Abstract
Self-regulation is one primary mechanism in interventions for health behaviour change and has been examined in numerous recent meta-analyses, which this meta-review systematically synthesizes. The meta-review protocol was pre-registered in PROSPERO (CRD42017074018): Meta-analyses of any intervention and health behaviour/outcome were eligible if they quantitatively assessed self-regulation and appeared between January 2006 and August 2017. Following a systematic literature search, we identified 12,198 abstracts, and 66 meta-analyses were ultimately eligible; 27% reported a protocol, 11% used GRADE; 58% focused on RCTs. Reviews satisfied only a moderate number of items on the AMSTAR 2 (M = 45.45%, SD = 29.57%). Only 6% of meta-analyses directly examined whether changes in self-regulation predicted behaviour change (i.e., self-efficacy and physical activity, l = 2; frequency of self-monitoring and goal attainment, l = 1; cognitive bias modification and addiction, l = 1). Meta-analyses more routinely assessed self-regulation by comparing the efficacy of intervention components (97%), such as those from behaviour change taxonomies. Meta-analyses that focused on intervention components identified several as successful, including personalized feedback, goal setting, and self-monitoring; however, none were consistently successful in that each worked only for some health behaviours and with particular populations. There was also inconclusive evidence for some components given that they were only examined in low quality reviews. Future reviewers should utilize advanced methods to assess mechanisms, and study authors should report hypothesized mechanisms to facilitate synthesis.
Keywords: behaviour change techniques, health, intervention, meta-review, self-regulation
Introduction
Chronic disease accounts for 2/3 of deaths worldwide (WHO, 2011, 2014). Chronic disease risk factors include health behaviours linked to lifestyle-related actions in which people commonly must engage (e.g., exercise, healthy eating) or avoid (e.g., alcohol use, smoking) as well as related physical health risks, including high blood pressure or high body-mass index (Office of Disease Prevention and Health Promotion, 2017). As such, many behavioural interventions have been developed to address changing the behaviours that lead to chronic disease or to improve its management. As a result, thousands of primary studies and hundreds of research syntheses have attempted to catalogue whether, and if so, how, these interventions work to improve health and reduce the burden of chronic disease. Given the mountain of evidence already accumulated, the purpose of this meta-review of extant meta-analyses is to compile knowledge about the mechanisms underlying health behaviour change, with a specific focus on self-regulation for behaviours related to the incidence of chronic disease. By compiling self-regulation mechanisms across a wide range of interventions and health outcomes related to chronic disease, this meta-review seeks to inform future research and policies by identifying optimal strategies to change important health behaviours.
Changing Health Behaviours
One key mechanism involved in behaviour change is self-regulation or the “ability to flexibly activate, monitor, inhibit, persevere, and/or adapt one’s behaviour, attention, emotions, and cognitive strategies in response to direction from internal cues, environmental stimuli and feedback from others, in an attempt to attain personally-relevant goals” (Moilanen, 2007 in Van Genugten, Dusseldorp, Massey, & Van Empelen, 2017). Self-regulation is a broad construct, but it can be characterized with three main dimensions: (a) emotion regulation, which is the ability to manage and respond to emotional experiences including acceptance, experiential avoidance, expressive suppression, mindfulness, problem solving, and reappraisal; (b) cognitive regulation, such as attention shifting, delay discounting, future orientation, and impulsivity; and (c) self-related processes such as self-affirmation and self-efficacy (Naragon-Gainey, McMahon, & Chacko, 2017). Evidence implicates self-regulation in many health behaviours. For example, research indicates that individuals with high trait self-control are more likely to habitually engage in healthy behaviours and have fewer poor health habits (Hagger, 2018); alternatively, those with poor self-control are liable to engage in a range of unhealthy behaviours (Bickel, Quisenberry, Moody, & Wilson, 2015).
In the context of intervention programming, practitioners could aim to increase self-regulation and foster health(ier) behaviours and maintenance of desired outcomes via differing components and strategies. For example, interventions seeking to enhance adherence to a healthier diet may include components that lead to initiating self-regulation, such as setting goals (e.g., eat three vegetables a day) and reviewing progress on those goals, self-monitoring behaviours (e.g., keep a diary of meal choices) or outcomes (e.g., changes in blood pressure), or by providing personalized feedback. Interventions could also include other components that might enhance self-regulation such as identifying potential barriers to meeting one’s goal (e.g., local lunch places only have fast food options) and creating action plans to address these barriers (e.g., grocery shopping to create healthy bagged lunches). Each of these components could, in theory, be independently assessed for effectiveness, provided the study is designed to do so. Indeed, recently scholars have succeeded in extracting into a taxonomy the most basic units of interventions that can be implemented and measured, including as many as 93 different behaviour change techniques (BCTs) (Michie et al., 2013). In addition to comparison of components, intervention studies could more directly examine whether self-regulation changes are linked to behaviour change by measuring initial and post-intervention self-regulation skills using established measures (e.g., Barratt Impulsiveness Scale; (Barratt, 1959), Brief Self-Control Survey (Tangney, Baumeister, & Boone, 2004)) and further examining whether these changes were linked to subsequent behaviour changes.
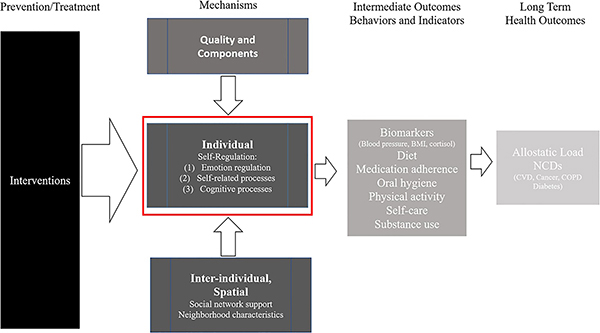
Considering the “black box” of present-day interventions with multiple components, and the many aspects of self-regulation that could be incorporated in health behaviour change interventions, as suggested by research synthesis experts (Anderson et al., 2011), we developed a logic model to guide our examination of mechanisms in this meta-review (Figure 1). In this model, interventions are broadly considered, and the focus is on the self-regulation component, acknowledging from an ecological perspective (Bronfenbrenner, 1979; Giles-Corti & Donovan, 2003; Johnson et al., 2010), the importance of the surrounding environment and other study- and intervention-specific factors. Thus, while our focus is on self-regulation mechanisms, it is within the context of interventions and the study of intermediate health behaviours (diet, exercise, medication adherence, substance use) and health indicators (blood pressure, BMI, cholesterol) as well as a range of potential long-term health outcomes (cardiovascular disease, diabetes).
Figure 1.
Logic model of review aims and proposed behaviour change pathways. Note. NCD: non-communicable disease; CVD: cardiovascular disease; COPD: chronic obstructive pulmonary disease; BMI: body-mass index
Need for Comprehensive Synthesis
Given the rapidly accumulating literature base of primary studies and research syntheses, it is not surprising that numerous meta-reviews on health behaviours and outcomes exist. These meta-reviews are limited, nonetheless, as they focused on specific health behaviours or interventions, and ignored mechanisms. For example, meta-reviews were often narrowly focused on particular health behaviour domains or intervention targets, such as mental health in the general population (Enns et al., 2016), dietary and physical activity interventions (Brand et al., 2014; Greaves et al., 2011), or the association between interventions and environmental and genomic factors that influence smoking cessation (de Viron, Malats, Van, Van Oyen, & Brand, 2013). Similarly, more comprehensive meta-reviews addressing behavioural change interventions examined multiple health behaviours but minimally focused on mechanisms (Jepson, Harris, Platt, & Tannahill, 2010; Johnson, Scott-Sheldon, & Carey, 2010); these reviews clearly indicate that interventions do improve numerous health behaviours but do not identify what factors mediate these changes. In addition to the variety of populations, behaviours, and health outcome agendas, these meta-reviews have variable quality and many limitations. Given the resource-intensive nature of meta-analyses and their influence on research, practice, and policy, understanding the quality of the existing research syntheses, alongside the findings they highlight, is vital to the field. Issues around whether the research identified is representative of the body of existing work, whether review authors have addressed and integrated study quality with the presentation of findings, and whether review authors have adequately examined heterogeneity among populations, interventions, and outcomes are all integral to mapping the state of the literature. Thus, the current meta-review is unique in scope in that using our logic model, we have identified and synthesized information on self-regulation mechanisms and intervention components across a broad range of related interventions and health behaviours linked to chronic diseases. Additionally, using established tools and processes, we have identified the sources of the strongest and weakest evidence and specific areas for improvement, providing information for reviewers and primary authors alike.
Aims
The primary objective of this meta-review was (a) to compile all self-regulation factors examined as target mechanisms of behaviour change (e.g., self-efficacy, emotion regulation and attention control) in previous meta-analyses that have focused on intervention trials to promote health behaviour change. It also aimed (b) to evaluate the methodological quality of recent meta-analyses related to self-regulation mechanisms and (c) to identify the limitations of existing meta-analyses and the underlying literature via methodological quality assessments. Given these limitations, other goals were (d) to determine the extent to which interventions engage target mechanisms of action, (e) identify where these links are most robust across varying methods (e.g., do different interventions engage similar mechanisms?), and (f) to determine which BCTs link to target mechanisms and/or behaviour.
Health behaviour interventions do not always work for all as intended, and outcomes may vary by differing population-level characteristics. Thus, as secondary objectives, we sought to determine whether self-regulation mechanisms of health behaviour change differ depending on key life conditions, including by stage of life (i.e., child, adolescent, adult, elderly), social adversity (e.g., low socioeconomic status), or clinical populations (e.g., asthma, diabetes). We also sought to examine overall trends in the literature, including factors related to higher quality meta-analyses, such as whether funding was available, the size of the author team, journal impact factor, and other review characteristics that previous research has identified as linked to review quality (Johnson et al., 2010; Polanin, Hennessy, & Tanner-Smith, 2017; Polanin, Tanner-Smith, & Hennessy, 2016). In addition to self-regulation mechanisms, we aimed to identify any environmental mechanisms (e.g., social support, neighbourhood context) that the synthesis literature examined.
Method
In 2017, prior to beginning this review, the team registered a protocol in PROSPERO (CRD42017074018). We describe any methodological deviations from the protocol in the appropriate methods sub-section, below; if no deviations are mentioned, then there were none in the conduct of that part of the review. Prior to each stage of the review, researchers (EAH, BTJ, RLA) experienced in systematic review processes trained undergraduate and graduate students on the protocol, screening instruments and data extraction and the assessment of meta-analysis quality process. Members of this trained team then conducted all review processes, supervised by the project investigators and facilitated by weekly meetings. Literature screening was facilitated by Eppi-Reviewer (Thomas, Brunton, & Graziosi, 2010), and data extraction, including AMSTAR 2 quality assessment, was conducted using the survey function in RedCAP (Harris et al., 2009). Analysis was conducted using Stata 15.1 (Stata Corp., 2017).
Inclusion Criteria
This review sought to identify specific self-regulation mechanisms between interventions/treatments and health behaviour change and/or resulting health outcomes. Thus, we included any self-regulation mechanisms addressed in existing meta-analyses of health behaviour interventions. Meta-analyses that evaluated trials of interventions applicable to the general public or non-institutionalized individuals were eligible. Meta-analyses that included restrictions to population diagnoses of certain disorder(s) were not excluded: For example, if the population was restricted to individuals diagnosed with a depressive disorder, but individuals were not institutionalized due to the diagnosis, then the review was included.
Meta-analyses that focus on interventions meant to improve health behaviours were eligible for this meta-review and thus encompassed a wide range of potential interventions and could include, but was not limited to, the following: computerized games, goal setting, health coaching, implementation intentions (a type of self-regulatory, if-then planning intervention), mindfulness training, mobile technology, motivational interviewing, and virtual reality. Meta-analyses of drug-based treatments were excluded unless a component of the intervention addressed improving medication adherence, rather than the effects of the drug itself (e.g., an intervention aiming to improve adherence to hypertension medication). Meta-analyses that focus on self-regulation strategies outside the context of an intervention/treatment and behaviour change were excluded (e.g., the impact of physical activity on cognition). Meta-analyses with multiple types of comparator groups were eligible. Comparator groups could contain another intervention of equal, lesser, or greater intensity than the primary one of study (e.g., treatment as usual, wait-list control, intervention + additional components).
The behaviour change domains of focus in this meta-review are those that are linked to chronic lifestyle diseases and include health behaviours and health outcomes resulting from those behaviours (Office of Disease Prevention and Health Promotion, 2017). These domains include the following: nutrition, physical activity, and obesity; oral health; and substance abuse and tobacco use. Specific health outcomes of interest include health behaviours and outcomes related to health behaviours such as diet, medical regimen adherence, obesity, oral hygiene, physical activity, self-care, sleep, and substance use.
The primary outcomes were the relationship between any self-regulation mechanisms that could feasibly influence health behaviour changes and/or health behaviour outcomes. We were broad in our original definition of self-regulation mechanism and included any report that examined one of the three dimensions outlined in the Introduction (i.e., emotion regulation, cognitive regulation and self-related processing). One adjustment was made to this criterion after initial screening began; we identified a number of meta-analyses that explored specific intervention features that could be considered relevant proxies of self-regulation behaviour (e.g., “self-monitoring of behaviour”, “goal setting of behaviour”, or “problem solving”). As a result, senior research team members (EAH, BTJ, RLA) reviewed the most current (and most exhaustive) behaviour change taxonomy (Michie et al., 2013) for any single intervention components that are considered related to self-regulation and, after discussion, identified 11 components that were particularly relevant to self-regulation as well as the broader category of “inhibitory control training” interventions: goal setting, prompt review of goals, prompt self-monitoring, emotional control training, prompt self-talk, stress management, action planning, barrier identification/problem solving, relapse prevention/coping planning, time management, provide feedback, and inhibitory control training. In keeping with our interest to map self-regulation mechanisms in the review literature, we also allowed for additional potential self-regulation mechanisms to be included if screeners identified new mechanisms that had not been specified during this process: We discussed any potential mechanisms identified by team members during our weekly meetings. The focus of our review precluded meta-analyses that focused on other mechanisms of behaviour change such as the role of motivation or intentions. For example, Webb and Sheeran’s (2006) meta-analysis did not qualify because it did not directly examine any self-regulation-focused mechanisms or BCTs. (See Table A1 in the Appendix for detailed information on how we defined and categorized each self-regulation intervention component for coding.)
Meta-analyses that compared the presence/absence of the specific components of interest were also only eligible if the meta-analysis made the comparison quantitatively. Thus, meta-analyses were included if they identified and quantitatively explored (e.g., via meta-analysis, subgroup analysis, meta-regression, or sensitivity analysis) at least one self-regulation mechanism directly or one of the self-regulation intervention components involved in health behaviour change interventions. Mere descriptive reporting of these characteristics did not warrant the review’s inclusion in our meta-review. Given the variable quality of systematic reviews and their lack of a quantitative synthesis, systematic reviews were ineligible.
Secondary outcomes were of interest in this meta-review, but a review was not excluded if it did not provide one. Although we had planned to collect other secondary mechanisms of interest if provided by review authors (e.g., other individual characteristics and intervention characteristics such as intervention duration, dose, theoretical underpinning, practical components, intervention delivery, and practitioner experience and training), this goal was too unwieldy given the diversity of behaviours, intervention types, and secondary targets presented by review authors. Thus, we elected to focus on any inter-individual characteristics (e.g., social network support) and neighbourhood characteristics such as levels of crime and violence, environmental presence of health-promoting factors (e.g., walkable streets), or risk-promoting factors (e.g., availability of tobacco or alcohol via local shops). For any of these secondary outcomes to be included in our meta-review, the review authors should have identified these mechanisms and included them in a quantitative analysis (e.g., subgroup or sensitivity analysis or by meta-regression).
To be the most relevant to current practice and to ensure up-to-datedness of the meta-review (Pieper, Antoine, Neugebauer, & Eikermann, 2014; Thomson, Russell, Becker, Klassen, & Hartling, 2010), meta-analyses published before 2006 were ineligible for this review unless they were linked to an updated review published after that date. There were no exclusion criteria for publication year of the primary studies in the meta-analyses; that is, meta-analyses that included studies from any year were still eligible for the meta-review.
Search and Screening Process
We reviewed the literature on self-regulation, health behaviours, and chronic diseases to compile an initial list of search terms. These terms were finalized after consulting with two reference librarians and piloting searches to refine the search strategy. Two team members (EAH, JSJ) searched the following electronic databases (hosts) in August 2017: PubMed, Education Resources Information Center, EMBASE (Scopus), PsycINFO, CINAHL, Cochrane Database of Abstracts of Reviews of Effects, and the Cochrane Database of Systematic Reviews. The search strategy for PubMed is included in the supplemental files (S1) and was modified to suit other databases. The bibliographies of identified reviews and meta-reviews were reviewed for additional eligible reviews. We did not exclude any meta-analyses based on publication status or language of publication.
Given the large search return and resulting time constraints as well as a change in review scope after electronic searches were conducted, we used a modified screening schedule. First, all team members screened the same 25 titles and abstracts: Discrepancies were reviewed, and the screening protocol was clarified. Next, 10% of the reports were screened independently and in duplicate at the title/abstract level (approximately 411 per person). Discrepancy statistics were calculated, and discrepancies were discussed and resolved; screeners were instructed to be overly inclusive at this stage to ensure that meta-analyses with potentially eligible mechanisms that were not discussed in the abstracts were still included for full-text review. This decision meant significant additional work, but it was deemed necessary to ensure that all relevant meta-analyses were obtained. Prior to commencing full-text review, to reduce the size of the review, the scope of the meta-review was modified to focus on outcomes related to health behaviours only and not mental health outcomes. Three of the senior project members (EAH, KM, RLA) re-reviewed potentially eligible titles/abstracts and categorized them as focusing on mental health outcomes only (to save for another review focused solely on mental health) or behavioural outcomes. If a meta-analysis focused on behaviour and mental health outcomes, it was eligible for this meta-review, but only the behaviour outcomes were used in the descriptive synthesis. This subset of potentially eligible titles/abstracts concerned with behaviour outcomes was used to generate a list of full-text articles to retrieve and screen. Training for full text screening then commenced with eight team members independently screening the same 10 reports. The screening of these 10 reports were reviewed; discrepancies were discussed, and the screening manual was updated. Everyone was then assigned to screen another set of the same five reports. Screening of these five was again reviewed, discrepancies discussed, and the screening manual was updated with clarifications. The remaining set of full texts was split between the team: The first 25 in each set was used to assess agreement between screeners (who screened independently and in duplicate), when senior team members were paired with junior team members. Junior team members were then paired with another team member for double-screening and senior team members screened the remaining full texts independently.
Data Extraction
Three independent reviewers used a standardized coding form to extract data from eligible reviews. The first third (l = 22) of meta-analyses were coded independently and in duplicate. Discrepancies between independent reviewers were resolved through discussion and consensus. For the remaining studies, one trained coder (JSJ) independently extracted data that were checked for accuracy by one senior team member (EAH). Discrepancies were discussed and resolved. Two additional variables were coded by a single reviewer (JSJ) after the meta-analyses were identified and coded for other pre-specified items: (1) the number of citations of each review (according to Google Scholar, January 2019) and (2) the impact factor of the journal in which individual meta-analyses were published according to the InCites Journal Citation Reports for the year the review was published (Clarivate Analytics, 2018).
Assessment of Meta-Analysis Quality
To assess the quality of the included meta-analytic reviews, two independent reviewers (EAH, JSJ) used the AMSTAR 2 instrument (Shea et al., 2017). We originally planned to use the original AMSTAR (Shea et al., 2009; Shea et al., 2007), but in the meantime an updated version was published. The first third (l = 22) of meta-analyses were rated independently and in duplicate. Discrepancies between reviewers were resolved through discussion and consensus. For the remaining studies, one coder independently assessed risk of bias (JSJ) and ratings were checked for accuracy by one senior team member (EAH). Discrepancies were discussed and resolved.
We used the AMSTAR 2 tool to estimate a threshold of review quality for reporting purposes. For the purpose of this meta-review, meta-analyses that satisfied at least 70% of the eligible AMSTAR 2 items were considered higher quality meta-analyses, while those with 50–69% completion were considered medium quality, and reviews less than 50% completion were considered low quality. Although these cut-points are somewhat arbitrary, they serve the qualitative purpose of helping to organize the literature. Items with No/Partial Yes/Yes options were given scores of 0/1/2, respectively, while items with No/Yes options were given scores of 0/2. The total calculation for each study was estimated out of the applicable items only; for example, in the case of a review that excluded non-randomized trials, the assessment of risk of bias of non-randomized trials is considered not applicable (NA). At present, there are no clear guidelines on how to address meta-analytic quality in meta-reviews; previous meta-review authors have used this method or a similar approach, although some have elected to remove meta-analyses of lower quality. To be fully transparent and remain rigorous, we chose to compare the highest quality of evidence with reviews of medium and low quality and to report individual ratings for all included meta-analyses (in the Appendix); doing so permits a determination of the robustness with which particular patterns appeared across meta-analyses (i.e., patterns that appear regardless of methodological quality are more robust than those that do not so appear). Additionally, we report our coding of subsets of items that enabled a “Yes/Probably yes/No” distinction for each included review in the Appendix (e.g., whether authors detailed the five PICO components, and whether specific search strategies were reported).
Synthesis
We conducted a narrative synthesis, guided by our logic model and the AMSTAR 2 quality ratings. Given the diversity of analyses across the included reviews, we present quantitative results in the metric originally presented in the reviews. When review authors reported heterogeneity statistics for particular outcomes, this information is also included with the narrative as appropriate (note that only 25% of the coded outcomes included heterogeneity data). We supplement this synthesis with quantitative descriptive summaries and examine trends over time and across topics.
The corrected covered area (CCA: Pieper, Antoine, Mathes, Neugebauer, & Eikermann, 2014) was estimated to determine the relative coverage, or overlap of primary studies, of the included meta-analyses. According to the authors, CCA scores between 0–5% are considered slight overlap, 6–10% are considered moderate overlap, 11–15% are considered high overlap, and scores greater than 15% are considered to have very high overlap (Pieper et al., 2014). The results from meta-analyses with moderate or high overlap, but no shared authorship team, are reported and compared. Meta-analyses with very high overlap in primary study by the same authors/study team (i.e., if there was a primary meta-analysis and then additional meta-analyses of secondary questions) were reviewed as a single review, and unique outcomes with unique samples were prioritized so as to ensure independence (e.g., van Genugten, Dusseldorp, Webb, & van Empelen, 2016; Webb, Joseph, Yardley, & Michie, 2010). Meta-analyses with slight or moderate overlap (< 10%) and unique outcome data were considered as separate reviews, although if the meta-analyses were reported by the same author teams and indicated use of similar methods, then information from all relevant reports was used in assessing meta-analysis quality and other review characteristics (e.g., Tanner-Smith & Lipsey, 2015; Tanner-Smith et al., 2015).
Results
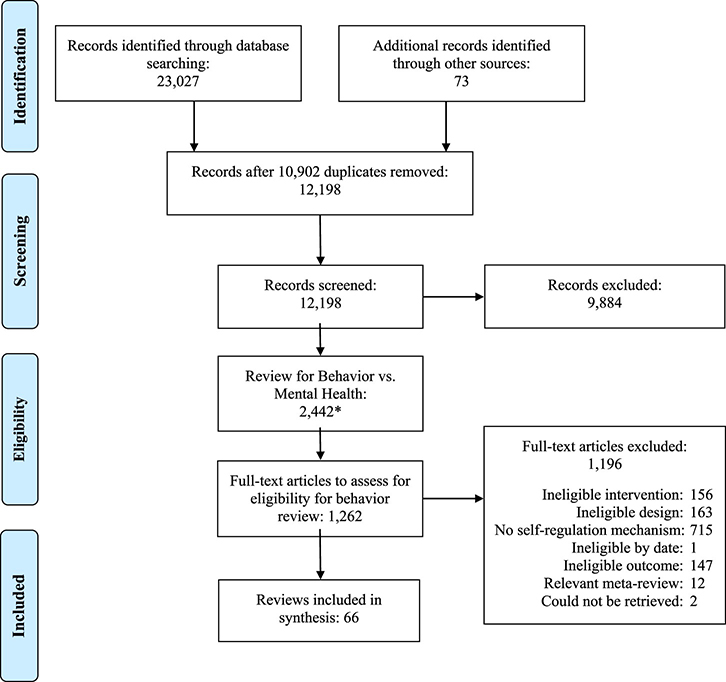
As Figure 2 shows, the initial search generated 23,027 records from electronic databases and 73 records from other sources. After removal of duplicates, 12,198 abstracts remained for screening, of which 9,884 were initially excluded. A second round of screening to differentiate the mental health-focused from the health-behaviour-focused meta-analyses resulted in 1,262 articles for full text screening. Of these, 66 meta-analyses were eligible for the meta-review (the supplemental files, S2, include a bibliography that lists each eligible meta-analysis). Across the included reviews, searches for eligible studies were on average conducted in 2012 (range: 2004 to 2017) and the lag from search to publication was 1.71 years (SD = 1.32; range: 0 to 8). On average, meta-analyses included 65 primary studies (SD = 103.57, range: 6 to 379); among the 48 meta-analyses that reported total participant sample size, there were an average of 37,578 participants per meta-analysis (SD = 101,776; range: 1,081 to 568,811). The majority of meta-analyses (l = 38; 58%) limited eligible study designs to randomized controlled trials (RCTs), although authors rarely reported a rationale for this decision. Overall, the literature assessed appears very comprehensive, given that primary studies included in meta-analyses were conducted as early as 1968. As Table A1 indicates, 45% of included meta-analyses were primarily in the area of promoting healthy behaviours; the remaining were broadly focused on preventing/reducing risky behaviours (23%), cardiovascular disease (CVD) prevention and treatment (11%), medication regimen/medication adherence (12%), and diabetes management (9%). Overlap across these meta-analyses according to the CCA was minimal (< 1%) and was minimal within each health behaviour domain: promoting healthy behaviours (< 1%), preventing risky behaviours (1%), CVD prevention and treatment (2%), medication regimen/medication adherence (4%), and diabetes management (3%).
Figure 2.
Flow of reports and meta-analyses into the meta-review. Note. The asterisk indicates that some additional titles/abstracts were deemed ineligible and excluded at certain points in the screening process.
Methodological Quality of the Meta-Analyses
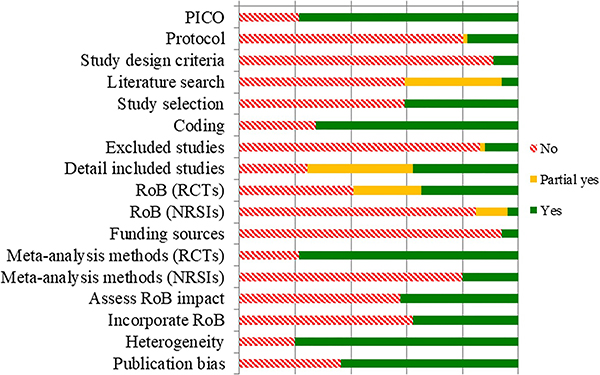
On average, meta-analyses achieved 45.45% completion of all AMSTAR 2 items (SD = 19.57%), ranging from 8.33–87.5% completion. Only 27 (41%) meta-analyses scored over 50% on the AMSTAR 2 and only 6 (9%) had 70% or more item completion; two (3%) had the highest score of 88%. The remaining 39 (59%) meta-analyses had an average of 32% completion of items (range 8 to 47%). Approximately 9% of meta-analyses were Cochrane reviews, and another 32% described using the PRISMA reporting standards. Less than half (27%) of the meta-analyses registered a protocol or discussed creating a protocol to guide the review. All meta-analyses reported searching at least two electronic databases, and 91% reported their search strategy, but only 6% fully satisfied all eight criteria for literature searching. Meta-analyses that justified restrictions (e.g., language, publication status; 44%) and that searched trial registries (45%) completed significantly more AMSTAR 2 items (p < .05). The majority (62%) reported contacting authors for missing data (e.g., data needed to calculate effect sizes). Approximately 32% of meta-analyses did not use any formalized tool to assess risk of bias; of the 45 that assessed study risk of bias, most used the Cochrane Risk of Bias scale (51%) and only a few used Jadad (8%), Downs and Black (4%) or another standardized scale (13%), and 24% reported creating their own tool. Similarly, approximately 32% of the meta-analyses did not assess potential for publication or small study bias. Of those that did, 64% used multiple approaches including funnel plots (86%), contour-enhanced funnel plots (4%), a regression-based test (44%), Trim and Fill (24%), and Failsafe N (20%). Only a minority of meta-analyses used GRADE to present findings (11%). Figure 3 summarizes quality assessments across all studies (supplemental files include a rating for each item for each study in Table 2, correlations between quality items and publication characteristics in Tables 3 and 4, and a comparison of AMSTAR 2 items met for reviews that achieved AMSTAR 2 completion of 70% or more versus reviews under that bar in Figure 1.).
Figure 3.
Quality Assessment Results according to AMSTAR 2 ratings, across all included meta-analyses. Note. NSRI: non-randomized studies of interventions; PICO: specification of inclusion criteria including the population, intervention, comparison, outcome; RCT: randomized controlled trial; RoB: risk of bias.
Visually, meta-analysis quality appears to increase over time, but this association did not reach statistical significance (see supplemental files, Figure 2, panel a). Most (55%) meta-analyses reported receiving funding, and 9% reported no funds, but 36% of meta-analyses left this fact unclear; there was no statistically significant correlation between receipt of funding and proportion of AMSTAR 2 completion. On average, meta-analyses had five authors (SD = 2.42, range: 2 to 12); as the number of meta-analysis authors increased, the proportion of AMSTAR 2 items completed increased, r = 0.50, p < .001, indicating improved methodological quality. Journal impact factor was on average 5.08 (SD = 4.16, range: 1.828 to 25.547); it was significantly correlated with the number of Google Scholar citations per year, but neither Google Scholar citations nor journal impact factor associated with total meta-analysis quality. Additionally, more recent reviews have better reported the flow of studies into the meta-analysis, risk of bias (in terms of RCTs), and were more likely to report conflicts of interest.
Meta-Analysis Scope and Focus
Reviews most often focused on broad health intervention categories (l = 36), although some were limited to studies focused on the following: self-monitoring or self-management (l = 8), self-monitoring and a broad/other intervention type (l = 5), mHealth/online (l = 3), inhibitory control training/cognitive bias modification (l = 4), implementation intentions (l = 3), brief interventions (l = 2), psychological (l = 1), worksite-based interventions (l = 1), behavioural/cognitive change strategies (l = 2), pedometer use (l = 1), and relapse prevention (l = 1). Meta-analyses focused on a range of health behaviours and, at times, overlapped in health focus: physical activity (33%), diet/healthy eating (23%), lifestyle or multiple behaviours (18%), smoking (15%), medication adherence (14%), blood pressure/hypertension (14%), alcohol consumption (12%), diabetes (9%), weight change (8%), sexual health behaviours (5%), substance use (5%), other behaviours related to specific disease management (3%; i.e., asthma, chronic obstructive pulmonary disease [COPD], hand hygiene (2%). On average, meta-analyses quantitatively examined the relationship between 1.59 health outcomes and self-regulation (SD = 1.02; range: 1 to 6).
Approximately 41% of the meta-analyses used selection criteria to specifically include studies of populations with a clinical diagnosis; of those, six focused on Type 2 diabetes, seven on individuals with hypertension, coronary heart disease or coronary artery disease (CAD), two on overweight or obese individuals, two on pregnant/post-partum women, six on those with chronic illness like COPD or MS, three on those with an unspecified illness, and one on healthy individuals or those at risk of an illness.1 Meta-analyses with a specified clinical population focus on average had slightly higher AMSTAR 2 completion than those without a specified clinical population (54% versus 40%). The majority of meta-analyses included an age, gender, or ethnicity restriction for eligible population samples, of which most of these were for age: Five meta-analyses focused on youth (AMSTAR 2 M = 26%, range: 8 to 44%), three on young adults (AMSTAR 2 M = 48%, range: 44 to 53%), and 33 on adults (AMSTAR 2 M = 46%, range: 11 to 78%), of which seven were older adults (AMSTAR 2 M = 49%, range: 11 to 78%). Two meta-analyses focused solely on women (AMSTAR 2 M = 72%, range: 56 to 88%), and one focused only on African American samples (AMSTAR 2 = 59%). For further descriptive characteristics of individual reviews, see Table A1.
Regarding our target of the review, self-regulation mechanisms, only four meta-analyses directly examined the relationship between a measure of self-regulation and health behaviours/outcomes. The remaining meta-analyses explored relationships between specific intervention components and whether the presence/absence of these components was a significant effect modifier. (See Table 5 in the online supplement for information on which components each included meta-analysis examined.)
Direct Tests of the Linkage between Self-Regulation and Behaviour Change (l = 4)
Of the four meta-analyses that directly tested the association between a self-regulation mechanism and related behaviour change, two focused on self-efficacy and health behaviours, one focused on the frequency of progress monitoring and goal attainment, and the fourth examined cognitive bias and substance use behaviours.
First, Sheeran and colleagues (2016) reviewed 240 reports of “health-related” behaviours—that is, “overt behavioural patterns, actions, or habits that relate to health maintenance, to health restoration and to health improvement” (p. 1180) within studies that experimentally induced significant changes in attitudes, norms, or self-efficacy between treatment and control groups. They found that changes in self-efficacy were significantly associated with behaviour change (d = 0.47, 95% CI [0.39, 0.56], Q = 962.42, p < .001; k = 90). There was significant heterogeneity in this effect that could not be completely explained by the moderator variables, although the effects were larger for interventions designed to increase (versus to decrease) behavioural performance. This meta-analysis satisfied 50% of the AMSTAR 2 items; problems included possible selective inclusion/reporting due to a lack of a pre-registered protocol, potential screening errors (as there was only a single screener), and the authors did not test for publication bias. Yet, the report provided detailed inclusion criteria and rationale, described duplicate data extraction, incorporated the risk of bias assessment into results and implications, and addressed heterogeneity to the degree that primary study reporting and theory allowed.
Second, one meta-analysis of 24 studies addressed self-efficacy and focused on its relationship with physical activity among non-clinical community-dwelling adults, aged 60 or older (French, Olander, Chisholm, & Mc Sharry, 2014). Across a subset of 16 studies, the authors found a positive, but non-significant relationship between the change in self-efficacy and change in physical activity (Spearman’s rho = 0.439, p = 0.089). Unfortunately, this meta-analysis satisfied only 11% of the AMSTAR 2 criteria, and, while the study detailed adequate inclusion criteria and reported funding/conflict of interest, there were many potential issues with the methods employed including screening, data extraction, and the synthesis approach. In addition, risk of bias among the primary studies was not formally assessed, and no details were provided about the heterogeneity of findings.
Third, one meta-analysis of 138 studies examined the relationship between the frequency of progress monitoring and rates of goal attainment (Harkin et al., 2016). Within a subset of 21 studies, changes in the frequency of progress monitoring significantly reduced the association between the intervention and goal attainment (Z = 13.09, p < .001), suggesting that the frequency of progress monitoring was a key mediator in the process of change. That is, findings indicate that interventions which increased the frequency of progress monitoring had stronger effects on health behaviour change. This meta-analysis satisfied 47% of AMSTAR 2 items, with potential bias in inclusion/reporting given its lack of a pre-registered protocol and unclear selection processes, a search that was less than comprehensive, provided few details on included primary studies, and used an inadequate assessment of risk of bias. The report did, however, provide detailed inclusion criteria, described duplicate data extraction, incorporated risk of bias assessment into results and implications, and addressed heterogeneity.
Last, one meta-analysis examined cognitive bias modification in 25 RCTs and addictive behaviour including alcohol consumption and smoking (Cristea, Kok, & Cuijpers, 2016). The included cognitive bias modification interventions (attention bias modification, Stop-Signal task, and Inhibition via the Go/No Go task) did not significantly reduce addiction at immediate post-test, but did reduce cognitive bias. There was also a non-significant relationship between cognitive bias and addiction (k = 19; b = 0.18, 95% CI [−0.07, 0.44]). This meta-analysis satisfied 44% of AMSTAR 2 items, with potential bias in inclusion/reporting given the lack of a pre-registered protocol, potential errors in screening or data extraction, and missing data. The strengths of this meta-analysis are that the authors incorporated risk of bias and publication bias assessments into the results and implications, reported funding/conflicts of interest, and addressed heterogeneity using subgroup and moderator analysis.
Target Self-regulation via Tests of Intervention Components (l = 64)
Of the 64 meta-analyses that analysed intervention components (two also appeared in the previous section), 36% used an established classification system including the 26-BCT version (12.5%, Abraham & Michie, 2008), 40-BCT version (12.5%, Michie et al., 2011), 93-BCT version (4.69%, Michie et al., 2013), and other (6.25%, e.g., Cugelman, Thelwall, & Dawes, 2009: Communication-Based Influence Component), while the remaining discussed similar interventions/components but did not reference any established taxonomy. Some of the meta-analyses that did not address BCTs instead focused on self-regulation intervention components added after the meta-review screening was initiated (i.e., self-management programs, implementation intention interventions, and self-affirmation interventions).
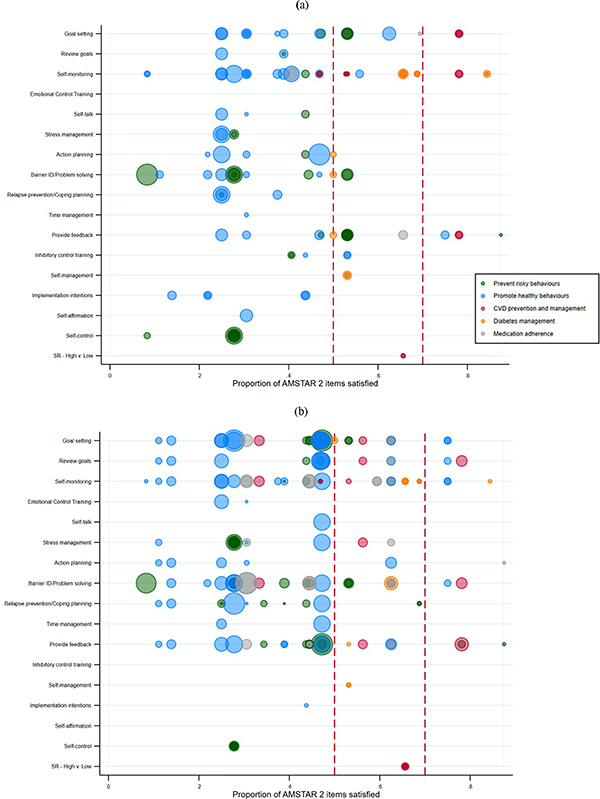
Meta-analyses examined between one and 10 BCTs/intervention components (M = 2.98, SD = 2.58). The most often analysed self-regulation intervention components in meta-analyses were prompting self-monitoring (52%), goal setting (42%), personalized feedback (41%), and barrier identification/problem solving (39%). Other self-regulation intervention components assessed included prompt review of goals (23%), relapse prevention/coping planning (20%), action planning (19%), stress management (14%), emotional control training (6%), prompt self-talk (6%), and time management (5%). Smaller numbers of meta-analyses examined inhibitory control training (l = 2), self-management programs (l = 2), implementation intention interventions (l = 3), and self-affirmation interventions (l = 1). Two meta-analyses broadly examined interventions that incorporated self-control. Four meta-analyses examined multiple self-regulation components at once (e.g., comparing interventions with high versus low numbers of self-regulation components) or interactions with self-regulation components and other factors. Figure 4 visually depicts each self-regulation intervention component for each review across the AMSTAR 2 quality scores and according to the five broad health behaviour domains; panel (a) indicates which components were identified as effective, while panel (b) indicates which components were not identified as improving the effectiveness of interventions. By the word “effective” we signify that a component results in a significant and positive outcome for participants when quantitatively compared to either (a) interventions without that component or (b) a control group that did not utilize the intervention/component. (Our Open Science Framework project page includes a dataset of all 349 self-regulation components coded from included reviews: https://osf.io/9usxt/?view_only=f104bf0d8a6b4dd595c9ccb4dd3327d1).
Figure 4.
Quality and supportiveness of meta-analyses supplied in favour (a) or opposed (b) for individual self-regulation mechanisms across all reviews. Bubbles for each meta-analysis are sized proportional to the numbers of studies each included. The vertical, green line shows the cut-point for higher versus lower quality meta-analyses.
We organize our synthesis of findings in reverse order of the frequency with which they were examined in meta-analyses. For each BCT, we examine the extent to which results generalize across levels of methodological quality and indicate consistency of results according to heterogeneity statistics (although, as previously noted, only 25% of meta-analyses provided heterogeneity statistics). We also note instances where both groups demonstrated improvement (positive outcome), but the inclusion of the BCT did not significantly improve outcomes beyond groups without the BCT. Where applicable, we indicate overlap of primary studies for particular domains.
Self-monitoring
Three high quality, eight medium quality, and 23 low quality meta-analyses examined self-monitoring.
There were three high and medium quality meta-analyses that focused on populations with diabetes and on diabetes-related outcomes (k = 6, Farmer et al., 2012; k = 12, Malanda et al., 2012; k = 15, Zhu, Zhu, & Leung, 2016) none had low quality. CCA was 38%, indicating that overlap between the three reviews was high; all of the studies in the individual participant data meta-analysis (Farmer et al., 2012) were also included in the other two reviews. Given the high degree of overlap, it is reassuring that the three meta-analyses were consistent in indicating that self-monitoring significantly reduced HBA1c and this finding was somewhat stronger for newly diagnosed patients; however, there was remaining additional heterogeneity, suggesting that some populations would not respond to self-monitoring in the same way. Other outcomes, such as blood pressure and cholesterol, had mixed results, with one meta-analysis of eight studies indicating a significant reduction in total cholesterol (Zhu et al., 2016) and two meta-analyses indicating that benefits did not translate to systolic or diastolic blood pressure (Farmer et al., 2012; Zhu et al., 2016).
Four medium quality and seven low quality reviews addressed outcomes for patients with other chronic conditions and on clinical measures of health. Two medium quality reviews (Fletcher, Hartmann-Boyce, Hinton, & McManus, 2015; Glynn, Murphy, Smith, Schroeder, & Fahey, 2010) found that self-monitoring of blood pressure consistently improved SBP (k = 9, k = 12) and DBP (k = 11, k = 14) with some remaining heterogeneity (I2 ranged from 0–67%). Similarly, a low quality review (Bray, Holder, Mant, & McManus, 2010) found that self-measuring of blood pressure lowered SBP and DBP (k = 21 and k = 23, respectively) assessed in office contexts, but also had substantial remaining heterogeneity (I2 = 71.9% and I2 = 42.1%, respectively). Participants also did not improve their mean daytime ambulatory measures of blood pressure (k = 3). Two medium and four low quality reviews focused on whether self-monitoring of medications or medication effects improved medication adherence; of these, only one medium quality review of hypertensive patients (k = 13, I2 = 43%, Fletcher et al., 2015) and one low quality review that examined older adults with at least one physical health condition (k = 3, Conn, et al., 2009) found that self-monitoring aids medication adherence. The other three reviews found no evidence of improvement of medication adherence by including self-monitoring among hypertensive patients (k = 108, Conn, Ruppar, Chase, Enriquez, & Cooper, 2015), black patients with hypertension (k = 45, Ruppar, Dunbar-Jacob, Mehr, Lewis, & Conn, 2017), patients with CAD (k = 28, Chase, Bogener, Ruppar, & Conn, 2016), or among an unspecified population (k = 98, Conn, Vicki S. & Ruppar, 2017). There was higher overlap across five of these reviews (CCA = 6%; Chase et al., 2016; Conn, et al., 2015; Conn & Ruppar, 2017; Conn, et al., 2009; Ruppar et al., 2017), likely due to the one very large and broad review whose topic definition seemed to encompass the scope of the smaller reviews (l = 771, Conn & Ruppar, 2017); thus, inconsistencies between findings are likely due to differences in the populations sampled. One final low quality review focused on whether self-monitoring of a behaviour or behavioural outcome improved any type of health behaviour among participants with a high BMI and found that both types of self-monitoring produced significant improvement (k = 42, Olander et al., 2013). One medium quality meta-analysis of asthma patients found no significant difference for a reduction in asthma symptoms with self-monitoring (k = 38, k = 27 for this outcome; Denford et al., 2014). One of the low quality reviews found that self-monitoring, but not interventions that focus on “maximizing self-regulatory skills,” significantly improved smoking cessation outcomes among patients with COPD (k = 17; Bartlett, Sheeran, & Hawley, 2014).
Two high quality and two medium meta-analyses focused on self-monitoring and weight loss and/or physical activity outcomes: Outcomes varied by population of focus. Reviews indicated that self-monitoring produced significant changes for the physical activity and/or diet outcomes in both the short-term (k = 50) and the long-term (k = 32) among overweight adults (Samdal, Eide, Barth, Williams, & Meland, 2017) and for body weight among postpartum women (k = 46, k = 17 for this outcome, Lim et al., 2015). However, there was substantial heterogeneity in the effect size for self-monitoring of body weight (I2 = 87%), suggesting that while effective for body weight, it may not be effective for all post-partum women. In contrast, self-monitoring was not found to produce significant effects on weight loss and associated activities outcomes among adults in the general population (k = 30, Sykes-Muskett, Prestwich, Lawton, & Armitage, 2015) and not among older adults (k = 19, O’Brien et al., 2015). There were mixed results among the 15 low quality reviews of similar outcomes. Self-monitoring was effective for increasing steps per day among adults (k = 26, (Bravata et al., 2007), increasing physical activity (k = 34) but not fitness outcomes (k = 18) among adults (Abraham & Graham-Rowe, 2009), increasing physical activity among adults with a chronic illness (k = 163, k = 91 for this outcome; Conn, , Hafdahl, Brown, & Brown, 2008), and for reducing weight among adults with cardio-metabolic risk factors (k = 44, k = 23 for this outcome; Dombrowski et al., 2012). Among adolescents, keeping a record of specified behaviours improved physical activity (k = 74, Brannon & Cushing, 2015). Yet, among overweight children, self-monitoring through mHealth techniques did not improve physical activity, but did improve weights status and dietary outcomes (k = 9 and k = 8, respectively; Darling & Sato, 2017). The other four reviews focused on adults found no significant improvements for self-monitoring on physical activity (Conn, Hafdahl, & Mehr, 2011; Higgins, Middleton, Winner, & Janelle, 2014), physical activity and/or healthy eating (Michie, Abraham, Whittington, McAteer, & Gupta, 2009), or any type of health behaviour (French et al., 2014).
Finally, the remaining five lower quality reviews focused on self-monitoring for unspecified populations and primarily indicated a lack of support for self-monitoring, although two reviews (Cugelman, Thelwall, & Dawes, 2011; Harkin et al., 2016) indicated support for “behaviour change” from self-monitoring of the behaviour (k = 16 and k = 17, respectively). These two reviews shared only a single primary study, so overlap was low, and it appears they sampled different populations of interventions; specifically, Cugelman and colleagues (2011) focused on mHealth/online interventions, whereas Harkin and colleagues (2016) had broader inclusion criteria. These two reviews differed in whether self-monitoring of the behavioural outcome was effective, and both effects from the Cugelman and colleagues (2011) review left substantial heterogeneity unexplained.
In sum, there is a great deal of interest in the research community on examining self-monitoring, but the quality of the evidence synthesis of this intervention component is quite variable across health behaviours, with the exception of the three reviews that examined diabetes and were all medium or high quality. These reviews overall indicate that self-monitoring can result in health behaviour change, but that success is highly variable and depends on the particular population and health outcome of study.
Goal setting
Two high-quality meta-analyses, eight medium quality, and 18 poor-quality reviews assessed the goal setting BCT and demonstrated goal setting to be inconsistently effective at yielding health behaviour change.
Both of the high-quality reviews (k = 19, O’Brien et al., 2015; k = 48, Samdal, Eide, Barth, Williams, & Meland, 2017) assessed goal setting in the context of physical activity. Both reviews found that interventions that included goal setting increased physical activity in overweight and older adults; yet, O’Brien and colleagues (2015) found no significant additional benefit to intervention efficacy versus interventions without goal setting for older adults. Two other medium-quality reviews similarly assessed physical activity (k = 45, McEwan et al., 2015) or weight-loss behaviour (k = 30, Sykes-Muskett et al., 2015) among non-specific or general adult populations. Only one found that goal-setting interventions were effective at changing physical activity (McEwan et al., 2015), whereas the other did not find increased benefit for goal setting in the context of monetary contingency contracts (Sykes-Muskett et al., 2015). Yet, McEwan and colleagues (2015) also reported high heterogeneity for this outcome indicating the presence of samples for which goal setting would not work. Although five low quality reviews found that the use of goal setting was associated with increased physical activity (k = 26, Bravata et al., 2007; k = 26, Casey et al., 2017; k = 20, Higgins et al., 2014 k = 58, Olander et al., 2013) or fitness (k = 20, Abraham & Graham-Rowe, 2009), only three reviews demonstrated that goal setting yielded additional benefits by inclusion in an intervention (Abraham & Graham-Rowe, 2009; Bravata et al., 2007; Olander et al., 2013), while two others found no additional benefit for adults with a chronic illness (k = 129, Conn, et al., 2008) or healthy adults (k = 206, Conn, et al., 2011). One low quality review found no evidence of efficacy of goal setting interventions to promote physical activity for older community-dwelling adults (k = 24, French et al., 2014).
Of the remaining six medium-quality reviews, one supported the use of goal setting interventions in improving hand hygiene (k = 41, k = 3 for this analysis, Luangasanatip et al., 2015) and two for reducing alcohol consumption (k = 41, Scott-Sheldon, Carey, Elliott, Garey, & Carey, 2014; k =185, Tanner-Smith & Lipsey, 2015). Specifically, Tanner-Smith and Lipsey (2015) found that interventions with goal setting reduced alcohol consumption and alcohol-related problems for both adolescents and young adults but were only more effective than interventions without goal setting in reducing alcohol consumption for adolescents. Scott-Sheldon et al. (2014) found a relatively nuanced pattern such that interventions with goal setting significantly reduced the quantity of alcohol use for first-year university students in terms of number of drinking weeks per month but did not reduce other measures of alcohol consumption. However low quality reviews of similar outcomes had mixed findings: One found no evidence of goal setting to change alcohol or drug use in youth (k = 23, Tanner-Smith, Steinka-Fry, Hennessy, Lipsey, & Winters, 2015), and another found that interventions with goal setting had reduced smoking rates, but not significantly more than conditions without it (k = 17, Bartlett et al., 2014).
Three further medium-quality reviews did not find goal-setting to be effective in promoting medication adherence in asthmatic patients (k = 38, Denford, Taylor, Campbell, & Greaves, 2014), improving glycaemic control in patients with Type 2 Diabetes (k = 16, Cheng et al., 2017), or reducing mortality in patients with cardiovascular heart disease (k = 22, Goodwin, Ostuzzi, Khan, Matthew, & Rona Moss-Morris, 2016). These findings were consistent with two low-quality reviews that focused on medication adherence: There was no evidence that goal setting improved medication adherence for non-specific patient populations (k = 87, Conn & Ruppar, 2017) and while goal-setting interventions improved mediation adherence for patients with CAD, it was not significantly more effective than interventions without this component (k=28, Chase et al., 2016).
Five low-quality reviews considered general health behaviour change, all focused on non-specific or general adult populations. One review found goal-setting interventions were associated with broad health behaviour changes, but high rates of heterogeneity (I2 = 70%) imply the existence of samples for which goal setting did not work (k = 16, Cugelman et al., 2011). Three other reviews found that whereas goal-setting in interventions yielded broad health behaviour change, they were not more effective than other interventions without these conditions (k = 34, McDermott, Oliver, Iverson, & Sharma, 2016; k = 122, Michie et al., 2009; k = 52, van Genugten et al., 2016), while a fourth found no efficacy of goal-setting interventions for broad health behaviour change, either when considering goal-setting for behaviour or for outcome (k = 138, Harkin et al., 2016). Finally, one review found that, compared to interventions without goal setting, outcome goal setting yielded greater fruit and vegetable consumption in adults of retirement age, whereas behaviour goal setting did not (k = 22, Lara et al., 2014).
Taken together, these reviews show inconsistent evidence for goal setting. Higher-quality meta-analyses show a higher proportion of analyses supporting goal setting, but even for these, results are disparate.
Personalized feedback
Four high quality, nine medium quality, and 14 low quality reviews assessed feedback. In two high quality meta-analyses of physical activity and physical activity and/or diet behaviours, personalized feedback was effective specifically for older adults (k = 19, O’Brien et al., 2015) and for personalized feedback on outcome (but not behaviour) among overweight or obese adults (k = 50 for short term, k = 32 for long-term, Samdal et al., 2017). However, O’Brien reported high heterogeneity (I2 = 80%). Yet, in another medium quality meta-analysis (k = 52), interventions with personalized feedback were no more effective at improving physical activity than interventions without unless combined with another effective component such as goal setting (McEwan et al., 2015). Notably, despite the similar focus, overlap between these three reviews was low, with only one shared primary study. Three low quality reviews found that personalized feedback was effective in improving physical activity (k = 129, Conn et al., 2008; k = 61, Michie et al., 2009; k = 42, Olander et al., 2013), whereas two other low quality reviews found no evidence that including personalized feedback was more effective than interventions without this technique (k = 37, Abraham & Graham-Rowe, 2009; k = 24, French et al., 2014).
Of the two remaining high quality reviews, one found that feedback interventions were effective for changing use of smokeless tobacco, but not more so than other interventions (k = 17, Ebbert, Elrashidi, & Stead, 2015), and there was high heterogeneity (I2 = 72%) for this outcome. Similarly, another review with low heterogeneity found feedback interventions to promote smoking cessation but only added benefits over usual care and not other interventions (k = 3 and k = 2, respectively, Chamberlain et al., 2017). Two low quality reviews also found that smoking cessation improved with personalized feedback but not more than interventions without this technique (k = 29, Bartlett et al., 2014; k = 13, Spohr et al., 2015).
Of the eight remaining medium quality reviews that examined feedback, two found that interventions with feedback effectively improved medication adherence for those using electronic event-monitoring systems (k = 22, Demonceau et al., 2013) and asthmatic patients (k = 16, Denford et al., 2014), and two provided evidence for personalized feedback on alcohol consumption in adolescents and young adults (k = 41, Scott-Sheldon et al., 2014; k = 185, Tanner-Smith & Lipsey, 2015). Two of these reviews specifically found that feedback interventions demonstrated added benefit over interventions without feedback (Demonceau et al., 2013; Scott-Sheldon et al., 2014), while Tanner-Smith & Lipsey (2015) found feedback interventions more effective than other interventions only for reducing alcohol-related problems in adolescents. Four reviews found that feedback did not change other health behaviours including weight-loss (k = 30, Sykes-Muskett et al., 2015), HbA1c levels in patients with Type 2 diabetes (k = 2, Sherifali, Bai, Kenny, Warren, & Ali, 2015; k = 8, Cheng et al., 2017), or mortality in patients with cardiovascular heart disease (k = 22, Goodwin et al., 2016). Denford and colleagues (2014, k = 27) further showed that feedback interventions yielded worse asthma symptoms in asthmatic patients compared to interventions without feedback.
Of the remaining low quality reviews that assessed feedback, four reviews found interventions with feedback to improve the following health behaviours: fruit and vegetable consumption (k = 25, Lara et al., 2014), medication adherence and blood pressure in hypertensive patients (k = 101, Conn, et al., 2015), and variable health behaviours (k = 18, Cugelman et al., 2011; k = 59, van Genugten et al., 2016). Yet, feedback interventions were significantly more effective than interventions without feedback only in one review (Lara et al., 2014), whereas five reviews showed no added benefit of feedback above other intervention components (Bartlett et al., 2014; Conn, et al., 2008; Conn, et al., 2015; Michie et al., 2009; van Genugten et al., 2016). Two reviews demonstrated no efficacy of feedback-based interventions for reducing alcohol or drug use (Tanner-Smith et al., 2015) or improving medication adherence (Conn & Ruppar, 2017), and one review found that interventions with feedback performed worse than interventions without this component for improving general health behaviours (McDermott et al., 2016).
The quality of the reviews examining feedback was variable, with the high quality reviews indicating only minimal improvement for feedback, often not even reaching significance compared to groups without this component; in contrast, some of the medium quality reviews indicated feedback could be effective for medication adherence and alcohol consumption. These results caution against the use of feedback in certain health behaviour change interventions; for a variety of outcomes, feedback does not seem to yield added benefit over other interventions, and in some cases, feedback interventions show reduced efficacy compared to other interventions.
Barrier identification and problem solving
Of the two high and six medium quality meta-analyses that addressed barrier identification and/or problem solving, only a few identified this component as producing better health outcomes. Identifying high-risk situations was effective in reducing alcohol quantity and frequency of heavy drinking among university students (k = 42 and k = 28, respectively, Scott-Sheldon et al., 2014) and subsequent illicit drug use among youth ages 11 to 25 with alcohol use (k = 23, Tanner-Smith et al., 2015). Among individuals with poorly controlled Type 2 diabetes, problem solving significantly reduced HbA1c levels (k = 7; Cheng et al., 2017). The other high and medium quality meta-analyses did not identify this component as a significant moderator of effects or as significantly more effective when compared to interventions without this component. These reviews examined the following health outcomes: physical activity and/or diet (k = 48; k = 32 for this relationship, Samdal et al., 2017), physical activity among healthy individuals (k = 19; k = 18 for this outcome, O’Brien et al., 2015), weight loss or associated weight loss behaviour (k = 30, Sykes-Muskett et al., 2015), reducing asthma symptoms and increasing adherence to preventive medications among asthma patients (k = 38; ks = 14 and 16, for these outcomes, Denford et al., 2014), and A1c among individuals with diabetes (k = 138; k = 33 for this outcome, Bolen et al., 2014). Similar to the high and medium quality meta-analyses, the low quality meta-analyses of parallel outcomes primarily had null effects: Barrier identification did not significantly increase physical activity among adults (k = 20, Bélanger-Gravel, Godin, & Amireault, 2013), among “healthy” adults (k = 206, (Conn et al., 2011), among older adults (k = 16, French et al., 2014), or among adults with chronic illness (k = 129, Conn et al., 2008). However, one review found that barrier identification/problem solving did significantly improve physical activity among obese adults (k = 42, Olander et al., 2013).
Of the 11 remaining low quality reviews, only four indicated that barrier identification was effective: for fruit and vegetable intake among adults of retirement age (k = 7, Lara et al., 2014), for condom use among populations with fewer than 49% Latinos (k = 142, Albarracín, Albarracín, & Durantini, 2008), for smoking, alcohol and drug use within universal programmes for early adolescents and for alcohol use within universal programmes for older adolescents (k = 91, k = 56, k = 39, and k = 6, respectively, (Onrust, Otten, Lammers, & Smit, 2016), and for voluntary behaviour change among unspecified populations (k = 10, Cugelman et al., 2011).
These results suggest that barrier identification and/or problem solving can be effective for certain behaviours including substance use among youth, Type 2 diabetes management, physical activity among obese adults, and healthier diet habits among older adults; yet, broad application is not yet warranted as much of the evidence is generated from lower quality reviews and heterogeneity of findings is mostly unknown.
Review goals
Two high quality reviews, three medium quality, and 10 low quality reviews assessed reviewing goals as a technique for behaviour change interventions. Both high quality reviews considered physical activity and neither found it to be effective for yielding improvements beyond interventions without this approach, including among overweight or obese adults (k = 32, Samdal et al. 2017) or older adults (k = 19, O’Brien et al., 2015). Similarly, a low quality review found reviewing goals did not effectively change physical activity in older adults, compared to other interventions (k = 6, French et al., 2014) while one medium quality review found no evidence that reviewing goals yielded changes in addition to monetary contingency contracts for weight loss behaviour (k = 30, Skyes-Muskett et al., 2015). In contrast, two low quality reviews demonstrated added benefit of goal reviewing over other interventions for improving physical activity in obese adults (Olander et al., 2013, k = 42) and for increasing fitness in non-clinical adults (Abraham & Graham-Rowe, 2009, k = 20).
Two other medium quality reviews found no evidence that reviewing goals yielded health behaviour change, for either asthma symptoms (k = 27, Denford et al., 2014), or mortality in cardiovascular health disease patients (k = 15, Goodwin et al., 2016). Of the seven remaining low-quality reviews, four considered general health behaviours in a non-specific population. These four reviews (k = 138, Harkin et al., 2016; k = 34, McDermott et al., 2016; k = 122, Michie et al., 2009; k = 85, van Genugten et al., 2016;) found goal reviewing to be associated with behaviour change, but not more so than interventions without this component. Lara and colleagues (2014, k = 25) and Bartlett and colleagues (2014, k=17) reported similar outcomes for fruit and vegetable consumption in older adults and smoking cessation in COPD patients, respectively. Finally, one low quality review found goal reviewing to be associated with improved condom use (k = 3, Tyson et al., 2014).
Across the meta-analyses that examined review goals, the highest support for this BCT results from low-quality reviews; even then, goal reviewing was not often found to be more effective than other intervention components.
Relapse prevention/coping planning
Relapse prevention was examined in 13 meta-analyses, and all but one were considered low quality. One medium quality meta-analysis focused on smoking cessation (k = 63; Hajek, Stead, West, Jarvis, Hartmann-Boyce, & Lancaster, 2013) and found no significant benefits for individuals who smoked and had quit unaided (k = 5) or those who had quit smoking through treatment (k = 5). Three low quality reviews that also focused on smoking (Bartlett et al., 2014; Song, Huttunen-Lenz, & Holland, 2010; Spohr et al., 2015) were consistent with this medium quality review (k = 17, k = 19, and k = 13, respectively); Overlap across these four reviews was moderate (CCA = 9%). When reported, heterogeneity was minimal. The highest quality review for this component (yet still only of medium quality), Hajek and colleagues (2013), was also the largest, likely because of its comprehensive scope (relapse prevention interventions), while the other smaller reviews addressed SMS text-message based interventions (Spohr et al., 2015) or focused on smokers with a COPD diagnosis (Bartlett et al., 2014). The consistent findings and low heterogeneity indicate confidence in the results.
Of the remaining low quality reviews, four focused on physical activity and weight-related outcomes; relapse prevention did not significantly increase physical activity among healthy adults (k = 206, Conn, et al., 2011) or among older adults (k = 16, French et al., 2014) but did improve physical activity among obese adults (k = 36, Olander et al., 2013) and significantly reduced weight among adults with cardio-metabolic risk factors (k = 23, Dombrowski et al., 2012). One low quality review found that relapse prevention did not significantly increase the likelihood of protected sex (k = 32, only one study with this component, Tyson, Covey, & Rosenthal, 2014). The remaining four low quality reviews examined broad changes in health behaviour: Only one of these reviews found evidence for the effectiveness of relapse prevention (k = 85, van Genugten et al., 2016), while the rest did not (Cugelman et al., 2011; McDermott et al., 2016; Michie et al., 2009).
These results caution against the widespread use of relapse prevention in health behaviour change interventions because for a variety of outcomes, this technique does not seem to yield added benefit over other interventions. Additionally, the existing synthesis base is overall of low quality; further high quality reviews of this component are necessary to determine the actual effect of including this component in intervention research.
Action planning
One high quality, two medium quality, and seven low quality reviews examined action planning. The one high quality review did not support action planning as an effective BCT for reducing the number of COPD exacerbations experienced by patients with COPD (k = 4, Lenferink et al., 2017). Low levels of heterogeneity (Q = 1.47) bolster this finding.
One medium-quality review found that interventions with action planning improved physical activity in the general population, but not significantly more than other interventions (k = 52, McEwan et al., 2016). The same effect was reported by two low quality reviews of physical activity in older adults (k = 16, French et al., 2014) and obese adults (k = 42, Olander et al., 2013), while a further low quality review found action planning to be effective for physical activity in students (k = 6, Bélanger-Gravel et al. 2014). The remaining medium quality review demonstrated action planning to be associated with a reduction in HbA1c in patients with poorly controlled Type 2 diabetes but did not compare the effect to that of other interventions (k = 6, Cheng et al., 2017).
Of the remaining five low quality reviews, four considered broad health behaviour change. Two reviews demonstrated that interventions with action planning yielded increases in general behaviour change, but this was not greater than interventions without action planning (k = 13, Cugelman et al., 2011; k = 34, McDemott et al. , 2016) while two other reviews demonstrated that action planning yielded added benefit over other interventions for general health behaviour change (k = 138, Harkin et al., 2016; k = 85, van Genugten et al., 2016). The last low quality review indicated that action planning promoted smoking cessation in patients with COPD (k = 17, Bartlett et al., 2014).
Taken together, these reviews show inconsistent evidence for action planning. While the higher-quality meta-analyses do not tend to support action planning, these effects were for specific populations/behaviours and the results may not translate well to other health behaviours.
Stress management
Two medium quality reviews, seven low quality reviews, and no high quality reviews assessed stress management. Of the two medium quality reviews, one found no advantage of stress management in reducing mortality in patients with cardiovascular heart disease (k = 22, Goodwin et al., 2016); the other found that stress management was associated with worse asthma symptoms in asthmatic patients compared to interventions without stress management (k = 27, Denford et al., 2014).
Four low quality reviews considered stress management for general health behaviour change. Of the three reviews that considered a non-specific population, Only one found stress management to yield broad health behaviour change, more so than other interventions (k = 85, van Genugten et al., 2016), while the other two did not (k = 4, Cugelman et al., 2011; k = 122, Michie et al., 2009). Of the remaining two low quality reviews, one found no support for stress management in improving medication adherence (k = 33, Conn & Ruppar et al., 2017) and another which examined stress management in preventing smoking, alcohol use, and drug use found stress management to be associated with reduced rates of smoking in middle and late adolescence only (k = 8 and k = 25, respectively; Onrust et al., 2016).
Stress management may not be effective as a behaviour change intervention component, except with specific populations and behaviours/outcomes that are particularly impacted by stress. However, as none of the existing reviews were of high quality, and the majority of evidence is from low quality reviews, future high quality syntheses may provide evidence to the contrary.
Inhibitory control/Emotional control/Self-control training
Seven reviews examined some type of self-control training, and none were of high quality, although one was of medium quality. The medium quality review found that inhibitory control training (ICT) effectively improved alcohol and food consumption outcomes among adults, but with some remaining heterogeneity (k = 14, I2 = 11 and 76%, (Jones et al., 2016)). Two low quality reviews were consistent with these findings: ICT was effective in reducing food intake (k = 5, Turton, Bruidegom, Cardi, Hirsch, & Treasure, 2016), and emotional control training (ECT) was effective in reducing alcohol consumption (k = 5) and improving eating behaviours (k = 14, Allom, Mullan, & Hagger, 2015), with little to no remaining heterogeneity (I2 = 0 to 33%). Overlap across these three reviews was high (CCA = 11%). Although these findings indicate support for ICT, the heterogeneity indicates this approach may not be equally effective for all populations. Two lower quality reviews with unspecified populations found that ECT was ineffective for voluntary behaviour change (k = 2, Cugelman et al., 2011) and health-related behaviours (k = 85, van Genugten et al., 2016).
Two low quality reviews also examined self-control training among youth. One review of 11 studies found that self-control skills training among outpatient youth (12 years or younger) who had experienced sexual abuse were significantly less likely to engage in inappropriate sexual behaviour (St. Amand, Bard, & Silovsky, 2008). The other review of 241 studies examined alcohol and drug use and smoking among youth at different developmental stages and for different types of programming with mixed evidence (Onrust et al., 2016). Universal programs were effective in reducing smoking in elementary and early adolescent youth but not for older adolescents. Universal programs were also effective in reducing alcohol use in every stage but middle adolescence and were only effective in reducing drug use in early adolescence. Programmes that targeted high-risk students were only effective in reducing smoking and alcohol use in late adolescence and were also only effective in reducing drug use among elementary and late adolescent youth.
Overall, given the inconclusiveness of the reviews across a variety of behaviours and populations as well as the notable low quality of the existing review evidence, it is premature to make definitive conclusions about the effectiveness of inhibitory control/emotional control/self-control training.
Self-talk
Four low quality reviews assessed self-talk. Two reviews found that self-talk was associated with improvement on various health behaviour domains (Cugelman et al., 2011; Michie et al., 2009), but in one case, self-talk did not provide added benefit over other intervention features (k = 122, Michie et al., 2009)). Two reviews showed benefits of self-talk above that of other interventions, specifically for promoting physical activity in obese adults (k = 42, Olander et al., 2013), and for improving smoking cessation rates in patients with COPD (k = 17, Bartlett et al., 2014). Given this evidence, it seems possible that self-talk is effective for specific behaviours among clinical populations, but the review evidence is of such low quality that future evidence syntheses are necessary to make conclusive statements.
Implementation intention interventions
Three lower quality reviews examined the effects of implementation intentions on weight, diet quality, and physical activity, while a fourth examined the effects on goal achievement. Implementation intentions appear to be effective for making changes to both healthy eating and unhealthy eating activities (k = 23 and k = 15, respectively) according to one review among primarily college students (Turton et al., 2016) and according to another review of an unspecified population, but a mix of children, adolescents and adults (k = 21, Adriaanse, Vinkers, De Ridder, Hox, & De Wit, 2011). Implementation intentions were also found to be ineffective for weight change (k = 5, Turton et al., 2016) but effective for increasing physical activity at immediate post-intervention and follow-up (k = 19 and k = 9, respectively, Bélanger-Gravel et al., 2013). Finally, one review found no influence of action initiation in the context of implementation intention interventions on goal achievement for individuals with psychiatric diagnoses (k = 9, Toli, Webb, & Hardy, 2015). Thus, it seems possible that implementation intention interventions are effective for changing eating behaviours and physical activity, but the review evidence is of such low quality that future evidence syntheses are necessary to make conclusive statements.
Time management
Three low quality reviews assessed time management. Of these, all three found time management reviews to promote physical activity (k = 42, Olander et al., 2013) or various health behaviours (k = 4, Cugelman et al., 2011; k = 122, Michie et al., 2009), but two of these reviews did not find this effect to be greater than interventions without time management (Michie et al., 2009; Olander et al., 2013). Overall, it seems unlikely that time management is an effective component given the lack of significant effects of interventions utilizing this component versus those not using this component; yet, as there have been no high or medium quality reviews examining time management, future rigorous syntheses that examine time management are necessary.
Self-management
Two medium quality reviews assessed self-management among populations with chronic diseases. One review of five studies found that self-management programs for adults with stage 1–5 kidney disease effectively reduced interdialytic weight gain (Lin, Liu, Hsu, & Tsai, 2017) while another (Sherifali et al., 2015) found that self-management programs for patients with Type-2 diabetes reduced HbA1c levels (k = 13) as well as total cholesterol (k = 5), but did not impact blood pressure outcomes (k = 6). Thus, self-management programs appear to be effective for some outcomes among clinical populations, but the existing evidence syntheses do not meet high quality rigor and are from a small evidence base (with less than 15 studies), warranting caution.
High versus low self-regulation
One medium quality meta-analysis (k = 38; Janssen, De Gucht, Dusseldorp, & Maes, 2013) examined self-regulation among patients with poor cardiovascular health by rating studies as high or low in four self-regulation components (goal setting, self-monitoring, planning, and feedback) and conducting subgroup analyses between the two levels of self-regulation. High self-regulation interventions resulted in significantly greater change in exercise (k = 14) and fat intake (k = 13) at immediate post-test only but not smoking or energy intake at either post-treatment or follow-up. One low quality meta-analysis of adults with rheumatoid arthritis provided effect sizes for the effects of high (g = 0.40) and low (g = 0.32) self-regulation conditions on physical activity but did not provide further quantitative comparison (Knittle, Maes, & De Gucht, 2010). In that review, five self-regulation components were used to differentiate between high and low self-regulation interventions (goal setting, planning, self-monitoring, feedback, and relapse prevention). Given the medium-to-low quality of evidence and the varying definition of self-regulation in these reviews, it is premature to state conclusively whether “high” versus “low” self-regulation interventions are more/less effective on behaviour change; future synthesis work in this area should first address standardization of high and low self-regulation components.
Self-affirmation
One low quality review examined self-affirmation and found that self-affirmation interventions were associated with greater health behaviour change (k = 46, Epton, Harris, Kane, Van Koningsbruggen, & Sheeran, 2015), but there was significant remaining heterogeneity (Q = 108.93, I2 = 58.7%, p < 0.01), indicating this effect may not be present for all studies. As there was only one review of self-affirmation and it was of low quality, future synthesis work in this area should be conducted.
Contextual Variables
Regarding attention to social and environmental level variables, just under half of the included meta-analyses conducted additional analyses to examine contextual factors (45%), and of these, on average, 1.83 of these additional analyses were conducted per meta-analysis (SD = 1.21, range: 1 to 5). Meta-analyses reporting these variables satisfied an average of 47% of AMSTAR 2 items (range 11 to 88%); five of these meta-analyses satisfied above 70%. The majority (l = 24) explored social support through close relationships (e.g., parents, peers), 15 through better health care system support (e.g., improve communication, increase provider integration, build rapport), 11 through the use of social comparison or social norms, and five through environmental restructuring. The examination of these types of contextual variables were most common among meta-analyses addressing the promotion of healthy behaviours (l = 13), the prevention of risky behaviours (l = 8), and medication adherence (l = 6); medication adherence meta-analyses most often addressed health care system supports (64%).
Discussion
In this systematic meta-review of meta-analyses that have examined health behaviour change interventions, our search of literature between January 2006 and August 2017 identified 66 meta-analyses that quantitatively examined the effects of aspects of self-regulation on health behaviours. This effort had several broad aims, the first of which was to compile target self-regulation mechanisms of action that have been identified in meta-analyses focused on trials of interventions to promote health behaviour change. Our meta-review revealed decidedly mixed evidence: In terms of direct examinations of self-regulation mechanisms that may lead to health behaviour change, there have been only four meta-analyses, and these focused on self-efficacy, frequency of self-monitoring, and cognitive bias. Although these meta-analyses were of moderate to low quality, they indicate that changing self-efficacy can be effective for behaviour change, but perhaps not always in the context of particular behaviours (e.g., physical activity), that more frequent self-monitoring (indicating higher self-regulation) improves goal attainment, and that cognitive bias modification programs can change cognitive bias, but may not be enough to change resulting addictive behaviours. In terms of indirect examinations, wherein meta-analyses examine whether interventions’ presence of BCTs that logically affect self-regulation mechanisms indicate increased effectiveness, meta-analyses have examined at least 17 different strategies; some BCTs have received a great deal of attention (e.g., goal setting, prompt self-monitoring, barrier identification, problem solving), but most have received scant attention to date (see Figure 4 and also in the online supplements, Table 5).
This meta-review also aimed to evaluate the methodological quality of recent meta-analyses related to self-regulation mechanisms and to identify the limitations of existing meta-analyses and the underlying literature using methodological quality assessments. Overall, as indicated by scores on the recently updated AMSTAR 2 instrument, meta-analysis (reporting) quality is mixed, and there are few rigorous meta-analyses (e.g., Figures 3 and 5). The facts that (a) meta-analysis quality in more recent meta-analyses remains highly variable; and (b) the correlation between quality scores and the impact of the meta-analysis was non-significant all are suggestive of a strong conclusion: Contemporary meta-analysts, editors, and reviewers of meta-analytic studies are yet to fully incorporate the AMSTAR 2 standards, which, granted, only appeared in 2018 (though an earlier form appeared in 2007 as the original AMSTAR). One bit of advice in ensuring a higher quality meta-analysis seems to be in the review team size, as larger author teams tended to score higher on AMSTAR 2. This result highlights the resource-intensive nature of such endeavours given the large number of studies in some domains (Table A1) and suggests that external funding may be necessary, especially to ensure an appropriately sized team and high rigour of meta-analysis work.
Given these limitations, other goals were (d) to determine the extent to which interventions engage target mechanisms of action; identify where these links are most robust across varying methods and (e) to determine which BCTs link to target mechanisms and/or behaviour. There is strong evidence from rigorous meta-analyses of the following results (see Figure 4): (a) self-monitoring and goal setting are effective for producing changes in exercise, especially for certain populations including adults who need to lose weight for clinical reasons; (b) as might be expected from clinical guidelines for managing diabetes, self-monitoring improves diabetes outcomes such as by lowering HBA1c, although other health-related findings may be limited (e.g., cholesterol, blood pressure); and (c) feedback appears effective for medication adherence and physical activity. The consistent findings between the higher and lower quality meta-analyses lends additional support to these findings. One important clinical finding and a potential outlier from other clinical conditions is that some of the self-regulation BCTs do not appear effective for asthma patients, and in some cases, may exacerbate symptoms. Specifically, the current analysis highlights the need for further investigation into why, at least at the aggregate level, stress management appears detrimental for asthma, as the comparison only included four studies that used stress management (versus 23 that did not), and differences in the underlying studies may better explain these findings. Additionally, there was some evidence that some of the BCTs, including personalized feedback were less effective for certain populations (e.g., older adults), again indicating the need for careful attention to the target population when designing interventions built of multiple components. However, we should note that we do not know the actual nature of the feedback provided in these primary studies, and that is another key factor in its success/failure that should be considered (Archer, 2010). Strong evidence for the success of barrier identification/problem solving, reviewing goals, action planning, and stress management has not yet materialized, although this gap may stem from a lack of primary studies addressing these components. Although relapse prevention was not focused on in any high quality reviews, the existing reviews concur in the lack of evidence for its effect on smoking cessation. Similarly, the intervention components of emotional control, self-talk, time management, inhibitory control, implementation intentions, and self-affirmations are of future interest; while they have not yet been included in rigorous meta-analyses (Figure 4), some of their results (inhibitory control/emotional control) have been replicated across multiple lower quality reviews. Thus, additional rigorous reviews of these components especially are necessary.
Overall, most of the higher quality meta-analyses focused on adults; thus, there is less evidence of self-regulation in the context of health behaviour changes among children, adolescents, and older adults. Indeed, all of the meta-analyses focused on youth had low AMSTAR 2 completion and meta-analyses for young adults were of similarly moderate and low quality. There were some reviews, however, of older adult populations that met higher standards as well as the two meta-analyses of women populations and African American populations. The fact that meta-analyses that specified clinical populations of interest were also more likely to be of higher quality indicates that these meta-analyses may be better at detailing their inclusion criteria, and thus possibly their scope, and that these meta-analyses may be better planned from the beginning than other meta-analyses of lower quality.
Meta-analyses that are broader in defining their scope are naturally larger, and may thus be unable to report as many of the individual study details and other methods steps that the AMSTAR 2 tool requires given resource limitations or journal space. Moreover, the literature does not yet make clear how different quality dimensions may relate to important results that meta-analyses reach. It is possible, therefore, that some of these broader meta-analyses still hold value for the field even if they lack implications for particular subgroups given heterogeneity in findings. For example, although some of the largest reviews (Harkin et al., 2016; Michie et al., 2009; Sheeran et al., 2016) were rated as lower quality, the greater size and thus statistical power of each review enabled a more comprehensive examination of not only the effectiveness of a single component, but of additional study and population characteristics, attempting to address the important questions of what works for whom and in what conditions. These meta-analyses in particular may be worthy candidates for updates to address unexplained heterogeneity and parse mechanisms of outcomes into smaller, meaningful sub-literatures (e.g., based on population, intervention, or other features).
We found that very few meta-analyses directly test the relationship between change in a self-regulation mechanism and change in behaviour, and those that have, have only focused on self-efficacy, frequency of self-monitoring, and cognitive bias. As has been suggested by others wishing to examine mechanisms via evidence synthesis, this gap may be a limitation of primary study reporting (Webb & Sheeran, 2006), but is certainly an area for further development in both the primary study literature as well as synthesis literature. Meta-analyses in this meta-review primarily addressed identifying key intervention components via use of coding for certain specific elements and making comparisons across studies that did and did not include these elements. Although a strength of the literature using this strategy is that authors have found a way to consistently identify unique components, especially by using established taxonomies and coding systems, it limits our ability to make generalizations about behaviour change mechanisms and rather identifies elements that may be important to include in some interventions for some behaviours. Furthermore, while only one lower quality meta-analysis (AMSTAR 2 = 25%) used data mining methods to identify the best predictors of behaviour change across multiple BCTs (van Genugten et al., 2016), this method holds potential in its ability to explore multiple components and to better address the real-life complexity of behaviour change interventions.
Of note are the interventions that meta-analyses have not yet focused on quantitatively examining self-regulation mechanisms; for example, this meta-review did not identify any relevant meta-analyses addressing self-regulation mechanisms in mindfulness-based interventions. Because primary study reporting is often inadequate for answering complex meta-analytic questions (Tanner-Smith & Grant, 2018), this gap in meta-analyses may be due to primary study availability and reporting. Given the rise in studies of mindfulness (Creswell, 2017), we anticipate that assessing mechanisms from these alternative interventions will be possible in the near future. Lastly, although a secondary element of this review, we found a limited assessment of environmental mechanisms – most, again, seemed to address the presence of specific intervention components (e.g., include practitioners to build rapport) or to focus on a generic measure of social support.
Limitations and Future Directions
Given the broad scope and multi-disciplinary nature of the questions posed in this meta-review, one of its limitations includes a possibility that some relevant meta-analyses were not identified and incorporated into this synthesis. Although we used results from the AMSTAR 2 standardized tool for decision-making, some items about whether authors reported sufficient rationale and justification for decisions (i.e., rationale for study design type) may be limited by journal space for reporting, as many journals have strict word limits. Similarly, it is possible that meta-analytic review teams satisfied many items on the AMSTAR 2 but neglected to detail this information in their reports; of note, the AMSTAR 2 has been available only since 2018, after the entire sample of meta-analyses were published (although its more critical items were also included in the first version of the AMSTAR). The AMSTAR 2 also asks whether authors list citations for excluded studies and the exclusion criterion used, which may be burdensome to require authors and journals to include in publications. The addition of online repositories may solve this problem, but this task may be asking too much of the typical meta-analysis team and use of this information by most readers may be minimal. Thus, we assert that some of the meta-analyses have been misjudged as of poorer quality due to the nuance of the tool but may still afford trustworthy results for some outcomes. To manage the enormous amount of information collected, we created a summary score of AMSTAR 2 items and categorized reviews as low, medium, or high quality. We acknowledge that these are somewhat arbitrary cut-point criteria but presented and contrasted all the results across each of the quality levels. Doing so sometimes permitted us to show that certain patterns appeared even in lower quality meta-analyses, thereby helping to show these were particularly robust, such as the beneficial effect of self-monitoring on blood pressure. It may be beneficial to examine more closely the findings of the meta-analyses with more varying quality, as long as the quality concerns do not discount meta-analyses completely. Further exploration is also required within each topic area (e.g., risky behaviours, CVD prevention and management, and medication adherence) to better understand why some self-regulation intervention components were effective for some health outcomes, while others were not. Additionally, although we chose to simplify the presentation of results by referring to intervention components as “effective” and “ineffective,” most of the included primary studies reviewed in these meta-analyses are controlled trials; thus, outcomes related to these self-regulation BCTs are more appropriately considered efficacy evidence rather than effectiveness evidence (Flay et al., 2005).
Our choice of coding of contextual variables was also limited in detail, but necessary given the broad diversity of meta-analyses included; thus, further examination of contextual details is a further area for future synthesis within particular health behaviour domains so that the identification of the most salient variables maximize coding resources. Finally, we set a limit of meta-analyses to as early as 2006, which means earlier meta-analyses are excluded; yet, as included meta-analyses have synthesized primary studies published in the 1960s, it seems likely that there are very few relevant intervention studies that were not included in the eligible sample of meta-analyses.
Conclusions
This meta-review of 66 meta-analyses that examined the link between self-regulation and health behaviour change indicates there are many further areas for research. Although some intervention components are promising, others may be ineffective for certain health behaviours, and still, others require further empirical examination to assess whether they are indeed worth resource expenditure, especially with some of the high levels of heterogeneity presented in this meta-review. The quality of the included meta-analyses is mixed, but evidence suggests meta-analysis methods have improved over time with additional reporting and quality guidelines. Yet, given the importance of meta-analyses for practice and policy changes, the lack of rigor in the literature is problematic, especially when examining how often even lower quality reviews are cited. Improving rigor is an issue for research synthesists, journal editors, and peer reviewers alike to address, beginning with pre-registration and continuing throughout the submission process.
Supplementary Material
S1. PubMed search terms.
S2. References of included reviews.
- Table 1. Coding definitions and examples for self-regulation intervention components.
- Table 2. Results of methodological quality for each meta-analysis using the AMSTAR 2 Instrument
- Table 3. Correlations of methodological quality scores (proportion of relevant AMSTAR 2 items satisfied) with other methodological variables of interest (l = 66)
- Table 4. Correlations of individual AMSTAR 2 items satisfied with other methodological variables of interest (l = 66)
- Table 5. Intervention components examined across reviews, for each individual review.
- Figure 1. AMSTAR 2 item completion split by higher quality and medium to lower quality reviews.
- Figure 2. Total meta-analytic study quality as a function (a) of the year the review was published, (b) of the number of authors named for the meta-analysis and (c) of the number of studies meta-analysed.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) Science of Behavior Change Common Fund Program through an award administered by the National Institute on Aging (U.S. PHS grant 5U24AG052175). The views presented here are solely the responsibility of the authors, and do not necessarily represent the official views of the NIH. We thank our literature screeners and coders: Emily Betterton, Meiko Howell, Sahar Iqbal, Kiana McDavid, and Lindsay Roethke. We also thank reference librarians for help with creating electronic searches: Louise Falzon and Valerie Banfi. We thank Cleo Protogerou for her thoughtful comments on our manuscript. Finally, we are thankful for the constructive commentary of the editors and the anonymous reviewers.
Appendix Table A1.
Meta-analyses included in the meta-review, clustered by primary focus, and, within each cluster, ordered from highest to lowest methodological quality (AMSTAR 2 score).
| Citation | Brief aim(s) (year of literature search) | Intervention type(s) | Population diagnosis | Included studies (k) | Participants (n) | Proportion of AMSTAR 2 items satisfied |
|---|---|---|---|---|---|---|
| Primary Focus: Prevent Risky Behaviours (l = 15) | ||||||
| Chamberlain, O’Mara-Eves, Porter, Coleman, Perlen, Thomas, & McKenzie (2017) | Evaluate psychosocial interventions for smoking cessation in pregnancy; compare intervention strategies (i.e., counselling, health education, feedback, social support, incentives, and exercise). (2015) | Broad | Female smokers or recent quitters who are pregnant or seeking pre-pregnancy consultation | 102 | 30,000 | 0.88 |
| Ebbert, Elrashidi, & Stead (2015) | Assess the effects of behavioural and pharmacotherapeutic interventions to treat smokeless tobacco use. (2015) | Broad | Users of smokeless tobacco | 20 | 9,982 | 0.78 |
| Hajek, Stead, West, Jarvis, Hartmann-Boyce, & Lancaster (2013) | Assess whether specific interventions for relapse prevention reduce the proportion of recent quitters who return to smoking. (2013) | Relapse prevention | Former smokers | 63 | NR | 0.69 |
| Scott-Sheldon, Carey, Elliott, Garey, & Carey (2014) | Evaluate alcohol interventions and identify intervention components that increase their efficacy. (2013) | Broad | First-year university students | 41 | 24,294 | 0.53 |
| Tanner-Smith & Lipsey (2015) | Synthesize brief alcohol interventions and assess whether effects are associated with intervention and participant characteristics; examine persistence of the effects. (2013) | Brief | Adolescents (age 11–18) and young adults (age 19–30) | 185 | NR | 0.47 |
| Cristea, Kok, & Cuijpers (2016) | Evaluate CBM interventions for addiction-related outcomes. (2015) | CBM/ICT | People with addiction(s) | 24 | 3,175 | 0.44 |
| Tanner-Smith, Steinka-Fry, Hennessy, Lipsey, & Winters (2015) | Synthesize brief alcohol-reduction interventions; examine BCTs associated with effects (e.g., decisional balance, goal-setting exercises); evaluate whether intervention duration and follow-up timing matter for effects. (2013) | Brief | Youth aged 11 to 25 who have alcohol and perhaps other drug use problems | 67 | NR | 0.44 |
| Bartlett, Sheeran, & Hawley (2014) | Evaluate BCTs most associated with more effective smoking cessation interventions. (2012) | Broad | Smokers with a diagnosis of COPD | 17 | 7,446 | 0.44 |
| Allom, Mullan, & Hagger (2016) | Evaluate inhibitory training effect and determine what moderators account for unique variance in this effect. (2015) | CBM/ICT | Not restricted to any population or diagnosis | 14 | NR | 0.41 |
| Tyson, Covey, & Rosenthal (2014) | Review interventions informed by the theory of planned behaviour or theory of reasoned action aimed at reducing heterosexual risk behaviours (prevention of STDs and unwanted pregnancies). (2013) | Broad | Not restricted to any population or diagnosis | 32 | NR | 0.39 |
| Spohr, Nandy, Gandhiraj, Vemulapalli, Anne, & Walters (2015) | Evaluate SMS text message-based interventions for individual smoking cessation. (2014) | mHealth/online | Smokers | 13 | 13,626 | 0.34 |
| Onrust, Otten, Lammers, & Smit (2016) | Synthesize school-based universal and targeted prevention programmes, examining which types of programmes are most effective for groups at various developmental stages. (2013) | Broad | Children and adolescents attending school | 241 | 436,180 | 0.28 |
| Song, Huttunen-Lenz, & Holland (2010) | Review RCTs on smoking relapse prevention; examine underlying theories or mechanisms; conduct exploratory meta-analysis. (2009) | Psychological | Former smokers or current smokers who wish to quit | 49 | NR | 0.25 |
| Albarracín, Albarracín, & Durantini (2008) | Evaluate HIV/AIDS prevention interventions. (2005) | Broad | U.S. Latinx and Latin American populations | 142 | 110,092 | 0.08 |
| St. Amand, Bard, & Silovsky (2008) | Evaluate success of treatments for child sexual abuse victims. (NR) | Broad | Outpatient children, 12 years and younger who had experienced a form of sexual abuse | 11 | 1,081 | 0.08 |
| Primary Focus: Promote Healthy Behaviours (l = 30) | ||||||
| O’Brien, McDonald, Araujo-Soares, Lara, Errington, Godfrey, Meyer, Rochester, Mathers, White, & Sniehotta (2015) | Examine whether PA interventions produce long-term effects; examine potential factors that may moderate these effects. | Broad | Free-living, healthy adults, those at risk of chronic disease, aged 55–70 years | 19 | 10,423 | 0.75 |
| Sykes-Muskett, Prestwich, Lawton, & Armitage (2015) | Evaluate evidence for weight-loss-related monetary contingency contracts. (2014) | Broad | Overweight and obese individuals | 30 | NR | 0.63 |
| McEwan, Harden, Zumbo, Sylvester, Kaulius, Ruissen, Dowd, & Beauchamp (2016) | Assess effect of goal setting interventions in relation to individual PA behaviour; examine moderator variables related to characteristics of the study, sample characteristics, and goal attributes. (2015) | Broad | Not restricted to any population or diagnosis | 45 | 5,912 | 0.63 |
| Lim, O’Reilly, Behrens, Skinner, Ellis, Dunbar (2015) | Determine effectiveness of various lifestyle intervention components (intervention type and duration, use of self-monitoring, delivery format, and delivery medium) on weight loss. (2014) | Broad | First year post-partum women | 46 | 4,342 | 0.56 |
| Lin, Liu, Hsu, & Tsai (2017) | Evaluate self-management programs on intradialytic weight gain, self-efficacy, anxiety, depression), and health-related quality of life. (2017) | Self-management | Patients with diagnosis of Stage 1–5 CKD | 18 | 1,647 | 0.53 |
| Jones, Di Lemma, Robinson, Christiansen, Nolan, Tudur-Smith, & Field (2016) | Evaluate laboratory studies of inhibition control training for appetitive behaviour change; investigate candidate mechanisms of action, individual differences that may moderate its effectiveness, and compare it to other psychological interventions. (2014) | CBM/ICT | Adults | 14 | 1,091 | 0.53 |
| Sheeran, Maki, Montanaro, Avishai-Yitshak, Bryan, Klein, Miles, & Rothman (2016) | Evaluate the extent to which changing attitudes, norms, or self-efficacy solely or in combination lead to changes in health-related intentions and behaviour; examine several factors (study quality, theoretical basis of the intervention, sample characteristics, measurement factors, and features of the targeted behaviour) that could moderate such effects. (2015) | Broad | Not restricted to any population or diagnosis | 151 | NR | 0.50 |
| Harkin, Webb, Chang, Prestwich, Conner, Kellar, Benn, & Sheeran (2016) | Evaluate impact of interventions on both the frequency of progress monitoring and rates of goal attainment; determine whether effects hinge on progress monitoring and behaviour changes; evaluate whether effects hinge on dimensions of progress monitoring and other intervention, methodological, and sample characteristics. (NR) | Broad | Not restricted to any population or diagnosis | 138 | 19,951 | 0.47 |
| Lara, Evans, O’Brien, Moynihan, Meyer, Adamson, Errington, Sniehotta, White, & Mathers (2014) | Identify the BCTs associated with more effective dietary interventions (especially for food and vegetable intake); evaluate whether behaviour theories were associated effectiveness. (2013) | Broad | Adults of retirement age | 22 | 63,189 | 0.47 |
| Michie, Abraham, Whittington, McAteer, & Gupta (2009) | Examine whether BCTs differentially relate to self-regulation success. (2008) | Behaviour and/or cognitive change strategies | Adults | 101 | 44,747 | 0.47 |
| Turton, Bruidegom, Cardi, Hirsch, & Treasure (2016) | Compare the effectiveness of methods useful to change eating behaviours (i.e., implementation intentions, food-specific inhibition training, and attention bias modification training). (2014) | CBM/ICT/II | Not restricted to any population or diagnosis | 44 | NR | 0.44 |
| Knittle, Maes, & de Gucht (2010) | Evaluate psychological interventions of increasing PA, as well as of reducing pain, disability, depressive symptoms, and anxiety; see if interventions succeed better if they include more self-regulation theory techniques. (2009) | Broad | Adults with rheumatoid arthritis | 27 | NR | 0.41 |
| Brannon & Cushing (2015) | Identify interventions to promote PA and healthy diet. (NR) | mHealth/online | Healthy children and adolescents without chronic illness or obesity | 74 | 75,541 | 0.41 |
| Abraham & Graham-Rowe (2009) | Evaluate effectiveness of worksite interventions to enhance PA. (2007) | Worksite | Working employees | 37 | 16,516 | 0.39 |
| Higgins, Middleton, Winner, & Janelle (2014) | Evaluate PA RCT interventions in terms of PA behaviour and EXSE or BSE; identify intervention characteristics associated with changes in EXSE, BSE, and PA. (2011) | Broad | Healthy adults | 20 | 3,941 | 0.38 |
| Dombrowski, Sniehotta, Avenell, Johnston, MacLennan, & Araújo-Soares (2012) | Examine whether mode of intervention delivery and particular BCTs used relate to intervention success. (2009) | Broad | Mean or median BMI ≥ 30 (plus comorbidity factor for morbidity or possess the risk for one) | 44 | NR | 0.38 |
| Cugelman, Thelwall, & Dawes (2011) | Evaluate online intervention features to guide the development of population-wide campaigns targeting voluntary lifestyle behaviours; evaluate the roles of intervention exposure (dose) and intervention efficacy. (2009) | mHealth/online | Not restricted to any population or diagnosis | 31 | 17,524 | 0.31 |
| Casey, Coote, Shirazipour, Hannigan, Motl, Martin Ginis, & Latimer-Cheung (2017) | Evaluate whether modifiable, individual-level psychosocial constructs in interventions improve PA participation in people with MS. (2015) | Broad | People with MS | 26 | 3,363 | 0.31 |
| Bravata, Smith-Spangler, Sundaram, Gienger, Lin, Lewis, Stave, Olkin, & Sirard (2007) | Evaluate whether pedometer use affects PA (as well as changes in body weight, serum lipid levels, fasting serum glucose and insulin, and blood pressure); evaluate whether setting daily step goals improves health outcomes. (2006) | Other | Outpatient adults | 26 | 2,767 | 0.31 |
| Toli, Webb, & Hardy (2016) | Investigate how implementation intentions affect goal attainment in clinical samples. (2014) | Broad | Clinical samples with DSM-IV/ICD-10 or other standardized clinical diagnosis | 29 | 1,652 | 0.31 |
| Epton, Harris, Kane, van Koningsbruggen, & Sheeran (2015) | Evaluate self-affirmation interventions to promote responsiveness to health-risk information in terms of accepting the information, intentions to adopt the recommended behaviours, and subsequent behaviour. (2013) | Broad | Not restricted to any population or diagnosis | 41 | NR | 0.31 |
| Conn, Hafdahl, & Mehr (2011) | Summarize the effects of interventions designed to increase PA among healthy adults. (NR) | Broad | Healthy adults | 358 | 99,011 | 0.28 |
| Conn, Hafdahl, Brown, & Brown (2008) | Integrate results interventions designed to increase PA and examine whether effects depend on characteristics of interventions, sample, or methodology. (2004) | Broad | Adults with chronic illnesses | 163 | 22,527 | 0.28 |
| van Genugten, Dusseldorp, Webb, & van Empelen (2016) | Evaluate effectiveness of online interventions designed to promote health-related behaviour; develop a taxonomy for coding the usability of online interventions; identify what combinations of BCTs, modes of delivery, and usability factors influence results. (2008) | Broad | Not restricted to any population or diagnosis | 52 | NR | 0.25 |
| Olander, Fletcher, Williams, Lou, Turner, & French (2013) | Identify which BCTs were associated with increases or decreases in self-efficacy for PA and assess whether a BCTs that improved self-efficacy also improved PA. (2011) | Broad | Sample mean BMI ≥ 30 | 58 | NR | 0.25 |
| Bélanger-Gravel, Godin, & Amireault (2013) | Investigate the effectiveness of implementation intentions on PA; explore potential conditions when implementation intentions have significantly increase PA. (2009) | II | Adults aged 18 to 64 | 24 | 6,366 | 0.22 |
| McDermott, Oliver, Iverson, & Sharma (2016) | Evaluate whether changes in intention relate to behaviour; identify BCTs most associated with these changes. (2016) | Broad | Not restricted to any population or diagnosis | 25 | 6,306 | 0.14 |
| Adriaanse, Vinkers, De Ridder, Hox, & De Wit (2011) | Examine whether implementation intentions help people put their intentions to eat a healthy diet into practice; investigate factors that influence implementation intentions’ effectiveness. (NR) | II | Not restricted to any population or diagnosis | 21 | NR | 0.14 |
| French, Olander, Chisholm, & McSharry (2014) | Identify BCTs that increase self-efficacy and PA; assess whether changes in self-efficacy are also associated with changes in PA. (2012) | Broad | Non-clinical, community-dwelling adults 60-years old or over | 24 | NR | 0.11 |
| Darling & Sato (2017) | Examine use of mHealth technologies on weight status and dietary choices or PA. (2016) | Self-monitoring and mHealth | Children or adolescents who are primary users of mobile technology | 14 | 2,369 | 0.08 |
| Primary Focus: Cardiovascular disease prevention and management (l = 7) | ||||||
| Samdal, Eide, Barth, Williams, & Meland (2017) | Evaluate behavioural interventions to increase PA and healthy eating in short- and long-term contexts; and examine if success depends on BCTs and other study characteristics. (2014) | Behaviour and/or cognitive change strategies | Overweight and obese adults | 48 | 11,183 | 0.78 |
| Janssen, De Gucht, Dusseldorp, & Maes (2012) | Examine whether recent lifestyle modification programmes improve CHD risk factors and related health behaviours, reduce mortality and cardiac recurrences; determine whether efficacy depends on particular BCTs or on aspects of the control condition. (NR) | Broad | CHD patients eligible for cardiac rehabilitation or with particular CHD-related diagnoses. | 38 | 11,085 | 0.66 |
| Goodwin, Ostuzzi, Khan, Hotopf, & Moss-Morris (2016) | Evaluate lifestyle behaviour change RCTs for health behaviours, BP, BMI, and CHD events and mortality (intermediate outcomes) and see whether these depend on particular BCTs and structure (length, format, theoretical basis). (2016) | Broad | CHD patients with varying diagnoses | 22 | 16,766 | 0.56 |
| Fletcher, Hartmann-Boyce, Hinton, & McManus (2015) | Synthesize the literature to determine the effect of self-monitoring of BP on MA, medication persistence, and lifestyle factors in people with hypertension. (2014) | Self-monitoring | Patients with hypertension who were receiving ambulatory or outpatient care | 28 | 7,021 | 0.53 |
| Glynn, Murphy, Smith, Schroeder, & Fahey (2010) | Summarise evidence from non-pharmacological RCT interventions to improve the management of hypertension in primary care. (2008) | Self-manage and broad/other | Patients with essential hypertension in an ambulatory setting | 72 | NR | 0.53 |
| Bray, Holder, Mant, & McManus (2010) | Evaluate evidence for self-monitoring in hypertension compared to usual care (no self-monitoring of blood pressure). (2009) | Self-management and self-monitoring | Not restricted to any population or diagnosis | 25 | 6,278 | 0.47 |
| Chase, Bogener Ruppar, & Conn (2016) | Evaluate effectiveness of MA intervention research; explore potential moderators of intervention effectiveness. (NR) | Broad | Patients with CAD diagnosis | 24 | 18,839 | 0.33 |
| Primary Focus: Diabetes (l = 6) | ||||||
| Malanda, Welschen, Riphagen, Dekker, Nijpel, & Bot (2012) | Evaluate effects of self-monitoring of blood glucose in patients with T2D who are not using insulin. (2011) | Self-management | Patients with noninsulin-treated T2D | 12 | 3,259 | 0.84 |
| Farmer, Perera, Ward, Heneghan, Oke, Barnett, Davidson, Guerci, Coates, Schwedes, & O’Malley (2012) | Evaluate effectiveness of self-monitoring blood glucose level in people with non-insulin treated T2D compared with clinical management without self-monitoring, and to explore the effects in specific patient groups. (2010) | Self-management and self-monitoring | Patients with non-insulin-treated T2D | 6 | 2,552 | 0.69 |
| Zhu, Zhu, & Leung (2016) | Examine how self-monitoring of blood glucose affects diabetes patients in RCTs; investigate whether ethnicity and living environment associates with effects of self-monitoring of blood glucose. (2015) | Self-monitoring | Patients with non-insulin-treated T2D | 15 | 3,383 | 0.66 |
| Bolen, Chandar, Falck-Ytter, Tyler, Perzynski, Gertz, Sage, Lewis, Cobabe, Ye, Menegay, & Windish (2014) | Evaluate the effectiveness and safety of patient-activating interventions for adults with T2D on a range of clinically relevant outcomes. (2011) | Self-management and broad/other | Non-pregnant persons with T2D | 138 | 33,124 | 0.63 |
| Sherifali, Bai, Kenny, Warren, & Ali (2015) | Evaluate the most effective T2D self-management education or support strategies in older adults, as measured by HbA1c, blood pressure, and lipids (total cholesterol, triglycerides, high-density and low-density lipoproteins). (NR) | Self-management and broad/other | Adults with T2D | 13 | 4,517 | 0.53 |
| Cheng, Sit, Choi, Chair, Li, & He (2017) | Evaluate effectiveness of interactive self-management interventions on glycaemic-control and patient-centred outcomes. (2015) | Self-management | Individuals with poorly controlled T2D | 16 | 3,545 | 0.50 |
| Primary Focus: Medical Regimen/Medication Adherence (l = 8) | ||||||
| Lenferink, Brusse-Keizer, van der Valk, Frith, Zwerink, Monninkhof, van der Palen, & Effing (2017) | Evaluate the efficacy of self-management interventions that include an action plan for exacerbations of COPD (vs. usual care) in terms of health-related quality of life, respiratory-related hospital admissions and other health outcomes. (2016) | Self-management and broad/other | Participants with COPD; people with compromised post-bronchodilator forced expiratory volume; none with primary diagnoses of asthma | 22 | 3,854 | 0.88 |
| Luangasanatip, Hongsuwan, Limmathurotsakul, Lubell, Lee, Harbarth, Day, Graves, & Cooper (2015) | Evaluate the relative efficacy of the World Health Organization 2005 campaign and other interventions to promote hand hygiene among healthcare workers in hospital settings and to summarize associated information on use of resources. (2014) | Broad | Healthcare workers in hospital settings | 41 | NR | 0.69 |
| Demonceau, Ruppar, Kristanto, Hughes, Fargher, Kardas, Geest, Dobbels, Lewek, Urquhart, & Vrijens (2013) | Integrate RCTs evaluating interventions to enhance MA to prescribed medications, as assessed by electronic medication-event monitoring methods. (2012) | Broad | Not restricted to any population or diagnosis | 79 | 5,237 | 0.66 |
| Denford, Taylor, Campbell, & Greaves (2014) | Review interventions targeting asthma self-care in adults with asthma; explore BCTs associated with change in asthma morbidity or symptoms, unscheduled health care use, and MA. (2013) | Other | Participants with a diagnosis of asthma | 38 | 7,883 | 0.63 |
| Ruppar, Dunbar-Jacob, Mehr, Lewis, & Conn (2017) | Review interventions to improve MA to BP medications. (2015) | Broad | Black adults with hypertension | 37 | 5,228 | 0.59 |
| Conn, Ruppar, Chase, Enriquez, & Cooper (2015) | Review intervention aimed at increasing MA; examine average effect, whether effects depend sample, study, and intervention characteristics. (NR) | Broad | Participants with hypertension | 101 | 34,272 | 0.44 |
| Conn, Hafdahl, Cooper, Ruppar, Mehr, Russell (2009) | Evaluate effectiveness of interventions to improve MA and whether these relate to participants’ knowledge about their medications, management of medications, disease symptoms, health outcomes, systolic and diastolic blood pressure, health care services utilization, and quality of life; evaluate whether sample demographics, intervention components, and adherence measurement methodologies moderate the effect of interventions on MA. (NR) | Broad | Older adults with a physical health condition and at least one medical prescription | 38 | 11,827 | 0.41 |
| Conn & Ruppar (2017) | Evaluate effects of interventions on MA and see whether these vary depending on study design, sample, and intervention characteristics. (2015) | Broad | Not restricted to any population or diagnosis | 739 | 568,811 | 0.31 |
Note. BSE = barrier self-efficacy. CAD = Coronary Artery Disease. CBM= Cognitive bias modification. CHD = Coronary Heart Disease. CKD = Chronic Kidney Disease. COPD = Chronic Obstructive Pulmonary Disease. EXSE = exercise self-efficacy. ICT = Inhibitory Control Training. II = Implementation Intentions. MA = Medical adherence. MS = Multiple Sclerosis. NR = Not reported. RA = Rheumatoid Arthritis. RCT = Randomised Controlled Trial. SMS = Short message service. T2D = Type 2 Diabetes. PA = Physical activity.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to disclose.
Based on our extraction of the stated aim of each of the reviews (see Table A1), other reviews may have had similar population criteria in place but did not specify these as study inclusion/exclusion criteria.
References
- Abraham C, & Graham-Rowe E (2009). Are worksite interventions effective in increasing physical activity? A systematic review and meta-analysis. Health Psychology Review, 3(1), 108–144. doi: 10.1080/17437190903151096 [DOI] [Google Scholar]
- Abraham C, & Michie S (2008). A taxonomy of behavior change techniques used in interventions. Health Psychology, 27(3), 379–387. doi: 10.1037/0278-6133.27.3.379 [DOI] [PubMed] [Google Scholar]
- Adriaanse MA, Vinkers CDW, De Ridder DTD, Hox JJ, & De Wit JBF (2011). Do implementation intentions help to eat a healthy diet? A systematic review and meta-analysis of the empirical evidence. Appetite, 56(1), 183–193. doi: 10.1016/j.appet.2010.10.012 [DOI] [PubMed] [Google Scholar]
- Albarracín J, Albarracín D, & Durantini M (2008). Effects of HIV-prevention interventions for samples with higher and lower percents of Latinos and Latin Americans: A meta-analysis of change in condom use and knowledge. AIDS and Behavior, 12(4), 521–543. doi: 10.1007/s10461-007-9209-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allom V, Mullan B, & Hagger M (2015). Does inhibitory control training improve health behaviour? A meta-analysis. Health Psychology Review, 10(2), 1–38. doi: 10.1080/17437199.2015.1051078 [DOI] [PubMed] [Google Scholar]
- Anderson LM, Petticrew M, Rehfuess E, Armstrong R, Ueffing E, Baker P, … Tugwell P (2011). Using logic models to capture complexity in systematic reviews. Research Synthesis Methods, 2(1), 33–42. doi: 10.1002/jrsm.32 [DOI] [PubMed] [Google Scholar]
- Archer JC (2010). State of the science in health professional education: Effective feedback. Medical Education, 44(1), 101–108. doi: 10.1111/j.1365-2923.2009.03546.x [DOI] [PubMed] [Google Scholar]
- Barratt ES (1959). Anxiety and impulsiveness related to psychomotor efficiency. Perceptual and Motor Skills, 9(3), 191–198. doi: 10.2466/pms.1959.9.3.191 [DOI] [Google Scholar]
- Bartlett YK, Sheeran P, & Hawley MS (2014). Effective behaviour change techniques in smoking cessation interventions for people with chronic obstructive pulmonary disease: A meta-analysis. British Journal of Health Psychology, 19(1), 181–203. doi: 10.1111/bjhp.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger-Gravel A, Godin G, & Amireault S (2013). A meta-analytic review of the effect of implementation intentions on physical activity. Health Psychology Review, 7(1), 23–54. doi: 10.1080/17437199.2011.560095 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Quisenberry AJ, Moody L, & Wilson AG (2015). Therapeutic opportunities for self-control repair in addiction and related disorders: Change and the limits of change in trans-disease processes. Clinical Psychological Science, 3(1), 140–153. doi: 10.1177/2167702614541260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen SD, Chandar A, Falck-Ytter C, Tyler C, Perzynski AT, Gertz AM, … Windish, D. M. (2014). Effectiveness and safety of patient activation interventions for adults with type 2 diabetes: Systematic review, meta-analysis, and meta-regression. Journal of General Internal Medicine, 29(8), 1166. doi: 10.1007/s11606-014-2855-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand T, Pischke CR, Steenbock B, Schoenbach J, Poettgen S, Florence Samkange-Zeeb, & Zeeb, H. (2014). What works in community-based interventions promoting physical activity and healthy eating? A review of reviews. International Journal of Environmental Research and Public Health, 11(6), 5866–5888. doi: 10.3390/ijerph110605866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon EE, & Cushing CC (2015). A systematic review: Is there an app for that? translational science of pediatric behavior change for physical activity and dietary interventions. Journal of Pediatric Psychology, 40(4), 373–384. doi: 10.1093/jpepsy/jsu108 [DOI] [PubMed] [Google Scholar]
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, … Sirard, J. R. (2007). Using pedometers to increase physical activity and improve health: A systematic review. Jama, 298(19), 2296. doi: 10.1001/jama.298.19.2296 [DOI] [PubMed] [Google Scholar]
- Bray EP, Holder R, Mant J, & McManus RJ (2010). Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Annals of Medicine, 42(5), 371–386. doi: 10.3109/07853890.2010.489567 [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U (1979). The ecology of human development: Experiments by nature and design. Cambridge, Mass: Harvard University Press. [Google Scholar]
- Casey B, Coote S, Shirazipour C, Hannigan A, Motl R, Martin Ginis K, & Latimer-Cheung A (2017). Modifiable psychosocial constructs associated with physical activity participation in people with multiple sclerosis: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation, 98(7), 1453–1475. doi: 10.1016/j.apmr.2017.01.027 [DOI] [PubMed] [Google Scholar]
- Chamberlain C, O’Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, & McKenzie JE (2017). Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews, (2). doi: 10.1002/14651858.CD001055.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DJ, Bogener LJ, Ruppar MT, & Conn SV (2016). The effectiveness of medication adherence interventions among patients with coronary artery disease: A meta-analysis. The Journal of Cardiovascular Nursing, 31(4), 357–366. doi: 10.1097/JCN.0000000000000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Sit JWH, Choi K, Chair S, Li X, & He X (2017). Effectiveness of interactive self-management interventions in individuals with poorly controlled type 2 diabetes: A meta-analysis of randomized controlled trials. Worldviews on Evidence-Based Nursing, 14(1), 65–73. doi: 10.1111/wvn.12191 [DOI] [PubMed] [Google Scholar]
- Clarivate Analytics. (2018). Journal impact factor Clarivate Analytics. [Google Scholar]
- Conn V, Ruppar T, Chase J, Enriquez M, & Cooper P (2015). Interventions to improve medication adherence in hypertensive patients: Systematic review and meta-analysis. Current Hypertension Reports, 17(12), 1–15. doi: 10.1007/s11906-015-0606-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Hafdahl AR, Brown SA, & Brown LM (2008). Meta-analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Education and Counseling, 70(2), 157–172. doi: 10.1016/j.pec.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, & Ruppar TM (2017). Medication adherence outcomes of 771 intervention trials: Systematic review and meta-analysis. Preventive Medicine, 99, 269–276. doi: 10.1016/j.ypmed.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Hafdahl AR, Cooper PS, Ruppar TM, Mehr DR, & Russell CL (2009). Interventions to improve medication adherence among older adults: Meta-analysis of adherence outcomes among randomized controlled trials. Gerontologist, 49(4), 447–462. doi: 10.1093/geront/gnp037 [DOI] [PubMed] [Google Scholar]
- Conn VS, Hafdahl AR, & Mehr DR (2011). Interventions to increase physical activity among healthy adults: Meta-analysis of outcomes. American Journal of Public Health, 101(4), 751–758. doi: 10.2105/AJPH.2010.194381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD (2017). Mindfulness interventions. Annual Review of Psychology, 68, 491–516. doi: 10.1146/annurev-psych-042716-051139 [DOI] [PubMed] [Google Scholar]
- Cristea IA, Kok RN, & Cuijpers P (2016). The effectiveness of cognitive bias modification interventions for substance addictions: A meta-analysis. PLoS One, 11(9), e0162226. doi: 10.1371/journal.pone.0162226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugelman B, Thelwall M, & Dawes P (2009). Communication-based influence components model. In Proceedings of the 4th International Conference on Persuasive Technology (p. 17). ACM. doi:10.1145/1541948.1541972 [Google Scholar]
- Cugelman B, Thelwall M, & Dawes P (2011). Online interventions for social marketing health behavior change campaigns: A meta-analysis of psychological architectures and adherence factors. Journal of Medical Internet Research, 13(1) doi: 10.2196/jmir.1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling KE, & Sato AF (2017). Systematic review and meta-analysis examining the effectiveness of mobile health technologies in using self-monitoring for pediatric weight management. Childhood Obesity, 13(5), 347. doi: 10.1089/chi.2017.0038 [DOI] [PubMed] [Google Scholar]
- de Viron S, Malats N, Van DH, Van Oyen H, & Brand A (2013). Environmental and genomic factors as well as interventions influencing smoking cessation: A systematic review of reviews and a proposed working model. Public Health Genomics, 16(4), 159–173. doi: 10.1159/000351453 [DOI] [PubMed] [Google Scholar]
- Demonceau J, Ruppar T, Kristanto P, Hughes D, Fargher E, Kardas P, … Vrijens, B. (2013). Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: A systematic literature review and meta-analysis. Drugs, 73(6), 545–562. doi: 10.1007/s40265-013-0041-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denford S, Taylor RS, Campbell JL, & Greaves CJ (2014). Effective behavior change techniques in asthma self-care interventions: Systematic review and meta-regression. Health Psychology, 33(7), 577–587. doi: 10.1037/a0033080 [DOI] [PubMed] [Google Scholar]
- Dombrowski SU, Sniehotta FF, Avenell A, Johnston M, Maclennan G, & Araújo-Soares V (2012). Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: A systematic review. Health Psychology Review, 6(1), 7–32. doi: 10.1080/17437199.2010.513298 [DOI] [Google Scholar]
- Ebbert JO, Elrashidi MY, & Stead LF (2015). Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews, (10) doi: 10.1002/14651858.CD004306.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns J, Holmqvist M, Wener P, Halas G, Rothney J, Schultz A, … Katz A (2016). Mapping interventions that promote mental health in the general population: A scoping review of reviews. Preventive Medicine, 87, 70–80. doi: 10.1016/j.ypmed.2016.02.022 [DOI] [PubMed] [Google Scholar]
- Epton T, Harris PR, Kane R, Van Koningsbruggen GM, & Sheeran P (2015). The impact of self-affirmation on health-behavior change: A meta-analysis. Health Psychology, 34(3), 187–196. doi: 10.1037/hea0000116 [DOI] [PubMed] [Google Scholar]
- Farmer AJ, Perera R, Ward A, Heneghan C, Oke J, Barnett AH, … O’Malley S (2012). Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes. BMJ, 344, e486. doi: 10.1136/bmj.e486 [DOI] [PubMed] [Google Scholar]
- Flay B, Biglan A, Boruch R, Castro F, Gottfredson D, Kellam S, … Ji, P. (2005). Standards of evidence: Criteria for efficacy, effectiveness and dissemination. Prevention Science, 6(3), 151–175. doi: 10.1007/s11121-005-5553-y [DOI] [PubMed] [Google Scholar]
- Fletcher BR, Hartmann-Boyce J, Hinton L, & McManus RJ (2015). The effect of self-monitoring of blood pressure on medication adherence and lifestyle factors: A systematic review and meta-analysis. American Journal of Hypertension, 28(10), 1209–1221. doi: 10.1093/ajh/hpv008 [DOI] [PubMed] [Google Scholar]
- French DP, Olander EK, Chisholm A, & Mc Sharry J (2014). Which behaviour change techniques are most effective at increasing older adults’ self-efficacy and physical activity behaviour? A systematic review. Annals of Behavioral Medicine. 48(2), 225–234. doi: 10.1007/s12160-014-9593-z [DOI] [PubMed] [Google Scholar]
- Giles-Corti B, & Donovan RJ (2003). Relative influences of individual, social environmental, and physical environmental correlates of walking. American Journal of Public Health, 93(9), 1583–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LG, Murphy AW, Smith SM, Schroeder K, & Fahey T (2010). Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: A systematic review. The British Journal of General Practice, 60(581), e476. doi: 10.3399/bjgp10X544113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin L, Ostuzzi G, Khan N, Matthew HH, & Moss-Morris Rona. (2016). Can we identify the active ingredients of behaviour change interventions for coronary heart disease patients? A systematic review and meta-analysis. PLoS One, 11(4), e0153271. doi: 10.1371/journal.pone.0153271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, … IMAGE Study Group. (2011). Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health, 11(1), 119. doi: 10.1186/1471-2458-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger MS (2018). Habit and physical activity: Theoretical advances, practical implications, and agenda for future research. Psychology of Sport & Exercise, doi: 10.1016/j.psychsport.2018.12.007 [DOI] [Google Scholar]
- Harkin B, Webb TL, Chang BPI, Prestwich A, Conner M, Kellar I, … Sheeran P (2016). Does monitoring goal progress promote goal attainment? A meta-analysis of the experimental evidence. Psychological Bulletin, 142(2), 198–229. doi: 10.1037/bul0000025 [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins TJ, Middleton KR, Winner L, & Janelle CM (2014). Physical activity interventions differentially affect exercise task and barrier self-efficacy: A meta-analysis. Health Psychology, 33(8), 891–903. doi: 10.1037/a0033864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson RG, Harris FM, Platt S, & Tannahill C (2010). The effectiveness of interventions to change six health behaviours: A review of reviews. BMC Public Health, 10(1), 538–538. doi: 10.1186/1471-2458-10-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BT, Redding CA, DiClemente RJ, Mustanski BS, Dodge B, Sheeran P, … Fishbein, M. (2010). A network-individual-resource model for HIV prevention. AIDS and Behavior, 14(2), 204–221. doi: 10.1007/s10461-010-9803-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BT, Scott-Sheldon LA, & Carey MP (2010). Meta-synthesis of health behavior change meta-analyses. American Journal of Public Health, 100(11), 2193–2198. doi: 10.2105/AJPH.2008.155200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Di Lemma LCG, Robinson E, Christiansen P, Nolan S, Tudur-Smith C, & Field M (2016). Inhibitory control training for appetitive behaviour change: A meta-analytic investigation of mechanisms of action and moderators of effectiveness. Appetite, 97, 16–28. doi: 10.1016/j.appet.2015.11.013 [DOI] [PubMed] [Google Scholar]
- Knittle K, Maes S, & De Gucht V (2010). Psychological interventions for rheumatoid arthritis: Examining the role of self-regulation with a systematic review and meta-analysis of randomized controlled trials. Arthritis Care & Research, 62(10), 1460–1472. doi: 10.1002/acr.20251 [DOI] [PubMed] [Google Scholar]
- Lara J, Evans EH, O’Brien N, Moynihan PJ, Meyer TD, Adamson AJ, … Mathers JC (2014). Association of behaviour change techniques with effectiveness of dietary interventions among adults of retirement age: A systematic review and meta-analysis of randomised controlled trials. BMC Medicine, 12(1), 177. doi: 10.1186/s12916-014-0177-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenferink A, Brusse-Keizer M, van d. V., Frith PA, Zwerink M, Monninkhof EM, … Effing TW (2017). Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews, (8). doi: 10.1002/14651858.CD011682.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, O’ Reilly S, Behrens H, Skinner T, Ellis I, & Dunbar JA (2015). Effective strategies for weight loss in post-partum women: A systematic review and meta-analysis. Obesity Reviews, 16(11), 972–978. doi: 10.1111/obr.12312 [DOI] [PubMed] [Google Scholar]
- Lin M, Liu MF, Hsu L, & Tsai P (2017). Effects of self-management on chronic kidney disease: A meta-analysis. International Journal of Nursing Studies, 74, 128–137. doi: 10.1016/j.ijnurstu.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Luangasanatip N, Hongsuwan M, Limmathurotsakul D, Lubell Y, Lee AS, Harbarth S, … Cooper, B. S. (2015). Comparative efficacy of interventions to promote hand hygiene in hospital: Systematic review and network meta-analysis. BMJ, 351, h3728. doi: 10.1136/bmj.h3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanda UL, Welschen LMC, Riphagen I, Dekker JM, Nijpels G, & Bot SDM (2012). Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database of Systematic Reviews, (1) ). doi: 10.1002/14651858.CD005060.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M, Oliver M, Iverson D, & Sharma R (2016). Effective techniques for changing physical activity and healthy eating intentions and behaviour: A systematic review and meta-analysis. British Journal of Health Psychology, 21(4), 827–841. doi: 10.1111/bjhp.12199 [DOI] [PubMed] [Google Scholar]
- McEwan D, Harden SM, Zumbo BD, Sylvester BD, Kaulius M, Ruissen GR, … Beauchamp MR (2015). The effectiveness of multi-component goal setting interventions for changing physical activity behaviour: A systematic review and meta-analysis. Health Psychology Review, 10(1), 1–44. doi: 10.1080/17437199.2015.1104258 [DOI] [PubMed] [Google Scholar]
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, … Wood CE (2013). The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine, 46(1), 81–95. doi: 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- Michie S, Abraham C, Whittington C, McAteer J, & Gupta S (2009). Effective techniques in healthy eating and physical activity interventions: A meta-regression. Health Psychology, 28(6), 690–701. doi: 10.1037/a0016136 [DOI] [PubMed] [Google Scholar]
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, & French DP (2011). A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychology & Health, 26(11), 1479–1498. doi: 10.1080/08870446.2010.540664 [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K, McMahon TP, & Chacko TP (2017). The structure of common emotion regulation strategies: A meta-analytic examination. Psychological Bulletin, 143(4), 384–427. doi: 10.1037/bul0000093 [DOI] [PubMed] [Google Scholar]
- O’Brien N, Mcdonald S, Araújo-Soares V, Lara J, Errington L, Godfrey A, … Sniehotta, F. F. (2015). The features of interventions associated with long-term effectiveness of physical activity interventions in adults aged 55 to 70 years: A systematic review and meta-analysis. Health Psychology Review, 9(4), 1–29. doi: 10.1080/17437199.2015.1012177 [DOI] [PubMed] [Google Scholar]
- Office of Disease Prevention and Health Promotion. (2017). Healthy people 2020. Retrieved from https://www.healthypeople.gov/
- Olander EK, Fletcher H, Williams S, Atkinson L, Turner A, & French DP (2013). What are the most effective techniques in changing obese individuals’ physical activity self-efficacy and behaviour: A systematic review and meta-analysis. The International Journal of Behavioral Nutrition and Physical Activity, 10, 29. doi: 10.1186/1479-5868-10-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onrust SA, Otten R, Lammers J, & Smit F (2016). School-based programmes to reduce and prevent substance use in different age groups: What works for whom? Systematic review and meta-regression analysis. Clinical Psychology Review, 44, 45–59. doi: 10.1016/j.cpr.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Pieper D, Antoine S, Mathes T, Neugebauer EAM, & Eikermann M (2014). Systematic review finds overlapping reviews were not mentioned in every other overview. Journal of Clinical Epidemiology, 67(4), 368–375. doi: 10.1016/j.jclinepi.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Pieper D, Antoine S, Neugebauer EAM, & Eikermann M (2014). Up-to-dateness of reviews is often neglected in overviews: A systematic review. Journal of Clinical Epidemiology, 67(12), 1302–1308. doi: 10.1016/j.jclinepi.2014.08.008 [DOI] [PubMed] [Google Scholar]
- Polanin JR, Hennessy EA, & Tanner-Smith EE (2017). A review of meta-analysis packages in R. Journal of Educational and Behavioral Statistics, 42(2), 206–242. [Google Scholar]
- Polanin JR, Tanner-Smith E, & Hennessy EA (2016). Estimating the difference between published and unpublished effect sizes: A meta-review. Review of Educational Research, 86(1), 207–236. doi: 10.3102/0034654315582067 [DOI] [Google Scholar]
- Ruppar MT, Dunbar-Jacob M, Mehr RD, Lewis SL, & Conn SV (2017). Medication adherence interventions among hypertensive black adults: A systematic review and meta-analysis. Journal of Hypertension, 35(6), 1145–1154. doi: 10.1097/HJH.0000000000001260 [DOI] [PubMed] [Google Scholar]
- Samdal GB, Eide GE, Barth T, Williams G, & Meland E (2017). Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. International Journal of Behavioral Nutrition and Physical Activity, 14(1) doi: 10.1186/s12966-017-0494-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon L, Carey KB, Elliott JC, Garey L, & Carey MP (2014). Efficacy of alcohol interventions for first-year college students: A meta-analytic review of randomized controlled trials. Journal of Consulting and Clinical Psychology, 82(2), 177–188. doi: 10.1037/a0035192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, … Boers M (2009). AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. Journal of Clinical Epidemiology, 62(10), 1013. doi: 10.1016/j.jclinepi.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, … Bouter LM (2007). Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology, 7, 10. doi: 10.1186/1471-2288-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeran P, Maki A, Montanaro E, Avishai-Yitshak A, Bryan A, Klein WMP, … Rothman AJ (2016). The impact of changing attitudes, norms, and self-efficacy on health-related intentions and behavior: A meta-analysis. Health Psychology, 35(11), 1178–1188. doi: 10.1037/hea0000387 [DOI] [PubMed] [Google Scholar]
- Sherifali D, Bai J −., Kenny M, Warren R, & Ali MU (2015). Diabetes self-management programmes in older adults: A systematic review and meta-analysis. Diabetic Medicine, 32(11), 1404–1414. doi: 10.1111/dme.12780 [DOI] [PubMed] [Google Scholar]
- Song F, Huttunen-Lenz M, & Holland R (2010). Effectiveness of complex psycho-educational interventions for smoking relapse prevention: An exploratory meta-analysis. Journal of Public Health, 32(3), 350–359. doi: 10.1093/pubmed/fdp109 [DOI] [PubMed] [Google Scholar]
- Spohr SA, Nandy R, Gandhiraj D, Vemulapalli A, Anne S, & Walters ST (2015). Efficacy of SMS text message interventions for smoking cessation: A meta-analysis. Journal of Substance Abuse Treatment, 56, 1–10. doi: 10.1016/j.jsat.2015.01.011 [DOI] [PubMed] [Google Scholar]
- St. Amand A, Bard DE, & Silovsky JF (2008). Meta-analysis of treatment for child sexual behavior problems: Practice elements and outcomes. Child Maltreatment, 13(2), 145. doi: 10.1177/1077559508315353 [DOI] [PubMed] [Google Scholar]
- Stata Corp. (2017). Stata statistical software [computer software]. College Station TX, USA: Stata Corp. [Google Scholar]
- Sykes-Muskett B, Prestwich A, Lawton RJ, & Armitage CJ (2015). The utility of monetary contingency contracts for weight loss: A systematic review and meta-analysis. Health Psychology Review, 9(4), 1–21. doi: 10.1080/17437199.2015.1030685 [DOI] [PubMed] [Google Scholar]
- Tangney JP, Baumeister RF, & Boone AL (2004). High Self-Control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of Personality, 72(2), 271–324. doi: 10.1111/j.0022-3506.2004.00263.x [DOI] [PubMed] [Google Scholar]
- Tanner-Smith E, & Grant S (2018). Meta-analysis of complex interventions. Annual Review of Public Health, 39(1), 135–151. doi: 10.1146/annurev-publhealth-040617-014112 [DOI] [PubMed] [Google Scholar]
- Tanner-Smith E, & Lipsey MW (2015). Brief alcohol interventions for adolescents and young adults: A systematic review and meta-analysis. Journal of Substance Abuse Treatment, 51, 1–18. doi: 10.1016/j.jsat.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner-Smith E, Steinka-Fry K, Hennessy E, Lipsey M, & Winters K (2015). Can brief alcohol interventions for youth also address concurrent illicit drug use? results from a meta-analysis. Journal of Youth and Adolescence, 44(5), 1011–1023. doi: 10.1007/s10964-015-0252-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Brunton J, & Graziosi S (2010). EPPI-reviewer 4: Software for research synthesis. EPPI-centre software. London: Social Science Research Unit, UCL Institute of Education. [Google Scholar]
- Thomson D, Russell K, Becker L, Klassen T, & Hartling L (2010). The evolution of a new publication type: Steps and challenges of producing overviews of reviews. Research Synthesis Methods, 1(3–4), 198–211. doi: 10.1002/jrsm.30 [DOI] [PubMed] [Google Scholar]
- Toli A, Webb TL, & Hardy GE (2015). Does forming implementation intentions help people with mental health problems to achieve goals? A meta-analysis of experimental studies with clinical and analogue samples. 55, 69–90. doi: 10.1111/bjc.12086 [DOI] [PubMed] [Google Scholar]
- Turton R, Bruidegom K, Cardi V, Hirsch CR, & Treasure J (2016). Novel methods to help develop healthier eating habits for eating and weight disorders: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 61, 132–155. doi: 10.1016/j.neubiorev.2015.12.008 [DOI] [PubMed] [Google Scholar]
- Tyson M, Covey J, & Rosenthal HES (2014). Theory of planned behavior interventions for reducing heterosexual risk behaviors: A meta-analysis. Health Psychology, 33(12), 1454–1467. doi: 10.1037/hea0000047 [DOI] [PubMed] [Google Scholar]
- Van Genugten L, Dusseldorp E, Massey EK, & Van Empelen P (2017). Effective self-regulation change techniques to promote mental wellbeing among adolescents: A meta-analysis. Health Psychology Review, 11(1), 53–71. doi: 10.1080/17437199.2016.1252934 [DOI] [PubMed] [Google Scholar]
- van Genugten L, Dusseldorp E, Webb TL, & van Empelen P (2016). Which combinations of techniques and modes of delivery in internet-based interventions effectively change health behavior? A meta-analysis. Journal of Medical Internet Research, 18(6) doi: 10.2196/jmir.4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TL, Joseph J, Yardley L, & Michie S (2010). Using the internet to promote health behavior change: A systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. Journal of Medical Internet Research, 12(1) doi: 10.2196/jmir.1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TL, & Sheeran P (2006). Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychological Bulletin, 132(2), 249–268. doi: 10.1037/0033-2909.132.2.249 [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhu Y, & Leung S (2016). Is self-monitoring of blood glucose effective in improving glycaemic control in type 2 diabetes without insulin treatment: A meta-analysis of randomised controlled trials. BMJ, 6(9) e010524. doi: 10.1136/bmjopen-2015-010524 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. PubMed search terms.
S2. References of included reviews.
- Table 1. Coding definitions and examples for self-regulation intervention components.
- Table 2. Results of methodological quality for each meta-analysis using the AMSTAR 2 Instrument
- Table 3. Correlations of methodological quality scores (proportion of relevant AMSTAR 2 items satisfied) with other methodological variables of interest (l = 66)
- Table 4. Correlations of individual AMSTAR 2 items satisfied with other methodological variables of interest (l = 66)
- Table 5. Intervention components examined across reviews, for each individual review.
- Figure 1. AMSTAR 2 item completion split by higher quality and medium to lower quality reviews.
- Figure 2. Total meta-analytic study quality as a function (a) of the year the review was published, (b) of the number of authors named for the meta-analysis and (c) of the number of studies meta-analysed.