Abstract
Whereas the majority of mammalian species are uni-parental with the mother solely providing care for young conspecifics, fathering behaviors can emerge under certain circumstances. For example, a great deal of individual variation in response to young pups has been reported in multiple inbred strains of laboratory male mice. Further, sexual experience and subsequent cohabitation with a female conspecific can induce caregiving responses in otherwise indifferent, fearful or aggressive males. Thus, a highly conserved parental neural circuit is likely present in both sexes, however the extent to which infants are capable of activating this circuit may vary. In support of this idea, fearful or indifferent responses toward pups in female mice are linked to greater immediate early gene (IEG) expression in a fear/defensive circuit involving the anterior hypothalamus than in an approach/attraction circuit involving the ventral tegmental area. However, experience with infants, particularly in combination with histone deacetylase inhibitor (HDACi) treatment, can reverse this pattern of pup-induced activation of fear/defense circuitry and promote approach behavior. Thus, HDACi treatment may increase the transcription of primed/poised genes that play a role in the activation and selection of a maternal approach circuit in response to pup stimuli. Here, we asked whether HDACi treatment would impact behavioral response selection and associated IEG expression changes in virgin male mice that are capable of ignoring, attacking or caring for pups. Our results indicate that systemic HDACi treatment induces spontaneous caregiving behavior in non-aggressive male mice and alters the pattern of pup-induced IEG expression across a fear/defensive neural circuit.
Keywords: paternal behavior, histone acetylation, immediate early gene expression, Npas4
Introduction
Mus musculus is a uni-parental rodent species in which the mother solely cares for her young in the wild. However maternal-like behavior (pup retrieval, sniffing/licking, crouching) along with the elimination of pup-directed aggression in males is reported in commonly used laboratory strains of mice1–5. This is observed in both sexually experienced males, which are often cohabited with females to optimally produce offspring, and sexually naïve male mice, albeit much less frequently. When exposed to pups, sexually naïve male mice tend to show highly variable responses to pups including aggressive, exploratory, avoidant, and even spontaneous caregiving behaviors6. Together these data support the idea that the neural circuit that regulates maternal behavior is conserved in male mice, but the extent to which infants stimulate this circuit varies considerably between individuals due to largely unknown mechanisms.
In females, seminal work uncovering the neural mechanisms that gate infant stimulation of the maternal neural circuit was conducted in postpartum rats7 and recent work has replicated some of these findings in mice8,9. Importantly, motivation to care for offspring first occurs around the time of birth. In non-parental animals, infants activate hypothalamic regions known to regulate anxiety/escape/attack behaviors such as the anterior hypothalamic nucleus (AHN) and ventromedial nucleus of the hypothalamus (VMN)6,10,11. Furthermore, lesions of these hypothalamic attack regions promote the onset of maternal behavior in sub-optimally hormonally-primed nulliparous female rats10,12. In contrast, the medial preoptic area (MPOA) of the rostral hypothalamus regulates caregiving behavior through its projection to the ventral tegmental area (VTA), which drives the release of dopamine into the nucleus accumbens (NA) causing high levels of maternal responding13–16. Thus, hormonal stimulation during late pregnancy and birth facilitates the onset of maternal behavior by increasing infant stimulation of this MPOA-VTA-NA circuit. Whereas plasticity within this circuit contributes to the maintenance of caregiving behavior across the postpartum period long after hormonal stimulation has waned17, caregiving behavior likely depends on changes in both anti-social and pro-social neural systems18,19. For example, the onset of mothering in rats also coincides with a reduction in the ability of infants to activate fear/defensive neural systems11 and experimentally induced reactivation of this system can turn mothering off20. Thus, the occurrence of caregiving behavior may depend on both a pup-induced activation of the maternal circuit and an inhibition of a competing fear/escape/attack neural system19.
Whereas the transition from pup avoidance to pup approach in female rats is typically uni-directional, male mice can revert back to an aggressive state under certain circumstances. For example, while males transition from aggressive or avoidant responses to approach and caregiving responses following sexual experience6, in the absence of continued pup exposure they can transition back to pup-directed aggression21. Therefore the male mouse model is useful for understanding the relationship between pup-induced activation of a neural system and pup-directed behavioral responses because males engage in aggressive, avoidant or caregiving responses under predictable circumstances. Most of what we know about the relationship between neuronal activity and behavioral response to pups comes from studies that have used immediate early gene (IEG) expression as an indicator of neuronal activity. IEGs are rapidly transcribed and translated in response to an extracellular stimulus because they do not require the de novo synthesis of transcription factors22. The protein products of IEGs are transcription factors themselves, which function to regulate the expression of late responding genes. Note that the pattern of gene expression induced by the same IEG transcription factor can vary greatly by cell23. Therefore, although IEG expression is ubiquitous across heterogenous populations of cells, the downstream effects are probably not. Recent work supports the idea that the reduced activation of a central aversion system (including AHN/VMN) in response to pups is also associated with the transition to paternal care6,24. Further, expression of the IEG, cFos, within the rhomboid part of the dorsal bed nucleus of the stria terminalis (dBNST) was found to be highly correlated with pup-directed aggression24, although the mechanism through which activation of a central aversion system mediates distinct types of aversive responses is presently unclear.
Whereas the role of pregnancy hormones in activating the maternal neural circuit has been well described, the mechanisms through which these neural systems are activated to promote caregiving behavior in non-lactating rodents are relatively unknown. Further, how a neural circuit is selected to mediate a specific behavioral response and how factors like sex, experience or reproductive status regulate the selection of a particular circuit over a competing circuit is unclear. One possibility is that transcriptional patterns within specific cell populations program the activation of a particular circuit. Sex may program a particular circuit for default selection from birth. Reproductive status (sexual experience in males or gestation in females) might re-program the pattern to set a new circuit as default. Repeated experience with pups may lead to neuronal activity-dependent transcriptional changes that result in differential circuit selection (specifically avoidance to approach). Histone deacetylase inhibitor (HDACi) drugs enhance the transcription of genes that are poised or primed for rapid transcription in response to an external stimulus25 and in this way may potentiate experience-driven behavioral modifications. Recently, we found that HDACi treatment in virgin female mice increased the likelihood that regions of the maternal neural circuit, rather than regions of the fear/avoidance circuit, were activated during the challenging task of pup retrieval in a novel T-maze26. Based on this finding, we hypothesized that experience-induced changes in behavioral response selection may depend on the extent to which IEGs are primed within neural regions regulating these responses to pups. Further, HDACi treatment may increase the transcription of primed genes that promote the activation and selection of approach circuits exclusively. Here, we investigate this hypothesis in pup-naïve virgin male mice because of the considerable variation they show in their default behavioral response to pups. To assay region-specific transcriptional response to pups we quantified mRNA expression of two IEGs, cFos and neuronal PAS domain protein 4 (Npas4)27,28. We measured Npas4 in addition to cFos because unlike cFos29, Npas4 is exclusively expressed in neurons and is a reliable indicator of neuronal activity30. In addition, Npas4 expression has been shown to be critical for plasticity31 and the regulation of inhibitory synapse formation on excitatory neurons32.
Methods and Materials
Subjects and drug treatment
All mice were C57BL/6J virgin adult males (45+ days of age) from our breeding colony, naive to pups, housed on a 12-hour reverse light cycle and given food and water ad libitum. The HDACi sodium butyrate (Sigma-Aldrich) was dissolved in sterile water and was administered at a dose of 8 mg/ml in the drinking water33. Control mice received standard drinking water. Drinking water containing sodium butyrate was given ad libitum beginning 24 hours prior to the start of testing and continued throughout testing. Daily drinking water was monitored for all sodium butyrate treated mice. All mice were housed individually for 3–7 days prior to and throughout testing. Behavioral testing was conducted one hour into the dark phase of the light/dark cycle under dim red light. Stimulus pups were obtained from lactating C57BL/6J or CD1 lactating dams in our donor-pup breeding colony. All procedures were in compliance with the University of California, Davis Institutional Animal Care and Use Committee.
Behavioral Procedures
Home cage parental behavior tests
Pup naïve virgin male mice were treated with sodium butyrate (N= 24) or water (N = 25). Behavioral testing began by scattering three stimulus pups (1–6 days old) in the home cage. Mice were rated using a 5 point scale based on their initial response to pups during a 15-minute test: 0- repeated biting of pups, 1- rough handled or stepped on pups, 2- spent less than 50% of the test investigating pups, 3- spent more than 50% of the test investigating pups, 4- retrieved at least one pup, 5- displayed full paternal care (retrieval, sniffing/licking and hovering over pups). Mice were then categorized based on their score as aggressive (0–1), indifferent (2), or paternal (4–5). None of the mice tested received a score of 3. For male mice that were not aggressive toward pups (scores 1–5), latencies to sniff, retrieve each pup to the nest, sniff/lick the grouped pups and hover over pups in the nest were recorded during the 15-minute test. Pups remained in the cage for a total of 2 hours and were then removed and returned to a lactating dam. In the event that a male attacked a pup, the test was stopped and the pups were immediately removed from the cage. Pups sustaining injuries (visible bite marks, blood or bruising) were euthanized immediately. Male mice that attacked pups on the first test were not tested again. Males that did not attack pups were tested for 2 consecutive days total.
Social interaction test
To investigate whether effects of HDACi on behavior are exclusive to interactions with pups, a separate cohort of pup-naïve virgin male mice treated with sodium butyrate (N= 8) or water (N= 8) was tested in the social interaction test. Sodium butyrate was given beginning 24 hours prior to the start of testing and continued throughout testing. Social interaction testing was conducted in a large Plexiglas open field that contained no bedding (89×63×60 cm), as described previously34. Briefly, the test consisted of 3 consecutive phases (open field, acclimation and interaction). During the open field phase of testing each mouse was introduced into the arena for 3 minutes. Time spent in the center of the arena, corners of the open field and total distance traveled was recorded (Any-Maze, Stoelting). Following the open field phase, a small wire cage was introduced against one wall of the arena (without removing the focal mouse from the arena). During this 3-minute acclimation phase, the time spent within 8 cm of the novel cage (time investigating novel object) or within the 2 corners (8×8 cm each) opposite the wire cage (time away from novel object) was recorded. During the last phase of testing, an unfamiliar same-sex stimulus mouse was placed into the wire cage for 3 min and the time spent investigating the novel mouse was recorded.
Region-specific Gene Expression in Aggressive and Non-Aggressive Males
Given that HDACi treatment significantly increased the proportion of animals showing paternal care, but had no effect on the proportion of animals responding aggressively toward pups, we hypothesized that HDACi treatment affects behavioral responses toward pups exclusively in male mice that are not aggressive to pups. In order to distinguish between the effects of HDACi treatment on activity-dependent gene expression in aggressive versus non-aggressive mice, we pre-screened naïve virgin males for their initial behavioral responses toward pups. A single pup was introduced into the cage and behavioral responses were recorded for 15 minutes. Mice that attacked were categorized as aggressive and mice that failed to attack within the 15-minute test were categorized as responsive. In order to match the 30-minute pup exposure time between groups while protecting the pups from infanticide a wire mesh ball (tea infuser; Norpro 1.75 inches in diameter) was used with 50 holes (3mm diameter). Males could make contact with pups but were not able to injure them. All males were habituated to the presence of the mesh ball prior to testing. Forty-eight hours prior to the start of testing a mesh ball was placed into each male’s cage. The mesh balls remained in the cage until the time of testing at which point the ball was removed and immediately replaced either empty or containing a pup. Gene expression was examined in 6 groups: pup-naïve virgin male control mice (N=6), pup-naïve virgin male control mice treated with HDACi (N=7), aggressive virgin males (N=7), aggressive virgin males treated with HDACi (N=7), responsive virgin males (N=9), and responsive virgin males treated with HDACi (N=11). On test day, the ball was removed from the cage and replaced with either a pup or no pup (control).
Quantification of mRNA by real time PCR
Following 30 minutes of pup exposure each male was placed in a bell jar containing isoflurane for approximately 15 seconds. To our knowledge, there are no reports of this brief exposure affecting gene expression, while it’s possible that isofluorane may have produced an effect, experimental and control groups were treated the same. Males were then euthanized by cervical dislocation and brains were immediately removed, frozen and later sectioned (120 microns) on a cryostat and frost-mounted onto slides. The MPOA (Bregma 0.37 to −0.35), AHN/VMN (Bregma −0.59 to −1.67), and VTA (Bregma −2.69 to −3.51) were dissected out using a blunted 15.5 gauge needle and the dBNST (Bregma 0.49 to −0.35) was dissected out using a blunted 18 gauge needle using coordinates from the Franklin and Paxinos Mouse Brain Atlas. Total RNA was isolated with Qiazol reagent (Qiagen) and purified with an RNeasy® Plus Micro Kit (74004; Qiagen, Valencia, CA) as well as the optional DNase digestion (Qiagen 129046). A Nanodrop™ Spectrophotometer was used to determine the quality (260/280 ratio > 1.8) and quantity of the RNA and 9 poor quality samples were not used. The cDNA templates were prepared using an Applied Biosystems cDNA Synthesis Kit (4368813) according to the manufacturer’s protocol. Quantitative real-time PCR was performed using the ABI Viia7 real-time PCR system. The PCR products of interest were detected using TaqMan® Gene Expression assays from (Applied Biosystems, Carlsbad, CA) (Table 1). All samples were normalized to beta-2 microglobulin (b2m). There were no statistically significant differences in the expression of the endogenous control gene between treatment groups. Target and endogenous control genes were measured in triplicate for each cDNA sample during each real-time run to avoid intra-sample variance. All genes of interest were analyzed with Viia7 Applied Biosystems software using the comparative cycle thresholds (delta delta CT) method. There were no statistically significant differences in relative gene expression between pup-naïve control mice treated with or without sodium butyrate for any gene tested (Table 2) and therefore these groups were collapsed and expression of experimental samples was normalized to the average expression of the combined no-pup control group.
Table 1. Taqman Primers.
Taqman primers used for qPCR reactions.
| Abbreviation | Gene Name | RefSeq | Assay ID |
|---|---|---|---|
| cFos | FBJ osteosarcoma oncogene | NM_010234.2 | Mm00487425_m1 |
| Npas4 | Neuronal PAS domain protein 4 | NM_153553.4 | Mm01227866_g1 |
| B2m | Beta-2-microglobulin | NM_009735.3 | Mm00437762_m1 |
Table 2. Immediate early gene expression in pup-naïve mice treated with HDACi.
Relative expression of cFos and Npas4 normalized to water-treated control mice. In the absence of pup stimulation, there was no effect of HDACi treatment on immediate early gene expression (Ns = 5–6).
| Transcript | Brain Region | Pup-naïve Control (Mean ± SEM) | Pup-naïve + HDACi (Mean ± SEM) | p-value |
|---|---|---|---|---|
| cFos | MPOA | 1 ± 0.09 | 1.03 ± 0.07 | 0.77 |
| AHN/VMN | 1 ± 0.22 | 1.10 ± 0.08 | 0.71 | |
| VTA | 1 ± 0.09 | 0.93 ± 0.24 | 0.79 | |
| dBNST | 1 ± 0.12 | 0.90 ± 0.09 | 0.51 | |
| Npas4 | MPOA | 1 ± 0.16 | 1.28 ± 0.13 | 0.23 |
| AHN/VMN | 1 ± 0.08 | 0.72 ± 0.22 | 0.25 | |
| VTA | 1 ± 0.11 | 1.07 ± 0.12 | 0.65 | |
| dBNST | 1 ± 0.13 | 1.05 ± 0.13 | 0.86 |
Serum Testosterone Assay
To assess whether HDACi treatment could have affected circulating levels of testosterone at the time of pup presentation, a separate cohort of pup-naïve virgin male mice was treated with sodium butyrate (N=7) or water (N=7) for 24 hours. Cardiac blood was collected in anesthetized mice at the time when pups would have been presented (1 hour after lights go off). Blood was left to coagulate at room temperature for ≥ 30 minutes before centrifugation at 3000g for 10 minutes at 4 degrees Celsius. Supernatant was transferred to a clean microcentrifuge tube and stored at −80 degrees Celsius until assayed. A DRG ELISA kit (EIA-1559) was used to assay serum testosterone according to the manufacturer’s protocol. The manufacturer reports the monoclonal antibody has a dynamic range between 0.083 and 16 ng/mL and the intra assay variance across an n of 20 is 4.16%, 3.28%, and 3.34% at low, mid, and high concentrations, respectively. A standard curve was fit using the 4-parameter logistics method. Experimental samples were assayed in triplicate on a single plate and the intra assay variance was 3.41%.
Statistical Analysis
Probability data were analyzed using Chi Square and Fisher’s Exact tests. The frequency of pup retrieval (number of pups retrieved) was analyzed by a mixed two-way ANOVA (Treatment x Time), with repeated measures on the second factor. Latency to the first pup contact (sniff) on the first test day was analyzed using a student’s T-test because all of the subjects completed the task in the duration of the test. Survival analyses were used to analyze all other latency data (pup retrieval and sniff/lick)35. This method takes into account that some subjects did not retrieve pups during the 15-minute test and censor those data. These latency data are plotted using Kaplan–Meier survival curves in which the fraction of mice that have retrieved (or sniff/licked) pups at each time point is calculated using the product limit (Kaplan-Meier) method. We used the Mantel-Cox log-rank test to statistically compare survival curves on each test day. In addition, hazard ratio and confidence intervals are reported for each variable. The hazard ratio, which is calculated from all the data in the survival curve, indicates the rate at which one group retrieves or licks pups compared to the other. Relative gene expression data were analyzed using two way ANOVAs (Behavior X Treatment). To determine whether IEGs were induced relative to no-pup controls, a one-sample T-test was used to compare each group to the hypothetical value “1”. All other experiments comparing two independent groups were analyzed using a student’s T-test. All statistical tests were two tailed. For ANOVA data, planned comparisons (HDACi versus control within each behavior) were analyzed using Fisher’s LSD post hoc tests. All data were analyzed using GraphPad Prism 7 software (GraphPad, Inc., La Jolla, CA).
Results
Effects of HDACi on Behavioral Response to Pups
Although the consumption of drinking water was consistent with what has been reported for C57BL/6J mice36, males tended to consumer more water if it was treated with sodium butyrate [t(47) = 6.185, p<0.0001, η2 = 0.4487; Fig.1b]. HDACi treatment significantly affected the probability of aggressive, indifferent or paternal responses toward pups in virgin male mice on the first test day [X2 (2) = 7.906, p = 0.0192, V=0.40; Fig.1c]. Specifically, HDACi treatment induced spontaneous paternal behavior in non-aggressive male mice (indifferent versus paternal, p=0.0108, Fisher’s Exact Test). All non-aggressive males retrieved more pups as a result of pup experience [main effect of time [F(1,17) = 6.434, p = 0.0213, η2 = 0.12] and HDACi treated males retrieved more pups than control males [main effect of treatment [F(1,17) = 10.95, p = 0.0042, η2 = 0.22], particularly on the first test day (p<0.05, d = 1.22; Fig.1e). There were no significant differences in latency to first approach pups on test day 1. HDACi treated males were faster to retrieve the first pup on test day 1 [X2 (1) = 5.894, p = 0.0152, HR 4.134; 95% CI,1.314, 13.00; Fig. 2]. On the second test day, HDACi treated males were faster to retrieve all pups to the nest [X2 (1) = 4.506, p = 0.0338, HR 3.309; 95% CI, 1.096, 9.988] and lick pups in the nest [X2 (1) = 5.689, p = 0.0171, HR 3.999; 95% CI, 1.280, 12.49] when compared to control males.
Figure 1.
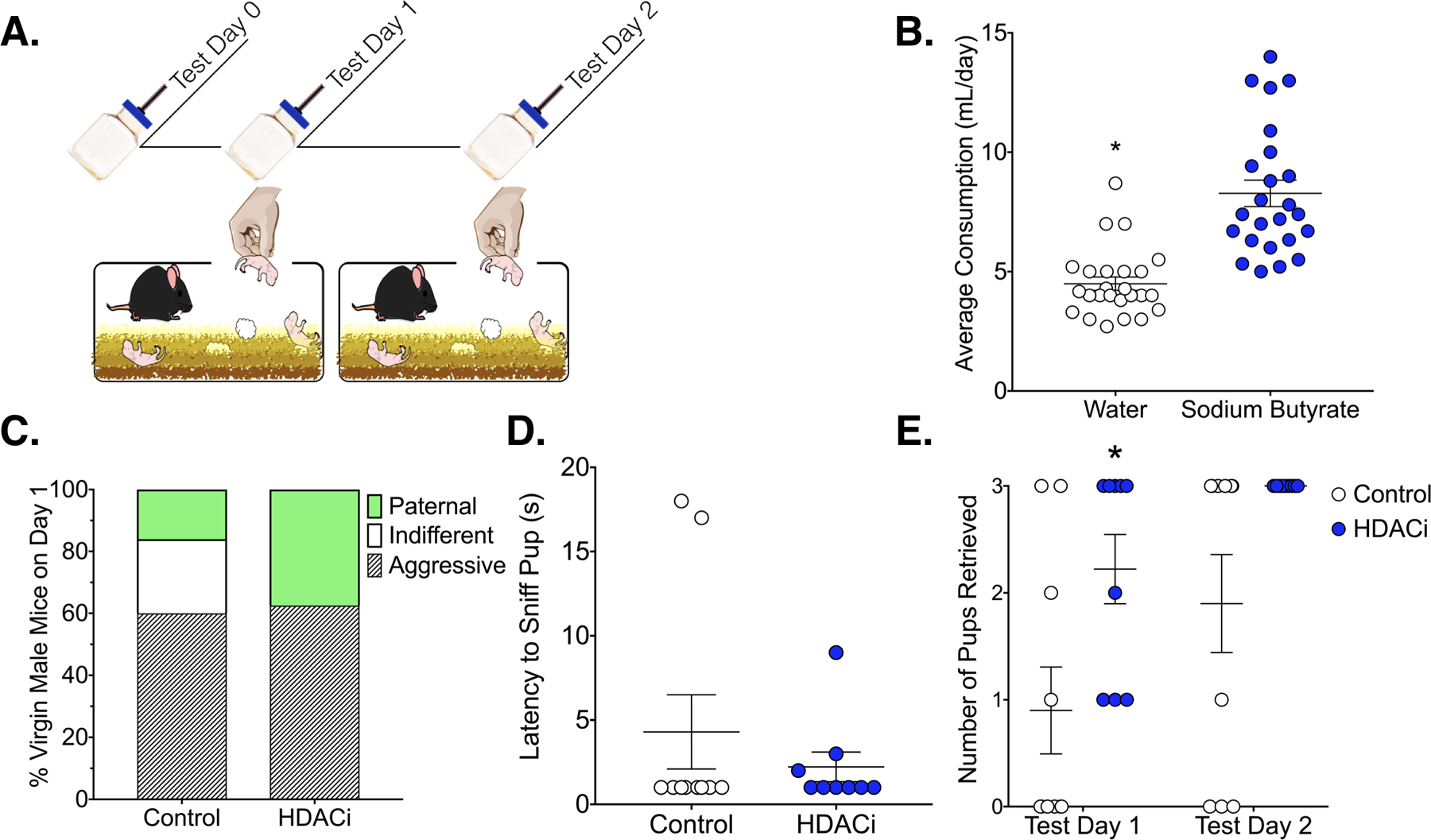
Effects of HDACi treatment on behavioral response selection in the home cage. A.) Timeline for Experiment 1: Mice were treated with HDACi (N = 24) or water (N = 25) for 24 hours prior to the start of testing. Only mice that did not show pup-directed aggression were tested on day 2. B.) Males readily consume sodium butyrate-treated water. Average consumption (ml/day) is represented as Mean ± SEM *Significantly different from HDACi group, p<0.0001 C.) Probability of behavioral response to pups varied significantly by treatment (Fisher’s Exact Test, p = 0.02). All non-aggressive HDACi treated mice showed spontaneous caregiving behavior compared to 40% of control mice (Fisher’s Exact Test, p = 0.01). D.) Mean ± SEM latency to approach and contact a pup on the first test day did not vary by HDACi treatment. E.) HDACi treated males retrieved more pups than controls and all males showed experience-induced improvements in retrieval (Main effects of treatment and time). *Significantly different than control, planned comparison, p< 0.05, d = 1.22
Figure 2.
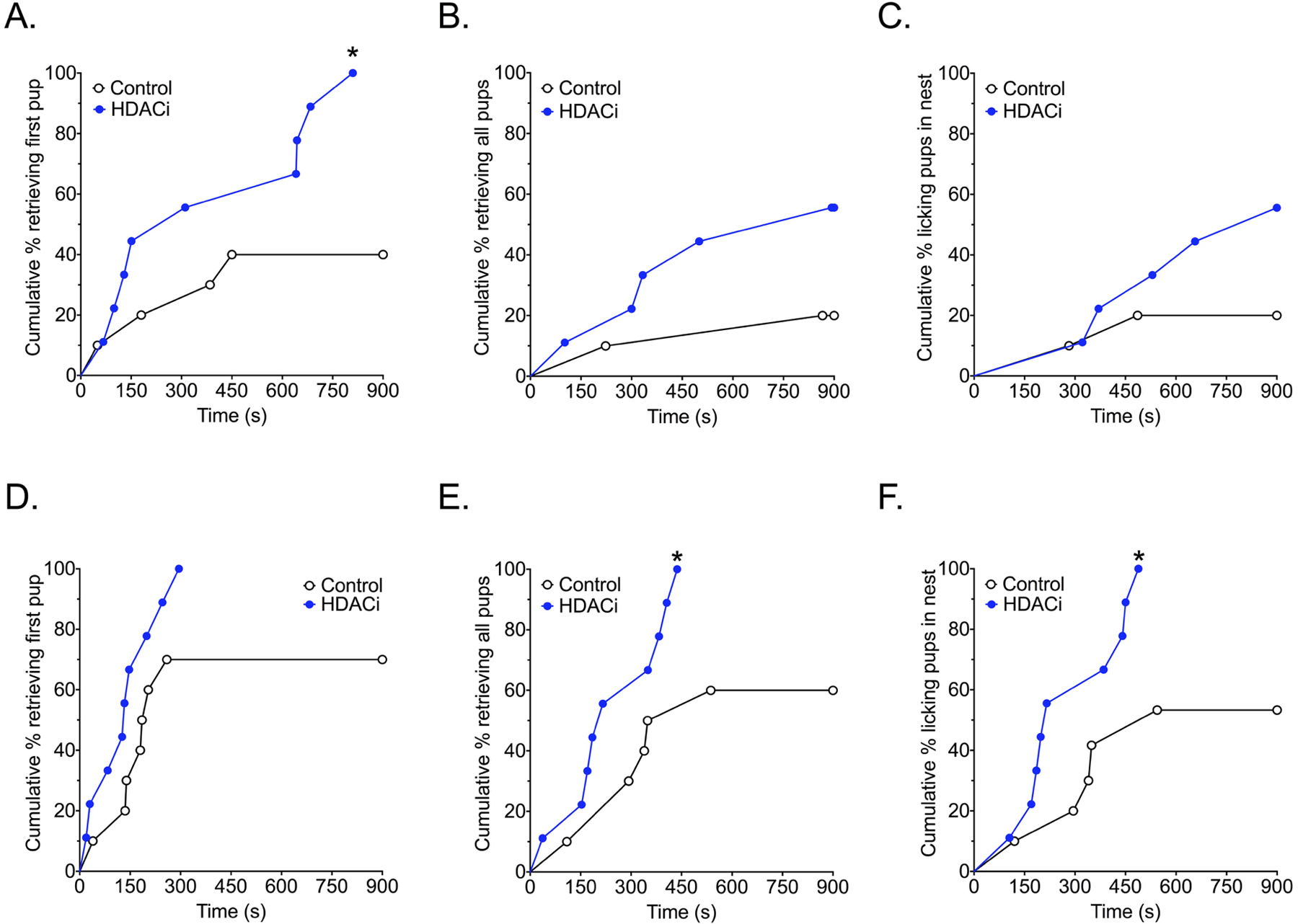
Effects of HDACi treatment in non-aggressive males on the latency to respond to pups. Kaplan-Meier survival curves show the proportion of animals completing the retrieval tasks (retrieving first or last pup) or licking retrieved pups in the nest at each time point on the X-axis in the home cage. A-C) HDACi treated male mice were faster to retrieve the first pup on test day 1. D-E) HDACi treated males were faster to retrieve all pups and lick retrieved pups in the nest on test day 2.
*Significantly different from control group, Chi Square tests, p< 0.05
Effects of HDACi on Social Interaction with a Novel Adult Conspecific
There were no significant differences in locomotion (total distance travelled, p = 0.964), thigmotaxis (time in the corners, p = 0.5025) or exploration (time in the center, p = 0.5256) during the open field phase of the social interaction test (Fig. 3). During the acclimation phase, HDACi treated mice spent more time in the corners [t(14)= 2.307, p = 0.0369, d = 1.23] and less time investigating the novel empty cage [t(14)= 2.2448, p = 0.0282, d = −1.31]. However, during the social interaction phase of the test, there were no group differences in locomotion (p = 0.3777), thigmotaxis (time in corners, p = 0.4177) or social interaction time (p = 0.6552).
Figure 3.
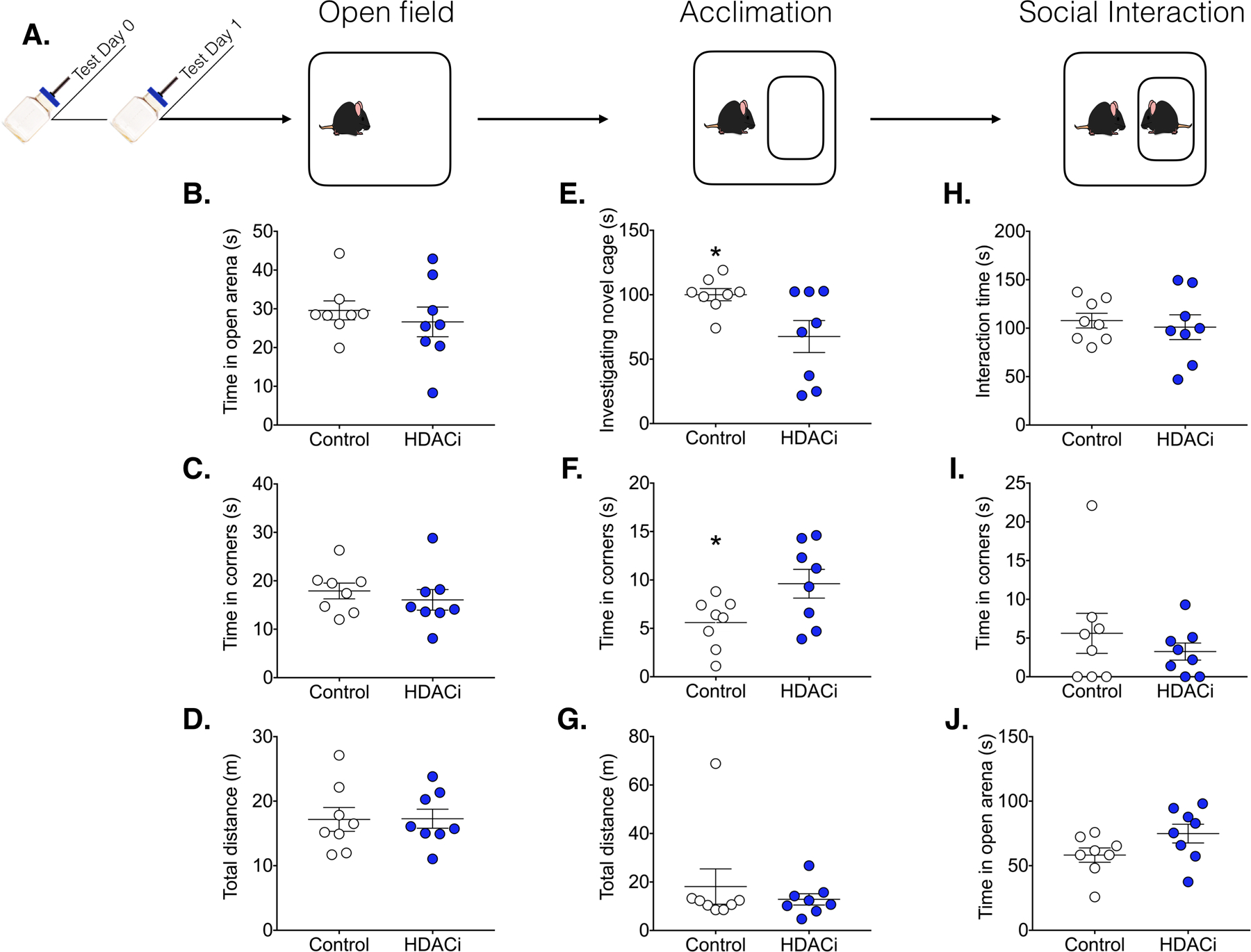
HDACi treatment had no effect on social behavior. A.) Timeline for Experiment 2: Males were treated with sodium butyrate in the drinking water or normal water 24 hours before the start of testing (Ns= 8). The social interaction test consisted of 3 phases (each lasting 3 min). All data are presented as Mean± SEM. B-D) Activity during the 3 min open field test was not altered by HDACi treatment. E-G) Upon introduction of a novel empty cage, HDACi treated males spent significantly more time in the corners of the arena and less time investigating the empty cage. H-J) HDACi treatment had no effect on activity or investigation of a novel adult conspecific
*Significantly different from control group, p< 0.05, ds > 1.2.
Effects of HDACi on Circulating Testosterone
We tested the possibility that effects of HDACi treatment on spontaneous caregiving behavior were related to a treatment-induced change in the circulating level of testosterone by assaying plasma testosterone in male mice exposed to sodium butyrate (or regular water) for 72 hours (Fig. 4). There was no significant effect of HDACi treatment on testosterone levels in virgin male mice (p = 0.2728)
Figure 4.
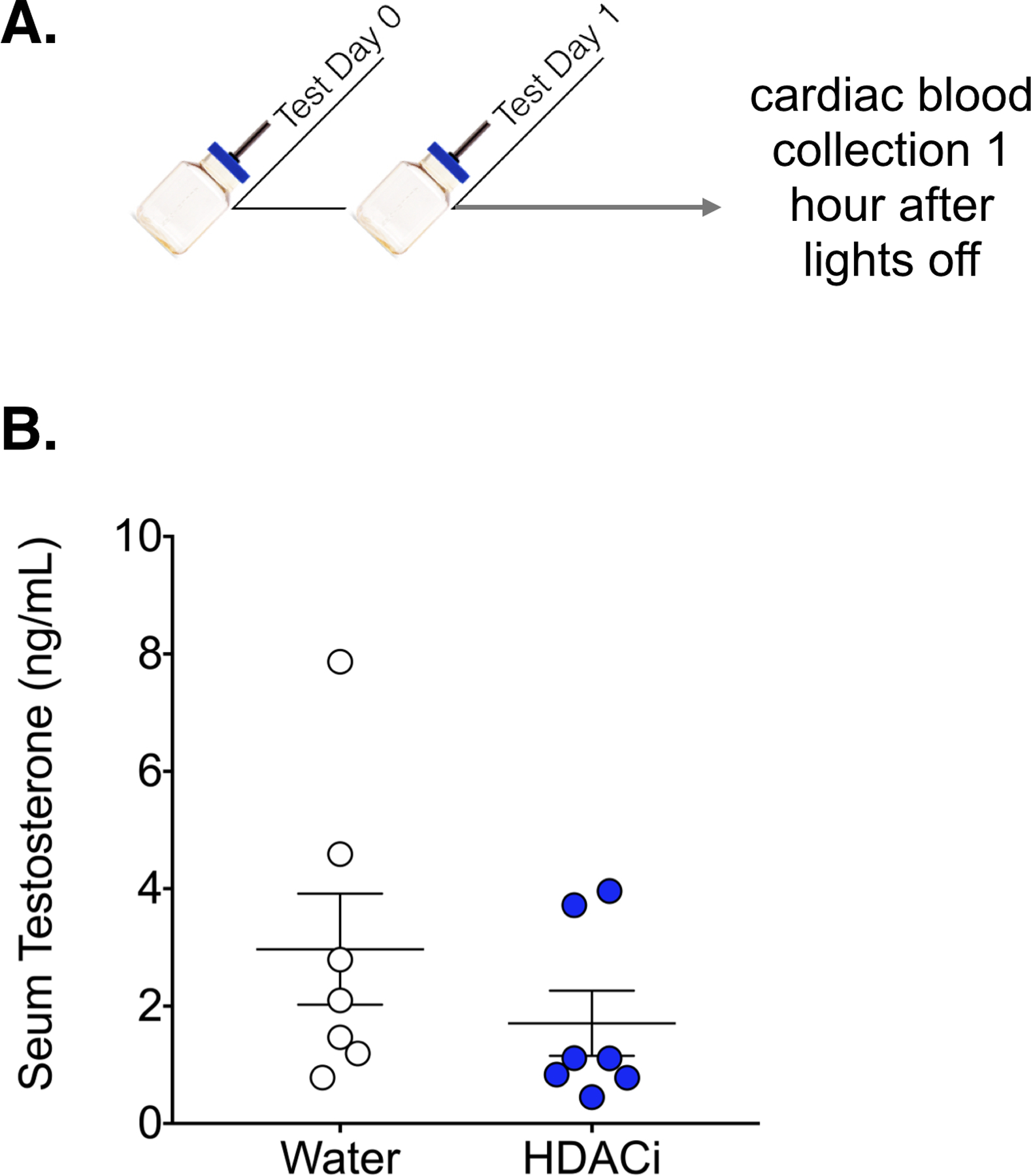
HDACi treatment had no effect on serum testosterone. A.) Timeline for Experiment 3: Males were given sodium butyrate in the drinking water or normal drinking water for 24 hours prior to cardiac blood collection (Ns = 7). B.) Concentration of testosterone was not significantly different between groups (p = 0.27)
Effects of HDACi on Activity-Dependent Gene Expression in Approach/Avoidance Nodes
cFos mRNA expression
cFos expression was significantly induced by pup exposure in all male mice within the MPOA, AHN/VMN and dBNST regardless of behavioral group or treatment (one sample T-test for each condition in each region, p< 0.05, ds> 0.5; Fig. 5). cFos expression in the VTA was significantly higher in aggressive versus non-aggressive males, regardless of HDACi treatment [Main effect of behavioral predisposition: F(1,24) = 4.762, p = 0.039, η2 = 0.16]. In fact, in the VTA pup-induced cFos expression failed to reach statistical significance in non-aggressive males compared to an empty mesh ball (p = 0.07, d = 0.75). In the AHN/VMN, there was a significant interaction effect between behavioral predisposition and HDACi treatment in relative cFos expression [F(1,24) = 6.714, p = 0.016, η2 = 0.22]. HDACi treatment reduced cFos expression in the AHN/VMN in males that were responsive, but not aggressive, toward pups (p< 0.05, d = −1.10).
Figure 5.
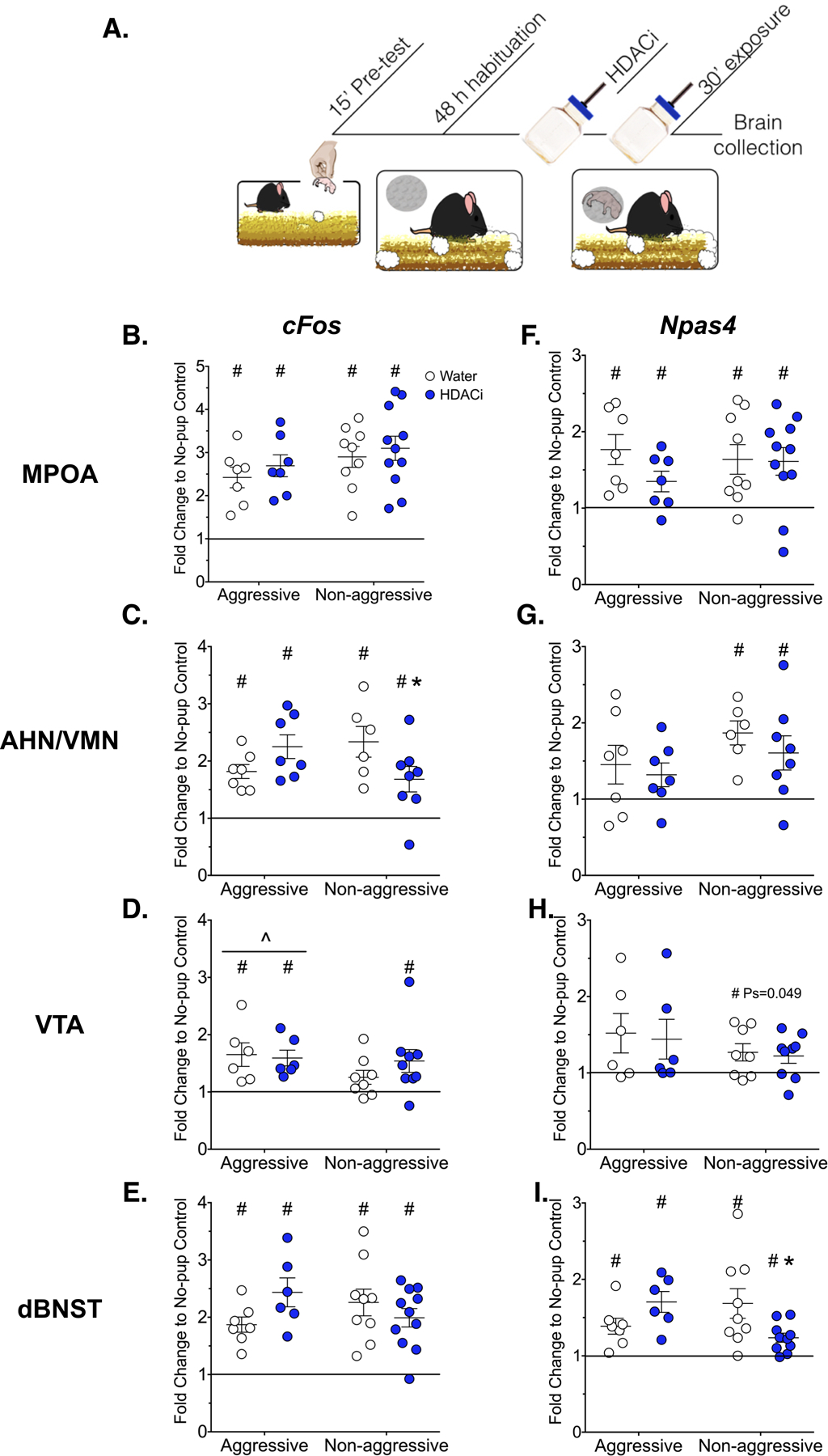
Effects of HDACi treatment on IEG expression A.) Timeline for Experiment 4: Males were given a brief pre-test to identify aggressive or responsive behaviors toward pups. Following the pre-test, males were habituated to the mesh ball for 48 hours. Twenty four hours prior to pup exposure, males were given HDACi-treated or regular water. On test day, a pup was placed into the mesh ball for 30 min before brain collection. Mean± SEM cFos (B-E) and Npas4 (F-I) mRNA expression within neural regions associated with pup avoidance/approach (Ns = 6–11). The black line represents normalization to the no-pup control group.
#Significantly different from no-pup control, One sample T-tests, p< 0.05
^Main effect of behavior, 2 way ANOVA, p< 0.05
*Significantly different from corresponding control-treated group, p< 0.05
Npas4 mRNA expression
The immediate early gene, Npas4, was also significantly induced by pup exposure in all male mice regardless of behavior or HDACi treatment, but only within the MPOA and dBNST, (one sample T-test for each condition in each region, p< 0.05, d> 0.9). Within the VTA, Npas4 induction was related to behavioral predisposition, with only non-aggressive mice showing a significant elevation of Npas4 over no-pup control (p< 0.05, d> 0.7). Similarly, induction of Npas4 by pup-exposure was limited to non-aggressive mice within the AHN/VMN (p < 0.05,d > 0.9). Within the dBNST, behavioral predisposition and HDACi treatment interacted to affect Npas4 expression [F(1,29) = 7.569, p = 0.01, η2 = 0.28]. HDACi treatment significantly reduced Npas4 expression in non-aggressive males (p< 0.05, d = −1.13).
Discussion:
Five important conclusions emerge from the results of the present study. First, HDACi treatment induced spontaneous caregiving behavior over pup avoidance, but had no effect on pup-directed aggression. For non-aggressive males, HDACi treatment reduced the latency to retrieve the first pup and increased the number of pups retrieved within 15 minutes of the first pup exposure. In addition to its effects on spontaneous care, HDACi treatment also amplified experience-induced changes in caregiving behavior. HDACi treated males were faster to group pups and lick pups in the nest compared to non-aggressive controls on test day 2. Second, the pro-social effects of HDACi treatment may be specific to pups because HDACi did not affect social investigation of an adult male conspecific. Further, HDACi treatment did not produce a reduction in general fearfulness as measured by exploration of a novel environment. If anything, HDACi treatment was associated with an avoidance of novel objects. Third, the induction of spontaneous caregiving behavior by HDACi treatment was probably not related to a reduction in testosterone because HDACi treatment had no significant effect on circulating levels of testosterone. Fourth, in line with the finding that HDACi treatment produces behavioral effects exclusively in non-aggressive mice, the effects of HDACi treatment on IEG expression were also limited to non-aggressive males. For example, cFos expression in response to pup cues was reduced in HDACi-treated non-aggressive males within the AHN/VMN. Further, HDACi treatment significantly reduced Npas4 expression in the dBNST, a region that includes the rhomboid nucleus, which may interfere with caregiving behavior through its direct inhibition of the central MPOA24. In contrast, no effects of HDACi treatment on IEG expression were detected within neural regions associated with pup approach. Both cFos and Npas4 were uniformly induced in the MPOA in all mice exposed to pups. In the VTA, cFos expression was induced in mice that show motivated behavioral responses toward pups (regardless of whether that response was pro or anti-social) and surprisingly cFos was higher in males that showed pup-directed aggression. Npas4 induction in the VTA, on the other hand, was limited to non-aggressive males. Finally, the two IEGs examined, Npas4 and cFos, did not show the same pattern of expressions in response to the same pup cues in most of the regions examined. Thus, further investigation of Npas4 in response to pup cues within these circuits may provide new insight into mechanisms of parental care and experience-induced plasticity.
The behavioral results presented here for control-treated male mice are consistent with other reports of the highly variable response of virgin C57BL/6J mice to foster pups6. The fact that the facilitatory effects of HDACi treatment on parental behavior were limited to non-aggressive mice suggests that there is an interaction between individual variation in response to pups and HDACi treatment. Although there is some evidence for a developmentally regulated onset of aggression in C57BL/6J mice, factors that contribute to the individual variation in the response of sexually naïve adult male mice to pups are mostly unknown37,38. In general there is weak support for a relationship between circulating testosterone and paternal responsiveness in rodents39, although castration does reduce infanticide in virgin male mice40. However, in the present study we found no significant effect of HDACi treatment on circulating levels of testosterone. These data fit with the finding that HDACi treatment also had no effect on aggressive behavior in virgin male mice.
The finding that HDACi treatment promotes caregiving behavior exclusively in non-aggressive males is consistent with our previous work, which has reported the facilitatory effects of HDACi in virgin female mice, which are typically non-aggressive26,41,42. Together these findings suggest that HDACi treatment acts on a conserved neural substrate. Of course the extent to which HDACi treatment would fail to promote caregiving behavior in aggressive female mice is unknown. Note that when rare instances of infanticide have occurred we have not found differences between HDACi treated and control female mice (unpublished findings). Although HDACi treatment promotes caregiving behavior in female and non-aggressive male mice, there is an important inconsistency between its effects in male versus female mice. Our previous work in female mice has emphasized the role of HDACi treatment in enhancing experience-dependent changes in caregiving behavior. Whereas the present data suggest that HDACi treatment promotes the initial onset of caregiving behavior in males. This difference could be related to the fact that the baseline level of maternal responding is much lower in male mice and therefore there is more room to detect a difference between HDACi and control groups. However, the fact that HDACi treatment was capable of inducing an onset of caregiving behavior in some, but not all male mice within a few minutes of pup exposure may imply something about the molecular mechanisms through which the drug produces its effect. HDAC inhibitor drugs are highly non-specific43. Most commonly used drugs (sodium butyrate, TSA, SAHA) inhibit nearly all HDAC proteins and since HDACs deacetylate non-histone proteins as well, the effects of these drugs likely extend beyond histone proteins. In spite of this, many labs, including our own have reported relatively specific molecular and behavioral effects of HDACi treatment41,44. Based on the finding that the distribution of HAT and HDAC proteins is largely overlapping and localized to regulatory regions of genes, one possibility is that HDACi shift the balance of HAT and HDAC activity such that a braking mechanism would be removed from active genes or sequences with active HATs25. In this way, stimulus-induced gene transcription would be amplified and therefore fewer experiences with the stimulus might be required for memory consolidation45,46. This explanation fits with our previous findings that HDACi treatment reduces the amount of pup experience required to produce long-lasting improvements in maternal care in female mice. However, the finding that HDACi treatment induced caregiving behavior on the first trial in a subset of male mice cannot be related to an HDACi amplification of experience-induced gene expression. Further, why would HDACi treatment affect some, but not all male mice? We speculate that this differential response to HDACi treatment is related to individual variation in chromatin accessibility. For example, in clinical studies testing the efficacy of HDACi drugs as cancer treatments, the pattern of transcription factor occupancy in cells from individual T-cell lymphoma patients predicts HDACi treatment efficacy47. In responsive patients, HDACi treatment is correlated with a rise in DNA accessibility, whereas non-responders show negligible changes in accessibility following treatment. If variation in chromatin accessibility is associated with the differential behavioral response to HDACi treatment in the present study, what might regulate variability in accessibility? One possibility is that genes associated with the maternal responsiveness are poised in non-aggressive males. Poised genes are not active but primed. Multiple mechanisms can mediate this poised or primed state48–52. For example, bivalent enhancer sequences are marked by the presence of both the activating (H3K4me1) and repressive (H3K27me3) marks48. These sites transition from a poised to active state as a result of a stimulus-induced swap of methylation for acetylation at H3K2751,52. Importantly, stimulus-induced cFos expression depends on whether RNA pol II is poised at the cFos promoter50, therefore the cell-specific pattern of IEG expression in response to pups could depend on which cells have a poised cFos promoter. Although we did not address the important issue of cell-specificity in this paper, there is good evidence that pups activate distinct populations of cells in aggressive versus non-aggressive male mice53.
In the present study we examined the expression of two IEG transcripts (cFos and Npas4) that show stimulus-driven transient expression in the brain. Although Npas4 expression has never been examined in response to pup cues, cFos expression (both mRNA and protein) has been investigated extensively in response to pup cues in both male and female mice as well as female rats6,9,11,24,54–56. Our data indicate a near global induction of cFos in response to pup cues, and this finding is consistent with other reports that have identified the MPOA, AHN, VMN, and dBNST as regions that are sensitive to pup stimulation. However, the finding that cFos induction in these regions was not, for the most part, related to the behavioral response to pups (as determined in the pre-test) was quite surprising. For example, we hypothesized that cFos in the AHN/VMN and dBNST would be exclusively induced in aggressive male mice, whereas cFos induction in the VTA and MPOA would be limited to non-aggressive males. Further, we predicted that HDACi treatment would amplify the cFos response in the MPOA and VTA of pup-responsive males. These hypotheses were based on previous reports of differential Fos expression in sexually naïve (aggressive) males compared with sexually experienced (paternal) C57BL/6J males. For example, compared to sexually experienced males, aggressive virgins had an exclusive induction of cFos in cells of the AHN, ventrolateral VMN, as well as some subregions of dBSNT6, and although Fos was induced (relative to non-pup control) in paternal males within some subregions of the dBNST, the Fos response of aggressive males was higher. In contrast, we did not find an exclusive relationship between cFos induction in the AHN/VMN and aggressive behavior. Instead, cFos was induced relative to no-pup control in all male mice exposed to pups. However, it should be noted that our study assayed sexually naïve males with spontaneous aggressive and non-aggressive responses to pups, whereas Tachikawa et al (2013) examined paternal males that had experience caring for pups prior to examining pup-induced Fos response. Certainly virgin males showing spontaneous care are less responsive to pups than pup-experienced fathers. Thus, one possibility is that as caregiving behavior increases the ability of pups to induce a Fos response in the AHN/VMN decreases. In support of this idea, males in our study that would have been most responsive to pups (those treated with an HDACi) showed significantly less cFos expression in the AHN/VMN in response to pup cues. Although it is unclear whether males without mating or pup experience would have also failed to show a pup-induced Fos response in the AHN/VMN, a recent investigation examined pup-induced Fos expression within multiple subregions of the dBNST and MPOA in sexually naïve male mice that were aggressive or spontaneously parental24. This work reported that the number of Fos positive cells in the central part of the MPOA and the rhomboid nucleus of the dBNST were highly predictive of paternal or infanticidal responses, respectively. However, when pup cues were presented indirectly (pups presented in a mesh ball) the differences in Fos expression between parental and infanticidal males in all subregions of the MPOA were eliminated and only the rhomboid and anterior lateral parts of the BNST were found to be significantly different between these groups. Our dBNST tissue punches included several subregions of dBNST in addition to the rhomboid/anterior lateral subregions and therefore any effect of the rhomboid region alone may have been washed out.
To our knowledge, this study is the first to examine differential cFos expression in aggressive and responsive virgin males within the VTA. Our hypothesis that non-aggressive males would have higher pup-induced cFos expression in the VTA was based on data from virgin female mice26. Again we were surprised to find that cFos was induced in both aggressive and non-aggressive HDACi-treated males. Further, aggressive males had significantly higher expression than non-aggressive males, regardless of HDACi treatment. One interpretation is mice that are least likely to approach and interact with pup (non-aggressive control-treated males) do not show a cFos response to pup cues in the VTA. Thus, motivation to approach pups, regardless of the intent to kill or care, is associated with cFos induction. In support of this idea, optogenetic stimulation of MPOA neurons that project to the VTA increased motivation to reach pups (by climbing over a physical barrier) in male and female mice even though males killed pups once they came into contact with them9. Therefore, perhaps it’s not surprising that cFos expression alone in the VTA doesn’t predict the intention to kill or care for pups. Together these findings fit nicely with the idea that hypothalamic interaction with the mesolimbic dopamine system regulates social motivation more broadly, including approach responses toward both appetitive and aversive57. It should be noted that HDACi-treated non-aggressive males have significantly reduced cFos expression in the AHN/VMN coupled with a pup-induced cFos response in the VTA, whereas non-aggressive control-treated males have a significantly higher cFos response in the AHN but no pup-induced cFos response in the VTA. Thus, perhaps it is the combination of these responses that is important for caregiving behavior.
In addition to cFos, we chose to examine Npas4, another IEG with a similar time course of induction to cFos31. Whereas cFos transcription is induced in brain cells by a number of different extracellular stimuli, Npas4 induction is specifically linked to depolarization of neurons, and therefore may provide some indication of the neuronal response to pups within these regions32. Further, Npas4 expression is induced in response to learning, rather than exposure to novel or robust stimuli. For example, Npas4 is induced in the hippocampus following contextual fear learning but unlike cFos, Npas4 expression is not induced by shock alone31. Once translated, Npas4 protein serves as a transcription factor, regulating the expression of several late-responding genes that are also critical for neuronal plasticity and particularly new synapse formation28. Thus, stimulus-induced expression of Npas4 might suggest a neuronal response to pups rather than an increased input to cells as a result of pup exposure. Our data indicate that Npas4 induction was limited to non-aggressive males in both the AHN/VMN and VTA, although the VTA data may be interpreted with some caution as this result barely reached statistical significance. HDACi treatment was without effect on Npas4 expression in these sites, thus Npas4 induction in these regions might be linked to the non-aggressive behavioral response rather than caregiving behavior per se. With respect to HDACi-induced changes in Npas4 expression, the dBNST was the only site affected. Therefore the HDACi-induced reduction in Npas4 expression may be related to the induction of paternal care. Finally, the dBNST and the MPOA may be particularly sensitive to pup stimuli given that we found a significant induction of both Npas4 and cFos in all males exposed to pups. The fact that HDACi treatment significantly lowered Npas4 in the dBNST fits with the idea that this region plays an inhibitory role in parental behavior, although the present data are not consistent with the idea that this role involves the exclusive regulation of pup-directed aggression.
In conclusion, the results of the present study indicate that HDACi treatment can induce spontaneous caregiving behavior in non-aggressive male mice. The facilitatory effect of HDACi treatment is robust and specific to parental behavior. All non-aggressive males with HDACi treatment responded to pups within 15 minutes of pup exposure and HDACi treatment did not reduce neophobia or increase social behavior generally. HDACi-induced reduction in IEG expression within two sites that inhibit caregiving behavior is likely related to the induction of spontaneous caregiving behavior, however the overall pattern of pup-induced IEG expression was not entirely supported by our predictions. An aggressive behavioral predisposition was not associated with the exclusive expression of cFos in regions of the brain linked to fearful/defensive behavior in response to pup cues. Similarly, we did not find greater activation of IEG expression in the MPOA of non-aggressive males in response to pup cues. Together these findings underscore the importance of understanding how the MPOA and its interaction with downstream neural sites regulate spontaneous care, indifference or pup-directed aggression. Finally, the present data support the idea that Npas4 expression may be a more specific marker for neuronal activation, as unlike cFos expression, Npas4 was differentially expressed in non-aggressive and aggressive mice. Future work will need to gain a cellular resolution of Npas4 activity in these regions in order to better understand its role in paternal experience-induced plasticity.
Table 3. Rates of paternal responsiveness by day.
Hazard ratios, calculated from all the data in the survival curves for pup retrieval and pup licking behaviors, indicate the rate at which HDACi treated males (N = 9) retrieve or lick pups compared to the control males (N = 10).
| Measure (HDACi v Control) | Hazard Ratio [95%CI] | p-value |
|---|---|---|
| Test Day 1 | ||
| Latency to retrieve the first pup | 4.13 [1.3, 13.0] | 0.01* |
| Latency to retrieve all pups | 3.31 [0.7, 14.9] | 0.12 |
| Latency to sniff/lick pups in nest | 2.90 [0.6, 12.9] | 0.16 |
| Test Day 2 | ||
| Latency to retrieve the first pup | 2.59 [0.9, 7.3] | 0.07 |
| Latency to retrieve all pups | 3.31 [1.1, 10.0] | 0.03* |
| Latency to sniff/lick pups in nest | 4.00 [1.3, 12.5] | 0.04* |
p<0.05
Acknowledgements:
This work was supported by the National Institute of Child Health and Human Development [1R01HD087709-01A1] and the National Institute of Mental Health [R01MH103322].
Footnotes
Disclosure of Conflicts of Interest:
The authors declare no conflicts of interest.
**This manuscript is submitted for consideration in the Parental Brain Special Issue
References
- 1.Jakubowski M, Terkel J. Infanticide and caretaking in non-lactating Mus musculus: influence of genotype, family group and sex. Anim Behav. 1982;30:1029–1035. [Google Scholar]
- 2.Huck UW, Soltis RL, Coopersmith CB. Infanticide in male laboratory mice: effects of social status, prior sexual experience, and basis for discrimination between related and unrelated young. Anim Behav. 1982;30:1158–1165. [Google Scholar]
- 3.Vom Saal FS, Howard LS. The regulation of infanticide and parental behavior: implications for reproductive success in male mice. Science. 1982;215:1270–1272. [DOI] [PubMed] [Google Scholar]
- 4.Elwood RW, Ostermeyer MC. Does copulation inhibit infanticide in male rodents? Anim Behav. 1984;32:293–294. [Google Scholar]
- 5.Leblond CP. Extra-hormonal factors in maternal behavior. Proc Soc Exp Biol Med. 1938;38:66–70. [Google Scholar]
- 6.Tachikawa KS, Yoshihara Y, Kuroda KO. Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. J Neurosci. 2013;33:5120–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Numan M, Stolzenberg DS. Hypothalamic interaction with the mesolimbic dopamine system and the regulation of maternal responsiveness. Neurobiol Parent Brain. 2008;3–22. [Google Scholar]
- 8.Fang Y-Y, Yamaguchi T, Song SC, et al. A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors. Neuron. 2018;98:192–207.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohl J, Babayan BM, Rubinstein ND, et al. Functional circuit architecture underlying parental behaviour. Nature. 2018;556:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheehan T, Paul M, Amaral E, et al. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106:341–356. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan TP, Cirrito J, Numan MJ, et al. Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behav Neurosci. 2000;114:337. [DOI] [PubMed] [Google Scholar]
- 12.Bridges RS, Mann PE, Coppeta JS. Hypothalamic involvement in the regulation of maternal behaviour in the rat: inhibitory roles for the ventromedial hypothalamus and the dorsal/anterior hypothalamic areas. J Neuroendocrinol. [DOI] [PubMed] [Google Scholar]
- 13.Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–727. [DOI] [PubMed] [Google Scholar]
- 14.Sheehan T, Numan M. Estrogen, progesterone, and pregnancy termination alter neural activity in brain regions that control maternal behavior in rats. Neuroendocrinology. 2002;75:12–23. [DOI] [PubMed] [Google Scholar]
- 15.Numan M, Numan MJ, Schwarz JM, et al. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005;158:53–68. [DOI] [PubMed] [Google Scholar]
- 16.Shahrokh DK, Zhang T-Y, Diorio J, et al. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afonso VM, King S, Chatterjee D, et al. Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup-and food-stimuli in the female rat. Horm Behav. 2009;56:11–23. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda KO, Numan M. The medial preoptic area and the regulation of parental behavior. Neurosci Bull. 2014;30:863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Numan M, Sheehan TP. Neuroanatomical Circuitry for Mammalian Maternal Behavior a. Ann N Y Acad Sci. 1997;807:101–125. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan TP, Numan M. Microinjection of the tachykinin neuropeptide K into the ventromedial hypothalamus disrupts the hormonal onset of maternal behavior in female rats. J Neuroendocrinol. 1997;9:677–687. [DOI] [PubMed] [Google Scholar]
- 21.Vom Saal FS. Time-contingent change in infanticide and parental behavior induced by ejaculation in male mice. Physiol Behav. 1985;34:15. [DOI] [PubMed] [Google Scholar]
- 22.Kovács KJ. Measurement of Immediate-Early Gene Activation- c-fos and Beyond. J Neuroendocrinol. 2008;20:665–672. [DOI] [PubMed] [Google Scholar]
- 23.Spiegel I, Mardinly AR, Gabel HW, et al. Npas4 Regulates Excitatory-Inhibitory Balance within Neural Circuits through Cell-Type-Specific Gene Programs. Cell. 2014;157:1216–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuneoka Y, Tokita K, Yoshihara C, et al. Distinct preoptic‐BST nuclei dissociate paternal and infanticidal behavior in mice. EMBO J. 2015;34:2652–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Zang C, Cui K, et al. Genome-wide Mapping of HATs and HDACs Reveals Distinct Functions in Active and Inactive Genes. Cell. 2009;138:1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer HS, Helton J, Torres LY, et al. Histone deacetylase inhibitor treatment induces postpartum-like maternal behavior and immediate early gene expression in the maternal neural pathway in virgin mice. Horm Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, Lin Y. Npas4: linking neuronal activity to memory. Trends Neurosci. 2016;39:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luckman SM, Dyball RE, Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci. 1994;14:4825–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bepari AK, Sano H, Tamamaki N, et al. Identification of optogenetically activated striatal medium spiny neurons by Npas4 expression. PloS One. 2012;7:e52783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramamoorthi K, Fropf R, Belfort GM, et al. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science. 2011;334:1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y, Bloodgood BL, Hauser JL, et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minamiyama M, Katsuno M, Adachi H, et al. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2004;13:1183–1192. [DOI] [PubMed] [Google Scholar]
- 34.Duque-Wilckens N, Steinman MQ, Busnelli M, et al. Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female California mice. Biol Psychiatry. 2018;83:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jahn-Eimermacher A, Lasarzik I, Raber J. Statistical analysis of latency outcomes in behavioral experiments. Behav Brain Res. 2011;221:271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachmanov AA, Reed DR, Beauchamp GK, et al. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svare B, Mann M. Infanticide: genetic, developmental and hormonal influences in mice. Physiol Behav. 1981;27:921–927. [DOI] [PubMed] [Google Scholar]
- 38.Amano T, Shindo S, Yoshihara C, et al. Development-dependent behavioral change toward pups and synaptic transmission in the rhomboid nucleus of the bed nucleus of the stria terminalis. Behav Brain Res. 2017;325:131–137. [DOI] [PubMed] [Google Scholar]
- 39.Wynne-Edwards KE, Timonin ME. Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–121. [DOI] [PubMed] [Google Scholar]
- 40.Gandelman R, Vom Saal FS. Pup-killing in mice: the effects of gonadectomy and testosterone administration. Physiol Behav. 1975;15:647–651. [DOI] [PubMed] [Google Scholar]
- 41.Stolzenberg DS, Stevens JS, Rissman EF. Experience-facilitated improvements in pup retrieval; evidence for an epigenetic effect. Horm Behav. 2012;62:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stolzenberg DS, Stevens JS, Rissman EF. Histone deacetylase inhibition induces long-lasting changes in maternal behavior and gene expression in female mice. Endocrinology. 2014;155:3674–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris MJ, Karra AS, Monteggia LM. Histone deacetylases govern cellular mechanisms underlying behavioral and synaptic plasticity in the developing and adult brain. Behav Pharmacol. 2010;21:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Intlekofer KA, Berchtold NC, Malvaez M, et al. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38:2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeh S-H, Lin C-H, Gean P-W. Acetylation of nuclear factor-κB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol. 2004;65:1286–1292. [DOI] [PubMed] [Google Scholar]
- 46.Levenson JM, O’Riordan KJ, Brown KD, et al. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. [DOI] [PubMed] [Google Scholar]
- 47.Qu K, Zaba LC, Satpathy AT, et al. Chromatin accessibility landscape of cutaneous T cell lymphoma and dynamic response to HDAC inhibitors. Cancer Cell. 2017;32:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernstein BE, Mikkelsen TS, Xie X, et al. A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell. 2006;125:315–326. [DOI] [PubMed] [Google Scholar]
- 49.Henriques T, Scruggs BS, Inouye MO, et al. Widespread transcriptional pausing and elongation control at enhancers. Genes Dev. 2018;32:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saha RN, Wissink EM, Bailey ER, et al. Rapid activity-induced transcription of Arc and other IEGs relies on poised RNA polymerase II. Nat Neurosci. 2011;14:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Creyghton MP, Cheng AW, Welstead GG, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci. 2010;107:21931–21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karmodiya K, Krebs AR, Oulad-Abdelghani M, et al. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics. 2012;13:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moffitt JR, Bambah-Mukku D, Eichhorn SW, et al. Molecular, spatial and functional single-cell profiling of the hypothalamic preoptic region. Science. 2018;eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Numan M, Numan MJ. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. J Neuroendocrinol. 1997;9:369–384. [DOI] [PubMed] [Google Scholar]
- 55.Numan M, Numan MJ, Marzella SR, et al. Expression of c-fos, fos B, and egr-1 in the medial preoptic area and bed nucleus of the stria terminalis during maternal behavior in rats. Brain Res. 1998;792:348–352. [DOI] [PubMed] [Google Scholar]
- 56.Tsuneoka Y, Maruyama T, Yoshida S, et al. Functional, anatomical, and neurochemical differentiation of medial preoptic area subregions in relation to maternal behavior in the mouse. J Comp Neurol. 2013;521:1633–1663. [DOI] [PubMed] [Google Scholar]
- 57.Numan M Neurobiology of social behavior: toward an understanding of the prosocial and antisocial brain. Academic Press, 2014. [Google Scholar]


