Rheumatology key message
Subclinical tenosynovitis of the biceps tendon is not an early feature of RA in clinically suspect arthralgia patients.
Sir, Multiple studies have demonstrated that shoulder complaints are frequent in RA [1, 2]. Recently, it has been shown that shoulder involvement is predictive of RA development in patients with undifferentiated arthritis and its value is comparable with that of small joint involvement [3]. The phase of clinically suspect arthralgia precedes the phase of clinically apparent arthritis. In this phase, subclinical tenosynovitis in small hand joints is associated with developing RA [4]. Given the similarities in predictive values between the shoulder and small joints in undifferentiated arthritis, and the predictive value of tenosynovitis in clinically suspect arthralgia, we hypothesized that tenosynovitis of the bicep tendon visualized by US is also associated with developing inflammatory arthritis (IA) in clinically suspect arthralgia patients. We examined the biceps tendon, since this is the only tendon of the shoulder that is enclosed by a synovial sheath as it passes through the bicipital groove (Fig. 1A–C) [5].
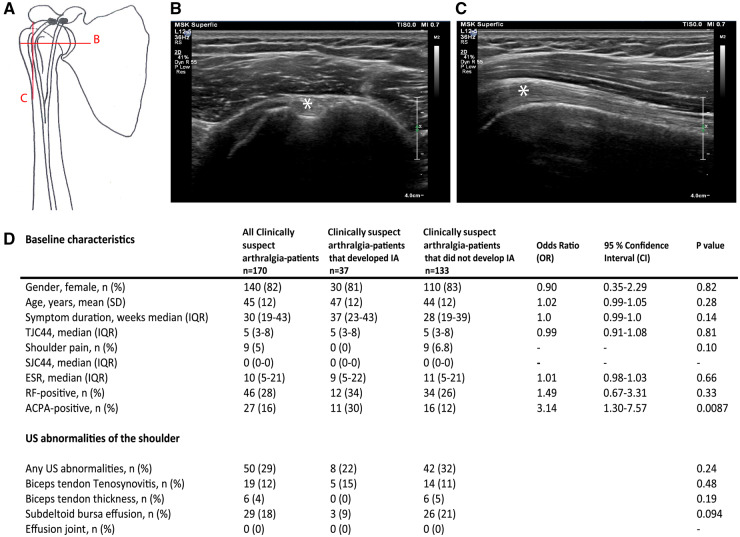
Fig. 1.
Anatomical representation of the biceps tendon and baseline characteristics including US abnormalities
(A) Anatomy of the shoulder joint and the course of the biceps tendon through the bicipital groove. The letters indicate the two perpendicular planes of the US probe for scanning the biceps tendon. (B) US of the transverse plane of the biceps tendon. (C) US of the longitudinal plane of the biceps tendon. (D) Baseline characteristics and US abnormalities at baseline in patients with clinically suspect arthralgia. Tub. Minus: tuberculum minus; Tub. majus: tuberculum majus; IA: inflammatory arthritis; TJC44: tender joint count in 44 joints; SCJ44: swollen joint count in 44 joints. White asterisk indicates biceps tendon.
To answer our research question we used data from the SONAR study, sonographic evaluation of hands, shoulders and feet in patients presenting with inflammatory arthralgia, to identify subclinical arthritis. This was a multicentre observational cohort study. In this study, US of both shoulders was made at baseline. US abnormalities of the biceps tendon [1], the glenohumeral joint [2] and the subdeltoid bursa [3] were assessed for tenosynovitis, arthritis and bursitis. Thereafter, patients were followed for the course of 1 year (with 6-monthly visits) for the development of clinically apparent IA, which was verified by the treating physician. The medical ethics committee of Erasmus University Medical Center (Erasmus MC), Rotterdam, The Netherlands approved the study (MEC-2010–353). Furthermore, the study was assessed for feasibility by the local ethical bodies of the other two participating hospitals (Maasstad Hospital and Vlietland Hospital). All patients gave written informed consent before inclusion according to the Declaration of Helsinki. Student’s t test and the Mann–Whitney test were used to compare baseline values. US abnormalities between groups were compared using a χ2 test. A detailed description of the cohort, US protocol, and statistics are presented in the Supplementary Material, Methods section, available at Rheumatology online.
A flowchart is presented as supplementary material, available at Rheumatology online. No significant differences in baseline characteristics were found between included and excluded patients (Supplementary Table S1, available at Rheumatology online). Of the participants, 140 patients (82%) were female, the mean age was 45 years and the median symptom duration was 30 weeks (Fig. 1D). After 1 year, 37 patients (22%) had developed IA and, of those patients, 17 (46%) fulfilled the 2010 criteria for RA (Supplementary Table S2, available at Rheumatology online). The remaining 20 patients were diagnosed with: undifferentiated arthritis (80%), OA (15%) and PsA (5%). Shoulder pain was infrequent (5%) and only observed in the non-IA group. ACPA positivity was associated with IA development [odds ratio (OR) 3.14, 95% confidence interval 1.30–7.57, P = 0.0087] (Fig. 1D).
Although shoulder pain was infrequent, we did find US shoulder abnormalities in 50 patients (29%, Fig. 1D). None were predictive for IA development. Subclinical tenosynovitis of the biceps tendon was present in 15% and 11% of clinically suspect arthralgia patients who, respectively, did and did not develop IA (P = 0.48). Also, bilateral tenosynovitis was evenly distributed between both groups (6% and 6%) (Supplementary Fig. S2, available at Rheumatology online). A thickened biceps tendon and subdeltoid bursa effusion were also not associated with IA development (P = 0.19 and P = 0.094, respectively). Joint effusion was absent (Fig. 1D). The subgroup analysis with RA as outcome showed similar results (Supplementary Table S3, available at Rheumatology online).
US abnormalities of the shoulder were less frequent in our study than in other studies: biceps tenosynovitis 23–44%, subdeltoid bursitis 18–67% and effusion of the joint 28–38% [6, 7]. However, these studies were performed in established RA. It is plausible that these patients had developed more abnormalities in the shoulder joint over the years due to active inflammation. On the other hand, US shoulder abnormalities are also seen in healthy individuals and prevalences vary widely. Iagnocco et al., for example, showed a prevalence of 28.9%, which is comparable with our overall prevalence of 29% [8].
A strength of the current study was that US was performed by two experienced ultrasonographers who had received training prior to the start of the study. Also, a standardized US protocol and scoring system was used for scanning. In addition, analyses on primary and secondary outcomes provided similar results.
A limitation is that the number of patients that developed IA or RA and the frequency of US abnormalities within this group were relatively low. Although this may have harboured the risk of false-negative findings, there was no tendency towards more US-detected inflammation, even in the clinically suspect arthralgia patients who progressed to IA. Therefore, further US studies on the shoulder in clinically suspect arthralgia would not seem to be valuable.
In conclusion, subclinical tenosynovitis of the biceps tendon, visualized with US, is not an early feature of RA and is also not predictive of the development of RA. Based on these results, standard US screening of the shoulder is not necessary in clinically suspect arthralgia patients for determining their risk of developing IA.
Funding: This work was supported by an investigator-initiated grant from Pfizer bv. and funding from the Dutch Arthritis Society.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Olofsson Y, Book C, Jacobsson LT.. Shoulder joint involvement in patients with newly diagnosed rheumatoid arthritis. Prevalence and associations. Scand J Rheumatol 2003;32:25–32. [DOI] [PubMed] [Google Scholar]
- 2. Bilberg A, Bremell T, Balogh I, Mannerkorpi K.. Significantly impaired shoulder function in the first years of rheumatoid arthritis: a controlled study. Arthritis Res Ther 2015;17:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brinkmann GH, Norli ES, Kvien TK. et al. Disease characteristics and rheumatoid arthritis development in patients with early undifferentiated arthritis: a 2-year followup study. J Rheumatol 2017;44:154–61. [DOI] [PubMed] [Google Scholar]
- 4. van Steenbergen HW, Mangnus L, Reijnierse M, Huizinga TW, van der Helm-van Mil AH.. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheum Dis 2016;75:1824–30. [DOI] [PubMed] [Google Scholar]
- 5. Streit JJ, Shishani Y, Rodgers M, Gobezie R.. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extra-articular biceps tendon and tenosynovium. Open Access J Sports Med 2015;6:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakellariou G, Iagnocco A, Filippucci E. et al. Ultrasound imaging for the rheumatologist XLVIII. Ultrasound of the shoulders of patients with rheumatoid arthritis. Clin Exp Rheumatol 2013;31:837–42. [PubMed] [Google Scholar]
- 7. Ottaviani S, Gill G, Palazzo E, Meyer O, Dieudé P.. Ultrasonography of shoulders in spondyloarthritis and rheumatoid arthritis: a case-control study. Joint Bone Spine 2014;81:247–9. [DOI] [PubMed] [Google Scholar]
- 8. Iagnocco A, Filippucci E, Sakellariou G. et al. Ultrasound imaging for the rheumatologist XLIV. Ultrasound of the shoulder in healthy individuals. Clin Exp Rheumatol 2013;31:165–71. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.