other NE Tumors Included | Other NE Tumors Excluded | Other NE Tumors Included | Other NE Tumors Excluded |
| All | 97 | 98 | 99 ± 5 | 99 ± 5 |
| LCNEC | 90 | NA | 98 ± 8 | NA |
| Non-LCNEC | 98 | 99 | 99 ± 5 | 99 ± 4 |
LCNEC, large cell neuroendocrine carcinoma; NA, not applicable; NE, neuroendocrine.
aOther NE tumors included typical and atypical carcinoids and small cell lung carcinoma.
Discussion
The most recent lung cancer classification statements suggest extreme caution in diagnosing LCNEC on biopsy material.3,13 Morphologic features such as palisading and organoid morphology are not readily seen, there are commonly not enough tumor cells (<2,000) to adequately count mitoses, and neuroendocrine stains by themselves are not considered specific for this entity. We here show that an approach combining morphology, neuroendocrine stains, and Ki-67 staining in a formal scoring system such as has previously been proposed might overcome these limitations.7
The utility of Ki-67 in the diagnosis and grading of lung neuroendocrine tumors has been examined in a number of studies. There is an overall agreement that Ki-67 LI is appropriate to assess in a hot spot area after thorough examination of the entire specimen.14 However, the need to assess a hot spot in LCNEC is unclear because minimal variability (<1.5%) has been shown in reproducibility studies of Ki-67 LI, with superior results to mitosis counting.15-20 In the current study, Ki-67 LI 40% or higher was highly specific for LCNEC (93%), which supports the utility of Ki-67 LI in lieu of mitotic count on specimens with low tumor cellularity, such as core biopsy and fine needle aspiration material, corroborating the aforementioned studies.
Reactivity with at least one neuroendocrine marker is required for diagnosis of LCNEC but these are generally acknowledged to be insufficient by themselves. Our results with neuroendocrine markers are in contrast to a recent study by Derks et al,21 which suggested that two or more positive neuroendocrine markers in a poorly differentiated tumor were sufficient for diagnosis of LCNEC irrespective of morphology. While the presence of immunoreactivity for two or more neuroendocrine markers may be a somewhat specific feature for LCNEC, the sensitivity of this approach is inadequate based on our study results.
Rekhtman et al4,22 has previously reported that strong napsin-A reactivity is not a feature of LNCEC and, as described in “Materials and Methods,” our cases that demonstrated strong reactivity were excluded from analysis on the basis of also being neuroendocrine marker negative. However, two of our 22 LCNEC cases (9%) showed weak, focal napsin-A immunoreactivity but also coexpressed neuroendocrine markers and were therefore considered true LCNEC. Napsin-A weakly positive LCNECs were reported to demonstrate morphologic and molecular features more similar to NSCLC, the latter of which include KRAS, STK11, and KEAP1 gene alterations.4,22 Conversely, most napsin-A–negative LCNECs were reported to be more similar to classic small cell carcinoma, which is defined by coaltered RB1 and TP53 genes.4,22
Although there is a degree of controversy and uncertainty regarding the clinical management of LCNEC, there has been growing evidence that platinum/etoposide-based regimens utilized for SCLC show similar efficacy in patients with LCNEC and are superior to NSCLC treatments in the adjuvant setting and as first-line therapy for advanced disease.23-29 The molecular subtype of LCNEC also appears to be important in predicting response to therapy.5 As we begin to better understand the natural history of LCNEC and its response to therapy, recognizing this entity and distinguishing it from NSCLC on biopsy specimens will become critical for molecular evaluation and optimal clinical management.
The proposed scoring criteria allowed accurate distinction of LCNEC from NSCLC with high overall sensitivity and specificity. However, it is important to emphasize that these criteria were not designed to differentiate LCNEC from SCLC or highly proliferative atypical carcinoids, as there is particular emphasis on neuroendocrine marker expression, which defines these tumors. Furthermore, SCLC and a subset of atypical carcinoids with high proliferative activity30 have Ki-67 LIs greater than 40%, which would make them indistinguishable from LCNEC based on the proposed scoring criteria. A similar difficulty may be encountered in differentiating LCNEC from NSCLC with focal neuroendocrine marker expression. In such instances the scoring criteria would have to be modified to place additional emphasis on morphologic features over neuroendocrine marker expression and proliferative activity.
In summary, we have characterized pulmonary LCNEC morphologically and immunophenotypically, demonstrated the utility of Ki-67 LI in distinguishing LCNEC from NSCLC, and established a reliable semiquantitative approach for this differential diagnosis by incorporating our findings with current knowledge and established WHO diagnostic criteria. The accuracy and reproducibility compare favorably to existing criteria for other WHO defined lung carcinoma categories. For instance, it is typically stated that 5% of cases of small cell carcinoma have moderate to high interobserver variability,31 and a recent study in NSCLCs has demonstrated a κ of only 0.45 for biopsy material using WHO 2015 classification.32
Limitations of the study include a small LCNEC sample size and lack of clinical follow-up due to restrictions imposed by the anonymous source of tissue. Thus, additional multi-institutional studies with large LCNEC cohorts are needed. Further validation of the proposed scoring criteria on paired biopsy and resection material of LCNEC and NSCLC is warranted, with a particular focus on solid adenocarcinoma and large cell carcinoma to further enhance the relevance of this semiquantitative approach. Future directions also include prospective analysis of biopsy samples with molecular correlation for additional validation. Although less critical for current therapeutic stratification, separate criteria for distinguishing LCNEC from SCLC and highly proliferative atypical carcinoid on biopsy may be of diagnostic utility. A combination of this method with targeted molecular profiling, such as that utilized by Rekhtman and others,4,5,33 could further provide additional information for clinical decision making at the time of biopsy.
Supplementary Material
This work was supported by the Yale School of Medicine Pathology Department. We extend special thanks to Dr David L. Rimm for providing the funding for construction of the LCNEC tissue microarrays.
References
- 1. Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma: an ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15:529-553. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Muller-Hermelink KH, et al. WHO Classification of Tumours: Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. 3rd ed. Lyon, France: IARC Press; 2004. [Google Scholar]
- 3. Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon, France: IARC Press; 2015. [DOI] [PubMed] [Google Scholar]
- 4. Rekhtman N, Pietanza MC, Hellmann MD, et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res. 2016;22:3618-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Derks JL, Leblay N, Thunnissen E, et al. ; PALGA-Group Molecular subtypes of pulmonary large-cell neuroendocrine carcinoma predict chemotherapy treatment outcome. Clin Cancer Res. 2018;24:33-42. [DOI] [PubMed] [Google Scholar]
- 6. Travis WD, Gal AA, Colby TV, et al. Reproducibility of neuroendocrine lung tumor classification. Hum Pathol. 1998;29:272-279. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe R, Ito I, Kenmotsu H, et al. Large cell neuroendocrine carcinoma of the lung: is it possible to diagnose from biopsy specimens? Jpn J Clin Oncol. 2013;43:294-304. [DOI] [PubMed] [Google Scholar]
- 8. Hiroshima K, Iyoda A, Shida T, et al. Distinction of pulmonary large cell neuroendocrine carcinoma from small cell lung carcinoma: a morphological, immunohistochemical, and molecular analysis. Mod Pathol. 2006;19:1358-1368. [DOI] [PubMed] [Google Scholar]
- 9. Kontogianni K, Nicholson AG, Butcher D, et al. CD56: a useful tool for the diagnosis of small cell lung carcinomas on biopsies with extensive crush artefact. J Clin Pathol. 2005;58:978-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng J, Sheng H, Zhu C, et al. Correlation of neuroendocrine features with prognosis of non-small cell lung cancer. Oncotarget. 2016;7:71727-71736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638. [DOI] [PubMed] [Google Scholar]
- 12. Cadioli A, Rossi G, Costantini M, et al. Lung cancer histologic and immunohistochemical heterogeneity in the era of molecular therapies: analysis of 172 consecutive surgically resected, entirely sampled pulmonary carcinomas. Am J Surg Pathol. 2014;38:502-509. [DOI] [PubMed] [Google Scholar]
- 13. Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pelosi G, Rindi G, Travis WD, et al. Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol. 2014;9:273-284. [DOI] [PubMed] [Google Scholar]
- 15. Walts AE, Ines D, Marchevsky AM. Limited role of Ki-67 proliferative index in predicting overall short-term survival in patients with typical and atypical pulmonary carcinoid tumors. Mod Pathol. 2012;25:1258-1264. [DOI] [PubMed] [Google Scholar]
- 16. Warth A, Fink L, Fisseler-Eckhoff A, et al. ; Pulmonary Pathology Working Group of the German Society of Pathology Interobserver agreement of proliferation index (Ki-67) outperforms mitotic count in pulmonary carcinoids. Virchows Arch. 2013;462:507-513. [DOI] [PubMed] [Google Scholar]
- 17. Aslan DL, Gulbahce HE, Pambuccian SE, et al. Ki-67 immunoreactivity in the differential diagnosis of pulmonary neuroendocrine neoplasms in specimens with extensive crush artifact. Am J Clin Pathol. 2005;123:874-878. [DOI] [PubMed] [Google Scholar]
- 18. Lin O, Olgac S, Green I, et al. Immunohistochemical staining of cytologic smears with MIB-1 helps distinguish low-grade from high-grade neuroendocrine neoplasms. Am J Clin Pathol. 2003;120:209-216. [DOI] [PubMed] [Google Scholar]
- 19. Pelosi G, Rodriguez J, Viale G, et al. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol. 2005;29:179-187. [DOI] [PubMed] [Google Scholar]
- 20. Rindi G, Klersy C, Inzani F, et al. Grading the neuroendocrine tumors of the lung: an evidence-based proposal. Endocr Relat Cancer. 2014;21:1-16. [DOI] [PubMed] [Google Scholar]
- 21. Derks JL, Dingemans AC, van Suylen RJ, et al. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathology. 2019;74:555-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rekhtman N, Pietanza CM, Sabari J, et al. Pulmonary large cell neuroendocrine carcinoma with adenocarcinoma-like features: napsin A expression and genomic alterations. Mod Pathol. 2018;31:111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glisson BS, Moran CA. Large-cell neuroendocrine carcinoma: controversies in diagnosis and treatment. J Natl Compr Canc Netw. 2011;9:1122-1129. [DOI] [PubMed] [Google Scholar]
- 24. Igawa S, Watanabe R, Ito I, et al. Comparison of chemotherapy for unresectable pulmonary high-grade non-small cell neuroendocrine carcinoma and small-cell lung cancer. Lung Cancer. 2010;68:438-445. [DOI] [PubMed] [Google Scholar]
- 25. Naidoo J, Santos-Zabala ML, Iyriboz T, et al. Large cell neuroendocrine carcinoma of the lung: clinico-pathologic features, treatment, and outcomes. Clin Lung Cancer. 2016;17:e121-e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol. 2005;23:8774-8785. [DOI] [PubMed] [Google Scholar]
- 27. Sarkaria IS, Iyoda A, Roh MS, et al. Neoadjuvant and adjuvant chemotherapy in resected pulmonary large cell neuroendocrine carcinomas: a single institution experience. Ann Thorac Surg. 2011;92:1180-1186; discussion 1186-1187. [DOI] [PubMed] [Google Scholar]
- 28. Shimada Y, Niho S, Ishii G, et al. Clinical features of unresectable high-grade lung neuroendocrine carcinoma diagnosed using biopsy specimens. Lung Cancer. 2012;75:368-373. [DOI] [PubMed] [Google Scholar]
- 29. Varlotto JM, Medford-Davis LN, Recht A, et al. Should large cell neuroendocrine lung carcinoma be classified and treated as a small cell lung cancer or with other large cell carcinomas? J Thorac Oncol. 2011;6:1050-1058. [DOI] [PubMed] [Google Scholar]
- 30. Rekhtman N, Desmeules P, Litvak AM, et al. Stage IV lung carcinoids: spectrum and evolution of proliferation rate, focusing on variants with elevated proliferation indices. Mod Pathol. 2019;32:1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. den Bakker MA, Willemsen S, Grünberg K, et al. Small cell carcinoma of the lung and large cell neuroendocrine carcinoma interobserver variability. Histopathology. 2010;56:356-363. [DOI] [PubMed] [Google Scholar]
- 32. Funkhouser WK Jr, Hayes DN, Moore DT, et al. Interpathologist diagnostic agreement for non-small cell lung carcinomas using current and recent classifications. Arch Pathol Lab Med. 2018;142:1537-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Derks JL, Leblay N, Lantuejoul S, et al. New insights into the molecular characteristics of pulmonary carcinoids and large cell neuroendocrine carcinomas, and the impact on their clinical management. J Thorac Oncol. 2018;13:752-766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.