Abstract
Background:
Environmental factors may contribute to the development of Kawasaki disease in children, but prenatal environmental exposures are understudied.
Objective:
We used a population-based cohort to investigate whether prenatal exposure to outdoor air pollution is associated with the incidence of Kawasaki disease in childhood.
Methods:
We performed a longitudinal cohort study of all children born in Quebec, Canada, between 2006 and 2012. Children were followed for Kawasaki disease from birth until 31 March 2018. We assigned prenatal air pollutant exposure according to the residential postal code at birth. The main exposure was annual average concentration of ambient fine particulate matter [PM in aerodynamic diameter () and nitrogen dioxide () from satellite-based estimates and land-use regression models. As secondary exposures, we considered industrial , , and sulfur dioxide () emissions estimated from dispersion models. We estimated hazard ratios (HRs) using Cox proportional hazards models, adjusted for maternal age, parity, sex, multiple birth, maternal smoking during pregnancy, socioeconomic status, birth year, and rural residence. We considered single and multipollutant models. We performed several sensitivity analyses, including assessing modifying effects of maternal comorbidities (e.g., diabetes, preeclampsia).
Results:
The cohort comprised 505,336 children, including 539 with Kawasaki disease. HRs for each interquartile range increase in ambient air pollution were 1.16 (95% CI: 0.96, 1.39) for and 1.12 (95% CI: 0.96, 1.31) for . For industrial air pollution, HRs were 1.07 (95% CI: 1.01, 1.13) for , 1.09 (95% CI: 0.99, 1.20) for , and 1.01 (95% CI: 0.97, 1.05) for . In multipollutant models, associations for ambient and (i.e., from all sources) were robust to adjustment for industrial pollution, and vice versa.
Discussion:
In this population-based cohort study, both prenatal exposure to ambient and industrial air pollution were associated with the incidence of Kawasaki disease in childhood. Further studies are needed to consolidate the observed associations. https://doi.org/10.1289/EHP6920
Introduction
Kawasaki disease is the leading cause of acquired heart disease in children in North America (Kawasaki Disease Canada 2020; McCrindle et al. 2017). Kawasaki is an autoimmune vasculitis, mostly affecting children 6 months to 5 years of age (Kawasaki Disease Canada 2020; McCrindle et al. 2017). In Canada, annual incidence of Kawasaki disease is estimated at 19.6, 6.4, and 1.3 cases per 100,000 children 0–4, 5–9, and 10–14 years of age, respectively, with boys more frequently affected (Manlhiot et al. 2018b). Although mostly targeting coronary arteries, inflammation due to Kawasaki disease can occur in multiple organs and tissues (Kato et al. 1996; McCrindle et al. 2017). If untreated, Kawasaki disease may cause coronary artery aneurysm in up to 25% of children (Yim et al. 2013; McCrindle et al. 2017). Other sequelae include increased risk of myocardial ischemia or infarction, premature atherosclerosis, and sudden death (McCrindle et al. 2017; Yim et al. 2013; McCrindle et al. 2017).
Despite more than five decades since Kawasaki disease was first reported (Burns 2002), the causes of this disease remain unclear (Burns and Glodé 2004; McCrindle et al. 2017; Newburger et al. 2016). Based on clinical and epidemiologic features, the current theory is that Kawasaki disease is an exaggerated inflammatory response to infectious or environmental agents among genetically susceptible individuals (Rowley 2011). Mechanisms include immune cell activation, endothelial cell damage, and systemic inflammation, along with increased markers of oxidative stress (Burns and Glodé 2004; McCrindle et al. 2017; Rowley 2011).
Seasonal and geographical variation in Kawasaki disease incidence provides support for the hypothesis that environmental factors may play an etiologic role (Manlhiot et al. 2018a; Rypdal et al. 2018). It is proposed that environmental factors such as air pollution may influence the susceptibility of children to Kawasaki disease who encounter a trigger (Manlhiot et al. 2018a; Rodó et al. 2014; Jung et al. 2017). Prenatal and early life exposure to air pollution are associated with health outcomes that have an immunologic component (Gawda et al. 2017). Air pollution is linked with oxidative stress and inflammation (Kelly 2003), processes involved in autoimmune disease (Zhao et al. 2019; Gawda et al. 2017), which could affect fetal development and inflammatory responses. As well, prenatal exposure to air pollution is associated with immune dysregulation during fetal growth, which could enhance autoimmune responses during childhood (Naoe 1991). Epigenetic changes induced by air pollution have also been postulated to affect immune programming and organ development, processes that could contribute to vasculitis (Korten et al. 2017; Veras et al. 2017; Renauer et al. 2016).
Studies of the association between Kawasaki disease and air pollution are scant (Lin et al. 2017; Rypdal et al. 2018; Yorifuji et al. 2018; Zeft et al. 2016). Three studies considered only short-term air pollution exposure during childhood and yielded mixed results (Lin et al. 2017; Rypdal et al. 2018; Zeft et al. 2016). In the only study of long-term air pollution exposure, a significantly increased risk of Kawasaki disease hospitalization was found in children with prenatal exposure to levels of suspended particulate matter of aerodynamic diameter relative to PM (Yorifuji et al. 2018). However, results were inconclusive when the exposure was treated as continuous, and the study was limited by air pollution estimates derived from fixed-site monitors and the use of survey data.
There is a need to elucidate risk factors, particularly modifiable ones, to improve prevention, treatment, and ultimately reduce the burden of Kawasaki disease and its long-term sequelae. Epidemiological studies may provide critical insights on causal agents or modulatory mechanisms that may increase the risk of developing Kawasaki disease. Owing to the limited data available, we sought to investigate the contribution of prenatal air pollution exposure to the development of Kawasaki disease. We conducted a longitudinal population-based birth cohort study in the province of Quebec, Canada, using spatially resolved air pollution exposure estimates. In addition to ambient air pollution, we addressed exposure to industrial air pollutant emissions. Industries are an important source of air pollution and their emissions may differ from ambient air pollution in terms of composition and properties responsible for inflammatory and immune responses (Wu et al. 2018; Lodovici and Bigagli 2011; Delfino et al. 2011).
Materials and Methods
Description of the Cohort
We used a retrospective birth cohort derived from health administrative databases (Auger et al. 2019). The study population comprised children who were born in Quebec between 2006 and 2012. We used hospital data compiled in the Maintenance and Use of Data for the Study of Hospital Clientele registry. The data contain discharge abstracts for all hospital admissions in Quebec, including pregnant women paired with their newborns, and are coded and validated by trained personnel using strict criteria. Given that 99% of infants are born in hospital in Quebec, the cohort captures most of the population.
Using health insurance numbers, newborns were followed from birth until admission for Kawasaki disease, death, or the end of the study if they did not develop the disease. Follow-up ended 31 March 2018, leaving at least 6 y of follow-up for all noncensored participants. Infants with invalid health insurance numbers were excluded because they could not be followed. As well, participants for whom residential location was unknown were excluded because air pollution exposure could not be assigned.
Case Ascertainment
Incident cases of Kawasaki disease were identified from population-based health administrative data using the diagnostic code M30.3 of the International Classification of Diseases, Tenth revision (ICD-10; WHO 2016) as a main or secondary diagnosis. Quebec follows guidelines of the American Heart Association for diagnosis of Kawasaki disease (McCrindle et al. 2017). The diagnosis is clinical given that there is no gold standard test for Kawasaki disease. Diagnostic criteria are prolonged fever () and at least four of five clinical signs: erythema of oral tissues, bilateral bulbar conjunctival injection without exudate, rash (maculopapular, diffuse erythroderma, or erythema multiforme-like), erythema/edema/desquamation of hands and feet, and cervical lymphadenopathy. The clinical diagnosis may be supplemented with echocardiography, chest X-ray, laboratory blood exams, and other imaging.
Prenatal Exposure to Ambient Air Pollution
In addition to ambient air pollution from all sources, we addressed exposure to industrial air pollution. Pollutant mixtures emitted by industries may differ in composition and properties responsible for inflammatory and immune responses (Wu et al. 2018; Lodovici and Bigagli 2011; Delfino et al. 2011). As an indicator of average prenatal exposure, we used annual average concentrations of air pollutants assigned to each child using the calendar year of birth and the mother’s six-digit residential postal code at time of delivery.
Ambient fine particulate matter and nitrogen dioxide.
Ambient air pollution data included annual average concentration of PM in aerodynamic diameter () and nitrogen dioxide () from national models. These data were available from the Canadian Urban Environmental Health Research Consortium (Brook et al. 2018).
For , spatially resolved annual average concentrations were derived from satellite observations of aerosol optical depth based on the Moderate Resolution Imaging Spectroradiometer from the National Aeronautics and Space Administration Terra satellite (van Donkelaar et al. 2015; Boys et al. 2014; DMTI Spatial 2015). Estimates of were calibrated using an optimal estimation algorithm in conjunction with a geographically weighted regression of urban land cover, elevation, and aerosol composition. Annual average concentrations of were available for each year of our study at a spatial resolution of by . satellite-based estimates correlate closely with ground measurements at fixed-site monitors in North America () (van Donkelaar et al. 2015).
For , annual average concentrations were estimated from a national land-use regression model (Hystad et al. 2011, 2015; Weichenthal et al. 2017; DMTI Spatial 2015). The model was developed from measurements at Environment Canada’s National Air Pollution Surveillance (NAPS) system, and included satellite estimates of for 2005–2011, road length, industrial land use, and summer rainfall. In addition, the model incorporated a distance–decay gradient based on proximity to highways and major roads to account for fine-scale geographic variability of from vehicle emissions. This model explained 73% of the variation in 2006 annual NAPS measurements (Hystad et al. 2011).
Industrial , , and sulfur dioxide.
Estimates of annual average concentration of industrial , , and sulfur dioxide () for each residential postal code and study year were obtained from previously developed atmospheric dispersion models that simulated emissions from industrial sources. A detailed description of the dispersion model is provided elsewhere (Buteau et al. 2020). Briefly, estimates were derived from a modeling system that combines a meteorological module with a dispersion module (Scire et al. 2000). The meteorological module interpolates winds and temperatures using higher-resolution terrain elevation and land-use data and creates detailed hourly meteorological fields as well as boundary layer parameters, such as mixing height. The dispersion module estimates the growth diffusion and transport of released puffs in the modeling domain, using spatiotemporally resolved meteorology and emissions data from industrial point sources in Quebec. Information about emissions were extracted from the National Pollutant Release Inventory, a legislated, publicly accessible database of pollutant releases, disposals, and transfers from Canadian facilities that meet reporting requirements (Government of Canada 2020).
Statistical Methods
We assessed the association between Kawasaki disease and prenatal exposure to air pollution using Cox proportional hazards models with age in days as the time scale. We tested single and multipollutant models, including models that simultaneously included terms for ambient air pollutants and industrial emissions to assess whether the effects of ambient air pollutants were independent of exposure to industrial pollution.
All models were adjusted for maternal age (continuous), parity, sex, multiple birth, birth year, maternal smoking during pregnancy, and neighborhood socioeconomic status (using quintiles of material deprivation) (Pampalon et al. 2009). We further controlled for urban or rural residence to account for potential differences in air pollution (mixture and levels) and health services. We verified the proportional hazards assumption for all models. We assessed linearity of the relationship between Kawasaki disease and exposure to air pollutants and continuous covariates by means of flexible modeling. We determined linearity by comparing the Akaike information criterion (AIC; Akaike 1974) from linear and nonlinear models (a lower AIC indicates better fit). To further assess linearity, we visually inspected the shape of the response function using natural cubic splines with two or three knots. Because we found no evidence of nonlinearity (Table S1), we report hazard ratios (HRs) for interquartile range (IQR) increments of air pollutant exposure ().
In sensitivity analysis, we assessed potential confounding for a number of additional covariates, including season of conception, preterm birth, maternal obesity (ICD-10 code E66), preexisting or gestational diabetes (ICD-10 code O24), and preeclampsia (ICD-10 codes O11, O13–O15). We ran the main model without adjusting for birth year, in the event of overadjustment due to a downward trend in ambient air pollution over time. To determine whether specific health problems or pregnancy complications linked with systemic inflammation, or possibly involved in autoimmune disorders, may modify the association between Kawasaki disease and prenatal air pollution exposure, we conducted subgroup analysis (i.e., separate models for each subgroup) and Cochran Q tests of heterogeneity (Kaufman and MacLehose 2013) by maternal smoking during pregnancy, diabetes (preexisting and gestational), preeclampsia, and preterm birth. We also assessed modifying effects of infant sex and season of conception dichotomized as warm (i.e., spring and summer) and cold (i.e., fall and winter), given that the incidence of Kawasaki disease is greater in boys (Manlhiot et al. 2018b; McCrindle et al. 2017) and that previous studies have reported seasonal patterns in incidence (Manlhiot et al. 2018b; Burns et al. 2013). The University of Montreal Hospital Centre’s Institutional Review Board waived the need for ethical review because the data were de-identified.
Results
Description of the Cohort
The cohort included 505,336 children who contributed 4,363,439 person-years of follow-up (Table 1). 51% of children were boys, and 18% were rural residents. Five hundred thirty-nine children were hospitalized for Kawasaki disease during follow-up. The overall average incidence rate over the study period was 12.4 new cases per 100,000 person-years. On average, children were 3 years of age (range: 0.1–10.4 y) at diagnosis, with 95% () years of age. All cases had different mothers, suggesting no familial aggregation. There was no clear indication of a disproportionate difference in the incidence rate between regions, including those that were primarily rural or urban (Table S2).
Table 1.
Incidence of Kawasaki disease according to characteristics of the birth cohort.
| Characteristics | Infants () | Kawasaki disease () | Person-years | Incidence rate per 100,000 person-years (95% CI) |
|---|---|---|---|---|
| Maternal age at delivery (y) | ||||
| 83,862 | 86 | 729,682 | 11.8 (9.5, 14.6) | |
| 25–34 | 340,833 | 357 | 2,946,683 | 12.1 (10.9, 13.4) |
| 80,641 | 96 | 687,074 | 14.0 (11.4, 17.1) | |
| Parity | ||||
| 0 | 248,472 | 282 | 2,151,611 | 13.1 (11.7, 14.7) |
| 1 | 177,290 | 181 | 1,530,972 | 11.8 (10.2, 13.7) |
| 79,574 | 76 | 680,856 | 11.2 (8.9, 14.0) | |
| Preterm birth | ||||
| Yes | 31,829 | 29 | 277,129 | 10.5 (7.3, 15.1) |
| No | 473,507 | 510 | 4,086,310 | 12.5 (11.4, 13.6) |
| Multiple birth | ||||
| Yes | 7,365 | 11 | 63,257 | 17.4 (9.6, 31.4) |
| No | 497,971 | 528 | 4,300,182 | 12.3 (11.3, 13.4) |
| Sex of infant | ||||
| Boy | 259,408 | 324 | 2,239,486 | 14.5 (13.0, 16.1) |
| Girl | 245,928 | 215 | 2,123,953 | 10.1 (8.9, 11.6) |
| Rural residence | ||||
| Yes | 92,100 | 76 | 799,016 | 9.5 (7.6, 11.9) |
| No | 413,236 | 463 | 3,564,423 | 13.0 (11.9, 14.2) |
| Socioeconomic deprivation | ||||
| Low | 92,048 | 103 | 801,510 | 12.9 (10.6, 15.6) |
| Low-moderate | 101,872 | 89 | 882,950 | 10.1 (8.2, 12.4) |
| Moderate | 99,545 | 106 | 861,361 | 12.3 (10.2, 14.9) |
| Moderate-high | 99,136 | 110 | 856,029 | 12.9 (10.7, 15.5) |
| High | 97,635 | 113 | 843,634 | 13.4 (11.1, 16.1) |
| Maternal smoking during pregnancy | ||||
| Yes | 5,587 | 9 | 45,350 | 19.8 (10.3, 38.1) |
| No | 499,749 | 530 | 4,318,089 | 12.3 (11.3, 13.4) |
| Maternal obesity | ||||
| Yes | 7,513 | 4 | 57,429 | 7.0 (2.6, 18.6) |
| No | 497,823 | 535 | 4,306,011 | 12.4 (11.4, 13.5) |
| Maternal diabetesa | ||||
| Yes | 34,053 | 46 | 286,446 | 16.1 (12.0, 21.4) |
| No | 471,283 | 493 | 4,076,993 | 12.1 (11.1, 13.2) |
| Preeclampsia | ||||
| Yes | 24,036 | 31 | 205,701 | 15.1 (10.6, 21.4) |
| No | 481,300 | 508 | 4,157,738 | 12.2 (11.2, 13.3) |
| Season of conception | ||||
| Spring | 112,886 | 119 | 969,303 | 12.3 (10.3, 14.7) |
| Summer | 136,623 | 139 | 1,196,288 | 11.6 (9.8, 13.7) |
| Fall | 143,376 | 146 | 1,220,028 | 12.0 (10.2, 14.1) |
| Winter | 112,451 | 135 | 977,819 | 13.8 (11.7, 16.3) |
| Total | 505,336 | 539 | 4,363,439 | 12.4 (11.4, 13.4) |
Maternal diabetes included preexisting (type 1 and type 2) and gestational diabetes. CI, confidence interval.
Table 2 shows the distribution of annual average concentrations of industrial emission-related air pollutants, as well as regional ambient air pollutants at residences of children at time of birth. For ambient and , mean annual exposures were () and (), respectively. For industrial air pollution, mean annual exposures to , , and were (), (), and (), respectively. Ambient and estimated from national models were also highly correlated [] (Table S3). Industrial was highly correlated with industrial () and with industrial ().
Table 2.
Prenatal exposure to ambient air pollutants and industrial air pollutant emissions.
| Air pollutant | Mean | SD | Percentiles of the distribution | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min. | 25th | 50th | 75th | 95th | 99th | Max. | |||
| Mean annual ambient concentration, estimated from satellite-based and land-use regression models | |||||||||
| Ambient () | 6.79 | 2.11 | 0.95 | 5.03 | 6.80 | 8.80 | 9.70 | 10.33 | 14.20 |
| Ambient () | 11.16 | 7.42 | 0.54 | 4.92 | 8.97 | 16.86 | 24.65 | 30.25 | 51.01 |
| Mean annual ambient concentration from industrial emissions, estimated from dispersion modeling | |||||||||
| Industrial () | 0.19 | 0.23 | 0 | 0.10 | 0.17 | 0.24 | 0.39 | 0.73 | 17.83 |
| Industrial () | 1.14 | 0.91 | 0 | 0.50 | 0.96 | 1.58 | 2.72 | 3.95 | 33.47 |
| Industrial () | 2.28 | 1.86 | 0 | 1.22 | 2.03 | 2.94 | 5.07 | 8.23 | 89.10 |
Note: Prenatal exposures correspond to annual average concentrations of air pollutants assigned using calendar year of birth and the six-digit residential postal code at time of delivery. Max., maximum; Min., minimum; , nitrogen dioxide; , fine particulate matter; SD, standard deviation; , sulfur dioxide.
Association between Prenatal Air Pollution Exposure and Kawasaki Disease
Table 3 shows the results of single and multipollutant models for the association between incident childhood Kawasaki disease and residential prenatal air pollution exposure. Both prenatal exposure to ambient and were positively associated with Kawasaki disease. HRs from single-pollutant models were 1.16 [95% confidence interval (CI): 0.96, 1.39] and 1.12 (95% CI: 0.96, 1.31) for every IQR increase in average prenatal () and (), respectively.
Table 3.
Adjusted association [adjusted HR (95% CI) per IQR] between prenatal exposure to air pollution and incidence of childhood Kawasaki disease in Quebec, Canada.
| Models | Ambient exposure | Industrial exposure | |||
|---|---|---|---|---|---|
| Single-pollutant | 1.16 (0.96, 1.39) | 1.12 (0.96, 1.31) | 1.01 (0.97, 1.05) | 1.09 (0.99, 1.20) | 1.07 (1.01, 1.13) |
| Two-pollutant | |||||
| Ambient and ambient | 1.06 (0.85, 1.32) | 1.10 (0.85, 1.43) | — | — | — |
| Ambient and industrial | 1.15 (0.96, 1.39) | — | 1.01 (0.97, 1.05) | — | — |
| Ambient and industrial | — | 1.07 (0.89, 1.28) | — | 1.07 (0.95, 1.20) | — |
| Ambient and industrial | 1.11 (0.92, 1.34) | — | — | — | 1.06 (1.01, 1.13) |
| Ambient and industrial | — | 1.08 (0.93, 1.27) | — | — | 1.06 (1.00, 1.13) |
| Three-pollutant | |||||
| Industrial , , and | — | — | 0.91 (0.83, 1.00) | 0.97 (0.84, 1.12) | 1.23 (1.06, 1.42) |
| Ambient , ambient , and industrial | 1.09 (0.84, 1.42) | 1.03 (0.83, 1.28) | — | — | 1.06 (1.00, 1.13) |
Note: Cox models are adjusted for maternal age (linear), parity, sex, multiple birth, maternal smoking during pregnancy, material deprivation, birth year (linear), and rural/urban residence. IQR increments are for ambient , for ambient , for industrial , for industrial , and for industrial . —, not applicable; CI, confidence interval; IQR, interquartile range; , nitrogen dioxide; , fine particulate matter; , sulfur dioxide.
In single air pollutant models for industrial pollution, we found positive associations for [ (95% CI: 0.99, 1.20)] and [ (95% CI: 1.01, 1.13)], but not [ (95% CI: 0.97, 1.05)] (Table 3). When all three industrial air pollutants were included simultaneously in the regression model, the association for prenatal exposure strengthened [ (95% CI: 1.06, 1.42)], whereas associations for and were attenuated.
In the two-pollutant model that simultaneously included terms for ambient and industrial , estimated effects did not change compared with the single-pollutant model (Table 3), indicating that the effects of ambient and industrial were independent. In the two-pollutant model for ambient and industrial , mean estimates of association were slightly attenuated compared with the single-pollutant model but confidence intervals overlapped substantially. Associations for industrial remained statistically significant in multipollutant models with ambient and/or .
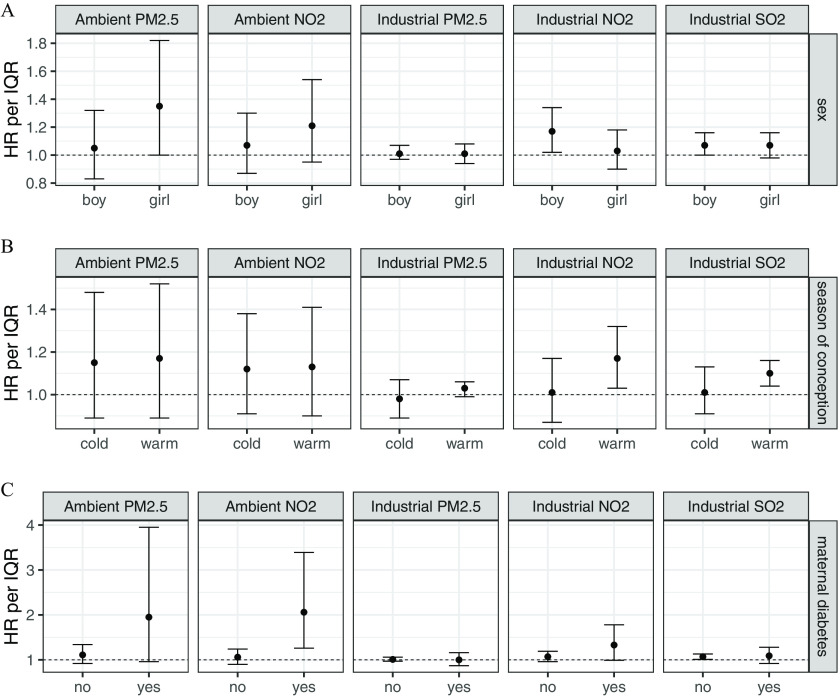
Findings from subgroup analysis (i.e., separate models for each subgroup) for child sex, season of conception, and diabetes are presented in Figure 1 (numeric values are provided in Table S4). Estimates suggest heterogeneity in the effect of ambient (Cochran’s Q ) and possibly ambient (Cochran’s Q ) and industrial (Cochran’s Q ) according to maternal diabetes, although the number of Kawasaki disease cases in the maternal diabetes subgroup was small (). Specifically, adjusted HRs from single-pollutant models for ambient were 2.06 (95% CI: 1.26, 3.39) for maternal diabetes and 1.06 (95% CI: 0.90, 1.24) for no diabetes, per IQR increase (). There was no evidence of effect modification with season of conception and child sex (Figure 1). As well, there was no evidence of heterogeneity by maternal smoking during pregnancy, preterm birth, and preeclampsia (Table S4); however, there were few Kawasaki disease cases for preterm birth (), preeclampsia (), and maternal smoking () subgroups. Adjustment for additional covariates had no influence on estimated effects or model fit (Table S5).
Figure 1.
Adjusted hazard ratio (HR) between prenatal exposure to ambient and industrial air pollution and incidence of childhood Kawasaki disease, according to (A) child sex, (B) season of conception, and (C) maternal diabetes. Dots represent the mean HR for an interquartile (IQR) increment in air pollutant exposure, and bars represent 95% confidence intervals (CIs), estimated separately for each subgroup using single-pollutant Cox models adjusted for maternal age, parity, sex, multiple birth, maternal smoking during pregnancy, material deprivation, birth year, and rural/urban residence. The horizontal axis indicates binary categories for sex (girl/boy), season of conception (cold/warm), and maternal diabetes (no/yes). IQRs are for industrial fine particulate matter (), for industrial nitrogen dioxide (), for industrial sulfur dioxide (), for ambient , and for ambient . Numeric values for HRs and 95% CIs, as well as number of cases in each subgroup and -value of Cochran Q tests, are provided in Table S4.
Discussion
In this population-based longitudinal cohort study, we found a positive association between Kawasaki disease in childhood and prenatal exposure to ambient and industrial air pollution. Both ambient () and ambient () were associated with the risk of Kawasaki disease, although confidence intervals were wide and included the null. The capacity to detect statistically significant associations was limited by the small number of incident cases in the study population ( children with Kawasaki disease, or 0.1% of the cohort). As well, the spatial resolution of ambient and in our data, which was less refined than estimates from dispersion models for industry emissions, may have attenuated differences between exposures and further reduced power. Industrial and were also both associated with a greater risk of Kawasaki disease. In the multipollutant analysis, the association with strengthened when we adjusted for other industrial air pollutants. This suggests that is the strongest predictor and, plausibly, the better surrogate of the effect of industrial emissions on risk of Kawasaki disease (Tolbert et al. 2007). is mainly emitted by industries and is a commonly used proxy of air emissions from industrial sources. However, the inclusion of multiple industrial pollutant exposures that are substantially correlated (Spearman’s coefficients between industrial pollutants ranging from 0.39 to 0.81) and not independent risk factors may lead to biased risk estimates (Tolbert et al. 2007). Thus, it seems plausible to assume that the true effects of industries may be in between the risk estimates for from the single-pollutant [ (95% CI: 1.01, 1.13)] and three-pollutant model [; (95% CI: 1.06, 1.42)]. Furthermore, associations for industrial were robust to adjustment for ambient and (i.e., from all sources) and vice versa. This finding suggests that the effects are independent. Thus, both ambient (i.e., all sources) and industrial air pollution may contribute to Kawasaki disease. Because ambient (i.e., total ) derives mainly from traffic-related and industrial emissions (CCME 2017), the estimated association for ambient in the two-pollutant model that adjusted for industrial pollution may reflect the contribution of traffic-related pollution.
The etiology of Kawasaki disease remains unknown (McCrindle et al. 2017; Newburger et al. 2016). Air pollution may play a role in autoimmune diseases such as Kawasaki disease by inducing systemic inflammation, oxidative stress, and epigenetic changes (Zhao et al. 2019). Pregnancy is a state of enhanced susceptibility to oxidative stress and inflammation, with evidence suggesting that air pollution may lead to placental inflammation (Liu et al. 2003). Moreover, our analyses suggested that maternal diabetes (preexisting and gestational) may modify the association between Kawasaki disease and prenatal air pollution exposure; however, we had limited power due to the small number () of diabetic women in the Kawasaki disease subgroup, most of which () were gestational. Inflammatory mechanisms are common features of Kawasaki disease and air pollution and are also involved in preexisting and gestational diabetes, possibly explaining the enhanced susceptibility in children with a maternal history of diabetes (Zhao et al. 2019; O’Neill et al. 2007; Radaelli et al. 2003). Furthermore, gestational diabetes has been associated with a greater risk of type 1 diabetes (Blotsky et al. 2019), which is an autoimmune disorder, as is Kawasaki disease (Sakurai 2019). Further research will be needed to determine whether different variants of diabetes can affect the association between air pollution and Kawasaki disease.
The present study contributes to the scarce epidemiological literature on air pollution and Kawasaki disease in childhood. Only four studies have considered an association between Kawasaki disease and air pollution exposure (Jung et al. 2017; Lin et al. 2017; Yorifuji et al. 2018; Zeft et al. 2016). Three studies, including one time-series (Lin et al. 2017) and two case-crossover analyses (Jung et al. 2017; Yorifuji et al. 2018), investigated short-term postnatal exposure to air pollution, measures that are not directly comparable with our study findings. The remaining study investigated long-term prenatal and early childhood exposure to pollutants in Japan (Yorifuji et al. 2018). Children exposed to a mean pregnancy concentration of of had a significantly increased risk of Kawasaki disease compared with [odds ratio (95% CI: 1.06, 2.38)]. However, findings were inconclusive when exposure was measured continuously using a linear term; the OR per increment was 1.13, with a 95% CI that included the null (95% CI: 0.79, 1.61). The study also used a questionnaire survey to ascertain Kawasaki disease cases, and exposure was assessed at the municipality level from fixed-site monitors (Yorifuji et al. 2018). In contrast, we ascertained cases from health administrative data with no possibility of recall bias, and exposure estimates were derived from refined spatiotemporal models and assigned using the full six-digit postal code of residential addresses (the most precise information available).
One of the unique features of this study was the potential contribution of industrial emissions. Pollutant mixtures emitted by different sources can differ in composition (metal vs. organic components), which may alter toxicity, oxidizing property, or capacity to induce inflammation and immune response to autoantigens (Wu et al. 2018; Lodovici and Bigagli 2011; Delfino et al. 2011). Another strength of the present study relates to universal access to health care in Quebec, which minimizes potential for selection bias. Diagnostic codes for Kawasaki disease have been used in previous research (Belkaibech et al. 2020). Health administrative data have been shown to provide valid estimates of Kawasaki disease given that hospitalization is obligatory in Canada (Manlhiot et al. 2018b). A study in Ontario suggested that health administrative data may overestimate the incidence of Kawasaki disease by 7% in regions that include unconfirmed cases of Kawasaki disease (Manlhiot et al. 2018b); however, this is not possible in Quebec, where only confirmed cases are coded and validated in the data (Santé et Services Sociaux Québec 2020). We also benefited from a rich record of individual-level information for both the mother and child. Consequently, we were able to investigate several covariates for potential confounding and modifying effects. Such analyses are of particular importance because the etiology of Kawasaki disease remains elusive. Overall, the estimated associations from our main model were robust to various sensitivity analyses.
This study nonetheless has limitations. Despite better recognition and greater awareness, misdiagnosis is possible given that there is no diagnostic test for Kawasaki disease and no clinical feature is pathognomonic. We may have missed atypical cases with less severe symptoms if families did not seek care. Although air pollutant concentrations from national models are the best available data for a large cohort in terms of geographical area covered, the spatial resolution is limited for local sources of air pollution and small-scale spatial variability in pollutant concentration. National models tend to average concentrations in the higher ranges, thus attenuating differences between exposure levels and reducing the statistical power of an epidemiological analysis. This limitation also contributes to exposure misclassification, further attenuating associations toward the null. may be subject to greater exposure misclassification than given its greater fine-scale spatial variability. is mainly emitted by motor vehicles and its concentration varies considerably with proximity to roadways and traffic (Karner et al. 2010; Crouse et al. 2009; Deville Cavellin et al. 2016). In contrast, there is less evidence of significant variation in mass concentration at the local spatial scale because a large proportion of is secondary in origin (Brauer et al. 2011; Smargiassi et al. 2005; Pinto et al. 2004). Nonetheless, components may vary substantially (Bell et al. 2007, 2011).
Regarding exposure to industrial emissions, we could not validate the dispersion model against a gold standard, but a similar modeling system simulating ambient from road-traffic emissions in Montreal was found to adequately account for the spatial distribution of air pollutants (Fallah-Shorshani et al. 2017; Shekarrizfard et al. 2015). Prenatal exposure was based on the mother’s residence at time of delivery, and we could not account for residential mobility during pregnancy or for the influence of early childhood exposure to air pollution. We could not assess trimester-specific associations between prenatal air pollution exposure and Kawasaki disease because we did not have exposure estimates at a finer temporal scale. Exploring critical windows of exposure should be considered in future studies. Despite the array of covariates in our analyses, we cannot rule out residual confounding from unmeasured risk factors. We did not have access to individual-level data about income, genetic susceptibility, or ethnicity. Underreporting of maternal smoking is possible. However, by controlling for neighborhood socioeconomic status and several maternal characteristics, we may have accounted for unmeasured confounders.
In summary, this population-based longitudinal cohort study supports a possible link between Kawasaki disease and prenatal exposure to ambient air pollution, including from industrial sources. Findings also suggest that maternal diabetes may enhance the effects of air pollution, particularly , on the risk of Kawasaki disease. Further studies that aim to replicate these results are needed to consolidate the observed associations between prenatal air pollution exposure and the incidence of Kawasaki disease. Additional recommendations include use of larger cohorts, health administrative data for disease ascertainment to rule out recall bias, and exposure estimates derived from refined spatiotemporal models that reduce misclassification and allow the investigation of multiple windows of exposure.
Supplementary Material
Acknowledgments
The data for ambient fine particulate matter and nitrogen dioxide from national models, indexed to DMTI Spatial Inc. postal codes, were provided by the Canadian Urban Environmental Health Research Consortium. Sources of financial support were the Heart & Stroke Foundation of Canada (G-18-0021776) and Fonds de Recherche du Québec-Santé (34695).
References
- Akaike H. 1974. A new look at the statistical model identification. IEEE Trans Automat Contr 19(6):716–723, 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- Auger N, Bilodeau-Bertrand M, Marcoux S, Kosatsky T. 2019. Residential exposure to electromagnetic fields during pregnancy and risk of child cancer: a longitudinal cohort study. Environ Res 176:108524, PMID: 31226625, 10.1016/j.envres.2019.108524. [DOI] [PubMed] [Google Scholar]
- Belkaibech S, Potter BJ, Kang H, Lee GE, Bilodeau-Bertrand M, Auger N, et al. 2020. Maternal autoimmune disorders and risk of Kawasaki disease in offspring. J Pediatr 222:240–243.e1, PMID: 32171556, 10.1016/j.jpeds.2020.02.016. [DOI] [PubMed] [Google Scholar]
- Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. 2007. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect 115(7):989–995, PMID: 17637911, 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD. 2011. Community-level spatial heterogeneity of chemical constituent levels of fine particulates and implications for epidemiological research. J Expo Sci Environ Epidemiol 21(4):372–384, PMID: 20664652, 10.1038/jes.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blotsky AL, Rahme E, Dahhou M, Nakhla M, Dasgupta K. 2019. Gestational diabetes associated with incident diabetes in childhood and youth: a retrospective cohort study. CMAJ 191(15):E410–E417, PMID: 30988041, 10.1503/cmaj.181001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boys BL, Martin RJ, Donkelaar AV, MacDonell RJ, Hsu NC, Cooper MJ, et al. 2014. Fifteen-year global time series of satellite-derived fine particulate matter. Environ Sci Technol 48(19):11109–11118, PMID: 25184953, 10.1021/es502113p. [DOI] [PubMed] [Google Scholar]
- Brauer M, Hystad P, Poplawski K. 2011. Assessing the spatial representativeness of the PM2.5 and O3: measurements from National Air Pollutant Surveillance System. Prepared for Environment Canada. https://circle.ubc.ca/handle/2429/41543 [accessed 11 September 2020].
- Brook JR, Setton EM, Seed E, Shooshtari M, Doiron D, CANUE (Canadian Urban Environmental Health Research Consortium). 2018. The Canadian Urban Environmental Health Research Consortium—a protocol for building a national environmental exposure data platform for integrated analyses of urban form and health. BMC Public Health 18(1):114, PMID: 29310629, 10.1186/s12889-017-5001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC. 2002. Commentary: translation of Dr. Tomisaku Kawasaki’s original report of fifty patients in 1967. Pediatr Infect Dis J 21(11):993–995, PMID: 12442017, 10.1097/00006454-200211000-00002. [DOI] [PubMed] [Google Scholar]
- Burns JC, Glodé MP. 2004. Kawasaki syndrome. Lancet 364(9433):533–544, PMID: 15302199, 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- Burns JC, Herzog L, Fabri O, Tremoulet AH, Rodó X, Uehara R, et al. 2013. Seasonality of Kawasaki disease: a global perspective. PLoS One 8(9):e74529, PMID: 24058585, 10.1371/journal.pone.0074529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteau S, Shekarrizfard M, Hatzopolou M, Gamache P, Liu L, Smargiassi A, et al. 2020. Air pollution from industries and asthma onset in childhood: a population-based birth cohort study using dispersion modeling. Environ Res 185:109180, PMID: 32278153, 10.1016/j.envres.2020.109180. [DOI] [PubMed] [Google Scholar]
- CCME (Canadian Council of Ministers of the Environment). 2017. Air quality: emissions and ambient trends. http://airquality-qualitedelair.ccme.ca [accessed 20 June 2020].
- Crouse DL, Goldberg MS, Ross NA. 2009. A prediction-based approach to modelling temporal and spatial variability of traffic-related air pollution in Montreal, Canada. Atmos Environ 43(32):5075–5084, 10.1016/j.atmosenv.2009.06.040. [DOI] [Google Scholar]
- Delfino RJ, Staimer N, Vaziri ND. 2011. Air pollution and circulating biomarkers of oxidative stress. Air Qual Atmos Health 4(1):37–52, PMID: 23626660, 10.1007/s11869-010-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deville Cavellin L, Weichenthal S, Tack R, Ragettli MS, Smargiassi A, Hatzopoulou M, et al. 2016. Investigating the use of portable air pollution sensors to capture the spatial variability of traffic-related air pollution. Environ Sci Technol 50(1):313–320, PMID: 26606504, 10.1021/acs.est.504235. [DOI] [PubMed] [Google Scholar]
- DMTI Spatial (Desktop Mapping Technologies, Inc.). 2015. CanMap Postal Code Suite. Version 2015.3. Markham, ON, Canada: DMTI Spatial Inc; https://mdl.library.utoronto.ca/collections/geospatial-data/canmap-postal-code-suite-0 [accessed 8 October 2020]. [Google Scholar]
- Fallah-Shorshani M, Shekarrizfard M, Hatzopoulou M. 2017. Evaluation of regional and local atmospheric dispersion models for the analysis of traffic-related air pollution in urban areas. Atmos Environ 167:270–282, 10.1016/j.atmosenv.2017.08.025. [DOI] [Google Scholar]
- Gawda A, Majka G, Nowak B, Marcinkiewicz J. 2017. Air pollution, oxidative stress, and exacerbation of autoimmune diseases. Cent Eur J Immunol 42(3):305–312, PMID: 29204097, 10.5114/ceji.2017.70975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Canada. 2020. National Pollutant Release Inventory. https://www.canada.ca/en/services/environment/pollution-waste-management/national-pollutant-release-inventory.html [accessed 17 January 2020].
- Hystad P, Setton E, Cervantes A, Poplawski K, Deschenes S, Brauer M, et al. 2011. Creating national air pollution models for population exposure assessment in Canada. Environ Health Perspect 119(8):1123–1129, PMID: 21454147, 10.1289/ehp.1002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hystad P, Villeneuve PJ, Goldberg MS, Crouse DL, Johnson K, Canadian Cancer Registries Epidemiology Research Group. 2015. Exposure to traffic-related air pollution and the risk of developing breast cancer among women in eight Canadian provinces: a case–control study. Environ Int 74:240–248, PMID: 25454241, 10.1016/j.envint.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Jung C-R, Chen W-T, Lin Y-T, Hwang B-F. 2017. Ambient air pollutant exposures and hospitalization for Kawasaki disease in Taiwan: a case-crossover study (2000–2010). Environ Health Perspect 125(4):670–676, PMID: 27458717, 10.1289/EHP137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner AA, Eisinger DS, Niemeier DA. 2010. Near-roadway air quality: synthesizing the findings from real-world data. Environ Sci Technol 44(14):5334–5344, PMID: 20560612, 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. 1996. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 94(6):1379–1385, PMID: 8822996, 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- Kaufman JS, MacLehose RF. 2013. Which of these things is not like the others? Cancer 119(24):4216–4222, PMID: 24022386, 10.1002/cncr.28359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Disease Canada. 2020. About Kawasaki Disease. https://kdcanada.org/en/about-kawasaki-disease/ [accessed 11 January 2020].
- Kelly FJ. 2003. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 60(8):612–616, PMID: 12883027, 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korten I, Ramsey K, Latzin P. 2017. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev 21:38–46, PMID: 27665510, 10.1016/j.prrv.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Lin Z, Meng X, Chen R, Huang G, Ma X, Chen J, et al. 2017. Ambient air pollution, temperature and Kawasaki disease in Shanghai, China. Chemosphere 186:817–822, PMID: 28822259, 10.1016/j.chemosphere.2017.08.054. [DOI] [PubMed] [Google Scholar]
- Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. 2003. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ Health Perspect 111(14):1773–1778, PMID: 14594630, 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodovici M, Bigagli E. 2011. Oxidative stress and air pollution exposure. J Toxicol 2011:1–487074, PMID: 21860622, 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manlhiot C, Mueller B, O’Shea S, Majeed H, Bernknopf B, Labelle M, et al. 2018a. Environmental epidemiology of Kawasaki disease: linking disease etiology, pathogenesis and global distribution. PLoS One 13(2):e0191087, PMID: 29415012, 10.1371/journal.pone.0191087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manlhiot C, O’Shea S, Bernknopf B, LaBelle M, Chahal N, Dillenburg RF, et al. 2018b. Epidemiology of Kawasaki disease in Canada 2004 to 2014: comparison of surveillance using administrative data vs periodic medical record review. Can J Cardiol 34(3):303–309, PMID: 29395706, 10.1016/j.cjca.2017.12.009. [DOI] [PubMed] [Google Scholar]
- McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. 2017. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 135(17):e927–e999, PMID: 28356445, 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- Naoe S, Takahashi K, Masuda H, Tanaka N. 1991. Kawasaki disease. With particular emphasis on arterial lesions. Acta Pathol Jpn 41(11):785–797, PMID: 1785339, 10.1111/j.1440-1827.1991.tb01620.x. [DOI] [PubMed] [Google Scholar]
- Newburger JW, Takahashi M, Burns JC. 2016. Kawasaki disease. J Am Coll Cardiol 67(14):1738–1749, PMID: 27056781, 10.1016/j.jacc.2015.12.073. [DOI] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, et al. 2007. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med 64(6):373–379, PMID: 17182639, 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampalon R, Hamel D, Gamache P, Raymond G. 2009. A deprivation index for health planning in Canada. Chronic Dis Can 29(4):178–191, PMID: 19804682, 10.24095/hpcdp.29.4.05. [DOI] [PubMed] [Google Scholar]
- Pinto JP, Lefohn AS, Shadwick DS. 2004. Spatial variability of PM2.5 in urban areas in the United States. J Air Waste Manag Assoc 54(4):440–449, PMID: 15115373, 10.1080/10473289.2004.10470919. [DOI] [PubMed] [Google Scholar]
- Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. 2003. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes 52(12):2951–2958, PMID: 14633856, 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- Renauer P, Coit P, Sawalha AH. 2016. Epigenetics and vasculitis: a comprehensive review. Clin Rev Allergy Immunol 50(3):357–366, PMID: 26093659, 10.1007/s12016-015-8495-6. [DOI] [PubMed] [Google Scholar]
- Rodó X, Curcoll R, Robinson M, Ballester J, Burns JC, Cayan DR, et al. 2014. Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan. Proc Natl Acad Sci USA 111(22):7952–7957, PMID: 24843117, 10.1073/pnas.1400380111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley AH. 2011. Kawasaki disease: novel insights into etiology and genetic susceptibility. Annu Rev Med 62:69–77, PMID: 20690826, 10.1146/annurev-med-042409-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypdal M, Rypdal V, Burney JA, Cayan D, Bainto E, Skochko S, et al. 2018. Clustering and climate associations of Kawasaki disease in San Diego County suggest environmental triggers. Sci Rep 8(1):16140, PMID: 30420674, 10.1038/s41598-018-33124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y. 2019. Autoimmune aspects of Kawasaki disease. J Investig Allergol Clin Immunol 29(4):251–261, PMID: 30183655, 10.18176/jiaci.0300. [DOI] [PubMed] [Google Scholar]
- Santé et Services Sociaux Québec. 2020. Cadre normatif du système MED-ÉCHO (Maintenance et Exploitation des Données pour l’Étude de la Clientèle Hospitalière. [In French.] https://publications.msss.gouv.qc.ca/msss/document-000170/ [accessed 18 September 2020].
- Scire JS, Strimaitis DG, Yamartino RJ. 2000. A User’s Guide for the CALPUFF Dispersion Model. Version 5. Concord, MA: Earth Tech, Inc; http://www.ihamodel.com/wp-content/uploads/2018/11/CALPUFF_UsersGuide.pdf [accessed 8 October 2020]. [Google Scholar]
- Shekarrizfard M, Valois M-F, Goldberg MS, Crouse D, Ross N, Parent M-E, et al. 2015. Investigating the role of transportation models in epidemiologic studies of traffic related air pollution and health effects. Environ Res 140:282–291, PMID: 25885116, 10.1016/j.envres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Baldwin M, Pilger C, Dugandzic R, Brauer M. 2005. Small-scale spatial variability of particle concentrations and traffic levels in Montreal: a pilot study. Sci Total Environ 338(3):243–251, PMID: 15713332, 10.1016/j.scitotenv.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Tolbert PE, Klein M, Peel JL, Sarnat SE, Sarnat JA. 2007. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J Expo Sci Environ Epidemiol 17(suppl 2):S29–S35, PMID: 18079762, 10.1038/sj.jes.7500625. [DOI] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Spurr RJD, Burnett RT. 2015. High-resolution satellite-derived PM2.5 from optimal estimation and geographically weighted regression over North America. Environ Sci Technol 49(17):10482–10491, PMID: 26261937, 10.1021/acs.est.5b02076. [DOI] [PubMed] [Google Scholar]
- Veras MM, de Oliveira Alves N, Fajersztajn L, Saldiva P. 2017. Before the first breath: prenatal exposures to air pollution and lung development. Cell Tissue Res 367(3):445–455, PMID: 27726025, 10.1007/s00441-016-2509-4. [DOI] [PubMed] [Google Scholar]
- Weichenthal S, Pinault LL, Burnett RT. 2017. Impact of oxidant gases on the relationship between outdoor fine particulate air pollution and nonaccidental, cardiovascular, and respiratory mortality. Sci Rep 7(1):16401, PMID: 29180643, 10.1038/s41598-017-16770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2016. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en [accessed 8 October 2020].
- Wu W, Jin Y, Carlsten C. 2018. Inflammatory health effects of indoor and outdoor particulate matter. J Allergy Clin Immunol 141(3):833–844, PMID: 29519450, 10.1016/j.jaci.2017.12.981. [DOI] [PubMed] [Google Scholar]
- Yim D, Curtis N, Cheung M, Burgner D. 2013. Update on Kawasaki disease: epidemiology, aetiology and pathogenesis. J Paediatr Child Health 49(9):704–708, PMID: 23560706, 10.1111/jpc.12172. [DOI] [PubMed] [Google Scholar]
- Yorifuji T, Tsukahara H, Kashima S, Doi H. 2018. Intrauterine and early postnatal exposure to particulate air pollution and Kawasaki disease: a nationwide longitudinal survey in Japan. J Pediatr 193:147–154.e2, PMID: 29212623, 10.1016/j.jpeds.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Zeft AS, Burns JC, Yeung RS, McCrindle BW, Newburger JW, Dominguez SR, et al. 2016. Kawasaki disease and exposure to fine particulate air pollution. J Pediatr 177:179–183.e1, PMID: 27496266, 10.1016/j.jpeds.2016.06.061. [DOI] [PubMed] [Google Scholar]
- Zhao C-N, Xu Z, Wu G-C, Mao Y-M, Liu L-N, Qian-Wu, et al. 2019. Emerging role of air pollution in autoimmune diseases. Autoimmun Rev 18(6):607–614, PMID: 30959217, 10.1016/j.autrev.2018.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.