Abstract
PURPOSE
To investigate quality-adjusted progression-free survival (QA-PFS) and quality-adjusted time without symptoms or toxicity (Q-TWiST) in a post hoc exploratory analysis of the phase III ARIEL3 study of rucaparib maintenance treatment versus placebo.
PATIENTS AND METHODS
Patients with platinum-sensitive, recurrent ovarian carcinoma were randomly assigned to rucaparib (600 mg twice per day) or placebo. QA-PFS was calculated as progression-free survival function × the 3-level version of the EQ-5D questionnaire (EQ-5D-3L) index score function. Q-TWiST analyses were performed defining TOX as the mean duration in which a patient experienced grade ≥ 3 treatment-emergent adverse events (TEAEs) or the mean duration in which a patient experienced grade ≥ 2 TEAEs of nausea, vomiting, fatigue, and asthenia. Q-TWiST was calculated as μTOX × TOX + TWiST, with μTOX calculated using EQ-5D-3L data.
RESULTS
The visit cutoff was Apr 15, 2017. Mean QA-PFS was significantly longer with rucaparib versus placebo in the intent-to-treat (ITT) population (375 randomly assigned to rucaparib v 189 randomly assigned to placebo; difference, 6.28 months [95% CI, 4.85 to 7.47 months]); BRCA-mutant cohort (130 rucaparib v 66 placebo; 9.37 months [95% CI, 6.65 to 11.85 months]); homologous recombination deficient (HRD) cohort (236 rucaparib v 118 placebo; 7.93 months [95% CI, 5.93 to 9.53 months]); and BRCA wild-type/loss of heterozygosity (LOH) low patient subgroup (107 rucaparib v 54 placebo; 2.71 months [95% CI, 0.31 to 4.44 months]). With TOX defined using grade ≥ 3 TEAEs, the difference in mean Q-TWiST (rucaparib v placebo) was 6.88 months (95% CI, 5.71 to 8.23 months), 9.73 months (95% CI, 7.10 to 11.94 months), 8.11 months (95% CI, 6.36 to 9.49 months), and 3.35 months (95% CI, 1.66 to 5.40 months) in the ITT population, BRCA-mutant cohort, HRD cohort, and BRCA wild-type/LOH low patient subgroup, respectively. Q-TWiST with TOX defined using select grade ≥ 2 TEAEs also consistently favored rucaparib.
CONCLUSION
The significant differences in QA-PFS and Q-TWiST confirm the benefit of rucaparib versus placebo in all predefined cohorts.
INTRODUCTION
Although most women with ovarian cancer respond to first-line treatment (typically surgery plus platinum-based chemotherapy), many experience relapse and may receive multiple lines of chemotherapy.1,2 Among those initially diagnosed with advanced ovarian cancer, only 29% survive for ≥ 5 years.3
CONTEXT
Key Objective
We evaluated the effect of rucaparib maintenance treatment on patient-centered outcomes, which incorporate measures of quality and quantity of life, in patients with recurrent ovarian cancer.
Knowledge Generated
Quality-adjusted progression-free survival and quality-adjusted time without symptoms or toxicity (Q-TWiST) were longer with rucaparib than with placebo in the intent-to-treat population and in all other analysis groups, irrespective of BRCA mutation status.
Relevance
To our knowledge, this is the first report of quality-adjusted patient-centered outcomes for rucaparib maintenance treatment and the first report of these outcomes for a poly(ADP-ribose) polymerase (PARP) inhibitor in an all-comer population that includes patients with ovarian cancer without a BRCA mutation. To our knowledge, our report is also the first to include Q-TWiST analyses for a PARP inhibitor in ovarian cancer. Across analysis groups, including patients with BRCA wild-type carcinomas, rucaparib maintenance treatment provided a significant benefit despite the impact of toxicities on patients’ health status, and rucaparib-treated patients had longer periods without clinically relevant symptoms compared with those receiving placebo.
Recently, maintenance treatment with a targeted agent such as bevacizumab or a poly(ADP-ribose) polymerase (PARP) inhibitor has become the standard of care for patients with ovarian cancer after a response to chemotherapy.4 Maintenance treatment aims to extend clinically meaningful survival by delaying disease progression and to prolong the period between chemotherapy treatments, thereby allowing patients to avoid the associated toxicities that can affect quality of life (QoL).5,6 Consequently, it is important to evaluate whether adding maintenance treatment to a patient’s therapeutic regimen prolongs survival at the expense of toxicities that compromise the patient’s overall health status.6
The PARP inhibitor rucaparib is approved in the United States and European Union for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who have a complete or partial response to platinum-based chemotherapy.7,8 Approval was based on the results from the ARIEL3 trial (CO-338-014; ClinicalTrials.gov identifier: NCT01968213), in which the primary efficacy end point of investigator-assessed progression-free survival (PFS) was significantly improved with rucaparib maintenance treatment versus placebo in all 3 prespecified, nested cohorts: patients with a BRCA1 or BRCA2 (BRCA)-mutated carcinoma (germline, somatic, or unknown origin); patients with a homologous recombination deficient (HRD) carcinoma (BRCA mutation plus BRCA wild type/high loss of heterozygosity [LOH]); and the intent-to-treat (ITT) population.9
Quality-adjusted PFS (QA-PFS) and quality-adjusted time without symptoms or toxicity (Q-TWiST) are methods that incorporate both the quality and quantity of life to provide additional insight into the impacts of a therapy. QA-PFS represents the duration of survival without disease progression adjusted for the value the patient placed on their health status (ie, it is a measure that adjusts for treatment toxicity and any associated detrimental effects as reported by the patient). TWiST is an outcome in which periods of treatment toxicity or disease symptoms are subtracted from the survival end point, and the Q-TWiST variation of this outcome incorporates patients’ assessments of their QoL in a health state (eg, time with toxicity of treatment) by weighting it with a patient-derived utility value.10 Assessments that draw on patient-centered quality adjustments are particularly relevant for targeted oncology therapies that are given continuously and for therapies, such as PARP inhibitors, administered to asymptomatic patients.11
Here we present analyses of QA-PFS and Q-TWiST from ARIEL3 to further evaluate the clinical benefits of rucaparib maintenance treatment from a patient-centered perspective. To our knowledge, our analyses are the first report of a Q-TWiST analysis for a PARP inhibitor in ovarian cancer and are the first report of quality-adjusted outcomes for a PARP inhibitor that includes patients with ovarian cancer without a known deleterious BRCA mutation.
PATIENTS AND METHODS
Study Design, Patients, and Procedures
The design of this randomized, double-blind, multicenter, international, phase III trial (ARIEL3; ClinicalTrials.gov identifier: NCT01968213) has been reported previously.9 Patients were enrolled between April 7, 2014, and July 19, 2016.
Eligible patients were ≥ 18 years of age, had platinum-sensitive, high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube carcinoma, had received ≥ 2 previous platinum-based chemotherapy regimens, and had achieved any of the following: a complete response according to RECIST version 1.1, a partial response according to RECIST, or a serologic response based on Gynecologic Cancer InterGroup (GCIG) cancer antigen 125 response criteria to their last platinum-based regimen. Full eligibility criteria have been reported previously.9
National or local institutional review boards approved the trial, which was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Council for Harmonisation. Patients provided written informed consent before participation.
Central testing of DNA derived from patient archival tumor tissue samples was performed to detect mutations in homologous recombination pathway genes and to assess genomic LOH. A cutoff of ≥ 16% for ARIEL3 was prespecified as a discriminator for high genomic LOH.9 Full details of the testing protocol have been reported previously.9
Patients were randomly assigned 2:1 to receive oral rucaparib (600 mg twice per day) or matched placebo with stratification factors of homologous recombination repair gene mutation status (based on gene mutation only: mutation in BRCA1 or BRCA2, mutation in a non-BRCA gene associated with homologous recombination, or no mutation in BRCA or a homologous recombination gene); progression-free interval after penultimate platinum-based regimen (6 to ≤ 12 months or > 12 months); and best response to most recent platinum-based regimen (complete or partial response). Rucaparib or placebo was administered in continuous 28-day cycles until disease progression (as assessed by RECIST), death, or other reason for discontinuation. Patients completed the 3-level version of the EQ-5D questionnaire (EQ-5D-3L) at screening, on day 1 of each treatment cycle, at the treatment discontinuation visit, and at the 28-day follow-up visit.
Outcomes
The primary efficacy end point in ARIEL3, investigator-assessed PFS, and secondary end points of PFS by blinded, independent central review, time to worsening in the FOSI-18, and safety have been reported previously.9 Here we report post hoc analyses of QA-PFS and Q-TWiST, both using utility values derived from the EQ-5D-3L questionnaire. For all analyses, the EQ-5D-3L index score was calculated using the UK value set obtained using time–trade-off methodology. Only questionnaires with all 5 EQ-5D-3L items completed were eligible for inclusion.
Statistical Analysis
The rationale for the sample size has been reported previously.9 Analyses were performed for the 3 prespecified, nested cohorts: the ITT population, patients with an HRD carcinoma (BRCA mutation or BRCA wild type/LOH high), and patients with a BRCA-mutated carcinoma. Analyses were also conducted in subgroups of patients with BRCA wild-type carcinomas based on LOH status: BRCA wild type/LOH high, BRCA wild type/LOH low, and BRCA wild type/LOH indeterminate.
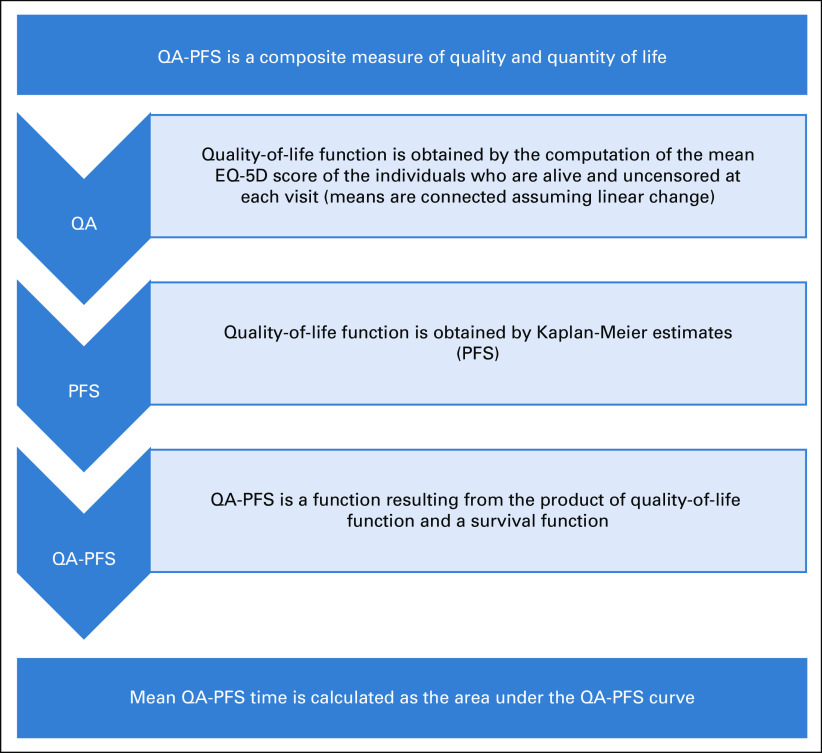
QA-PFS was calculated as the product of the investigator-assessed PFS function and the EQ-5D-3L index score function. Mean QA-PFS was obtained by computing the area under the quality-survival product function up to the last follow-up date available in each group. Because differences in censoring and/or follow-up time in the rucaparib and placebo groups could introduce bias, a sensitivity analysis was also performed in which the area under the quality-survival product function was computed using a follow-up time of 24 months for both groups. Additional details are provided in the Appendix and in Appendix Fig A1 (online only).
Mean time without toxicity or symptoms of disease progression (TWiST state) was calculated as the mean PFS time minus the mean time with toxicities (TOX state). Mean time with symptoms of disease (REL state), usually calculated as the mean overall survival (OS) time minus the mean PFS time, was not included because ARIEL3 OS data were not mature at the time of this analysis.
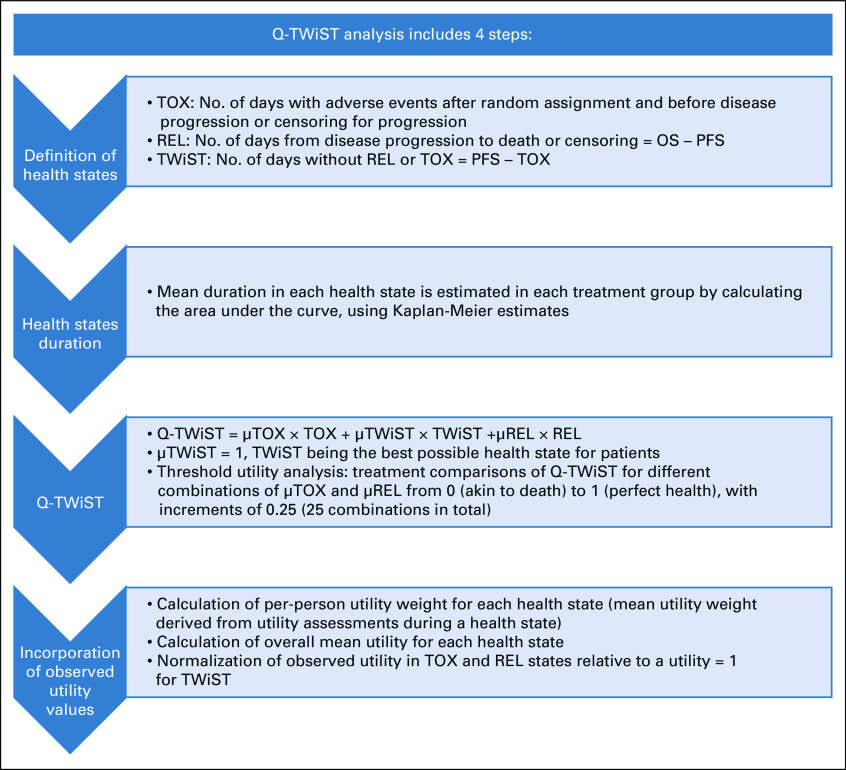
Q-TWiST was calculated as μTOX × TOX + TWiST. µTOX denotes the utility weight for the TOX state, and the utility weight for the TWiST state was set to 1 (highest possible), because this state is the best state for patients in the clinical trial (additional details are provided in the Appendix and in Appendix Fig A2 (online only).
For each patient, time with toxicity of treatment was defined as the number of days with grade ≥ 3 treatment-emergent adverse events (TEAEs) after random assignment and before disease progression or censoring for progression. An additional analysis was conducted in which time with toxicity of treatment was defined using grade ≥ 2 TEAEs of nausea, vomiting, fatigue, and asthenia only, because these TEAEs are frequently observed with rucaparib and other PARP inhibitors. Additional details on calculations are included in the Appendix.
The level of significance was set to 5%, and CIs were calculated using 2-sided bootstrap methods. No method to control for multiple testing was applied because this was an exploratory, post hoc analysis. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patients
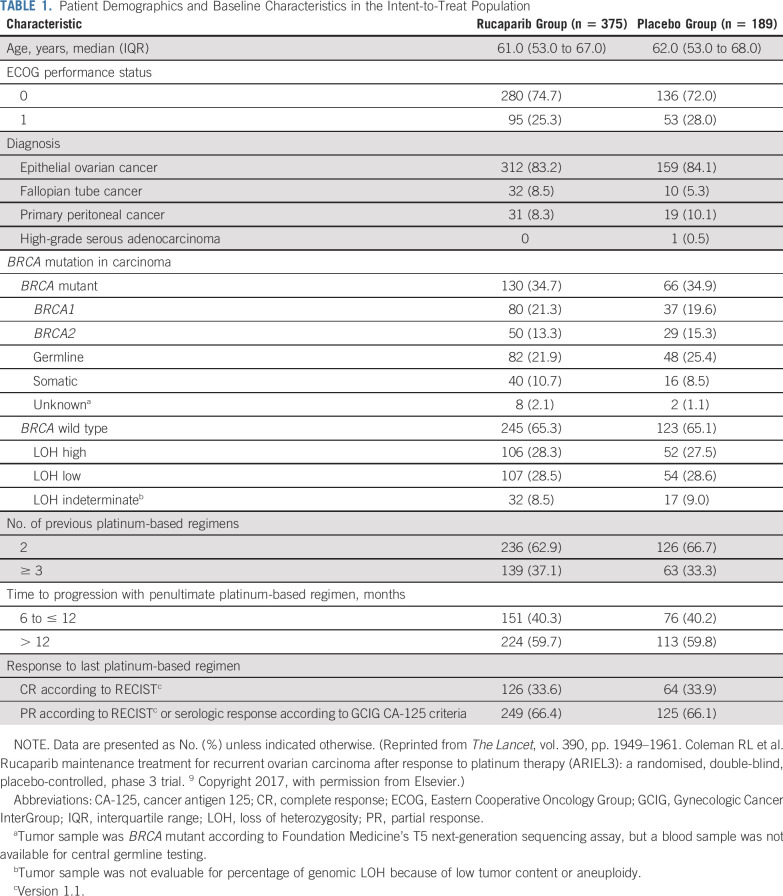
As reported previously,9 we randomly allocated 564 patients in ARIEL3: 375 (66%) to rucaparib and 189 (34%) to placebo. The safety population included 372 patients (99.2%) who received rucaparib (3 patients [0.8%] withdrew before receiving rucaparib), and 189 patients (100%) who received placebo. The analyses presented here used the primary efficacy data after unblinding (April 15, 2017, visit cutoff). Baseline characteristics were balanced between treatment groups (Table 1); full details have been reported previously.9
TABLE 1.
Patient Demographics and Baseline Characteristics in the Intent-to-Treat Population
Adverse Events
As of the April 15, 2017, visit cutoff, the most frequent TEAEs (reported in ≥ 35% of patients in either group) of any grade were nausea, asthenia/fatigue, dysgeusia, anemia/decreased hemoglobin concentration, constipation, and vomiting (Fig 1).9 The most frequent TEAEs of grade ≥ 3 (reported in ≥ 3% of patients) included anemia/hemoglobin decreased, alanine aminotransferase/aspartate aminotransferase increased, asthenia/fatigue, neutropenia/neutrophil count decreased, thrombocytopenia/platelet count decreased, vomiting, and nausea (Fig 1). The following TEAEs of interest were reported at grade ≥ 2: asthenia/fatigue (130 patients [34.9%] in the rucaparib group v 25 [13.2%] in the placebo group), nausea (108 [29.0%] v 12 [6.3%]), and vomiting (48 [12.9%] v 9 [4.8%]).
FIG 1.
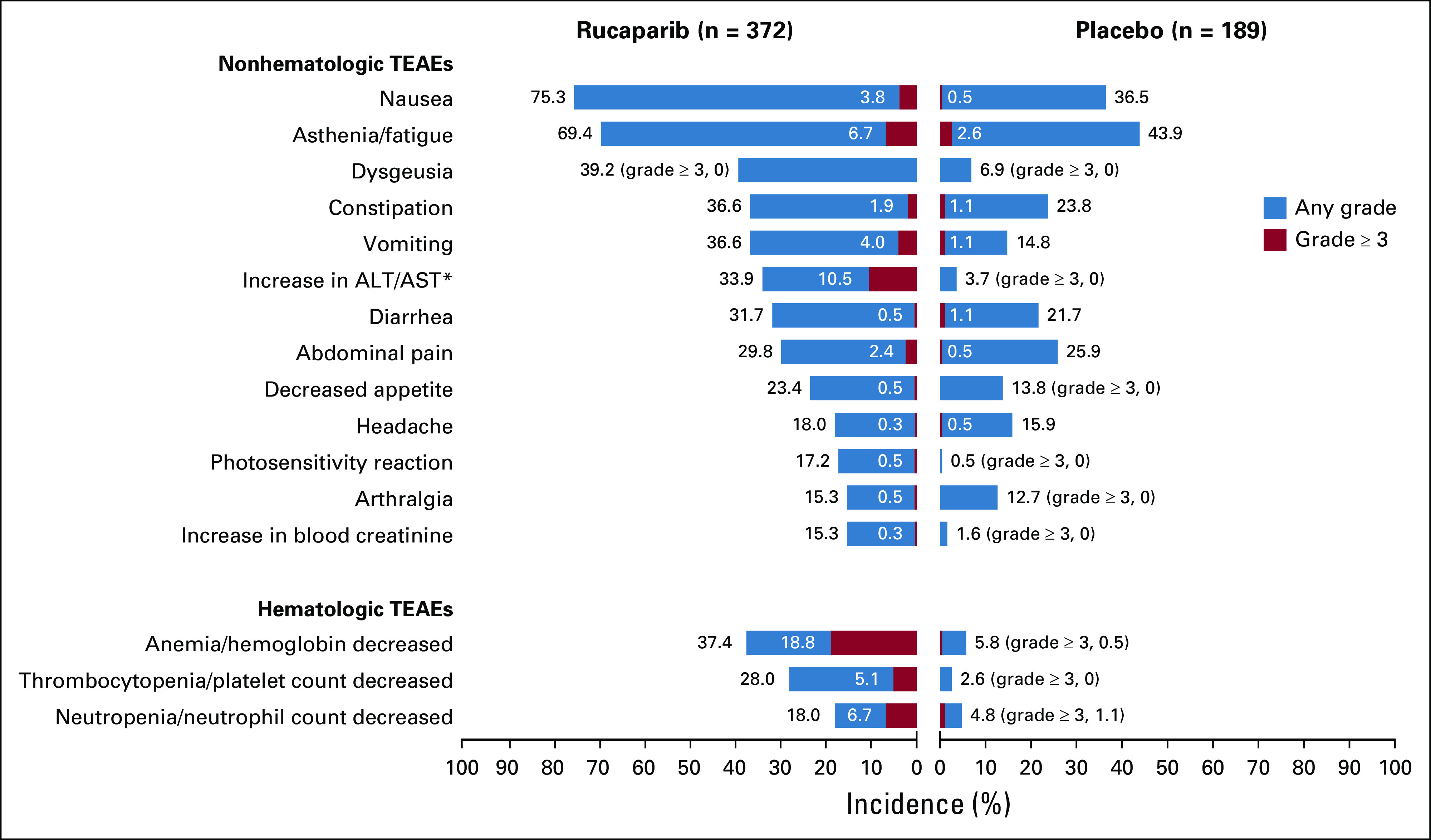
Most frequent treatment-emergent adverse events (TEAEs; reported in ≥ 35% of patients) in ARIEL3. (*) Elevations were transient, self-limiting, and not associated with other signs of liver toxicity. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Patient-Centered Outcomes
Details of EQ-5D-3L records included in this analysis are provided in the Appendix. In the ITT population, mean EQ-5D-3L index scores were relatively stable over the course of the study in both groups (Appendix Fig A3, online only); similar trends in mean EQ-5D-3L index scores were observed in all other analytical cohorts.
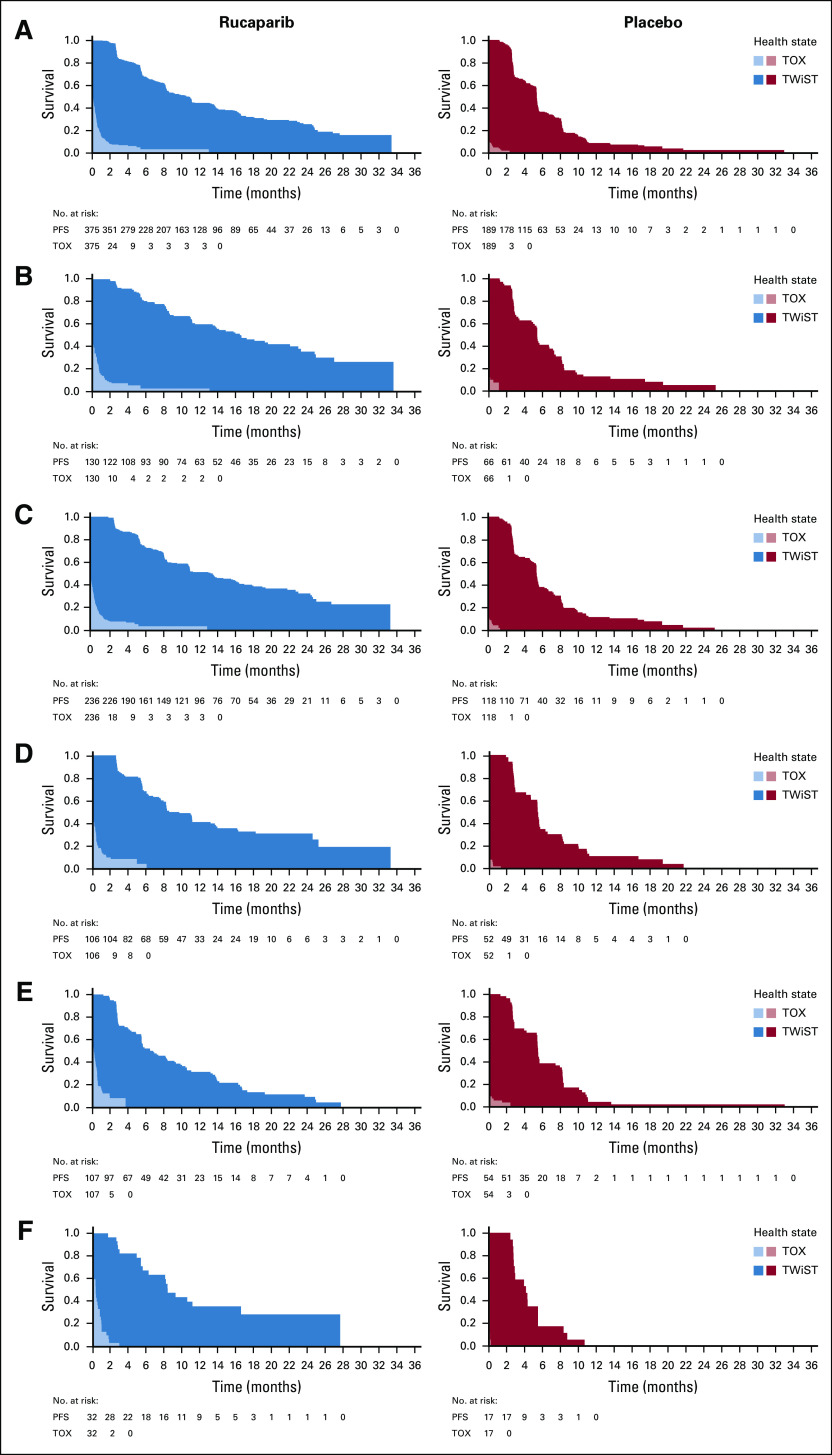
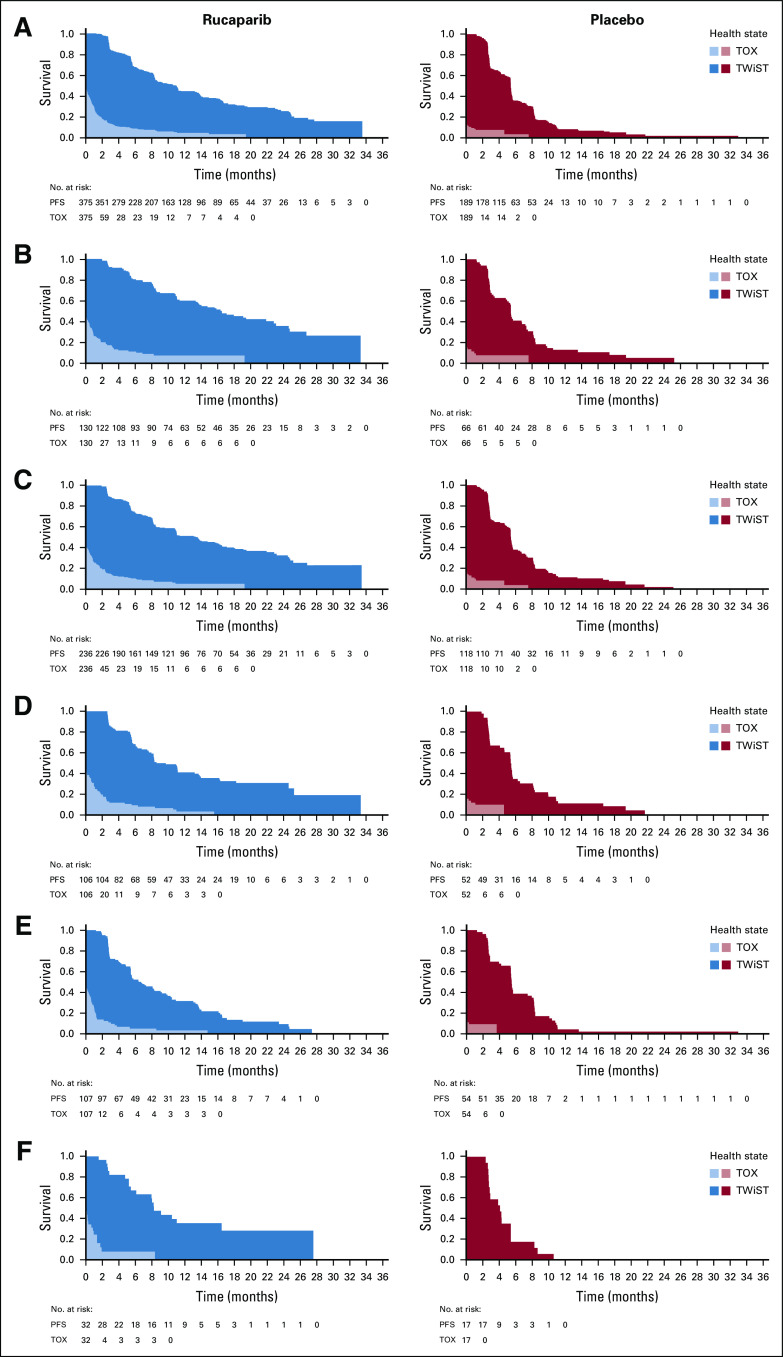
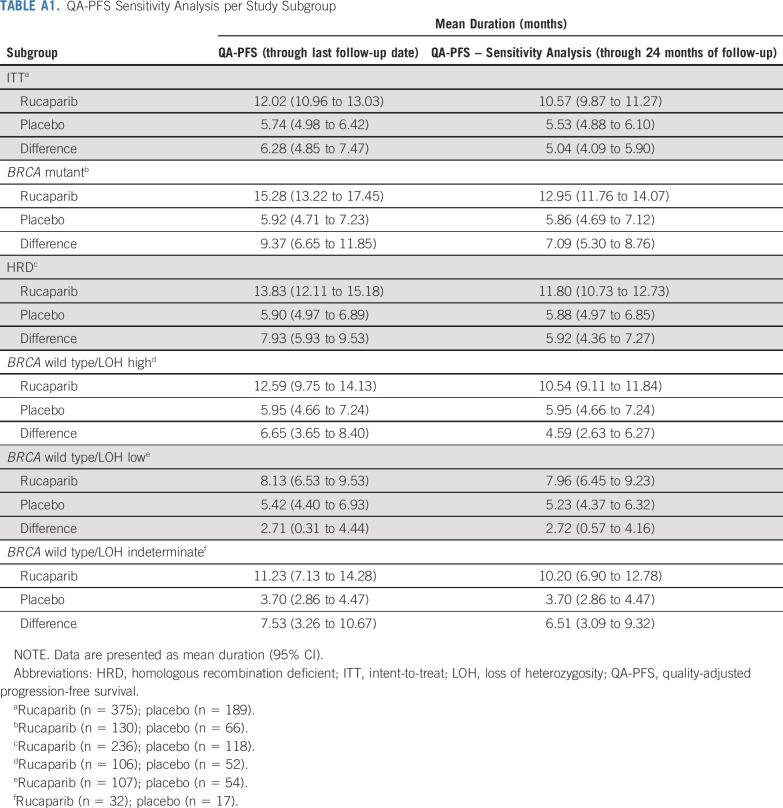
Mean QA-PFS was significantly longer in the rucaparib group than in the placebo group in the 3 prespecified, nested cohorts, with a mean difference of 6.28 months (95% CI, 4.85 to 7.47 months) in the ITT population (Fig 2A), 9.37 months (95% CI, 6.65 to 11.85 months) in the BRCA-mutant cohort (Fig 2B), and 7.93 months (95% CI, 5.93 to 9.53 months) in the HRD cohort (Fig 2C). Mean QA-PFS was also longer with rucaparib than with placebo in the BRCA wild-type/LOH high (difference, 6.65 months [95% CI, 3.65 to 8.40 months]), BRCA wild-type/LOH low (2.71 months [95% CI, 0.31 to 4.44 months]), and BRCA wild-type/LOH indeterminate (7.53 months [95% CI, 3.26 to 10.67 months]) patient subgroups (Figs 2D-2F). Consistent results were obtained in a sensitivity analysis of QA-PFS calculated based on 24 months of follow-up, with longer mean QA-PFS with rucaparib than with placebo in all prespecified cohorts and BRCA wild-type subgroups (Appendix Table A1, online only).
FIG 2.
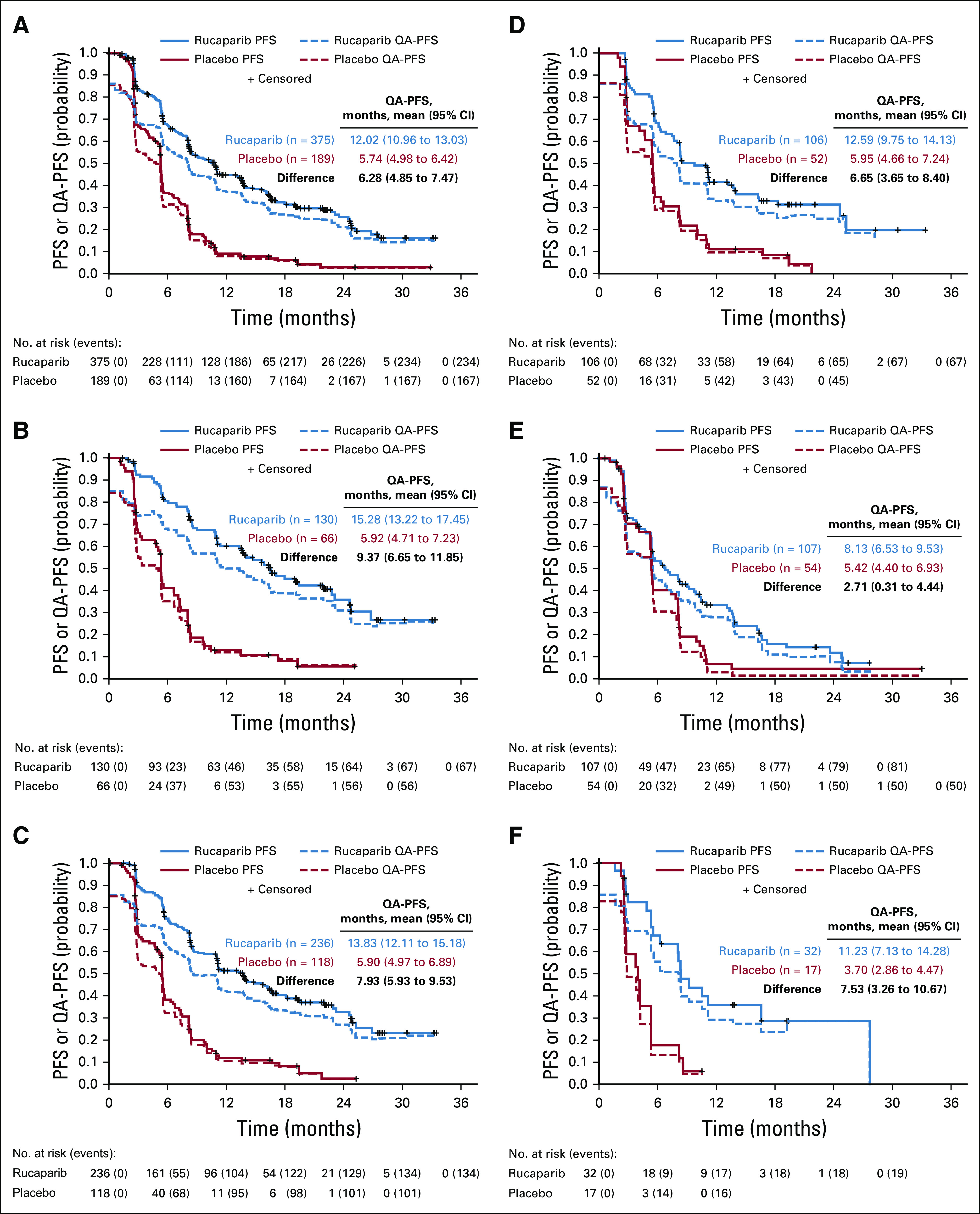
Quality-adjusted progression-free survival (QA-PFS) in the intent-to-treat population (A), BRCA-mutant cohort (B), homologous recombination deficient cohort (C), BRCA wild-type/loss of heterozygosity (LOH) high (D), BRCA wild-type/LOH low (E), and BRCA wild-type/LOH indeterminate (F) patient subgroups. Patients at-risk data are shown for the progression-free survival (PFS) analysis.
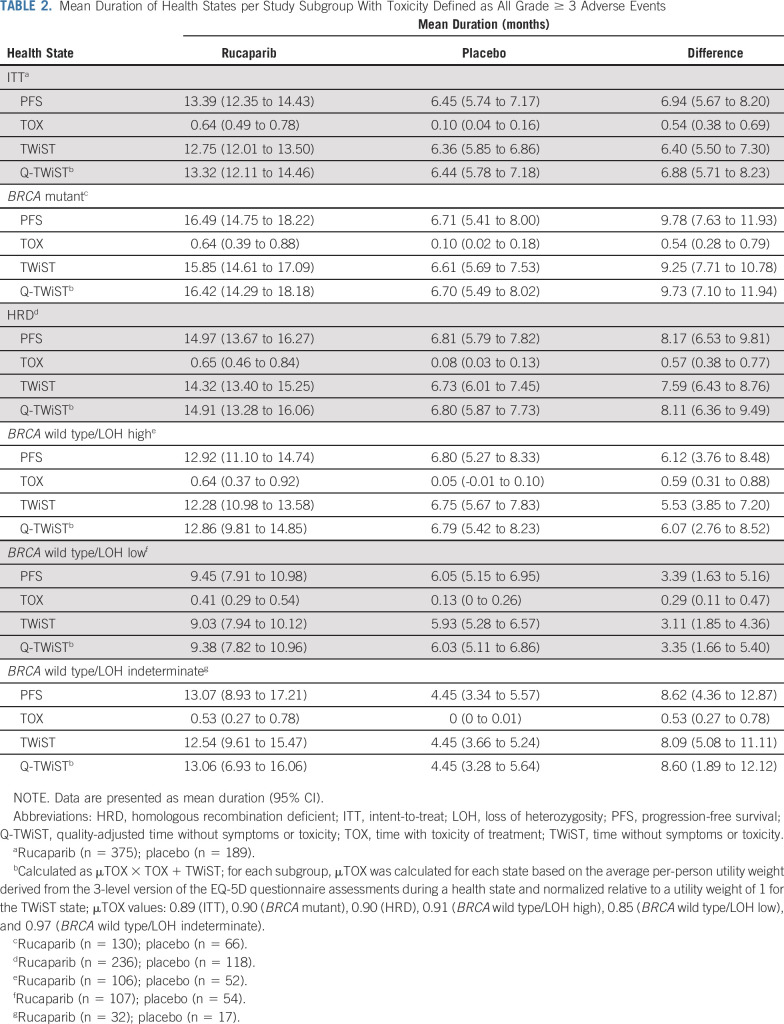
In the ITT population, mean PFS was significantly longer with rucaparib than with placebo (mean difference, 6.94 months [95% CI, 5.67 to 8.20 months]; Table 2). Although mean duration with grade ≥ 3 TEAEs (TOX state) was also significantly longer in the rucaparib group than in the placebo group (mean difference, 0.54 months [95% CI, 0.38 to 0.69 months]), mean TWiST remained significantly longer with rucaparib (mean difference, 6.40 months [95% CI, 5.50 to 7.30 months]; Table 2 and Fig 3). In the quality-adjusted analysis, the mean difference in mean Q-TWiST was 6.88 months (95% CI, 5.71 to 8.23 months; Table 2). In the BRCA-mutant and HRD cohorts, the difference in mean Q-TWiST was 9.73 months (95% CI, 7.10 to 11.94 months) and 8.11 months (95% CI, 6.36 to 9.49 months), respectively (Table 2). In the subgroups of patients with a BRCA wild-type ovarian carcinoma, Q-TWiST consistently favored rucaparib, with a mean difference of 6.07 months (95% CI, 2.76 to 8.52 months), 3.35 months (95% CI, 1.66 to 5.40 months), and 8.60 months (95% CI, 1.89 to 12.12 months) in patients with LOH high, LOH low, and LOH indeterminate, respectively (Table 2).
TABLE 2.
Mean Duration of Health States per Study Subgroup With Toxicity Defined as All Grade ≥ 3 Adverse Events
FIG 3.
Time without symptoms or toxicity (TWiST) analysis, with toxicity defined as all grade ≥ 3 treatment-emergent adverse events in the intent-to-treat population (A), BRCA-mutant cohort (B), homologous recombination deficient cohort (C), BRCA wild-type/loss of heterozygosity (LOH) high (D), BRCA wild-type/LOH low (E), and BRCA wild-type/LOH indeterminate (F) patient subgroups. PFS, progression-free survival. TOX, time with toxicity of treatment.
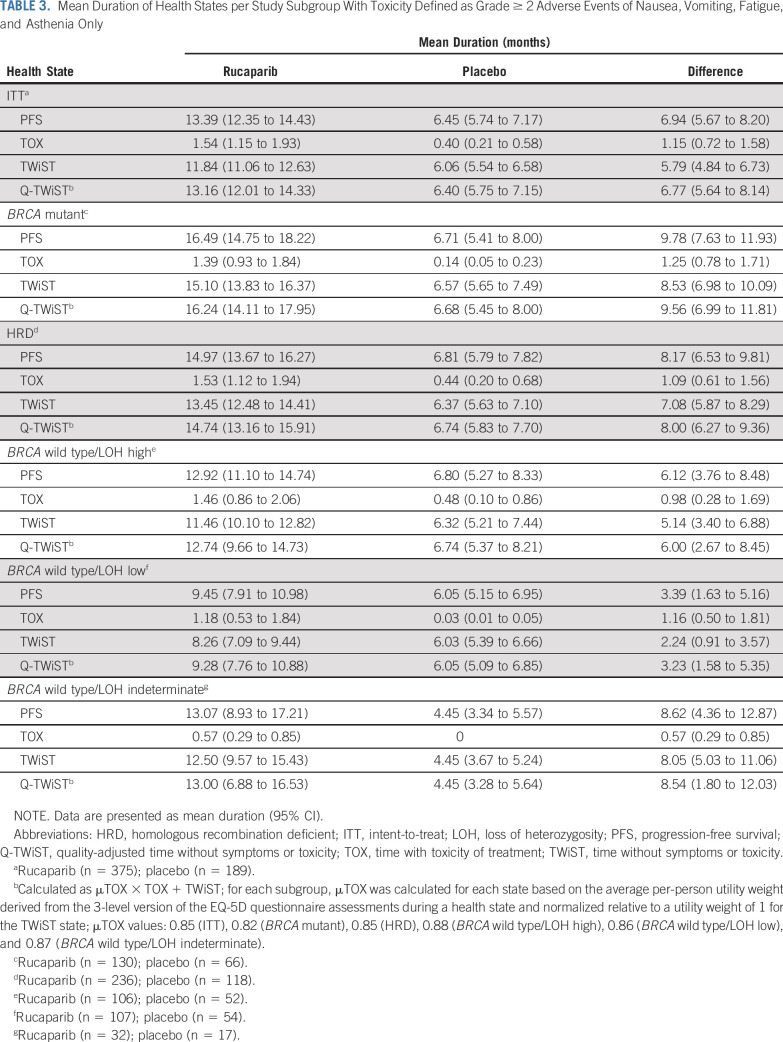
Q-TWiST analyses in which the TOX state was defined using grade ≥ 2 TEAEs of nausea, vomiting, fatigue, and asthenia were consistent with the Q-TWiST analyses in which the TOX state was defined as any grade ≥ 3 TEAEs. Outcomes favored rucaparib in all subgroups, as listed in Table 3 and Appendix Fig A4 (online only).
TABLE 3.
Mean Duration of Health States per Study Subgroup With Toxicity Defined as Grade ≥ 2 Adverse Events of Nausea, Vomiting, Fatigue, and Asthenia Only
DISCUSSION
By evaluating quality-adjusted survival, which incorporates assessments of quality and quantity of life, we demonstrated that rucaparib maintenance treatment provided significant benefit despite the impact of toxicities on patients’ health status during rucaparib treatment and that patients receiving rucaparib had longer periods without clinically relevant symptoms.
QA-PFS was 2.1-fold longer in the rucaparib group than in the placebo group among patients in the ITT population, and ranged from approximately 1.5-fold (BRCA wild type/LOH low) to 3.0-fold (BRCA wild type/LOH indeterminate) longer in the other analytical groups. This showed that, when weighted by patients’ perceptions of their health status, the PFS benefit of rucaparib persisted.
Results for Q-TWiST also consistently favored rucaparib over placebo in the ITT population and other analytical groups, ranging from approximately 1.5-fold (BRCA wild type/LOH low) to 2.9-fold (BRCA wild type/LOH indeterminate) longer durations of Q-TWiST in both the grade ≥ 3 and selected grade ≥ 2 TEAE-based analyses. The Q-TWiST results also indicate that rucaparib maintenance treatment extended the time in which patients had good health status or QoL without cancer-related symptoms, which is a key objective for patients.12,13
The QA-PFS and Q-TWiST findings together suggest that rucaparib maintenance treatment provides a broad clinical benefit to women with recurrent ovarian cancer. Notably, clinical benefit was observed in all cohorts analyzed, with the greatest benefit (ie, largest mean differences) observed in patients with a documented BRCA mutation. Analyses in the subgroups of patients with a BRCA wild-type carcinoma demonstrate that the benefits observed in the HRD cohort and the ITT population were not driven solely by the improvements in the BRCA-mutant and HRD cohorts. QA-PFS and Q-TWiST are able to align the impact of toxicity on patient outcomes with the time that toxicity is experienced by patients and, therefore, reflect more faithfully the overall experience of patients.
Other clinical trials of PARP inhibitors as second-line maintenance treatment of ovarian cancer have also assessed patient-centered outcomes using EQ-5D and toxicity data. In the SOLO2/ENGOT-Ov21 study comparing maintenance olaparib with placebo in women with platinum-sensitive, recurrent ovarian cancer and a BRCA1/2 mutation, QA-PFS was almost twice as long in the olaparib group (13.96 v 7.28 months; P < .0001). Based on TWiST analysis, patients who received olaparib also had approximately 2-fold longer survival with good health status than those who received placebo (15.03 v 7.70 months; P < .0001; toxicity defined as grade ≥ 2 TEAEs of nausea, vomiting, or fatigue).14 In the ENGOT-OV16/NOVA study, mean TWiST was at least 2-fold longer with niraparib than with placebo; the mean difference in TWiST was 2.95 years (35.4 months) in patients with a germline BRCA mutation and 1.34 years (16.1 months) in patients without a germline BRCA mutation (including patients with somatic BRCA mutations).15 The TWiST analysis in NOVA was limited to grade ≥ 2 TEAEs of nausea, vomiting, and fatigue. Furthermore, the NOVA analysis calculated mean PFS with extrapolated survival curves under the assumption that patients could remain progression free for up to 20 years. The differences in how each of these analyses were conducted demonstrate the need for consistency in reporting TWiST analyses in studies of maintenance therapies for recurrent ovarian cancer, to enable the results to be compared across clinical trials.
Patient-centered outcome assessments are particularly important as health-related QoL is of great importance to women with ovarian cancer because of the significant morbidity they experience as a result of the disease and its treatment.16 Indeed, organizations such as the GCIG, the Society of Gynecologic Oncology, the European Society for Gynaecological Oncology, and the European Society for Medical Oncology recognize that the benefits of PFS can be supported by QoL measures.4,17,18 New treatments that increase PFS may not be of sufficient value to patients with advanced-stage cancer unless they also convey tangible QoL benefits.19 Moreover, women with recurrent or advanced ovarian cancer may be willing to tolerate treatment toxicities if the goal is curative but may be less tolerant when the goal is a PFS benefit18; therefore, physicians and patients must carefully consider a wide variety of factors including expectations about efficacy, treatment toxicities, QoL, frequency of clinic visits and blood tests, and direct and indirect treatment costs when choosing whether to initiate maintenance therapy.20,21 Of particular note, physicians and patients must be aware of the potential trade-offs between quality and quantity of life,19,22 and data such as that presented here may be helpful in discussing this particular aspect. Equally important, disease relapse has a negative psychological and physical impact, with a subsequent deterioration in QoL, underlining the importance of prolonging time without recurrence or progression.23
The strengths of these analyses include the incorporation of a direct measurement of EQ-5D-3L (from which utility values were derived) and the consistency of outcomes favoring rucaparib over placebo in the context of a randomized clinical trial. The QA-PFS and Q-TWiST analyses did not rely on extrapolation or assumptions with respect to survival time, and the current analysis was conservative in that it penalized time with toxicities yet still showed results in PFS time similar to those in the original ITT analysis. The QA-PFS and Q-TWiST analyses consistently favored rucaparib even in subgroups of patients with BRCA wild-type carcinomas, a population in which clinical benefits are less pronounced than in those with BRCA-mutant carcinomas. Importantly, to our knowledge, Q-TWiST data have not been reported previously for PARP inhibitors in the maintenance setting for ovarian cancer, and the incorporation of quality-adjusted methodology in our analysis demonstrates the impact of patients’ perceptions of QoL on the TWiST analyses.
Limitations of this post hoc, retrospective analysis include the lack of adjustment for multiple analyses, the small sample sizes for some of the subgroup analyses, and the fact that the TWiST analysis with TOX defined as grade ≥ 2 TEAEs was restricted to nausea, vomiting, fatigue, and asthenia. Another limitation is that the analyses presented here are based on EQ-5D-3L and toxicity data, rather than on other QoL assessments, such as FOSI-18. Because EQ-5D-3L data were collected on the first day of each treatment cycle and were not designed to be collected during adverse events (AEs), EQ-5D-3L data were not available at the time of each AE. Therefore, our methods required the assumption of interpolation between 2 assessments to define values; for our analysis, a linear function was used. Thus, if only 1 assessment was available during the period of a patient’s TEAE, we assumed that the EQ-5D-3L value was constant for the duration of the TEAE. Some patients may also have had TEAEs that occurred between EQ-5D-3L assessments, resulting in missing data. Furthermore, the mean time difference estimates should be interpreted in the light of the maximum length of follow-up (eg, a mean difference of 6 months is not interpreted in the same way when the global timeframe of the analysis is approximately 30 months [as was the case here] as it would be for a follow-up duration of 10 years). In addition, the OS data for ARIEL3 were not mature at the time of these analyses and, therefore, could not be incorporated into the Q-TWiST analysis; however, the analysis could be repeated after OS maturation. Last, these results require confirmation in larger, prospective studies, which could include observational cohort studies that evaluate the effects of rucaparib maintenance therapy on QA-PFS in daily clinical practice.
In our analyses, rucaparib provided significant benefits to patient health status even when accounting for toxicities, as demonstrated by QA-PFS and Q-TWiST analyses. These benefits were observed in the ITT population and in subgroups of patients with a BRCA-mutant carcinoma and those with a BRCA wild-type carcinoma. Taken together, these findings demonstrated that rucaparib extended PFS in the maintenance setting without detrimental effects on patient health status.
ACKNOWLEDGMENT
We thank all the patients and their families and caregivers for their participation in ARIEL3 and the ARIEL3 investigators for their contributions to the administration and execution of the trial. Medical writing and editorial support funded by Clovis Oncology were provided by Nathan Yardley and Shannon Davis of Ashfield Healthcare Communications.
Appendix
Methods
Calculation of quality-adjusted progression-free survival.
Quality-adjusted progression-free survival (QA-PFS) was calculated as the product of the investigator-assessed progression-free survival function, obtained by Kaplan-Meier estimation up to the April 15, 2017, visit cutoff and the 3-level version of the EQ-5D questionnaire (EQ-5D-3L) index score function (flowchart in Appendix Fig A1). The EQ-5D-3L index score function was obtained by computation of the mean EQ-5D-3L index score of patients who were alive and uncensored at each visit scheduled in the double-blind treatment period. No adjustment was made for patient dropout, and there was no imputation in the EQ-5D-3L data. To create a quality-of-life function over continuous time, estimates of the mean EQ-5D-3L index score at each visit were connected assuming a linear change. Mean QA-PFS was obtained by computing the area under the quality-survival product function. The 95% CI for the mean QA-PFS in the rucaparib and placebo groups and for the difference between groups was computed using the bootstrap method,24 with 200 replications of the sample.
Calculation of quality-adjusted time without symptoms or toxicity.
Quality-adjusted time without symptoms or toxicity (Q-TWiST) was calculated as μTOX × TOX + TWiST. µTOX denotes the utility weight for the TOX state and was determined as described later in the Appendix (flowchart in Appendix Fig A2). The mean durations for the TOX and TWiST states were estimated by the area under each survival curve and calculated using Kaplan-Meier estimates. In Q-TWiST analyses based on all grade ≥ 3 TEAEs, time with toxicity for treatment of each patient was defined as the number of days with grade ≥ 3 treatment-emergent adverse events (TEAEs) after random assignment and before disease progression or censoring for progression. All grade ≥ 3 TEAEs before progression were included in the calculation of time with toxicity of treatment. If several adverse events (AEs) overlapped, the number of days was calculated between the start date of the first AE and the end date of the last AE. For analyses that were based on grade ≥ 2 TEAEs of nausea, vomiting, fatigue, and asthenia, the same methods were used for inclusion and treatment of overlap.
Determination of µTOX.
Observed utility data from the EQ-5D-3L, EuroQol’s 5-dimension questionnaire 3-level version, were incorporated in the Q-TWiST analysis. For each patient, the average utility weight derived from EQ-5D-3L assessments during a health state was assigned as a per-person utility weight for the TOX and TWiST states. The overall average utility was then calculated for each state to determine the µTOX utility weight for the TOX state, and the µTWiST utility weight for the TWiST state. The utility weight for the TOX state was then normalized relative to a utility weight of 1 (best possible utility weight) for the TWiST state.
Results
In the April 15, 2017, cut of the ARIEL3 trial data, a total of 5,503 EQ-5D-3L nonmissing records (4,042 from the rucaparib group; 1,461 from the placebo group) were analyzed. These comprised 5,084 records (3,796 rucaparib; 1,288 placebo) from a maximum of 39 treatment cycles, 245 records (144 rucaparib; 101 placebo) from the end of treatment, and 174 records (102 rucaparib; 72 placebo) from the day 28 follow-up visit after treatment discontinuation.
FIG A1.
Flowchart for calculation of quality-adjusted (QA) progression-free survival (PFS; QA-PFS).
FIG A2.
Flowchart for calculation of quality-adjusted time without symptoms or toxicity (TWiST; Q-TWiST). The mean time with symptoms of disease (REL state) was not included in these analyses because ARIEL3 OS data were not mature at the time of this analysis. AEs, adverse events; OS, overall survival; PFS, progression-free survival; TOX, time with toxicity of treatment.
FIG A3.
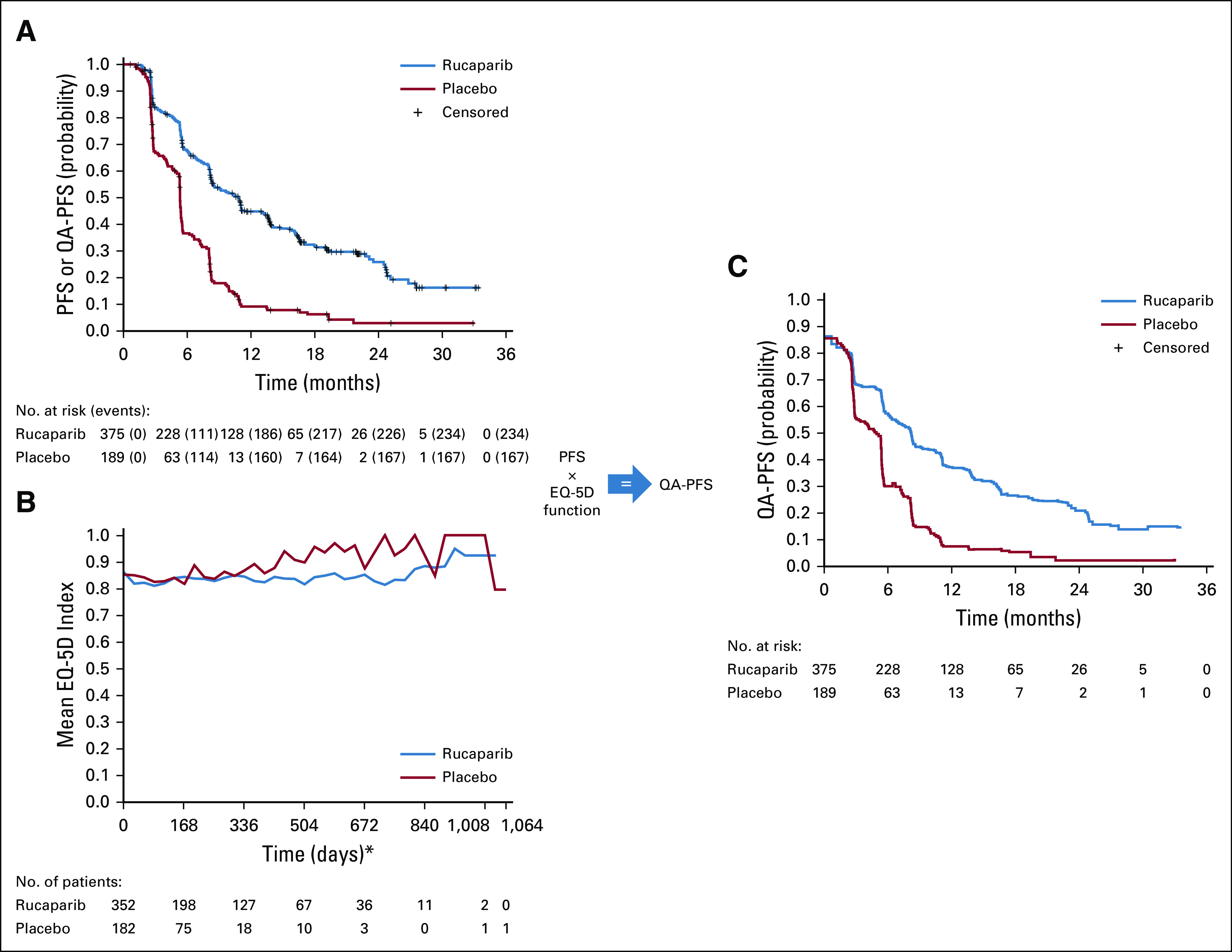
Quality-adjusted progression-free survival (QA-PFS) for the intent-to-treat population determined by multiplying the investigator-assessed progression-free survival (PFS) function (A) by the EQ-5D-3L index score function (B) to obtain a QA-PFS function (C). (*) EQ-5D-3L data were collected on day 1 of each 28-day treatment cycle. PFS, progression-free survival; QA-PFS, quality-adjusted progression-free survival.
FIG A4.
Time without symptoms or toxicity (TWiST) analysis with toxicity defined as grade ≥ 2 treatment-emergent adverse events of nausea, vomiting, fatigue, and asthenia only in the intent-to-treat population (A), BRCA-mutant cohort (B), homologous recombination deficient cohort (C), BRCA wild-type/loss of heterozygosity (LOH) high (D), BRCA wild-type/LOH low (E), and BRCA wild-type/LOH indeterminate (F) patient subgroups. TOX, time with toxicity of treatment.
TABLE A1.
QA-PFS Sensitivity Analysis per Study Subgroup
PRIOR PRESENTATION
Presented at the International Society for Pharmacoeconomics and Outcomes Research 24th International Meeting, May 20, 2019, New Orleans, LA.
SUPPORT
Supported by Clovis Oncology. Additional support was provided in part by the Ann Rife Cox Chair in Gynecology and the Judy Reis/Albert Pisani, MD, Ovarian Cancer Research Fund (to R.L.C.) and by the National Institute of Health Research Biomedical Research Centre at University College London (to J.A.L.). C.A. is supported in part by Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748. Funding was also provided by US Department of Defense Ovarian Cancer Research Program OC120506, a V Foundation Translational Award, and a Stand Up To Cancer–Ovarian Cancer Research Fund Alliance–National Ovarian Cancer Coalition Dream Team Translational Research Grant (Grant No. SU2C-AACR-DT16–15; all to E.M.S.). Stand Up to Cancer is a program of the Entertainment Industry Foundation; research grants are administered by the American Association for Cancer Research, a scientific partner of Stand Up To Cancer.
NOTEA.-C.M. and J.B. have declared their affiliations based on the time during which the analyses were conducted.
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth M. Swisher, David Cella, Sandra Goble, Terri Cameron, Josh Bedel, Jonathan A. Ledermann, Robert L. Coleman
Provision of study material or patients: Amit M. Oza, Domenica Lorusso, Carol Aghajanian, Ana Oaknin, Andrew Dean, Nicoletta Colombo, Johanne I. Weberpals, Andrew R. Clamp, Giovanni Scambia, Alexandra Leary, Robert W. Holloway, Margarita Amenedo Gancedo, Peter C. Fong, Jeffrey C. Goh, David M. O’Malley, Deborah K. Armstrong, Susana Banerjee, Jesus García-Donas, Elizabeth M. Swisher, Jonathan A. Ledermann, and Robert L. Coleman.
Collection and assembly of data: Amit M. Oza, Domenica Lorusso, Carol Aghajanian, Ana Oaknin, Andrew Dean, Nicoletta Colombo, Johanne I. Weberpals, Andrew R. Clamp, Giovanni Scambia, Alexandra Leary, Robert W. Holloway, Margarita Amenedo Gancedo, Peter C. Fong, Jeffrey C. Goh, David M. O’Malley, Deborah K. Armstrong, Susana Banerjee, Jesus García-Donas, Elizabeth M. Swisher, Juliette Meunier, Sandra Goble, Josh Bedel, Jonathan A. Ledermann, Robert L. Coleman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient-Centered Outcomes in ARIEL3, a Phase III, Randomized, Placebo-Controlled Trial of Rucaparib Maintenance Treatment in Patients With Recurrent Ovarian Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Amit M. Oza
Uncompensated Relationships: Ozmosis Research
Domenica Lorusso
Honoraria: Roche, AstraZeneca, Tesaro, Clovis Oncology, Merck, Genmab, Immunogen
Consulting or Advisory Role: PharmaMar
Speakers' Bureau: AstraZeneca, Clovis Oncology, Tesaro, PharmaMar
Research Funding: PharmaMar (Inst), Clovis Oncology (Inst), Tesaro (Inst), Merck (Inst)
Expert Testimony: Clovis Oncology
Travel, Accommodations, Expenses: Tesaro, Roche, PharmaMar, AstraZeneca, Clovis Oncology
Carol Aghajanian
Consulting or Advisory Role: Tesaro, Mersana, Eisai, Roche, AbbVie
Research Funding: Genentech/Roche (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
Ana Oaknin
Consulting or Advisory Role: Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab
Research Funding: AbbVie Deutschland (Inst), Ability Pharmaceuticals (Inst), Advaxis (Inst), Aeterna Zentaris (Inst), Amgen (Inst), Aprea Therapeutics (Inst), Clovis Oncology (Inst), Eisai (Inst), Hoffmann La Roche (Inst), Regeneron Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Andrew Dean
Travel, Accommodations, Expenses: Novartis
Nicoletta Colombo
Honoraria: Roche/Genentech, AstraZeneca, Tesaro, PharmaMar
Consulting or Advisory Role: Roche/Genentech, PharmaMar, AstraZeneca, Clovis Oncology, Pfizer, MSD Oncology, Takeda, Tesaro, BioCad, GlaxoSmithKline
Johanne I. Weberpals
Consulting or Advisory Role: AstraZeneca
Research Funding: AstraZeneca
Andrew R. Clamp
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Speakers' Bureau: Clovis Oncology
Research Funding: AstraZeneca (Inst), Clovis Oncology (Inst), Millennium (Inst), Pfizer (Inst), Immunogen (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Tesaro
Giovanni Scambia
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, PharmaMar, Roche, Tesaro
Speakers' Bureau: Clovis Oncology Italy, MSD Italia
Alexandra Leary
Honoraria: AstraZeneca, Clovis Oncology
Consulting or Advisory Role: Clovis Oncology (Inst), AstraZeneca (Inst), Tesaro (Inst), BioCad, Gritstone Oncology, Seattle Genetics, Ability Pharma (Inst), MSD (Inst), GlaxoSmithKline (Inst), Merck Serono (Inst)
Research Funding: Merus (Inst), GamaMabs Pharma (Inst), Inivata (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Tesaro
Robert W. Holloway
Consulting or Advisory Role: Clovis Oncology, AbbVie
Speakers' Bureau: AstraZeneca, Clovis Oncology, Tesaro, Bard/Davol
Margarita Amenedo Gancedo
Consulting or Advisory Role: Clovis Oncology
Speakers' Bureau: AstraZeneca, PharmaMar, Roche, Tesaro
Travel, Accommodations, Expenses: Roche, Lilly
Peter C. Fong
Consulting or Advisory Role: MSD, Pfizer
Travel, Accommodations, Expenses: Pfizer
Jeffrey C. Goh
Stock and Other Ownership Interests: Immutep
Honoraria: Ipsen Australia
Consulting or Advisory Role: Tesaro, AstraZeneca
Speakers' Bureau: Novartis, Ipsen, Janssen, Mundipharma, AstraZeneca India
Travel, Accommodations, Expenses: Astellas Pharma, AstraZeneca
David M. O'Malley
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Clovis Oncology, Tesaro, Novocure, AbbVie, Genentech/Roche, OncoQuest, Immunogen, GOG Foundation, Translational Genomics/Cordgenics, Agenus, Marker Therapeutics, Eisai, Genelux, Iovance Biotherapeutics, Ambry Genetics, Tarveda Therapeutics, Leap Therapeutics, Myriad Genetics, GlaxoSmithKline, Regeneron
Research Funding: Amgen (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Regeneron (Inst), Immunogen (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Immunogen (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Array BioPharma (Inst), Bristol Myers Squibb (Inst), Agenus (Inst), Tesaro (Inst), Tracon Pharma (Inst), Genmab (Inst), Seattle Genetics (Inst), Iovance Biotherapeutics (Inst), Leap Therapeutics (Inst), Merck (Inst), AbbVie/Stemcentrx (Inst), AbbVie (Inst)
Deborah K. Armstrong
Consulting or Advisory Role: Cue Biopharma, AbbVie, Eisai
Research Funding: Clovis Oncology (Inst), AstraZeneca (Inst), Advaxis (Inst), Syndax (Inst), Pfizer (Inst), Tesaro (Inst), Eisai (Inst)
Other Relationship: AstraZeneca
Susana Banerjee
Honoraria: Roche
Consulting or Advisory Role: AstraZeneca/MedImmune, Tesaro, Clovis Oncology, Merck, Seattle Genetics, Genmab, Carrick Therapeutics (Inst), Amgen, Roche, GlaxoSmithKline, MSD Oncology
Research Funding: AstraZeneca (Inst), Janssen-Cilag (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: NuCana BioMed, AstraZeneca
Jesús García-Donas
Consulting or Advisory Role: Bristol Myers Squibb, Clovis Oncology
Speakers' Bureau: Roche, Bristol Myers Squibb, AstraZeneca, PharmaMar, GlaxoSmithKline, Amgen, Clovis Oncology, Janssen-Cilag
Research Funding: Pfizer, Bristol Myers Squibb, Roche, AstraZeneca, Merck, GamaMabs, InvitroCue
Travel, Accommodations, Expenses: Roche
Elizabeth M. Swisher
Leadership: Ideaya Biosciences
David Cella
Stock and Other Ownership Interests: FACIT.org
Consulting or Advisory Role: AbbVie, GlaxoSmithKline, Pfizer, Astellas Pharma, Novartis, PledPharma, IDDI, Bristol Myers Squibb, Asahi Kasei Pharma, Ipsen, Mei Pharma
Research Funding: Novartis (Inst), Genentech (Inst), Ipsen (Inst), Pfizer (Inst), Bayer (Inst), GlaxoSmithKline (Inst), PledPharma (Inst), Bristol Myers Squibb (Inst), AbbVie (Inst), Regeneron (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Ipsen, PledPharma
Juliette Meunier
Employment: Modus Outcomes
Sandra Goble
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Teresa Cameron
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Patents, Royalties, Other Intellectual Property: Patent application submitted January 2020 for dosing methodology (Inst)
Lara Maloney
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Ann-Christin Mörk
Employment: Clovis Oncology, UCB Pharma
Stock and Other Ownership Interests: UCB Pharma, Clovis Oncology
Josh Bedel
Employment: Clovis Oncology
Stock and Other Ownership Interests: Clovis Oncology
Travel, Accommodations, Expenses: Clovis Oncology
Jonathan A. Ledermann
Honoraria: AstraZeneca/MedImmune
Consulting or Advisory Role: AstraZeneca/MedImmune, Clovis Oncology, Pfizer, Cristal Therapeutics, Artios, Seattle Genetics, Tesaro, Merck, Eisai
Speakers' Bureau: Clovis Oncology, Pfizer, Tesaro/GlaxoSmithKline
Research Funding: AstraZeneca (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Clovis Oncology
Other Relationship: Regeneron
Robert L. Coleman
Consulting or Advisory Role: Clovis Oncology, Genentech/Roche, Esperance Pharmaceuticals, AstraZeneca/MedImmune, Genmab, Tesaro, OncoMed, Sotio, Oncolytics, AbbVie/Stemcentrx, Immunogen, AbbVie, Agenus, OncoSec, Novocure
Research Funding: AstraZeneca/MedImmune, Esperance Pharmaceuticals, OncoMed, Array BioPharma, Clovis Oncology, Johnson & Johnson, Merck, Roche/Genentech, Abbott/AbbVie
Travel, Accommodations, Expenses: Merck, AstraZeneca/MedImmune, Array BioPharma, Clovis Oncology, Roche/Genentech, Research to Practice, GOG, Clovis Oncology, Sotio, Vaniam Group
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23:2605–2612. doi: 10.1093/annonc/mds203. [DOI] [PubMed] [Google Scholar]
- 2.Bouberhan S, Pujade-Lauraine E, Cannistra SA. Advances in the management of platinum-sensitive relapsed ovarian cancer. J Clin Oncol. 2019;37:2424–2436. doi: 10.1200/JCO.19.00314. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 4.Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30:672–705. doi: 10.1093/annonc/mdz062. [DOI] [PubMed] [Google Scholar]
- 5.Chase DM, Wenzel L. Health-related quality of life in ovarian cancer patients and its impact on clinical management. Expert Rev Pharmacoecon Outcomes Res. 2011;11:421–431. doi: 10.1586/erp.11.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiSilvestro P, Alvarez Secord A. Maintenance treatment of recurrent ovarian cancer: Is it ready for prime time? Cancer Treat Rev. 2018;69:53–65. doi: 10.1016/j.ctrv.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Rubraca (rucaparib) tablets. Boulder, CO,: Clovis Oncology, Inc; 2020. [prescribing information] [Google Scholar]
- 8. Rubraca (rucaparib) tablets [summary of product characteristics]. Swords, Ireland, Clovis Oncology Ireland, Ltd, 2019.
- 9.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billingham LJ, Abrams KR, Jones DR. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess. 1999;3:1–152. [PubMed] [Google Scholar]
- 11.Cabarrou B, Boher JM, Bogart E, et al. How to report toxicity associated with targeted therapies? Ann Oncol. 2016;27:1633–1638. doi: 10.1093/annonc/mdw218. [DOI] [PubMed] [Google Scholar]
- 12.Oskay-Özcelik G, Alavi S, Richter R, et al. Expression III: Patients’ expectations and preferences regarding physician-patient relationship and clinical management-results of the international NOGGO/ENGOT-ov4-GCIG study in 1830 ovarian cancer patients from European countries. Ann Oncol. 2018;29:910–916. doi: 10.1093/annonc/mdy037. [DOI] [PubMed] [Google Scholar]
- 13. Rohr I, Keller M, Chekerov R, et al: What are the expectations and preferences of patients with ovarian cancer to a maintenance therapy? A NOGGO/ENGOT-OV22 survey (EXPRESSION IV) in 2101 patients. Presented at the European Gynaecological Oncology Congress. Vienna, Austria, November 4-7, 2017. [Google Scholar]
- 14.Friedlander M, Gebski V, Gibbs E, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): A placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018;19:1126–1134. doi: 10.1016/S1470-2045(18)30343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matulonis UA, Walder L, Nøttrup TJ, et al. Niraparib maintenance treatment improves time without symptoms or toxicity (TWiST) versus routine surveillance in recurrent ovarian cancer: A TWiST analysis of the ENGOT-OV16/NOVA trial. J Clin Oncol. 2019;37:3183–3191. doi: 10.1200/JCO.19.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson MK, Mercieca-Bebber R, Friedlander M. A practical guide to understanding, using and including patient reported outcomes in clinical trials in ovarian cancer. J Gynecol Oncol. 2018;29:e81. doi: 10.3802/jgo.2018.29.e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joly F, Hilpert F, Okamoto A, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: Recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138. doi: 10.1016/j.ejca.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Herzog TJ, Armstrong DK, Brady MF, et al. Ovarian cancer clinical trial endpoints: Society of Gynecologic Oncology white paper. Gynecol Oncol. 2014;132:8–17. doi: 10.1016/j.ygyno.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallowfield LJ, Fleissig A. The value of progression-free survival to patients with advanced-stage cancer. Nat Rev Clin Oncol. 2011;9:41–47. doi: 10.1038/nrclinonc.2011.156. [DOI] [PubMed] [Google Scholar]
- 20.Minion LE, Coleman RL, Alvarez RD, et al. Endpoints in clinical trials: What do patients consider important? A survey of the Ovarian Cancer National Alliance. Gynecol Oncol. 2016;140:193–198. doi: 10.1016/j.ygyno.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 21.Frey MK, Ellis AE, Koontz LM, et al. Ovarian cancer survivors’ acceptance of treatment side effects evolves as goals of care change over the cancer continuum. Gynecol Oncol. 2017;146:386–391. doi: 10.1016/j.ygyno.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Hilpert F, Du Bois A. Patient-reported outcomes in ovarian cancer: Are they key factors for decision making? Expert Rev Anticancer Ther. 2018;18:3–7. doi: 10.1080/14737140.2018.1516146. [DOI] [PubMed] [Google Scholar]
- 23.Colombo N, Lorusso D, Scollo P. Impact of recurrence of ovarian cancer on quality of life and outlook for the future. Int J Gynecol Cancer. 2017;27:1134–1140. doi: 10.1097/IGC.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene WH. Econometric Analysis. ed 5) Upper Saddle River, NJ: Prentice Hall; 2003. [Google Scholar]