Abstract
Radiation therapy is capable of directing adaptive immune responses against tumors by stimulating the release of endogenous adjuvants and tumor-associated antigens. Within the tumor, conventional type 1 dendritic cells (cDC1s) are uniquely positioned to respond to these signals, uptake exogenous tumor antigens and migrate to the tumor draining lymph node (dLN) to initiate cross-priming of tumor reactive cytotoxic CD8+ T cells. Here we report that radiation therapy promotes the activation of intratumoral cDC1s in radio-immunogenic murine tumors and this process fails to occur in poorly radio-immunogenic murine tumors. In poorly radio-immunogenic tumors, the adjuvant poly I:C overcomes this failure following radiation and successfully drives intratumoral cDC1 maturation, ultimately resulting in durable tumor cures. Depletion studies revealed that both cDC1 and CD8+ T cells are required for tumor regression following combination therapy. We further demonstrate that treatment with radiation and poly I:C significantly expands the proportion of proliferating CD8+ T cells in the tumor with enhanced cytolytic potential and requires T cell migration from LNs for therapeutic efficacy. Thus, we conclude that lack of endogenous adjuvant release or active suppression following radiation therapy may limit its efficacy in poorly radio-immunogenic tumors, and co-administration of exogenous adjuvants that promote cDC1 maturation and migration can overcome this limitation to improve tumor control following radiation therapy.
INTRODUCTION
Radiation therapy is used to treat over half of all cancer patients at some point during the course of their treatment [1, 2]. However, the treatment response varies significantly across cancer pathologies and mechanisms describing why particular cancers respond poorly to radiation are lacking. Traditionally, the efficacy of radiation has been attributed to direct killing of cancer cells following radiation induced DNA damage [3]. Recently this paradigm has shifted, as studies have demonstrated that radiation can trigger immunogenic cancer cell death capable of igniting tumor specific immunity [4–6]. Treatment with radiation leads to the release of endogenous adjuvants and tumor associated antigens that can be recognized by the immune system to direct anti-tumor immune responses [7–9]. Conversely, it has also been reported that radiation therapy can promote upregulation of molecules that foster immunosuppression following treatment [10–14]. Thus, the cumulative integration of these signals within individual tumors likely plays a significant role in determining whether a successful anti-tumor immune response is generated following radiation. A better understanding for how individual tumor microenvironments shape the immune response following radiation is needed to improve patient outcomes following treatment.
Dendritic cells (DCs) are key sentinels of the immune system, capable of processing and presenting antigens, sensing innate danger signals and integrating microenvironmental cues to regulate whether an adaptive immune response is mounted towards foreign invaders. In particular, conventional type 1 DCs (cDC1s) have the specialized ability to uptake exogenous cell-associated antigens and potently cross-prime antigen specific CD8+ T cell responses [15–18]. Cross-presenting cDC1s are defined by their expression of the transcription factors BATF3, ZBTB46, ID2 and IRF8 [19]. cDC1 can be further divided into those capable of migrating from tissues (CD103+ cDC1) and those resident to lymphoid organs (CD8α+ cDC1) [20, 21]. CD103+ cDC1s are present in many murine tumors, and are thought to be the predominant cell type capable of trafficking intact tumor-associated antigens to the draining lymph node (dLN) to initiate cross-priming of tumor reactive CD8+ T cells [22, 23].
In preclinical models, cDC1s are required for the rejection of immunogenic tumors and they are known to play an important role in promoting anti-tumor immune responses following treatment with many immunotherapies [15, 24–26]. Moreover, it has been reported that increased cDC1 signatures in patient tumors correlates with improved outcomes in a range of cancers [23, 27, 28]. Activation of intratumoral cDC1 is proposed to support the development of anti-tumor immunity through two key mechanisms; 1) cDC1 migration to the dLN to deliver tumor-associated antigen and initiate priming of tumor reactive CD8+ T cells, and 2) cDC1 function within the tumor to recruit and re-prime tumor reactive CD8+ T cells locally. The role of cDC1s activation and migration in radiation mediated tumor regression remains to be determined. In certain tumor models the efficacy of radiation has been shown to depend on the presence of cDC1s [29, 30]. However, these studies utilized mice that lack cDC1s (Batf3−/−) throughout the course of tumor development, as opposed to only during therapy, making it difficult to draw conclusions regarding the mechanism. Thus, the question remains whether cDC1 activation and migration is required to successfully promote anti-tumor immune responses following radiation therapy and whether this differs across cancers.
In this study, we investigated mechanisms that regulate why particular cancer types are either highly or poorly responsive to radiation. Using tumor models with equivalent radiosensitivity in vitro, but differing responsiveness to radiation in vivo, we demonstrate that poorly radio-immunogenic tumors fail to activate intratumoral cDC1 following treatment. Poly I:C has been shown to successfully combine with radiation therapy to improve tumor control [31]. We similarly show that by combining radiation with the exogenous adjuvant poly I:C this successfully drives cDC1 maturation resulting in tumor cures. We determine the combined efficacy of radiation and poly I:C is dependent on cDC1s, which promote the development of tumor specific effector CD8+ T cells. Finally, we establish that trafficking of CD8+ T cells from LNs to the tumor is necessary for treatment efficacy. Taken together these data demonstrate that intratumoral cDC1 activation and migration following radiation is one potential mechanistic factor that limits the response to radiation therapy across different cancer pathologies.
MATERIALS & METHODS
Animals and cell lines
Experiments utilized 6–8 week old C57BL/6 (#000664), B6.SJL (#002014) and Zbtb46-DTR (#019506) mice that were obtained from The Jackson Laboratories. 2C TCR transgenic mice were kindly provided by Dr. Thomas Gajewski at the University of Chicago. Survival experiments were performed with 5–8 mice per experimental group, and mechanistic experiments with 4–6 mice per group. Animal protocols were approved by the Earle A. Chiles Research Institute (EACRI) Institutional Animal Care and Use Committee (Animal Welfare Assurance No. A3913–01). The Panc02-SIY pancreatic adenocarcinoma line expressing the model antigen SIY was kindly provided by Dr. Ralph Weichselbaum at the University of Chicago. MC38 colorectal carcinoma line was obtained from Dr. Kristina Young at EACRI. Moc1 and Moc2 oral squamous cell carcinoma lines were kindly provided by Dr. Ravindra Uppaluri at the Dana Faber Cancer Institute. Panc02-SIY, Moc1 and Moc2 cell lines were grown in complete RPMI containing 10% heat inactivated fetal bovine serum (FBS), 100U/mL penicillin, 100μg/mL streptomycin. MC38 cell lines were grown in DMEM containing 10% heat inactivated FBS, 100U/mL penicillin, 100μg/mL streptomycin. Pathogen and mycoplasma contamination testing were performed on all cell lines within the past 6 months using the IMPACT II Mouse PCR Profiling from IDEXX BioAnalytics.
Clonogenic assay
Tumor cells lines were treated with indicated dose of radiation using a cesium irradiator. After treatment 5 × 102 cells were seeded in a 6-well plate and allowed to grow for 5 days. On day 5 media was removed, plates were washed with PBS and cells were fixed with methanol. The number of tumor cell colonies was counted for each well and normalized by dividing by the number of colonies in the untreated well to get the percent of surviving cells for each dose of radiation.
Tumor treatments
Tumors were implanted subcutaneously into the right flank as follows; 2 × 105 MC38, 5 × 106 Panc02-SIY, 1 × 106 Moc1 and 1 × 105 Moc2. When tumors were approximately 5mm in average diameter, mice were randomized to receive treatment with CT-guided radiation using the Small Animal Radiation Research Platform (SARRP) from XStrahl. Dosimetry was performed using Murislice software from XStrahl. The SARRP delivered a single dose of 12Gy to an isocenter within the tumor using a 10mm x 10mm collimator and a 45° beam angle to minimize dose delivery to normal tissues. For poly I:C treatments vaccine grade reagent from InvivoGen (#vac-pic) was administered intratumorally at 50 μg/tumor in a total volume of 10ul. Control mice received 10μl of vehicle. The 1st dose of poly I:C was administered concurrently with radiation and the 2nd dose was given 5 days later. For CD8 depletion, 200 μg of α-CD8β antibodies from BioXCell (clone 53–5.8) were given intraperitoneally one day prior to radiation and again 7 days later. To block T cell egress during treatment, FTY720 from Cayman Chemical Company (#10006292) was administered at 1 mg/kg/day intraperitoneally, starting 1 day prior to radiation for a total of 7 consecutive days. For Flt3L experiments, compound was provided by Bristol Myers-Squibb and administered intraperitoneally at 30μg/mouse/day for 9 consecutive days. In all survival experiments, tumor length and width were measured 2–3 times per week using calipers. Mice were euthanized when tumor size exceeded 12 mm in any dimension, or when body condition score declined 1 level.
Tissue processing
Following dissection, tumors were weighed and minced into small fragments, then transferred into C tubes from Miltenyi Biotec containing enzyme digest mix with 250U/mL collagenase IV (Worthington Biochemical, #LS004188), 30U/mL DNase I (Millipore-Sigma, #4536282001), 5mM CaCl2, 5% heat inactivated FBS and HBSS. Tissue was dissociated using a GentleMACS tissue dissociator from Miltenyi Biotech. This was followed by incubation at 37°C for 30 min with agitation. For the dLNs, capsules were cut open and incubated with enzymatic mix described above at 37°C for 15 min with agitation. Enzyme mix containing dLNs was then vigorously pipet mixed and incubated at 37°C for an additional 15 min. Enzymatic reactions for both the tumor and dLN were quenched using ice cold RPMI containing 10% FBS and 2mM EDTA. Single cell suspensions were then filtered through 100μm (tumor) or 40μm (dLN) nylon cell strainers to remove macroscopic debris. Cells were washed and counted as described above.
Flow cytometry
For staining, 2 × 106 cells were stained with Zombie Aqua Viability Dye from BioLegend (#423102) in PBS for 10 min on ice, then Fc receptors were blocked with α-CD16/CD32 antibodies from BD Biosciences (2.4G2) for an additional 10 min. After centrifugation, the supernatant was removed and cell were stained with a surface antibody cocktail containing in FACS buffer (PBS, 2mM EDTA, 2% FBS) and Brilliant Stain Buffer Plus from BD Biosciences (#566385) for 20 min on ice. The following antibodies were purchased from BioLegend; F4/80-PerCP/Cy5.5 (BM8), CD11c-PE/Cy7 (N418), CCR7-PE (4B12), CD90.2-A700 (30-H12), CD19-A700 (6D5), MHC-II-BV421 (M5/114.14.2), CD11b-BV605 (M1/70), CD8α-BV650 (53–6.7), Ly-6C-BV711 (HK1.4) and IL-12 PE (C15.6). CD40-FITC (HM40–3), CD103-APC (2E9), CD24-APC e780 (M1/69) and Granzyme B eFluor450 (NGZB) were obtained from Thermo Fisher Scientific. CD80-PE CF594 (16–10A1), CD45-BV786 (30-F11) and Ki-67 FITC (B56) were purchased from BD Biosciences. PE-conjugated Kb - SIYRYYGL pentamers (#F1803–2B) were purchased from Proimmune. After surface staining, cells were washed in FACS buffer and fixed for 20 min on ice with Fixation/Permeabilization Buffer from BD Biosciences (#554722). For intracellular and intranuclear cytokine analysis, single cell suspensions from tumors were incubated in complete RPMI +/− 50μg/mL poly I:C and 10 μg/mL GolgiPlug from BD Biosciences (#555029) at 37°C for 6 hrs. Cells were then stained as described above, except fixation and permeabilization was performed using the Foxp3/Transcription Factor Staining Buffer Set from Thermo Fisher Scientific (#00–5523-00) and then cells were incubated with intracellular antibodies for 30 min on ice. All samples were resuspended in FACS buffer and acquired on a BD Fortessa flow cytometer. Data were analyzed using FlowJo software from Tree Star, v10.5. cDC1 were gated as leukocytes/single cells/Live/CD45+/CD90.2−CD19−/Ly-6C−/MHC-II+/CD24+F4–80−/CD11b−/CD103+. CD8+ T cells were gated as single cells/Live/CD45+/CD90.2+ CD19−/CD8+CD4−.
Bone marrow chimeras
Bone marrow chimeras were generated using B6.SJL (CD45.1+) recipient mice that were irradiated with 1000 rads. Bone marrow cells were isolated from WT C57BL/6 (CD45.2+) or Zbtb46-DTR (CD45.2+) donor mice femurs and tibias using a 27G needle. Cells were filtered through a 70 μm cell strainer to generate a single cell suspension and resuspended in PBS. Recipient mice received 3–5 × 106 donor bone marrow cells by retro-orbital injection. Tumors were implanted 8 weeks following bone marrow reconstitution. Diphtheria toxin from Millipore-Sigma (#D0564) was administered 3 days prior to radiation at 20 ng/g intraperitoneally for initial DC depletion. This was followed by an additional 3 doses of 5 ng/g of diphtheria toxin that were given every 3 days to maintain depletion.
Cytokine Luminex assay
Tumors were harvested on ice, weighed and homogenized in PBS containing 4.5 μl HALT Protease Inhibitor Cocktail from Thermo Fisher Scientific (#78440) per mg tissue. The cell debris was removed by centrifugation at 14,000g for 15 minutes at 4°C, and supernatants were stored in aliquots at −80°C until analyzed. Cytokines and chemokines were detected using 25 μl of supernatant and the Cytokine & Chemokine 26-Plex Mouse ProcartaPlex Panel 1 kit from Life Technologies (#EPX260–26088-901). Data was acquired on a Luminex 100 array reader and cytokine/chemokine concentrations for each tumor sample was calculated using standard curves for each analyte.
Statistics
Data were analyzed and graphed using Prism from GraphPad Software (v7.0). Individual data sets were compared using Student’s T-test and analysis across multiple groups was performed using ANOVA with individual groups assessed using Tukey’s comparison. Kaplan Meier survival curves were compared using a log-rank test.
RESULTS
In radio-immunogenic tumors CD8+ T cells control the response to radiation independent of tumor cell intrinsic radiosensitivity.
First, we set out to identify murine tumor models with equivalent radiosensitivity in vitro, but differing responsiveness to the same dose of radiation in vivo. We compared the radiosensitivity of the murine colon tumor cell line, MC38 and the pancreatic tumor cell line, Panc02-SIY. In vitro, both tumor cell lines had comparable sensitivity to a range of radiation doses (Fig 1A), These cell lines were then used to establish syngeneic flank tumors in mice and further evaluate their response to radiation in vivo. When tumors reached an average diameter of 5 mm, they were treated with CT-guided radiation to prevent indirect targeting of the tumor dLN (Fig 1B i–ii). Both tumors types showed delayed tumor growth kinetics in response to radiation, as compared to untreated controls. Despite displaying equivalent radiosensitivity to Panc02-SIY in vitro, MC38 tumors exhibited considerable tumor regression and, in some instances, tumor cures (Fig 1C i). We also tested the head and neck tumor cell lines Moc1 and Moc2, which had comparable radiosensitivity in vitro, but differing responsiveness in vivo (Fig S1A–B). Taken together these data indicate that tumor cell intrinsic radiosensitivity is not the limiting factor controlling the response to radiation in vivo in these tumor models. To determine if the improved tumor control in MC38 tumors following radiation was dependent on the adaptive immune response, we depleted CD8+ T cells prior to treatment and found that CD8+ T cell depletion significantly abrogated the enhanced survival benefit of radiation in MC38 tumors, but had no impact on Panc02-SIY (Fig 1C ii, Fig S1B). We observed similar results in Moc1 tumors which required CD8+ T cells for their enhanced response to radiation, whereas Moc2 tumors did not require CD8+ T cells (Fig S1B). Given that MC38 and Moc1 tumors exhibited a CD8+ T cell-dependent survival advantage in response to radiotherapy, we will refer to them as “radio-immunogenic” tumors from this point forward, while Panc02-SIY and Moc2 will be referred to as a “poorly radio-immunogenic” tumors, in the context of radiation.
Figure 1: Radio-immunogenic tumors require CD8+ T cells for enhanced response to radiation.
(A) MC38 or Panc02-SIY (P2SIY) tumors were treated in vitro with indicated dose of radiation, cultured for 5d and the number of surviving colonies was quantified. The colony number was then normalized to untreated control (0Gy) for each tumor type. Data represent the mean ± SD from 3 independent experiments. (B) i) MC38 or P2SIY tumors were established and allowed to grow to ~5mm average diameter before being treated with 12Gy of CT-guided radiation therapy (RT). ii) Representative CT image with targeting of tumor (large dotted line) within field of radiation (solid white box) to avoid indirect targeting of the tumor dLN (TdLN) (small dotted line). (C) i) MC38 and P2SIY tumor growth curves for tumors that were untreated (NT), ii) treated with 12Gy focal radiation (RT), or iii) treated with αCD8β depleting antibodies one day prior 12Gy focal RT. iv) Overall survival. n = 5 animals per treatment group. Results shown are representative of two independent experiments. *p < 0.05. **p < 0.01.
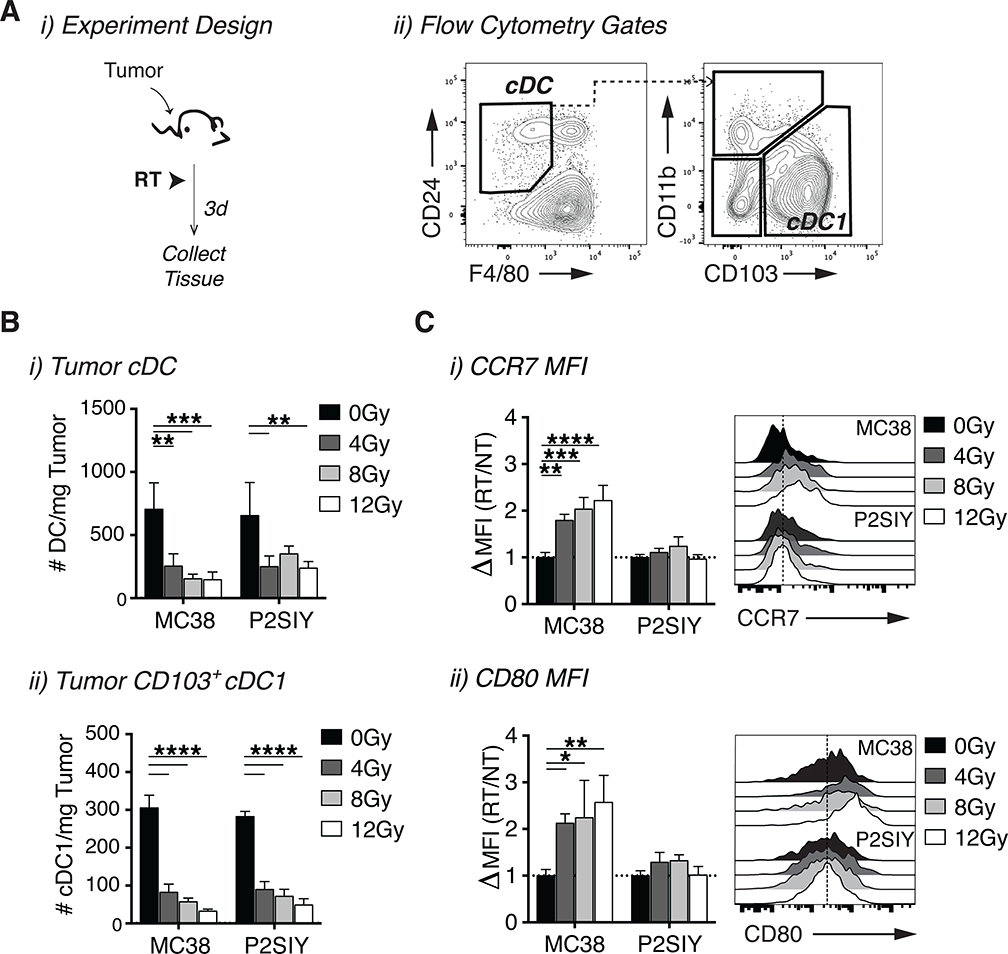
Radiation induces cDC1 maturation in radio-immunogenic tumors but not poorly radio-immunogenic tumors.
In radio-immunogenic MC38 tumors, improved tumor control following radiation therapy required CD8+ T cells, suggesting a potential failure to generate an effective anti-tumor CD8+ T cell response in poorly radio-immunogenic Panc02-SIY tumors. Since cDC1 are known to play an important role in cross-priming CD8+ T cell responses, this led us to evaluate whether cDC1 were being activated equivalently in both tumor models following radiation [15]. We used flow cytometry to assess changes in both the quantity and maturation state of DC subsets within the tumor after treatment with a range of radiation doses (Fig 2A, Fig S2A). There was a significant reduction in total DCs, particularly within the CD103+ cDC1 compartment following radiation in both tumor models (Fig 2B i–ii). Interestingly, the remaining intratumoral cDC1s in MC38 tumors expressed higher levels of markers associated with DC maturation, including CCR7, which is important for migration to the dLN (Fig 2C i) and the co-stimulatory molecule CD80 (Fig 2C i–ii) [32]. Moreover, expression of these activation markers increased in a dose dependent manner with higher doses of radiation (Fig 2C i–ii). Similarly, there was a trend towards increased intratumoral cDC1 activation following 12Gy of radiation in the radio-immunogenic Moc1 tumors, but not in the poorly radio-immunogenic Moc2 tumors (Fig S1C i–iii). To determine whether increased accumulations of intratumoral cDC1s could improve the efficacy of radiation in poorly radio-immunogenic Panc02-SIY tumors, we administered the cytokine Fms-like tyrosine kinase 3 ligand (FLT3L) in combination with radiation (Fig S3A) [33]. Treatment with FLT3L significantly increased the accumulation of intratumoral cDC1s, but DC maturation was still impaired (Fig S3B i–ii), and treatment had no impact on animal survival following radiation (Fig S3C i–ii). Thus, while radiation is clearly capable generating signals to promote cDC1 maturation in particular tumor types, these signals are either lacking or actively suppressed in poorly radio-immunogenic tumors, leading to impaired tumor control after radiation. Importantly, these results provide one potential explanation for why equivalent doses of radiation are capable of inducing varying degrees of tumor regression across different tumor types.
Figure 2: Radio-immunogenic tumors successfully activate intratumoral cDC1s following radiation.
(A) i) Experiment setup for B-C and ii) flow cytometry gating strategy for cDCs and cDC1s from Live CD45+ CD90.2− CD19− Ly-6C− MHC-II+. When tumors reached an average diameter of 5mm they were treated +/− RT and tumor infiltrating immune cells were phenotyped three days following treatment. (B) i) The number of cDCs and ii) CD103+ cDC1s per mg of tumor tissue in MC38 and P2SIY tumors treated with 0Gy, 4Gy, 8Gy or 12Gy of radiation. (C) The average expression (MFI) of i) CCR7 and ii) CD80 on intratumoral CD103+ cDC1s for each radiation dose was divided by the average MFI for 0Gy samples in each tumor type to calculate the fold increase in expression following treatment with radiation. n = 5 animals/group. Data represent the mean ± SD of each group. Results shown are representative of two independent experiments. *p < 0.05. **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Adjuvants that target cDC1s overcome the failure of radiation to induce intratumoral cDC1 maturation in poorly radio-immunogenic tumors, resulting in tumor cures.
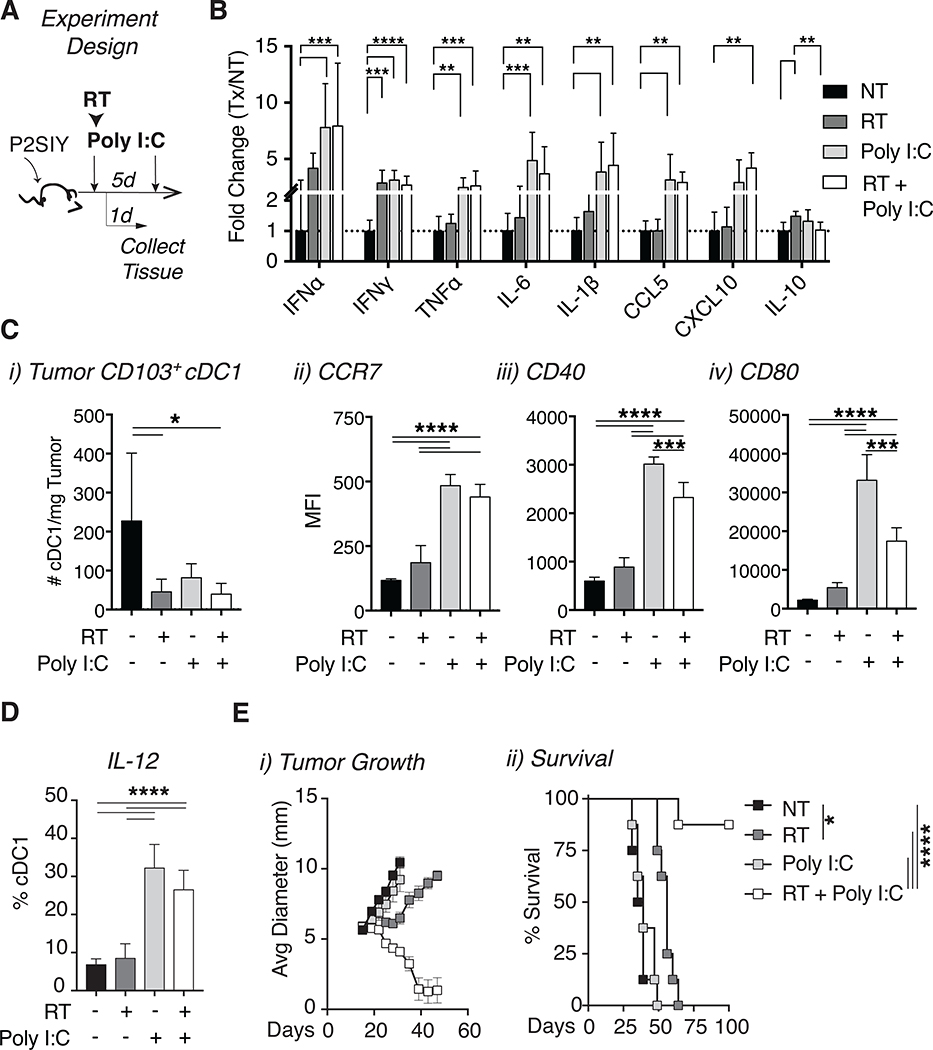
Our results thus far had suggested that radiation alone is unable to drive cDC1 activation in poorly radio-immunogenic tumors and this failure may limit the extent of tumor control following radiation. We hypothesized that externally driving DC maturation by administration of adjuvants directly to the tumor would restore T cell mediated tumor control. To identify an optimal adjuvant, we examined toll-like receptor (TLR) expression on DCs and found TLR3 expression to be highly enriched on cross-presenting cDC1s (Fig S2B–D). Importantly, signaling through this innate receptor has been shown to induce cDC1 maturation [34, 35]. Previous work has demonstrated improved tumor control in murine models when radiation is combined with poly I:C, suggesting that this agent may restore cDC1 function in tumors [31, 36, 37]. We administered intratumoral poly I:C concurrently with radiation and then again 5 days later and assessed tumors for cytokine responses and DC maturation (Fig 3A). Analysis of cytokines in tumors revealed increased levels of type I interferon (IFN⍺), pro-inflammatory cytokines (TNF⍺, IL-6, IL-1β) and chemokines known to recruit T cells (CCL5, CXCL10) in both single agent poly I:C or the combination of radiation and poly I:C treated tumors (Fig 3B). Thus, treatment with poly I:C transforms the milieu within the tumor into an environment that is more favorable for the development of anti-tumor immunity in the context of radiation therapy.
Figure 3: The adjuvant poly I:C induces intratumoral cDC1 activation resulting in tumor cures when combined with radiation.
(A) Experiment setup for B-D. P2SIY tumor bearing animals were treated with 12Gy of RT and 50ug of intratumoral poly I:C on day 15, followed by a second dose of intratumoral poly I:C on day 20. Tumors were harvested and analyzed on day 16. (B) Tumors were homogenized, and cytokines were quantified using a multiplex Luminex assay. (C) i) The number of CD103+ cDC1s per mg of tumor tissue was quantified. Intratumoral CD103+ cDC1 expression of ii) CCR7 MFI, iii) CD40 MFI and iv) CD80 MFI. (D) Treated tumors were harvested one day following treatment in vivo, processed into a single cell suspension and cultured with brefeldin A +/− poly I:C in vitro for 6 hours before intracellular cytokine staining. The percentage of CD103+ cDC1 expressing IL-12 was quantified using FACS. (E) i) Tumor growth curves and ii) animal survival following treatment with radiation and poly I:C. n = 5–8 animals/group. Data represent the mean ± SD of each group. Data are representative of 2–3 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Earlier data indicated that radiation effectively induced cDC1 maturation only in radio-immunogenic tumors (MC38, Moc1) and this process did not occur in poorly radio-immunogenic tumors (Panc02-SIY, Moc2). To address whether poly I:C was able to induce cDC1 maturation in poorly radio-immunogenic Panc02-SIY tumors, we used flow cytometry to monitor changes in the quantity and activation state of cDC1s within the tumor. Our analysis revealed that all treatment groups had fewer intratumoral cDC1s as compared to untreated controls one day following treatment (Fig 3C i). However, of the cDC1s that remained in the tumor, we noted increased expression of markers associated with DC maturation and migration (CCR7, CD40, CD80) when poly I:C was given alone or in combination with radiation (Fig 3C ii–iv). Treatment with poly I:C significantly increased production of IL-12 specifically in intratumoral cDC1s (Fig 3D), a cytokine associated with enhanced DC priming [35]. Interestingly, while single agent poly I:C induced changes in cDC1 maturation and generated a favorable cytokine environment within tumors, it failed to impact tumor growth, whereas the combination of radiation and poly I:C resulted in tumor regression (Fig 3E i). Unlike earlier studies, our dosing regimen also resulted in durable tumor cures (Fig 3E ii) [31]. These data demonstrate in tumor models where cDC1 maturation is impaired either due to active suppression or a failure for radiotherapy to release sufficient signals, we can overcome this deficit by administering exogenous adjuvants to promote cDC1 maturation following radiation therapy and this leads to durable tumor cures. Importantly, these results suggest that adjuvant signal in the form of poly I:C alone is insufficient to induce tumor cures.
cDCs are required for combined efficacy of radiation and poly I:C.
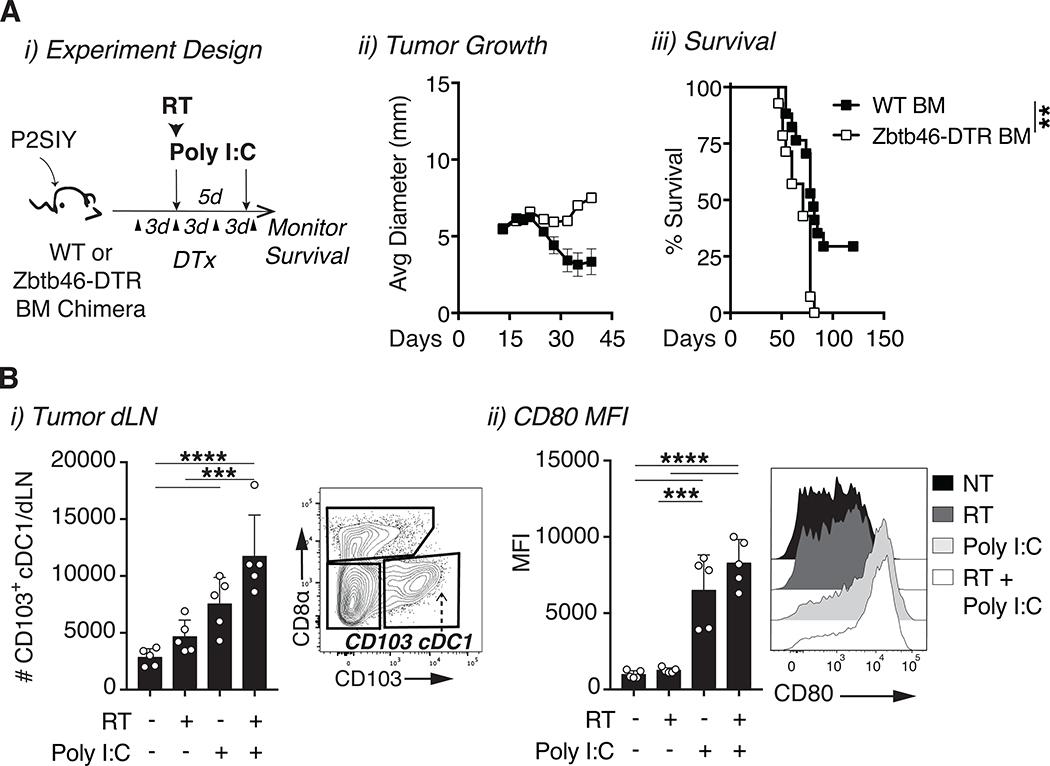
Since macrophages in the tumor express some TLR3 (Fig S2C), and tumor associated macrophages can impact tumor control following radiation therapy [38], we evaluated the importance of tumor macrophages to the treatment response. We found that macrophage depletion using anti-CSF1 did not significantly impact tumor control by the combination of radiation therapy and poly I:C (Fig S4A i–ii), suggesting that cDC1s may be the critical target for TLR3 ligands. Although cDC1s were successfully activated by poly I:C when combined with radiation, the question remained whether these cells were required for treatment efficacy. One widely used approach to deplete cDC1 in murine models are Batf3−/− mice; however, these mice lack DC through all stages of tumor development, which changes the baseline tumor immune environment prior to treatment initiation [15]. To isolate the effect of treatment on DC populations, we required an approach to selectively deplete cDCs at the time of treatment. Zbtb46-DTR mice express the diphtheria toxin receptor selectively in cDCs and permits their depletion at any time point by administration of diphtheria toxin [39]. To deplete cDCs, we established Panc02-SIY tumors in Zbtb46-DTR bone or wild-type (WT) C57BL/6J marrow chimeras and treated them with diphtheria toxin three days prior to treatment with radiation and poly I:C (Fig 4A i). Treatment with diphtheria toxin resulted in a loss of cross-presenting DCs in both the tumor (Fig S4B) and in the tumor dLN (Fig S4C i–ii) of Zbtb46-DTR bone marrow chimeras, but not in WT control bone marrow chimeras. Depletion of cDCs immediately prior to radiation significantly impaired tumor control and abrogated the enhanced survival benefit of radiation and poly I:C when compared to control WT bone marrow chimeras treated with diphtheria toxin (Fig 4A ii–iii). Notably, in bone marrow chimeras given the combination of poly I:C and radiation therapy without DC depletion the overall efficacy of treatment was consistently reduced compared to that observed in WT mice (Fig 3), suggesting some general loss of immune function through development of bone marrow chimeras. While cDC1s were clearly important for the efficacy of combination therapy, the mechanism by which they promoted tumor regression remained unclear. To determine whether DC migration was important for therapy we first quantified the total number of migratory CD103+ cDC1s in the tumor dLN following treatment. The data revealed more migratory CD103+ cDC1s with an activated phenotype (CD80) in the dLNs of combination treated animals as compared to untreated or single agent controls (Fig 4B i–ii), suggesting increased migration following treatment. These data demonstrate that cDC1s play important role in the anti-tumor efficacy of radiation and poly I:C.
Figure 4: The efficacy of radiation and poly I:C is dependent on cDCs.
(A) i) Bone marrow (BM) chimeras were generated by transferring wild-type (WT) C57BL/6 or Zbtb46-DTR donor bone marrow into lethally irradiated B6.SJL hosts. P2SIY tumors were established ~8 weeks following bone marrow reconstitution. Each chimeric group was treated with diphtheria toxin (DTx) starting 3 days prior to 12Gy radiation and poly I:C. n = 12–16 animals/group ii) Tumor growth and iii) animal survival following cDC depletion with diphtheria toxin. (B) i) The tumor dLN was harvested one day following radiation and poly I:C to quantify the number of migratory CD103+ cDC1s and ii) CD80 MFI on migratory CD103+ cDC1s. n = 5–8 animals/group. Data represent the mean ± SD of each group. Data is representative of two independent experiments. **p < 0.01, ***p < 0.001, ****p < 0.0001.
Adjuvant combined with radiation therapy promotes the development of effector CD8+ T cells and requires T cell trafficking from the LN.
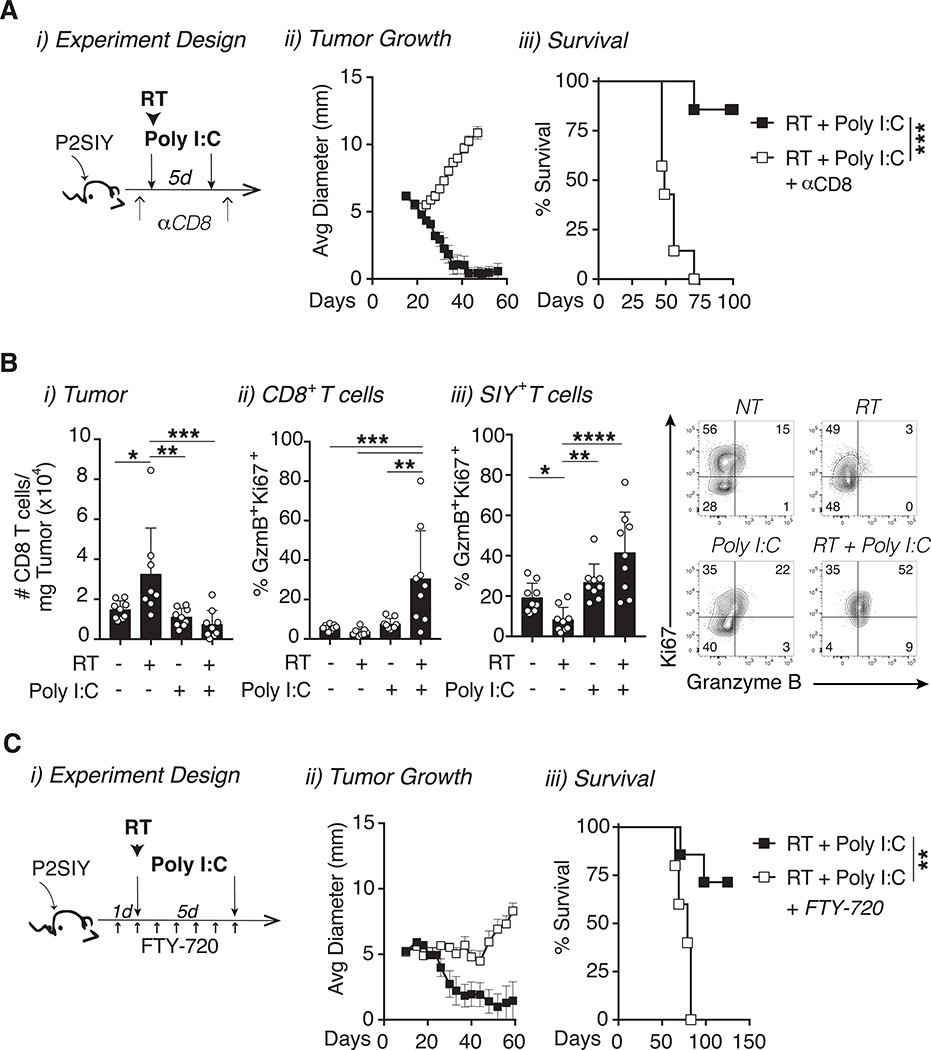
Our data thus far suggested that CD103+ cDC1 migration to the dLN is increased following combination therapy. While antigen recognition serves as signal 1 for T cell priming, DCs are known to provide additional signals in the form of co-stimulation (signal 2) and cytokines (signal 3) that further promote the expansion and quality of antigen specific T cells [40]. This led us to first evaluate whether CD8+ T cells were required for the combined efficacy of radiation and poly I:C by depleting CD8+ T cells (Fig 5A i, Fig S4D). Depletion of CD8+ T cells completely abolished the efficacy of treatment, indicating that these cells were indeed important for treatment (Fig 5A ii–iii). Next we used flow cytometry to assess the phenotype of CD8+ T cells in the tumor 7 days after treatment. While radiation alone increased the number of CD8+ T cells in tumors compared to all other treatment groups, this was not the case in the combination of treated animals (Fig 5B i). Instead the combination of radiation and poly I:C significantly expanded the proportion of proliferating (Ki67+) CD8+ T cells in the tumor with enhanced cytotoxic potential as identified by the protease granzyme B (Fig 5B ii). This pattern was also observed in antigen specific 2C CD8+ T cells which recognize the SIYRYYGL (SIY) peptide expressed by Panc02-SIY tumor cells (Fig 5B iii). Moreover, we observed a similar increase in CD8+ Ki67+ Granzyme B+ cells following radiation alone in radio-immunogenic MC38 tumors (Fig S4E). These data suggest that following radiation and poly I:C, cDC1s prime CD8+ T cells that have improved cytolytic potential as compared to controls. The question then remained whether these T cells were being activated within the tumor or were instead being primed by cDC1 within the dLN. To address this question, we used S1P receptor agonist FTY720 to sequester T cells in the LN, thereby preventing their migration to the tumor following priming in the dLN (Fig 5C i, Fig S4D) [41]. When T cell egress from the LNs was impaired with FTY720, the combined efficacy of radiation and poly I:C was completely abrogated (Fig 5C ii–iii). Taken together, these results demonstrate that tumor regression following treatment radiation and poly I:C is dependent on cDC1s which play an important role in generating tumor reactive effector CD8+ T cells within the tumor dLN, and these T cells must be free to migrate through the circulation to the treatment site to result in tumor cure.
Figure 5: Combination therapy increases the recruitment of effector CD8+ T cells to the tumor.
(A) i) P2SIY tumor bearing mice were treated with CD8 depleting antibodies one day prior to treatment with 12Gy radiation and intratumoral poly I:C. ii) Tumor growth and iii) animal survival were monitored following treatment. (B) i) Tumors were harvested 7 days following treatment with radiation plus poly I:C and CD8+ T cells were gated as Live CD45+ CD19− CD90.2+ CD4− CD8+. The number of intratumoral CD8+ T cells per mg of tumor tissue were quantified. ii) The expression of Ki67 and Granzyme B was assessed on all intratumoral CD8+ T cells and iii) within tumor antigen SIY+ CD8 T cells within P2SIY tumors. (C) i) P2SIY tumor bearing mice were treated daily with intraperitoneal FTY720 injections starting one day before treatment with radiation and poly I:C. ii) Tumor growth and iii) animal survival following radiation and poly I:C with FTY720 treatment. n = 4–7 animals/group. Data represent the mean ± SD of each group. Data represent two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
DISCUSSION
The treatment response to radiation is highly variable across different cancer pathologies. While radiation is capable of directly killing tumor cells, this is not the sole mechanism responsible for tumor shrinkage following treatment [7]. Our studies confirm that tumor cell intrinsic radiosensitivity in vitro is a poor predictor for the overall response to radiation in vivo and instead implicates other mechanisms. Given that radiation has been shown to elicit tumor-specific adaptive immune responses, we investigated immune-related mechanisms that might explain this variable response across cancer pathologies [8, 42]. Our findings demonstrate that when a range of tumor types were treated with equivalent doses of radiation in vivo, improved treatment responses were dependent on the presence of CD8+ T cells only in radio-immunogenic tumors (MC38, Moc1), and independent of tumor cell intrinsic radiosensitivity. These data highlight the importance of generating a productive tumor-specific adaptive immune response following radiation and provide useful insight into the potential immune-related mechanisms that explain the differential response to radiation across different cancers.
cDC1s are a critical cross-presenting cell type capable of linking the innate and adaptive immune system [15]. We discovered that intratumoral cDC1 activation following radiation is not uniform across different tumor types. Instead, radiation induces cDC1 maturation only in particular tumor types (MC38, Moc1) that corresponds with the tumor types reliant on CD8+ T cells for an improved response to radiation. These data suggest that cDC1 maturation fails to occur in poorly radio-immunogenic tumors either due to active suppression or the absence of adequate signals following radiation therapy. Ultimately, this failure results in impaired generation of tumor specific CD8+ T cell responses and limits the extent of tumor control following radiation. While we did see a modest increase in the DC-suppressive cytokine IL-10 following radiation in the poorly radio-immunogenic Panc02-SIY tumors [43], each tumor type may have its own unique pathways or cell types potentially responsible for DC suppression following radiation. These could include other cytokines or metabolites such as PGE2 or IDO that are increased following radiation and function to suppress intratumoral cDC1 activation [28]. Additional studies are needed to identify the specific factors and signaling pathways within various tumors that prevent cDC1 maturation after treatment in order to improve responses to radiation.
Previous studies have demonstrated that bone marrow-derived DC injected into irradiated tumors can take up antigens and cross-present in the draining lymph nodes, but have a limited ability to recruit activated T cells back to the irradiated site [44]. Similarly, Jahns et al demonstrated that radiation of monocyte-derived DC in vitro did not directly cause DC maturation, but also did not prevent their maturation following exposure to appropriate stimuli [45]. One approach to overcome the failure of radiation to induce intratumoral cDC1 activation is to provide exogenous adjuvants that drive DC maturation. In this study we used the adjuvant poly I:C to target the innate receptor, TLR3, which is highly expressed by cDC1s [35]. Yoshida et al. previously demonstrated that poly-I:C in combination with radiation improved tumor control, resulting in DC activation in the tumor-draining lymph node [31]. We similarly demonstrate that concurrent administration of poly I:C and radiation with a second dose of poly I:C given 5 days later successfully drives intratumoral cDC1 maturation in poorly radio-immunogenic Panc02-SIY tumors. Importantly this treatment combination leads to durable tumor cures that are dependent on cDCs. The prior reports have suggested that when poly I:C is given one day prior to radiation can temporarily delay tumor growth, but treatment ultimately fails to cure tumors [31]. Timing adjuvant delivery with radiation-mediated tumor cell death is likely critical in coordinating the release of tumor associated antigens with the adjuvant signals that function to promote DC maturation.
Our data suggest that while radiation alone is capable of generating signals that promote cDC1 maturation in radio-immunogenic tumors, these signals are either absent or suppressed in poorly radio-immunogenic tumors. We have previously demonstrated that macrophages suppress T cell control of tumors following radiation therapy [11, 46], and others have shown they can secrete factors such as IL-10 that suppress DC maturation in tumors [43]. In addition, other cell populations present in the tumor environment can alter patterns of DC maturation following radiation therapy [47], suggesting that the immune milieu may regulate the ability of DCs to mature. In poorly radio-immunogenic tumors a bolus of innate adjuvant was sufficient to provide the missing signal or overcome suppressive mechanisms. In our studies in poorly radio-immunogenic tumors we provided this signal in the form of poly I:C which was selected based on the enriched expression of its receptor TLR3 in cDC1s, but other innate adjuvants that activate DC maturation have also shown synergy with radiation therapy [48–50]. While we see no evidence of other cells contributing to cDC1 maturation following TLR3 ligation, this possibility has not been excluded. While TLR3 is expressed by cDC1 and necessary for their activation by poly I:C, cDC1 maturation to full antigen presenting and processing capacity following TLR3 ligation is dependent on their production and response to type I IFN [34, 35, 51]. Thus, TLR3 ligation likely causes additional positive pro-inflammatory effects in the tumor environment secondary to TLR3 ligation in DC. Together, these data indicate that the presence of immunological adjuvant in the tumor and the capability of DCs to respond to these released adjuvants are critical determinants for the success of radiation therapy.
A long-standing question within the field of radiation therapy is whether treatment can lead to the development of new tumor reactive CD8+ T cell responses and essentially function as an endogenous cancer vaccine. Here we provide evidence that radiation fails to drive intratumoral cDC1 maturation in poorly radio-immunogenic tumors, one of the first steps in developing a productive anti-tumor CD8+ T cell response. However, by combining radiation with poly I:C, we overcome this barrier and demonstrate that when T cells have been sequestered in the LNs during treatment tumors fail to cure. DC maturation through signals such as TLR3 ligation results in a decreased phagocytosis and a shift to a migratory and antigen presentation phenotype via expression of markers such as CCR7 and CD80, respectively [52, 53]. Our data suggests that in poorly radio-immunogenic tumors DC are actively phagocytosing material from irradiated cancer cells, but fail to receive the signals that allow them to mature. In radio-immunogenic tumors, or in poorly radio-immunogenic tumors given adjuvants, these cells complete their cycle and travel to the dLN to prime T cells [52, 53]. These data suggest that in these circumstances that combination therapy is generating new CD8+ T cells responses within the dLN and indicate that under optimal conditions radiation therapy can function as an endogenous cancer vaccine. Importantly, this work also demonstrates the importance of selecting diverse tumor models to evaluate treatments. The non-responsive tumors may provide the greatest source of information to understand how treatments succeed, and critically guide novel interventions to help patient populations who currently do not respond to treatment.
In patients, CD8+ T cell infiltration within tumors tends to correlate with improved outcomes across a range of malignancies [54–56]. Even in the absence of radiation, recent studies have demonstrated that the presence of DCs within tumors is highly impactful to the success of other therapies [25, 57]. We propose that patients with a poor immune environment are similar to our poorly responsive murine models, whereby radiation therapy fails to drive DC maturation either due to absence of adjuvant signals or by active suppression within the tumor microenvironment. In these patients, radiation would be unable to generate high quality tumor reactive T cell responses despite the release of tumor antigens that have the potential to be recognized by the immune system. Thus, these unresponsive patients may benefit from the addition of adjuvants that enable radiation therapy to fully function as an endogenous cancer vaccine by driving cDC1 maturation and effective cross-presentation of tumor antigens to CD8+ T cells. We believe that by combining radiation therapy with adjuvants that target these deficiencies, we can restart the cycle of immunity and convert otherwise dismal radiation responses into more favorable outcomes.
Supplementary Material
KEY POINTS.
Radiation fails to promote cDC1 maturation in poorly radio-immunogenic tumors.
Impaired cDC1 activation following radiation limits the response to treatment.
Adjuvants that drive cDC1 maturation improve tumor responses to radiation.
Acknowledgments
Supported in part by research grants from:
NIH R01CA182311, R01 CA244142, NIH R01CA208644.
REFERENCES
- 1.Delaney G, Jacob S, Featherstone C, and Barton M, The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer, 2005. 104(6): p. 1129–37. [DOI] [PubMed] [Google Scholar]
- 2.Begg AC, Stewart FA, and Vens C, Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer, 2011. 11(4): p. 239–53. [DOI] [PubMed] [Google Scholar]
- 3.Bernier J, Hall EJ, and Giaccia A, Radiation oncology: a century of achievements. Nature Reviews Cancer, 2004. 4(9): p. 737–747. [DOI] [PubMed] [Google Scholar]
- 4.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, and Lord EM, Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol, 2008. 180(5): p. 3132–9. [DOI] [PubMed] [Google Scholar]
- 5.Gough MJ, Crittenden MR, Sarff M, Pang P, Seung SK, Vetto JT, Hu HM, Redmond WL, Holland J, and Weinberg AD, Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother, 2010. 33(8): p. 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, and Fu YX, Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood, 2009. 114(3): p. 589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golden EB and Apetoh L, Radiotherapy and immunogenic cell death. Semin Radiat Oncol, 2015. 25(1): p. 11–7. [DOI] [PubMed] [Google Scholar]
- 8.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, and Neefjes JJ, Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med, 2006. 203(5): p. 1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, Tesniere A, Martins I, Ly A, Haynes NM, Smyth MJ, Kroemer G, and Zitvogel L, Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol, 2010. 22(3): p. 113–24. [DOI] [PubMed] [Google Scholar]
- 10.Crittenden MR, Savage T, Cottam B, Baird J, Rodriguez PC, Newell P, Young K, Jackson AM, and Gough MJ, Expression of arginase I in myeloid cells limits control of residual disease after radiation therapy of tumors in mice. Radiat Res, 2014. 182(2): p. 182–90. [DOI] [PubMed] [Google Scholar]
- 11.Crittenden MR, Cottam B, Savage T, Nguyen C, Newell P, and Gough MJ, Expression of NF-kappaB p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS One, 2012. 7(6): p. e39295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, and Wu L, CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res, 2013. 73(9): p. 2782–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, and Brown JM, Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A, 2010. 107(18): p. 8363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, and Coussens LM, TH2-Polarized CD4(+) T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol Res, 2015. 3(5): p. 518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, and Murphy KM, Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science, 2008. 322(5904): p. 1097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel PM, Gurka S, and Kroczek RA, Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med, 2010. 207(6): p. 1273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, Vulink AJ, Hart DN, and Radford KJ, Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med, 2010. 207(6): p. 1247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anandasabapathy N, Feder R, Mollah S, Tse SW, Longhi MP, Mehandru S, Matos I, Cheong C, Ruane D, Brane L, Teixeira A, Dobrin J, Mizenina O, Park CG, Meredith M, Clausen BE, Nussenzweig MC, and Steinman RM, Classical Flt3L-dependent dendritic cells control immunity to protein vaccine. J Exp Med, 2014. 211(9): p. 1875–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M, Gautier EL, Jakubzick C, Randolph GJ, Best AJ, Knell J, Goldrath A, Miller J, Brown B, Merad M, Jojic V, Koller D, Cohen N, Brennan P, Brenner M, Shay T, Regev A, Fletcher A, Elpek K, Bellemare-Pelletier A, Malhotra D, Turley S, Jianu R, Laidlaw D, Collins J, Narayan K, Sylvia K, Kang J, Gazit R, Rossi DJ, Kim F, Rao TN, Wagers A, Shinton SA, Hardy RR, Monach P, Bezman NA, Sun JC, Kim CC, Lanier LL, Heng T, Kreslavsky T, Painter M, Ericson J, Davis S, Mathis D, and Benoist C, Deciphering the transcriptional network of the dendritic cell lineage. Nature Immunology, 2012. 13(9): p. 888–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, and Murphy KM, Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med, 2010. 207(4): p. 823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitano M, Yamazaki C, Takumi A, Ikeno T, Hemmi H, Takahashi N, Shimizu K, Fraser SE, Hoshino K, Kaisho T, and Okada T, Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc Natl Acad Sci U S A, 2016. 113(4): p. 1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, and Krummel MF, Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell, 2016. 30(2): p. 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S, Van’t Veer LJ, Sperling AI, Wolf DM, and Krummel MF, Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell, 2014. 26(5): p. 638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, Chakarov S, Rivera C, Hogstad B, Bosenberg M, Hashimoto D, Gnjatic S, Bhardwaj N, Palucka AK, Brown BD, Brody J, Ginhoux F, and Merad M, Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity, 2016. 44(4): p. 924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spranger S, Dai D, Horton B, and Gajewski TF, Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell, 2017. 31(5): p. 711–723 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, and Gajewski TF, Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med, 2011. 208(10): p. 2005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michea P, Noel F, Zakine E, Czerwinska U, Sirven P, Abouzid O, Goudot C, Scholer-Dahirel A, Vincent-Salomon A, Reyal F, Amigorena S, Guillot-Delost M, Segura E, and Soumelis V, Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat Immunol, 2018. 19(8): p. 885–897. [DOI] [PubMed] [Google Scholar]
- 28.Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, and Reis e Sousa C, NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell, 2018. 172(5): p. 1022–1037 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S, Shizuru JA, Negrin RN, Engleman EG, and Strober S, Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res, 2015. 21(16): p. 3727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, and Demaria S, DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun, 2017. 8: p. 15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida S, Shime H, Takeda Y, Nam JM, Takashima K, Matsumoto M, Shirato H, Kasahara M, and Seya T, Toll-like receptor 3 signal augments radiation-induced tumor growth retardation in a murine model. Cancer Sci, 2018. 109(4): p. 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, and Forster R, CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity, 2004. 21(2): p. 279–88. [DOI] [PubMed] [Google Scholar]
- 33.Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, and Liu K, Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med, 2011. 208(8): p. 1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varthaman A, Moreau HD, Maurin M, and Benaroch P, TLR3-Induced Maturation of Murine Dendritic Cells Regulates CTL Responses by Modulating PD-L1 Trafficking. PLoS One, 2016. 11(12): p. e0167057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jelinek I, Leonard JN, Price GE, Brown KN, Meyer-Manlapat A, Goldsmith PK, Wang Y, Venzon D, Epstein SL, and Segal DM, TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J Immunol, 2011. 186(4): p. 2422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le UM, Kaurin DG, Sloat BR, Yanasarn N, and Cui Z, Localized irradiation of tumors prior to synthetic dsRNA therapy enhanced the resultant anti-tumor activity. Radiother Oncol, 2009. 90(2): p. 273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hammerich L, Marron TU, Upadhyay R, Svensson-Arvelund J, Dhainaut M, Hussein S, Zhan Y, Ostrowski D, Yellin M, Marsh H, Salazar AM, Rahman AH, Brown BD, Merad M, and Brody JD, Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat Med, 2019. 25(5): p. 814–824. [DOI] [PubMed] [Google Scholar]
- 38.Gough MJ, Young K, and Crittenden M, The impact of the myeloid response to radiation therapy. Clin Dev Immunol, 2013. 2013: p. 281958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, and Nussenzweig MC, Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med, 2012. 209(6): p. 1153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, and Schoenberger SP, Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol, 2003. 4(4): p. 361–5. [DOI] [PubMed] [Google Scholar]
- 41.Mandala S, Alteration of Lymphocyte Trafficking by Sphingosine-1-Phosphate Receptor Agonists. Science, 2002. 296(5566): p. 346–349. [DOI] [PubMed] [Google Scholar]
- 42.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, and Minn AJ, Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature, 2015. 520(7547): p. 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, and Coussens LM, Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell, 2014. 26(5): p. 623–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teitz-Tennenbaum S, Li Q, Okuyama R, Davis MA, Sun R, Whitfield J, Knibbs RN, Stoolman LM, and Chang AE, Mechanisms Involved in Radiation Enhancement of Intratumoral Dendritic Cell Therapy. Journal of Immunotherapy, 2008. 31(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jahns J, Anderegg U, Saalbach A, Rosin B, Patties I, Glasow A, Kamprad M, Scholz M, and Hildebrandt G, Influence of low dose irradiation on differentiation, maturation and T-cell activation of human dendritic cells. Mutat Res, 2011. 709–710: p. 32–9. [DOI] [PubMed] [Google Scholar]
- 46.Crittenden MR, Baird J, Friedman D, Savage T, Uhde L, Alice A, Cottam B, Young K, Newell P, Nguyen C, Bambina S, Kramer G, Akporiaye E, Malecka A, Jackson A, and Gough MJ, Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget, 2016. 7(48). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malecka A, Wang Q, Shah S, Sutavani RV, Spendlove I, Ramage JM, Greensmith J, Franks HA, Gough MJ, Saalbach A, Patel PM, and Jackson AM, Stromal fibroblasts support dendritic cells to maintain IL-23/Th17 responses after exposure to ionizing radiation. J Leukoc Biol, 2016. 100(2): p. 381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baird JR, Friedman D, Cottam B, Dubensky TW Jr., Kanne DB, Bambina S, Bahjat K, Crittenden MR, and Gough MJ, Radiotherapy Combined with Novel STING-Targeting Oligonucleotides Results in Regression of Established Tumors. Cancer Res, 2016. 76(1): p. 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medler T, Patel JM, Alice A, Baird JR, Hu H-M, and Gough MJ, Chapter Six - Activating the Nucleic Acid-Sensing Machinery for Anticancer Immunity, in International Review of Cell and Molecular Biology, Vanpouille-Box C and Galluzzi L, Editors. 2019, Academic Press; p. 173–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mason KA and Hunter NR, CpG plus radiotherapy: a review of preclinical works leading to clinical trial. Front Oncol, 2012. 2: p. 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pantel A, Teixeira A, Haddad E, Wood EG, Steinman RM, and Longhi MP, Direct type I IFN but not MDA5/TLR3 activation of dendritic cells is required for maturation and metabolic shift to glycolysis after poly IC stimulation. PLoS Biol, 2014. 12(1): p. e1001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banchereau J and Steinman RM, Dendritic cells and the control of immunity. Nature, 1998. 392(6673): p. 245–252. [DOI] [PubMed] [Google Scholar]
- 53.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, and Lanzavecchia A, Maturation, Activation, and Protection of Dendritic Cells Induced by Double-stranded RNA. Journal of Experimental Medicine, 1999. 189(5): p. 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pagès F, Mlecnik B, Marliot F, Bindea G, Ou F-S, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină A-M, Scripcariu D-V, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, and Galon J, International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. The Lancet, 2018. 391(10135): p. 2128–2139. [DOI] [PubMed] [Google Scholar]
- 55.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, and Green AR, Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol, 2011. 29(15): p. 1949–55. [DOI] [PubMed] [Google Scholar]
- 56.Tang ES, Newell PH, Wolf RF, Hansen PD, Cottam B, Ballesteros-Merino C, and Gough MJ, Association of Immunologic Markers With Survival in Upfront Resectable Pancreatic Cancer. JAMA Surgery, 2018. 153(11): p. 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Byrne KT, Yan F, Yamazoe T, Chen Z, Baslan T, Richman LP, Lin JH, Sun YH, Rech AJ, Balli D, Hay CA, Sela Y, Merrell AJ, Liudahl SM, Gordon N, Norgard RJ, Yuan S, Yu S, Chao T, Ye S, Eisinger-Mathason TSK, Faryabi RB, Tobias JW, Lowe SW, Coussens LM, Wherry EJ, Vonderheide RH, and Stanger BZ, Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy. Immunity, 2018. 49(1): p. 178–193.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.