Abstract
HIV-1 selectively disrupts neuronal integrity within specific brain regions, reflecting differences in viral tropism and/or the regional differences in the vulnerability of distinct neuronal subpopulations within the CNS. Deficits in prefrontal cortex (PFC)-mediated executive function and the resultant loss of behavioral control are a particularly debilitating consequence of neuroHIV. To explore how HIV-1 disrupts executive function, we investigated the effects of 48 h, 2 and/or 8 weeks of HIV-1 Tat exposure on behavioral control, synaptic connectivity, and neuroimmune function in the anterior cingulate cortex (ACC) and associated cortico-basal ganglia (BG)-thalamocortical circuitry in adult, Tat transgenic male mice. HIV-1 Tat exposure increased novelty-exploration in response to novel food, flavor, and environmental stimuli, suggesting that Tat triggers increased novelty-exploration in situations of competing motivation (e.g., drive to feed or explore vs. fear of novel, brightly lit open areas). Furthermore, Tat induced adaptability in response to an environmental stressor and pre-attentive filtering deficits. The behavioral insufficiencies coincided with decreases in the inhibitory pre- and post-synaptic proteins, synaptotagmin 2 and gephyrin, respectively, in the ACC, and alterations in specific pro- and anti-inflammatory cytokines out of 23 assayed. The interaction of Tat exposure and the resultant time-dependent, selective alterations in CCL4, CXCL1, IL-12p40, and IL-17A levels in the PFC predicted significant decreases in adaptability. Tat decreased dendritic spine density and cortical VGLUT1 inputs, while increasing IL-1β, IL-6, CCL5, and CCL11 in the striatum. Alternatively, IL-1α, CCL5, and IL-13 were decreased in the mediodorsal thalamus despite the absence of synaptic changes. Thus, HIV-1 Tat appears to uniquely and systematically disrupt immune regulation and the inhibitory and excitatory synaptic balance throughout the ACC-BG-thalamocortical circuitry resulting in a loss of behavioral control.
Keywords: C-C motif chemokine ligand 4, Interleukin-17A, Cortical layer V pyramidal neurons, HIV-associated neurocognitive disorders, Glutamic acid decarboxylase 67, Striatal medium spiny neurons
Highlights
-
•
HIV-1 Tat impairs anterior cingulate cortical (ACC) mediated behavioral control.
-
•
HIV-1 Tat selectively decreases inhibitory synapses in the ACC.
-
•
Tat-induced ACC alterations in CCL4, CXCL1, IL-12p40, and IL-17A predict behavior.
-
•
Tat-dependent changes in cytokines in the striatum and thalamus differ from the ACC.
-
•
Tat-induced decreases in VGLUT1 suggest diminished corticostriatal projections.
1. Introduction
The advent of combination antiretroviral therapy (cART) has shifted HIV-associated neurocognitive disorders (HAND) from more severe deficits to milder neurocognitive losses in learning and executive functions (Heaton et al., 2011; Saylor et al., 2016). Executive functions, including behavioral control, are a set of top-down cognitive processes essential for coordinating thoughts and actions to maintain decision-making ability and execute appropriate behaviors in different contexts. Executive functions are markedly altered with many neurodegenerative and cognitive disorders (e.g. Parkinson’s and Alzheimer’s diseases, depression, and impulse control disorder) (Chamorro et al., 2012; Diamond, 2013). The prefrontal cortex (PFC) is the primary region associated with executive functions, but the synaptic connections within the prefrontal pathways of the cortico-basal ganglia (BG)-thalamocortical circuits are integral in maintaining these higher-order cognitive functions. Aberrant connections within the PFC and these associated circuits can decrease behavioral control over decision-making, leading to impulsivity, compulsivity, and inattentiveness (Alexander et al., 1986; Duncan and Owen, 2000; Napier and Persons, 2018; Peters et al., 2016).
Persons living with HIV (PLWH) typically act more impulsively than seronegative individuals in a variety of PFC-mediated behavioral measures (Chang et al., 2017; Fujiwara et al., 2015; Hardy et al., 2006). Nevertheless, even in the absence of behavioral deficits, PLWH display increased fMRI activity in the anterior cingulate cortex (ACC) region of the PFC and striatum when making risky choices (Connolly et al., 2014; du Plessis et al., 2015). Furthermore, viral load is increased within the PFC, striatum, and thalamus (Nath, 2015), while brain volume within these regions (Janssen et al., 2015; Nichols et al., 2019; Sanford et al., 2018), and fronto-striatal resting state connectivity (Ipser et al., 2015; Janssen et al., 2017; Ortega et al., 2015; Plessis et al., 2014), are decreased in PLWH. Accordingly, HIV-1 may decrease the executive function of behavioral control by altering the morphology and function of prefrontal cortico-BG-thalamocortical loops.
Sublethal, region-specific neuronal dysfunction likely underlies HAND (Ellis et al., 2007; Everall et al., 1999; Masliah et al., 1997; McArthur et al., 2010; Nath, 2015). HIV-1-induced synaptodendritic degeneration in the frontal cortex and putamen is correlated with neuropsychological impairment (Levine et al., 2016; Moore et al., 2006). Furthermore, in PLWH, decreased GABAergic synaptic connections within the PFC correlate with increases in immune markers (Buzhdygan et al., 2016). HIV-1 selectively decreases the expression of GABAergic and glutamatergic synaptic markers in the PFC and striatum, but to a lesser extent in other brain areas examined, suggesting prefrontal cortico-BG-thalamocortical circuits are selectively damaged (Buzhdygan et al., 2016; Gelman et al., 2012; Gelman and Nguyen, 2010; Guha et al., 2018). Indeed, although virus is detected throughout the prefrontal cortico-BG-thalamocortical circuitry, viral loads are particularly high in the striatum, although why HIV-1 targets certain central nervous system (CNS) regions and why some neuronal subtypes are preferentially vulnerable are questions that remain (Nath, 2015).
Despite cART, regulatory HIV-1 proteins, including trans-activator of transcription (Tat) can be elevated in spinal fluid of aviremic individuals (Henderson et al., 2019; Johnson et al., 2013) and secreted by infected cells (Chopard et al., 2018; Debaisieux et al., 2012; Rayne et al., 2010; Schatz et al., 2018), disrupting bystander neuron function (Chopard et al., 2018; Hategan et al., 2017). In fact, cART may increase Tat expression by attenuating feedback inhibition of proviral transcription (Henderson et al., 2019; Johnson et al., 2013; Mbonye and Karn, 2017). To explore the role of Tat per se in the pathophysiology of HIV, the present (Bruce-Keller et al., 2008) and another closely related Tat transgenic model (Kim et al., 2003) have been extensively used. Both models express Tat mRNA under the glial fibrillary acidic protein (GFAP) promoter, leading to protein expression throughout the cerebral cortex, striatum, hippocampus, and spinal cord (Bruce-Keller et al., 2008; Carey et al., 2012; Dickens et al., 2017; Fitting et al., 2012); however, one mouse line has 3–7 tat copies (Kim et al., 2003) and the present thought to have a single copy (Bruce-Keller et al., 2008; Duncan et al., 2008). In the present model, Tat induction activates astrocytes (as well as bystander microglia) in the striatum (Bruce-Keller et al., 2008; Fitting et al., 2010a; Zou et al., 2011) and causes modest gliosis within 48 h following expression (Bruce-Keller et al., 2008) that can be sustained for as long as 1 year (Dickens et al., 2017). In preclinical rodent studies, CNS exposure to HIV-1 Tat induces behavioral impairments similar to HAND, including deficits in pre-attentive filter processing (Fitting et al., 2006; Paris et al., 2015), emotionality (Fu et al., 2011; Hahn et al., 2015, 2016; Lawson et al., 2011; Paris et al., 2014, 2016), motivation (Kesby et al., 2018), and memory (Carey et al., 2012; Fitting et al., 2013; Marks et al., 2016).
We previously determined that 3-months of Tat induction was more detrimental to the dorsal striatum of males than females for most outcome measures (Hahn et al., 2015). Based on these earlier findings, this study initially surveyed the ACC and mediodorsal (MD) thalamus, which synapse directly with the dorsal striatum (Hunnicutt et al., 2016) in males to assess whether the ACC or MD thalamus might be reciprocally affected, with the intent of pursuing more detailed studies of the most susceptible region in both sexes. To investigate the effects of HIV-1 within the PFC and associated circuitry at the systems level to determine their role in HAND, we took a reductionist approach by experimentally manipulating Tat. We hypothesized that Tat exposure per se would impair aspects of behavioral control that would coincide with disrupted synaptic connectivity and increased inflammation in one or more components of prefrontal cortico-BG-thalamocortical circuitry. Although the existence of a rodent PFC is debated, the medial PFC (mPFC), particularly the ACC, in rodents (especially mice) has some cytoarchitectural homology with the mPFC in humans and monkeys (Carlen, 2017; Laubach et al., 2018; Vogt and Paxinos, 2014). Lesions to the ACC in rodents and monkeys impairs sustained attention and behavioral flexibility (Bissonette et al., 2013). Furthermore, the ACC is involved in other neurocognitive deficits evident in PLWH, including losses in motivation, goal-directed behavior, adaptability, and sociability, all of which can be tested in rodents (Devinsky et al., 1995; Holroyd and Yeung, 2012; Kolling et al., 2016). Accordingly, we explored the behavioral, structural, and neuroimmune effects of Tat on brain regions associated with ACC-BG-thalamocortical circuits in mice. Our results indicated that Tat increases novelty-exploration while impairing adaptability and pre-attentive filtering, which is associated with regional- and Tat exposure duration-dependent alterations in the balance between excitatory/inhibitory neurotransmission and in the production of cytokines. In addition, multiple linear regressions indicated that the interaction of Tat exposure and cytokine expression within the intact PFC significantly predicted coping ability.
2. Materials and methods
All procedures and experiments were approved by the Virginia Commonwealth University Animal Care and Use Committee and conducted in accordance with the National Institutes of Health (NIH Publication No. 85–23) ethical guidelines.
2.1. Subjects and treatment
Adult male doxycycline (DOX)-inducible GFAP-driven tet-on HIV–1IIIB Tat1–86 transgenic mice (n = 91) were generated in the vivarium of Virginia Commonwealth University as previously described (Bruce-Keller et al., 2008; Hauser et al., 2009; Paris et al., 2016). Mice (3-7-months-old) were fed a standard chow supplemented with DOX (6 g/kg, Harlan Laboratories Madison, WI; DOX-chow) for 48 h - 8 weeks to induce CNS expression of HIV-1 Tat. Control, Tat(−) mice also received chow containing DOX to control for off-target DOX effects. Mice were housed 1–5 per cage in a temperature- and humidity-controlled, AAALAC-accredited facility, with ad libitum access to food and water, on a 12:12 light:dark cycle.
2.2. Behavioral assays
Mice were repeatedly tested in assays of behavioral control (exploratory behavior, adaptability, pre-attentive filtering) and social interaction at 2, 4, 6, and 8 weeks of Tat exposure to determine the timing of Tat-induced functional deficits associated with the ACC. Mice were also tested for compulsivity, another aspect of behavioral control associated with the orbitofrontal cortex, to assess possible Tat-induced functional deficits associated with other frontal regions. To minimize animal numbers, mice were run through one of the two following batteries of behavioral tests: (1) hole-board exploratory test and novelty-induced hypophagia or (2) marble burying, social interaction, and the forced swim test. Testing was conducted in ascending order of presumed stress to reduce carry-over effects. A subset of mice was also tested in the following alternate behavioral control measures, nestlet shredding, novelty-suppressed feeding, and prepulse inhibition tasks after 8 weeks of DOX exposure to increase validity. Mice were habituated to the testing room for at least 1 h prior to behavioral testing and experimenters were blinded to treatment conditions throughout. At the conclusion of behavioral testing, mice were humanely euthanized and brain tissues were collected to assess morphological damage and inflammatory markers in discrete regions of the ACC-BG-thalamocortical circuitry.
2.2.1. Exploratory hole-board test
Tests of novelty-exploration in mice measure the overt expression of a fear (neophobia) or preference for novel stimuli (e.g. objects, food, flavors, or environments) and often rely on competing motivations of mice to explore or to avoid potential dangers. Due to these competing motivations, neophobia is often interpreted as anxiety- or depressive-like behavior; whereas a longer time spent with novel stimuli is interpreted as increased novelty-seeking or exploration. The exploratory hole-board test measures the degree to which rodents engage in novelty-exploration behavior by nose poking into a previously unexplored environment (Kliethermes and Crabbe, 2006; Pogorelov et al., 2005; Takeda et al., 1998). Rodents that engage in more frequent nose pokes into the novel holes display an increase in exploratory behavior. A hole-board insert (Stoelting Co., Wood Dale, IL, USA) with 16 equidistant holes was placed into an open field chamber (40 × 40 × 35 cm; Stoelting Co.). At 2-week intervals during Tat exposure, mice were placed in the middle of the board and allowed to freely explore for 10 min. The number of nose pokes into the holes and movement were recorded using the ANY-maze system (Stoelting Co.) as a measure of novelty-exploration and mobility.
2.2.2. Novelty-induced hypophagia
Hypophagia is the fear of eating in a novel environment (Dulawa and Hen, 2005). In mice, increased hypophagia indicates increased anxiety-like behavior; whereas decreased hypophagia, or preference for palatable food in a novel environment suggests increased novelty-exploration (Kliethermes and Crabbe, 2006; Loos et al., 2009). Mice were tested for novelty-induced hypophagia with modifications (Dulawa and Hen, 2005). Briefly, a 60 mm-diameter Petri dish containing 10 Fruit Rings cereal (Kroger, Cincinnati, OH) was placed in the center of an open field chamber (40 × 40 × 35 cm; Stoelting Co.). Mice were placed in the corner of the chamber and allowed to explore for 10 min every 2 weeks. As a measure of hypophagia and novelty-exploration, each mouse’s movements and the time spent with the cereal were recorded using a digital camera, and analyzed using ANY-maze software (Stoelting Co.) (Rudebeck et al., 2007).
2.2.3. Novelty-suppressed feeding
Due to possible apparatus habituation effects in the novelty-induced hypophagia test, a separate group of mice were also tested in the novelty-suppressed feeding test. Although similar to the novelty-induced hypophagia test, the novelty-suppressed feeding test measures novelty-exploration in response to standard chow after food deprivation, instead of palatable food without deprivation (Morris et al., 2016) and was used to further validate novelty-exploration in the Tat(+) mice. After 8 weeks of Tat exposure, mice were food deprived for 14 h. Mice were then placed in the corner of an open field chamber (40 × 40 × 35 cm; Stoelting Co.) with a 60 mm-diameter Petri dish filled with 6–8 food pellets in the center and allowed to explore for 10 min. Movement and time spent interacting with the food were recorded and analyzed by ANY-maze (Stoelting Co.) as measures of mobility, and hypophagia and novelty-exploration, respectively (Bodnoff et al., 1988; Morris et al., 2016).
2.2.4. Forced swim test (FST)
Although the FST has been used as an anti-depressant screen, it is best suited to measuring coping behavior and adaptability in response to an acute, inescapable stressor (Commons et al., 2017; Molendijk and de Kloet, 2015). Engaging in appropriate, while inhibiting inappropriate behaviors in different contexts is an important aspect of adaptability and behavioral control modulated by the ACC-BG-thalamocortical circuitry (Hamilton and Brigman, 2015; Jahanshahi et al., 2015). As previously described, mice were placed in a 2 L glass cylinder filled with 1.8 L of water for 6 min total (Porsolt et al., 1978; Toma et al., 2017). Mice were acclimated to the water for 2 min where they perform active coping behaviors (i.e., swimming and climbing). Immediately after acclimation, the time spent swimming or climbing (i.e., struggling) was then assessed for 4 min per mouse as a measure of adaptive coping behavior. It is expected that during the 4 min testing period mice will switch to passive coping behaviors (i.e., floating) due to the impossibility of escape (Commons et al., 2017; Molendijk and de Kloet, 2015).
2.2.5. Prepulse inhibition (PPI)
Prepulse inhibition (PPI) is a test of the ability to filter and disregard unnecessary stimuli, and is regulated by multiple brain regions, including the mPFC, striatum, and MD thalamus (Swerdlow et al., 2001, 2016). Pre-attentive filter processing of sensory stimuli was measured by PPI of the acoustic startle response as previously described (Geyer and Dulawa, 2003; Trexler et al., 2018). During PPI, a lower-decibel (dB) non-startling tone (prepulse) is presented promptly before a higher dB startling tone, reducing the response to the startle tone. In many neurocognitive disorders and corresponding animal models, PPI is diminished, indicating an attenuation of pre-attentive filtering. After 8 weeks of DOX, mice were habituated to the startle response chamber and restrainer (Kinder Scientific Startle Monitor; Poway, CA) with 65 dB background noise for 2 days. During testing, mice were placed in the startle response chamber and subjected to startle pulse stimuli of 120 dB for 64 trials with randomized ITIs averaging 15 s. During PPI trials, prepulse stimuli (4, 8, or 16 dB above background) were presented 100 ms before the startle pulse. The PPI was measured as the force exerted in Newtons (N) when jumping/displaying the startle response.
2.2.6. Marble burying
In humans and animal models, abnormalities in the orbitofrontal cortex and striatum are associated with compulsive behavior (Adams et al., 2018; Burguiere et al., 2015; Tedford et al., 2015). Compulsive-like behavior was assessed in the marble burying test, which is based on the natural digging behavior of rodents (Thomas et al., 2009). To decrease confounding anxiety effects, housing cages (33 × 21 × 19 cm) were placed in dimly-lit, enclosed chambers. The cages were filled with 3 cm of sawdust bedding (7090 Teklad Sani-Chips; Envigo, Somerset, NJ) and 20 black-glass marbles were placed on top of the bedding, in an evenly spaced 4 x 5 arrangement. Individual mice were placed into testing cages for 20 min and the number of marbles buried was calculated as a proxy measure for stereotyped digging behavior (Broekkamp et al., 1986; Kinsey et al., 2011). A marble was considered buried if at least 2/3 of the marble was covered with sawdust (Trexler et al., 2018). Movement was recorded by video camera and time spent mobile was analyzed by ANY-maze (Stoelting Co.) software.
2.2.7. Nestlet shredding
The nestlet shredding test is often used in conjunction with the marble burying test as a rodent model of obsessive-compulsive disorder because both tests measure repetitive rodent behaviors (Angoa-Perez et al., 2013; Li et al., 2006; Rose et al., 2018; Witkin, 2008). Nestlet shredding measures goal directed, compulsive-like behavior and was performed as previously described (Angoa-Perez et al., 2013; Rose et al., 2018). Briefly, mice were individually placed in housing cages (28 × 16 × 13 cm) filled with corn cob bedding and 1 pre-weighed cotton nestlet for 3 h. Intact pieces of nestlet were removed and dried overnight before being weighed again. The percentage of the nestlet shredded was calculated as a measure of compulsion.
2.2.8. Social interaction
Social interaction with novel, same-sex conspecifics was tested as previously described (Jamain et al., 2008; Morris et al., 2016). Mice were habituated to an open field chamber (40 × 40 × 35 cm; Stoelting Co.) for 5 min. Mice were removed and then immediately placed back in the chamber with a novel mouse that was either free to interact reciprocally (2–6 weeks of DOX/Tat exposure duration) or restrained under a mesh cup (~8 cm diameter) to test non-reciprocal social interactions (8 weeks of DOX/Tat exposure duration) for 5 min. Time spent interacting (i.e., direct physical contact, sniffing, following) initiated by the test mouse was video-recorded and coded by a blinded experimenter using ANY-maze software (Stoelting Co.). All observed interactions were non-aggressive.
2.3. Spine density assessment
Golgi impregnations were performed with the FD Rapid GolgiStain™ kit (FD Neurotechnologies, Columbia, MD) to assess dendritic pathology in the ACC, striatum, and MD thalamus. Dendritic length, branching, and spine density were quantified as previously described using a Golgi-Kopsch procedure (Fitting et al., 2013; Hahn et al., 2015; Hauser et al., 1989). Details are provided in supplemental methods.
2.4. Synaptic protein assessment
Pre- and postsynaptic protein markers were assessed by immunoblotting tissue from the PFC, striatum, and thalamus of Tat(+) and Tat(−) mice after 8 weeks of DOX exposure as previously described (Fitting et al., 2013). The inhibitory synaptic markers included (1) synaptotagmin 2 (Syt2), a presynaptic Ca2+ sensor (Chen et al., 2017), (2) gephyrin, an inhibitory postsynaptic scaffolding protein (Tyagarajan and Fritschy, 2014), and (3) glutamic acid decarboxylase 67 (GAD67), a GABA synthetic enzyme (Kanaani et al., 2010). The excitatory synaptic markers included (1) synapsin-1 that labels presynaptic excitatory terminals, (2) vesicular glutamate transporter 1 (VGLUT1), (3) vesicular glutamate transporter 2 (VGLUT2), and (4) postsynaptic density protein 95 (PSD-95) that labels excitatory postsynaptic densities. ACC colocalization of inhibitory pre- and post-synaptic markers, Syt2 and gephyrin, respectively, were also assessed by immunohistochemistry. For detailed methods, see supplemental methods.
2.5. Cytokine assessment
To assess chemokine/cytokine levels in the PFC, striatum, and thalamus we used Bio-Plex Pro Mouse Cytokine 23-plex assay kits (cat. no. M60009RDPD Bio-Rad Laboratories, Inc., Hercules, CA) per the manufacturer’s instructions as previously described (Gonek et al., 2018). See supplemental methods for complete list and brief details.
2.6. Statistical analyses
Data were analyzed by analysis of variance (ANOVA), followed by the Bonferroni correction for multiple comparisons to delineate significant interactive and main DOX/Tat exposure duration effects using Prism version 8.2.1 (GraphPad Software, Inc.). Behavioral timepoint data were analyzed using repeated measures ANOVA. Dendritic morphological changes (genotype × DOX/Tat exposure duration or dendritic length/branching) and cytokines (genotype × DOX/Tat exposure duration) were analyzed by two-way ANOVA for each separate brain region. Multiple linear regressions were performed to assess the amount of variance that Tat, cytokine expression within the PFC, and exposure time accounted for in the behavioral changes. All other analyses were made using Student’s t-test. All data are presented as the mean ± the S.E.M. Differences were considered statistically significant if p < 0.05.
3. Results
3.1. HIV-1 Tat decreases anterior cingulate cortico-BG-thalamocortical mediated behavioral control
Behavioral control, the ability to execute appropriate actions in response to changes in the environment, is altered by neurological and psychiatric disorders, including HAND (Chang et al., 2017; Fujiwara et al., 2015; Hardy et al., 2006; Jahanshahi et al., 2015). However, whether HIV-1 Tat contributes to aberrant behavioral control is less clear. Therefore, Tat(−) and Tat(+) mice were placed on DOX chow for 8 weeks and tested on a variety of measures of behavioral control (novelty-exploration, adaptability, pre-attentive filtering, compulsive-like behavior) and sociability. Prior studies in Tat transgenic mice have shown that Tat mRNA and/or protein is expressed within 48 h of induction throughout the brain (including the cortex, striatum, and hippocampus) and spinal cord and has been sustained for 1 year (Bruce-Keller et al., 2008; Carey et al., 2012; Dickens et al., 2017; Duncan et al., 2008; Fitting et al., 2010a, 2012).
3.1.1. HIV-1 Tat induces aberrant novelty-exploration, adaptability, and pre-attentive filtering
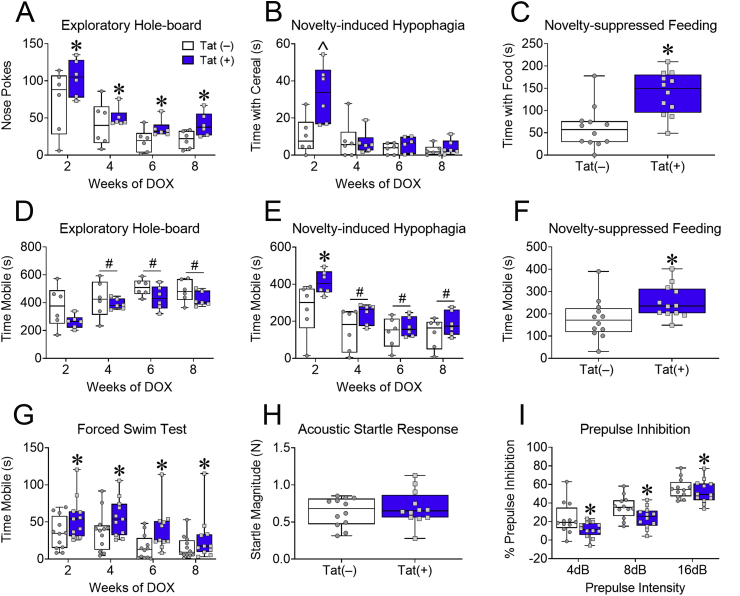
Novelty-exploration was tested once every 2 weeks in the exploratory hole-board and novelty-induced hypophagia tests. Tat exposure increased nose pokes in the exploratory hole-board test (Tat main effect [F(1,10) = 5.58, p < 0.05; Fig. 1A]) over the course of 8 weeks, but only increased time spent with the sugary cereal (Interaction [F(3,30) = 7.09, p < 0.001; Fig. 1B]) after 2 weeks. Similarly, Tat exposure did not alter mobility in the exploratory hole-board test (p = 0.16; Fig. 1D), despite an increase in mobility in both Tat(+) and Tat(–) mice over time (Time main effect [F(3,30) = 24.61, p < 0.0001; Fig. 1D]), but did increase mobility in Tat(+) mice at 2 weeks during the novelty-induced hypophagia test (Interaction [F(3,30) = 4.10, p < 0.05; Fig. 1E]). Due to possible habituation effects in the novelty-induced hypophagia test, a separate group of mice were tested in a different hypophagia test with a food pellet novel stimulus, novelty-suppressed feeding. Eight weeks of Tat exposure also increased time spent with food [t(22) = 3.96, p < 0.001; Fig. 1C] and mobility [t(22) = 2.25, p < 0.05; Fig. 1F] in the novelty suppressed-feeding test. Together these data indicate that Tat increases exploration in tests of novelty-seeking with different novel stimuli (environment, flavor, and food).
Fig. 1.
HIV-1 Tat increases novelty-exploration, while decreasing adaptability and pre-attentive filtering in novel environments. Tat(+) and Tat(−) mice were fed DOX chow for 8 weeks and assessed in ACC-mediated measures of behavioral control. Measures of novelty-exploration (A–C). Tat(+) mice exhibited increased exploratory hole-board nose pokes following 2–8 weeks of DOX (A). Tat exposure increased interactions with palatable food after 2 weeks (B) and regular food interactions in a novel environment following overnight deprivation after 8 weeks of DOX (C). Locomotor activity in novelty-exploration tests (D-F). Tat(+) and Tat(−) mice displayed increased mobility in the exploratory hole-board test at 4–8 weeks compared to 2 weeks of DOX exposure (D); whereas in the novelty-induced hypophagia test Tat exposure increased mobility at 2 weeks, but regardless of genotype, mice exhibited decreased mobility at 4–8 weeks (E). In the novelty-suppressed feeding test, Tat increased locomotor activity (F). Tat exposure for 2–8 weeks increased mobility in the forced swim test (FST) (G). After 8 weeks of DOX administration, Tat exposure did not affect acoustic startle response (H), but did decrease prepulse inhibition (PPI) or the ability to ignore irrelevant stimuli (I). Data are presented as mean ± SEM; (A-B&D-E) n = 6 mice per group; (C&F–I) n = 12 mice per group; ˆp < 0.05 vs Tat(−) (control) mice at 2 week exposure time. Main effect of Tat, ∗p < 0.05 vs Tat(−) (control) mice. Main effect of exposure time, #p < 0.05 vs 2 weeks of DOX.
Tat(−) and Tat(+) mice were also tested in the FST to assess adaptive coping in response to an acute stressor. Tat increased mobility in the FST (Tat main effect [F(1,21) = 11.75, p < 0.05; Fig. 1G]) after repeated exposures, indicating Tat interferes with the ability to behaviorally adapt to potentially stressful environmental changes.
Pre-attentive filtering, another important aspect of behavioral control, can be measured by assessing the PPI of the acoustic startle response. After 8 weeks of exposure, Tat decreased PPI (Tat main effect [F(2,44) = 107.3, p < 0.001; Fig. 1I]), but not the acoustic startle response (p = 0.5; Fig. 1H). These data suggest that Tat interferes with sensorimotor filter processing, but not the startle reflex.
3.1.2. HIV-1 Tat exposure does not affect compulsivity or sociability
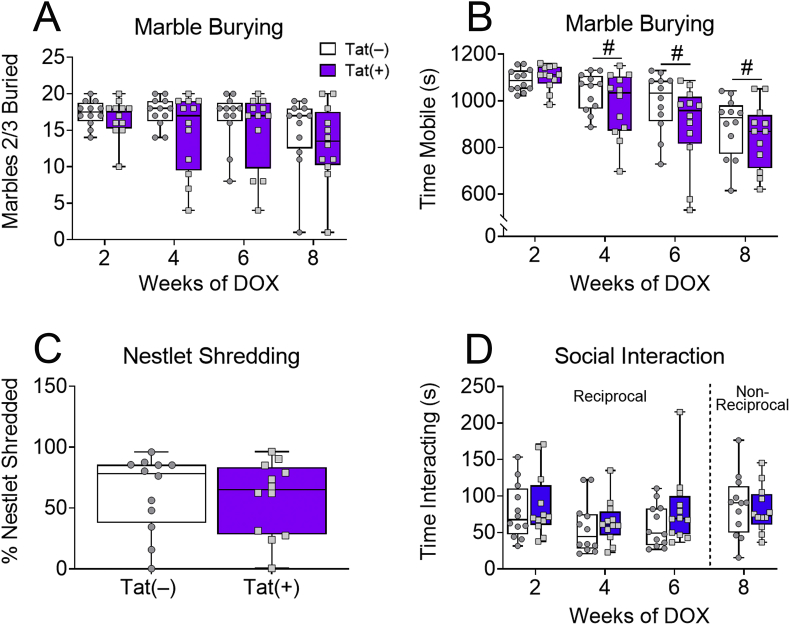
Due to Tat-induced alterations in the above aspects of behavioral control, we also assessed the effects of Tat on compulsivity and social interaction. Tat exposure did not affect either the number of marbles buried (p = 0.66; Fig. 2A) after 2–8 weeks, or the percentage of nestlets shredded (p = 0.74; Fig. 2C) after 8 weeks, suggesting that Tat does not affect compulsive-like behavior. Tat also did not affect mobility in the marble burying test (p = 0.14; Fig. 2B), although both Tat(+) and Tat(–) mice exhibited decreased mobility across testing weeks (Time main effect [F(3,66) = 33.9, p < 0.0001; Fig. 2B]).
Fig. 2.
HIV-1 Tat does not affect compulsive-like or social behaviors in novel environments. After 8 weeks of DOX administration, Tat exposure did not affect orbitofrontal cortex-mediated compulsive measures of marbles buried (A) or percentage of nestlet shredded (C). Tat exposure for up to 8 weeks also did not change mobility in the marble-burying test, but in both Tat(–) and Tat(+) mice mobility did decrease over time (B). Tat(+) and Tat(−) mice did not differ in time spent interacting in reciprocal (novel mouse free; 2–6 weeks) and non-reciprocal (novel mouse was restrained under a pencil cup; 8 weeks) social interaction assays (D). Data are presented as mean ± SEM, n = 12 mice per group. Main effect of exposure time, #p < 0.05 vs 2 weeks of DOX.
The ACC is implicated in social cognition and behavior (Mao et al., 2017). Therefore, to assess other behaviors mediated by the ACC, we tested Tat(+) and Tat(−) mice in reciprocal (2–6 weeks of DOX) and non-reciprocal (8 weeks of DOX) social interaction tasks. Similar to compulsive behavior, Tat did not change sociability when the novel mouse was free to reciprocally interact with the test mouse (p = 0.54; Fig. 2D), or when the novel mouse was restrained from interacting with the test mouse (p = 0.54; Fig. 2D). The behavioral data together suggest that Tat impairs aspects of behavioral control related to ACC, but not orbitofrontal cortical function.
3.2. HIV-1 Tat decreases inhibitory, but not excitatory synaptic markers in the anterior cingulate cortex
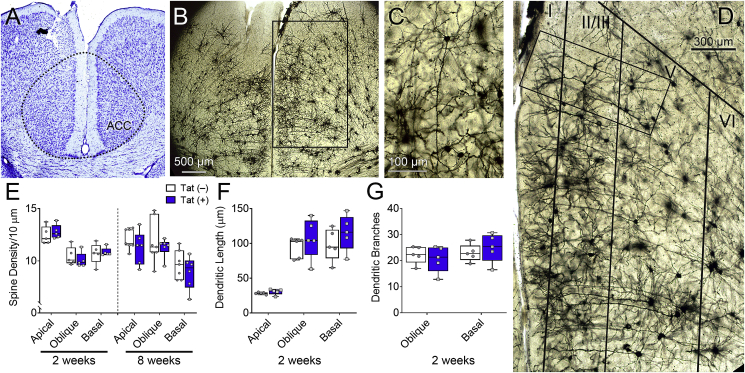
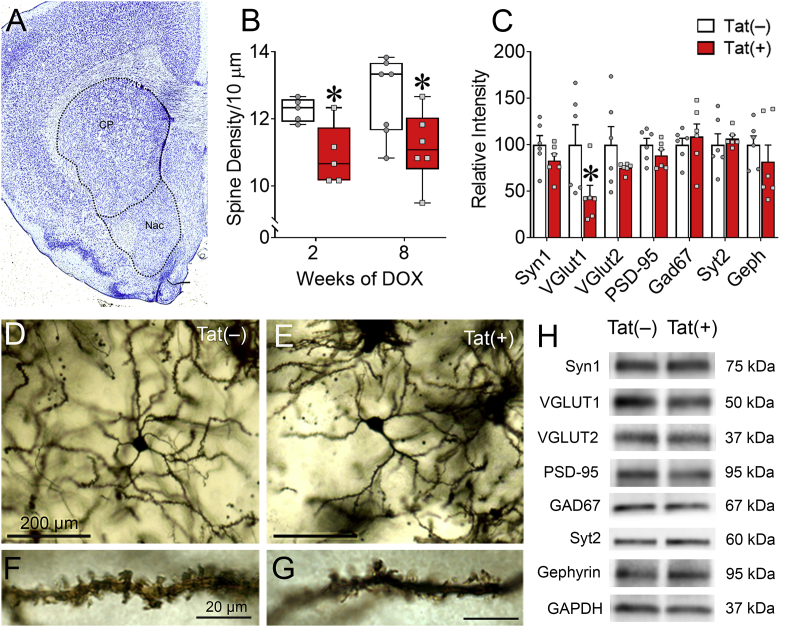
Since Tat impaired ACC-mediated behavioral control, we next investigated dendritic morphology and synaptic connections within distinct regions of the ACC-BG-thalamocortical circuit. Dendritic spine density, complexity, and length of ACC layer V pyramidal neurons were assessed in Golgi-impregnated brain sections to determine the effects of Tat on synaptodendritic morphology of neurons projecting to the striatum. Tat exposure for 2 or 8 weeks did not affect overall ACC layering (Fig. 3A–D), morphology (Fig. 3C), or dendritic spine density (spines/10 mm) of Golgi-impregnated layer V pyramidal neurons (p = 0.78; Fig. 3E). In addition, no difference was detected in ACC layer V pyramidal neuronal dendritic length (p = 0.58; Fig. 3F) or dendritic branching (p = 0.94; Fig. 3G) between Tat(+) and Tat(−) mice.
Fig. 3.
HIV-1 Tat exposure does not affect ACC layer V pyramidal neuron dendritic complexity or spine density. Dendritic morphology of ACC layer V pyramidal neurons of Tat(+) and Tat(−) mice were assessed after 2 or 8 weeks of DOX. Representative image of Nissl staining of the ACC (A). Representative images of Golgi-impregnated neurons in the ACC illustrating cortical layering (B&D) and layer V pyramidal neurons (C). Layer V pyramidal neurons (C) from Tat(−) and Tat(+) (not shown) mice displayed similar morphology and showed no differences in dendritic spine density (E) or dendritic complexity (F–G). Data are presented as mean ± SEM, multiple neurons (≥ 6) were sampled in each n = 5–7 mice per group; dashed-line, approximate borders of the ACC.
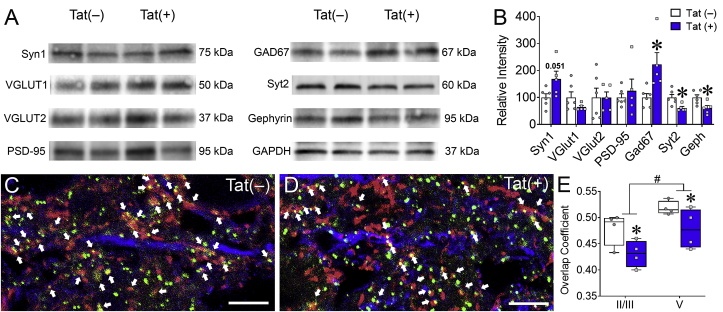
Based on previous data indicating that Tat may induce specific synaptic deficits in hippocampal area CA1 pyramidal neurons (Fitting et al., 2013; Marks et al., 2016), and more severe synaptodendritic injury in striatal MSNs (Fitting et al., 2010a; Schier et al., 2017), the effects of Tat on excitatory and inhibitory synaptic proteins in the intact PFC were evaluated. Whole PFC tissues were isolated from Tat(+) and Tat(−) mice after 8 weeks of DOX and synaptic proteins were assessed by immunoblot (Fig. 4A and B). The lack of differences seen in the density of excitatory dendritic spines between Tat(−) and Tat(+) mice in layer V pyramidal neurons was supported by a similar lack of changes in the levels of the excitatory vesicular glutamate transporters VGLUT1 (t(9) = 1.52, p = 0.16) and VGLUT2 (t(9) = 0.01, p = 0.99) or in the excitatory postsynaptic density protein PSD-95 (t(9) = 0.58, p = 0.57) in Tat(−) and Tat(+) mice. Furthermore, there was no significant difference with Tat treatment in the level of the excitatory presynaptic protein synapsin-1 (t(9) = 2.3, p = 0.051). In contrast, Tat exposure decreased the inhibitory pre- and post-synaptic proteins Syt2 (t(9) = 2.99, p < 0.05) and gephyrin (t(9) = 2.82, p < 0.05), respectively, and increased the inhibitory GABA synthesizing enzyme GAD67 (t(9) = 2.9, p < 0.05). These data suggest that Tat preferentially interferes with inhibitory interneurons and synapses throughout the PFC. To extend this finding to the ACC subregion, immunofluorescence intensity of Syt2 and gephyrin colocalization along dendrites stained for MAP2 within different layers of the ACC were also measured in Tat(−) (Fig. 4C) and Tat(+) (Fig. 4D) mice. In the ACC, 8 weeks of Tat exposure decreased Syt2 and gephyrin colocalization in layers II/III and V (Tat main effect [F(1,12) = 9.71, p < 0.01; Fig. 4E]), suggesting that Tat decreases inhibitory synapses within both the input and output layers of the ACC.
Fig. 4.
HIV-1 Tat exposure disrupts the inhibitory pre- and post-synaptic colocalization in the ACC. After 8 weeks of Tat exposure, excitatory and inhibitory synaptic markers were assessed within the PFC/ACC. In the whole PFC, 8 weeks of Tat induction decreased inhibitory pre- and post-synaptic proteins Syt2 and gephyrin, respectively, and increases GAD67, which is involved in the synthesis of GABA, as measured by immunoblotting (A–B). Representative images of IHC stained ACC neurons (C–D). Colocalization of inhibitory pre- and post-synaptic proteins Syt2 (red) and gephyrin (green), respectively, along pyramidal neuron dendrites indicated by the marker MAP2 (blue) in layer V of the ACC in Tat(−) (C) and Tat(+) (D) mice. Tat induction decreased Syt2 and gephyrin colocalization in layers II/III and V of the ACC after 8 weeks of DOX administration as measured by the overlap coefficient, the ratio of colocalized to non-colocalized pixels (E). Data are presented as mean ± SEM; n = 4–6 mice per group; ∗p < 0.05 vs Tat(−) mice; #p < 0.05 main effect of ACC layer; ↑ indicate colocalized inhibitory puncta; scale bar = 10 μm.
3.3. HIV-1 Tat decreases excitatory cortical inputs into the striatum
We next determined the effects of Tat exposure on synaptic morphology and markers within the striatum (Fig. 5), the main input region within the BG. Tat(+) mice (Fig. 5E&G) exhibited decreased striatal MSN dendritic spine density compared to Tat(−) mice (Fig. 5D&F) irrespective of the 2- or 8-week exposure time (Tat main effect [F(1,19) = 12.19, p < 0.01; Fig. 5B]). These results support previous data showing that Tat reduces the dendritic spine density of striatal MSNs after 1 or 12 weeks of exposure (Fitting et al., 2010a; Hahn et al., 2015).
Fig. 5.
HIV-1 Tat exposure decreases MSN dendritic spine density and VGLUT1 in the striatum. Synaptodendritic morphology in the striatum after 2 or 8 weeks of Tat exposure was altered. Representative image of Nissl staining in the area of the striatum examined (A). Representative images of Golgi-impregnated MSNs (D–G). Appearance of striatal MSNs (D) and tertiary (3rd order) dendrites (F) in Tat(−) mice. Appearance of striatal MSNs (E) and tertiary dendrites (G) in Tat(+) mice. Tat induction decreased the density of dendritic spines on tertiary branches of MSNs after 2 and 8 weeks of DOX administration (B). In the whole striatum, 8 weeks of Tat induction decreased the excitatory VGLUT1 protein levels, as measured by immunoblotting (C&H). Data are presented as mean ± SEM, multiple neurons (≥ 6) were sampled in each of n = 5–7 mice per group; ∗p < 0.05 vs Tat(−) mice; dashed-lines, approximate borders of the CP and NAc; caudate putamen (CP); n. accumbens (NAc).
The effects of 8 weeks of Tat exposure on excitatory and inhibitory synaptic markers in the striatum were also assessed (Fig. 5C&H). Interestingly, Tat decreased striatal inputs from the cortex as measured by VGLUT1 (t(10) = 2.25, p < 0.05), but not from the thalamus, as measured by VGLUT2 (t(10) = 1.26, p = 0.24) (Kaneko and Fujiyama, 2002). However, there was no difference between Tat(+) and Tat(−) in striatal levels of the excitatory pre- and post-synaptic proteins synapsin-1 (t(10) = 1.39, p = 0.2) and PSD-95 (t(10) = 1.23, p = 0.25), respectively. Similarly, Tat exposure did not affect the inhibitory pre- and post-synaptic proteins, Syt2 (t(10) = 0.54, p = 0.6) and gephyrin (t(10) = 0.9, p = 0.4), respectively, or the GABA synthesizing enzyme, GAD67 (t(10) = 0.59, p = 0.57). These data suggest that Tat alters excitatory cortical input connections to striatal MSNs.
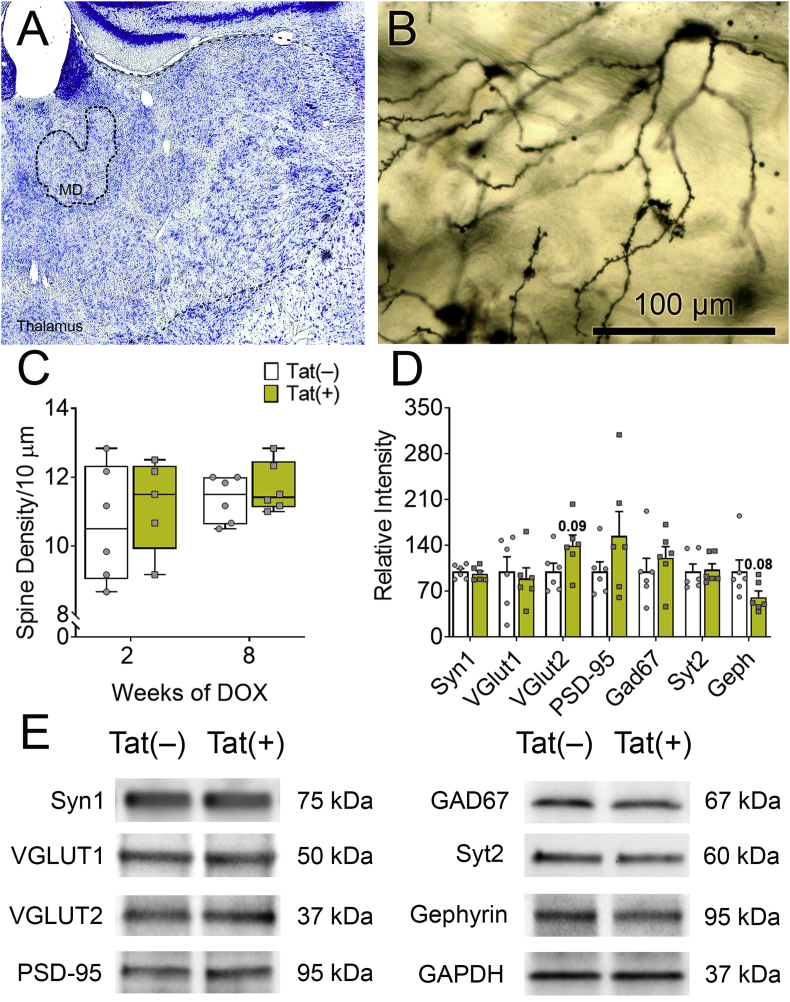
3.4. Tat does not affect thalamic spine density or synaptic markers
Since the thalamus provides a myriad of inputs and outputs within the ACC-BG-thalamocortical circuit (Phillips et al., 2019), we also assessed the effects of Tat on thalamic synaptic morphology. Tat exposure did not affect the density of dendritic spines of stellate neurons in the MD thalamus (p = 0.82; Fig. 6A–C), a key output subregion within the circuit (Halassa and Sherman, 2019). Similarly, within the whole thalamus, Tat did not change levels of the excitatory synaptic protein markers VGLUT1 (t(10) = 0.38, p = 0.71), VGLUT2 (t(10) = 1.90, p = 0.09), synapsin-1 (t(10) = 0.56, p = 0.59), or PSD-95 (t(10) = 1.35, p = 0.21). Furthermore, the inhibitory synaptic proteins Syt2 (t(10) = 0.20, p = 0.84), gephyrin (t(10) = 1.9, p = 0.08), and GAD67 (t(10) = 0.79, p = 0.45) were unaffected by Tat (Fig. 6D and E). Together, these data suggested that Tat may not affect synaptic morphology in the MD thalamus directly, but instead indirectly influence it through connections between the BG and PFC.
Fig. 6.
HIV-1 Tat does not affect MD thalamic stellate neuronal spine density or synaptic markers. In the thalamus, synaptodendritic morphology was measured after 2 or 8 weeks of Tat exposure. Representative Nissl staining showing the approximate left MD thalamic nucleus (MD) (A). Appearance of Golgi-impregnated stellate neurons in the MD thalamus (although dendritic spines populate the more distal dendrites, the cell body of origin is shown instead to better confirm its identity as a MD thalamic stellate neuron) of Tat(−) (B) and Tat(+) (not shown) mice displayed similar morphology and showed no differences in dendritic complexity (see C). No genotypic differences in dendritic spine density were seen in the MD thalamus after 2 or 8 weeks of Tat exposure (C). In the whole thalamus, Tat(+) did not significantly alter excitatory or inhibitory postsynaptic connections after 8 weeks of DOX (D–E). Data are presented as mean ± SEM, multiple neurons (≥ 6) were sampled in each of n = 5–7 mice per group; dashed-lines, approximate borders of the MD thalamus and thalamus.
3.5. HIV-1 Tat differentially affects cytokine levels in separate regions associated with frontal cortico-BG-thalamocortical circuitry
We next assessed the effects of Tat on cytokine levels in the intact PFC, striatum, and thalamus of Tat(+) and Tat(−) mice exposed to DOX for 48 h, 2 weeks, or 8 weeks to determine whether temporal changes in neuroimmune signaling might accompany Tat-induced behavioral dysfunction and the systematic dysregulation of synaptic connections within ACC-BG-thalamocortical circuitry. Basal cytokine levels differ among different brain regions (Miller et al., 2013; Silverman et al., 2015). Therefore, in the present study, chemokines and cytokines were analyzed in each brain region separately. For clarity, only chemokines (Fig. 7), proinflammatory cytokines (Fig. 8A–F), and anti-inflammatory cytokines (Fig. 8G–I) displaying significant Tat-dependent differences are displayed graphically, while detailed statistical parameters and outcomes corresponding to Figs. 7, 8A-F, and 8G-I, respectively, are reported in Table 1, Table 2, Table 3. Means ± SEM of cytokines that were not significantly affected by Tat at any time tested in any brain regions are reported in Table 4.
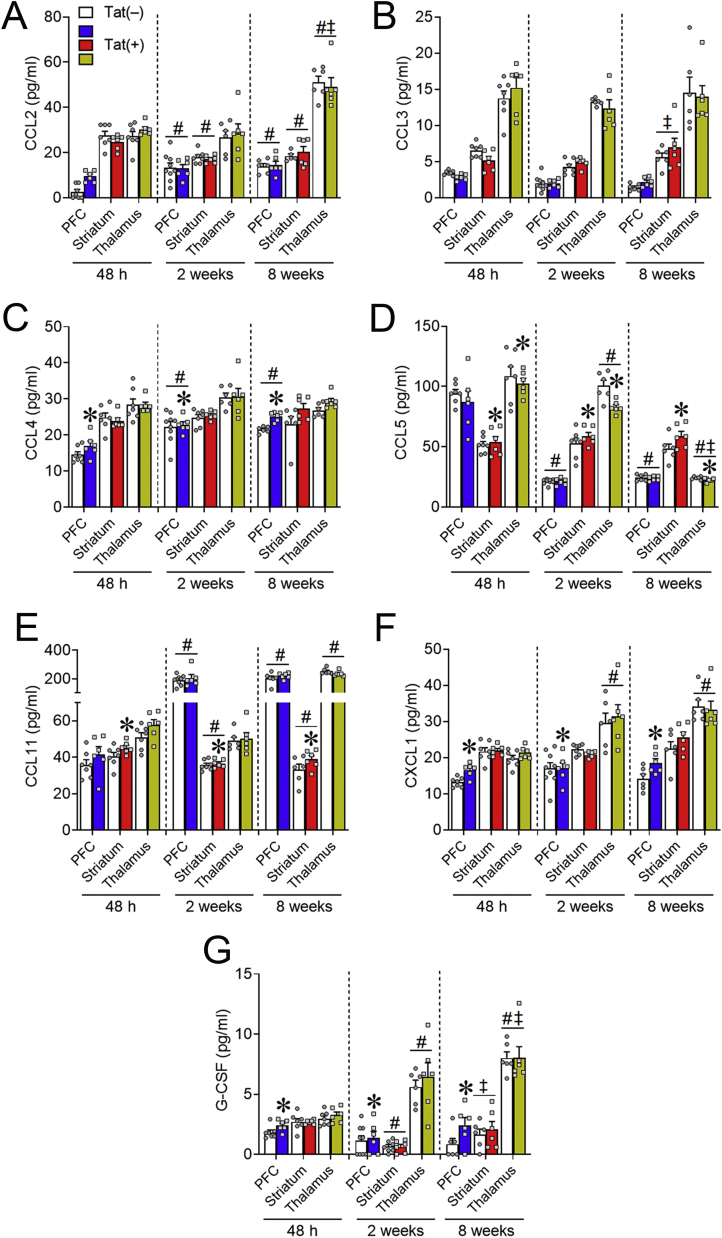
Fig. 7.
Chemokines are differentially altered by HIV-1 Tat in distinct regions of the frontal cortico-BG-thalamocortical circuit. Chemotactic cytokine protein expression in Tat(+) mice (color bars) was inversely changed in discrete regions of the frontal cortico-BG-thalamocortical circuit after 48 h, 2 weeks, or 8 weeks of DOX. CCL4 (C), CXCL1 (F), and G-CSF (G) levels were increased in the PFC after 48 h to 8 weeks of Tat exposure. Regardless of exposure time, Tat increased CCL5 (D) expression in the striatum; whereas in the thalamus expression was decreased. CCL11 (E) levels were also increased in the striatum after 48 h to 8 weeks of Tat (D). Data are presented as mean ± SEM; n = 6–9 mice per group. Main effect, ∗p < 0.05 vs respective Tat(−) (control) (white bars) within discrete regions. Main effect of exposure time within each separate region, #p < 0.05 vs respective 48 h exposure time; ‡p < 0.05 vs respective 2-week exposure time.
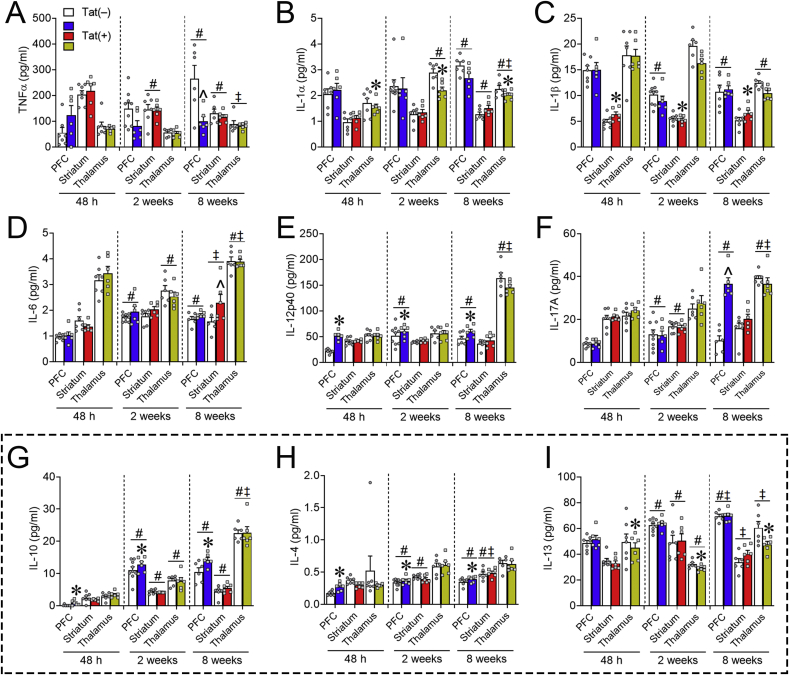
Fig. 8.
Proinflammatory and anti-inflammatory cytokine expression in the frontal cortico-BG-thalamocortical circuit is partially altered by HIV-1 Tat exposure. Proinflammatory and anti-inflammatory cytokine expression of Tat(+) (color bars) compared to Tat(−) (white bars) mice in the PFC, striatum, and thalamus after 48 h, 2 weeks, or 8 weeks of DOX exposure was largely unchanged. Proinflammatory IL-1β levels (C) were increased in the striatum after 48 h to 8 weeks of Tat exposure and IL-12p40 levels (E) were increased in the PFC; whereas in the thalamus IL-1α (B) levels were decreased. PFC expression of proinflammatory TNF-α (A) decreased, while IL-17A (F) increased in the PFC, and striatal IL-6 (D) expression increased in Tat(+) compared to Tat(−) mice after only 8 weeks of DOX. Expression of anti-inflammatory IL-10 (G) and IL-4 (H) was increased in the PFC; whereas IL-13 expression (I) was decreased in the thalamus after 48 h, 2 weeks, or 8 weeks of Tat exposure. Data are presented as mean ± SEM; n = 6–9 mice per group. Main effect, ∗p < 0.05 vs respective Tat(−) (control) within discrete regions. Main effect of exposure time within each separate region, #p < 0.05 vs respective 48 h exposure time; ‡p < 0.05 vs respective 2-week exposure time. ˆp < 0.05, interaction of Tat and exposure time within discrete regions. Dashed line separates anti-inflammatory cytokines.
Table 1.
Effects of brain region, Tat, and the duration of DOX exposure on chemokine levels.
| Brain Region | Main Effect of Tat | Tat/DOX Duration | Interaction | ||||
|---|---|---|---|---|---|---|---|
| PFC | Chemokine | F(1,34) | p | F(2,34) | p | F(2,34) | p |
| CCL2 | 2.96 | 0.95 | 14.35 | <0.01# | 2.95 | 0.07 | |
| CCL3 | 0.01 | 0.91 | 16.24 | <0.01# | 3.41 | 0.04 | |
| CCL4 | 6.48 | 0.02 | 29.46 | <0.01# | 1.20 | 0.32 | |
| CCL5 | 0.78 | 0.38 | 213.80 | <0.01# | 0.54 | 0.59 | |
| CCL11 | 1.36 | 0.25 | 101.90 | <0.01# | 0.12 | 0.89 | |
| CXCL1 | 6.06 | 0.02 | 1.49 | 0.24 | 1.72 | 0.19 | |
| G-CSF | 5.03 | 0.03 | 2.00 | 0.15 | 1.27 | 0.29 | |
| GM-CSF | 0.72 | 0.40 | 13.47 | <0.01# | 0.38 | 0.69 | |
| Striatum | Chemokine | F(1,32) | p | F(2,32) | p | F(2,32) | p |
| CCL2 | 0.06 | 0.80 | 13.12 | <0.01# | 2.09 | 0.14 | |
| CCL3 | 0.12 | 0.73 | 3.98 | 0.03‡ | 2.51 | 0.10 | |
| CCL4 | 1.76 | 0.19 | 0.22 | 0.80 | 2.25 | 0.12 | |
| CCL5 | 4.17 | 0.05 | 0.32 | 0.73 | 0.62 | 0.55 | |
| CCL11 | 5.94 | 0.02 | 8.96 | <0.01# | 0.85 | 0.44 | |
| CXCL1 | 0.51 | 0.48 | 2.56 | 0.09 | 1.90 | 0.17 | |
| G-CSF | 0.22 | 0.64 | 18.52 | <0.01@‡ | 0.27 | 0.77 | |
| GM-CSF | 1.20 | 0.28 | 1.55 | 0.23 | 1.77 | 0.19 | |
| Thalamus | Chemokine | F(1,31) | p | F(2,31) | p | F(2,31) | p |
| CCL2 | 0.17 | 0.68 | 35.20 | <0.01#‡ | 0.37 | 0.70 | |
| CCL3 | 0.00 | 0.98 | 0.84 | 0.44 | 0.45 | 0.64 | |
| CCL4 | 0.56 | 0.46 | 1.95 | 0.16 | 0.50 | 0.61 | |
| CCL5 | 5.25 | 0.03 | 191.00 | <0.01$ | 1.64 | 0.21 | |
| CCL11 | 0.57 | 0.46 | 985.20 | <0.01+‡ | 3.16 | 0.06 | |
| CXCL1 | 0.23 | 0.64 | 21.45 | <0.01# | 0.24 | 0.79 | |
| G-CSF | 0.46 | 0.50 | 26.13 | <0.01$ | 0.18 | 0.83 | |
| GM-CSF | 0.13 | 0.72 | 33.76 | <0.01$ | 0.05 | 0.95 | |
The F-values, degrees of freedom, and P-values from the chemokine ANOVA results. Bolded values denote significant differences at α = 0.05 (Bonferroni correction for multiple comparisons). Main effect of exposure time within each separate region, $ All exposure time-points (48 h, 2-week, and 8-week) are significantly different, # 48 h exposure time-point significantly differs from respective 2- and 8-week DOX exposure time-points; @ 48 h exposure time-point differs from the respective 2-week exposure time-point; + 48 h exposure time-point differs from its respective 8-week exposure time-point; ‡ 2-week exposure time-point differs from the respective 8-week exposure time-point.
Table 2.
Effects of brain region, Tat, and the duration of DOX exposure on proinflammatory cytokine levels.
| Brain Region | Main Effect of Tat | Tat/DOX Duration | Interaction | ||||
|---|---|---|---|---|---|---|---|
| PFC | Cytokine | F(1,34) | p | F(2,34) | p | F(2,34) | p |
| TNFα | 5.06 | 0.03 | 4.96 | 0.01+ | 7.48 | <0.01ˆ | |
| IFN-γ | 0.00 | 0.98 | 24.63 | <0.01# | 1.73 | 0.19 | |
| IL-1α | 0.44 | 0.51 | 4.67 | 0.02+ | 0.71 | 0.50 | |
| IL-1β | 0.08 | 0.78 | 15.57 | <0.01# | 0.45 | 0.64 | |
| IL-2 | 0.00 | 0.98 | 30.64 | <0.01# | 0.16 | 0.85 | |
| IL-3 | 2.22 | 0.15 | 18.39 | <0.01# | 1.86 | 0.17 | |
| IL-6 | 3.58 | 0.07 | 39.77 | <0.01# | 0.21 | 0.81 | |
| IL-9 | 2.10 | 0.16 | 39.87 | <0.01# | 0.68 | 0.51 | |
| IL-12p40 | 17.83 | <0.01 | 8.70 | <0.01# | 2.93 | 0.07˅ | |
| IL-12p70 | 0.48 | 0.49 | 48.42 | <0.01# | 0.64 | 0.53 | |
| IL-17A | 29.34 | <0.01 | 28.39 | <0.01# | 29.37 | <0.01ˆ | |
| Striatum | Cytokine | F(1,32) | p | F(2,32) | p | F(2,32) | p |
| TNFα | 0.01 | 0.91 | 14.52 | <0.01# | 0.34 | 0.71 | |
| IFN-γ | 0.26 | 0.62 | 0.30 | 0.75 | 1.31 | 0.28 | |
| IL-1α | 2.72 | 0.11 | 4.86 | 0.01+ | 0.26 | 0.77 | |
| IL-1β | 4.76 | 0.04 | 0.37 | 0.69 | 2.05 | 0.15 | |
| IL-2 | 0.40 | 0.53 | 6.68 | <0.01# | 0.79 | 0.46 | |
| IL-3 | 0.75 | 0.39 | 1.31 | 0.28 | 3.11 | 0.06 | |
| IL-6 | 3.43 | 0.07 | 4.39 | 0.02+ | 4.07 | 0.03ˆ | |
| IL-9 | 0.54 | 0.47 | 1.21 | 0.31 | 0.44 | 0.65 | |
| IL-12p40 | 2.40 | 0.13 | 0.28 | 0.76 | 0.56 | 0.57 | |
| IL-12p70 | 0.32 | 0.58 | 20.54 | <0.01# | 0.50 | 0.61 | |
| IL-17A | 1.73 | 0.20 | 3.70 | 0.04@ | 1.19 | 0.32 | |
| Thalamus | Cytokine | F(1,31) | p | F(2,31) | p | F(2,31) | p |
| TNFα | 0.67 | 0.42 | 4.47 | 0.02‡ | 0.09 | 0.92 | |
| IFN-γ | 0.82 | 0.37 | 83.84 | <0.01# | 0.16 | 0.86 | |
| IL-1α | 9.31 | <0.01 | 21.46 | <0.01$ | 2.05 | 0.15 | |
| IL-1β | 3.70 | 0.06 | 20.58 | <0.01+‡ | 1.01 | 0.37 | |
| IL-2 | 1.72 | 0.20 | 52.72 | <0.01+‡ | 1.28 | 0.29 | |
| IL-3 | 0.12 | 0.73 | 208.20 | <0.01+‡ | 0.33 | 0.72 | |
| IL-6 | >0.01 | 0.95 | 19.58 | <0.01$ | 0.83 | 0.45 | |
| IL-9 | 0.35 | 0.56 | 128.40 | <0.01$ | 0.43 | 0.65 | |
| IL-12p40 | 1.64 | 0.21 | 193.70 | <0.01+‡ | 1.51 | 0.24 | |
| IL-12p70 | 0.18 | 0.68 | 125.30 | <0.01+‡ | 0.03 | 0.97 | |
| IL-17A | 0.20 | 0.66 | 24.07 | <0.01+‡ | 0.82 | 0.45 | |
The F-values, degrees of freedom, and P-values from the proinflammatory cytokine ANOVA results. Bolded values denote significant differences at α = 0.05 (Bonferroni correction for multiple comparisons). Interactive effects of Tat and DOX exposure time within discrete brain regions, ˆ Tat(+) mice significantly differ from Tat(–) mice at 8-week exposure time-point only; ˅ Tat(+) mice significantly differ from Tat(–) mice at 48 h exposure time-point only. Main effect of exposure time within each separate region, $ All exposure time-points (48 h, 2-week, and 8-week) are significantly different, # 48 h exposure time-point significantly differs from its respective 2- and 8-week DOX exposure time-points; @ 48 h exposure time-point differs from its respective 2-week exposure time-point; + 48 h exposure time-point differs from its respective 8-week exposure time-point; ‡ 2-week exposure time-point differs from its respective 8-week exposure time-point.
Table 3.
Effects of brain region, Tat, and the duration of DOX exposure on anti-inflammatory cytokine levels.
| Brain Region | Main Effect of Tat | Tat/DOX Duration | Interaction | ||||
|---|---|---|---|---|---|---|---|
| PFC | Cytokine | F(1,34) | p | F(2,34) | p | F(2,34) | p |
| IL-4 | 9.88 | <0.01 | 28.82 | <0.01# | 1.77 | 0.19 | |
| IL-5 | 0.10 | 0.32 | 1.98 | 0.15 | 0.25 | 0.78 | |
| IL-10 | 8.17 | <0.01 | 4.67 | <0.01# | 1.41 | 0.26 | |
| IL-13 | 0.84 | 0.37 | 47.64 | <0.01$ | 0.23 | 0.80 | |
| Striatum | Cytokine | F(1,32) | p | F(2,32) | p | F(2,32) | p |
| IL-4 | 3.10 | 0.09 | 19.61 | <0.01$ | 2.32 | 0.11 | |
| IL-5 | 0.41 | 0.52 | 3.23 | 0.05 | 3.16 | 0.06 | |
| IL-10 | 0.02 | 0.88 | 20.19 | <0.01# | 1.97 | 0.16 | |
| IL-13 | 0.40 | 0.53 | 9.68 | <0.01@‡ | 0.61 | 0.55 | |
| Thalamus | Cytokine | F(1,31) | p | F(2,31) | p | F(2,31) | p |
| IL-4 | 0.58 | 0.45 | 2.22 | 0.13 | 0.69 | 0.51 | |
| IL-5 | 0.11 | 0.75 | 4.60 | 0.02@‡ | 0.00 | 0.99 | |
| IL-10 | 0.05 | 0.83 | 199.40 | <0.01$ | 0.05 | 0.95 | |
| IL-13 | 4.50 | 0.04 | 17.90 | <0.01@‡ | 0.81 | 0.45 | |
The F-values, degrees of freedom, and P-values from the anti-inflammatory cytokine ANOVA results. Bolded values denote significant differences at α = 0.05 (Bonferroni correction for multiple comparisons). Main effect of exposure time within each separate region, $ All exposure time-points (48 h, 2-week, and 8-week) are significantly different, # 48 h exposure time-point significantly differs from its respective 2- and 8-week DOX exposure time-points; @ 48 h exposure time-point differs from its respective 2-week exposure time-point; + 48 h exposure time-point differs from its respective 8-week exposure time-point; ‡ 2-week exposure time-point differs from its respective 8-week exposure time-point.
Table 4.
Means ± SEM of chemokines and cytokines not significantly altered by Tat in this study.
| Tat(–) | Tat(+) | ||||||
|---|---|---|---|---|---|---|---|
| Brain Region | Cytokine (pg/ml) | 48 h | 2 weeks | 8 weeks | 48 h | 2 weeks | 8 weeks |
| Mean ± SEM | |||||||
| PFC | GM-CSF | 0.00 ± 0.00 | 3.07 ± 0.85# | 2.58 ± 0.82# | 0.00 ± 0.00 | 3.34 ± 0.89# | 3.78 ± 0.72# |
| IFN-γ | 13.78 ± 0.45 | 25.50 ± 1.52# | 29.17 ± 1.43# | 14.61 ± 0.66 | 28.92 ± 4.04# | 24.78 ± 2.94# | |
| IL-2 | 25.55 ± 1.43 | 45.55 ± 2.95 | 47.62 ± 5.02 | 25.21 ± 1.99 | 44.19 ± 3.29 | 49.57 ± 2.43 | |
| IL-3 | 0.49 ± 0.05 | 2.31 ± 0.41 | 1.51 ± 0.30 | 0.48 ± 0.04 | 2.38 ± 0.40 | 2.68 ± 0.43 | |
| IL-9 | 12.65 ± 0.60 | 23.22 ± 0.87 | 24.65 ± 2.02 | 13.08 ± 0.98 | 27.16 ± 3.29 | 25.97 ± 0.84 | |
| IL-12p70 | 49.13 ± 4.08 | 123.22 ± 9.84# | 138.46 ± 14.57# | 48.79 ± 2.38 | 125.33 ± 10.38# | 121.20 ± 6.01# | |
| IL-5 | 0.57 ± 0.05 | 0.70 ± 0.05 | 0.71 ± 0.07 | 0.66 ± 0.06 | 0.70 ± 0.08 | 0.78 ± 0.09 | |
| Striatum | GM-CSF | 5.38 ± 0.63 | 5.40 ± 0.42 | 5.31 ± 1.12 | 5.16 ± 0.43 | 5.34 ± 0.19 | 7.43 ± 0.94 |
| IFN-γ | 34.85 ± 1.83 | 48.95 ± 5.75 | 33.55 ± 2.93 | 32.83 ± 1.97 | 50.40 ± 5.37 | 40.10 ± 2.82 | |
| IL-2 | 14.95 ± 0.92 | 19.45 ± 1.10# | 20.27 ± 1.95# | 17.22 ± 0.95 | 18.78 ± 1.15# | 20.52 ± 0.97# | |
| IL-3 | 1.03 ± 0.08 | 0.85 ± 0.04 | 1.79 ± 0.12 | 0.92 ± 0.04 | 0.85 ± 0.02 | 1.07 ± 0.12 | |
| IL-9 | 20.12 ± 1.70 | 17.64 ± 1.33 | 19.96 ± 3.08 | 19.34 ± 0.64 | 19.23 ± 0.75 | 22.18 ± 1.46 | |
| IL-12p70 | 95.19 ± 7.47 | 62.98 ± 3.14# | 67.98 ± 7.49# | 96.86 ± 1.78 | 60.42 ± 1.81# | 76.54 ± 7.24# | |
| IL-5 | 1.89 ± 0.14 | 2.03 ± 0.12 | 1.92 ± 0.28 | 1.64 ± 0.10 | 1.95 ± 0.05 | 2.55 ± 0.30 | |
| Thalamus | GM-CSF | 2.98 ± 0.22$ | 5.60 ± 0.57$ | 8.01 ± 0.51$ | 3.27 ± 0.26$ | 6.44 ± 1.19$ | 8.03 ± 0.93$ |
| IFN-γ | 25.57 ± 0.96 | 24.18 ± 1.77# | 45.23 ± 1.35# | 28.00 ± 1.16 | 24.98 ± 2.28# | 45.92 ± 2.66# | |
| IL-2 | 28.01 ± 2.07 | 28.22 ± 1.45 | 46.27 ± 2.12#‡ | 29.43 ± 1.59 | 24.51 ± 1.67 | 42.49 ± 2.18#‡ | |
| IL-3 | 0.93 ± 0.06 | 1.43 ± 0.12 | 10.01 ± 0.55#‡ | 1.03 ± 0.05 | 1.51 ± 0.20 | 9.43 ± 1.05#‡ | |
| IL-9 | 26.13 ± 1.03$ | 21.32 ± 1.21$ | 41.65 ± 1.23$ | 27.89 ± 1.46$ | 21.96 ± 1.15$ | 41.08 ± 1.47$ | |
| IL-12p70 | 98.69 ± 6.90 | 98.43 ± 7.52 | 263.48 ± 14.61#‡ | 96.07 ± 4.79 | 90.87 ± 10.39 | 261.27 ± 21.36#‡ | |
| IL-5 | 2.25 ± 0.11 | 2.91 ± 0.29# | 2.28 ± 0.18‡ | 2.34 ± 0.12 | 2.96 ± 0.40# | 2.34 ± 0.24‡ | |
Chemokine and cytokine protein levels within discrete brain regions of the frontal cortico-BG-thalamocortical circuit in Tat(+) and Tat(–) mice after 48 h, 2 weeks, or 8 weeks of DOX expressed as mean ± SEM in pg/ml. Bolded values denote significant differences at α = 0.05 (Bonferroni correction for multiple comparisons). Main effect of exposure time within each separate region, $p < 0.05 vs all other respective exposure time-points (48 h, 2-week, and 8-week), #p < 0.05 vs the respective 48 h exposure time-point, ‡p < 0.05 vs the respective 2-week exposure time-point.
3.5.1. Chemokines
Within the PFC, Tat induction significantly increased levels of the chemokines, CCL4 [Table 1; Fig. 7C], CXCL1 [Table 1; Fig. 7F], and G-CSF [Table 1; Fig. 7G] compared to Tat(−) mice. Tat exposure also significantly increased the expression of CCL11 [Table 1; Fig. 7E] and CCL5 in the striatum [Table 1; Fig. 7D]; whereas thalamic expression of CCL5 [Table 1; Fig. 7D] was markedly decreased. Duration of DOX exposure also significantly altered chemokine levels in discrete brain regions in Tat(–) and Tat(+) mice (see Table 1, Table 4, and Fig. 7). Together, these data indicate that Tat differentially alters chemokine expression within the intact PFC, striatum, and thalamus.
3.5.2. Proinflammatory cytokines
Regarding proinflammatory cytokines (Fig. 8), there was a main effect of Tat associated with significant overall increases in PFC levels of IL-12p40 [Table 2; Fig. 8E] and striatal levels of IL-1β [Table 2; Fig. 8C], and decreases in the thalamic levels of IL-1α [Table 2; Fig. 8B] across exposure durations. Interactive effects indicated that 8 weeks of Tat decreased PFC expression of TNFα [Table 2; Fig. 8A] while increasing PFC expression of IL-17A [Table 2; Fig. 8F] and striatal expression of IL-6 [Table 2; Fig. 8D]. In Tat(–) and Tat(+) mice, the duration of DOX exposure also altered the expression of proinflammatory cytokine levels within the PFC, striatum, and thalamus (Table 2, Table 4, and Fig. 8). These data suggest that Tat differentially increases and decreases pro-inflammatory cytokines within separate regions of the frontal cortico-BG-thalamocortical circuit.
3.5.3. Anti-inflammatory cytokines
Among Tat(+) mice, there was a significant increase in the anti-inflammatory cytokines IL-10 [Table 3; Fig. 8G] and IL-4 [Table 3; Fig. 8H] in the PFC, and a decrease in IL-13 [Table 3; Fig. 8I] expression in the thalamus. In discrete brain regions, the duration of DOX exposure also significantly altered the expression of anti-inflammatory cytokine levels in Tat(–) and Tat(+) mice (Table 3, Table 4, and Fig. 8).
3.6. Tat and PFC CCL4, CXCL1, IL-12(p40), or IL-17A interactions predict coping behavior
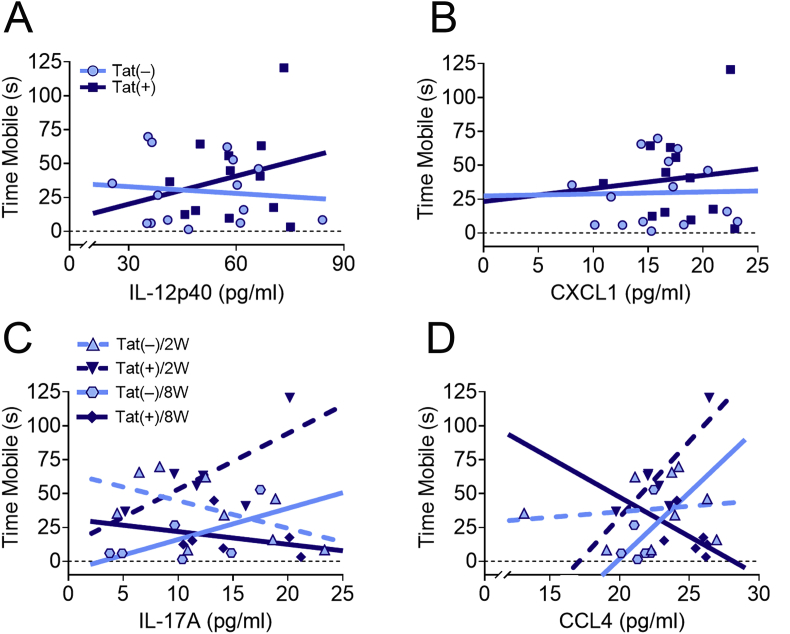
We used multiple linear regression analyses to investigate whether cytokine expression within the PFC predicted behavioral deficits after different lengths of Tat exposure. The regression results indicate that the interaction models of IL-12p40 [R2 = 0.6, F(7,19) = 3.4, p < 0.05; Fig. 9A], CXCL1 [R2 = 0.57, F(7,19) = 3.7, p < 0.05; Fig. 9B], IL-17A [R2 = 0.63, F(7,19) = 4.7, p < 0.01; Fig. 9C], or CCL4 [R2 = 0.60, F(7,19) = 4.1, p < 0.01; Fig. 9D] expression within the PFC, Tat, and exposure time significantly predict FST mobility. There was a significant interaction between Tat and IL-12p40 [β = 3.09, t(19) = 2.4, p < 0.05], CXCL1 [β = 11.06, t(19) = 2.6, p < 0.05], IL-17A [β = 9.30, t(19) = 3.2, p < 0.005], or CCL4 [β = 19.03, t(19) = 2.5, p < 0.05] and adaptability in response to an acute environmental stressor in the FST. Furthermore, the interaction of Tat, IL-17A, exposure time, and adaptability was significant [β = −0.22, t(19) = 2.8, p < 0.05], while the interaction between Tat, CCL4, exposure time, and adaptability was not significant [β = −0.62, t(19) = 1.9, p = 0.08]. The interaction plots suggest that in Tat(+) mice an increase in IL-12p40 or CXCL1 in the PFC correlates with increased FST mobility across exposure time; whereas an increase in IL-17A or CCL4 in Tat(+) mice correlates with increased FST mobility after 2 weeks exposure, but decreased mobility at 8 weeks of exposure. No additional significant interactions were seen between Tat, exposure time, and altered PFC cytokine expression to predict other behavioral deficits.
Fig. 9.
HIV-1 Tat-dependent alterations in CCL4, CXCL1, IL-12p40, and IL-17A levels in the PFC interact to predict mobility in the forced swim test (FST). Multiple linear regression interaction plots demonstrate that Tat and IL-12p40 (A) or CXCL1 (B) expression in the PFC significantly interacted to predict an increase in FST mobility irrespective of Tat exposure time. Interaction plots of Tat, exposure duration, and IL-17A (C) or CCL4 (D) expression indicate that Tat-dependent increases in either cytokine in the PFC at 2 weeks following Tat exposure predict an increase in mobility, whereas at 8 weeks of exposure predict a decrease in mobility. Other Tat-dependent alterations in cytokine levels in the PFC did not significantly predict behavioral outcomes. Data are presented as mean ± SEM; n = 6–9 mice per group.
4. Discussion
Despite cART, many PLWH experience executive functioning deficits that can decrease quality of life (Heaton et al., 2011). Since Tat levels can be elevated in the CSF of PLWH (Henderson et al., 2019; Johnson et al., 2013) and trigger TH17 immunological responsiveness in cART-suppressed, aviremic patients (Johnson et al., 2013), Tat appears unique from most other viral proteins. In fact, cART may increase Tat expression by failing to prevent feedback inhibition or host suppression of proviral transcription (Henderson et al., 2019; Johnson et al., 2013; Mbonye and Karn, 2017). Our goal was to begin to take a systems approach toward examining the effects of HIV-1 Tat on ACC and circuit-wide deficits in CNS structure and function. Inducible Tat-transgenic mice display many of the neurocognitive deficits seen in PLWH with HAND (Bruce-Keller et al., 2008; Fitting et al., 2010a, 2013; Gonek et al., 2018; Hahn et al., 2015, 2016; Marks et al., 2016; Paris et al., 2014, 2016). The Tat transgenic line we used resembles the slower onset, chronic pathology exhibited by PLWH on cART, including less outright neuronal death and more subtle structural and physiological impacts (Dickens et al., 2017).
Behavioral control is the ability to override impulses and adjust behavior to respond appropriately to different stimuli (Diamond, 2013; Kliethermes and Crabbe, 2006). Two to eight weeks of Tat exposure increased novelty-exploration in response to a novel environment, flavor, or food—coinciding with findings in HIV-1 transgenic rats (expressing multiple HIV proteins including Tat, Nef, and Vpr) demonstrating increased exploration in a novel environment (Wingo et al., 2016; Yang et al., 2017), and extending prior findings by revealing that increased novelty-exploration seen in preclinical HAND models is observed across the presentation of different novel stimuli. Thus, exposure to Tat alone (versus other viral proteins or protein combinations) is sufficient to increase novelty-exploration. Tat(+) mice also exhibited increased mobility in the FST after 2–8 weeks of DOX. Accordingly, Tat appears to alter adaptability to an inescapable stress-inducing environment. Although a single intracerebroventricular (icv) Tat injection decreased FST mobility after 24 h (Fu et al., 2011; Lawson et al., 2011), the present study was conducted at 2-8 weeks following sustained Tat induction, making comparisons difficult. We speculate that the neuronal dysfunction and chronic, low levels of inflammation following sustained Tat exposure differ notably from what is seen after an acute icv Tat injection. Interestingly, Tat also increased overall mobility in the novelty-induced hypophagia and novelty-suppressed feeding tests, corresponding with increases in novelty-exploration. However, Tat-induced mobility changes were not seen in the exploratory hole-board or the marble burying tests suggesting that the increased novelty-exploration and FST mobility we observed did not result from a Tat-induced hypermotoric phenotype. HIV-1 (Heaton et al., 2011; Robinson-Papp et al., 2008; Saylor et al., 2016) and Tat induction (Fitting et al., 2012; Hahn et al., 2015; Paris et al., 2016) often decrease motor coordination and locomotor activity, and the increase in mobility in response to novel food and flavor stimuli might indicate an enhancement of their rewarding properties (Kesby et al., 2016; Paris et al., 2014). Eight weeks of Tat exposure also decreased PPI of the acoustic startle response, confirming previous findings in PLWH and other rodent models of HAND that Tat can interfere with the pre-attentive filtering of stimuli (Fitting et al., 2006; McLaurin et al., 2016; Moran et al., 2013a, 2013b; Paris et al., 2015). Thus, we find that Tat can disrupt other facets of behavioral control besides PPI.
The balance of excitatory and inhibitory tone within frontal cortico-BG-thalamocortical loops is essential for modulating behavioral control (Christakou et al., 2001; Muir et al., 1996; Naaijen et al., 2015), and HAND is associated with the dysregulation of both glutamatergic and GABAergic systems (Buzhdygan et al., 2016; Gelman et al., 2012; Musante et al., 2010; Potter et al., 2013; Xu and Fitting, 2016; Xu et al., 2016). Accordingly, Tat-induced dysregulation of the ACC, striatum, and thalamus may result in decreased control of novelty-exploration, adaptability, and pre-attentive filtering. Although previous findings indicate ~1 month of Tat exposure increases PFC excitatory postsynaptic currents (Jacobs et al., 2019; Xu et al., 2017), the 8 weeks of Tat exposure in the current study did not affect the spine density, length, or branching of ACC layer V pyramidal neuronal dendrites. This disparity may relate to, (i) Tat-induced reductions in KCC2 (Barbour et al., 2020) altering chloride concentrations and reducing GABA potency, (ii) increased excitatory postsynaptic potentials preceding changes in dendritic spine density or dendritic complexity, which are likely to be altered with more prolonged Tat exposure, and (iii) in one instance, a difference in the model (rats) and Tat exposure (added acutely to PFC ex vivo slices) (Wayman et al., 2015). In contrast, HIV-transgenic rats exhibit dendritic alterations in infralimbic/prelimbic area layer II/III pyramidal neurons (Festa et al., 2015; McLaurin et al., 2019) suggesting that similar to other neurodegenerative disorders (Penzes et al., 2011), HIV proteins may also selectively damage dendrites within specific cortical layers and subdivisions in the PFC.
Aligning with previous reports in individuals with HAND (Buzhdygan et al., 2016; Gelman et al., 2012), Tat decreased whole PFC levels of the inhibitory synaptic markers, Syt2 and gephyrin, respectively. Furthermore, within layers II/III and V of the ACC, Tat decreased Syt2 and gephyrin colocalization, indicating that Tat may selectively target inhibitory synapses within this mPFC subregion. Tat decreases the frequency and amplitude of pyramidal neuron miniature inhibitory postsynaptic currents (mIPSCs) in layer II/III of the infralimbic/prelimbic mPFC, further suggesting impaired GABAergic function (Xu et al., 2016). The corresponding increase in GAD67 levels in the PFC may be a compensatory response and suggests that Tat-induced interneuronal dysfunction is more nuanced than simply a net decrease in inhibition. The lack of a corresponding change in dendritic morphology was unexpected; however, it is anticipated that subtle disruptions to inhibitory neurotransmission may precede the eventual reductions in dendritic spine density and complexity. Previous findings show that 10 days of Tat exposure decreased Syt2 specifically within the stratum radiatum and increased gephyrin throughout CA1, with only modest reductions in dendritic spine density without changes in dendritic length in hippocampal CA1 pyramidal cells (Fitting et al., 2013), which show greater decreases in dendritic spines with more prolonged exposure to Tat. Within the ACC, we speculate that the microcircuitry underlying behavioral control, but not sociability is more vulnerable to Tat. Marks et al. (2016) and Schier et al. (2017) found distinct subsets of neurons within hippocampal area CA1 and striatum, respectively, to be preferentially vulnerable to Tat, while surrounding interneurons seemed unaffected. Based on the present and other studies, we speculate that the intrinsic susceptibility of the neurocircuitry underlying a specific behavioral deficit to be as important as regional increases in viral loads/Tat expression.
In Tat(+) mice, striatal MSNs exhibit synaptodendritic damage after 1 or 12 weeks of DOX administration (Fitting et al., 2010a; Hahn et al., 2015), which agrees with the present findings. The dendritic spines of GABAergic MSNs within the striatum receive cortical and thalamic glutamatergic projections, indicated by VGLUT1 and VGLUT2, respectively (Kaneko and Fujiyama, 2002; Ni et al., 1995; Reiner et al., 2010). Tat-dependent decreases in striatal VGLUT1 suggest that corticostriatal afferents are also preferentially vulnerable. While the reasons other synaptic excitatory markers are unaffected are unclear, the timing of pre- and postsynaptic remodeling are often asynchronous, suggesting Tat-dependent decreases in VGLUT1 in the striatum precede decreases in synapsin-1 or PSD-95 (Fisher-Lavie and Ziv, 2013). In support, we previously demonstrated decreases in synapsin-1 and PSD-95 and concomitant decreases in dendritic spine density in the striatum of Tat(+) mice after 12 weeks of DOX (Hahn et al., 2015), compared to the reductions in spine density seen at 2 or 8 weeks in the present study. This suggests that, similar to GABAergic dysregulation within the PFC, Tat-induced glutamatergic dysregulation in the striatum differs depending on the duration of Tat exposure.
Although there is a paucity of research on the effects of HIV-1 in the thalamus, reports suggest that PLWH exhibit a loss in thalamic volume (Janssen et al., 2015; Nichols et al., 2019; Sanford et al., 2018) and increased viral loads (Nath, 2015). HIV-1 transgenic rats also exhibit decreased thalamic MAP2 levels (Pang and Panee, 2014). Although Tat exposure did not change dendritic spine density of stellate neurons in the MD thalamus, the main output region to layer V inhibitory neurons in the PFC (Halassa and Sherman, 2019), a recent study demonstrated a selective loss in the ventral lateral posterior region of the thalamus of PLWH, but not in other thalamic areas including the MD region (Zahr et al., 2019).
Novelty-exploration and compulsive-like behaviors are regulated by different frontal cortico-BG-thalamocortical circuits: the ACC and orbitofrontal, respectively (Chudasama et al., 2003; Fineberg et al., 2010; Napier and Persons, 2018). In the present study, Tat did not affect marble burying and nestlet shredding behavior, suggesting that Tat decreases novelty-exploration, but does not affect engagement in repetitive or stereotypical behaviors (Naaijen et al., 2015; Napier and Persons, 2018) and may not affect the orbitofrontal cortex. However, this hypothesis currently remains speculative. Due to possible Tat-induced increases in anxiety-like behavior (Hahn et al., 2015, 2016; Paris et al., 2014, 2016; Schier et al., 2017), environmental changes were made in the present study (e.g. enclosed chamber vs. open room with overhead light) to decrease anxiety in the marble burying test (Broekkamp et al., 1986; Crawley, 1985; Holter et al., 2015; Thomas et al., 2009). Notably, the reduction of aversive environmental conditions (e.g., brightly lit open space) may explain the lack of Tat-induced changes in the marble burying test used to assess compulsive-like behavior, which diverge from previous studies in which marble burying assessed anxiety-like behavior and Tat induction increased marble burying behavior (Paris et al., 2014, 2016). HIV-1 transgenic rats spent less time exploring the center of an open field with an overhead light, but more time exploring an open field when the center is dark (Rowson et al., 2016), suggesting changes in environmental stimuli can alter behavioral control or anxiety-like behaviors.
Social interactions did not differ between Tat(+) and Tat(−) mice, suggesting Tat-induced excitatory and inhibitory imbalances interfere with behavioral control, but not social motivation. However, in another Tat transgenic model (Kim et al., 2003), Tat exposure transiently decreased social interactions at 1 and 7 days following induction, which became normal after 14 days (Paris et al., 2014). The decreased social interactions with short-term Tat exposure may be due to “sickness behavior” caused by the initial release of proinflammatory cytokines including IL-1β, IL-6, and TNFα (Dantzer and Kelley, 2007; Hennessy et al., 2014; Lawson et al., 2011). We found nominal changes in cytokine expression within the PFC, striatum, and thalamus after 48 h to 8 weeks of Tat exposure, which supports previous findings suggesting that cytokine levels peak shortly (≤48 h) following Tat exposure (Gonek et al., 2018). Alternatively, sickness behavior may be regulated by complex temporal and regional differences in cytokine expression. Declines in IL-1α in the thalamus at 2–8 weeks and TNFα in the PFC at 8 weeks of Tat exposure were accompanied by marked increases in IL-1β at 2–8 weeks and IL-6 at 8 weeks of Tat exposure in the striatum suggesting considerable underlying complexity. Assuming “sickness behavior” results from the transient increases in key cytokines, which return to pretreatment levels with sustained Tat exposure, this may partially explain the lack of diminished social interactions in the present study.
Regional differences in cytokine levels differ with age, onset of insult, and duration (Deverman and Patterson, 2009; Drabek et al., 2015). The Tat-induced changes in cytokines were regionally specific, which agrees with previous in vitro findings in astrocyte-enriched cultures (Fitting et al., 2010b), and may reflect glial heterogeneity or regional alterations in blood-brain barrier integrity (Leibrand et al., 2017, 2019). Tat induction in the PFC increased expression of proinflammatory cytokines (i.e., IL-12p40), chemokines (i.e., CCL4, CXCL1, and G-CSF), and anti-inflammatory cytokines (i.e., IL-4 and IL-10). A substantial increase in IL-17A levels accompanied by a decrease in TNFα in the PFC of Tat(+) mice after 8 weeks, but not earlier, may indicate a shift to T-cell mediated regulatory responses. IL-17A is overexpressed in chronic inflammatory diseases (Rea et al., 2018), and simian immunodeficiency virus-infected macaques administered cART exhibited increased CNS infiltration of T and B lymphocytes (Mangus et al., 2018). Interestingly, Tat and increased PFC expression of CXCL1 and IL-12p40 interacted to predict mobility in the FST, suggesting that Tat-dependent increases in PFC levels of these two cytokines leads to a decrease in adaptability in response to an acute environmental stressor. Furthermore, Tat-induced expression of CCL4 and IL-17A interacted with exposure time to predict increased FST mobility after 2 weeks of exposure, while predicting a decrease after 8 weeks of Tat. The correlation of IL-12p40 and IL-17A expression in the PFC with Tat-induced dysfunctional coping aligns with previous reports indicating their importance in potentially mediating depression and cognitive impairment and suggest they might be therapeutic targets for HAND (Johansson et al., 2017; McGeachy et al., 2019). Prolonged Tat induction in the present study increased CCL5, CCL11, IL-1β, and IL-6 levels in the striatum. Previously, striatal increases in CCL3, CCL4, CCL11, IL-1α, and IL-9 expression were seen after 4 weeks of Tat induction, suggesting that cytokine expression differs dynamically depending on the duration of Tat expression, or is influenced by the stress of i.p. injections and/or alterations to the microbiome due to DOX (Gonek et al., 2018).
Regardless of genotype, many cytokine levels increased, particularly within the thalamus, throughout the duration of the study. Although regional CNS differences in cytokine expression can occur during normal aging, and may not coincide with neurodegenerative changes (Lopez-Gonzalez et al., 2017), some alterations to the microbiome introduced by continous DOX exposure may play a role. DOX administration can alter the gut microbiota compared to non-treated mice (Boynton et al., 2017). Vascular leakiness at the subfornical organ, adjacent to the thalamus, may result in net increases in cytokine levels due to the increased presence of blood-borne microbial products, such as lipopolysaccharides, translocated from the gut especially following prolonged DOX administration (Knoop et al., 2016; Kustova et al., 1999; Schulz and Engelhardt, 2005). Interestingly, not all cytokines within the thalamus increased; IL-1α, CCL5, and IL-13 decreased in Tat(+) mice after 2 or 8 weeks of DOX agreeing with decreased thalamic cytokine levels in HIV transgenic rats that may result from enhanced glutathione production (Pang and Panee, 2014; Pang et al., 2013).
5. Conclusions
Studies in humans and rodents have demonstrated that HIV-1 and several of its viral proteins, including Tat, disrupt aspects of behavioral control and induce aberrant synaptodendritic connections and inflammation within the frontal cortex, striatum, and thalamus. The present study extends previous findings by demonstrating that HIV-1 Tat exposure alters behavioral control and systematic, circuit-wide morphological and neuroimmune changes within the ACC-BG-thalamocortical circuitry in a highly complex and coordinated manner—including decreased control of novelty-exploration, adaptability, and pre-attentive filtering, but not altered compulsive-like or social behaviors. Tat itself may underlie reductions in impulse control, the ability to cope in stressful environments, and attentional filtering deficits seen in individuals with HAND. Tat-induced PFC expression of CCL4, CXCL1, IL-12p40, and IL-17A significantly predicted a decrease in adaptability in response to an unescapable stressor. The loss of specific behavioral control appears to be mediated by unique and systematic alterations in neuroimmune regulation and in the net balance of inhibitory and excitatory synapses within circuitry responsible for executive functions.
Declaration of competing interest
The authors declare they have no conflicts of interest.
Acknowledgments
This work was supported by funds from the National Institute on Drug Abuse grants F32 DA047193 (VDM), R01 DA034231, R01 DA044939 (PEK and KFH), K02 DA027374 (KFH), and R01 DA045588 (KFH). SRN, PEK, and KFH conceptualized and designed the experiments. SRN, YKH, VDM, NBV, and MID acquired the data. SRN, VDM, and KFH carried out data analysis and interpretation. Drafting the article for important intellectual content was undertaken by SRN, VDM, PEK, and KFH. All authors participated in proofing, revising, and approving the final version of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100077.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adams T.G., Kelmendi B., Brake C.A., Gruner P., Badour C.L., Pittenger C. The role of stress in the pathogenesis and maintenance of obsessive-compulsive disorder. Chronic Stress (Thousand Oaks) 2018;2:1–11. doi: 10.1177/2470547018758043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Angoa-Perez M., Kane M.J., Briggs D.I., Francescutti D.M., Kuhn D.M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 2013;82:50978. doi: 10.3791/50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A.J., Hauser K.F., McQuiston A.R., Knapp P.E. HIV and opiates dysregulate K+-Cl− cotransporter 2 (KCC2) to cause GABAergic dysfunction in primary human neurons and Tat-transgenic mice. Neurobiology of disease. 2020 doi: 10.1016/j.nbd.2020.104878. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette G.B., Powell E.M., Roesch M.R. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav. Brain Res. 2013;250:91–101. doi: 10.1016/j.bbr.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnoff S.R., Suranyi-Cadotte B., Aitken D.H., Quirion R., Meaney M.J. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berlin) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Boynton F.D.D., Ericsson A.C., Uchihashi M., Dunbar M.L., Wilkinson J.E. Doxycycline induces dysbiosis in female C57BL/6NCrl mice. BMC Res. Notes. 2017;10:644. doi: 10.1186/s13104-017-2960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekkamp C.L., Rijk H.W., Joly-Gelouin D., Lloyd K.L. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur. J. Pharmacol. 1986;126:223–229. doi: 10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller A.J., Turchan-Cholewo J., Smart E.J., Geurin T., Chauhan A., Reid R., Xu R., Nath A., Knapp P.E., Hauser K.F. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguiere E., Monteiro P., Mallet L., Feng G., Graybiel A.M. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr. Opin. Neurobiol. 2015;30:59–65. doi: 10.1016/j.conb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhdygan T., Lisinicchia J., Patel V., Johnson K., Neugebauer V., Paessler S., Jennings K., Gelman B. Neuropsychological, neurovirological and neuroimmune aspects of abnormal GABAergic transmission in HIV infection. J. Neuroimmune Pharmacol. 2016;11:279–293. doi: 10.1007/s11481-016-9652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey A.N., Sypek E.I., Singh H.D., Kaufman M.J., McLaughlin J.P. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav. Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M. What constitutes the prefrontal cortex? Science. 2017;358:478–482. doi: 10.1126/science.aan8868. [DOI] [PubMed] [Google Scholar]
- Chamorro J., Bernardi S., Potenza M.N., Grant J.E., Marsh R., Wang S., Blanco C. Impulsivity in the general population: a national study. J. Psychiatr. Res. 2012;46:994–1001. doi: 10.1016/j.jpsychires.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Lim A., Lau E., Alicata D. Chronic tobacco-smoking on psychopathological symptoms, impulsivity and cognitive deficits in HIV-infected individuals. J. Neuroimmune Pharmacol. 2017;12:389–401. doi: 10.1007/s11481-017-9728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Arai I., Satterfield R., Young S.M., Jr., Jonas P. Synaptotagmin 2 is the fast Ca2+ sensor at a central inhibitory synapse. Cell Rep. 2017;18:723–736. doi: 10.1016/j.celrep.2016.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopard C., Tong P.B.V., Toth P., Schatz M., Yezid H., Debaisieux S., Mettling C., Gross A., Pugniere M., Tu A., Strub J.M., Mesnard J.M., Vitale N., Beaumelle B. Cyclophilin A enables specific HIV-1 Tat palmitoylation and accumulation in uninfected cells. Nat. Commun. 2018;9:2251. doi: 10.1038/s41467-018-04674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A., Robbins T.W., Everitt B.J. Functional disconnection of a prefrontal cortical-dorsal striatal system disrupts choice reaction time performance: implications for attentional function. Behav. Neurosci. 2001;115:812–825. doi: 10.1037//0735-7044.115.4.812. [DOI] [PubMed] [Google Scholar]
- Chudasama Y., Passetti F., Rhodes S.E., Lopian D., Desai A., Robbins T.W. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav. Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Commons K.G., Cholanians A.B., Babb J.A., Ehlinger D.G. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem. Neurosci. 2017;8:955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C.G., Bischoff-Grethe A., Jordan S.J., Woods S.P., Ellis R.J., Paulus M.P., Grant I., TMARC Group Altered functional response to risky choice in HIV infection. PloS One. 2014;9 doi: 10.1371/journal.pone.0111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J.N. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Dantzer R., Kelley K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaisieux S., Rayne F., Yezid H., Beaumelle B. The ins and outs of HIV-1 Tat. Traffic (Copenhagen, Denmark) 2012;13:355–363. doi: 10.1111/j.1600-0854.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- Deverman B.E., Patterson P.H. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens A.M., Yoo S.W., Chin A.C., Xu J., Johnson T.P., Trout A.L., Hauser K.F., Haughey N.J. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Sci. Rep. 2017;7:7748. doi: 10.1038/s41598-017-07570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabek T., Wilson C.D., Janata A., Stezoski J.P., Janesko-Feldman K., Garman R.H., Tisherman S.A., Kochanek P.M. Unique brain region-dependent cytokine signatures after prolonged hypothermic cardiac arrest in rats. Ther. Hypothermia Temp. Manag. 2015;5:26–39. doi: 10.1089/ther.2014.0013. [DOI] [PubMed] [Google Scholar]
- du Plessis S., Vink M., Joska J.A., Koutsilieri E., Bagadia A., Stein D.J., Emsley R. HIV infection is associated with impaired striatal function during inhibition with normal cortical functioning on functional MRI. J. Int. Neuropsychol. Soc. 2015;21:722–731. doi: 10.1017/S1355617715000971. [DOI] [PubMed] [Google Scholar]
- Dulawa S.C., Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci. Biobehav. Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Duncan J., Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan M.J., Bruce-Keller A.J., Conner C., Knapp P.E., Xu R., Nath A., Hauser K.F. Effects of chronic expression of the HIV-induced protein, transactivator of transcription, on circadian activity rhythms in mice, with or without morphine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1680–R1687. doi: 10.1152/ajpregu.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R., Langford D., Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Everall I.P., Heaton R.K., Marcotte T.D., Ellis R.J., McCutchan J.A., Atkinson J.H., Grant I., Mallory M., Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa L., Gutoskey C.J., Graziano A., Waterhouse B.D., Meucci O. Induction of interleukin-1beta by human immunodeficiency virus-1 viral proteins leads to increased levels of neuronal ferritin heavy chain, synaptic injury, and deficits in flexible attention. J. Neurosci. 2015;35:10550–10561. doi: 10.1523/JNEUROSCI.4403-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg N.A., Potenza M.N., Chamberlain S.R., Berlin H.A., Menzies L., Bechara A., Sahakian B.J., Robbins T.W., Bullmore E.T., Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Lavie A., Ziv N.E. Matching dynamics of presynaptic and postsynaptic scaffolds. J. Neurosci. 2013;33:13094–13100. doi: 10.1523/JNEUROSCI.2144-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S., Booze R.M., Mactutus C.F. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int. J. Dev. Neurosci. 2006;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S., Ignatowska-Jankowska B.M., Bull C., Skoff R.P., Lichtman A.H., Wise L.E., Fox M.A., Su J., Medina A.E., Krahe T.E., Knapp P.E., Guido W., Hauser K.F. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol. Psychiatr. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S., Scoggins K.L., Xu R., Dever S.M., Knapp P.E., Dewey W.L., Hauser K.F. Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice. Eur. J. Pharmacol. 2012;689:96–103. doi: 10.1016/j.ejphar.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S., Xu R., Bull C., Buch S.K., El-Hage N., Nath A., Knapp P.E., Hauser K.F. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am. J. Pathol. 2010;177:1397–1410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S., Zou S., Chen W., Vo P., Hauser K.F., Knapp P.E. Regional heterogeneity and diversity in cytokine and chemokine production by astroglia: differential responses to HIV-1 Tat, gp120, and morphine revealed by multiplex analysis. J. Proteome Res. 2010;9:1795–1804. doi: 10.1021/pr900926n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Lawson M.A., Kelley K.W., Dantzer R. HIV-1 Tat activates indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures in a p38 mitogen-activated protein kinase-dependent manner. J. Neuroinflammation. 2011;8:88. doi: 10.1186/1742-2094-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara E., Tomlinson S.E., Purdon S.E., Gill M.J., Power C. Decision making under explicit risk is impaired in individuals with human immunodeficiency virus (HIV) J. Clin. Exp. Neuropsychol. 2015;37:733–750. doi: 10.1080/13803395.2015.1057481. [DOI] [PubMed] [Google Scholar]
- Gelman B.B., Chen T., Lisinicchia J.G., Soukup V.M., Carmical J.R., Starkey J.M., Masliah E., Commins D.L., Brandt D., Grant I., Singer E.J., Levine A.J., Miller J., Winkler J.M., Fox H.S., Luxon B.A., Morgello S., National Neuro A.T.C. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PloS One. 2012;7 doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman B.B., Nguyen T.P. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J. Neuroimmune Pharmacol. 2010;5:92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M.A., Dulawa S.C. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr. Protoc. Neurosci. 2003;24:8.17.1–8.17.15. doi: 10.1002/0471142301.ns0817s24. [DOI] [PubMed] [Google Scholar]
- Gonek M., McLane V.D., Stevens D.L., Lippold K., Akbarali H.I., Knapp P.E., Dewey W.L., Hauser K.F., Paris J.J. CCR5 mediates HIV-1 Tat-induced neuroinflammation and influences morphine tolerance, dependence, and reward. Brain Behav. Immun. 2018;69:124–138. doi: 10.1016/j.bbi.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha D., Wagner M.C.E., Ayyavoo V. Human immunodeficiency virus type 1 (HIV-1)-mediated neuroinflammation dysregulates neurogranin and induces synaptodendritic injury. J. Neuroinflammation. 2018;15:126. doi: 10.1186/s12974-018-1160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y.K., Paris J.J., Lichtman A.H., Hauser K.F., Sim-Selley L.J., Selley D.E., Knapp P.E. Central HIV-1 Tat exposure elevates anxiety and fear conditioned responses of male mice concurrent with altered mu-opioid receptor-mediated G-protein activation and beta-arrestin 2 activity in the forebrain. Neurobiol. Dis. 2016;92:124–136. doi: 10.1016/j.nbd.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y.K., Podhaizer E.M., Farris S.P., Miles M.F., Hauser K.F., Knapp P.E. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct. Funct. 2015;220:605–623. doi: 10.1007/s00429-013-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa M.M., Sherman S.M. Thalamocortical circuit motifs: a general framework. Neuron. 2019;103:762–770. doi: 10.1016/j.neuron.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton D.A., Brigman J.L. Behavioral flexibility in rats and mice: contributions of distinct frontocortical regions. Gene Brain Behav. 2015;14:4–21. doi: 10.1111/gbb.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D.J., Hinkin C.H., Levine A.J., Castellon S.A., Lam M.N. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20:355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hategan A., Bianchet M.A., Steiner J., Karnaukhova E., Masliah E., Fields A., Lee M.H., Dickens A.M., Haughey N., Dimitriadis E.K., Nath A. HIV Tat protein and amyloid-β peptide form multifibrillar structures that cause neurotoxicity. Nat. Struct. Mol. Biol. 2017;24:379–386. doi: 10.1038/nsmb.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser K.F., Hahn Y.K., Adjan V.V., Zou S., Buch S.K., Nath A., Bruce-Keller A.J., Knapp P.E. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57:194–206. doi: 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser K.F., McLaughlin P.J., Zagon I.S. Endogenous opioid systems and the regulation of dendritic growth and spine formation. J. Comp. Neurol. 1989;281:13–22. doi: 10.1002/cne.902810103. [DOI] [PubMed] [Google Scholar]
- Heaton R.K., Franklin D.R., Ellis R.J., McCutchan J.A., Letendre S.L., Leblanc S., Corkran S.H., Duarte N.A., Clifford D.B., Woods S.P., Collier A.C., Marra C.M., Morgello S., Mindt M.R., Taylor M.J., Marcotte T.D., Atkinson J.H., Wolfson T., Gelman B.B., McArthur J.C., Simpson D.M., Abramson I., Gamst A., Fennema-Notestine C., Jernigan T.L., Wong J., Grant I., CHARTER Group, HNRC Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.J., Johnson T.P., Smith B.R., Reoma L.B., Santamaria U.A., Bachani M., Demarino C., Barclay R.A., Snow J., Sacktor N., McArthur J., Letendre S., Steiner J., Kashanchi F., Nath A. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS. 2019;33(Suppl. 2):S145–S157. doi: 10.1097/QAD.0000000000002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy M.B., Deak T., Schiml P.A. Sociality and sickness: have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain Behav. Immun. 2014;37:15–20. doi: 10.1016/j.bbi.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C.B., Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cognit. Sci. 2012;16:122–128. doi: 10.1016/j.tics.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Holter S.M., Einicke J., Sperling B., Zimprich A., Garrett L., Fuchs H., Gailus-Durner V., Hrabe de Angelis M., Wurst W. Tests for anxiety-related behavior in mice. Curr. Protoc. Mouse Biol. 2015;5:291–309. doi: 10.1002/9780470942390.mo150010. [DOI] [PubMed] [Google Scholar]
- Hunnicutt B.J., Jongbloets B.C., Birdsong W.T., Gertz K.J., Zhong H., Mao T. A comprehensive excitatory input map of the striatum reveals novel functional organization. eLife. 2016;5:e19103. doi: 10.7554/eLife.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser J.C., Brown G.G., Bischoff-Grethe A., Connolly C.G., Ellis R.J., Heaton R.K., Grant I., TMARC Group HIV infection is associated with attenuated frontostriatal intrinsic connectivity: a preliminary study. J. Int. Neuropsychol. Soc. 2015;21:203–213. doi: 10.1017/S1355617715000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs I.R., Xu C., Hermes D.J., League A.F., Xu C., Nath B., Jiang W., Niphakis M.J., Cravatt B.F., Mackie K., Mukhopadhyay S., Lichtman A.H., Ignatowska-Jankowska B.M., Fitting S. Inhibitory control deficits associated with upregulation of CB1R in the HIV-1 Tat transgenic mouse model of hand. J. Neuroimmune Pharmacol. 2019;14:661–678. doi: 10.1007/s11481-019-09867-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M., Obeso I., Rothwell J.C., Obeso J.A. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat. Rev. Neurosci. 2015;16:719–732. doi: 10.1038/nrn4038. [DOI] [PubMed] [Google Scholar]
- Jamain S., Radyushkin K., Hammerschmidt K., Granon S., Boretius S., Varoqueaux F., Ramanantsoa N., Gallego J., Ronnenberg A., Winter D., Frahm J., Fischer J., Bourgeron T., Ehrenreich H., Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen M.A., Meulenbroek O., Steens S.C., Goraj B., Bosch M., Koopmans P.P., Kessels R.P. Cognitive functioning, wellbeing and brain correlates in HIV-1 infected patients on long-term combination antiretroviral therapy. AIDS. 2015;29:2139–2148. doi: 10.1097/QAD.0000000000000824. [DOI] [PubMed] [Google Scholar]
- Janssen M.A.M., Hinne M., Janssen R.J., van Gerven M.A., Steens S.C., Goraj B., Koopmans P.P., Kessels R.P.C. Resting-state subcortical functional connectivity in HIV-infected patients on long-term cART. Brain Imag. Behav. 2017;11:1555–1560. doi: 10.1007/s11682-016-9632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P., Almqvist E.G., Wallin A., Johansson J.O., Andreasson U., Blennow K., Zetterberg H., Svensson J. Reduced cerebrospinal fluid concentration of interleukin-12/23 subunit p40 in patients with cognitive impairment. PloS One. 2017;12 doi: 10.1371/journal.pone.0176760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.P., Patel K., Johnson K.R., Maric D., Calabresi P.A., Hasbun R., Nath A. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc. Natl. Acad. Sci. U. S. A. 2013;110:13588–13593. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaani J., Kolibachuk J., Martinez H., Baekkeskov S. Two distinct mechanisms target GAD67 to vesicular pathways and presynaptic clusters. J. Cell Biol. 2010;190:911–925. doi: 10.1083/jcb.200912101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci. Res. 2002;42:243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kesby J.P., Fields J.A., Chang A., Coban H., Achim C.L., Semenova S., TMARC Group Effects of HIV-1 TAT protein and methamphetamine exposure on visual discrimination and executive function in mice. Behav. Brain Res. 2018;349:73–79. doi: 10.1016/j.bbr.2018.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby J.P., Markou A., Semenova S. The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology. 2016;109:205–215. doi: 10.1016/j.neuropharm.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.O., Liu Y., Ruan Y., Xu Z.C., Schantz L., He J.J. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey S.G., O’Neal S.T., Long J.Z., Cravatt B.F., Lichtman A.H. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol. Biochem. Behav. 2011;98:21–27. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes C.L., Crabbe J.C. Genetic independence of mouse measures of some aspects of novelty seeking. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5018–5023. doi: 10.1073/pnas.0509724103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop K.A., McDonald K.G., Kulkarni D.H., Newberry R.D. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut. 2016;65:1100–1109. doi: 10.1136/gutjnl-2014-309059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N., Behrens T., Wittmann M.K., Rushworth M. Multiple signals in anterior cingulate cortex. Curr. Opin. Neurobiol. 2016;37:36–43. doi: 10.1016/j.conb.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustova Y., Grinberg A., Basile A.S. Increased blood-brain barrier permeability in LP-BM5 infected mice is mediated by neuroexcitatory mechanisms. Brain Res. 1999;839:153–163. doi: 10.1016/s0006-8993(99)01734-5. [DOI] [PubMed] [Google Scholar]
- Laubach M., Amarante L.M., Swanson K., White S.R. What, if anything, is rodent prefrontal cortex? eNeuro. 2018;5 doi: 10.1523/ENEURO.0315-18.2018. ENEURO.0315-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M.A., Kelley K.W., Dantzer R. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain Behav. Immun. 2011;25:1569–1575. doi: 10.1016/j.bbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrand C.R., Paris J.J., Ghandour M.S., Knapp P.E., Kim W.K., Hauser K.F., McRae M. HIV-1 Tat disrupts blood-brain barrier integrity and increases phagocytic perivascular macrophages and microglia in the dorsal striatum of transgenic mice. Neurosci. Lett. 2017;640:136–143. doi: 10.1016/j.neulet.2016.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrand C.R., Paris J.J., Jones A.M., Masuda Q.N., Halquist M.S., Kim W.K., Knapp P.E., Kashuba A.D.M., Hauser K.F., McRae M. HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity. J. Neurovirol. 2019;25:560–577. doi: 10.1007/s13365-019-00757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A.J., Soontornniyomkij V., Achim C.L., Masliah E., Gelman B.B., Sinsheimer J.S., Singer E.J., Moore D.J. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J. Neurovirol. 2016;22:431–441. doi: 10.1007/s13365-015-0410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Morrow D., Witkin J.M. Decreases in nestlet shredding of mice by serotonin uptake inhibitors: comparison with marble burying. Life Sci. 2006;78:1933–1939. doi: 10.1016/j.lfs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Loos M., van der Sluis S., Bochdanovits Z., van Zutphen I.J., Pattij T., Stiedl O., Neuro B.M.P.c., Smit A.B., Spijker S. Activity and impulsive action are controlled by different genetic and environmental factors. Gene Brain Behav. 2009;8:817–828. doi: 10.1111/j.1601-183X.2009.00528.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Gonzalez I., Tebe Cordomi C., Ferrer I. Regional gene expression of inflammation and oxidative stress responses does not predict neurodegeneration in aging. J. Neuropathol. Exp. Neurol. 2017;76:135–150. doi: 10.1093/jnen/nlw117. [DOI] [PubMed] [Google Scholar]
- Mangus L.M., Beck S.E., Queen S.E., Brill S.A., Shirk E.N., Metcalf Pate K.A., Muth D.C., Adams R.J., Gama L., Clements J.E., Mankowski J.L. Lymphocyte-dominant encephalitis and meningitis in simian immunodeficiency virus-infected macaques receiving antiretroviral therapy. Am. J. Pathol. 2018;188:125–134. doi: 10.1016/j.ajpath.2017.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C.V., Araujo M.F., Nishimaru H., Matsumoto J., Tran A.H., Hori E., Ono T., Nishijo H. Pregenual anterior cingulate gyrus involvement in spontaneous social interactions in primates-evidence from behavioral, pharmacological, neuropsychiatric, and neurophysiological findings. Front. Neurosci. 2017;11:34. doi: 10.3389/fnins.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks W.D., Paris J.J., Schier C.J., Denton M.D., Fitting S., McQuiston A.R., Knapp P.E., Hauser K.F. HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J. Neurovirol. 2016;22:747–762. doi: 10.1007/s13365-016-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Heaton R.K., Marcotte T.D., Ellis R.J., Wiley C.A., Mallory M., Achim C.L., McCutchan J.A., Nelson J.A., Atkinson J.H., Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann. Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Mbonye U., Karn J. The molecular basis for human immunodeficiency virus latency. Annu. Rev. Virol. 2017;4:261–285. doi: 10.1146/annurev-virology-101416-041646. [DOI] [PubMed] [Google Scholar]
- McArthur J.C., Steiner J., Sacktor N., Nath A. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann. Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., Cua D.J., Gaffen S.L. The IL-17 family of cytokines in Health and disease. Immunity. 2019;50:892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin K.A., Booze R.M., Mactutus C.F. Progression of temporal processing deficits in the HIV-1 transgenic rat. Sci. Rep. 2016;6:32831. doi: 10.1038/srep32831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin K.A., Li H., Booze R.M., Mactutus C.F. Disruption of timing: NeuroHIV progression in the post-cART era. Sci. Rep. 2019;9:827. doi: 10.1038/s41598-018-36822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Haroon E., Raison C.L., Felger J.C. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress. Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk M.L., de Kloet E.R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology. 2015;62:389–391. doi: 10.1016/j.psyneuen.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Moore D.J., Masliah E., Rippeth J.D., Gonzalez R., Carey C.L., Cherner M., Ellis R.J., Achim C.L., Marcotte T.D., Heaton R.K., Grant I., Group H. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Moran L.M., Booze R.M., Mactutus C.F. Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J. Neuroimmune Pharmacol. 2013;8:988–997. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran L.M., Booze R.M., Webb K.M., Mactutus C.F. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp. Neurol. 2013;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M.J., Na E.S., Autry A.E., Monteggia L.M. Impact of DNMT1 and DNMT3a forebrain knockout on depressive- and anxiety like behavior in mice. Neurobiol. Learn. Mem. 2016;135:139–145. doi: 10.1016/j.nlm.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir J.L., Everitt B.J., Robbins T.W. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cerebr. Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Musante V., Summa M., Neri E., Puliti A., Godowicz T.T., Severi P., Battaglia G., Raiteri M., Pittaluga A. The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cerebr. Cortex. 2010;20:1974–1984. doi: 10.1093/cercor/bhp274. [DOI] [PubMed] [Google Scholar]
- Naaijen J., Lythgoe D.J., Amiri H., Buitelaar J.K., Glennon J.C. Fronto-striatal glutamatergic compounds in compulsive and impulsive syndromes: a review of magnetic resonance spectroscopy studies. Neurosci. Biobehav. Rev. 2015;52:74–88. doi: 10.1016/j.neubiorev.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Napier T.C., Persons A.L. Pharmacological insights into impulsive-compulsive spectrum disorders associated with dopaminergic therapy. Eur. J. Neurosci. 2018;50:2492–2502. doi: 10.1111/ejn.14177. [DOI] [PubMed] [Google Scholar]
- Nath A. Eradication of human immunodeficiency virus from brain reservoirs. J. Neurovirol. 2015;21:227–234. doi: 10.1007/s13365-014-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni B., Wu X., Yan G.M., Wang J., Paul S.M. Regional expression and cellular localization of the Na+-dependent inorganic phosphate cotransporter of rat brain. J. Neurosci. 1995;15:5789–5799. doi: 10.1523/JNEUROSCI.15-08-05789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols M.J., Gates T.M., Soares J.R., Moffat K.J., Rae C.D., Brew B.J., Cysique L.A. Atrophic brain signatures of mild forms of neurocognitive impairment in virally suppressed HIV infection. AIDS. 2019;33:55–66. doi: 10.1097/QAD.0000000000002042. [DOI] [PubMed] [Google Scholar]
- Ortega M., Brier M.R., Ances B.M. Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. AIDS. 2015;29:703–712. doi: 10.1097/QAD.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X., Panee J. Roles of glutathione in antioxidant defense, inflammation, and neuron differentiation in the thalamus of HIV-1 transgenic rats. J. Neuroimmune Pharmacol. 2014;9:413–423. doi: 10.1007/s11481-014-9538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X., Panee J., Liu X., Berry M.J., Chang S.L., Chang L. Regional variations of antioxidant capacity and oxidative stress responses in HIV-1 transgenic rats with and without methamphetamine administration. J. Neuroimmune Pharmacol. 2013;8:691–704. doi: 10.1007/s11481-013-9454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J.J., Singh H.D., Carey A.N., McLaughlin J.P. Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav. Brain Res. 2015;291:209–218. doi: 10.1016/j.bbr.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J.J., Singh H.D., Ganno M.L., Jackson P., McLaughlin J.P. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein. Tat. Psychopharmacology (Berl) 2014;231:2349–2360. doi: 10.1007/s00213-013-3385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J.J., Zou S., Hahn Y.K., Knapp P.E., Hauser K.F. 5α-reduced progestogens ameliorate mood-related behavioral pathology, neurotoxicity, and microgliosis associated with exposure to HIV-1 Tat. Brain Behav. Immun. 2016;55:202–214. doi: 10.1016/j.bbi.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P., Cahill M.E., Jones K.A., VanLeeuwen J.E., Woolfrey K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S.K., Dunlop K., Downar J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front. Syst. Neurosci. 2016;10:104. doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.W., Schulmann A., Hara E., Winnubst J., Liu C., Valakh V., Wang L., Shields B.C., Korff W., Chandrashekar J., Lemire A.L., Mensh B., Dudman J.T., Nelson S.B., Hantman A.W. A repeated molecular architecture across thalamic pathways. Nat. Neurosci. 2019;22:1925–1935. doi: 10.1038/s41593-019-0483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessis S.D., Vink M., Joska J.A., Koutsilieri E., Stein D.J., Emsley R. HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS. 2014;28:803–811. doi: 10.1097/QAD.0000000000000151. [DOI] [PubMed] [Google Scholar]
- Pogorelov V.M., Rodriguiz R.M., Insco M.L., Caron M.G., Wetsel W.C. Novelty seeking and stereotypic activation of behavior in mice with disruption of the Dat1 gene. Neuropsychopharmacology. 2005;30:1818–1831. doi: 10.1038/sj.npp.1300724. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Anton G., Blavet N., Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Potter M.C., Figuera-Losada M., Rojas C., Slusher B.S. Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders. J. Neuroimmune Pharmacol. 2013;8:594–607. doi: 10.1007/s11481-013-9442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayne F., Debaisieux S., Yezid H., Lin Y.L., Mettling C., Konate K., Chazal N., Arold S.T., Pugniere M., Sanchez F., Bonhoure A., Briant L., Loret E., Roy C., Beaumelle B. Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 2010;29:1348–1362. doi: 10.1038/emboj.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea I.M., Gibson D.S., McGilligan V., McNerlan S.E., Alexander H.D., Ross O.A. Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A., Hart N.M., Lei W., Deng Y. Corticostriatal projection neurons - dichotomous types and dichotomous functions. Front. Neuroanat. 2010;4:142. doi: 10.3389/fnana.2010.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Papp J., Byrd D., Mindt M.R., Oden N.L., Simpson D.M., Morgello S., Manhattan HIV Brain Bank Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Arch. Neurol. 2008;65:1096–1101. doi: 10.1001/archneur.65.8.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S.J., Pack T.F., Peterson S.M., Payne K., Borrelli E., Caron M.G. Engineered D2R variants reveal the balanced and biased contributions of G-protein and β-arrestin to dopamine-dependent functions. Neuropsychopharmacology. 2018;43:1164–1173. doi: 10.1038/npp.2017.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowson S.A., Harrell C.S., Bekhbat M., Gangavelli A., Wu M.J., Kelly S.D., Reddy R., Neigh G.N. Neuroinflammation and behavior in HIV-1 transgenic rats exposed to chronic adolescent stress. Front. Psychiatr. 2016;7:102. doi: 10.3389/fpsyt.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck P.H., Walton M.E., Millette B.H., Shirley E., Rushworth M.F., Bannerman D.M. Distinct contributions of frontal areas to emotion and social behaviour in the rat. Eur. J. Neurosci. 2007;26:2315–2326. doi: 10.1111/j.1460-9568.2007.05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R., Ances B.M., Meyerhoff D.J., Price R.W., Fuchs D., Zetterberg H., Spudich S., Collins D.L. Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin. Infect. Dis. 2018;67:1697–1704. doi: 10.1093/cid/ciy362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D., Dickens A.M., Sacktor N., Haughey N., Slusher B., Pletnikov M., Mankowski J.L., Brown A., Volsky D.J., McArthur J.C. HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016;12:309–348. doi: 10.1038/nrneurol.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz M., Tong P.B.V., Beaumelle B. Unconventional secretion of viral proteins. Semin Cell Dev Biol. 2018;83:8–11. doi: 10.1016/j.semcdb.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Schier C.J., Marks W.D., Paris J.J., Barbour A.J., McLane V.D., Maragos W.F., McQuiston A.R., Knapp P.E., Hauser K.F. Selective vulnerability of striatal D2 versus D1 dopamine receptor-expressing medium spiny neurons in HIV-1 Tat transgenic male mice. J. Neurosci. 2017;37:5758–5769. doi: 10.1523/JNEUROSCI.0622-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M., Engelhardt B. The circumventricular organs participate in the immunopathogenesis of experimental autoimmune encephalomyelitis. Cerebrospinal Fluid Res. 2005;2:8. doi: 10.1186/1743-8454-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman H.A., Dancho M., Regnier-Golanov A., Nasim M., Ochani M., Olofsson P.S., Ahmed M., Miller E.J., Chavan S.S., Golanov E., Metz C.N., Tracey K.J., Pavlov V.A. Brain region-specific alterations in the gene expression of cytokines, immune cell markers and cholinergic system components during peripheral endotoxin-induced inflammation. Mol. Med. 2015;20:601–611. doi: 10.2119/molmed.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N.R., Braff D.L., Geyer M.A. Sensorimotor gating of the startle reflex: what we said 25 years ago, what has happened since then, and what comes next. J. Psychopharmacol. 2016;30:1072–1081. doi: 10.1177/0269881116661075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N.R., Geyer M.A., Braff D.L. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berlin) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Takeda H., Tsuji M., Matsumiya T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur. J. Pharmacol. 1998;350:21–29. doi: 10.1016/s0014-2999(98)00223-4. [DOI] [PubMed] [Google Scholar]
- Tedford S.E., Persons A.L., Napier T.C. Dopaminergic lesions of the dorsolateral striatum in rats increase delay discounting in an impulsive choice task. PloS One. 2015;10 doi: 10.1371/journal.pone.0122063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A., Burant A., Bui N., Graham D., Yuva-Paylor L.A., Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berlin) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma W., Kyte S.L., Bagdas D., Alkhlaif Y., Alsharari S.D., Lichtman A.H., Chen Z.J., Del Fabbro E., Bigbee J.W., Gewirtz D.A., Damaj M.I. Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology. 2017;117:305–315. doi: 10.1016/j.neuropharm.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler K.R., Nass S.R., Crowe M.S., Gross J.D., Jones M.S., McKitrick A.W., Siderovski D.P., Kinsey S.G. Novel behavioral assays of spontaneous and precipitated THC withdrawal in mice. Drug Alcohol Depend. 2018;191:14–24. doi: 10.1016/j.drugalcdep.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan S.K., Fritschy J.M. Gephyrin: a master regulator of neuronal function? Nat. Rev. Neurosci. 2014;15:141–156. doi: 10.1038/nrn3670. [DOI] [PubMed] [Google Scholar]
- Vogt B.A., Paxinos G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct. 2014;219:185–192. doi: 10.1007/s00429-012-0493-3. [DOI] [PubMed] [Google Scholar]
- Wayman W.N., Chen L., Napier T.C., Hu X.T. Cocaine self-administration enhances excitatory responses of pyramidal neurons in the rat medial prefrontal cortex to human immunodeficiency virus-1 Tat. Eur. J. Neurosci. 2015;41:1195–1206. doi: 10.1111/ejn.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo T., Nesil T., Chang S.L., Li M.D. Interactive effects of ethanol and HIV-1 proteins on novelty-seeking behaviors and addiction-related gene expression. Alcohol Clin. Exp. Res. 2016;40:2102–2113. doi: 10.1111/acer.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin J.M. Animal models of obsessive-compulsive disorder. Curr. Protoc. Neurosci. 2008;45:9.30.1–9.30.9. doi: 10.1002/0471142301.ns0930s45. [DOI] [PubMed] [Google Scholar]
- Xu C., Fitting S. Inhibition of GABAergic neurotransmission by HIV-1 Tat and opioid treatment in the striatum involves μ-opioid receptors. Front. Neurosci. 2016;10:497. doi: 10.3389/fnins.2016.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Hermes D.J., Mackie K., Lichtman A.H., Ignatowska-Jankowska B.M., Fitting S. Cannabinoids occlude the HIV-1 Tat-induced decrease in GABAergic neurotransmission in prefrontal cortex slices. J. Neuroimmune Pharmacol. 2016;11:316–331. doi: 10.1007/s11481-016-9664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Hermes D.J., Nwanguma B., Jacobs I.R., Mackie K., Mukhopadhyay S., Lichtman A.H., Ignatowska-Jankowska B., Fitting S. Endocannabinoids exert CB1 receptor-mediated neuroprotective effects in models of neuronal damage induced by HIV-1 Tat protein. Mol. Cell. Neurosci. 2017;83:92–102. doi: 10.1016/j.mcn.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Nesil T., Wingo T., Chang S.L., Li M.D. HIV-1 proteins influence novelty-seeking behavior and alter region-specific transcriptional responses to chronic nicotine treatment in HIV-1Tg rats. Nicotine Tob. Res. 2017;19:1024–1032. doi: 10.1093/ntr/ntx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr N., Saranathan M., Pohl K., Sullivan E.V. vol. 14. 2019. Thalamic substructures in HIV: volume deficits and correlates; pp. 326–365. (The 25th Scientific Conference of the Society on Neuroimmune Pharmacology: Program and Abstracts. J Neuroimmune Pharmacol). [DOI] [PubMed] [Google Scholar]
- Zou S., Fitting S., Hahn Y.K., Welch S.P., El-Hage N., Hauser K.F., Knapp P.E. Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at μ-opioid receptor-expressing glia. Brain. 2011;134:3613–3628. doi: 10.1093/brain/awr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.